Abstract
Gestational diabetes mellitus (GDM) prevalence is higher among racially/ethnically diverse groups compared to non-Hispanic white populations. While race has been shown to modify other cardiovascular disease risk factors in postpartum women, the role of race/ethnicity on GDM and subsequent hypertension has yet to be examined. The aim of this study was to evaluate the impact of race/ethnicity in relation to GDM and subsequent hypertension in a retrospective analysis of women who delivered at Massachusetts General Hospital from 1998–2007. Multivariate analyses were used to determine the associations between GDM and 1) race/ethnicity; 2) hypertension; and 3) the interaction with hypertension and race/ethnicity. Women were followed for a median of 3.8 years from the date of delivery. In our population of 4,010 women, GDM was more common among non-white participants (p < 0.0001). GDM was also associated with hypertension subsequent to delivery after adjusting for age, race, parity, first-trimester systolic blood pressure, BMI, maternal gestational weight gain, and preeclampsia (HR 1.75, 95% CI 1.28–2.37; p = 0.0004). Moreover, Hispanic (HR 3.25, 95% CI 1.85–5.72; p < 0.0001) and white (1.68, 95% CI 1.10–2.57; p = 0.02) women with GDM had greater hypertension risk relative to their race/ethnicity-specific counterparts without GDM in race-stratified multivariable analyses. In conclusion, Hispanic women compared to white women have an increased risk of hypertension. Hispanic and white women with GDM are at a greater risk for hypertension compared to those without GDM. Because the current study may have had limited power to detect effects among black and Asian women with GDM, further research is warranted to elucidate the need for enhanced hypertension risk surveillance among these young women.
Key terms: gestational diabetes mellitus, race/ethnicity, hypertension
Several studies report that gestational diabetes mellitus (GDM) prevalence is higher among racially/ethnically diverse populations compared to non-Hispanic white populations.(1–2) For example, the prevalence of GDM among women in the United States has been reported as 7.4% among Asian, 3.9% among Hispanic, 3.1% among non-Hispanic black, and 2.4% among non-Hispanic white populations.(3) Although race has been shown to modify other cardiovascular disease (CVD) risk factors such as type 2 diabetes (4) and the metabolic syndrome,(5) the role of race/ethnicity in mediating the relationship between GDM and hypertension risk has not been fully examined. Consequently, our investigation tests the hypothesis that a population of women with clinically-confirmed GDM will demonstrate an increased risk of future hypertension, independent of the development of type 2 diabetes. Furthermore, we hypothesize that, among non-diabetic women with a history of GDM, the association between GDM and hypertension will be greater among non-white compared to white women.
Methods
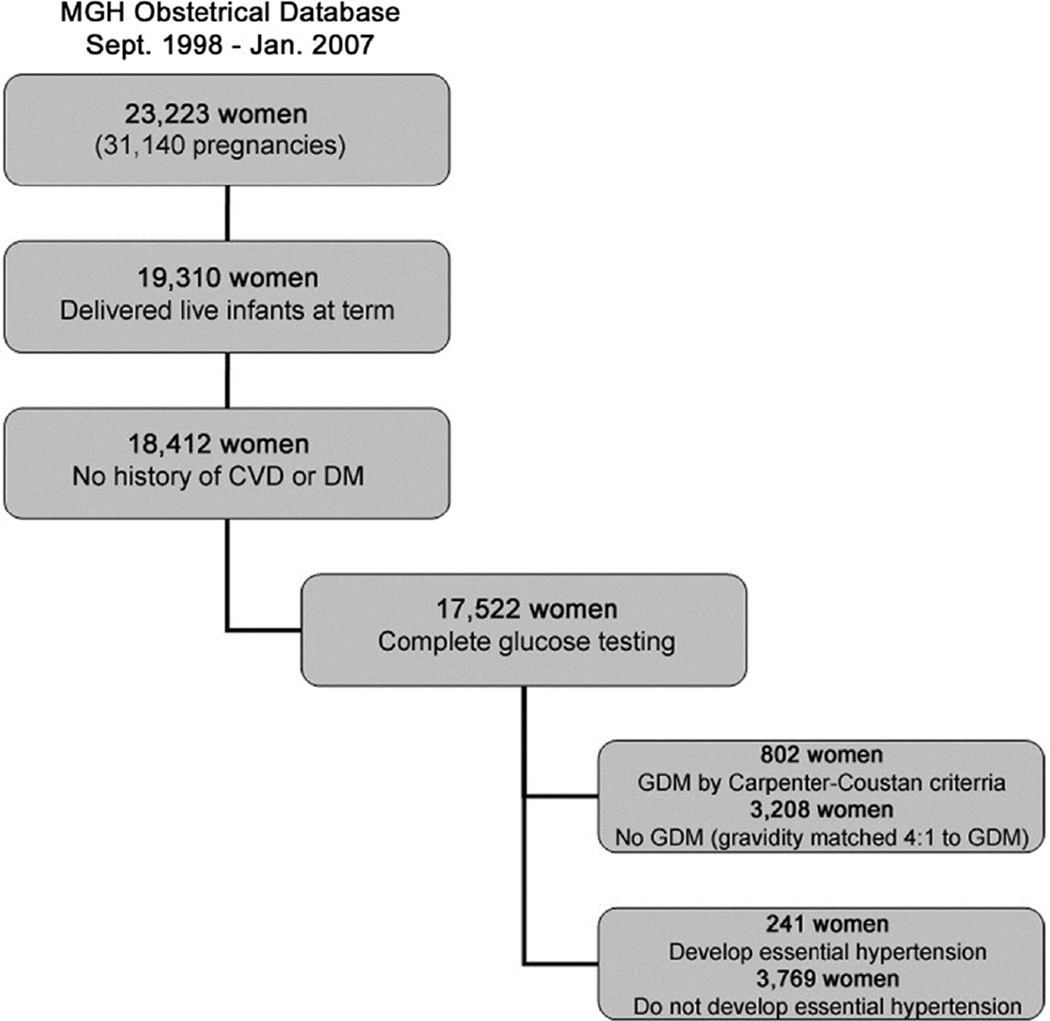
We conducted a retrospective study of women who presented for prenatal care to the Massachusetts General Hospital (MGH) Obstetrical Department between September 1998 and January 2007. These 23,223 women (representing 31,140 pregnancies) were subsequently included in the study population if they delivered full-term (gestational age ≥37 weeks) live infants (n=19,310); had no history of pregestational diabetes; had no history of CVD prior to delivery, including cerebrovascular disease, ischemic heart disease, or hypertension as identified by medical record review and ICD-9 codes (n=18,412); and had complete third trimester (24–28 weeks gestational age) biochemical data to diagnose GDM, including a 1-hour glucose load test and a 3-hour oral glucose tolerance test with complete data available (n=17,522; Figure 1). Race/ethnicity was obtained by self-report and was categorized during this study period as singularly white, black, Asian, Hispanic, or other. Institutional review board approval was granted by the Partners Human Research Committee (PHRC) prior to initiating the study. All study participants provided informed written consent to allow Partners Healthcare to use their health information for PHRC-approved research.(6)
