Abstract
Aim
Both muscle mass and strength decline with ageing, but the loss of strength far surpasses what is projected based on the decline in mass. Interestingly, the accumulation of fat mass has been shown to be a strong predictor of functional loss and disability. Furthermore, there is a known attenuated hypertrophic response to skeletal muscle overload with ageing. The purpose of this study was to determine the effect of 28 days of overload on the storage of intramuscular triglycerides (IMTG) and metabolic regulators of lipid synthesis in young and old skeletal muscle.
Methods
The phosphorylation and expression of essential lipogenic regulators were determined in the plantaris of young (YNG; 6-month-old) and aged (OLD; 30-month-old) rats subjected to bilateral synergist ablation (SA) of two-thirds of the gastrocnemius muscle or sham surgery.
Results
We demonstrate that age-induced increases in IMTG are associated with enhancements in the expression of lipogenic regulators in muscle. We also show that the phosphorylation and concentration of the 5′AMP-activated protein kinase (AMPK) isoforms are altered in OLD. We observed increases in the expression of lipogenic regulators and AMPK signalling after SA in YNG, despite no increase in IMTG. Markers of oxidative capacity were increased in YNG after SA. These overload-induced effects were blunted in OLD.
Conclusion
These data suggest that lipid metabolism may be altered in ageing skeletal muscle and is unaffected by mechanical overload via SA. By determining the role of increased lipid storage on skeletal muscle mass during ageing, possible gene targets for the treatment of sarcopenia may be identified.
Keywords: lipid metabolism, mechanical overload, sarcopenia, skeletal muscle
The loss of muscle mass and function with age, or sarcopenia, is associated with substantial social and economic costs as a result of impairments in strength and function, which ultimately lead to physical disability and institutionalization (Frontera et al. 1991, Baumgartner et al. 1998). Despite these observations, the idea that a decline in physical functioning is fundamentally related to a decrease in muscle mass remains controversial. Both muscle mass and strength decline with age, but the loss of strength far surpasses what is projected based on the decline in mass (Hughes et al. 2001, Lauretani et al. 2003, Goodpaster et al. 2006, Stenholm et al. 2008). This may be explained by deterioration in muscle quality including decreased fibre size and number, reduced contractility of fibres, motor neurone loss and intramyocellular lipid infiltration (Lexell et al. 1988, Sipila & Suominen 1994, Larsson et al. 1997, Visser et al. 2000, Goodpaster et al. 2001, Delbono 2003, Cree et al. 2004). Interestingly, in several epidemiological studies the accumulation of fat mass has been shown to be a strong independent predictor of subsequent functional loss and disability (Sipila & Suominen 1994, Visser et al. 2000, Goodpaster et al. 2001).
The master transcription factor, sterol regulatory element-binding protein (SREBP) 1, mediates transcriptional effects on lipogenic genes including fatty-acid synthase (FAS), acetyl-CoA carboxylase (ACC) and stearoyl-CoA desaturase (SCD) (Osborne 2000, Dobrzyn & Dobrzyn 2006, Sampath et al. 2007, Wang et al. 2009). These enzymes catalyse the synthesis of fatty acids that are the key substrates for the formation of complex lipids such as triglycerides, diglycerides and ceramides, which have been shown to be upregulated in obesity and insulin resistance (Hyde et al. 2005, Summers & Nelson 2005, Brownsey et al. 2006, Dobrzyn & Dobrzyn 2006, Holland et al. 2007, Lessard et al. 2007, Sampath et al. 2007, Erion & Shulman 2010). However, their function in age-associated skeletal muscle loss and anabolic resistance are currently unknown.
The 5′AMP-activated protein kinase (AMPK) is a sensor of cellular energy homeostasis and has well-established roles in the regulation of glucose and lipid metabolism (Hawley & Lessard 2008, Osler & Zierath 2008, Witczak et al. 2008, Steinberg & Kemp 2009). Recently, it has been recognized that AMPK activation is increased in both aged skeletal muscle (Thomson & Gordon 2005, Thomson et al. 2008, 2009) and in the presence of increased lipids or a high-fat diet (Watt et al. 2006, Lessard et al. 2007) and may have a function in the inhibition of the response of aged skeletal muscle to contraction-induced anabolic stimulation (Thomson et al. 2009). In support of this contention, the AMPKα1 catalytic subunit is thought to have a role in the prevention of excess muscle growth by the inhibition of the mammalian target of rapamycin (mTOR) anabolic pathway (Mounier et al. 2009, McGee et al. 2008, Rivas et al. 2009a).
We (Parkington et al. 2004, Funai et al. 2006, Chale-Rush et al. 2009) and others (Cutlip et al. 2006, Thomson & Gordon 2006, Baker et al. 2008, Dennis et al. 2008, Drummond et al. 2008, Kumar et al. 2009) have previously demonstrated that the ability of aged humans (Dennis et al. 2008, Drummond et al. 2008, Kumar et al. 2009) and rodents (Parkington et al. 2004, Thomson & Gordon 2005, 2006, Cutlip et al. 2006, Funai et al. 2006, Baker et al. 2008, Chale-Rush et al. 2009) to adapt (i.e. hypertrophy, increased anabolic signalling, etc.) following overload is blunted compared with young. Therefore, the aim of the present study was to determine what role age-related lipid accumulation in skeletal muscle has on muscle growth in response to 28 days of muscle overload. For this purpose, we determined the storage of triglycerides in skeletal muscle, the concentration of SREBP1 and its transcriptional target genes, the activation of AMPK and the concentration of the isoforms of AMPKα. We hypothesized that the storage of lipids would be increased in aged skeletal muscle and this would be associated with an increase in lipogenic capacity leading to the activation of AMPK even in animals that have undergone chronic skeletal muscle overload.
Methods
Experimental animals
Young adult (YNG; 6-month-old, n = 16) and aged (OLD; 30-month-old, n = 16) male Fischer 344 × Brown Norway rats were purchased from the National Institute on Aging. The Fischer 344 × Brown Norway rat strain is less susceptible to disease (Lipman et al. 1996) and shows muscle atrophy that is similar to aged skeletal muscle in humans (Blough & Linderman 2000). Rats were housed in a temperature-controlled animal room (21 °C) maintained on a 12-h light–dark cycle. Animals were provided with standard chow diets and water ad libitum. Rats were acclimatized for 14 days and fasted overnight before initiation of experimental protocol. All animal experimentation procedures were carried out with the approval of Institutional Animal Use and Care Committee of the Jean Mayer USDA Human Nutrition Research Center at Tufts University.
Experimental design and synergist ablation
Following the 14-day acclimatization period, YNG and OLD rats were randomly assigned to one of the four groups (n = 8/group): YNG control (YNG CON), YNG surgically ablated (YNG SA), OLD control (OLD CON) and OLD surgically ablated (OLD SA). Surgical procedures were performed under aseptic conditions after rats were anaesthetized with 2–3% isofluorane gas supplemented with oxygen. The animals underwent either bilateral SA (YNG, n = 8; OLD, n = 8) of two-thirds of the gastrocnemius muscle or sham operation (YNG CON, n = 8; OLD CON, n = 8). SA was performed to induce overload for 28 days and promote compensatory hypertrophy in the plantaris (PLAN) as previously described (Chale-Rush et al. 2009).
