Abstract
Background
Previous assessments of colon morphology have relied on tests which were either invasive or used ionizing radiation. We aimed to measure regional volumes of the undisturbed colon in healthy volunteers (HV) and patients with diarrhea-predominant irritable bowel syndrome (IBS-D).
Methods
3D regional (ascending, transverse, and descending) colon volumes were measured in fasting abdominal magnetic resonance (MR) images of 75 HVs and 25 IBS-D patients. Thirty-five of the HV and all 25 IBS-D subjects were fed a standard meal and postprandial MRI data obtained over 225 min.
Key Results
Colonic regions were identified and 3D maps from cecum to sigmoid flexure were defined. Fasted regional volumes showed wide variation in both HVs being (mean ± SD) ascending colon (AC) 203 ± 75 mL, transverse (TC) 198 ± 79 mL, and descending (DC) 160 ± 86 mL with no difference from IBS-D subjects (AC 205 ± 69 mL, TC 232 ± 100 mL, and DC 151 ± 71 mL, respectively). The AC volume expanded by 10% after feeding (p = 0.007) in the 35 HV possibly due to increased ileo-colonic inflow. A later rise in AC volume occurred from t = 90 to t = 240 min as the meal residue entered the cecum. In contrast, IBS-D subjects showed a much reduced postprandial response of the AC (p < 0.0001) and a greater increase in TC volume after 90 min (p = 0.0244) compared to HV.
Conclusions & Inferences
We have defined a normal range of the regional volumes of the undisturbed colon in fasted and fed states. The AC in IBS-D appeared less able to accommodate postprandial inflow which may account for faster colonic transit.
Keywords: Colon, IBS-D, MRI, volume
Introduction
Disorders of colonic function with associated disturbances of bowel habit are common causes of chronic abdominal pain. We hypothesized that the ability to directly image the undisturbed colon in patients using MRI may provide novel insight into phenotypes and potential mechanisms of a range of symptoms, including pain, bloating, and abdominal distension, which are often triggered by feeding in IBS patients.1 In addition, there is an increasing interest in the use of colon-targeted drug delivery systems2 requiring knowledge of colonic volumes and water content3 which can vary considerably within the healthy population and may be altered in disease states.
Basic physiological data on colon morphology have previously been obtained from invasive tests and tests using ionizing radiation. To date, measurements of regional colon dimensions have mainly been obtained from resected cadaver specimens,4,5 interoperative measurements6 or from radiological assessments of the prepared, distended colon during barium examination7 or CT colonoscopy.8 All these methods disturb the natural morphology of the bowel. Cadaver fixing and dissection can shrink and stretch the bowel affecting both length and volume measurements. Surgical intervention affects the morphology of the excised bowel. Gas insufflation of the bowel for in vivo radiological imaging results in larger bowel diameters and possible increased looping (leading to greater apparent lengths) and this is common to both 2D and 3D imaging. However, Bourgouin et al.9 used contrast enhanced CT of the bowel (without bowel preparation or air insufflation) to establish a numerical model of the variability in colonic landmarks (including hepatic and splenic flexures) for use in studies of virtual trauma and possible surgical simulations. Nuclear scintigraphy has also been used to obtain qualitative 3D images of the ascending colon using a timed release capsule10 but SPECT spatial resolution is usually limited.
In contrast to these methods, MRI has the potential both to image and measure in vivo parameters of the undisturbed bowel without exposure to ionizing radiation in both the fasted and postprandial state11 and to assess the colonic volumes, gas volumes, and transit characteristics in baseline and interventional studies.12–14 We use here a dual echo MRI pulse sequence for the imaging and identification of the large bowel structures enabling 3D reconstruction and measurement of colonic morphology.
Methods
Subjects and study design
We report the colonic volume data from healthy volunteers (HV) as well as IBS-D patients whose small bowel data have been reported previously.15 The study protocol for the 25 patients was approved by the NREC (Ethic approvals 06GM006 and 06/Q2404/74). Control fed data were provided from an identical protocol involving 35 healthy controls with LREC Ethic approval K/6/2009. The current study also includes the control fasting data acquired on the above 35 HV and an additional 40 HV who attended only for a fasting MRI scan with the same criteria and restrictions (NREC Ethic approvals 08/H0408/134 and 10/H0906/50). The studies were carried out according to Good Clinical Practice principles and the Declaration of Helsinki. All subjects gave written informed consent. There were no adverse events during the studies.
