Abstract
Many animals engage in spectacular courtship displays, likely recruiting specialized neural, hormonal and muscular systems to facilitate these performances. Male golden-collared manakins (Manacus vitellinus) of Panamanian rainforests perform physically elaborate courtship displays that include novel forms of visual and acoustic signaling. We study the behavioral neuroendocrinology of this male’s courtship, combining field behavioral observations with anatomical, biochemical and molecular laboratory-based studies. Seasonally, male courtship is activated by testosterone with little correspondence between testosterone levels and display intensity. Females prefer males whose displays are exceptionally frequent, fast and accurate. The activation of androgen receptors (AR) is crucial for optimal display performance, with AR expressed at elevated levels in several neuromuscular tissues. Apparently, courtship enlists an elaborate androgen-dependent network that includes spinal motoneurons, skeletal muscles and somatosensory systems. This work highlights the value of studying non-traditional species to illuminate physiological adaptations and, hopefully, stimulates future research on other species with complex behaviors.
Keywords: Sex steroids, Androgens, Estrogens, Androgen receptors, Spinal cord, Neuroendocrinology, Songbirds, Behavioral ecology, Aromatase, 5Alpha-reductase
1. I Introduction
The courtship displays of some birds are spectacular wonders of nature. Worldwide, across virtually all avian Orders, birds combine physical strength, stamina and coordination, with colorful, unusual ornamentation and vocal and non-vocal sound production to produce these natural spectacles. The extraordinary diversity of avian displays is underscored by the fact that they can occur while in flight, while running on the ground, while jumping amongst branches of trees, while swimming on the water or while using combinations of these settings and forms of locomotion. Even more intriguing is that some species enhance their displays by altering the physical environment in which this behavior is performed, with bowerbirds being supreme examples (Johnsgard, 1994; Höglund and Alatalo, 1995).
Animal displays have long attracted the attention of biologists and the study of avian courtship has served to shape modern biological thought. The core concept of sexual selection, a fundamental tenet of evolutionary theory, was formed as Charles Darwin pondered avian courtship (Darwin, 1871). The science of ethology grew out of the comprehensive descriptions of many avian courtship displays (Pycraft, 1913), notably the elegant and sexually coordinated dances of some grebes and ducks (Huxley, 1914; Dane and Van Der Kloot, 1964). The origin and maintenance of elaborate courtship displays is one of the most challenging problems of modern evolutionary biology (Zahavi, 2007; Candolin, 2003; Hau et al., 2010; Kotiaho et al., 2007; Jones and Ratterman, 2009; Prum, 2012). To this day, scientists continue to explore avian courtship in order to increase the breadth and depth of our understanding of many biological principles.
1.1. A brief history of the neuroendocrinology of courtship
Courtship has also long attracted the interest of behavioral endocrinologists, and recent comprehensive reviews describe this interesting history and the relatively current state of the field (i.e. Fusani, 2008; Hau et al., 2008; Wade, 2011; Zornik and Kelley, 2011). The earliest era of research on birds established a role for the testes by showing that castration of males eliminated or reduced masculine courtship phenotypes (physical ornaments, courtship displays and song), while subsequent replacement with the testes restored these masculine phenotypes. The testes were thought to make “stimulating” and “mysterious” “juices” that were only recognized to be “hormones” after they were first described by Starling at the beginning of the 20th century (Pycraft, 1913). The discovery, purification and synthesis of testosterone (Koch, 1938) made it possible to confirm its primary role in the activation of courtship (e.g. Beach and Inman, 1965; Erpino, 1969; Brockway, 1974). A subsequent phase of investigation showed that the activation of male courtship by the testicular hormone testosterone (T) occurred directly, or after T was converted in brain either to the more potent androgen 5α-dihydrotestosterone or to estradiol for action on androgen and estrogen receptors, respectively (Pietras and Wenzel, 1974; Hutchison and Steimer, 1984). Indeed the complexity of courtship seems to have arisen as the result of a collection of motivational states (Huxley, 1914) that include aggressive and sexual features where androgenic and estrogenic pathways work somewhat independently to activate these distinct arousal states (Hutchison, 1970; Belle et al., 2005; Fusani et al., 2001). These sex-steroids were then shown to act on discrete sex-steroid receptors expressed by neurons within regions of what we now refer to as the “social” brain (Adkins-Regan, 1998; Newman, 1999; Goodson, 2005; Goodson and Kabelik, 2009; O’Connell and Hofmann, 2011), such as the medial preoptic area and septum, to boost the overall motivation for males to court females (Hutchison, 1967).
1.2. Courtship and avian vocalizations
Many male birds employ vocalizations in courtship and the study of these songs and calls has been a particularly active area of neuroendocrine investigation and is of special relevance in this review. In most birds, vocalizations are simple “calls” and there is good evidence that midbrain nucleus intercollicularis (nICO) is a conserved steroid-sensitive pre-motor site for the activation of calling behavior (Brown, 1965; Zigmond et al., 1973; Cohen, 1981). Thus, sex-steroids increase the motivation to perform behavior, but are also directly involved in motor control of vocal output. This dual view of steroid action on the brain achieved even greater realization with the discovery of the neural sites controlling song of oscine songbirds (Nottebohm et al., 1976). Oscine song can serve in a variety of social and aggressive functions, but is especially important as a male courtship signal. Multiple brain areas are required for the learning, memory and performance of song as well as in the processing of song-related acoustic signals, and most of these regions express receptors for androgens and/or estrogens (Arnold et al., 1976; Gahr et al., 1993). Thus, sex steroids act on the avian brain to control courtship via sensory, motor and cognitive pathways. The study of this neural song system has generated new concepts regarding steroid action on the central nervous system, such as their role in promoting large scale neural plasticity (Tramontin et al., 2003). In the end, singing and calling arise from coordinated contractions of intricate syringeal musculature and respiratory muscles; much of what we know about steroidal control of motor aspects of avian courtship come from studies of the relatively minute steroid-sensitive syrinx (Goller and Suthers, 1996; Suthers et al., 1999). We will return to a discussion of the oscine neural song system as it more directly relates to more complex courtship displays.
1.3. General considerations
Of course birds are not the only animals to participate in elaborate courtship e.g. (Pycraft, 1913); indeed, male courtship displays have arisen in virtually all vertebrate and invertebrate taxa with some invertebrates serving as exceptional animal models for study (Greenspan and Ferveur, 2000). Our focus here on birds serves not to ignore these other species, but rather to provide an overview of concepts gained from our avian studies that can be applied to other organisms. When this rich history is considered, we believe that a number of significant questions remain unanswered or unexplored. Table 1 summarizes what we believe are some of important open questions that have, in part, driven our own research program described below.
Table 1.
Questions underlying our research on the neuroendocrinology of complex courtship.
| 1 | Courtship displays can require extraordinary motor skill, so what is known about the motor control of the many neuromuscular systems involved in complex courtship? |
| 2 | What role do spinal circuits play in the intensively coordinated performances of some courtship displays? |
| 3 | Are premotor and motor circuits in the brain and spinal cord regulated by gonadal hormones? |
| 4 | As observed in the syrinx, are peripheral muscles involves in courtship sensitive to gonadal hormones? |
| 5 | Much like the brain regions controlling song of oscine songbirds, are courtship muscles, and their central control neural circuits, sexually dimorphic and do they demonstrate significant seasonal plasticity? |
| 6 | Are neural circuits controlling vocal acoustic communication related to those involved in non-vocal sound production? |
| 7 | Like oscine bird song, do males need to learn behavioral elements of their courtship displays and are there specific neural circuits devoted to motor learning? |
| 8 | Some males perform their courtship behaviors outside of the breeding season. Do traditional sex steroids activate courtship at these times and, if so, where are the steroids produced? |
| 9 | It is also now well established that neural steroid receptors can affect behavior by both the relatively slow process of modulating gene expression in the nucleus or by more rapid modulation of cell membranes and intracellular signaling systems. This raises the question as to whether there are discrete roles for these relatively slow and fast mechanisms in the control of male courtship displays. |
| 10 | Inasmuch as females choose males for mating in part based on these courtship displays, do females express unique sensory or cognitive attributes that enable their discrimination of male motor function? |
Driven by these questions and others, our laboratories have focused our attention on the remarkable courtship display of one species in particular, the golden-collared manakin (Manacus vittelinus). Courtship of this bird (and related bearded manakins of the genus Manacus) is physically intensive, acrobatic and involves vocal and non-vocal sound production. This species is also reasonably common in certain Central American forests, so it is readily studied in the field and in captivity through facilities operated by the Smithsonian Tropical Research Institute of Panama. It is our hope that by understanding more about the biology of this species that we will contribute answers to some questions of neuroendocrine function with broad application to all courting animals. This chapter will review our work to date on these questions and we will return to these questions at the conclusion of the review to see where advances have been made. Of course, the neuroendocrine mechanisms that influence male courtship have not developed in a vacuum, and a more comprehensive understanding requires consideration of the evolutionary pressures that influence golden-collared manakin courtship and neuroendocrinology, as well as the ecological implications of their anatomy and physiology. Our primary focus, however, will be how the study of golden-collared manakin behavioral neuroendocrinology has advanced the field and also raised new questions that might drive future research. To fully appreciate the neural, hormonal, muscular and ecological scope of the neuroendocrine mechanisms at work, it is essential for the reader to possess a clear sense of golden-collared manakin biology and of the complexity of the male golden-collared manakin courtship display, which is described in the next sections.
2. Manacus behavioral ecology
The great ornithologist Frank Chapman originally described these birds and their extraordinary displays (Chapman, 1935). Additional insight has been provided by studies from our lab as well as the study of related Manacus species (Bostwick and Prum, 2003; Lill, 1974; DuVal and Goymann, 2011; Snow, 1962). All males in the genus Manacus perform a similar visually and acoustically stunning display (Fig. 1; Supplementary video). The speedy, gymnastic routine is noisy, with the production of “chee-poo” vocalizations and a variety of non-vocal sounds, or sonations. The latter is largely a collection of remarkably loud “wing-snaps” produced by the rapid and powerful lifting of their wings (Bostwick and Prum, 2003). The males of this species are strikingly colored in lemon-yellows, blacks and greens, whereas females and juvenile males are a cryptic dull-green. All birds have conspicuous orange legs. Males also possess elongated throat feathers (their “beard”) that they erect and present to females during their displays (Schlinger et al., 2008a).
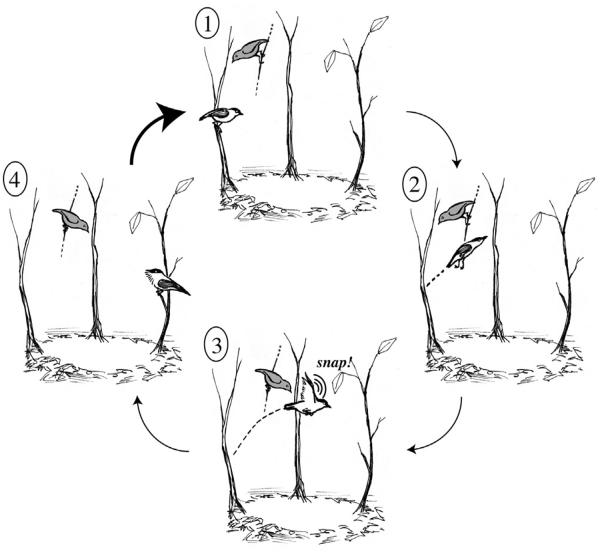
Fig. 1.
A schematic moving clockwise of the courtship dance of the male golden-collared manakin (Manacus vitellinus) being observed by a female, the solid gray bird perched above the male. The display begins (1) with the male sitting rigid on a upright stem. He launches his body from the perch with a powerful thrust of his legs (2). Midflight, he makes a single powerful and rapid wing stroke (3) that produces the loud single snap. He retracts his wings and holds them in place, sailing across the arena while still holding his “beard” solidly out in front. Just before landing, he makes a quick partial wing flap that turns him in midair and he lands squarely on the second stem (4). He holds this position briefly rigidly until embarking on the next jump and wingsnap. The thick arrow indicates that this complete sequence is repeated, on average, about nine times per complete courtship display. Note that in some cases, the female joins the male in a “duo dance” but she flies, not jumps, across the arena and her movements appear more awkward.
Male manakins court females via a lek breeding system and thereby provide no parental care duties. Approximately 2–15 males occupy a single ‘exploded’ lek of about an acre of forest within which each male clears organic material on the forest floor (fallen leaves mostly) from one or more elliptical arenas (∼30 × 50 cm). It is around these arenas that the males display. In our study area, golden-collared manakins begin displaying at the beginning of the dry season, usually in mid January (Fig. 2). Outside of their brief forays to feed on fruit laden-trees near the lek, adult males remain close to their own individual arenas for months, displaying daily, for as much as 6 or 7 months. They prefer second growth woods with a relatively low canopy and thick ground vegetation. They also require forest with many small upright roots or thin saplings that they use for their displays and dances (Schlinger et al., 2008a).
Fig. 2.