Analysis of intramuscular lipid storage and citrate synthase activity
Portions of PLAN muscle were freeze–dried, powdered and analysed for the content of glycerol and maximal citrate synthase (CS) activity as previously described (Lessard et al. 2011, Rivas et al. 2011). Briefly, freeze–dried muscle was powdered and cleaned of all visible connective tissue and blood under magnification. Portions (4–5 mg) of the sample (8–10/group) were used to fluorometrically determine skeletal muscle triacylglycerol (total glycerol) content, following Folch lipid extraction and saponification. Portions (3–4 mg) of the sample (8–10/group) were homogenized in 100 mm potassium phosphate buffer (pH 7.3, 1 : 400 dilution), and CS was assayed spectrophotometrically at 25 °C by the reduction of DTNB as previously described (Srere 1969, Chi et al. 1983).
Western blotting analysis
The phosphorylation and concentration of signalling proteins were quantified with Western blot analyses as previously described (Rivas et al. 2009b). Muscle samples were cut and weighed, frozen and homogenized in an ice-cold homogenization buffer (1 : 10 wt/vol) containing 50 mm Tris–HCl (pH 7.5), 5 mm Na-pyrophosphate, 50 mm NaF, 1 mm EDTA, 1 mm EGTA, 10% glycerol (v/v), 1% Triton-X, 1 mm DTT, 1 mm benz-amidine, 1 mm PMSF, 10 μg mL−1 trypsin inhibitor and 2 μg mL−1 aprotinin. Following centrifugation (21 000 g, 4 °C) for 15 min, the supernatant was collected and assayed for protein content. PLAN (30 μg) were solubilized in Laemmli buffer, separated by SDS-PAGE, and transferred to nitrocellulose membranes. The membranes were then blocked (5% NFDM), and incubated overnight at 4 °C with primary antibodies specific for SREBP1 (ab3259; Abcam, Cambridge, MA, USA), SCD1 (ab19862; Abcam), FAS1 (ab22759; Abcam), Phospho-ACC Ser79 (3661; Cell Signaling Technology, Danvers, MA, USA), ACC (3676; Cell Signaling), AMPKα1 (2795, Cell Signaling), AMPKα2 (2757; Cell Signaling), Phospho-AMPKα Thr172 (3562; Cell Signaling), Cytochrome c (4280; Cell Signaling), Cytochrome c Oxidase IV (COX IV; 4850; Cell Signaling). Membranes were probed with α/β-tubulin (T6074; Sigma, St Louis, MO, USA) antibody to monitor protein loading. The immunoreactive proteins were detected with Supersignal Chemiluminescent Substrate (Thermo Scientific, Rockford, IL, USA). Images were scanned and band intensities quantified by optical density using standardized bandwidths (Alpha Innotech Corporation, San Leandro, CA, USA).
Quantitative mRNA analysis
The quantification of mRNA was determined on SCD1, FAS1, ACC1 and ACC2 as previously described (Lessard et al. 2009). RNA was extracted from muscle using RNeasy Fibrous Tissue Mini Kit (74704; Qiagen, Germantown, MD, USA). cDNA levels of SCD1 (QT02285493), FAS1 (QT00371210), ACC1 (QT00190946) and ACC2 (QT01082655) were measured using commercially available primer mixtures (QuantiTect Primer Assays; Qiagen). All reactions were run using a commercially available reaction mixture (QuantiFast SYBR Green RT-PCR Kit; Qiagen) on a Stratagene MX3000P (Agilent, Santa Clara, CA, USA). Changes in gene expression were normalized to the housekeeping gene glyceraldehyde 3-phosphate dehydrogenase (QT00199633). Efficiencies of each primer set were assessed using a standard curve, analysed using 0.001–10 ng of control cDNA.
Statistical analysis
Differences between groups were identified using a two-way analysis of variance (anova) with Bonferroni posttest performed with graphpad prism version 5.00 for Windows (GraphPad Software, CA, USA, http://www.graphpad.com). Results are expressed as mean ± SEM and statistical significance was accepted at P < 0.05.
Results
Animal body weights, muscle wet weights and total protein contents
OLD animals had a significant decrease in body weight from day 0 to day 28 compared with YNG (P < 0.0001 vs. YNG; Table 1) and SA were significantly different after 28 days of overload compared with CON (P = 0.01 vs. CON; Table 1). Older animals had significantly smaller PLAN muscle wet weights (P < 0.0001 vs. YNG; Table 1) and 28 days of overload significantly increased PLAN wet weight in both YNG and OLD (P = 0.0002 vs. CON; Table 1). However, we have previously reported in these animals that the PLAN muscle weights when normalized to body weight were significantly higher in YNG animals with SA and this effect was attenuated in OLD (Chale-Rush et al. 2009). The total protein content of the PLAN muscles was also lower in OLD (P = 0.0036 vs. YNG; Table 1) while overload caused a significantly higher total protein content of 80% in SA and 64% in YNG and OLD respectively (P = 0.0004 vs. CON; Table 1).
Table 1.
Change in whole-body weights (g) from day 0 to day 28, plantaris muscle wet weights and plantaris total protein content of young (YNG), old (OLD) and surgically ablated (SA) Fischer 344 × Brown Norway rats
| YNG |
OLD |
|||
|---|---|---|---|---|
| CON | SA | CON | SA | |
| Δ Body weight (g) | 27.9 ± 4.0 | −0.04 ± 3.7† | −46.3 ± 3.7* | −76.4 ± 4.2*† |
| Plantaris wet weight (mg) | 434.0 ± 13.8 | 506.5 ± 19.7† | 352.8 ± 6.4* | 402.1 ± 14.4*† |
| Total protein content (mg) | 25.13 ± 1.8 | 45.16 ± 5.7† | 17.30 ± 1.9* | 28.52 ± 4.0*† |
Significant differences between groups P < 0.05 vs. YNG
Significant differences between groups P < 0.05 vs. CON; n = 8/group.
Intramuscular triglycerides
Ageing led to a 52% higher storage of intramuscular triglycerides (IMTG) (P = 0.0002 vs. YNG; Fig. 1).
Figure 1.
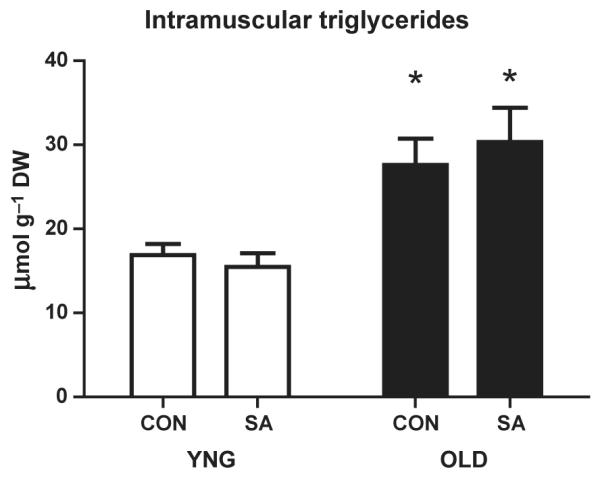
Intramuscular lipid storage and mitochondrial enzyme activity in the skeletal muscle of young (YNG), old (OLD) and surgically ablated (SA) Fischer 344 × Brown Norway rats. Intramuscular triglyceride content was determined in plantaris on aliquots of freeze–dried/powdered muscle and expressed per mg of dry weight (DW). *P < 0.05.