The primary endpoint of this work was the regional volume of the undisturbed colon. The 75 HV with no history of gastrointestinal disease underwent fasting MRI scanning. There were 31 males and 44 females, aged 29 ± 14 years with mean height 171 ± 10 cm and body mass index (BMI) 23 ± 4 kg/m2. Of these, 35 received a test meal and subsequently underwent serial postprandial MRI scanning. These comprised 16 males and 19 females, 21 ± 2 years old, with height 1.71 ± 10 cm and BMI 21 ± 3 kg/m2.
Twenty-five IBS-D patients (10 males/15 females) had the fasting and serial postprandial MRI scanning after the same test meal. They were 49 ± 11 years old, with height 168 ± 9 cm and BMI 26 ± 5 kg/m2. All satisfied the Rome III criteria for IBS-D and other causes of diarrhea were excluded by prior investigations including colonoscopy, lactose tolerance test, and screening for celiac disease as previously described.15 One additional patient completed the study but was excluded from this analysis as image artefacts caused by large amounts of gas in some segments of the colon made the image segmentation unfeasible. No subject had contraindications to MRI such as metal implants in the body.
All subjects were asked to fast from 2000 h the previous evening and to avoid alcohol for 24 h and caffeine and strenuous exercise from the night before the experiment. They were only allowed a small glass of water on waking on the day of the experiment. They completed a questionnaire to investigate adherence to the study restrictions. All underwent a baseline, fasted MRI scan at 09:00 h (defined as t = −45 min time point). At 09:25 h they were asked to eat their study meal within 20 min so that at 09:45 h all subjects underwent an immediate postprandial scan (defined as t = 0 min). This was followed by a scan every 45 min up to 225 min.
The test meal consumed by the fed subjects was a standard rice pudding meal as used previously.15 This consisted of 220 g creamed rice pudding (J. Sainsbury Plc, London, UK), 34 g seedless raspberry jam (Robertsons, Addlestone, Surrey, UK) with 15 g coarse wheat bran (Holland and Barrett Health Foods, Hinckley, Leicestershire, UK) mixed together uniformly, and a glass of 100 mL pure smooth orange juice from concentrate providing 2.9 g fructose, 3.1 g sucrose, and 2.8 g glucose (J. Sainsbury Plc). The test meal had 362 kcal energy 10% from fat, 81% from carbohydrate, and 9% from protein.
Magnetic resonance imaging
All MRI scans were carried out using a 1.5 T Philips Achieva MRI scanner (Philips, Best, The Netherlands). The subjects were positioned supine with a SENSE 4-element body coil wrapped around the abdomen. Their abdomen was then scanned fasted (baseline) and, if fed, postprandially at 45 min intervals for 225 min. The abdomen was imaged using a coronal dual echo fast field echo sequence (24 contiguous slices with TE = 2.3/4.6 ms TR = 158 ms, in-plane resolution 1.76 × 1.76 mm, slice thickness of 7 mm, SENSE factor = 2) during an expiration breath hold of 13 s and then a transverse dual echo FFE sequence (45 contiguous slices with TE = 2.3/4.6 ms TR = 296 ms with in-plane resolution 1.76 × 1.76 mm, slice thickness 7 mm, SENSE factor = 2) under a 20-s expiration breath hold. The subjects spent only a few minutes per time point inside the scanner and, if fed, they were asked to spend the rest of the time sitting upright in an adjacent room.
Data analysis and statistics
Individual regional colon volumes were manually segmented by SEP from the coronal data on each image slice using Analyze9™ software (Mayo Foundation, Rochester, MN, USA). The data analysis was not blinded toward the groups. Regional boundaries commenced at cecum (ascending) and were fixed in a coronal plane at the superior point of the hepatic flexure (ascending to transverse) and splenic flexure (transverse to descending), and terminated at the sagittal plane of commencement of sigmoid colon where the descending colon deviates posteriorly or medially. Each colon region was identified within each coronal image slice, building a 3D representation of the morphology from which the volume of each region was measured. Where anatomy was ambiguous information from axial data guided the definition of the regions. The time series for each individual was expressed as percentage changes from fasting baseline. The % change values at each time point were then averaged and plotted.