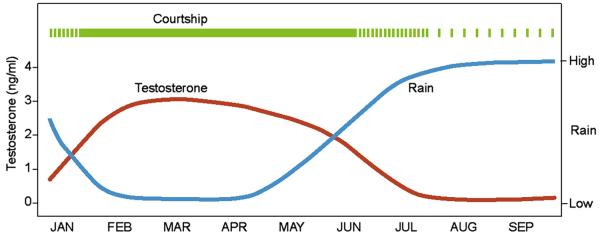
Schematic illustrating the male golden-collared manakin courtship season in central Panama (green) that peaks between mid-January to mid-June relative to relative mean plasma testosterone levels (orange) and relative monthly rainfall amounts (blue). Note plasma T levels are very variable during the dry-season when courting is at its peak.
2.1. Courtship behavior and mate choice
2.1.1. Advertisement
During months of courtship, even when females are absent from the lek, males can be quite active vocalizing frequently and producing their distinctive and conspicuous mechanical sounds. Using their wings, males create a very loud single “snap”, a “snapping whirr” or “rollsnap”, a soft “snip” and an unusual “reedy whir” or “grunt” that sounds a bit like a duck-quack. Wingsnaps are exceptionally loud; Chapman wrote that he could hear males wingsnapping at a distance of 300 yards [∼274 m] over still water on a calm morning (Chapman, 1935). The snap is produced by a powerful upward lift of the wings (Bostwick and Prum, 2003), while the rollsnap is produced by a rapid series of wingsnaps (∼50 Hz). The snap is produced as the morphologically unique radii of the wings collide (Bostwick and Prum, 2003; our unpublished data). Whereas single wingsnaps are part of the courtship display, described below, the rollsnap may hold both agonistic and advertisement functions (McDonald et al., 2001). All of these activities produce a great deal of noise that is thought to attract females to the lek. Presumably, larger leks are noisier than smaller leks and attract more females (see also Höglund and Alatalo, 1995). Once a female is at the lek, males increase their activity as she moves about, eventually choosing a male to observe closely and with whom she may eventually mate (Lill, 1974).
2.1.2. The display
Males produce single wingsnaps and “grunts” as part of their acrobatic courtship display (Fig. 1). This display is performed alone, but if a female finds the male to be of interest, she will join him in his dance (“duo dance”). When viewed in the field, the male appears to bounce somewhat mechanically from stem-to-stem (on average 9 times/display) in a somewhat frenzied sequence, with the entire display sequence lasting about 9 s. Powerful single wingsnaps are emitted with each male jump, and less frequently the grunt is heard when a male jumps to the ground and back to a stem, often before copulating with the female. Before landing at the end of each individual jump-snap, the male rapidly turns in midair with a precise landing that allows him to rapidly turn his head to face the female, thereby showing off his extended throat feathers (the beard-up display). All of these elements of the display are difficult to discern with the naked eye, so we use very high-speed digital photography (up to 1000 frames per second) to study the kinematics of the behavior. When these videos are viewed in slow motion, the displays are seen to be strikingly acrobatic, graceful and well-controlled (Fusani et al., 2007b; see Supplementary video). Presumably, females are able to see the details of these courtship displays, given that birds have a relatively higher flicker fusion frequency than humans (birds: 100–120 Hz; humans: 25–30 Hz) and thus likely exhibit faster visual processing speed than humans (Jones et al., 2007).
Frame-by-frame analysis of multiple high-speed video sequences from ten adults reveals considerable individual variability in male performance (Fusani et al., 2007b). For example, we documented an approximate 3-fold difference in the amount of time that males remained on the perch before initiating a second jump as well as in the speed of the jump itself (in m/s). Because many of the individual display elements are performed so quickly, performance differences were often on the order of tens to hundreds of msecs. Although we do not know why males differ from one another (i.e. differences in age, experience, genetics), these differences in speed and/or quality of the male display likely provide information to females as they consider males for potential mating.
2.1.3. Female preference
Mate choice in golden-collared manakins has served as a powerful driving force behind the evolution of the male phenotype. For example, the brightness of the male’s yellow collar and the cleanliness of the court contribute to male mating success (Stein and Uy, 2006; Uy and Endler, 2004). Using behavioral analysis of both conventional and high-speed videos of individual males in which copulations with females were observed, we found that just a few individual display parameters predicted over 60% of the variance in male mating success (Barske et al., 2011). Notably, females preferred to mate with males that performed their display bouts more frequently and also more rapidly. For example, males that achieved a “beard-up” posture upon landing just tens of milliseconds faster than other males obtained considerably more copulations (Barske et al., 2011). Presumably, the speed and accuracy of these moves are physically challenging and -provide information on male quality to females. Furthermore, our evidence that courtship behavior predicts mating success fits well with the hypothesis that the courtship display is the primary focus upon which females select males and that plumage color may have evolved to enhance the intensity of the behavioral signal (e.g. Prum, 1998).
If speed and quality of the male’s performance is crucial for mating success, we would predict improvement in malecourtship when females are watching. This is just what we find. Males enhance their display performance when the female is present at the display court, increasing both the number of wingsnaps per display and the speed of certain display elements (Barske et al., unpublished data). Moreover, as in some other birds (e.g. Patricelli et al., 2002; Akre and Ryan, 2011) females appear to challenge the male performance by initiating jumps during their “duo” dance. These various analyses provide evidence that the elaborate courtship display of the male golden-collared manakin evolved under considerable sexual selection pressure, pressures that strongly influence male anatomy and physiology, including the neuroendocrine basis of male courtship behavior.
3. Neuroendocrinology of Manacus courtship
3.1. Hormones and courtship
Unlike male birds breeding in temperate or arctic latitudes that exhibit elevated levels of testosterone when breeding (Wingfield and Silverin, 2002), reproductive levels of testosterone are more variable in males of tropical breeding species (Moore et al., 2002; Chastel et al., 2005; Osorno et al., 2010), with levels in birds of lowland rainforests tending to be relatively low (Goymann et al., 2004). In golden-collared manakins, testosterone does fluctuate seasonally (as depicted in Fig. 2) but breeding males have quite variable levels of testosterone with many having levels as described for some other birds of wet tropical lowlands (Goymann et al., 2004). Wikelski et al. (2003) reported that when compared to other periods, males had relatively higher concentrations of testosterone during April, when they were “territorial”. We sampled testosterone levels of adult males and females, as well as juvenile males, from July–April (Day et al., 2007; Fusani et al., 2007a). Adult males during the active courtship season consistently had higher levels of testosterone than any other group. Nevertheless, some adult displaying males had undetectable levels of testosterone in blood whereas other males had testosterone levels up to 6 ng/ml.
In one group of males, we investigated plasma testosterone concentrations at two time points, at the onset of displaying season, when males were re-establishing and clearing their arenas, and then again three-to-four weeks later. As expected, testosterone levels were initially elevated (on average ∼1 ng/ml), but then decreased to near basal concentrations at the second bleeding. Importantly, this decrease occurred even though the birds were actively displaying (Fusani et al., 2007a). This suggests that testosterone is important for activating the display behavior at the onset of the breeding season, but that maintaining elevated levels of this hormone is not necessary for continuous production of the display itself. The golden-collared manakin may therefore stand in contrast to other lekking males in temperate regions, as testosterone levels remain elevated in males of these species throughout the entire displaying season, even though the season is much shorter (∼2 or 3 weeks) (Lisano and Kaennamer, 1977; Alatalo et al., 1996). Although somewhat controversial, prolonged high testosterone concentrations may be costly to males, such as in reduced immune function and increased oxidative stress (Folstad and Karter, 1992; Roberts et al., 2004; Mougeot et al., 2009; Edler et al., 2011) so manakins may need to limit such costs by reducing peripheral testosterone secretion. One possibility is that aggressive or competitive interactions between and among males within a lek, or even sexual interactions with females, produce transient pulses in circulating testosterone that 58 do not always detect but which serve to maintain testosterone-dependent properties in males at this time of year. More work is needed to address this idea.
3.2. Hormone treatments
In most bird species investigated so far, courtship displays have been shown to be dependent on androgens (Fusani, 2008). We suspected this would be the case for manakins as well. To test whether testosterone influenced golden-collared manakin display behavior, we implanted adult males, juvenile males and females in the non-breeding season (with naturally low testosterone levels; Wikelski et al., 2003) with testosterone implants and measured their behavioral response. Treatment with testosterone successfully induced the expression of several components of courtship, such as wingsnapping, in all of these birds (Day et al., 2007). These findings confirm that testosterone can activate courtship. Interestingly, testosterone treatment in adult males that were already actively displaying at their leks had no effects on behavior (Day et al., 2007). This finding was not surprising, because we knew already that adult males display intensely even with low circulating levels of testosterone (Fusani et al., 2007b). As discussed previously, male golden-collared manakin courtship could be activated by androgen at the onset of the breeding season and then maintained by alternate mechanisms, such that increases in testosterone above some threshold level would yield no further increases in courtship. This concept that a threshold level of testosterone activates behavior, but does not determine the magnitude of the behavior, has been described many times for various male behaviors (Hews and Moore, 1997; Adkins-Regan, 2005; Fusani, 2008) and seems also to apply to some avian courtship displays (Fusani and Hutchison, 2003).
3.3. Treatment with AR antagonists
As will be discussed in more detail below, testosterone can serve a signaling function through androgen receptors (AR) or, after its conversion into 17β-estradiol, via estrogen receptors (ER). Previous work on species like quail and doves, indicated a significant role for AR in the activation of male courtship (Adkins and Mason, 1974). To test the involvement of AR in the maintenance of male manakin courtship activity we treated adult courting males with flutamide, a competitive androgen receptor antagonist that had been used previously to successfully inhibit territorial and sexual behavior of male birds (Hegner and Wingfield, 1987; Schwabl and Kriner, 1991; Canoine and Gwinner, 2002). As predicted, treatment of wild courting male manakins with a relatively low dose of flutamide significantly decreased courtship activity for the first week after treatment. Surprisingly, this effect disappeared during the second week of treatment, while several measures of courtship actually increased during the third week after treatment (Fusani et al., 2007b). The paradoxical results could not be explained by a failure of the treatment, as we confirmed the efficacy of our flutamide implants (Fusani et al., 2007b). This led us to conclude that flutamide (i) began to function as an AR agonist after 2 weeks of treatment (Shet et al., 1997; Wirth and Froschermaier, 1997), (ii) up-regulated AR or other downstream signaling mechanisms (Chen et al., 2004), (iii) or both. Regardless of these considerations, the data clearly showed that disruption of AR impacts the expression of male manakin courtship. Although we have some evidence that ER may synergize with AR to activate the full courtship display (Day et al., unpublished data), our recent studies showing inhibition of male courtship by use of another AR antagonist (Casodex) confirms that AR play a crucial role in the activation, and perhaps maintenance, of the male manakin courtship display (Fuxjager et al., unpublished data).
4. Anatomy and physiology of courtship
4.1. Oscine vs sub-oscine
Before proceeding further, it is important to describe more about the taxonomic position of manakins with regard to other birds. Manakins of the family Pipridae, are members of the Order Passeriformes that is generally further divided into the oscine and sub-oscine clades (Barker, 2011). As described previously, the oscine songbird brain exhibits a highly specialized circuitry (the neural song system) that is the basis for the learning and production of the complex, learned syringeal-based vocalizations, or songs, produced by this group of birds. Like other suboscine species (Kroodsma and Konishi, 1991; Gahr et al., 1993), the golden-collared manakin is not thought to learn its vocalizations and does not possess a recognizable song system when the brain is stained by a conventional Nissl stain or by immunocytochemical-staining for the neuron-specific marker MAP5 (Saldanha et al., 2000). Specifically, when directly compared to the zebra finch, we saw no evidence in the golden-collared manakin for HVC, n. robustus arcopallii (RA), lMAN (lateral magnocellular nucleus of the anterior neopallium) or area X, all prominent song nuclei within the zebra finch forebrain. These data argue that the relatively simple “chee-poo” vocalization of the manakin is not acquired by learning and is produced in the manner of the calls of non-passeriform species with pre-motor control in the nucleus ICO (Brown, 1965; Cohen, 1981). Although a song circuitry is not visible in the sub-oscine brain, the neurons in these brain regions may still participate in sub-oscine acoustic communication by invoking calls or even non-vocal sonations, such as wing-snaps, in contexts that would invoke songs in oscine species. Data on this latter point will be discussed in the section that follows on brain sex-differences.
4.2. Brain aromatase
An especially prominent neurochemical feature of the oscine songbird brain is that the estrogen synthetic enzyme aromatase is expressed at exceptionally high levels in the forebrain when compared to non-passeriform species (Saldanha et al., 2013). This enzyme is a conserved feature of the vertebrate brain where it steers the actions of circulating testosterone along estrogen-dependent pathways (Balthazart and Ball, 2013). In oscine songbirds, high expression is most notable in caudal regions of the telencephalon within or adjacent to auditory processing areas, the lateral ventricles and song system nuclei. In these regions, aromatase contributes to song perception (Remage-Healey et al., 2010) and production (Meitzen et al., 2007), and contributes to the conspicuous neuroplasticity demonstrated by this group of birds (Tramontin et al., 2003). In contrast, nonpasseriform species exhibit aromatase in more conventional regions of the “social” brain, including nuclei of the diencephalon and septum (Schlinger and Balthazart, 2013).