Figure 1. Study Population Flow Diagram.
We began with a study population of 23,223 women and after including only those women with who delivered live-born, term infants at term (n=19,310) and had no history of cardiovascular disease or diabetes prior to delivery (n=18,412), we were left with 802 women with gestational diabetes mellitus and 241 women who developed hypertension. CVD = cardiovascular disease; DM = diabetes; GDM = gestational diabetes mellitus.
Women were diagnosed with GDM by a 1-hour glucose load test value ≥7.8 mmol/L (140 mg/dl) and 2 abnormal values on a 3-hour 100-gram glucose tolerance test using Carpenter-Coustan criteria.(7) In the event that the participant had multiple pregnancies, we selected the first pregnancy with confirmed GDM in order to eliminate violations of independence for statistical testing. To examine the implications of eliminating repeated pregnancies, a sensitivity analysis using generalized estimating equations was performed utilizing all pregnancies. Univariate and multivariable Cox proportional hazards models predicting hypertension were then repeated and we found that the hazard ratios differed by <5% and had significantly overlapping confidence intervals (results not shown). Our Cox proportional hazards models did not use a marginal approach, which is normally used for multiple time-to-event analysis where each event is dependent on each other. Of note, the GDM participant may have had pregnancies before or after the selected pregnancy without diagnosed GDM, but this was not considered an exclusion or inclusion criterion for selection. For women without a pregnancy history of GDM and with multiple pregnancies, one pregnancy was randomly selected for each mother and they were matched on maternal gravidity to women with GDM in a 4:1 ratio in order to optimize statistical precision.(8)
The primary outcome, hypertension identified by the International Classification on Diseases, 9th Revision, Clinical Modification (ICD-9-CM) code 401.xx, was obtained from electronic medical records comprised of inpatient and outpatient data. Progress notes and other narrative sources of information were not accessed for the purposes of this study. Prior data supports the use of ICD-9 codes for identifying cardiovascular and stroke risk factors with a positive predictive value for hypertension (401.x—405.x; 437.2) of 0.97 and negative predictive value of 0.52, suggesting that hypertension may be ruled in but may not be ruled out when using only ICD-9-CM codes.(9)
Baseline measurements for height, weight, and seated blood pressure were performed and pregnancy history, including gravidity information, was captured at the first prenatal visit for each participant. Body mass index (BMI) was calculated as weight (kg)/height (m2) from the first prenatal visit measurements. Gestational weight gain was calculated as the difference from the initial first trimester prenatal visit weight to the third trimester prenatal visit weight measured proximate to delivery. Lipid profiles and smoking history data were abstracted through electronic medical record review of each woman’s encounter with the MGH system within one year after delivery. Length of follow-up was calculated from time of delivery to the last visit encounter at MGH.