The primary endpoint was the regional colonic volumes for which there was no previous data on which to power this study. However, given the SD of 190 mL for total colonic volume in HV, we calculate that we had an 80% power to detect a difference of 124 mL between IBS-D and controls which represents a moderate effect size of 0.65.
The data are expressed as mean ± SD (with the range indicated in brackets). Statistical analysis was carried out using Prism 5 (GraphPad Software Inc., La Jolla, CA, USA). Normality of the data was checked using D'Agostino Pearson's normality test. Comparisons within group were performed using two-tailed Student's t-test or Wilcoxon's matched-pairs signed rank test. Comparisons between groups for non-normal data were performed using Mann–Whitney rank sum test. Two-way anova was used to assess the significance of differences in normally distributed data. Possible correlations of the fasted colonic volumes and subjects’ age, weight, and BMI were assessed using Spearman non-parametric correlation analysis. Differences were considered significant at p < 0.05.
Results
Good quality images were obtained in most subjects and it was possible to identify and map regional colon structures as shown in Fig. 1. However, data from one HV was discarded because excessive movement during data acquisition prevented reliable identification of colon regions. In addition, one IBS-D patient showed substantial gaseous distension of the transverse and descending colon, not seen in any other subject, and so was excluded from further analysis.
Figure 1.
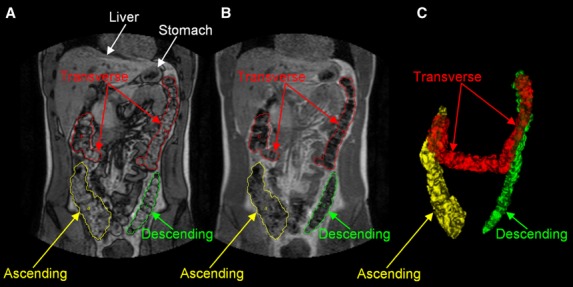
Representative example of the anatomical segmentation of the colon on coronal magnetic resonance imaging (MRI) images. The left panel (A) shows the dual echo MRI image with water and fat imaged out-of-phase and the manual drawings of the regions of interest around the colon; the central panel (B) shows the corresponding MRI image with water and fat imaged in-phase; the right panel (C) shows the 3D reconstruction of the colon.
Considerable intersubject variation in colon geometry (e.g., curvature, tortuosity in transverse and descending colon, and relative haustra size) was seen in both HV and IBS-D subjects. However, intrasubject geometry was generally maintained between postprandial scans albeit with changes seen in size. In three subjects, large pockets of gas were observed in the transverse and descending colon.
Fasting colon volumes
Colon volumes of the fasted subjects are shown schematically in Fig. 2. The regional volumes are reported as mean ± SD (range) showed wide interindividual variation but were very similar for both HV and IBS-D cohorts as shown in Table 1.
Figure 2.
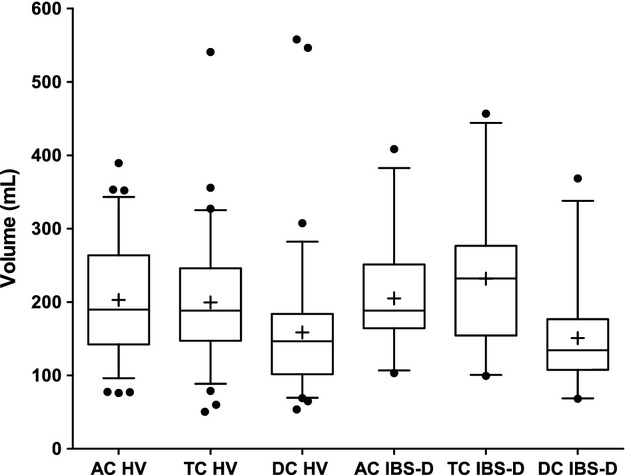
Fasted regional colon volumes (ascending colon [AC], transverse colon [TC], and descending colon [DC]) for 75 healthy volunteers (HV) and 25 diarrhea-predominant irritable bowel syndrome (IBS-D) patients. The box and whiskers plot shows the mean (+) and median (−) values of the regional ascending, transverse, and descending colon volumes. The box represents the 25th–75th centile and the whiskers represent the 5th–95th centile ranges, respectively.