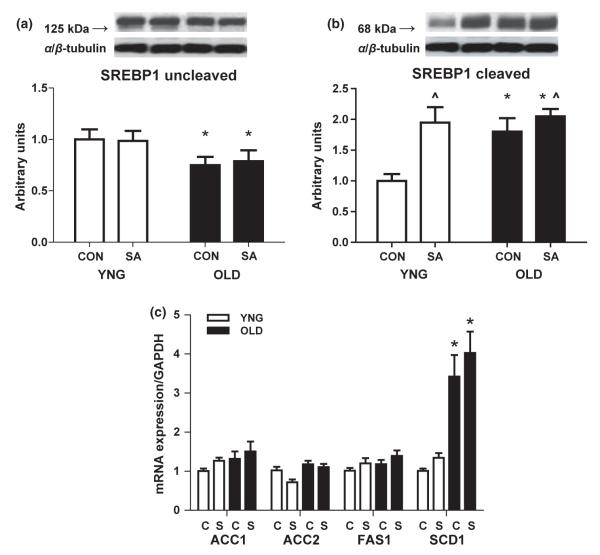
Concentration of active SREBP1 and lipid synthesis genes
There was a significantly lower concentration of the inactive uncleaved form of SREBP1 in OLD (P = 0.037 vs. YNG; Fig. 2a) while there was no change in SA in either age group (Fig. 2a). The active cleaved form of SREBP1 was higher with ageing (P = 0.024 vs. YNG; Fig. 2b) and after muscle overload (P = 0.004 vs. CON; Fig. 2b). In addition, there was a trend for an interaction between age and overload (P = 0.073, CON vs. SA; Fig. 2b) with a 90% increase in YNG and a 17% increase in OLD.
Figure 2.
Total protein content of the lipogenic transcription factor SREBP1 in the plantaris muscle of YNG, OLD and SA rats. Relative protein levels of the inactive uncleaved SREBP1 (a) and the active cleaved SBREP1 (b) were quantified using Western blot analysis and densitometry in plantaris muscle. mRNA expression (c) of the SREBP1 target genes ACC1/2, FAS1 and SCD1 differences between groups (*P < 0.05 vs. YNG, ^P < 0.05 vs. CON; n = 8/group).
We determined the gene expression of selected transcriptional targets of SREBP1. The gene expression of SCD1 was fourfold higher in OLD (P < 0.05 vs. YNG; Fig. 2c), although there was no effect in either age group by SA. However, there was no change in the expression of ACC1, ACC2 and FAS1 with ageing or muscle overload (Fig. 2c).
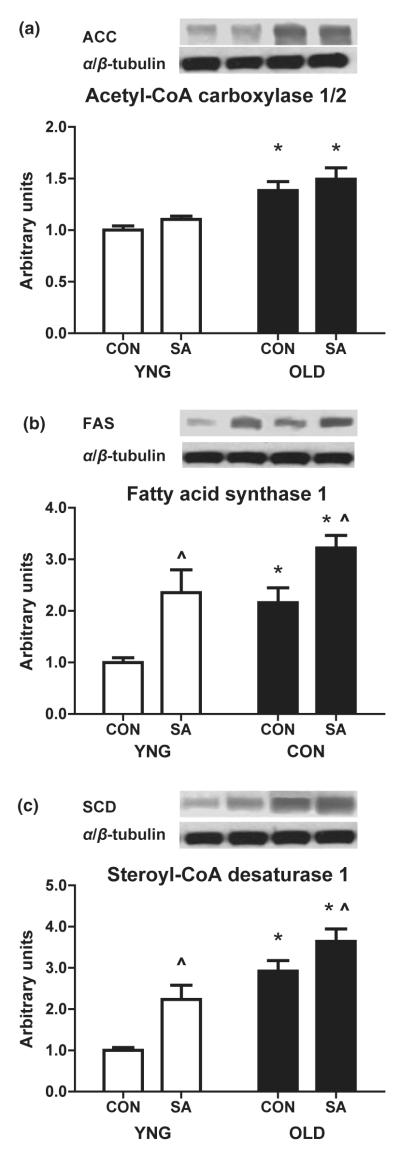
Protein expression of ACC1/2, FAS1 and SCD1
We next determined the protein concentration of ACC1/2, FAS1 and SCD1 by Western blotting. There was a 32% higher concentration of ACC1/2 in OLD (P < 0.0001 vs. YNG; Fig. 3a), which was not affected by SA (Fig. 3a). FAS1 was also higher in OLD (P = 0.003 vs. YNG; Fig. 3b) and was augmented by SA (P = 0.0008 vs. CON; Fig. 3b). The protein expression of SCD1 mirrored the gene expression results in the older animals (P < 0.0001 vs. YNG; Fig. 3c) and was further increased in SA (P = 0.002 vs. CON; Fig. 3c) in both groups.
Figure 3.
Total protein content of lipid synthesis regulators in the skeletal muscle of YNG, OLD and SA Fischer 344 × Brown Norway rats. Relative protein levels of ACC1/2 (a), FAS1 (b) and SCD1 (c) were quantified using Western blot analysis in plantaris muscle. Significant differences between groups (*P < 0.05 vs. YNG; ^P < 0.05 vs CON; n = 8/group).
Phosphorylation of AMPKα and concentration of the AMPKα isoforms
We determined the phosphorylation of the AMPKα activation site, Thr172, and the total protein concentration of AMPKα1 and AMPKα2. The phosphorylation of AMPKα on Thr172 was 40% higher in OLD (P = 0.001 vs. YNG; Fig. 4a) and with overload (P = 0.0008 vs. CON; Fig. 4a). The total protein expression of the AMPKα1 isoform was higher in OLD (P = 0.01 vs. YNG; Fig. 4b). The concentration of AMPKα1 was further increased by SA (P = 0.006 vs. CON; Fig. 4b). In addition, there was a significant interaction between age and overload (P = 0.032, CON vs. SA; Fig. 4b) with a 50% increase in YNG and a ~20% increase in OLD. The protein concentration of AMPKα2 was 20% lower in OLD (P = 0.001 vs. YNG; Fig. 4c), but was unaffected in either group by SA.
Figure 4.