Characteristics of the study population were described by % (n) for categorical variables and mean ± standard deviation of the mean for continuous variables. Independent samples t-tests and Chi-Squared tests were used to compare demographic and clinical characteristics by outcome and exposure status. One-way analysis of variance was used to summarize race- and GDM-stratified characteristics. Multiple comparisons were performed as necessary using post-hoc adjustments. Follow-up time was summarized using medians. Multivariable Cox proportional hazards models examined the development of hypertension with terms for GDM, age, baseline systolic blood pressure and BMI, parity, race, maternal gestational weight gain, and preeclampsia. Covariates were selected based on statistical significance in age-adjusted analyses. To examine effect modification between GDM and race, race-stratified Cox proportional hazards models were performed. Multivariable, race-stratified models retained the same terms as overall models except for race. Women were censored if they developed type 2 diabetes prior to the development of hypertension in order to minimize confounding. If no hypertension developed, women were censored at the time of their last MGH encounter. The assumptions of proportional hazards and linearity of continuous covariates were inspected for all final models. A sensitivity analysis was conducted with a total of 5 imputations using the Markov Chain Monte Carlo (MCMC) method (10) to account for any differential loss of data for certain minorities. Race, age, parity, systolic blood pressure, weight gain, BMI and preeclampsia status were used to impute data for all missing values. Sample size calculations were not performed during the study design process. Additionally, as this is a retrospective, observational study, no interim analyses were performed. P-values < 0.05 were considered statistically significant. The statistical analyses were performed using the SAS for Windows version 9.2 statistical software package (SAS Institute Inc., Cary, NC).
Results
From the initial population of 23,223 women (Figure 1), 236 (1.0%) had diabetes and 1,126 (4.8%) had CVD prior to delivery and were excluded from analyses. Baseline characteristics are described in Table 1. Women with GDM were significantly older; had higher baseline BMI, systolic and diastolic blood pressures; had heavier offspring; had lower gestational weight gain; and were more likely to be of non-white race/ethnicity compared to women without GDM. Regarding traditional risk factors for hypertension, we observed no significant difference between GDM and non-GDM women in the frequency of smoking. However, lipid profile analysis revealed that women with GDM had higher triglyceride levels and lower high-density lipoprotein levels compared to non-GDM women. Comparing within each racial/ethnic group, women were more likely to have GDM if they were Hispanic or Asian and more likely not to have GDM if they were white or black (Table 1). Notably, regarding hypertension risk factors, Hispanic women were less likely to be past or current smokers compared to white women but had elevated triglyceride levels compared to white, black and Asian women with GDM (Table 2).
Table 1.
Baseline Characteristics of Study Population by Gestational Diabetes Mellitus Status
| Gestational Diabetes Mellitus | |||
|---|---|---|---|
| Variable | No (n=3,208) | Yes (n=802) | p-value |
| Age (years) | 30.3 ± 6.1 | 32.2 ± 5.4 | <0.0001 |
| Body Mass Index (kg/m2) | 25.7 ± 5.5 | 28.7 ± 7.0 | <0.0001 |
| Systolic Blood Pressure (mmHg) | 110 ± 11 | 113 ± 13 | <0.0001 |
| Diastolic Blood Pressure (mmHg) | 68 ± 8 | 71 ± 9 | <0.0001 |
| Live Births | 1 ± 2 | 1 ± 1 | 0.26 |
| Total Pregnancies1 | 3 ± 2 | 3 ± 2 | --- |
| Gestational Age at First Prenatal Visit (weeks) | 12.5 ± 5.5 | 12.2 ± 5.7 | 0.17 |
| Gestational Age at Delivery (weeks) | 39.4 ± 1.7 | 38.8 ± 1.9 | <0.0001 |
| Weight Gain (kg) | 12.66 ± 5.58 | 10.89 ± 5.35 | <0.0001 |
| Baby Weight (grams) | 3,364.1 ± 539.6 | 3,449.5 ± 634.4 | 0.001 |
| Birth Weight for Gestational Age (Percentile) | 49 ± 28 | 57 ± 29 | <0.0001 |
| Preeclampsia | 23 (0.7%) | 14 (1.8%) | 0.006 |
| Total Cholesterol (mmol/L) | 5.15 ± 1.3 | 5.12 ± 1.1 | |
| [mg/dL] | 199 ± 50 | 198 ± 44 | 0.68 |
| High-Density Lipoprotein (HDL) (mmol/L) | 1.50 ± 0.4 | 1.37 ± 0.4 | |
| [mg/dL] | 58 ± 15 | 53 ± 14 | 0.0004 |
| Low-Density Lipoprotein (LDL) (mmol/dL) | 2.92 ± 1.0 | 2.77 ± 0.8 | |
| [mg/dL] | 113 ± 39 | 107 ± 32 | 0.11 |
| Triglycerides (mmol/dL) | 1.83 ± 1.3 | 2.22 ± 1.5 | |
| [mg/dL] | 162 ± 112 | 197 ± 135 | 0.004 |
| Smoking Status | 0.08 | ||
| Never | 1,254 (39.1%) | 336 (41.9%) | |
| Past | 497 (15.5%) | 129 (16.1%) | |
| Current | 252 (7.9%) | 46 (5.7%) | |
| Missing | 1,205 (37.6%) | 291 (36.3%) | |
| Race | <0.0001 | ||
| White | 1,688 (52.6%) | 369 (46.0%) | |
| Black | 193 (6.0%) | 46 (5.7%) | |
| Asian | 207 (6.5%) | 83 (10.4%) | |
| Hispanic | 828 (25.8%) | 217 (27.1%) | |
| Other | 163 (5.1%) | 56 (7.0%) | |
| Missing | 129 (4.0%) | 31 (3.9%) | |
| Breast Feed at Discharge | 2,384 (74.3%) | 599 (74.7%) | 0.77 |
P-value is not provided for total pregnancies due to matching scheme
Table 2.