Aromatase expression in the sub-oscine golden-collared manakin brain exhibits a pattern that is somewhat intermediate between the oscine and non-passeriform species (Saldanha et al., 2000). As determined by both biochemical measures of activity as well as immunocytochemical staining for protein, aromatase was present in the hypothalamus-preoptic area at levels comparable to what is seen in other bird species. By contrast, areas rich in aromatase in oscine songbirds (the hippocampus and a forebrain auditory processing region called NCM), but aromatase-poor in non-passeriform species, had readily detectable levels of aromatase in the manakin, albeit lower than what is seen in typical oscine songbirds (Saldanha et al., 2000). Given that estrogens facilitate song learning and production in oscine birds (Schlinger and Brenowitz, 2009), it is possible that elevated expression of aromatase in the forebrain evolved early in passerifrom species and in a manner that is similar to the current sub-oscine clade. In turn, such elevated estrogenic signaling may have predisposed the evolution of song and the neural song system in the oscine clade. This idea needs to be confirmed by studies of aromatase in other suboscine species.
As has been observed in other species (Balthazart et al., 1996), aromatase activity was greater in the hypothalamus-preoptic area (HPOA) of male than female golden-collared manakins (Saldanha et al., 2000). Presumably, estrogens synthesized in the male HPOA are responsible for activating copulatory and aggressive behaviors (Schlinger and Balthazart, 2013), though we cannot dismiss a role in courtship as well (Hutchison, 1967) and we will discuss this possibility in the next section.
Although our focus in this segment has been the brain, we have looked for aromatase elsewhere as well. Aromatase is detected in the manakin spinal cord (Fuxjager et al., 2012b), as has been seen in quail (Evrard et al., 2000), but we found no evidence for aromatase in manakin skeletal muscles (Feng et al., 2010).
4.3. A role for estrogens in male manakin courtship
Although we have direct and convincing data that AR signaling is crucial for the activation and maintenance of manakin courtship, we cannot exclude a role for estrogens. We have seen that treatment of non-breeding birds with a combination of estradiol and the potent androgen, 5α-dihydrotestosterone (DHT) activated displays more potently than either steroid alone, but only when DHT followed treatment with estradiol (Day et al., unpublished data). Moreover, simultaneous treatment of non-breeding birds with testosterone and fadrozole, an inhibitor of aromatase, reduced several display elements compared to males treated with testosterone alone. These data suggest that estrogens participate in the activation of the golden-collared manakin display behavior by combining with androgens; yet, exactly how the signaling pathways of these two sex steroids synergize to fully activate courtship remains an active area of investigation. We do detect ER expression in the male manakin spinal cord (Fuxjager et al., 2012b), skeletal muscle (Feng et al., 2010) and in brain (Fusani et al., unpublished data), though the amounts at which this receptor is expressed remain lower than that of AR. Moreover, the distribution of ER in the manakin brain and spinal cord resembles what has been described previously for ER expression for non-passerine species having courtship displays of limited motoric complexity (Metzdorf et al., 1999; Evrard and Balthazart, 2002). Thus, our work examining the distribution of ER throughout the manakin brain provides little insight into the role for ER in the male golden-collared manakin display and the remainder of this review will focus on androgen signaling pathways.
4.4. Tissue distribution of androgen action
4.4.1. Brain
Using in situ hybridization and/or quantitative PCR procedures, we find that AR are expressed abundantly in a variety of golden-collared manakin tissues, and this suggests that androgenic action can occur in important positions throughout the body to influence the neuromuscular control of the male’s courtship display. In the brain (Fusani et al., 2003), we find significant AR expression in classical androgen-sensitive regions such as the septum, preoptic area, and hypothalamus. Yet, we also found AR in the nucleus subrotundus (SR) and in the cerebellum (Fusani et al. unpublished data). Notably, we detected a large field of AR-labeled cells in the arcopallium, which is the area which contains the RA of songbirds and the nucleus taeniae (nT), a homologue of the mammalian amygdala. To our knowledge, this is the first report of AR expression in the forebrain of a non-oscine passerine outside of nT (Gahr et al., 2000) suggesting a possible functional relationship between AR expression in arcopallium and the acrobatic and/or acoustic properties of courtship. The arcopallium has established pre-motor functions in passerines (Suthers and Margoliash, 2002); thus, androgens might act in this part of the central nervous system to facilitate the fine motor control required for jump-snap displays, wingsnaps, and roll-snaps.
The cerebellum has a crucial role in complex motor function (Paula-Barbosa and Sobrinho-Simoes, 1976; Tohyama, 1976; Paula-Barbosa et al., 1980; Smith et al., 1993; Molinari et al., 2001; Rodriguez et al., 2005; Yoshida et al., 2007) and was found to express significant AR in the golden-collared manakin. As in other vertebrates, limb movements of passeriform species activate cerebellar immediate-early gene expression (Feenders et al., 2008), and lesions of the cerebellum disturb motor and cognitive performance (Spence et al., 2009). Studies have also found that the volume of the cerebellum is associated with complex motor and cognitive functions related to avian courtship (Day et al., 2005). Thus, androgens may exert considerable influence over manakin courtship by their actions on the cerebellum. It is also worth mentioning that in several birds and mammals, cerebellar Purkinje neurons and the deep cerebellar express steroidogenic enzymes (Tsutsui and Ukena, 1999; London et al., 2006) including the androgen synthetic enzyme cytochrome P450 17α-hydroxylase (London et al., 2003). Cerebellar AR may therefore bind locally produced androgens as part one component of androgen signaling in golden-collared manakins.
AR expressing cells in the nT and the preoptic area have been described in other oscine and suboscine birds and probably function to motivate the performance of courtship (Hutchison, 1967; Jarvis et al., 1998; Heimovics and Riters, 2007; Riters and Ball, 1999; Alger and Riters, 2006). In fact, these areas are thought to be part of a conserved network of steroid-sensitive brain regions that underlie the basic components of animal sociality (Newman, 1999; Goodson, 2005; Goodson and Kabelik, 2009; O’Connell and Hofmann, 2011), including courtship (Balthazart et al., 1998; Riters and Ball, 1999; Absil et al., 2002).
4.4.2. Spinal cord
The brain communicates with most peripheral muscle tissues by way of the spinal cord. These circuits can be relatively simple; that is, descending neural fibers from central motor centers can synapse directly onto spinal motoneurons, which in turn project onto muscles in the periphery. At the same time, some of these circuits can be complex, polysynaptic pathways, whereby descending fibers synapse onto spinal interneurons, rather than motoneurons. While these interneurons eventually connect to motoneurons, they also receive input from other descending pathways and somatosensory fibers. Thus, spinal interneuron pools are capable of integrating various sources of information from the brain and from the body to control movement, balance, and reflexes (Kiehn, 2006).
Because of the spinal cord’s role in guiding physical movement, this part of the nervous system is a prime target on which androgenic hormones might act to potentially modulate male golden-collared manakin motor skills. Wild, adult males and females given tritiated-testosterone (3H-T) accumulate 3H-steroid throughout much of their spinal cord, though most of this accumulation is observed in the cervical and lumbosacral spinal enlargements (Schultz and Schlinger, 1999). These areas of the spinal cord contain the motoneurons that innervate the muscles that control the wings and legs, respectively; thus, the data suggest that testosterone is able to act within the golden-collared manakins’ spinal tissues and exert its most concentrated effects at the motor circuitry responsible for controlling motions of the appendages and reflexes.
Equally interesting is that this study also shows that males accumulate higher levels of 3H-T in their spinal cord than females (Schultz and Schlinger, 1999). This sex difference of course suggests that the enhanced ability to detect androgens in spinal tissues is associated with the sexually dimorphic ability to produce wing-snap and jump-snap displays. Androgen-spinal interactions may therefore represent a physiological phenotype that has evolved to facilitate complex motor coordination for courtship.
Subsequent work from our lab suggests that effects of testosterone on the spinal cord likely occur through AR, rather than through ER (after testosterone is aromatized to E; see above). To show this, wild male and female manakins were injected in their wing and leg muscles with retrograde, fluorescent tracers that back-fill (and thus label) motoneuron somas in the spinal cord. AR mRNA in these tissues was then labeled using in situ hybridization, and the results showed that the motoneurons controlling wing and leg muscles expressed considerable amounts of AR (Fuxjager et al., 2012b) (Fig. 3a). Thus, androgenic hormones can signal directly on the cell bodies of motoneurons involved in activating the complex movements associated with their display signals. To this end, this same data set also revealed that AR was expressed in the dorsal root ganglia, which convey information of sensory afferent fibers from the wing and leg muscles to spinal cord interneurons (Fig. 3b). It, thus, appears that androgens are capable of modulating the neural fibers that transmit somatosensory feedback from the periphery to the CNS.
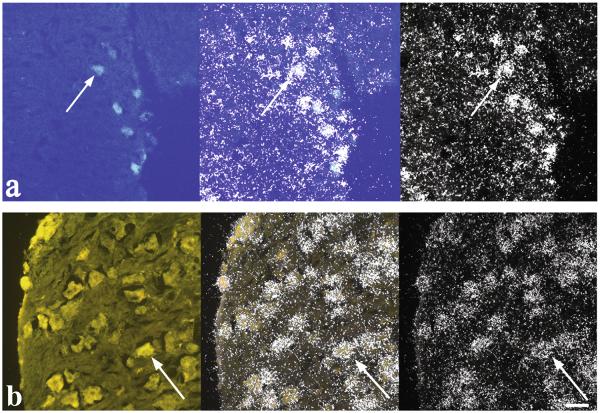
Fig. 3.
Photomicrographs of androgen receptor (AR) expressing cells in the golden-collared manakin spinal cord. The top panel (a) shows spinal motoneurons that innervate the wing-lifting supracoracoideus (SC) muscle, whereas the bottom panel (b) shows cells in the dorsal root ganglia, which relay somatic information from the SC to the spinal cord. On the left, photomicrographs show cells labeled with fluorescent tracers under ultraviolet illumination. On the right, photomicrographs show the same tissue under dark-field illumination to illustrate hybridization of the AR in situ probe. In the center, photomicrographs show an overlay of the images from the left and right; this image indicates that fluorescent cells express abundant AR mRNA. White arrows point to the same cell in all three images. Reference bar = 40 μm. Figures are modified from (Fuxjager et al., 2012a).
Somewhat surprisingly, we found that male and female golden-collared manakins do not differ in their relative levels or patterns of spinal cord AR expression. In fact, females appear to express slightly more AR in their spinal cords than males (Fuxjager et al., 2012b). This result at first seems at odds with our prior studies showing that females do not accumulate as much 3H-steroid in their spinal cord as males after 3H-T injections (Schultz and Schlinger, 1999). However, it is possible that this discrepancy results from sex differences in androgen metabolism (Jurman et al., 1982; Breedlove and Arnold, 1983) or AR protein half-lives. The enzyme 5α-reductase (see below) is expressed at high levels in the male golden-collared manakin spinal cord (Fuxjager et al., unpublished data) suggesting that testosterone metabolism may be the more important consideration. We are currently optimizing conditions for use of AR antibodies to assess their protein levels in golden-collared manakin tissues. From a functional perspective, it is also intriguing that female golden-collared manakins maintain high levels of AR in their spinal tissues, particularly if this phenotype contributes to male-typical sexual display behavior. It is likely that the abundant AR in females has minimal function, because birds of this sex have low levels of circulating T and thus little capacity to actually activate these receptors (Wikelski et al., 2003; Day et al., 2007).
Exactly how does androgenic action within the male manakin spinal cord affect behavioral output? The answer to this question is not completely clear, mostly because there are few studies that examine how sex steroids influence avian spinal tissues and function (but see Evrard and Balthazart, 2002). Our findings suggest that the enhanced ability of the golden-collared manakin spinal cord to detect androgenic hormones is unique among passerine birds (Fuxjager et al., 2012b), though more comparative work is required. Nonetheless, these findings are consistent with the idea that this special spinal phenotype underlies some of the complex motor characteristics of displaying males. There is certainly a precedent for this line of thinking, especially in mammals. For example, in rodents, AR modulates functional and morphological features of motoneurons in the spinal nucleus of the bulbocavernosus (SBN) (Breedlove and Arnold, 1981; Kurz et al., 1986; Matsumoto et al., 1988). These cells control the bulbocavernosus (BC) and levator ani (LA) muscles (Rand and Breedlove, 1992), which in turn govern penile reflexes critical for successful copulation (Holmes and Sachs, 1994). Thus, similar to findings in golden-collared manakins, studies of the SBN-BC/LA neuromuscular circuitry suggest that selection has linked the regulatory effects of masculine sex steroids with sex-related reflexes and locomotor ability.
