Abstract
Most eukaryotes show uniparental inheritance of mitochondrial DNA (mtDNA). DeLuca and O’Farrell (2012) now show that in Drosophila active elimination of mtDNA during sperm development ensures that that mature spermatozoa are devoid of DNA.
Mitochondria are unusual organelles that contain their own genome. Though small, mitochondrial DNA (mtDNA) encodes essential components of the respiratory chain and therefore is required for respiratory chain function and oxidative energy metabolism. Most eukaryotes show uniparental inheritance of mtDNA. Although this phenomenon is widespread, its advantages, and the evolutionary pressures driving it, remain poorly understood. Some models have proposed that co-existence of paternal and maternal mtDNAs may be incompatible, that uniparental inheritance may reduce the spread of harmful mtDNA mutations, or that it is simply a byproduct of another factor, such as the unequal size of gametes (Birky, 1995). In humans, offspring inherit mtDNA strictly from the mother. Because of this unusual feature, diseases caused by mtDNA mutations typically display a maternal inheritance pattern. In addition, evolutionary biologists have exploited this feature to date important events during human evolution through the use of mtDNA as a molecular clock.
To ensure uniparental mtDNA inheritance, mechanisms exist to remove paternal mtDNA from the fertilized egg. In this issue, however, DeLuca and O'Farell find that the fruit fly Drosophila melanogaster avoids this problem altogether by removing mtDNA from the spermatozoa during their development (DuLuca and O'Farrell, 2012). The authors demonstrate that mature Drosophila sperm do not contain appreciable amounts of mtDNA and uncover two novel mechanisms by which this occurs. During spermatogenesis, mtDNA nucleoids (aggregates of mtDNAs and their associated proteins) are progressively lost from spermatids, starting from the head to the tail. By the time the spermatids have fully elongated (and they are indeed long, extending for up to 2 mm), the mtDNA molecules have been largely removed. The authors find that mitochondrial endonuclease EndoG is important for this loss of mtDNA, as mutation of EndoG resulted in persistence of mtDNA in fully elongated sperm. However, even in EndoG mutants, the remaining mtDNA is ultimately removed by a second mechanism. During