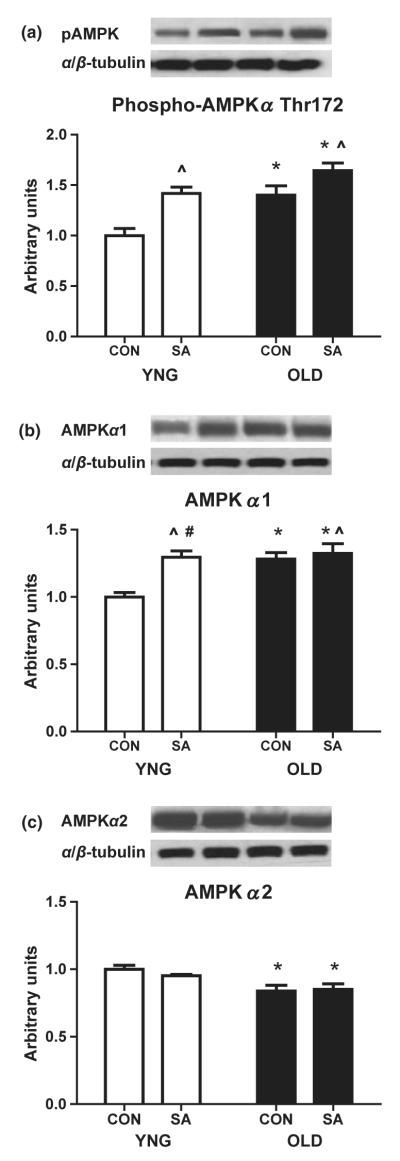
Phosphorylation and total protein content of the metabolic regulator 5′ AMP-activated protein kinase (AMPK) in the skeletal muscle of YNG, OLD and SA Fischer 344 × Brown Norway rats. Relative protein levels of Phospho-AMPKα (a), the AMPK α1 isoform (b) and the AMPK α2 isoform (c) were quantified using Western blot analysis in plantaris muscle. Significant differences between groups (*P < 0.05 vs. YNG, ^P < 0.05 vs. CON, #P < 0.05. vs. YNG CON; n = 8/group).
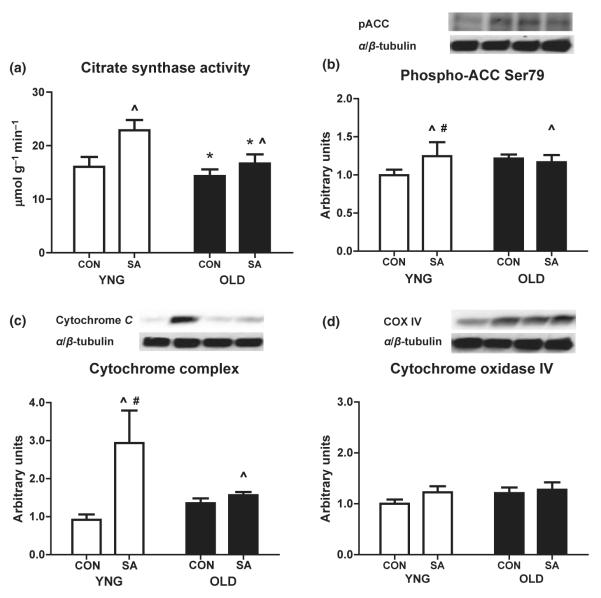
Markers of skeletal muscle oxidative capacity
Citrate synthase activity in PLAN muscle was lower in OLD (P = 0.036 vs. YNG) while SA increased CS activity in both groups (P = 0.017 vs. CON; Fig. 5a). There was a significant increase in ACC Ser79 phosphorylation after SA in YNG (P = 0.04; Fig. 5b) and a significant interaction between age and SA (P = 0.005; Fig. 5b). However, there was no difference in the phosphorylation of ACC on Ser79 between age groups (Fig. 5b). The levels of cytochrome c were unaffected with ageing (Fig. 5c). Conversely, there was a significant increase after overload in both groups (P = 0.009; Fig. 5c) and a further significant interaction between age and SA (P < 0.05; Fig. 5c). In contrast to cytochrome c, there were no difference in the protein expression of COX IV with ageing and SA (Fig. 5d).
Figure 5.
Marker of mitochondrial enzyme activity and markers of mitochondrial protein expression in the skeletal muscle of young, old and surgically ablated Fischer 344 × Brown Norway rats. Citrate synthase activity (a) as determined by enzymatic assay. Relative protein levels of Phospho-ACC (b), Cytochrome c (c) and COX IV (d) were quantified using Western blot analysis in plantaris muscle. Significant differences between groups (*P < 0.05 vs. YNG, ^P < 0.05 vs. CON, #P < 0.05. vs. YNG CON; n = 8/group).
Discussion
Here, we demonstrate for the first time that age-induced increases in intramuscular lipids are associated with increased lipid synthetic regulators including SREBP1, ACC1/2, FAS1 and SCD1 (Figs 1–3). We also demonstrate that AMPK activation (Fig. 4c) and concentration of the AMPKα1 isoform are higher in aged skeletal muscle (Fig. 4a,b). Furthermore, we observed increases in the protein expression of these same lipogenic regulators in YNG after SA, despite no increase in IMTG. Markers of oxidative capacity (CS activity and cytochrome c) were also increased after 28 days of muscle overload in YNG, but the response in OLD was blunted (Fig. 5).
We have recently reported that after 28 days of muscle overload the hypertrophic response in the skeletal muscle of aged animals was attenuated compared with young adult animals (Chale-Rush et al. 2009). We now report a similar blunted response to the levels of total protein content of PLAN in OLD (Table 1). This is in agreement with previous studies employing overload that have revealed an attenuation of muscle hypertrophy and the activation of the mTOR anabolic pathway in older animals (Blough & Linderman 2000, Thomson & Gordon 2006, Hwee & Bodine 2009).
In addition to decreases in muscle mass, fibre cross-sectional area and changes in fibre type composition, ageing is also associated with an increased accumulation of IMTG that is likely a result of increased lipid uptake and decreased lipid oxidation (Blaak 2000, Tucker & Turcotte 2003, Cree et al. 2004, Goodpaster et al. 2006, Nakagawa et al. 2007). Furthermore, there is an age-related decline in the capacity of skeletal muscle to oxidize fatty acids in the fasting state and during exercise (Coggan et al. 1992, Sial et al. 1996, Blaak 2000, Tucker & Turcotte 2002). This is in agreement with the current study where we report higher levels of IMTG stored in aged muscle even after 28 days of mechanical overload (Fig. 1). We further report a 44% higher maximal CS activity in YNG, while only 13% higher in OLD after SA (Fig. 1). The cytochrome c concentration mirrors CS activity; we report a twofold increase in protein expression of cytochrome c in YNG and only a 10% increase in OLD after SA (Fig. 5c). However, the expression of COXIV was unchanged with age or SA (Fig. 5d). Taken together, these data demonstrate there is a reduced ability of aged skeletal muscle to increase oxidative capacity after chronic skeletal muscle overload.
