Abstract
Endocannabinoids (eCBs) are natural lipids regulating a large array of physiological functions and behaviors in vertebrates. The eCB system is highly conserved in evolution and comprises several specific receptors (type-1 and type-2 cannabinoid receptors), their endogenous ligands (e.g., anandamide and 2-arachidonoylglycerol), and a number of biosynthetic and degradative enzymes. In the last few years, eCBs have been described as critical signals in the control of male and female reproduction at multiple levels: centrally, by targeting hypothalamic gonadotropin-releasing-hormone-secreting neurons and pituitary, and locally, with direct effects on the gonads. These functions are supported by the extensive localization of cannabinoid receptors and eCB metabolic enzymes at different levels of the hypothalamic–pituitary–gonadal axis in mammals, as well as bonyfish and amphibians. In vivo and in vitro studies indicate that eCBs centrally regulate gonadal functions by modulating the gonadotropin-releasing hormone–gonadotropin–steroid network through direct and indirect mechanisms. Several proofs of local eCB regulation have been found in the testis and male genital tracts, since eCBs control Sertoli and Leydig cells activity, germ cell progression, as well as the acquisition of sperm functions. A comparative approach usually is a key step in the study of physiological events leading to the building of a general model. Thus, in this review, we summarize the action of eCBs at different levels of the male reproductive axis, with special emphasis, where appropriate, on data from non-mammalian vertebrates.
Keywords: GnRH, hypothalamus, pituitary, spermatogenesis, chromatin remodeling, male fertility
Introduction
Since the discovery of Δ9-tetrahydrocannabinol (THC) as the main psychoactive ingredient in marijuana, the subsequent cloning of cannabinoid receptors and the identification of their endogenous ligands [i.e., endocannabinoids (eCBs)], our understanding of the functions of the eCB system (ECS) has evolved considerably. It has become evident that most components of the mammalian ECS are highly conserved in evolution, pointing to a fundamental modulatory role in basic cellular and organismic functions (1, 2). Accordingly, the ECS is widely expressed in vertebrates, central and peripheral organs, and regulates a large array of physiological functions and behaviors.
The basic eCB signaling system consists of (1) at least two G-protein-coupled receptors, known as the cannabinoid type-1 and type-2 receptors (CB1 and CB2); (2) the endogenous ligands, of which anandamide (AEA) and 2-arachidonoylglycerol (2-AG) are the best characterized; and (3) synthetic and degradative enzymes and transporters that regulate eCB levels and action at receptors. CB1 receptors are abundant in the whole vertebrate central nervous system (CNS) and some peripheral tissues (3–5), whereas CB2 receptors are mostly expressed in peripheral tissues and immune cells, but they have recently been found also in the CNS (6–8). Research in mammals has provided evidence that eCBs can also bind to and activate type-1 transient receptor potential vanilloid (TRPV1) channels (9).
An enormous amount of information on the general properties of the ECS has accumulated over the last two decades [for general reviews on the ECS, see Ref. (10–14)]. In the past years, growing evidence has been accumulating to show the central role of the ECS in controlling vertebrate reproductive functions at both central and gonadal level (15). This review will summarize the action of eCBs at different levels of the reproductive axis, including data from non-mammalian vertebrates.
Effects of eCBs on Hypothalamic–Pituitary Control of Reproduction
Reproductive functions are under neuroendocrine control and require a tight crosstalk between the hypothalamus, pituitary, and gonads. Gonadotropin-releasing-hormone (GnRH) is a key molecule in reproductive behavior and physiology. This neuropeptide is synthesized by hypothalamic neurons mostly located, in mammals, in the preoptic area and in the arcuate nucleus. GnRH axons project to the median eminence, where pulsatile release of GnRH into the hypophysial portal circulation drives the synthesis and secretion of follicle-stimulating hormone (FSH) and luteinizing hormone (LH) from anterior pituitary gonadotropic cells. Circulating FSH and LH, in turn, stimulate gametogenesis and the synthesis and secretion of the gonadal steroid hormones, androgens, estrogens, and progesterone. Under various physiological and pathological conditions, hormonal and metabolic signals regulate GnRH neurons both directly or through upstream neuronal circuitries to influence the pattern of GnRH secretion. The emerging picture from studies in different vertebrate models is that eCBs can modulate both GnRH and gonadotropic cell function, in other words that eCBs can influence the regulation of reproduction at both hypothalamic and pituitary levels (16, 17).