the cellularization process that produces individualized sperm, the remaining nucleoids and other debris are "swept" into a waste compartment near the sperm's tail for elimination. As a result of these two mechanisms, Drosophila sperm are devoid of mtDNA before ever encountering an egg. While the data presented here contradict earlier results indicating that mature Drosophila sperm do contain mtDNA, they are nevertheless compelling, as the combination of PCR, genetic, and cell biological approaches all point to the elimination of mtDNA from sperm prior to fertilization.
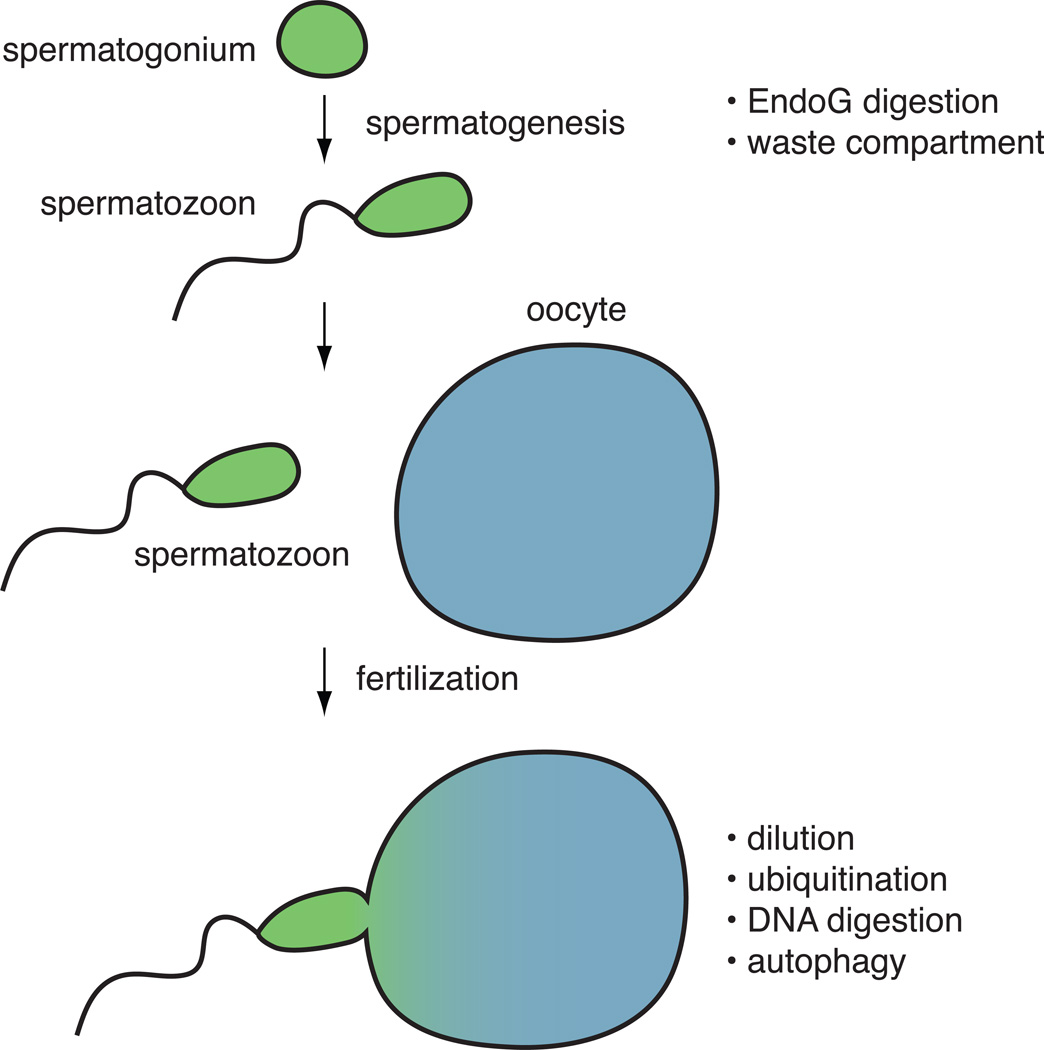
The results of this study are striking because most studies of uniparental mtDNA inheritance have focused on post-fertilization mechanisms. In many organisms, the spermatozoa are much smaller than oocytes, so that dilution effects may facilitate maternal inheritance of mtDNA (Figure 1). However, a number of studies indicate that active mechanisms for paternal mtDNA removal do exist. In fertilized primate and cow eggs, sperm mitochondria are tagged with ubiquitin (Sutovsky et al., 1999), which is thought to target the organelles for destruction by the ubiquitin proteasome system. There is also evidence for active degradation of paternal mtDNA in fertilized eggs of several vertebrates. In fish, mtDNA disappears before loss of the mitochondria, suggesting that the DNA is enzymatically digested (Nishimura et al., 2006). Recent studies in the nematode C. elegans have implicated the autophagy pathway in degradation of paternal mitochondria in the early embryo (Al Rawi et al., 2011; Sato and Sato, 2011). Markers of the autophagosome colocalize with paternal mitochondria, which in this case are not ubiquitinated. This association appears functionally important, because worm mutants defective in the autophagy pathway show prolonged persistence of paternal mitochondria. Autophagosome markers also colocalize with paternal mitochondria in fertilized mouse oocytes, hinting that removal of paternal mitochondria by autophagy may be conserved in mammals.
Figure 1.
Mechanisms to ensure uniparental mtDNA inheritance. The different modes of mtDNA removal have been identified in diverse systems and may not all co-exist in a single organism. The DeLuca and O'Farrell study identified the two pre-fertilization mechanisms listed at the top.