As we observed increased levels of IMTG in aged skeletal muscle, we next determined the role of lipogenic regulators such as SREBP1. This is a membrane-bound transcription factor that regulates the expression of genes involved in the production and uptake of fatty acids, triglycerides and phospholipids (Osborne 2000, Hagen et al. 2010). In response to various stimuli (e.g. sterol-repletion, insulin, ER-stress, etc.), SREBP1 is escorted from the ER to the Golgi where it is then cleaved releasing the amino terminus of SREBP1 (68 kDa), which is able to enter the nucleus and transcriptionally upregulate SREBP1 target genes (Sampath et al. 2007, Hagen et al. 2010). It has been previously reported that cleaved active SREBP1 is upregulated in high-fat fed diabetic rats and its inhibition was associated with reduced levels of IMTG in this model (Bi et al. 2009). We now show an increased expression of mature active SREBP1 (Fig. 2b) in aged skeletal muscle that mirrors the increases in IMTG levels in these animals (Fig. 1). We also noted increases in the protein expression of SREBP1 transcription targets ACC1/2, FAS1 and SCD1 in OLD (Fig. 3a,b,c). Contrary to the protein results, there was a fourfold higher mRNA expression of SCD1 in OLD with no change of any other SREBP1 target gene (Fig. 2c). SCD1 is highly associated with increased lipid accumulation (Dobrzyn & Dobrzyn 2006, Jiang et al. 2008) and its inhibition is protective against high-fat diet induced obesity and insulin resistance (Dobrzyn & Dobrzyn 2006, Sampath et al. 2007, Miyazaki et al. 2009). Although mRNA expressions for the other gene targets are somewhat unexpected it is not unprecedented. Changes in the gene expression may be transient and differences are dependent on a variety of physiological events.
The response of SREBP1 to exercise training is equivocal, with some studies showing an increased expression (Boonsong et al. 2007) and others reporting a decreased expression (Nadeau et al. 2006). Our study is in agreement with Nadeau et al. (2006) who found an exercise training adaptive response of increased SREBP1 in skeletal muscle. We now report a 95% increase in cleaved SREBP1 in YNG and only a 20% increase in OLD after 28 days of SA (Fig. 2b). We observed a 150% and 90% increase in FAS1 protein expression following SA (Fig. 3b) in YNG and OLD animals, respectively. These results were also found in SCD1 protein expression, which increased 150% and 50% after SA in YNG and OLD, respectively. However, there were no changes in the mRNA expression of any measured SREBP1 targets after SA (Fig. 2c).
In addition, we examined the role of the isoforms of the energy regulator, AMPK, in ageing skeletal muscle. Recent studies provide evidence for the existence of distinct regulatory functions for AMPKα1 and AMPKα2 catalytic subunits (McGee et al. 2008, Mounier et al. 2009). Furthermore, there is support for the idea that AMPK ‘hyperphosphorylation’ has a function in the loss of skeletal muscle mass with ageing (Thomson & Gordon 2005). However, these studies have failed to show a mechanism for these increases of AMPK activation. In agreement with Thomson & Gordon (2005, 2006) and Thomson et al. (2008, 2009), we now show significant increases in the Thr172 phosphorylation of AMPKα (Fig. 4a) in the skeletal muscle of aged animals. We (Lessard et al. 2007, Yeo et al. 2008) and others (Fediuc et al. 2006, Watt et al. 2006) have previously reported increases in the activation of AMPK in response to a high-fat diet or in the presence of increased lipids. Of interest, previous studies have found that rodent obesity is characterized by a decrease in muscle mass and an impaired response to overload (Almond & Enser 1984, Sitnick et al. 2009). In the current study, the increases in AMPK phosphorylation (Fig. 4a) in OLD are associated with the increases of IMTG (Fig. 1) and decreases in muscle mass (Table 1) in this group.
In agreement with Thomson et al. (2009), we observed a significant increase in AMPKα1 concentration (Fig. 4b) in OLD animals. This is of interest because, Mounier et al. (2009) using mice deleted for AMPKα1 observed a more pronounced hypertrophy after overload in this model. The authors hypothesized it was likely from increases to the activation of components of mTOR pathway, which regulate protein synthesis (Mounier et al. 2009). We have previously reported in these animals a decrease of mTOR activity in OLD after SA (Chale-Rush et al. 2009). AMPK, because of its role in suppressing energy consuming processes, is a known physiological inhibitor of components of the energy consuming mTOR pathway (Rivas et al. 2009b). Our results provide evidence to show an association of increases in AMPKα1 concentration to the attenuation in muscle mass growth observed in OLD even after SA.
AMPK phosphorylation and activation is highly regulated by exercise and contraction (Hawley & Lessard 2008, Witczak et al. 2008, Rivas et al. 2009b). In contrast to AMPKα1, the AMPKα2 catalytic subunit is hypothesized to regulate metabolic processes (lipid oxidation and glucose uptake) (Hardie & Sakamoto 2006). We now show a 50 and 20% increase in the phosphorylation of Thr172 site of AMPKα (Fig. 4c) after SA in YNG and OLD, respectively. We believe that the increases of AMPKα phosphorylation in OLD are a result of an increase of the AMPKα1 and not the AMPKα2. This is likely the result of lowered total protein concentration of AMPKα2 levels (Fig. 4c) and higher levels of AMPKα1 (Fig. 4b) in the OLD. The AMPKα phosphorylation levels closely mirrored the level of ACC phosphorylation (Fig. 5b) in the young animals after SA. Increases of Ser79 phosphorylation and the subsequent inhibition of ACC are highly associated with the increased lipid oxidation in skeletal muscle (Witczak et al. 2008).
In conclusion, we demonstrate that age-induced increases in intramuscular lipids are associated with the increased lipid synthetic regulators including SREBP1, ACC1/2, FAS1 and SCD1. We also demonstrate that the AMPK activation and concentration of the AMPKα1 isoform are higher in aged skeletal muscle. Furthermore, we observed increases in the protein expression of these same lipogenic regulators in YNG after SA, despite no increase in IMTG. Markers of oxidative capacity and lipid metabolism (CS activity, cytochrome c, and Phospho-ACC) were also increased after 28 days of muscle overload in YNG, but this increase was attenuated in OLD. This indicates that our results of lower IMTG levels in YNG SA, despite higher concentrations of lipogenic regulators is possibly a result of a significant adaptive increase in skeletal muscle oxidative capacity. Future studies should examine the management of IMTG levels in aged skeletal muscle by inhibition of lipogenic regulators such as, SREBP1 and/or SCD1 via pharmacological treatments or gene manipulation. By determining the role of increased lipid storage on skeletal muscle mass during ageing, possible gene targets for the treatment of sarcopenia maybe identified.
Acknowledgments
This material is based upon the work supported by the USDA, under agreement No. 58-1950-7-707 Any opinions, findings, conclusion, or recommendations expressed in this publication are those of the authors and do not necessarily reflect the view of the U.S. Department of Agriculture. Also supported by NIA Grant No. R03AG025270 (RAF) and the Boston Claude D. Pepper Center OAIC (1P30AG031679). The authors thank Mauricio Morais, Allistair Mallillin and Tracy Kendall for their excellent technical assistance.
Footnotes
Conflict of interest The authors declare to have no conflicts of interest.