Baseline Characteristics of Study Population by Gestational Diabetes Mellitus Status and Race/Ethnicity
| GDM | Non-GDM | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Characteristic | White (n=369) |
Black (n=46) |
Asian (n=83) |
Hispanic (n=217) |
p-value | White (n=1,688) |
Black (n=193) |
Asian (n=207) |
Hispanic (n=828) |
p-value |
| Age (years) | 33.3±5.0 | 32.9±5.1 | 32.6±4.7 | 30.3±6.2 | <0.0001 | 32.1 ± 5.3 | 28.8 ± 6.3 | 30.2 ± 5.6 | 27.0 ± 6.2 | <0.0001 |
| Body Mass Index (kg/m2) | 28.8±7.3 | 30.0±7.4 | 23.6±3.2 | 31.5±6.4 | <0.0001 | 25.0 ± 5.0 | 27.9 ± 6.4 | 22.5 ± 3.5 | 27.9 ± 6.3 | <0.0001 |
| Systolic Blood Pressure (mmHg) | 116±12 | 114±12 | 111±14 | 111±15 | <0.0001 | 112 ± 11 | 111 ± 13 | 105 ± 11 | 107 ± 11 | <0.0001 |
| Diastolic Blood Pressure (mmHg) | 72±9 | 72±13 | 69±8 | 68±7 | <0.0001 | 70 ± 8 | 69 ± 9 | 66 ± 8 | 65 ± 8 | <0.0001 |
| Live Births | 1±1 | 1±1 | 1±1 | 1±2 | <0.0001 | 1 ± 2 | 2 ± 2 | 1 ± 1 | 1 ± 2 | <0.0001 |
| Total Pregnancies | 2±2 | 3±2 | 2±2 | 3±2 | <0.0001 | 3 ± 2 | 3 ± 2 | 2 ± 2 | 3 ± 2 | <0.0001 |
| Gestational Age at First Prenatal Visit (weeks) | 11.6±4.7 | 12.8±6.0 | 13.2±6.0 | 12.2±6.5 | 0.01 | 12.0 ± 4.6 | 13.6 ± 5.8 | 14.0 ± 6.1 | 12.7 ± 6.2 | <0.0001 |
| Gestational Age at Delivery (weeks) | 38.7±2.1 | 38.4±2.6 | 38.9±1.5 | 39.1±1.5 | 0.16 | 39.3 ± 1.7 | 39.5 ± 1.6 | 39.1 ± 1.7 | 39.4 ± 1.7 | 0.14 |
| Weight Gain (kg) | 11.52±5.31 | 8.62±5.35 | 11.07±4.81 | 10.21±5.49 | 0.001 | 13.56± 5.26 | 10.48±6.40 | 12.20±4.72 | 11.52±5.72 | <0.0001 |
| Baby Weight (grams) | 3.427±644 | 3,328±740 | 3,351±565 | 3,588±623 | 0.01 | 3,410 ± 550 | 3,340 ± 496 | 3,211 ± 515 | 3,320 ± 525 | <0.0001 |
| Birth Weight for Gestational Age (Percentile) | 58±28 | 54±27 | 51±31 | 61±30 | 0.025 | 52 ± 28 | 46 ± 27 | 41 ± 26 | 46 ± 27 | <0.0001 |
| Preeclampsia | 9 (2.4%) | 0 (0.0%) | 1 (1.2%) | 4 (1.8%) | 0.65 | 9 (0.5%) | 1 (0.5%) | 1 (0.5%) | 11 (1.3%) | 0.65 |
| Total Cholesterol (mmol/L) [mg/dL] |
11.1±2.6 [200±46] |
10.8±2.2 [194±40] |
10.7±2.6 [193±47] |
11.4±2.1 [206±37] |
0.52 | 11.5±3.0 [208 ± 54] |
9.9±2.5 [178 ± 45] |
10.5±2.5 [189 ± 45] |
10.4±2.2 [187 ± 40] |
<0.0001 |
| High-Density Lipoprotein (mmol/L) [mg/dL] |
3.1±0.78 [55±14] |
3.0±0.83 [54±15] |
3.1±0.89 [55±16] |
2.8±0.78 [51±14] |
0.48 | 3.3±0.8 [59 ± 15] |
3.3±0.8 [59 ± 15] |
3.2±1.2 [58 ± 22] |
3.0±0.8 [54 ± 14] |
0.02 |
| Low-Density Lipoprotein (mmol/L) [mg/dL] |
5.88±1.9 [106±35] |
6.55±1.3 [118±23] |
6.10±1.6 [110±28] |
6.10±1.6 [110±29] |
0.53 | 6.4±2.4 [115 ± 43] |
5.6±2.3 [101 ± 41] |
5.8±1.8 [105 ± 33] |
6.1±1.7 [110 ± 31] |
0.33 |
| Triglycerides (mmol/L) [mg/dL] |
11.2±7.4 [201±133] |
6.22±3.4 [112±62] |
7.71±3.9 [139±70]c |
13.3±9.0 [240±162] |
0.008 | 9.5±7.0 [171 ± 127] |
6.4±2.8 [115 ± 51] |
8.9±5.7 [161 ± 102] |
8.2±4.8 [147 ± 86] |
0.07 |
| Smoking Status | <0.0001 | <0.0001 | ||||||||