4.4.3. Skeletal muscles
The jumping, twisting, turning and many postural adjustments that are employed during the manakin courtship display involve coordinated contractions of a diversity of skeletal muscle systems. What stands out, however, are those skeletal muscles that control wing kinematics leading to the wing- and roll-snap behaviors. Birds lift their wings largely by contraction of the ventral supracoracoideus (SC) and retract the wings by contraction of the ventral pectoralis (PEC) muscle (Dial, 1992). During wingsnaps, manakins likely also lift their wings, or control the wing upon lift, by contraction of the scapulohumeralis caudalis (SH) muscle, a muscle noticeably hypertrophied in the golden-collared manakin (Lowe, 1942). Each of these muscles shows specializations that indicate that they are adapted for the production of the powerful and fast movements of the wing- and roll-snaps (Lowe, 1942; Schultz et al., 2001). One such feature of the muscle tissue itself is its remarkable sensitivity to androgen hormones, as golden-collared manakin wing muscles express considerably more AR mRNA than other oscine and suboscine passerine species (Fig. 4a) (Feng et al., 2010). Indeed, AR expression in golden-collared manakin musculature is relatively more robust than its expression in other known androgen targets, like the brain, spinal cord, and gonads.
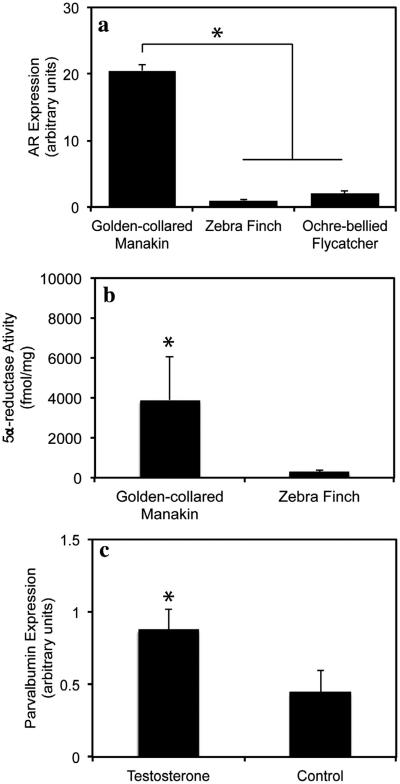
Fig. 4.
Expression and function of androgen receptors (AR) in the male golden-collared manakin supracoricoideus (SC) wing muscle. The top figure (a) shows that AR mRNA expression is significantly higher in golden-collared manakin SC than in the SC of either the ochre-bellied flycatcher (a tropical suboscine) or the zebra finch (a classic oscine model). The middle figure (b) shows that activity levels of the 5α-reductase enzyme are greater in the golden-collared manakin SC as compared to the zebra finch SC. As such, the manakin SC has a greater capacity to locally covert testosterone to the more potent 5α-DHT. The bottom graph (c) shows that, in golden-collared manakins, testosterone increases expression of parvalbumin mRNA in the SC. This indicates that muscular AR in this species is functional. All significant differences are denoted by asterisks (*) above the relevant bars, and data represent mean ± 1SEM. Data are taken from (Feng et al., 2010; Fuxjager et al., 2012a).
This unique pattern of AR expression in golden-collared manakins is detected in both breeding and non-breeding males, as well as in females. The relative stability of high AR expression in skeletal muscle across reproductive state and sex implies that a permanent genetic (or even epigenetic) mechanism evolved to constitutively sustain robust production of this protein. Again, because females maintain low levels of circulating T, it is unlikely that muscular AR is ever activated in this sex in a way that induces strong physiological effects to the female musculoskeletal system.
The activation of skeletal muscle AR seems to have functional consequences in golden-collared manakins. Testosterone treatment of non-breeding male manakins that have naturally basal levels of circulating androgen causes an increase in mRNA expression of parvalbumin (Fig. 4c) and IGF-I, two genes that are important for modulating basic muscle physiology (Fuxjager et al., 2012a). Parvalbumin enhances the way in which Ca2+ is shuttled from the muscle’s contractile machinery to its sarcoplasmic reticulum, effectively increasing the speed of muscle relaxation and hastening the muscle contraction cycle (Heizmann et al., 1982; Muntener et al., 1995; Arif, 2009). IGF-I acts on IGF-I receptors expressed locally in muscle tissues to promote muscle fiber hypertrophy and increase the strength of effected muscle (Coleman et al., 1995; Sacheck et al., 2004). Thus, androgen-dependent up-regulation of these gene products may represent functional mechanisms by which androgens optimize muscle performance in a manner consistent with this species’ muscular needs for courtship. From a broader perspective, this study provides a template from which we can deduce how trade-offs in the transcriptional program of a cell or tissue may ultimately facilitate and coordinate shifts in life history tactics, such as the transition from non-breeding to breeding states.
Androgen action on skeletal muscle AR may influence more than simply the muscle itself; that is, it may also change spinal motoneurons that innervate the targeted muscle. This has been widely demonstrated in rodents, using the AR-sensitive SBN-BC/LA neuromuscular circuit as a model. This work shows that activation of AR within the LA muscles itself changes the morphology of the innervating motoneuron cell bodies within the SBN (Rand and Breedlove, 1995). This is thought to occur in part as a result of AR-dependent expression of brain-derived neurotrophic factor (BDNF) in the muscle cells, which is retrogradely transported from the muscle to the motoneuron soma (Verhovshek et al., 2010; Verhovshek et al., 2013) where it then acts via its receptor, tyrosine-related kinase B (trkB), to enhance motoneuron size and dendritic arbor (Verhovshek and Sengelaub, 2010). IGF-I produced locally in muscle tissue is also retrogradely transported to the spinal cord to induce neurotrophic effects (Kaspar et al., 2003; Dobrowolny et al., 2005). Thus, given that androgens up-regulate muscle signaling hormones, like IGF-I (Fuxjager et al., 2012a), in the golden-collared manakin, androgenic signaling in the peripheral wing muscles may similarly impact general maintenance and plasticity in spinal motor circuits that guide these tissues. This hypothesis supports the notion that androgen action outside of the central nervous system plays a critical role in modulating the properties that underlie activation and/or production of acrobatic manakin display maneuvers.
4.4.4. Muscle 5α-reductase
The enzyme 5α-reductase converts testosterone into 5α-dihydrotestosterone (DHT), a compound that binds and activates androgen receptors more potently than testosterone and is crucial for androgen action on many tissues (Martini et al., 1996). Research in mammals suggests that the local 5α-reduction of circulating testosterone is an important step in the regulation of facilitating androgen-dependent muscle function (Aizawa et al., 2010, 2011). Thus, we hypothesized that this enzyme influences how androgens affect manakin muscles used in courtship. Therefore, we examined this enzyme in two muscles involved in the male manakin courtship display, the wing-lifting SC and the gluteal muscle used for jumping and we compared activity in manakins to that seen in the zebra finch, a species that lacks a complex physical courtship (Feng et al., 2010). Interestingly, 5α-reductase was readily measureable in both muscles of both species and, in the manakins, the enzyme was present at comparable levels in males and females (Feng et al., 2010). Across species, 5α-reductase was more active in the SC muscle of the manakins compared to zebra finches (Fig. 4b), with the opposite pattern observed in the gluteal. Thus, when circulating T levels are elevated in male manakins, 5α-reductase may increase the local concentration of DHT in the SC to promote the wingsnap display. Why 5α-reductase is elevated in the zebra finch gluteal is unknown. However, androgens do regulate gene expression in zebra finch skeletal muscle, as we have seen in golden-collared manakins (Fuxjager et al., 2012a), so 5α-reductase may play an important role in the skeletal muscles of these birds as well. The widespread action of androgens on avian skeletal muscle is ripe for further study.
4.5. Neuroanatomical specializations
4.5.1. Sexual dimorphism in brain
Emboldened by our previous experience visualizing the highly conspicuous neural song system of oscine songbirds (that controls the relatively small but complex muscular syrinx (Nottebohm et al., 1976), we assumed that an examination of the male golden-collared manakin brain would reveal a conspicuous neural circuitry controlling the numerous and massive muscles that produce their courtship display. Inspection of the manakin brain, however, revealed no gross morphological features of motor control brain regions that might subserve complex courtship (Day et al., 2011). As is the case of oscine songbirds (Nottebohm and Arnold, 1976) and most other vertebrates, the brains of male and females can differ markedly in structure and function and, whereas sex steroid hormones are known to participate in this sexual differentiation process, sex-chromosome effects cannot be ignored (Wade et al., 1999; Arnold, 2004; Juntti et al., 2010; McCarthy et al., 2012). When we directly compared the brains of adult male and female manakin, several sexually dimorphic features were detectable that give us some clues as to regions that might participate in the courtship circuitry.
Most notably,we found that relative to the telencephalon, the arcopallium was larger in volume in males manakins compared to females (Day et al., 2011). Recall that we find AR widely distributed throughout the golden-collared manakin arcopallium further piquing our interest in this brain region. Sub-regions of the avian arcopallium are known to contribute to sensorimotor (anterior) and limbic (posterior) functions (Zeier, 1971; Sadananda et al., 2007; Saint-Dizier et al., 2009) including involvement in copulation and courtship displays (Charlier et al., 2005; Sadananda et al., 2007). Thus, we measured soma sizes and density in five sub-regions of the golden-collared manakin arcopallium to look for sex-differences that might provide clues into arcopallial function and/or specializations in this species. We found no overall sex differences in any regions of the arcopallium but we found that regions were cytoarchitecturally distinct. Furthermore, the pattern of cytoarchitectural differences was different across the sexes with nT most neuron dense in females and least cell dense in males. The significance of these differences in cell density requires further study.
We also found that relative to the telencephalon, the hippocampus of male manakins was larger than that of females. This regions is closely associated with spatial abilities in birds and mammals (Brownlie and Sherry, 1996; Hampton and Shettleworth, 1996; Clayton and Reboreda, 1997; Biegler et al., 2001; Bingman and Able, 2002; Day et al., 2008; Sherry and Hoshooley, 2010). Although the hippocampus may not be involved in courtship per se, golden-collared manakin males have extreme site fidelity with the same bird being found displaying at the same arena for several years. These sites are abandoned during the rainiest time of year (Fig. 2) when birds migrate elsewhere (Chapman, 1935) and the birds return for breeding the following year. Relocating this small patch of forest for courtship might require exceptional spatial memory capabilities in males, and thus an enlarged hippocampus. Females also disperse and return to leks as well, but females may be guided by the acoustic signals of the males rather than relying on their own spatial memory.
We were struck by finding sex differences in visual processing areas of the brain, with the volume of a downstream nucleus of the tectofugal visual pathway, the ventrolateral mesopallium (MVL) being larger in females than in males. As pointed out previously, females are able to discriminate minute temporal and perhaps postural features of the male displays and use different visual cues to select males for mating (Uy and Endler, 2004; Barske et al., 2011). These abilities may be associated with MVL specialization in the female manakins as the MVL is thought to modulate visually guided behavior via striatal-brainstem pathways (Krutzfeldt and Wild, 2004) and to have associative functions. Thus, it is tempting to speculate that MVL is larger in females to assist the visually tracking males during their display. Perhaps use of a rapid visual processing capability by females in selecting males for copulations has driven the evolution of the rapid physical movements of the males’ courtship display. Studies to evaluate visual processing speed in manakins, especially compared to other bird species, could shed light on this possibility.
4.6. New directions
4.6.1. Cardiovascular system and steroids in manakins
The preceding material has focused on tissues and systems that have conserved functions in courtship, as well as some new perspectives, such as the elevated androgen sensitivity of golden-collared manakin skeletal muscles. There are likely other anatomical and physiological systems that might be targets of sex-steroids to enable male courtship. As one example, we focus on the heart. During male golden-collared manakin courtship, heart rates rise to unprecedented levels, as high as 1300 beats/minute (Barske et al., 2011) almost six times their resting heart rate. The most successful males perform up to 140 such displays per day suggesting they might possess cardiovascular specializations (Höglund and Alatalo, 1995). Mammalian studies show that the heart is a target of sex steroids (McGill et al., 1980; Malhotra et al., 1990; Nahrendorf et al., 2003; Handelsman, 2009; Bhupathy et al., 2010). We are currently investigating steroid sensitivity, morphometrics and patterns of gene expression in the golden-collared manakin heart to ascertain if steroids impact optimal function. If plasma testosterone does impact heart rate, it could do so directly on the myocardium or indirectly via the CNS. AR are found in the central cardiovascular regulatory regions in mammals(Peuler et al., 1990; Pouliot et al., 1996) and androgens can influence sympathetic tone and parasympathetic control the heart (El-mas et al., 2001). Future studies might investigate such links in avian heart.
5. Conclusions
So, let us return to some of the issues that were raised at the outset and delineated in Table 1 and ask how our research on manakins can offer insight into more general current questions regarding the neuroendocrine control of courtship behavior.