References
- Almond RE, Enser M. A histochemical and morphological study of skeletal muscle from obese hyperglycaemic ob/ob mice. Diabetologia. 1984;27:407–413. doi: 10.1007/BF00304859. [DOI] [PubMed] [Google Scholar]
- Baker BA, Hollander MS, Mercer RR, Kashon ML, Cutlip RG. Adaptive stretch-shortening contractions: diminished regenerative capacity with aging. Appl Physiol Nutr Metab. 2008;33:1181–1191. doi: 10.1139/H08-110. [DOI] [PubMed] [Google Scholar]
- Baumgartner RN, Koehler KM, Gallagher D, Romero L, Heymsfield SB, Ross RR, Garry PJ, Lindeman RD. Epidemiology of sarcopenia among the elderly in New Mexico. Am J Epidemiol. 1998;147:755–763. doi: 10.1093/oxfordjournals.aje.a009520. [DOI] [PubMed] [Google Scholar]
- Bi Y, Cai M, Liang H, Sun W, Li X, Wang C, Zhu Y, Chen X, Li M, Weng J. Increased carnitine palmitoyl transferase 1 expression and decreased sterol regulatory element-binding protein 1c expression are associated with reduced intramuscular triglyceride accumulation after insulin therapy in high-fat-diet and streptozotocin-induced diabetic rats. Metabolism. 2009;58:779–786. doi: 10.1016/j.metabol.2009.01.011. [DOI] [PubMed] [Google Scholar]
- Blaak EE. Adrenergically stimulated fat utilization and ageing. Ann Med. 2000;32:380–382. doi: 10.3109/07853890008995942. [DOI] [PubMed] [Google Scholar]
- Blough ER, Linderman JK. Lack of skeletal muscle hypertrophy in very aged male Fischer 344 × Brown Norway rats. J Appl Physiol. 2000;88:1265–1270. doi: 10.1152/jappl.2000.88.4.1265. [DOI] [PubMed] [Google Scholar]
- Boonsong T, Norton L, Chokkalingam K, Jewell K, Macdonald I, Bennett A, Tsintzas K. Effect of exercise and insulin on SREBP-1c expression in human skeletal muscle: potential roles for the ERK1/2 and Akt signalling pathways. Biochem Soc Trans. 2007;35:1310–1311. doi: 10.1042/BST0351310. [DOI] [PubMed] [Google Scholar]
- Brownsey RW, Boone AN, Elliott JE, Kulpa JE, Lee WM. Regulation of acetyl-CoA carboxylase. Biochem Soc Trans. 2006;34:223–227. doi: 10.1042/BST20060223. [DOI] [PubMed] [Google Scholar]
- Chale-Rush A, Morris EP, Kendall TL, Brooks NE, Fielding RA. Effects of chronic overload on muscle hypertrophy and mTOR signaling in young adult and aged rats. J Gerontol A Biol Sci Med Sci. 2009;64:1232–1239. doi: 10.1093/gerona/glp146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chi MM, Hintz CS, Coyle EF, Martin WH, III, Ivy JL, Nemeth PM, Holloszy JO, Lowry OH. Effects of detraining on enzymes of energy metabolism in individual human muscle fibers. Am J Physiol. 1983;244:C276–C287. doi: 10.1152/ajpcell.1983.244.3.C276. [DOI] [PubMed] [Google Scholar]
- Coggan AR, Spina RJ, King DS, Rogers MA, Brown M, Nemeth PM, Holloszy JO. Histochemical and enzymatic comparison of the gastrocnemius muscle of young and elderly men and women. J Gerontol. 1992;47:B71–B76. doi: 10.1093/geronj/47.3.b71. [DOI] [PubMed] [Google Scholar]
- Cree MG, Newcomer BR, Katsanos CS, Sheffield-Moore M, Chinkes D, Aarsland A, Urban R, Wolfe RR. Intramuscular and liver triglycerides are increased in the elderly. J Clin Endocrinol Metab. 2004;89:3864–3871. doi: 10.1210/jc.2003-031986. [DOI] [PubMed] [Google Scholar]
- Cutlip RG, Baker BA, Geronilla KB, Mercer RR, Kashon ML, Miller GR, Murlasits Z, Alway SE. Chronic exposure to stretch-shortening contractions results in skeletal muscle adaptation in young rats and maladaptation in old rats. Appl Physiol Nutr Metab. 2006;31:573–587. doi: 10.1139/h06-033. [DOI] [PubMed] [Google Scholar]
- Delbono O. Neural control of aging skeletal muscle. Aging Cell. 2003;2:21–29. doi: 10.1046/j.1474-9728.2003.00011.x. [DOI] [PubMed] [Google Scholar]
- Dennis RA, Przybyla B, Gurley C, Kortebein PM, Simpson P, Sullivan DH, Peterson CA. Aging alters gene expression of growth and remodeling factors in human skeletal muscle both at rest and in response to acute resistance exercise. Physiol Genomics. 2008;32:393–400. doi: 10.1152/physiolgenomics.00191.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobrzyn A, Dobrzyn P. Stearoyl-CoA desaturase – a new player in skeletal muscle metabolism regulation. J Physiol Pharmacol. 2006;57(Suppl 10):31–42. [PubMed] [Google Scholar]
- Drummond MJ, Dreyer HC, Pennings B, Fry CS, Dhanani S, Dillon EL, Sheffield-Moore M, Volpi E, Rasmussen BB. Skeletal muscle protein anabolic response to resistance exercise and essential amino acids is delayed with aging. J Appl Physiol. 2008;104:1452–1461. doi: 10.1152/japplphysiol.00021.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erion DM, Shulman GI. Diacylglycerol-mediated insulin resistance. Nat Med. 2010;16:400–402. doi: 10.1038/nm0410-400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fediuc S, Gaidhu MP, Ceddia RB. Regulation of AMP-activated protein kinase and acetyl-CoA carboxylase phosphorylation by palmitate in skeletal muscle cells. J Lipid Res. 2006;47:412–420. doi: 10.1194/jlr.M500438-JLR200. [DOI] [PubMed] [Google Scholar]
- Frontera WR, Hughes VA, Lutz KJ, Evans WJ. A cross-sectional study of muscle strength and mass in 45- to 78-yr-old men and women. J Appl Physiol. 1991;71:644–650. doi: 10.1152/jappl.1991.71.2.644. [DOI] [PubMed] [Google Scholar]
- Funai K, Parkington JD, Carambula S, Fielding RA. Age-associated decrease in contraction-induced activation of downstream targets of Akt/mTor signaling in skeletal muscle. Am J Physiol Regul Integr Comp Physiol. 2006;290:R1080–R1086. doi: 10.1152/ajpregu.00277.2005. [DOI] [PubMed] [Google Scholar]
- Goodpaster BH, Carlson CL, Visser M, Kelley DE, Scherzinger A, Harris TB, Stamm E, Newman AB. Attenuation of skeletal muscle and strength in the elderly: The Health ABC Study. J Appl Physiol. 2001;90:2157–2165. doi: 10.1152/jappl.2001.90.6.2157. [DOI] [PubMed] [Google Scholar]
- Goodpaster BH, Park SW, Harris TB, Kritchevsky SB, Nevitt M, Schwartz AV, Simonsick EM, Tylavsky FA, Visser M, Newman AB. The loss of skeletal muscle strength, mass, and quality in older adults: the health, aging and body composition study. J Gerontol A Biol Sci Med Sci. 2006;61:1059–1064. doi: 10.1093/gerona/61.10.1059. [DOI] [PubMed] [Google Scholar]