Table 1.
Measured regional colon volumes
| Cohort | Ascending colon volume Mean ± SD (range) | Transverse colon volume Mean ± SD (range) | Descending colon volume Mean ± SD (range) |
|---|---|---|---|
| Healthy | 203 ± 75 mL (76–390) |
199 ± 79 mL (50–541) |
159 ± 85 mL (54–558) |
| IBS-D | 205 ± 70 mL (103–408) |
232 ± 100 mL (99–457) |
151 ± 71 mL (68–368) |
All data passed the D'Agostino Pearson normality test with the exception of the descending colon where two HVs and one IBS-D had large amounts of gas in this region resulting in the outlier values shown in Fig. 2. However, no significant differences in regional or total colon volumes were found between the fasted HV and IBS-D cohorts.
Influences of demographics
Males were taller than females both in healthy (179 ± 7 cm vs 164 ± 8 cm) and IBS-D (177 ± 9 cm vs 163 ± 4 cm) groups. We calculated a height-standardized regional colonic volume index (HSCV) dividing the colonic volumes by height in meters cubed. Height-standardized regional colonic volume index in healthy females was greater than in healthy males (128 ± 52 [57–301] mL/m3 vs 97 ± 24 [63–155] mL/m3, p = 0.0065), primarily due to the difference in ascending colon HSCV (46 ± 18 [16–91] mL/m3 vs 35 ± 13 [12–67] mL/m3, p = 0.0049). The total HSCV in IBS-D females was also greater than in IBS-D males (142 ± 55 [79–256] mL/m3 vs 99 ± 31 [67–170] mL/m3, p = 0.0475) also due to the significant difference in the ascending HSCV (50 ± 18 [25–86] mL/m3 vs 35 ± 13 [18–59] mL/m3, p = 0.0337).
There was no correlation of total colonic volumes with weight or BMI of the healthy subjects. We found a modest (Spearman's r = 0.23) non-significant (p = 0.08) correlation between age and total fasted colonic volumes for all the subjects of the fed cohort indicating just 4% of the variation in volume was accounted for by age. Including all other fasting HV makes the correlation still modest (Spearman's r = 0.27) but significant (p = 0.007). Analysis of the IBS-D cohort showed no such significant correlation between age and colonic volumes.
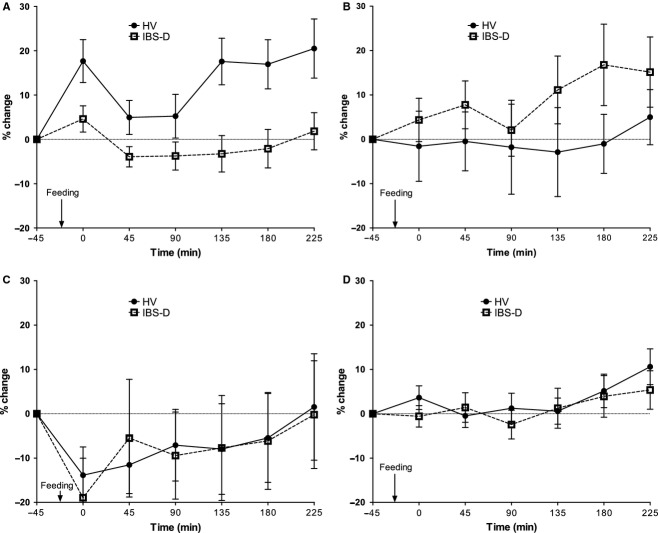
Fed colon volumes
The % change in postprandial regional colon volume is plotted in Fig. 3. There is an immediate increase in AC volume post feeding for both cohorts but this is only significant for the HV (mean difference 21 mL; p = 0.007 paired t-test) compared with the IBS-D subjects (mean difference 7 mL; p = 0.15 paired t-test). Expansion of the ascending colon (Fig. 3A) occurs with the arrival of the meal in the HVs but no such expansion is seen in the IBS-D cohort, but instead the transverse colon expands more quickly and to a greater extent (16% vs −1%; Fig. 3B). The descending colon responds in a similar manner for both cohorts reducing in volume post feeding (HV by 11 mL; p = 0.0633, IBS-D by 19 mL; p = 0.0437) then gradually returning to baseline values. anova analysis of the time dependence of the data shown in Fig. 3A demonstrated a significantly lower postprandial change (p < 0.001) in the ascending colon volume of IBS-D compared to HV. The postprandial change in transverse colon volume (Fig. 3B) was significantly higher in IBS-D compared to HV (p = 0.024).