There is general agreement on the inhibitory effect exerted by cannabinoids and eCBs on GnRH release. Early studies in rats demonstrated that the ECS influence gonadal androgens via effects on the hypothalamus and the anterior pituitary. THC, as well as eCBs, lowers not only circulating testosterone levels but also the levels of LH and FSH (18). Most of this negative effect appears to be exerted by inhibition of GnRH secretion into median eminence blood portal vessels (19, 20). Serum LH decreases in response to AEA administration in wild-type mice, whereas CB1 knockout mice (Cb1−/−) are unresponsive to the treatment (21) and show low levels of GnRH and FSH-beta mRNA at hypothalamic and pituitary levels (22), demonstrating the pivotal role exerted by CB1 in the regulation of GnRH and godanotropins synthesis and/or release.
The above effects require CB1 expression in ventro-medial telencephalic and hypothalamic regions. Early localization studies in rodents detected a low abundance of CB1-immunoreactive axons (23) and a low expression level of CB1 mRNA (24–26) in the rodent hypothalamus. However, more recent immunocytochemical studies (27) revealed a dense CB1-immunoreactive fiber network in the mouse hypothalamus. These data are consistent with studies in teleosts and amphibians, showing the expression of CB1-immunoreactive fibers and cell bodies in several hypothalamic regions of adult teleosts (Carassius auratus and Pelvicachromis pulcher) and anuran amphibians (Xenopus laevis and Rana esculenta) (4, 5, 28, 29), as well as in zebrafish and in embryos of X. laevis (30, 31). The expression of CB1 appears to be regulated in the diencephalon during the annual sexual cycle in anuran amphibians (32). Interestingly, CB1 fluctuations show an opposite trend compared to GnRH-I mRNA variations, suggesting that maximal GnRH release corresponds to minimal CB1 levels in the diencephalon. Both GnRH-I and GnRH-II expressions are inhibited in the frog diencephlaon by AEA administration, indicating that both molecular forms might be involved in the regulation of gonatropin discharge (33). Only few data so far indicate that CB2 and TRPV1 receptors might have a role in GnRH cell regulation. Profiling neurotransmitter receptor expression in mouse GnRH-secreting neurons revealed CB2 expression in diestrous adult females (34), and CB1/TRPV1 co-localization has been reported in mouse hypothalamic paraventricular nucleus (35).
An important question is whether eCBs exert their effect directly on GnRH neurons, or on neighboring cells that control GnRH release. Gammon et al. (36) demonstrated that immortalized GnRH neurons (GT1 cells) are both a source and target of eCBs; they produce and secrete 2-AG and AEA, are able to take up and degrade eCBs, and possess CB1 and CB2, whose activation leads to the inhibition of pulsatile GnRH release. Nevertheless, such observations have not been confirmed in vivo in mammals, although GnRH-secreting neurons are close to cannabinergic fibers in male mice (37) and few hypothalamic GnRH neurons seem to express CB1 receptors (36). Close proximity between CB1-expressing fibers and GnRH cells has been well documented in non-mammalian vertebrates. In P. pulcher, C. auratus, Solea solea, and Danio rerio, CB1-containing cell bodies and terminals codistribute with GnRHIII (also called salmon GnRH) cell bodies and fibers (38–40). Similarly, codistribution of CB1- and GnRH-I-immunoreactivity has been found in corresponding brain regions of X. laevis and R. esculenta (39, 41). Noteworthy, a subset of frog GnRH-I-immunoreactive neurons in the septum and preoptic area are also CB1 immunopositive (28), suggesting the existence of a CB1-mediated autocrine mechanism in the control of GnRH secretion, in addition to presynaptic mechanisms. Ultrastructural studies in mammals indicate that CB1-immunoreactive terminals establish symmetric as well as asymmetric synapses on GnRH neurons, suggesting that retrograde eCB signaling might influence GABAergic and glutamatergic synaptic transmission, respectively (27). It should be noted that most recent studies examining the effects of endogenous GABA release on GnRH neurons indicate that the predominant action is that of excitation (42). In line with this, Farkas et al. (37) provided electrophysiological and morphological evidence that retrograde eCB signaling reduces GABAergic excitatory drive onto GnRH neurons via activation of presynaptic CB1 receptors, and that the reduced GABAA receptor signaling in turn inhibits GnRH neuron firing activity. Besides the major afferent regulation exerted on GnRH neurons by GABAergic and glutamatergic inputs, available neuroanatomical literature describes afferent inputs by peptidergic and monoaminergic neuronal systems (43). However, whether the ECS interacts also with these systems has not been determined yet.