| Never | 111 (30.1%) | 29 (63.0%) | 50 (60.2%) | 102 (47.0%) | 580 (34.4%) | 94 (48.7%) | 118 (57.0%) | 359 (43.4%) | ||
| Past | 100 (27.1%) | 3 (6.5%) | 3 (3.6%) | 17 (7.8%) | 351 (20.8%) | 16 (8.3%) | 24 (11.6%) | 75 (9.1%) | ||
| Current | 29 (7.9%) | 1 (2.2%) | 1 (1.2%) | 10 (4.6%) | 163 (9.7%) | 15 (7.8%) | 9 (4.4%) | 39 (4.7%) | ||
| Breast Feed at Discharge | 263 (71.3%) | 37 (80.4%) | 65 (78.3%) | 162 (74.7%) | 0.21 | 1,230 (72.9%) | 145 (75.1%) | 136 (65.7%) | 641 (77.4%) | 0.21 |
GDM=Gestational Diabetes Mellitus
Women with GDM were 2.45 times more likely to develop hypertension compared to women without GDM in age-adjusted analysis (Table 3). After adjusting for age, race, parity, baseline systolic blood pressure and BMI, gestational weight gain, and preeclampsia, the relationship between GDM and hypertension was attenuated but still significant (Table 3). When considering the impact of race/ethnicity on the development of hypertension, black and Hispanic women had a higher risk while Asian women had a lower risk of developing hypertension subsequent to pregnancy compared to white women in age-adjusted analyses. These differences remained significant in multivariable analyses for both black and Hispanic women (Table 3).
Table 3.
Risk of Hypertension from Proportional-Hazards Regression Overall and by Race/Ethnicity
| Age-Adjusted | Multivariable | ||||
|---|---|---|---|---|---|
| Person Time (Years) |
HR (95% CI) | p-value | HR (95% CI) | p-value | |
| Overall Model | |||||
| GDM | 2.45 (1.89, 3.19) | <0.0001 | 1.75 (1.28, 2.37) | 0.0004 | |
| Black Race1 | 3.28 (2.25, 4.80) | <0.0001 | 2.48 (1.61, 3.83) | <0.0001 | |
| Asian Race1 | 0.32 (0.12, 0.86) | 0.02 | 0.45 (0.14, 1.45) | 0.18 | |
| Hispanic Race1 | 1.69 (1.24, 2.31) | 0.001 | 1.54 (1.07, 2.21) | 0.02 | |
| Other Race1 Live Births (n) |
14,899 | 1.02 (0.53, 1.95) 1.05 (1.01, 1.10) |
0.95 0.01 |
1.31 (0.63, 2.71) 1.05 (0.98, 1.12) |
0.47 0.16 |
| SBP (mmHg) | 1.03 (1.02, 1.03) | <0.0001 | 1.03 (1.02, 1.03) | <0.0001 | |
| BMI (kg/m2) | 1.10 (1.09, 1.12) | <0.0001 | 1.08 (1.06, 1.10) | <0.0001 | |
| Weight Gain (lbs) | 0.97 (0.96, 0.98) | <0.0001 | 0.99 (0.98, 1.01) | 0.30 | |
| Preeclampsia | 4.00 (1.97, 8.10) | 0.0001 | 4.82 (2.35, 9.89) | <0.0001 | |
| Race-Stratified Model2 | |||||
| White Race: GDM | 8,179 | 2.50 (1.72, 3.63) | <0.0001 | 1.68 (1.10, 2.57) | 0.02 |
| Black Race: GDM | 849 | 0.85 (0.40, 1.84) | 0.68 | 0.91 (0.37, 2.45) | 0.91 |
| Asian Race: GDM | 937 | 1.72 (0.24, 12.45) | 0.59 | 1.04 (0.05, 20.71) | 0.98 |
| Hispanic Race: GDM | 3,741 | 3.44 (2.11, 5.63) | <0.0001 | 3.25 (1.85, 5.72) | <0.0001 |
BMI=Body Mass Index; GDM=Gestational Diabetes Mellitus, SBP=Systolic Blood Pressure
Reference group is White
Models include terms (not shown) for all covariates listed in overall model (except for race). HR and 95% CI are for GDM term.