Figure 3.
The time courses of the % change in colonic volumes from fasted values for the subgroup of 35 healthy volunteers (HV) and 25 diarrhea-predominant irritable bowel syndrome (IBS-D) patients who were fed in this study (mean ± SEM): (A) ascending colon, (B) transverse colon, (C) descending colon, and (D) total colon volume. 3A anova p < 0.001 and 3B anova p = 0.024.
Discussion
A valuable database of three-dimensional in vivo undisturbed human colons has been obtained. In contrast to previously reported results, these data have been measured on the unprepared and undisturbed bowel and, since no ionizing radiation has been involved, have allowed repeated measurements investigating the postprandial behavior of the colon. We have shown that the undisturbed descending colon has lower volume than the ascending and transverse colon. We have also provided evidence that these volumes vary widely between individuals and that when adjusted for height females have relatively larger colons.
The regional volumes measured and the tendency toward increasing transverse volume with subject's age are compatible with previous findings where some relationships between fasted colon length and body height, weight, age, and gender have been reported. However, there have not been consistent conclusions between these previous studies which have a number of possible confounders. Khashab et al.8 measured CT regional volumes of an insufflated bowel and found an increase in the length of transverse colon with age and female gender, Postmortem studies are hard to interpret5 while measurements from barium enema7 are likely to be influenced by the volume infused which depends on patient tolerance. This study found that the total length of colon was longer in females (and female colon lengths tended to increase with height and weight), and tendency to increase with age for both genders. Intraoperative measurements of patients undergoing laparotomy are likely to be strongly influenced by anesthesia and so hard to relate to normal values.6 Previous scintigraphic data showed a negative correlation between fractional emptying rate of the proximal colon and proximal colonic volumes.16 We therefore explored our data post hoc to assess if there were any correlations between fasted regional colonic volumes and Bristol stool parameters. We observed only a modest positive correlation between fasting ascending colon volumes and stool consistency score in female IBS-D patients, but this was not significant due to small numbers and variability (r = 0.3, p < 0.3). There was no correlation between fasting ascending colon volumes and stool frequency in this group of female IBS-D patients (r = −0.14, p < 0.6). The relative constancy of colonic volumes, despite differences in stool output in different conditions, may well reflect the fact that overdistension produces unpleasant sensations of distension and bloating leading to defecation which keeps the colon volume relatively constant despite varying through put of stool. One limitation of this study was that the average age of the HV was lower than that of the D-IBS patients so we considered whether there might be any correlation of colonic volume with demographics. The correlation found between age and total fasted colonic volumes when including all subjects was modest and possibly driven by the larger number of younger/thinner people.
The postprandial effect on colon volume in HVs and IBS-D patients has not been previously reported. Despite the large differences in bowel habit reported by the IBS-D cohort, there is no drastic difference in the appearance of the colon regions, their relative size, shape or immediate response to feeding. However, the expansion of the ascending colon after feeding does not appear to occur in these patients. Previous work on the effect of feeding on small bowel water content (SWBC) in healthy and IBS-D patients15 from our group measured significantly smaller SBWC volumes in both fasted and postprandial IBS-D patients and a faster oro-cecal transit. The immediate postprandial fall in SBWC was not significantly different between the IBS-D and healthy controls so the lack of immediate postprandial increase in ascending colon volumes may reflect failure of receptive accommodation rather than decreased ileal inflow. This lack of relaxation might cause increase transfer of colonic contents to the transverse colon. This would be compatible with the known faster colonic transit in IBS-D17 and a correlation between fast ascending colon clearance and reduced ascending colon volumes and increased 24 h stool weight in IBS-D patients.16 The technical challenges to manometry in IBS-D patients mean that direct comparison of proximal colonic tone in IBS with HV has yet to be reported although a very early study suggests postprandial increase in descending colonic motility in IBS.18 This suggests that meal-induced sigmoid contractions, which are known to be largely non-propulsive in healthy humans,19,20 could drive a small amount of colonic contents retrogradely into the transverse colon. This would be compatible with the results of recent high-resolution manometry suggesting that retrograde propulsive motor patterns in the left colon can delay overall colonic transit.21 The undisturbed sigmoid colon was not visible well enough to allow accurate segmentation in the majority of subjects, hence it could not be reported here. The composition of the test meal used here may not be typical of common mixed meals and it remains to be investigated whether different responses might be obtained with meals of different nutrient composition.