Besides the effect on GnRH cells, eCBs could also modulate the activity of other hypothalamic cell types involved in reproduction. Cells containing aromatase, the enzyme that catalyzes the transformation of androgen into estrogens, are localized in the hypothalamus and are deeply involved in sexual differentiation of the brain and activation of male sexual behavior. Aromatase and CB1 are expressed in close contiguity in the goldfish preoptic area and periventricular gray of hypothalamic inferior lobes (16), suggesting a possible CB1-mediated regulation of aromatase activity, at least in bony fish.
Several lines of evidence indicate that eCBs may control adenohypophyseal hormone secretion also acting directly at pituitary level. Both AEA and 2-AG have been detected in the anterior pituitary, suggesting local synthesis (44). In addition, CB1 has been localized in the anterior pituitary within the gonadotroph and lactotroph cells in adult male rats (45, 46), in humans (47), and in X. laevis (48). CB1 expression in pituitary depends on steroids, since it is reduced in both orchidectomized male and estradiol-replaced OVX female rats (46). Recently, the presence of ECS has been demonstrated in mammalian pars tuberalis (49). This finding might be functionally significant also for GnRH release, since this pituitary region is a key station for the anterograde signaling toward the pars distalis.
Effects of eCBs at Gonadal Level
Beside the role exerted at hypothalamic level to control reproductive activity in both sexes, the discovery of eCBs in gonads and reproductive fluids – from seminal plasma in males to oviductal fluid and milk in females – (50–52) pointed out the importance of eCB signaling in the gonads. Gonads have the ability to synthesize eCBs which in turn exert differential effects activating both different types of receptors or tissue-/cell-specific receptor subtypes, the latter obtained by both alternative splicing or transcription sites (53–55). The content of eCBs is regulated by biosynthetic/hydrolyzing enzyme balance, and the appropriate “eCBs tone” in loco, is critical for spermatogenesis progression in male and follicle maturation in female, for sperm quality and the acquisition of sperm functions related to fertilization (motility and capacitation), for fertilization, early-embryo migration, implantation and placentation, for parturition onset and labor as well (15, 17, 56–63). Focusing on males, evidence of eCB direct action into the testis has been provided in most vertebrates [fish (8, 64), frogs (32, 57, 65–68), mammals (21, 69–74)), whereas an ECS has also been described in spermatozoa (SPZ) collected from sea urchin (75), amphibians (65), rodents (76–79), bull (80), boar (81), and human (82–85). A specific and significant association between the use of marijuana and the occurrence of non-seminomatous and mixed testicular germ cell tumors (TGCT) has been recently reported in humans (86–88); although a deep characterization of ECS has never been provided in TGCT patients yet, these data may suggest that the recreational and therapeutic use of cannabinoids may represent a risk factor for TGCT. In general, a relationship between the expression of cannabinoid receptors and the outcome of sex-steroid-dependent cancer has been documented, thus the imbalance in the ECS and its interaction with sex-steroid hormone homeostasis may promote cancer development, proliferation, and migration [for recent review, see Ref. (89)]. Defects in eCB signaling or eCB tone have recently been reported in rat treated with HU210 – a synthetic analog of THC – (90) as well as in clinical cases of male infertility in humans (85, 91). Consistently, genetic inactivation of the AEA-hydrolyzing enzyme, Faah (Fatty acid amide hydrolase) results in increased levels of AEA in the male reproductive system that negatively affect sperm motility and impair