With regard to motor control of complex courtship, it seems likely that androgens can indeed have widespread actions on the complete neuromuscular circuitry linking motor elements in the brain, through the spinal cord, to skeletal muscles, and returning back to the central nervous system via somatosensory systems. Whether and how signals might ultimately return to motor or cognitive circuits in the brain remains unexplored. Moreover, how central cortical, striatal, mid- and hind- brain circuits are linked to coordinate the numerous motor elements of complex courtship and how, or whether, androgens are involved remain an outstanding questions as well. Nevertheless, with regard to golden-collaed manakins, these birds have evolved numerous anatomical and physiological specializations to enable courtship, with androgens appearing to serve as a dominant switch to activate this fascinating behavior (Fig. 5).
The spinal cord may prove to be an especially interesting site of hormone action for enabling the specific motor patterns that are employed during courtship. There is good evidence from work on other species, including humans, that upon appropriate activation, spinal circuits hold a great deal of the patterning of motor behavior (Courtine et al., 2009). This may well be the case for features of complex courtship itself. Undeniably, sex steroids play a crucial role in facilitating muscular contractions involved in reproductive systems like the bulbocavernosus muscles (Sachs and Leipheimer, 1988). We believe it will be of value to explore hormone-dependent spinal circuits in the context of complex behaviors, like courtship.
Although we have not yet fully defined the motor control pathways of even the some of the most conspicuous elements of the manakin courtship display, our data point to hormone-dependent pre-motor and motor control pathways that likely contribute to performance of courtship displays. As discussed previously, there are likely multiple motivational influences that contribute to all of the display elements, so the connectivity from motivational to pre-motor areas, and the roles of androgens and estrogens on these pathways remain important unsolved pieces of the overall puzzle.
Whereas skeletal muscle has long been viewed as a target of androgens, especially in humans, its role as an androgen-dependent organ system controlling natural animal behavior has received less attention. In golden-collared manakins, skeletal muscle appears to be a key target for sex steroids in promoting complex physical movements of courtship. Not only do androgens appear to promote optimal gene expression in some muscles to enable contraction capabilities appropriate for displays, but hormone action or muscle use itself might serve to retrogradely signal to the spinal cord and brain to optimize and coordinate motor output.
We were surprised to discover that treatments with testosterone could activate some complex components of male courtship in female golden-collared manakins. We conclude from this that the neuromuscular systems have not undergone sexual differentiation and are readily available to be activated in the presence of the gonadal hormone signal. Do females utilize the same circuits, but in a sub-optimal way, when they dance with males? Does testosterone serve only to optimize the performance? Might it be problematic to create sexually dimorphic neuromuscular systems when most, if not all, of those muscles are used also in normal locomotion and postural control? We are attempting to explore the extent to which testosterone can activate other elements of male manakin courtship to perhaps shed light on some of these questions.
There is the tantalizing possibility that neurons in the arcopallium that have evolved in the pre-motor control of syringeal function (i.e. in one form of acoustic communication) are exploited in golden-collared manakins for the production of wingsnaps (a non-vocal form of acoustic communication). Our current results do not allow us to reach this conclusion yet, but we are working on this problem. There is the intriguing idea that neural control of vocal structures arose from circuits that served to control limb movements in other species (Bass and Chagnaud, 2012). In humans, this idea has been used to argue that gesturing is neuroanatomically related to speech in their communication functions. Perhaps wingsnapping in Manacus species arose in a similar fashion and from a similar neural circuitry as a secondary (or even primary) form of acoustic communication.
We have good evidence from our combined many hours of field observations of manakins that juvenile males must learn to excel at their acrobatic and speedy courtship displays. Young males, with a green-plumage like females (having not yet grown their adult male plumage), often create transient arenas near established leks where they perform shortened, relatively slow displays and produce weak snaps. In some cases, these juvenile males enter the lek and join an adult in a “duo dance”; the unsuspecting adult appears unable to discriminate juvenile males from reproductively accessible females. Whether the males simply improve by practicing an innate behavior or actually learn features of the dance from adult males is unknown but a question we are attempting to answer. No doubt, breeding experience can influence the frequency of courtship displays (Cheng et al., 1986), but whether display elements are acquired and perfected via experience is not well established.
Golden-collared manakins only perform courtship in a defined, if not lengthy, season of courtship and, as we have pointed out above, the exact role of circulating hormones is not entirely clear. Some birds, especially many species of waterfowl, engage in courtship throughout the winter (e.g. Dane and Van Der Kloot, 1964), even under conditions of extreme cold when it would be suboptimal for the gonads to be active and steroidogenic. Whether steroids play any role in courtship under these conditions is unknown but worthy of exploration. Perhaps, a pro-hormone like dehydroepiandrosterone (DHEA) circulates at elevated levels during the non-breeding season as has been detected in some birds (Soma and Wingfield, 2001). In the presence of the steroid-metabolic enzymes 3β-HSD, 17β-HSD and aromatase, DHEA could be converted locally in brain into active androgens and/or estrogens to promote the motivation to court with little impact on tissues lacking these enzymes (Schlinger et al., 2008b). Alternatively, steroids may play no role in courtship outside of the breeding season. This degree of steroid-independence would alter our conceptions about the reliance of these avian courtship behaviors on gonadal function. Dissociation between gonadal activity and courtship behavior has been described previously in snakes (Crews et al., 1984). Thus, we should not be surprised to find diverse neurohormonal mechanisms controlling courtship across the many varied species of birds.
Inasmuch as steroids employ actions on nuclear receptors and membrane receptors, and can regulate gene expression, intracellular second messenger systems and membrane ion channels to facilitate behavior (McCarthy, 2008), we have little doubt a multiplicity of mechanisms are employed to enable the numerous coordinated behaviors of complex courtship. Rapid actions of steroids on skeletal muscle may be a particularly promising area of research (Sachs and Leipheimer, 1988; Monks et al., 2004). This area of research is timely and worthy of our attention.
Finally, there is enormous body of work citing mate choice as the crucial impetus for the evolution of the male’s courtship display. Thus we can surmise that the female’s auditory and visual detection and processing of the male’s signals is central to considerations of how and why the male’s display evolves as it does. We can then easily image that the neurobiology of female sensory systems may be particularly suited for the male’s signals, or that his display evolves to meet the demands of her capabilities. Our results suggest that female manakins may indeed possess properties of the visual processing system that differ from males. Further work understanding the basis of these differences and whether they apply to courtship are now warranted. Although we are often biased to focusing on males when considering the neurendocrine basis of male behavior, perhaps it is time to place the spotlight on females and consider what it is about the neurobiology/endocrinology of females that makes males do what they do.
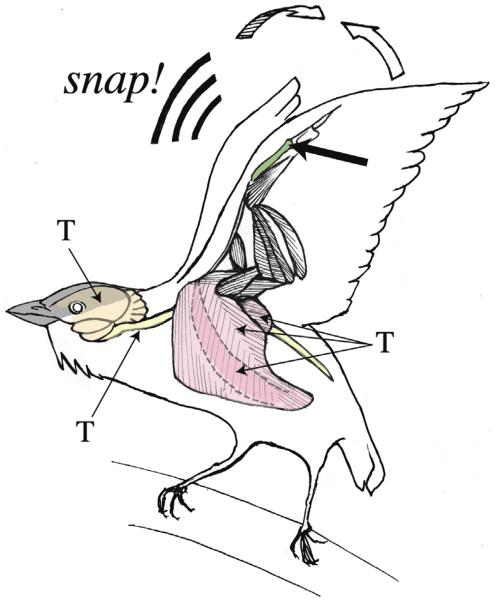
Fig. 5.
Summary schematic of a reproductively active male golden-collared manakin and an androgen sensitive neuromuscular circuit that presumably underlies his remarkable courtship display. The male is perched and is repeatedly snapping his wings together above its head to produce a roll-snap sonations. The thin arrows point to tissues within the manakin on which androgens, like testosterone (T), act to influence wing-snap behavior (by their high expression of androgen-receptors). These tissues include the brain (highlighted in orange), the spinal cord (highlighted in yellow), and the three main muscles that control the wing (highlighted in pink). The largest of the three highlighted muscles is the pectoralis (PEC), which retracts the wing upon contraction. Deep to the PEC, however, is the supracoicoideus (SC), which is depicted by a darker pink and the dashed lines running through the PEC. The SC helps raise the wing when it contracts. The final muscle is the dorsal scapulohumeralis caudalis (SH) that helps stabilize and elevate the wing upon contraction (Dial, 1992; Schultz et al., 2001; Bostwick and Prum, 2003). The thick arrow points to the manakin’s radius (highlighted in green). Preliminary evidence from our laboratory shows that this bone is flattened as compared to other species and thus may facilitate sounds production of wing-snaps.
Supplementary Material
Acknowledgments
International field work of this type can only be done as a team and that includes a vast number of people and institutions whose contributions big and small pervade our work. We are grateful for funding from NSF that has supported this work over many years (currently IOS-1147288). We are indebted to the staff at the Smithsonian Tropical Research Institute without whose help none of this would have been possible. Their devotion to the scientists is extraordinary. The government of Panama has graciously allowed us to work in their country and to study “their” birds and we thank the various government agencies for their approval. In particular, we thanks Autoridad Nacional del Ambiente and Autoridad del Canal de Panamá for permission to conduct research in Panama. We thank the Panamanian citizens for their hospitality. We have had numerous field assistants and collaborators over the years whose help has been invaluable.
Footnotes
Appendix A. Supplementary material
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.yfrne.2013.04.001.
References
- Absil P, Papello M, et al. The medial preoptic nucleus receives vasotocinergic inputs in male quail: a tract-tracing and immunocytochemical study. J. Chem. Neuroanat. 2002;24(1):27–39. doi: 10.1016/s0891-0618(02)00017-0. [DOI] [PubMed] [Google Scholar]
- Adkins EK, Mason P. Effects of cyproterone acetate in the male Japanese quail. Horm. Behav. 1974;5(1):1–6. doi: 10.1016/0018-506x(74)90001-4. [DOI] [PubMed] [Google Scholar]
- Adkins-Regan E. Hormonal mechanisms of mate choice. Am. Zool. 1998;38(1):166–178. [Google Scholar]
- Adkins-Regan E. Hormones and Animal Social Behavior. Princeton University Press; Princeton: 2005. [Google Scholar]
- Aizawa K, Iemitsu M, et al. Acute exercise activates local bioactive androgen metabolism in skeletal muscle. Steroids. 2010;75(3):219–223. doi: 10.1016/j.steroids.2009.12.002. [DOI] [PubMed] [Google Scholar]
- Aizawa K, Iemitsu M, et al. Endurance exercise training enhances local sex steroidogenesis in skeletal muscle. Med. Sci. Sports Exerc. 2011;43(11):2072–2080. doi: 10.1249/MSS.0b013e31821e9d74. [DOI] [PubMed] [Google Scholar]