- Hagen RM, Rodriguez-Cuenca S, Vidal-Puig A. An allostatic control of membrane lipid composition by SREBP1. FEBS Lett. 2010;584:2689–2698. doi: 10.1016/j.febslet.2010.04.004. [DOI] [PubMed] [Google Scholar]
- Hardie DG, Sakamoto K. AMPK: a key sensor of fuel and energy status in skeletal muscle. Physiology (Bethesda) 2006;21:48–60. doi: 10.1152/physiol.00044.2005. [DOI] [PubMed] [Google Scholar]
- Hawley JA, Lessard SJ. Exercise training-induced improvements in insulin action. Acta Physiol (Oxf) 2008;192:127–135. doi: 10.1111/j.1748-1716.2007.01783.x. [DOI] [PubMed] [Google Scholar]
- Holland WL, Brozinick JT, Wang LP, Hawkins ED, Sargent KM, Liu Y, Narra K, Hoehn KL, Knotts TA, Siesky A, Nelson DH, Karathanasis SK, Fontenot GK, Birnbaum MJ, Summers SA. Inhibition of ceramide synthesis ameliorates glucocorticoid-, saturated-fat-, and obesity-induced insulin resistance. Cell Metab. 2007;5:167–179. doi: 10.1016/j.cmet.2007.01.002. [DOI] [PubMed] [Google Scholar]
- Hughes VA, Frontera WR, Wood M, Evans WJ, Dallal GE, Roubenoff R, Fiatarone Singh MA. Longitudinal muscle strength changes in older adults: influence of muscle mass, physical activity, and health. J Gerontol A Biol Sci Med Sci. 2001;56:B209–B217. doi: 10.1093/gerona/56.5.b209. [DOI] [PubMed] [Google Scholar]
- Hwee DT, Bodine SC. Age-related deficit in load-induced skeletal muscle growth. J Gerontol A Biol Sci Med Sci. 2009;64:618–628. doi: 10.1093/gerona/glp026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyde R, Hajduch E, Powell DJ, Taylor PM, Hundal HS. Ceramide down-regulates System A amino acid transport and protein synthesis in rat skeletal muscle cells. FASEB J. 2005;19:461–463. doi: 10.1096/fj.04-2284fje. [DOI] [PubMed] [Google Scholar]
- Jiang Z, Michal JJ, Tobey DJ, Daniels TF, Rule DC, Macneil MD. Significant associations of stearoyl-CoA desaturase (SCD1) gene with fat deposition and composition in skeletal muscle. Int J Biol Sci. 2008;4:345–351. doi: 10.7150/ijbs.4.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar V, Selby A, Rankin D, Patel R, Atherton P, Hildebrandt W, Williams J, Smith K, Seynnes O, Hiscock N, Rennie MJ. Age-related differences in the dose–response relationship of muscle protein synthesis to resistance exercise in young and old men. J Physiol. 2009;587:211–217. doi: 10.1113/jphysiol.2008.164483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsson L, Li X, Frontera WR. Effects of aging on shortening velocity and myosin isoform composition in single human skeletal muscle cells. Am J Physiol. 1997;272:C638–C649. doi: 10.1152/ajpcell.1997.272.2.C638. [DOI] [PubMed] [Google Scholar]
- Lauretani F, Russo CR, Bandinelli S, Bartali B, Cavazzini C, Di Iorio A, Corsi AM, Rantanen T, Guralnik JM, Ferrucci L. Age-associated changes in skeletal muscles and their effect on mobility: an operational diagnosis of sarcopenia. J Appl Physiol. 2003;95:1851–1860. doi: 10.1152/japplphysiol.00246.2003. [DOI] [PubMed] [Google Scholar]
- Lessard SJ, Rivas DA, Chen ZP, Bonen A, Febbraio MA, Reeder DW, Kemp BE, Yaspelkis BB, III, Hawley JA. Tissue-specific effects of rosiglitazone and exercise in the treatment of lipid-induced insulin resistance. Diabetes. 2007;56:1856–1864. doi: 10.2337/db06-1065. [DOI] [PubMed] [Google Scholar]
- Lessard SJ, Rivas DA, Chen ZP, Van Denderen BJ, Watt MJ, Koch LG, Britton SL, Kemp BE, Hawley JA. Impaired skeletal muscle beta-adrenergic activation and lipolysis are associated with whole-body insulin resistance in rats bred for low intrinsic exercise capacity. Endocrinology. 2009;150:4883–4891. doi: 10.1210/en.2009-0158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lessard SJ, Rivas DA, Stephenson EJ, Yaspelkis BB, III, Koch LG, Britton SL, Hawley JA. Exercise training reverses impaired skeletal muscle metabolism induced by artificial selection for low aerobic capacity. Am J Physiol Regul Integr Comp Physiol. 2011;300:R175–R182. doi: 10.1152/ajpregu.00338.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lexell J, Taylor CC, Sjostrom M. What is the cause of the ageing atrophy? Total number, size and proportion of different fiber types studied in whole vastus lateralis muscle from 15- to 83-year-old men. J Neurol Sci. 1988;84:275–294. doi: 10.1016/0022-510x(88)90132-3. [DOI] [PubMed] [Google Scholar]
- Lipman RD, Chrisp CE, Hazzard DG, Bronson RT. Pathologic characterization of brown Norway, brown Norway × Fischer 344, and Fischer 344 × brown Norway rats with relation to age. J Gerontol A Biol Sci Med Sci. 1996;51:B54–B59. doi: 10.1093/gerona/51A.1.B54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGee SL, Mustard KJ, Hardie DG, Baar K. Normal hypertrophy accompanied by phosphoryation and activation of AMP-activated protein kinase alpha1 following overload in LKB1 knockout mice. J Physiol. 2008;586:1731–1741. doi: 10.1113/jphysiol.2007.143685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyazaki M, Sampath H, Liu X, Flowers MT, Chu K, Dobrzyn A, Ntambi JM. Stearoyl-CoA desaturase-1 deficiency attenuates obesity and insulin resistance in leptin-resistant obese mice. Biochem Biophys Res Commun. 2009;380:818–822. doi: 10.1016/j.bbrc.2009.01.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mounier R, Lantier L, Leclerc J, Sotiropoulos A, Pende M, Daegelen D, Sakamoto K, Foretz M, Viollet B. Important role for AMPKalpha1 in limiting skeletal muscle cell hypertrophy. FASEB J. 2009;23:2264–2273. doi: 10.1096/fj.08-119057. [DOI] [PubMed] [Google Scholar]
- Nadeau KJ, Ehlers LB, Aguirre LE, Moore RL, Jew KN, Ortmeyer HK, Hansen BC, Reusch JE, Draznin B. Exercise training and calorie restriction increase SREBP-1 expression and intramuscular triglyceride in skeletal muscle. Am J Physiol Endocrinol Metab. 2006;291:E90–E98. doi: 10.1152/ajpendo.00543.2005. [DOI] [PubMed] [Google Scholar]
- Nakagawa Y, Hattori M, Harada K, Shirase R, Bando M, Okano G. Age-related changes in intramyocellular lipid in humans by in vivo H-MR spectroscopy. Gerontology. 2007;53:218–223. doi: 10.1159/000100869. [DOI] [PubMed] [Google Scholar]
- Osborne TF. Sterol regulatory element-binding proteins (SREBPs): key regulators of nutritional homeostasis and insulin action. J Biol Chem. 2000;275:32379–32382. doi: 10.1074/jbc.R000017200. [DOI] [PubMed] [Google Scholar]