Within each racial/ethnic cohort, we determined the risk of hypertension development in women with GDM compared to those without GDM (Table 3, Race-Stratified Models). Both white and Hispanic women with GDM had a significantly greater risk of hypertension compared to women of the same race/ethnicity without GDM in age-adjusted analyses. Moreover, these differences were maintained after adjustment for age, parity, baseline systolic blood pressure and BMI, maternal gestational weight gain, and preeclampsia among both white and Hispanic women (Table 3, Race-Stratified Models). Additionally, these results remained significant in models utilizing multiple imputation techniques (results not shown). There was no significant distinction in the risk of developing hypertension in black and Asian women with GDM compared to women of the same race without GDM. When running the multivariable model from Table 3 with black women as the reference group instead of white women, we observed that Hispanic women had a significantly lower risk of hypertension compared to black women in multivariable analysis (HR: 0.62, 95% CI: 0.39–0.99, p = 0.04). Moreover, when looking at GDM women only, Hispanic women with GDM did not have a greater risk of developing hypertension compared to black women with GDM (p = 0.64).
Discussion
In our study population, we observed that GDM was associated with a 75% increased risk of hypertension after adjusting for age, race, parity, baseline systolic blood pressure and BMI, gestational weight gain, and preeclampsia. Black and Hispanic women with GDM had a higher risk of developing hypertension than white women with GDM. In a comparison of women with and without GDM within their respective racial/ethnic groups, we observed that Hispanic women had a significantly elevated likelihood of developing subsequent hypertension compared to Hispanic women without GDM. White women with GDM also had a higher risk of developing hypertension compared to white women without GDM. Additionally, the mean age of our GDM study population was 32 years old, suggesting that hypertension risk surveillance should be considered in a population younger than one may generally expect.
Prior investigations of CVD risk subsequent to GDM have similarly identified an association between GDM and increased risk of subsequent hypertension.(11–13)However, one study used ICD-9 code data to identify the GDM diagnosis;(13) two others identified GDM by self-report.(11–12) In addition, when following the women for development of CVD, Shah et al.(13) and Carr et al.(12) included women who had also developed type 2 diabetes, which potentially represents a different population of women since type 2 diabetes is independently associated with an increased risk of hypertension.
Although race/ethnicity has been associated with an increased risk of GDM,(1–3, 14) type 2 diabetes,(14) and hypertension,(15) to our knowledge, our study is the first published to exclusively examine the effect of race/ethnicity in the development of hypertension after GDM. We demonstrated that the association between hypertension and race/ethnicity is maintained even after adjustment for GDM and other factors such as age, parity, baseline systolic blood pressure and BMI, gestational weight gain, and preeclampsia. Moreover, in race-stratified analyses (Table 3), although both white and Hispanic women with GDM were more likely to develop hypertension than their non-GDM, race/ethnicity-congruent counterparts, the hazard ratio among Hispanics was nearly two-fold greater than that among white women. Although the limited sample size of black and Asian women may have potentially limited the signal of GDM, one may suggest that the impact of race was so great that it overwhelmed any potential effect that would have resulted from the GDM diagnosis.
The increased prevalence of preeclampsia in the setting of GDM has been previously reported(16) and the increased risk of hypertension in women who have had preeclampsia has been well-established.(17) Racial/ethnic differences in the prevalence of preeclampsia (18) and in the perinatal effects of GDM have also been reported.(19) Our data demonstrate that, although preeclampsia clearly increases the risk of hypertension subsequent to pregnancy, there is an increased risk of hypertension among white and Hispanic women with a history of GDM, independent of preeclampsia. This highlights the importance of GDM as a pregnancy complication that mediates CVD risk beyond what has typically been reported most prominently with preeclampsia.