At present, our derivation of colon volume is time consuming, requiring manual identification and segmentation of the colon regions within image planes. This, in addition to the requirement for multiple MRI scans to observe dynamic behavior, makes this a costly evaluation. Nevertheless MRI is becoming increasingly accessible and the relative safety of such a non-invasive, non-radiological method for assessing colon form and function has great potential to identify biomarkers and stratify patients and also for validating both pharmacological and dietary disease management. To allow this potential to be realised, further refinement, involving conjoined optimization of the imaging sequences and image analysis is required to allow semiautomatic segmentation with associated time and cost reductions.
In conclusion, MRI has great potential for the future evaluation of the physiological and pharmacological response of the human colon in both health and disease states with abnormalities of colonic motility. Its high patient acceptability aids recruitment and means that we can now study representative samples of patients in sufficient numbers to produce reliable results.
Key Messages.
Previous measurements of regional colonic volume have used invasive methods that could disturb natural morphology.
Using MRI, volume measurement of the undisturbed colon was achieved in 75 healthy volunteers and 25 IBS-D patients. Normal volume ranges were defined.
IBS-D patients showed less ability to accommodate post-prandial inflow in the ascending colon.
Acknowledgments
We are grateful for support from the Nottingham Digestive Diseases Biomedical Research Unit.
Glossary
- AC
ascending colon
- DC
descending colon
- HSCV
height-standardized regional colonic volume index
- MRI
magnetic resonance imaging
- TC
transverse colon
Funding
The study on IBS patients was partly funded by a NHS R&D Pump Priming grant and by a University of Nottingham Research Imaging Fund grant. The HV study was partly funded by the National Institute for Health Research (NIHR) under a Research for Patient Benefit (RfPB) grant. The views expressed are those of the authors and not necessarily those of the NHS, the NIHR or the Department of Health.
Conflicts of interest
The authors have no competing interests.
Author Contribution
RCS, PAG, LM, and CLH designed the research; SEP, LM, KG, WT, and ER performed the research; SEP analyzed the research; SEP, LM, PAG, and RCS written the manuscript.
References
- 1.Houghton LA. Bloating in constipation: relevance of intraluminal gas handling. Best Pract Res Clin Gastroenterol. 2011;25:141–50. doi: 10.1016/j.bpg.2010.12.009. [DOI] [PubMed] [Google Scholar]
- 2.Philip AK, Philip B. Colon targeted drug delivery systems: a review on primary and novel approaches. Oman Med J. 2010;25:79–87. doi: 10.5001/omj.2010.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Waterman KC, Sutton SC. A computational model for particle size influence on drug absorption during controlled-release colonic delivery. J Controlled Release. 2003;86:293–304. doi: 10.1016/s0168-3659(02)00418-2. [DOI] [PubMed] [Google Scholar]
- 4.Underhill BML. Intestinal length in man. Br Med J. 1955;2:1243–6. doi: 10.1136/bmj.2.4950.1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hounnou G, Destrieux C, Desme J, Bertrand P, Velut S. Anatomical study of the length of the human intestine. Surg Radiol Anat. 2002;24:290–4. doi: 10.1007/s00276-002-0057-y. [DOI] [PubMed] [Google Scholar]
- 6.Saunders BP, Masaki T, Sawada T, Halligan S, Phillips RKS, Muto T. A peroperative comparison of western and oriental colonic anatomy and mesenteric attachments. Int J Colorectal Dis. 1995;10:216–21. doi: 10.1007/BF00346222. [DOI] [PubMed] [Google Scholar]