- Osler ME, Zierath JR. Adenosine 5′-monophosphate-activated protein kinase regulation of fatty acid oxidation in skeletal muscle. Endocrinology. 2008;149:935–941. doi: 10.1210/en.2007-1441. [DOI] [PubMed] [Google Scholar]
- Parkington JD, Lebrasseur NK, Siebert AP, Fielding RA. Contraction-mediated mTOR, p70S6k, and ERK1/2 phosphorylation in aged skeletal muscle. J Appl Physiol. 2004;97:243–248. doi: 10.1152/japplphysiol.01383.2003. [DOI] [PubMed] [Google Scholar]
- Rivas DA, Lessard SJ, Coffey VG. mTOR function in skeletal muscle: a focal point for overnutrition and exercise. Appl Physiol Nutr Metab. 2009a;34:807–816. doi: 10.1139/H09-073. [DOI] [PubMed] [Google Scholar]
- Rivas DA, Yaspelkis BB, III, Hawley JA, Lessard SJ. Lipid-induced mTOR activation in rat skeletal muscle reversed by exercise and 5′-aminoimidazole-4-carboxamide-1-beta-D-ribofuranoside. J Endocrinol. 2009b;202:441–451. doi: 10.1677/JOE-09-0202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivas DA, Lessard SJ, Saito M, Friedhuber AM, Koch LG, Britton SL, Yaspelkis BB, 3rd, Hawley JA. Low intrinsic running capacity is associated with reduced skeletal muscle substrate oxidation and lower mitochondrial content in white skeletal muscle. Am J Physiol Regul Integr Comp Physiol. 2011;300:R835–R843. doi: 10.1152/ajpregu.00659.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sampath H, Miyazaki M, Dobrzyn A, Ntambi JM. Stearoyl-CoA desaturase-1 mediates the pro-lipogenic effects of dietary saturated fat. J Biol Chem. 2007;282:2483–2493. doi: 10.1074/jbc.M610158200. [DOI] [PubMed] [Google Scholar]
- Sial S, Coggan AR, Carroll R, Goodwin J, Klein S. Fat and carbohydrate metabolism during exercise in elderly and young subjects. Am J Physiol. 1996;271:E983–E989. doi: 10.1152/ajpendo.1996.271.6.E983. [DOI] [PubMed] [Google Scholar]
- Sipila S, Suominen H. Knee extension strength and walking speed in relation to quadriceps muscle composition and training in elderly women. Clin Physiol. 1994;14:433–442. doi: 10.1111/j.1475-097x.1994.tb00402.x. [DOI] [PubMed] [Google Scholar]
- Sitnick M, Bodine SC, Rutledge JC. Chronic high fat feeding attenuates load-induced hypertrophy in mice. J Physiol. 2009;587:5753–5765. doi: 10.1113/jphysiol.2009.180174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srere PA. Citrate Synthase. Academic Press; New York: 1969. p. 5. [Google Scholar]
- Steinberg GR, Kemp BE. AMPK in health and disease. Physiol Rev. 2009;89:1025–1078. doi: 10.1152/physrev.00011.2008. [DOI] [PubMed] [Google Scholar]
- Stenholm S, Harris TB, Rantanen T, Visser M, Kritchevsky SB, Ferrucci L. Sarcopenic obesity: definition, cause and consequences. Curr Opin Clin Nutr Metab Care. 2008;11:693–700. doi: 10.1097/MCO.0b013e328312c37d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summers SA, Nelson DH. A role for sphingolipids in producing the common features of type 2 diabetes, metabolic syndrome X, and Cushing’s syndrome. Diabetes. 2005;54:591–602. doi: 10.2337/diabetes.54.3.591. [DOI] [PubMed] [Google Scholar]
- Thomson DM, Gordon SE. Diminished overload-induced hypertrophy in aged fast-twitch skeletal muscle is associated with AMPK hyperphosphorylation. J Appl Physiol. 2005;98:557–564. doi: 10.1152/japplphysiol.00811.2004. [DOI] [PubMed] [Google Scholar]
- Thomson DM, Gordon SE. Impaired overload-induced muscle growth is associated with diminished translational signalling in aged rat fast-twitch skeletal muscle. J Physiol. 2006;574:291–305. doi: 10.1113/jphysiol.2006.107490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson DM, Fick CA, Gordon SE. AMPK activation attenuates S6K1, 4E-BP1, and eEF2 signaling responses to high-frequency electrically stimulated skeletal muscle contractions. J Appl Physiol. 2008;104:625–632. doi: 10.1152/japplphysiol.00915.2007. [DOI] [PubMed] [Google Scholar]
- Thomson DM, Brown JD, Fillmore N, Ellsworth SK, Jacobs DL, Winder WW, Fick CA, Gordon SE. AMP-activated protein kinase response to contractions and treatment with the AMPK activator AICAR in young adult and old skeletal muscle. J Physiol. 2009;587:2077–2086. doi: 10.1113/jphysiol.2008.166512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker MZ, Turcotte LP. Impaired fatty acid oxidation in muscle of aging rats perfused under basal conditions. Am J Physiol Endocrinol Metab. 2002;282:E1102–E1109. doi: 10.1152/ajpendo.00175.2001. [DOI] [PubMed] [Google Scholar]
- Tucker MZ, Turcotte LP. Aging is associated with elevated muscle triglyceride content and increased insulin-stimulated fatty acid uptake. Am J Physiol Endocrinol Metab. 2003;285:E827–E835. doi: 10.1152/ajpendo.00222.2002. [DOI] [PubMed] [Google Scholar]
- Visser M, Newman AB, Nevitt MC, Kritchevsky SB, Stamm EB, Goodpaster BH, Harris TB. Reexamining the sarcopenia hypothesis. Muscle mass versus muscle strength. Health, Aging, and Body Composition Study Research Group. Ann N Y Acad Sci. 2000;904:456–461. [PubMed] [Google Scholar]
- Wang GL, Fu YC, Xu WC, Feng YQ, Fang SR, Zhou XH. Resveratrol inhibits the expression of SREBP1 in cell model of steatosis via Sirt1-FOXO1 signaling pathway. Biochem Biophys Res Commun. 2009;380:644–649. doi: 10.1016/j.bbrc.2009.01.163. [DOI] [PubMed] [Google Scholar]
- Watt MJ, Steinberg GR, Chen ZP, Kemp BE, Febbraio MA. Fatty acids stimulate AMP-activated protein kinase and enhance fatty acid oxidation in L6 myotubes. J Physiol. 2006;574:139–147. doi: 10.1113/jphysiol.2006.107318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witczak CA, Sharoff CG, Goodyear LJ. AMP-activated protein kinase in skeletal muscle: from structure and localization to its role as a master regulator of cellular metabolism. Cell Mol Life Sci. 2008;65:3737–3755. doi: 10.1007/s00018-008-8244-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeo WK, Lessard SJ, Chen ZP, Garnham AP, Burke LM, Rivas DA, Kemp BE, Hawley JA. Fat adaptation followed by carbohydrate restoration increases AMPK activity in skeletal muscle from trained humans. J Appl Physiol. 2008;105:1519–1526. doi: 10.1152/japplphysiol.90540.2008. [DOI] [PubMed] [Google Scholar]