sperm fertilizing ability (92), whereas defects in the acquisition of sperm motility during the epididymal transit have been reported in Cb1−/− mice (76, 77). Thus, ECS is nowadays considered a potential therapeutic target in male infertility. ECS is widely expressed in testis in both germ and somatic cells, and a map of ECS localization in several species is provided in Table 1. The first intratesticular targets of eCBs to be identified were the Leydig cells (21, 93), consistent with the low basal testosterone production observed in both Cb1−/− mice and AEA-treated controls, providing evidence of mechanisms other than the AEA/THC-dependent downregulation exerted at hypothalamic/pituitary levels. The direct effect of Bhang (cannabis) on 3β-HSD, a well-known marker of Leydig cell activity, also confirmed this issue (79). The involvement of CB1 signaling in the control of Leydig cell activity is not restricted to steroid (both testosterone/estradiol) production (21, 22, 93), but also extends to Leydig cells ontogenesis. In fact, as reported by Cacciola et al. (72), CB1 expression in differentiating adult Leydig cells negatively correlates with cell division and the characterization of Cb1−/− mice phenotype revealed a 30% decrease in Leydig cells number (72), as well as low circulating estradiol level (22) [for recent review, see Ref. (94)].
Table 1.
Localization of ECS components [both mRNA and protein (Prot)] in testicular somatic and germ cells.
| Cell type | CB1 | CB2 | TRPV1 | FAAH | NAPE-PLD | MAGL | DAGLα/β | Species | Reference |
|---|---|---|---|---|---|---|---|---|---|
| Leydig cells | mRNA | Prot | Prot | mRNA | R. esculenta | (68, 69, 72, 79) | |||
| Prot | M. musculus | ||||||||
| Prot | R. norvegicus | ||||||||
| Sertoli cells | mRNA | mRNA/Prot | mRNA | mRNA/Prot | mRNA | R. esculenta | (68, 70–73) | ||
| Prot | M. musculus | ||||||||
| R. norvegicus | |||||||||
| ISPG | Prot | mRNA/Prot | mRNA | mRNA | mRNA | mRNA | mRNA | R. esculenta | (65, 69, 73) |
| mRNA/Prot | M. musculus | ||||||||
| IISPG | mRNA/Prot | mRNA/Prot | mRNA | mRNA | mRNA | mRNA | mRNA | R. esculenta | (65, 68, 69, 73) |
| mRNA/Prot | mRNA | M. musculus | |||||||
| ISCP | Prot | mRNA/Prot | mRNA | Prot | mRNA | mRNA | mRNA | R. esculenta | (65, 68, 69, 73) |
| mRNA/Prot | mRNA | mRNA | M. musculus | ||||||
| IISPC | Prot | mRNA/Prot | mRNA | Prot | mRNA | mRNA | mRNA | R. esculenta | (65, 73) |
| mRNA | mRNA | M. musculus | |||||||
| SPT | mRNA/Prot | mRNA/Prot | mRNA | Prot | mRNA | mRNA | mRNA | R. esculenta | (65, 68, 69, 72, 73) |
| mRNA | mRNA | M. musculus | |||||||
| Prot | R. norvegicus | ||||||||
| SPZ | mRNA/Prot | mRNA/Prot | mRNA/Prot | Prot | mRNA/Prot | mRNA/Prot | mRNA/Prot | R. esculenta | (65, 68, 72, 78, 81, 83, 84) |
| mRNA/Prot Prot | Prot | Prot | mRNA/Prot | Prot | M. musculus | ||||
| Prot | mRNA/Prot | Prot | Prot | R. norvegicus | |||||
| mRNA/Prot | Prot | Prot | S. scrofa | ||||||
| mRNA/Prot | Prot | B. taurus | |||||||
| H. sapiens |
In the germinal compartment, AEA reduces the spermatogenic output inducing the apoptosis of Sertoli cells (70) in a mechanism reversed by FSH-dependent activation of aromatase and by estradiol-dependent upregulation of Faah (71). Recent studies carried out by Grimaldi et al. (95) demonstrated that in mature Sertoli cells Faah gene is a direct target of estradiol whose promoter contains two proximal estrogen-responsive element (ERE) sequences named ERE2/3. In vivo, a mechanism involving the binding of ERβ to ERE 2/3 and the epigenetic modifications of Faah gene proximal promoter (demethylation of both DNA at CpG site and histone H3 at lysine 9) has been demonstrated (95); consistently FAAH silencing abolished estrogen protection against AEA-dependent apoptosis (95). Thus, AEA content finely toned by its hydrolyzing enzyme FAAH is a fundamental tool to prevent the apoptosis in Sertoli cells.