However, autophagy alone may be insufficient to fully eliminate paternal mtDNA, as paternal genomes are detected in the progeny of interspecific mouse crosses (Gyllensten et al., 1991), even though the autophagic machinery in those embryos presumably should be able to detect paternal mitochondria. Post-fertilization mechanisms must also operate in humans, as mtDNA has been observed in mature sperm (Manfredi et al., 1997). In a "one-off" event, a case of paternal inheritance in humans was demonstrated in a patient with a muscle-specific mitochondrial disease. In muscle from this patient, 90% of the mtDNA was paternally-derived and contained a mutation in NADH dehydrogenase 2 (ND2), an mtDNA-encoded subunit of the respiratory chain (Schwartz and Vissing, 2002). This unusual mtDNA inheritance likely resulted from a failure to eliminate the low levels of mtDNA normally present in human sperm.
Might the discovery of pre-fertilization mechanisms for uniparental inheritance in Drosophila be applicable to other organisms? A reduction in paternal mtDNA has indeed been documented in some vertebrates during sperm maturation. In the Japanese medaka fish, the number of mtDNA nucleoids is reduced five-fold during sperm maturation, leaving only ~100 mtDNA molecules in mature sperm to be removed by post-fertilization mechanisms (Nishimura et al., 2006). A similar ten-fold reduction in paternal mtDNA occurs during mouse spermatogenesis (Hecht et al., 1984). The molecular basis for this mtDNA reduction is unknown, and it will be important to determine whether EndoG or a related nuclease is involved in these systems.
Uniparental inheritance is a nearly universal feature of eukaryotes, but the mechanisms to achieve this result have evolved differently in different organisms. For example, mussels have a remarkable system of "doubly-uniparental inheritance" females inherit only maternal mtDNA, whereas males inherit mtDNA from both parents (Birky, 1995). The demonstration by DeLuca and O'Farrell of pre-fertilization elimination of paternal mtDNA highlights the strength of the evolutionary pressure to ensure uniparental inheritance. It will be interesting to see if other equally novel mechanisms to maintain the purity of mtDNA through the germline are lurking in other organisms.
References
- DuLuca SZ, O'Farrell PH. Developmental Cell. 2012 this issue. [Google Scholar]
- Al Rawi S, Louvet-Vallee S, Djeddi A, Sachse M, Culetto E, Hajjar C, Boyd L, Legouis R, Galy V. Science. 2011;334:1144–1147. doi: 10.1126/science.1211878. [DOI] [PubMed] [Google Scholar]
- Sato M, Sato K. Science. 2011;334:1141–1144. doi: 10.1126/science.1210333. [DOI] [PubMed] [Google Scholar]
- Nishimura Y, Yoshinari T, Naruse K, Yamada T, Sumi K, Mitani H, Higashiyama T, Kuroiwa T. Proc Natl Acad Sci U S A. 2006;103:1382–1387. doi: 10.1073/pnas.0506911103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz M, Vissing J. N Engl J Med. 2002;347:576–580. doi: 10.1056/NEJMoa020350. [DOI] [PubMed] [Google Scholar]
- Sutovsky P, Moreno RD, Ramalho-Santos J, Dominko T, Simerly C, Schatten G. Nature. 1999;402:371–372. doi: 10.1038/46466. [DOI] [PubMed] [Google Scholar]
- Manfredi G, Thyagarajan D, Papadopoulou LC, Pallotti F, Schon EA. Am J Hum Genet. 1997;61:953–960. doi: 10.1086/514887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birky CW., Jr Proc Natl Acad Sci U S A. 1995;92:11331–11338. doi: 10.1073/pnas.92.25.11331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gyllensten U, Wharton D, Josefsson A, Wilson AC. Nature. 1991;352:255–257. doi: 10.1038/352255a0. [DOI] [PubMed] [Google Scholar]
- Hecht NB, Liem H, Kleene KC, Distel RJ, Ho SM. Dev Biol. 1984;102:452–461. doi: 10.1016/0012-1606(84)90210-0. [DOI] [PubMed] [Google Scholar]