- Akre KL, Ryan MJ. Female tungara frogs elicit more complex mating signals from males. Behav. Ecol. 2011;22:846–853. [Google Scholar]
- Alatalo RV, Hoglund J, et al. Testosterone and male mating success on the black grouse leks. Proc. R. Soc. Lond. B. 1996;263(1377):1697–1702. [Google Scholar]
- Alger SJ, Riters LV. Lesions to the medial preoptic nucleus differentially affect singing and nest box-directed behaviors within and outside of the breeding season in European starlings (Sturnus vulgaris) Behav. Neurosci. 2006;120:1326–1336. doi: 10.1037/0735-7044.120.6.1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arif SH. A Ca2+-binding protein with numerous roles and uses: parvalbumin in molecular biology and physiology. BioEssays. 2009;31:410–421. doi: 10.1002/bies.200800170. [DOI] [PubMed] [Google Scholar]
- Arnold AP. Sex chromosomes and brain gender. Nat. Rev. Neurosci. 2004;5:701–708. doi: 10.1038/nrn1494. [DOI] [PubMed] [Google Scholar]
- Arnold AP, Nottebohm F, et al. Hormone concentrating cells in vocal control and other areas of brain of zebra finch (Poephila-Guttata) J. Comp. Neurol. 1976;165(4):487–511. doi: 10.1002/cne.901650406. [DOI] [PubMed] [Google Scholar]
- Balthazart J, Ball G. Brain Aromatase Estrogens and Behavior. Oxford University Press; 2013. [Google Scholar]
- Balthazart J, Tlemcani O, et al. Do sex differences in the brain explain sex differences in the hormonal induction of reproductive behavior? What 25 years of research on the Japanesequailtellsus. Horm.Behav. 1996;30(4):627–661. doi: 10.1006/hbeh.1996.0066. [DOI] [PubMed] [Google Scholar]
- Balthazart J, Absil P, et al. Appetitive and consummatory male sexual behavior in Japanese quail are differentially regulated by subregions of the preoptic medial nucleus. J. Neurosci. 1998;18(16):6512–6527. doi: 10.1523/JNEUROSCI.18-16-06512.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker FK. Phylogeny and diversification of modern passerines. In: Dyke GKG, editor. Living Dinosaurs: The Evolutionary History of Modern Birds. J. Wiley and Sons; 2011. pp. 235–256. [Google Scholar]
- Barske J, Schlinger B, et al. Female choice for male motor skills. Proc. R. Soc. B: Biol. Sci. 2011;278:3523–3528. doi: 10.1098/rspb.2011.0382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bass AH, Chagnaud BP. Shared developmental and evolutionary origins for neural basis of vocal–acoustic and pectoral–gestural signaling. Proc. Nat. Acad. Sci. U.S.A. 2012;109:10677–10684. doi: 10.1073/pnas.1201886109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beach FA, Inman NG. Effects of castration and androgen replacement on mating in male quail. Proc. Nat. Acad. Sci. U.S.A. 1965;54:1426–1431. doi: 10.1073/pnas.54.5.1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belle MDC, Sharp PJ, et al. Aromatase inhibition abolishes courtship behaviours in the ring dove (Streptopelia risoria) and reduces androgen and progesterone receptors in the hypothalamus and anterior pituitary gland. Mol. Cell. Biochem. 2005;276:193–204. doi: 10.1007/s11010-005-4060-6. [DOI] [PubMed] [Google Scholar]
- Bhupathy P, Haines CD, et al. Influence of sex hormones and phytoestrogens on heart disease in men and women. Womens Health. 2010;6:77–95. doi: 10.2217/whe.09.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biegler R, McGregor A, et al. A larger hippocampus is associated with longer-lasting spatial memory. Proc. Nat. Acad. Sci. U.S.A. 2001;98(12):6941–6944. doi: 10.1073/pnas.121034798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bingman VP, Able KP. Maps in birds: representational mechanisms and neural bases. Curr. Opin. Neurobiol. 2002;12(6):745–750. doi: 10.1016/s0959-4388(02)00375-6. [DOI] [PubMed] [Google Scholar]
- Bostwick KS, Prum RO. High-speed video analysis of wing-snapping in two manakin clades (Pipridae: Aves) J. Exp. Biol. 2003;206(20):3693–3706. doi: 10.1242/jeb.00598. [DOI] [PubMed] [Google Scholar]
- Breedlove SM, Arnold AP. Sexually dimorphic motor nucleus in the rat lumbar spinal cord: response to adult hormone manipulation, absence in androgen-insensitive rats. Brain Res. 1981;225(2):297–307. doi: 10.1016/0006-8993(81)90837-4. [DOI] [PubMed] [Google Scholar]
- Breedlove SM, Arnold AP. Sex-differences in the pattern of steroid accumulation by motoneurons of the rat lumbar spinal-cord. J. Comp. Neurol. 1983;215(2):211–216. doi: 10.1002/cne.902150208. [DOI] [PubMed] [Google Scholar]
- Brockway BF. The influence of some experientialandgeneticfactors, including hormones, on the visible courtship behavior of budgerigars(Melopsittacus) Behaviour. 1974;51(1–2):1–18. doi: 10.1163/156853974x00110. [DOI] [PubMed] [Google Scholar]
- Brown JL. Vocalization evoked from the optic lobe of a songbird. Science. 1965;149:1002–1003. doi: 10.1126/science.149.3687.1002. [DOI] [PubMed] [Google Scholar]
- Brownlie L, Sherry D. Dissociation of memory for spatial and non-spatial information by lesions of the chickadee hippocampus; A Synthetic Approach to Studying Animal Cognition, Flagstaff, AZ; 1996. [Google Scholar]
- Candolin U. The use of multiple cues in mate choice. Biol. Rev. 2003;78:575–595. doi: 10.1017/s1464793103006158. [DOI] [PubMed] [Google Scholar]
- Canoine V, Gwinner E. Seasonal differences in the hormonal control of territorial aggression in free-living European stonechats. Horm. Behav. 2002;41:1–8. doi: 10.1006/hbeh.2001.1720. [DOI] [PubMed] [Google Scholar]
- Chapman FM. The courtship of gould’s manakin (Manacus vitellinus vitellinus) on Barro Colorado Island, Canal Zone. Bull. Am. Mus. Nat. History. 1935;68:472–521. [Google Scholar]
- Charlier TD, Ball GF, et al. Sexual behavior activates the expression of the immediate early genes c-fos and Zenk (EGR-1) in catecholaminergic neurons of male Japanese quail. Neuroscience. 2005;131(1):13–30. doi: 10.1016/j.neuroscience.2004.09.068. [DOI] [PubMed] [Google Scholar]
- Chastel O, Barbraud C, et al. High levels of LH and testosterone in a tropical seabird with and elaborate courtship display. Gen. Comp. Endocrinol. 2005;140:33–40. doi: 10.1016/j.ygcen.2004.10.012. [DOI] [PubMed] [Google Scholar]
- Chen CC, Welsbie DS, et al. Molecular determinants of resistance to antiandrogen therapy. Nat. Med. 2004;10:33–39. doi: 10.1038/nm972. [DOI] [PubMed] [Google Scholar]
- Cheng MF, Klint T, et al. Breeding experience modulating androgen dependent courtship behavior in male ring doves (Streptopelia risoria) Physiol. Behav. 1986;36:625–630. doi: 10.1016/0031-9384(86)90344-6. [DOI] [PubMed] [Google Scholar]
- Clayton NS, Reboreda JC. Seasonal changes of hippocampus volume in parasitic cowbirds. Behav. Process. 1997;41:237–243. doi: 10.1016/s0376-6357(97)00050-8. [DOI] [PubMed] [Google Scholar]
- Cohen J. Hormones and midbrain mediation of courtship behavior in the male ring dove (Streptopelia risoria) J. Comp. Physiol. Psychol. 1981;95(4):512–526. doi: 10.1037/h0077797. [DOI] [PubMed] [Google Scholar]
- Coleman ME, Demayo F, et al. Myogenic vector expression of insulin-like growth factor I stimulates mucle cell differentiation and myofiberhypertrophy in transgenic mice. J. Biol. Chem. 1995;270:12109–12116. doi: 10.1074/jbc.270.20.12109. [DOI] [PubMed] [Google Scholar]
- Courtine G, Gerasimenko Y, et al. Transformation of nonfunctional spinal circuits into functional states after the loss ofbraininput. Nat.Neurosci. 2009;10:1333–1342. doi: 10.1038/nn.2401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crews D, Camazine B, Diamond M, Mason R, Tokarz RR, Garstka WR. Hormonal independence of courtship behavior in the male garter snake. Horm. Behav. 1984;18:29–41. doi: 10.1016/0018-506x(84)90048-5. [DOI] [PubMed] [Google Scholar]
- Dane B, Van Der Kloot WG. An analysis of the display of the goldeneye duck (Bucephala clangula) Behavior. 1964;22:282–327. [Google Scholar]
- Darwin C. The Descent of Man, and Selection in Relation to Sex. Appleton; New York: 1871. [Google Scholar]
- Day LB, Westcott DA, et al. Evolution of bower complexity and cerebllum size in bowerbirds. Brain Behav. Evol. 2005;66:62–72. doi: 10.1159/000085048. [DOI] [PubMed] [Google Scholar]
- Day L, Fusani L, et al. Testosterone and its effects on courtship in Golden-collared manakins: seasonal, sex and age differences. Horm. Behav. 2007;51:62–68. doi: 10.1016/j.yhbeh.2006.08.006. [DOI] [PubMed] [Google Scholar]
- Day LB, Guerra M, et al. Sex differences in the effects of captivity on hippocampus size in brown-headed cowbirds (Molothrus ater obscurys) Behav. Neurosci. 2008;122:527–534. doi: 10.1037/0735-7044.122.3.527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day LB, Fusani L, et al. Sexually dimorphic neural phenotypes in golden-collared manakins (Manacus vitellinus) Brain Behav. Evol. 2011;77(3):206–218. doi: 10.1159/000327046. [DOI] [PubMed] [Google Scholar]
- Dial KP. Activity petterns of the wing muscles of the pigeon (Columbia livia) during different modes of flight. J. Exp. Zool. 1992;262:357–373. [Google Scholar]
- Dobrowolny G, Giacinti C, et al. Muscle expression of a local Igf-1 isoform protects motor neurons in an ALS mouse model. J. Cell Biol. 2005;168:193–199. doi: 10.1083/jcb.200407021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DuVal EH, Goymann W. Hormonal correlates of social status and courtship display in the cooperatively lekking lance-tailed manakin. Horm. Behav. 2011;59:44–50. doi: 10.1016/j.yhbeh.2010.10.004. [DOI] [PubMed] [Google Scholar]
- Edler R, Goymann W, et al. Experimentally elevated testosterone levels enhance courtship behaviour and territoriality but depress acquired immune response in Red Bishops Euplectes orix. Ibis. 2011;153:46–58. [Google Scholar]
- El-Mas MM, Afify EA, et al. Testosterone facilitates the baroreceptor control of reflex bradycardia: role of cardiac sympathetic and parasympathetic components. J. Cardiovasc. Pharmacol. 2001;38(5):754–763. doi: 10.1097/00005344-200111000-00012. [DOI] [PubMed] [Google Scholar]
- Erpino MJ. Hormonal control of courtship behaviour in the pigeon (Columbalivia) Anim. Behav. 1969;17:401–405. doi: 10.1016/0003-3472(69)90139-0. [DOI] [PubMed] [Google Scholar]
- Evrard HC, Balthazart J. Localization of oestrogen receptors in the sensory and motor areas of the spinal cord in Japanese quail (Coturnix japonica) J. Neuroendocrinol. 2002;14(11):894–903. doi: 10.1046/j.1365-2826.2002.00857.x. [DOI] [PubMed] [Google Scholar]
- Evrard H, Baillen M, et al. Localization and control of aromatase in the quail spinal cord. J. Comp. Neurol. 2000;423:552–564. doi: 10.1002/1096-9861(20000807)423:4<552::aid-cne2>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- Feenders G, Liedvogel M, et al. Molecular mapping of movement-associated areas in the avian brain: a motor theory for vocal learning origin. Plos One. 2008;3 doi: 10.1371/journal.pone.0001768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng NY, Katz A, et al. Limb muscles are androgen targets in an acrobatic tropical bird. Endocrinology. 2010;151(3):1042–1049. doi: 10.1210/en.2009-0901. [DOI] [PubMed] [Google Scholar]
- Folstad I, Karter AJ. Parasites, bright males, and the immunocompetence handicap. Am. Natural. 1992;139(3):603–622. [Google Scholar]
- Fusani L. Testosterone control of male courtship in birds. Horm. Behav. 2008;54(2):227–233. doi: 10.1016/j.yhbeh.2008.04.004. [DOI] [PubMed] [Google Scholar]
- Fusani L, Hutchison JB. Lack of changes in the courtship behaviour of male ring doves after testosterone treatment. Ethol. Ecol. Evol. 2003;15(2):143–157. [Google Scholar]
- Fusani L, Gahr M, et al. Aromatase inhibition reduces specifically one display of the ring dove courtship behavior. Gen. Comp. Endocrinol. 2001;122(1):23–30. doi: 10.1006/gcen.2001.7608. [DOI] [PubMed] [Google Scholar]
- Fusani L, Donaldson Z, et al. The distribution of steroid receptors and aromatase in the brain and spinal cord of a suboscine bird with elaborate courtship display, the Golden-collared manakin. Horm. Behav. 2003;44:50. (Society for Behavioral Neuroendocrinology 2003 Annual Meeting) [Google Scholar]
- Fusani L, Day LB, et al. Androgen and the elaborate courtship behavior of a tropical lekking bird. Horm. Behav. 2007a;51(1):62–68. doi: 10.1016/j.yhbeh.2006.08.005. [DOI] [PubMed] [Google Scholar]