- 7.Sadahiro S, Ohmura T, Yamada Y, Saito T, Taki Y. Analysis of length and surface area of each segment of the large intestine according to age, sex and physique. Surg Radiol Anat. 1992;14:251–7. doi: 10.1007/BF01794949. [DOI] [PubMed] [Google Scholar]
- 8.Khashab MA, Pickhardt PJ, Kim DH, Rex DK. Colorectal anatomy in adults at computed tomography colonography: normal distribution and the effect of age, sex, and body mass index. Endoscopy. 2009;41:674–8. doi: 10.1055/s-0029-1214899. [DOI] [PubMed] [Google Scholar]
- 9.Bourgouin S, Bege T, Lalonde N, et al. Three-dimensional determination of variability in colon anatomy: applications for numerical modeling of the intestine. J Surg Res. 2012;178:172–80. doi: 10.1016/j.jss.2012.03.054. [DOI] [PubMed] [Google Scholar]
- 10.Perkins AC, Mann C, Wilson CG. 3-Dimensional visualization of the large bowel: a potential tool for assessing targeted drug delivery and colonic pathology. Eur J Nucl Med. 1995;22:1035–8. doi: 10.1007/BF00808416. [DOI] [PubMed] [Google Scholar]
- 11.Placidi E, Marciani L, Hoad CL, et al. The effects of loperamide, or loperamide plus simethicone, on the distribution of gut water as assessed by MRI in a mannitol model of secretory diarrhoea. Aliment Pharmacol Ther. 2012;36:64–73. doi: 10.1111/j.1365-2036.2012.05127.x. [DOI] [PubMed] [Google Scholar]
- 12.Garsed KC, Marciani L, Fields A, et al. Mode of action of a macrogol formulation on distribution of Intestinal fluid: a MRI study. Gastroenterology. 2012;142:S814. [Google Scholar]
- 13.Lam C, Chaddock G, Hoad CL, et al. A new validated MRI method for measuring whole gut transit time. Gastroentrology. 2013;144:S-920. [Google Scholar]
- 14.Murray K, Wilkinson-Smith V, Lam C, et al. Different effects of FODMAP (fermentable oligo-, di-, and mono-saccharides and polyols) components on small bowel water content: an MRI study. Gut. 2012;61:A39. [Google Scholar]
- 15.Marciani L, Cox EF, Hoad CL, et al. Postprandial changes in small bowel water content in healthy subjects and patients with irritable bowel syndrome. Gastroenterology. 2010;138:469–77. doi: 10.1053/j.gastro.2009.10.055. [DOI] [PubMed] [Google Scholar]
- 16.Vassallo M, Camilleri M, Phillips SF, Brown ML, Chapman NJ, Thomforde GM. Transit through the proximal colon influences stool weight in the irritable bowel syndrome. Gastroenterology. 1992;102:102–8. doi: 10.1016/0016-5085(92)91789-7. [DOI] [PubMed] [Google Scholar]
- 17.Cann PA, Read NW, Brown C, Hobson N, Holdsworth CD. Irritable bowel syndrome: relationship of disorders in the transit of a single solid meal to symptom patterns. Gut. 1983;24:405–11. doi: 10.1136/gut.24.5.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vassallo MJ, Camilleri M, Phillips SF, et al. Colonic tone and motility in patients with irritable bowel syndrome. Mayo Clin Proc. 1992;67:725–31. doi: 10.1016/s0025-6196(12)60796-4. [DOI] [PubMed] [Google Scholar]
- 19.Bazzocchi G, Ellis J, Villanuevameyer J, Reddy SN, Mena I, Snape WJ. Effect of eating on colonic motility and transit in patients with functional diarrhea: simultaneous scintigraphic and manometric evaluations. Gastroenterology. 1991;101:1298–306. doi: 10.1016/0016-5085(91)90080-5. [DOI] [PubMed] [Google Scholar]
- 20.Reddy SN, Bazzocchi G, Chan S, et al. Colonic motility and transit in health and ulcerative colitis. Gastroenterology. 1991;101:1289–97. doi: 10.1016/0016-5085(91)90079-z. [DOI] [PubMed] [Google Scholar]
- 21.Dinning PG, Zarate N, Hunt LM, et al. Pancolonic spatiotemporal mapping reveals regional deficiencies in, and disorganization of colonic propagating pressure waves in severe constipation. Neurogastroenterol Motil. 2010;22:E340–9. doi: 10.1111/j.1365-2982.2010.01597.x. [DOI] [PubMed] [Google Scholar]