The strengths of our study lie in 1) the examination of the association of GDM and hypertension by race/ethnicity; 2) the biochemically-confirmed clinical diagnosis of all women defined as GDM; 3) the elimination of potential confounding of hypertension development by the intercurrent development of type 2 diabetes; and 4) crude generalizability of our data.. We identified each case according to uniform clinical criteria and expertly-accepted Carpenter-Coustan diagnostic criteria for GDM.(7) We identified non-GDM women based on frequency- matching with GDM women in a ratio of 4:1 to maximize the ability to detect a statistically significant differences given the literature suggesting an increased CVD risk attributable to the number of pregnancies.(8) We reviewed each record for the presence of traditional hypertension risk factors, including lipid derangements or smoking. By censoring subjects who developed type 2 diabetes prior to the development of hypertension, we strengthened the likelihood that the association between GDM and hypertension is unbiased as type 2 diabetes has been widely accepted as an hypertension risk factor.(20) Finally, although we were unable to analyze socioeconomic data of the study participants, such as income or educational level, the racial/ethnic distribution of MGH’s population of pregnant women appeared comparable and therefore generalizable to that of the United States.(21)
Despite the rigorous methodology by which we obtained and analyzed our data, we do acknowledge several limitations with regards to 1) duration of follow-up; 2) missing data; and 3) racial/ethnic heterogeneity. Because this is a retrospective study, we were unable to determine if women with longer duration of follow-up and more medical encounters were more likely to be classified as hypertensive. Further prospective studies are warranted to determine if more encounters contribute to a hypertensive diagnosis. Also, we could not determine if we missed hypertension, pregnancy, or other medical encounters if the women presented for care at outside facilities during the study time frame prior to database censor. Because we captured information at one point in time, we cannot determine if there were lifestyle behaviors or anthropometric measurement variations over time captured in the data, such as smoking or BMI. Additionally, because we were limited by the capability of electronic medical records, we were subject to the customary risks associated with acquisition of data in this manner, including the use of ICD-9 codes for the inclusion and exclusion criteria of hypertension and the capture of smoking history which may not have been consistently ascertained. However, because we censored women after their last MGH clinical encounter, our data were complete on each woman to the point of analysis and we do not feel that the potential data lost would have influenced the results. In fact, our results may potentially be more compelling with additional encounter information. Finally, we had limited racial/ethnic heterogeneity beyond Hispanic women; a larger sample of black and Asian women may have revealed additional associations between these groups and the measured outcomes and may have enabled additional risk comparisons within the non-white population. Nevertheless, these data demonstrate the need for enhanced clinical monitoring for hypertension risk even among young, presumably healthy women, who have had a prior history of GDM.
Acknowledgments
The authors wish to thank Ms. Florence-Damilola Odufalu of the University of California, Irvine School of Medicine for her assistance with data collection; Ms. Annie Yang of Harvard University for her assistance with literature review; and Ms. Grace Xiong and Ms. Jennifer Huynh of the MGH Diabetes Research Center for their assistance with manuscript production.
Support/Grant Information: This study was supported in part by the following grants: NIH 1K23RR023333 and the Robert Wood Johnson Foundation Harold Amos Medical Faculty Development Program awarded to R.B-L.; the Howard Hughes Medical Institute Medical Research Fellowship, 2009–2010, awarded to C.E.P.; and NIH K24 DK094872 awarded to R.T. The study was conducted entirely in Boston/Massachusetts/United States.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
An abstract based on these data was presented at The Endocrine Society's 93rd Annual Meeting in Boston, Massachusetts, June 2011.
Disclosure Statement: The authors have nothing to disclose.
References
- 1.Lawrence JM, Contreras R, Chen W, Sacks DA. Trends in the prevalence of preexisting diabetes and gestational diabetes mellitus among a racially/ethnically diverse population of pregnant women, 1999–2005. Diabetes Care. 2008;31:899–904. doi: 10.2337/dc07-2345. [DOI] [PubMed] [Google Scholar]