- Fusani L, Giordano M, et al. High-speed video analysis reveals individual differences in the courtship display of male golden-collared manakins. Ethology. 2007b;113:964–972. [Google Scholar]
- Fuxjager MJ, Barske J, et al. Androgens regulategene expression in avianskeletalmsucles. PLoS ONE. 2012a;7:e51482. doi: 10.1371/journal.pone.0051482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuxjager MJ, Schultz JD, et al. Spinal motor and sensory neurons are androgen targets in an acrobaticbird. Endocrinology. 2012b;153:3780–3791. doi: 10.1210/en.2012-1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gahr M, Guttinger HR, et al. Estrogen receptors in the avian brain: survey reveals general distribution and forebrain areas unique to songbirds. J. Comp. Neurol. 1993;327(1):112–122. doi: 10.1002/cne.903270109. [DOI] [PubMed] [Google Scholar]
- Gahr M, Geuttinger HR, et al. Neural song control system of hummingbirds: comparison to swifts, vocal learning (songbirds) and non-learning (sub-oscines) passerines, and vocal learning (budgerigars) and nonlearning (dove, owl, gull, quail, chicken) nonpasserines. J. Comp. Neurol. 2000;426:182–196. [PubMed] [Google Scholar]
- Goller F, Suthers RA. Role of syringeal muscles in controlling the phonology of bird song. J. Neurophysiol. 1996;76(1):287–300. doi: 10.1152/jn.1996.76.1.287. [DOI] [PubMed] [Google Scholar]
- Goodson JL. The vertebrate social behavior network: evolutionary themes and variations. Horm. Behav. 2005;48:11–22. doi: 10.1016/j.yhbeh.2005.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodson JL, Kabelik D. Dynamic limbic networks and social diversity in vertebrates: from neural context to neuromodulatory patterning. Front. Neuroend. 2009;30:429–441. doi: 10.1016/j.yfrne.2009.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goymann W, Moore IT, et al. Testosterone in tropical birds: effects of environmental and social factors. Am. Natural. 2004;164(3):327–334. doi: 10.1086/422856. [DOI] [PubMed] [Google Scholar]
- Greenspan RJ, Ferveur JF. Courtship in Drosophila. Annu. Rev. Genet. 2000;34:205–232. doi: 10.1146/annurev.genet.34.1.205. [DOI] [PubMed] [Google Scholar]
- Hampton RR, Shettleworth SJ. Hippocampus and memory in a food-storing and in a nonstoring bird species. Behav. Neurosci. 1996;110(5):946–964. doi: 10.1037//0735-7044.110.5.946. [DOI] [PubMed] [Google Scholar]
- Handelsman DJ. Androgens and cardiovascular disease. Endocr. Rev. 2009;24:313–340. doi: 10.1210/er.2003-0005. [DOI] [PubMed] [Google Scholar]
- Hau M, Gill SA, et al. Tropical field endocrinology: ecology and evolution of testosterone concentrations in male birds. Gen. Comp. Endocrinol. 2008;157:241–248. doi: 10.1016/j.ygcen.2008.05.008. [DOI] [PubMed] [Google Scholar]
- Hau M, Ricklefs RE, et al. Corticosterone, testosterone and life-history strategies of birds; Proceedings of the Royal Society B: Biological Sciences; 2010. pp. 3203–3212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hegner RE, Wingfield JC. Effects of experimental manipulation of testosterone levels on parental investment and breeding success in male house sparrows. Auk. 1987;104:462–469. [Google Scholar]
- Heimovics SA, Riters LV. ZENK labeling within social behavior brain regions reveals breeding context-dependent patterns of neural activity associated with song in male European starlings (Sturnus vulgaris) Behav. Brain Res. 2007;176:333–343. doi: 10.1016/j.bbr.2006.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heizmann CW, Berchtold MW, et al. Correlation of parvalbumin concentration with relaxation speed in mammalian muscle. Proc. Nat. Acad. Sci. U.S.A. 1982;79:7243–7247. doi: 10.1073/pnas.79.23.7243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hews DK, Moore MC. Hormones and sex-specific traits: critical questions. In: Beckage NE, editor. Parasites and Pathogens: Effects on Host Hormones and Behavior. Chapman and Hall; New York: 1997. pp. 277–292. [Google Scholar]
- Höglund J, Alatalo RV. Leks. Princeton University Press; Princeton, NJ: 1995. [Google Scholar]
- Holmes GM, Sachs BD. Physiology and mechanics of rat levator ani muscle: evidence for sexual function. Physiol. Behav. 1994;55:255–266. doi: 10.1016/0031-9384(94)90131-7. [DOI] [PubMed] [Google Scholar]
- Hutchison JB. Initiation of courtship by hypothalamic implants of testosterone proprionate in castrated doves (Streptopelia risoria) Nature. 1967;216:591–592. doi: 10.1038/216591a0. [DOI] [PubMed] [Google Scholar]
- Hutchison JB. Differential effects of testosterone and oestradiol on male courtship in Barbary doves (Streptopelia risoria) Anim. Behav. 1970;18:41–52. doi: 10.1016/0003-3472(70)90068-0. [DOI] [PubMed] [Google Scholar]
- Hutchison JB, Steimer T. Androgen metabolism in the brain: behavioural correlates. Prog. Brain Res. 1984;61(3):23–51. doi: 10.1016/S0079-6123(08)64427-1. [DOI] [PubMed] [Google Scholar]
- Huxley JS. The courtship habits of the Great-crested Grebe. Proc. Zool. Soc. Lond. 1914;34:491–563. [Google Scholar]
- Jarvis ED, Scharff C, et al. For whom the bird sings: context-dependent gene expression. Neuron. 1998;21(4):775–788. doi: 10.1016/s0896-6273(00)80594-2. [DOI] [PubMed] [Google Scholar]
- Johnsgard PA. Arena Birds: Sexual Selection and Behavior. Smithsonian Institution Press; Washington: 1994. [Google Scholar]
- Jones AG, Ratterman NL. Mate choice and sexual selection: what have we learned since Darwin? Proc. Nat. Acad. Sci. U.S.A. 2009;106:10001–10008. doi: 10.1073/pnas.0901129106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones MP, Pierce KE, et al. Avian vision: a review of form and function. Test. 2007;16:69–87. [Google Scholar]
- Juntti SA, Tollkuhn J, et al. The androgen receptor governs the execution, but not programming, of male sexual and territorial behaviors. Neuron. 2010;66(2):260–272. doi: 10.1016/j.neuron.2010.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jurman ME, Erulkar SD, et al. Testosterone 5-α-reductase in the spinal cord of Xenopus laevis. J. Neurochem. 1982;38:657–661. doi: 10.1111/j.1471-4159.1982.tb08681.x. [DOI] [PubMed] [Google Scholar]
- Kaspar BK, Llado J, et al. Retrograde viral delivery of IGF-1 prolongs survival in a mouse ALS model. Science. 2003;301:839–842. doi: 10.1126/science.1086137. [DOI] [PubMed] [Google Scholar]
- Kiehn O. Locomotor circuits in the mammalian spinal cord. Ann. Rev. Neurosci. 2006;29:279–306. doi: 10.1146/annurev.neuro.29.051605.112910. [DOI] [PubMed] [Google Scholar]
- Koch FC. The chemistry and biology of male sex hormones. Bull. N.Y. Acad. Med. 1938;14:655–680. [PMC free article] [PubMed] [Google Scholar]
- Kotiaho JS, LeBas NR, et al. On the resolution of the lek paradox. Trends Ecol. Evol. 2007;23:1–3. doi: 10.1016/j.tree.2007.09.012. [DOI] [PubMed] [Google Scholar]
- Kroodsma DE, Konishi M. A suboscine bird (eastern phoebe, Sayornis phoebe) develops normal song without auditory feedback. Anim. Behav. 1991;42(3):477–487. [Google Scholar]
- Krutzfeldt NO, Wild JM. Definition and connections of the entopallium in the zebra finch (Taeniopygia guttata) J. Comp. Neurol. 2004;468:452–465. doi: 10.1002/cne.10972. [DOI] [PubMed] [Google Scholar]
- Kurz EM, Sengelaub DR, et al. Androgens regulate the dendritic length of mammalian motoneurons in adulthood. Science. 1986;232(4748):395–398. doi: 10.1126/science.3961488. [DOI] [PubMed] [Google Scholar]
- Lill A. Sexual behavior of the lek-forming White-bearded Manakin (Manacus manacus trinitatis Hartert) Z. Tierpsychol. 1974;36:1–36. doi: 10.1111/j.1439-0310.1974.tb02126.x. [DOI] [PubMed] [Google Scholar]
- Lisano ME, Kaennamer JE. Seasonal variations in plasma testosterone level in male eastern wild turkeys. J. Wildl. Manege. 1977;41:184–188. [Google Scholar]
- London SE, Boulter J, et al. Cloning of the zebra finch androgen synthetic enzyme CYP17: a study of its neural expression throughout posthatch development. J. Comp. Neurol. 2003;467(4):496–508. doi: 10.1002/cne.10936. [DOI] [PubMed] [Google Scholar]
- London SE, Monks DA, et al. Widespread capacity for steroid synthesis in the avian brain and song system. Endocrinology. 2006;147(12):5975–5987. doi: 10.1210/en.2006-0154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe PR. The anatomy of Gould’s manakin (Manacus vitellinus) in relation to its display. Ibis. 1942;6(14):50–83. [Google Scholar]
- Malhotra A, Buttrick P, et al. Effects of sex hormones on development of physiological and pathological cardiac hypertrophy in male and female rats. Am. J. Physiol. 1990;259:H866–871. doi: 10.1152/ajpheart.1990.259.3.H866. [DOI] [PubMed] [Google Scholar]
- Martini L, Celotti F, et al. Testosterone and progesterone metabolism in the central nervous system: cellular localization and mechanism of control of the enzymes involved. Cell. Mol. Neurobiol. 1996;16(3):271–282. doi: 10.1007/BF02088095. [DOI] [PubMed] [Google Scholar]
- Matsumoto A, Arnold AP, et al. Androgenic regulation of gap junctions between motoneurons in the rat spinal cord. J. Neurosci. 1988;8(11):4177–4183. doi: 10.1523/JNEUROSCI.08-11-04177.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy MM. Estradiol and the developing brain. Physiol. Rev. 2008;88(1):91–124. doi: 10.1152/physrev.00010.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy MM, Arnold AP, et al. Sex differences in the brain: the not so inconvenient truth. J. Neurosci. 2012;32(7):2241–2247. doi: 10.1523/JNEUROSCI.5372-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald DB, Clay RP, et al. Sexual selection on plumage and behavior in an avian hybrid zone: experimental tests of male-male interactions. Evolution. 2001;55:1443–1451. doi: 10.1111/j.0014-3820.2001.tb00664.x. [DOI] [PubMed] [Google Scholar]
- McGill HC, Anselmo VC, et al. The heart is a target organ for androgen. Science. 1980;02:1–4. doi: 10.1126/science.6766222. [DOI] [PubMed] [Google Scholar]
- Meitzen J, Moore IT, et al. Steroid hormones act transsynaptically within the Forebrain to regulate neuronal phenotype and song stereotypy. J. Neurosci. 2007;27(44):12045–12057. doi: 10.1523/JNEUROSCI.3289-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metzdorf R, Gahr M, et al. Distribution of aromatase, estrogen receptor, and androgen receptor mRNA in the forebrain of songbirds and nonsongbirds. J. Comp. Neurol. 1999;407(1):115–129. [PubMed] [Google Scholar]
- Molinari M, Leggio MG, et al. Cerebellar contribution to spatial event processing: involvement in procedural and working memory components. Eur. J. Neurosci. 2001;14:2011–2022. doi: 10.1046/j.0953-816x.2001.01819.x. [DOI] [PubMed] [Google Scholar]
- Monks DA, O’Bryant EL, et al. Androgen receptor immunoreactivity in skeletal muscle: enrichment at the neuromuscularjunction. J. Comp. Neurol. 2004;473(1):59–72. doi: 10.1002/cne.20088. [DOI] [PubMed] [Google Scholar]
- Moore IT, Perfito N, et al. Latitudinal variation in plasma testosterone levels in birds of the genus Zonotrichia. Gen. Comp.Endocrinol. 2002;129(1):13–19. doi: 10.1016/s0016-6480(02)00563-4. [DOI] [PubMed] [Google Scholar]
- Mougeot F, Martínez-Padilla J, Webster LM, Blount JD, Pérez-Rodríguez L, Piertney SB. Honest sexual signalling mediated by parasite and testosterone effects on oxidative balance. Proc. Biol. Sci. 2009;276:1093–1100. doi: 10.1098/rspb.2008.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muntener M, Kaser L, et al. Increase of skeletal muscle relaxation speed by direct injection of parvalbumin cDNA. Proc. Nat. Acad. Sci. U.S.A. 1995;92:6504–6508. doi: 10.1073/pnas.92.14.6504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nahrendorf M, Frantz S, et al. Effect of testosterone on post-myocardial infarction remodeling and function. Cardiovasc. Res. 2003;57:370–378. doi: 10.1016/s0008-6363(02)00701-0. [DOI] [PubMed] [Google Scholar]
- Newman SW. The medial extended amygdala in male reproductive behavior – a node in the mammalian social behavior network; Advancing from the Ventral Striatum to the Extended Amygdala: Implications for Neuropsychiatry and Drug Abuse: In Honor of Lennart Heimer; 1999. pp. 242–257. [DOI] [PubMed] [Google Scholar]
- Nottebohm F, Arnold AP. Sexual dimorphism in vocal control areas of the songbird brain. Science. 1976;194(4261):211–213. doi: 10.1126/science.959852. [DOI] [PubMed] [Google Scholar]