- 2.Dabelea D, Snell-Bergeon JK, Hartsfield CL, Bischoff KJ, Hamman RF, McDuffie RS. Increasing prevalence of gestational diabetes mellitus (GDM) over time and by birth cohort: Kaiser Permanente of Colorado GDM Screening Program. Diabetes Care. 2005;28:579–584. doi: 10.2337/diacare.28.3.579. [DOI] [PubMed] [Google Scholar]
- 3.Thorpe LE, Berger D, Ellis JA, Bettegowda VR, Brown G, Matte T, Bassett M, Frieden TR. Trends and Racial/Ethnic Disparities in Gestational Diabetes Among Pregnant Women in New York City, 1990–2001. Am J Public Health. 2005;95:1536–1539. doi: 10.2105/AJPH.2005.066100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brancati FL, Kao WH, Folsom AR, Watson RL, Szklo M. Incident type 2 diabetes mellitus in African American and white adults: the Atherosclerosis Risk in Communities Study. JAMA. 2000;283:2253–2259. doi: 10.1001/jama.283.17.2253. [DOI] [PubMed] [Google Scholar]
- 5.Ford ES, Giles WH, Dietz WH. Prevalence of the metabolic syndrome among US adults: findings from the third National Health and Nutrition Examination Survey. JAMA. 2002;287:356–359. doi: 10.1001/jama.287.3.356. [DOI] [PubMed] [Google Scholar]
- 6.Partners Healthcare Notice for Use and Sharing of Protected Health Information. Boston: Partners Healthcare; 2013. [Google Scholar]
- 7.Coustan DR, Carpenter MW. The diagnosis of gestational diabetes. Diabetes Care. 1998;21:B5–B8. [PubMed] [Google Scholar]
- 8.Rassen JA, Shelat AA, Myers J, Glynn RJ, Rothman KJ, Schneeweiss S. One-to-many propensity score matching in cohort studies. Pharmacoepidemiol Drug Saf. 2012;S2:69–80. doi: 10.1002/pds.3263. [DOI] [PubMed] [Google Scholar]
- 9.Birman-Deych E, Waterman AD, Yan Y, Nilasena DS, Radford MJ, Gage BF. Accuracy of ICD-9-CM codes for identifying cardiovascular and stroke risk factors. Med Care. 2005;43:480–485. doi: 10.1097/01.mlr.0000160417.39497.a9. [DOI] [PubMed] [Google Scholar]
- 10.Schafer JL. Analysis of Incomplete Multivariate Data. London: Chapman & Hall; 1997. [Google Scholar]
- 11.Tobias DK, Hu FB, Forman JP, Chavarro J, Zhang C. Increased Risk of Hypertension After Gestational Diabetes Mellitus. Diabetes Care. 2011;34:1582–1584. doi: 10.2337/dc11-0268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Carr DB, Utzschneider KM, Hull RL, Tong J, Wallace TM, Kodama K, Shofer JB, Heckbert SR, Boyko EJ, Fujimoto WY, Kahn SE. Gestational diabetes mellitus increases the risk of cardiovascular disease in women with a family history of type 2 diabetes. Diabetes Care. 2006;29:2078–2083. doi: 10.2337/dc05-2482. [DOI] [PubMed] [Google Scholar]
- 13.Shah BR, Retnakaran R, Booth GL. Increased risk of cardiovascular disease in young women following gestational diabetes mellitus. Diabetes Care. 2008;31:1668–1669. doi: 10.2337/dc08-0706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rosenberg TJ, Garbers S, Lipkind H, Chiasson MA. Maternal obesity and diabetes as risk factors for adverse pregnancy outcomes: differences among 4 racial/ethnic groups. Am J Public Health. 2005;95:1545–1551. doi: 10.2105/AJPH.2005.065680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sampson UK, Edwards TL, Jahangir E, Munro H, Wariboko M, Wassef MG, Fazio S, Mensah GA, Kabagambe EK, Blot WJ, Lipworth L. Factors Associated With the Prevalence of Hypertension in the Southeastern United States: Insights From 69 211 Blacks and Whites in the Southern Community Cohort Study. Circ Cardiovasc Qual Outcomes. 2013 doi: 10.1161/CIRCOUTCOMES.113.000155. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schneider S, Freerksen N, Rohrig S, Hoeft B, Maul H. Gestational diabetes and preeclampsia--similar risk factor profiles? Early Hum Dev. 2012;88:179–184. doi: 10.1016/j.earlhumdev.2011.08.004. [DOI] [PubMed] [Google Scholar]
- 17.Drost JT, Arpaci G, Ottervanger JP, de Boer MJ, van Eyck J, van der Schouw YT, Maas AH. Cardiovascular risk factors in women 10 years post early preeclampsia: the Preeclampsia Risk Evaluation in FEMales study (PREVFEM) Eur J Prev Cardiol. 2012;19:1138–1144. doi: 10.1177/1741826711421079. [DOI] [PubMed] [Google Scholar]
- 18.Gong J, Savitz DA, Stein CR, Engel SM. Maternal ethnicity and pre-eclampsia in New York City, 1995–2003. Paediatr Perinat Epidemiol. 2012;26:45–52. doi: 10.1111/j.1365-3016.2011.01222.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nguyen BT, Cheng YW, Snowden JM, Esakoff TF, Frias AE, Caughey AB. The effect of race/ethnicity on adverse perinatal outcomes among patients with gestational diabetes mellitus. Am J Obstet Gynecol. 2012;207:321–326. doi: 10.1016/j.ajog.2012.06.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Haffner SM, Lehto S, Rönnemaa T, Pyörälä K, Laakso M. Mortality from coronary heart disease in subjects with type 2 diabetes and in nondiabetic subjects with and without prior myocardial infarction. N Engl J Med. 1998;339:229–234. doi: 10.1056/NEJM199807233390404. [DOI] [PubMed] [Google Scholar]
- 21.Ventura SJ, Curtin SC, Abma JC, Henshaw SK. Estimated pregnancy rates and rates of pregnancy outcomes for the United States, 1990–2008. Natl Vital Stat Rep. 2012;60:1–21. [PubMed] [Google Scholar]