- Nottebohm F, Stokes TM, et al. Central control of song in the canary, Serinus canarius. J. Comp. Neurol. 1976;165(4):457–486. doi: 10.1002/cne.901650405. [DOI] [PubMed] [Google Scholar]
- O’Connell LA, Hofmann HA. The Vertebrate mesolimbic reward system and social behavior network: a comparative synthesis. J. Comp. Neurol. 2011;519:3599–3639. doi: 10.1002/cne.22735. [DOI] [PubMed] [Google Scholar]
- Osorno JL, Nunez-de la Mora A, et al. Hormonal correlates of breeding behavior and pouch coloration in the Magnificent Frigatebird, Fregata magnificens. Gen. Comp. Endocrinol. 2010;169:18–22. doi: 10.1016/j.ygcen.2010.06.011. [DOI] [PubMed] [Google Scholar]
- Patricelli GL, Uy AC, et al. Male displays adjusted to female’s response. Nature. 2002;415:279–280. doi: 10.1038/415279a. [DOI] [PubMed] [Google Scholar]
- Paula-Barbosa MM, Sobrinho-Simoes MA. An ultrastructural morphometric study of mossy fiber endings in pideon, rat and man. J. Comp. Neurol. 1976;170:365–379. doi: 10.1002/cne.901700307. [DOI] [PubMed] [Google Scholar]
- Paula-Barbosa MM, Sobrinho-Simoes MA, et al. Comparative morphometric study of cerebellar neurons. I. Granule cells. Acta Anat. (Basel) 1980;106:262–269. doi: 10.1159/000145189. [DOI] [PubMed] [Google Scholar]
- Peuler JD, Edwards GL, et al. Area postrema and differential reflex effects of vasopressin and phenylephrine in rats. Am. J. Physiol. Heart Circul. Physiol. 1990;258:1255–1259. doi: 10.1152/ajpheart.1990.258.4.H1255. [DOI] [PubMed] [Google Scholar]
- Pietras RJ, Wenzel BM. Effects of androgens on body weight, feeding, and courtship behavior in the pigeon. Horm. Behav. 1974;5:289–302. doi: 10.1016/0018-506x(74)90016-6. [DOI] [PubMed] [Google Scholar]
- Pouliot WA, Handa RJ, et al. Androgen modulates N-methyld-aspartate-mediated depolarization in CA1 hippocampal pyramidal cells. Synapse. 1996;23:10–19. doi: 10.1002/(SICI)1098-2396(199605)23:1<10::AID-SYN2>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Prum RO. Sexual selection and the evolution of mechanical sound production in manakins (Aves: Pipridae) Anim. Behav. 1998;55(4):977–994. doi: 10.1006/anbe.1997.0647. [DOI] [PubMed] [Google Scholar]
- Prum RO. Aesthetic evolution by mate choice: Darwin’s really dangerous idea. Philos. Trans. Roy. Soc. Lond. Ser. B: Biol. Sci. 2012;367:2253–2265. doi: 10.1098/rstb.2011.0285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pycraft WP. The Courtship of Animals. Hutchison and Co; London: 1913. [Google Scholar]
- Rand MN, Breedlove SM. Androgen locally regulates rat bulbocavernosus and levator ani size. J. Neurobiol. 1992;23(1):17–30. doi: 10.1002/neu.480230104. [DOI] [PubMed] [Google Scholar]
- Rand MN, Breedlove SM. Androgen alters the dendritic arbors of SNB motoneurons by acting upon their target muscles. J. Neurosci. 1995;15(6):4408–4416. doi: 10.1523/JNEUROSCI.15-06-04408.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remage-Healey L, Coleman MJ, et al. Brain estrogens rapidly strengthen auditory encoding and guide song preference in a songbird. Proc. Nat. Acad. Sci. U.S.A. 2010;107(8):3852–3857. doi: 10.1073/pnas.0906572107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riters LV, Ball GF. Lesions to the medial preoptic area affect singing in the male European starling (Sturnus vulgaris) Horm. Behav. 1999;36(3):276–286. doi: 10.1006/hbeh.1999.1549. [DOI] [PubMed] [Google Scholar]
- Roberts ML, Buchanan KL, Evans MR. Testing the immunocompetence hadnicap hypothesis: a review of the evidence. Anim. Behav. 2004;68:227–239. [Google Scholar]
- Rodriguez F, Duran E, et al. Cognitive and emotional functions of the teleost fish cerebellum. Brain Res. Bull. 2005;66:365–370. doi: 10.1016/j.brainresbull.2004.11.026. [DOI] [PubMed] [Google Scholar]
- Sacheck JM, Ohtsuka A, et al. IGF-I stimulates muscle growth by suppressing protein breakdown and expression of atrophy-related ubiquitin ligases, atrogin-1 and MuRF1. Am. J. Physiol. Endocrinol. Metab. 2004;287:E591–E601. doi: 10.1152/ajpendo.00073.2004. [DOI] [PubMed] [Google Scholar]
- Sachs BD, Leipheimer RE. Rapid effect of testosterone on striated muscle activity inrats. Neuroendocrinology. 1988;48(5):453–458. doi: 10.1159/000125049. [DOI] [PubMed] [Google Scholar]
- Sadananda M, Korte S, et al. Afferentiation of a caudal forebrain area activated during courtship behavior: a tracing study in the zebra finch (Taeniopygia guttata) BrainRes. 2007;1184:108–120. doi: 10.1016/j.brainres.2007.09.040. [DOI] [PubMed] [Google Scholar]
- Saint-Dizier H, Constantin P, et al. Subdivisions of the arcopallium/posterior pallial amygdala complex are differentially involved in the control of fear behaviour in the Japanese quail. Brain Res. Bull. 2009;79(5):288–295. doi: 10.1016/j.brainresbull.2009.03.004. [DOI] [PubMed] [Google Scholar]
- Saldanha CJ, Schultz JD, et al. Telencephalic aromatase, but not a song system, in a sub-oscine passerine, the golden collared manakin (Manacus vitellinus) Brain Behav. Evol. 2000;56:29–37. doi: 10.1159/000006675. [DOI] [PubMed] [Google Scholar]
- Saldanha CJ, Remage-Healey L, et al. Neuroanatomical Distribution of Aromatase in Birds: Cellular and Subcellular Analyses. Oxford University Press, J.B.a.G.F. Ball; 2013. pp. 100–114. [Google Scholar]
- Schlinger BA, Balthazart J. Aromatase and behavior: concepts gained from studies of aromatase in the avian brain. In: Balthazart J, Ball Brain G, editors. Aromatase Estrogens and Behavior. Oxford University Press; 2013. pp. 169–198. [Google Scholar]
- Schlinger BA, Brenowitz EA. Neural and hormonal control of birdsong. In: Pfaff DW, Arnold AP, Etgen AM, Fahrbach SE, Rubin RT, editors. Hormones, Brain and Behavior. Academic Press; San Diego: 2009. pp. 897–941. [Google Scholar]
- Schlinger BA, Day LB, et al. Behavior, natural history and neuroendocrinology of a tropical bird. Gen. Comp. Endocrinol. 2008a;157(3):254–258. doi: 10.1016/j.ygcen.2008.05.015. [DOI] [PubMed] [Google Scholar]
- Schlinger BA, Pradhan DS, et al. 3(beta)-HSD activates DHEA in the songbird brain. Neurochem. Int. 2008b;52:611–620. doi: 10.1016/j.neuint.2007.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz JD, Schlinger BA. Widespread accumulation of [(3)H]testosterone in the spinal cord of a wild bird with an elaborate courtship display. Proc. Nat. Acad. Sci. U.S.A. 1999;96(18):10428–10432. doi: 10.1073/pnas.96.18.10428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz JD, Hertel F, et al. Adaptations for rapid and forceful contraction in wing muscles of the male golden-collared manakin: sex and species comparisons. J. Comp. Physiol. A. 2001;187(9):677–684. doi: 10.1007/s00359-001-0239-9. [DOI] [PubMed] [Google Scholar]
- Schwabl H, Kriner E. Territorial aggression and song of male European robins (Erithacus rubecula) in autumn and spring: effects of antiandrogen treatment. Horm.Behav. 1991;25(2):180–194. doi: 10.1016/0018-506x(91)90049-n. [DOI] [PubMed] [Google Scholar]
- Sherry DF, Hoshooley JS. Seasonal hippocampal plasticity in food-storing birds. Philos. Trans. R. Soc. Lond. B Biol.Sci. 2010;365(1542):933–943. doi: 10.1098/rstb.2009.0220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shet MS, McPhaul M, et al. Metabolism of the antiandrogenic drug (flutamide) by human CYP1A2. Drug Metab. Dispos.: Biol. Fate Chem. 1997;25:1298–1303. [PubMed] [Google Scholar]
- Smith TG, Brauer K, et al. Quantitative phylogenetic constancy of cerebellar Purkinje cell morphological complexity. J. Comp. Neurol. 1993;331:402–406. doi: 10.1002/cne.903310309. [DOI] [PubMed] [Google Scholar]
- Snow DW. A field study of the Black and White Manakin, Manacus manacus, in Trinidad. Zoologica. 1962;47(8):65–104. [Google Scholar]
- Soma KK, Wingfield JC. Dehydroepiandrosterone in songbird plasma: Seasonal regulation and relationship to territorial aggression. Gen. Comp. Endocrinol. 2001;123(2):144–155. doi: 10.1006/gcen.2001.7657. [DOI] [PubMed] [Google Scholar]
- Spence RD, Zhen Y, et al. Recovery of motor and cognitive function after cerebellar lesions in a songbird: role of estrogens. Eur. J. Neurosci. 2009;29(6):1225–1234. doi: 10.1111/j.1460-9568.2009.06685.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein AC, Uy JAC. Plumage brightness predicts male mating success in the lekking golden-collared manakin, Manacus vitellinus. Behav. Ecol. 2006;17:41–47. [Google Scholar]
- Suthers RA, Margoliash D. Motor control of birdsong. Curr. Opin. Neurobiol. 2002;12(6):684–690. doi: 10.1016/s0959-4388(02)00386-0. [DOI] [PubMed] [Google Scholar]
- Suthers RA, Goller F, et al. The neuromuscular control of birdsong. Philos. Trans. R. Soc. Lond. B Biol. Sci. 1999;354(1385):927–939. doi: 10.1098/rstb.1999.0444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tohyama M. Comparative anatomy of cerebellar catecholamine innervation from teleosts to mammals. J. fur Hirnforsch. 1976;17:43–60. [PubMed] [Google Scholar]
- Tramontin AD, Wingfield JC, et al. Androgens and estrogens induce seasonal-like growth of song nuclei in the adult songbird brain. J. Neurobiol. 2003;57(2):130–140. doi: 10.1002/neu.10263. [DOI] [PubMed] [Google Scholar]
- Tsutsui K, Ukena K. Neurosteroids in the cerebellar Purkinje neuron and their actions (Review) Intl. J. Mol.Med. 1999;4:49–56. [PubMed] [Google Scholar]
- Uy JAC, Endler JA. Modification of the visualbackground increases the conspicuousness of golden-collared manakin displays. Behav.Ecol. 2004;15:1003–1010. [Google Scholar]
- Verhovshek T, Sengelaub DR. Trophic effects of brain-derivedneurotrophic factor blockade in an androgen-sensitive neuromuscular system. Endocrinology. 2010;151:5337–5348. doi: 10.1210/en.2010-0799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verhovshek T, Cai Y, et al. Androgen regulates brain-derived neurotrophic factor in spinal motoneurons and their target musculature. Endocrinology. 2010;151:253–261. doi: 10.1210/en.2009-1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verhovshek T, Rudolph LM, Sengelaub DR. Brain-derived neurotrophic factor and androgen interactions in spinal neuromuscular systems. Neuroscience. 2013;239:103–114. doi: 10.1016/j.neuroscience.2012.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wade J. Relationships among hormones, brain and motivated behaviors in lizards. Horm. Behav. 2011;59:637–644. doi: 10.1016/j.yhbeh.2010.08.014. [DOI] [PubMed] [Google Scholar]
- Wade J, Swender DA, et al. Sexual differentiation of the zebra finch song system parallels genetic, not gonadal, sex. Horm. Behav. 1999;36:141–152. doi: 10.1006/hbeh.1999.1537. [DOI] [PubMed] [Google Scholar]
- Wikelski M, Hau M, et al. Reproductive seasonality of seven neotropical passerine species. Condor. 2003;105(4):683–695. [Google Scholar]
- Wingfield JC, Silverin B. Ecophysiological studies of hormone-behavior relations in birds. In: Pfaff DW, editor. Hormones, Brain and Behavior. Vol. 2. Academic Press; Amsterdam: 2002. pp. 597–647. [Google Scholar]
- Wirth MP, Froschermaier SE. The antiandrogen withdrawal syndrome. Urol. Res. 1997;25:S67–S71. doi: 10.1007/BF00941991. [DOI] [PubMed] [Google Scholar]
- Yoshida T, Funabiki K, et al. Increased occurence of climbing fiber inputs to the cerebellar flocculus in a mutant mouse is correlated with the timeing delay of optokinetic response. Eur. J. Neurosci. 2007;25:1467–1474. doi: 10.1111/j.1460-9568.2007.05394.x. [DOI] [PubMed] [Google Scholar]
- Zahavi A. Sexual selection, signal selection and the handicap principle. In: Jamieson BGM, editor. Reproductive Biology and Phylogeny of Birds. Science Publishers Enfield; New Hampshire England: 2007. pp. 143–159. [Google Scholar]
- Zeier H. Archistriatal lesions and response inhibition in the pigeon. Brain Res. 1971;31(2):327–339. doi: 10.1016/0006-8993(71)90186-7. [DOI] [PubMed] [Google Scholar]
- Zigmond RE, Notteboh F, et al. Androgen-concentrating cells in midbrain of a songbird. Science. 1973;179(4077):1005–1007. doi: 10.1126/science.179.4077.1005. [DOI] [PubMed] [Google Scholar]
- Zornik E, Kelley DB. A neuroendocrine basis for the hierarchical control of frog courtship vocalizations. Front. Neuroendocrinol. 2011;32:353–366. doi: 10.1016/j.yfrne.2010.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.