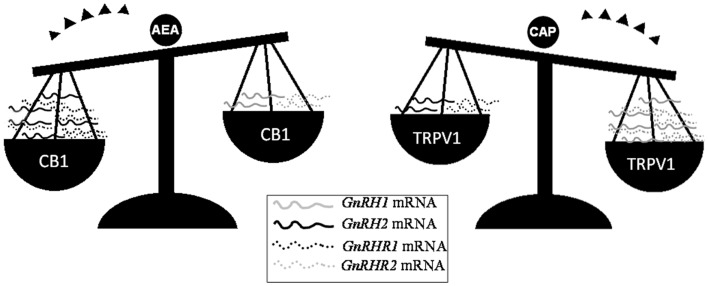
Beside the activity exerted on Sertoli cells, eCBs are critical for the progression of spermatogenesis from mitotic stages throughout the meiotic stages and spermiogenesis events. In such a context, the FAAH-dependent modulation of eCB tone and the cell-specific expression of CB1, CB2, and TRVP1 provide evidence of multiple, differential eCB-dependent signaling involved in the spermatogenetic events. In mouse, decreasing levels of 2-AG have been detected from spermatogonia (SPG) to spermatocytes (SPC) and spermatids (SPT), suggesting that 2-AG, through CB2 – the receptor highly expressed just in mitotic and meiotic stages, but retained in residual body during the spermiogenesis – may act as an autocrine/paracrine mediator during spermatogenesis (73). Conversely, the high expression of Trpv1 observed in meiotic stages (73) and the massive germ cell depletion detected in Trpv1 null mice (96) candidate TRPV1 as a controller of meiotic stages. Very recently, the involvement of both CB1 and TRPV1 in the opposite modulation of testicular GnRH signaling (15, 68, 97) – a master system involved in the control of both spermatogenesis progression and steroidogenetic activity – has been reported in the anuran amphibian, the frog R. esculenta (97), a seasonal breeder in which two GnRH molecular forms (GnRH-I and GnRH-II) and three GnRH receptors (GnRH-RI, -RII and -RIII) have been characterized in testis (68). In such a context, AEA might act as an autocrine/paracrine factor via CB1 and as an intracrine signal via TRPV1; thus, it might be hypothesized that AEA, through the activation of specific receptors, switches on/off testicular GnRH signaling, leading to germ cell progression (Figure 1).
Figure 1.
Differential expression of GnRH system components after in vitro incubation of frog testis collected in post-reproductive periods with AEA or capsaicin (CAP), a selective agonist of TRPV1 receptor. Since intracellular AEA also bind TRPV1, the involvement of AEA in the modulation of testicular GnRH signaling may occur via the selective activation of different eCB receptors.
However, in mammalian and non-mammalian vertebrates, CB1 activity is linked to the control of post-meiotic stages (32, 65, 69, 73). In particular, it has been suggested that ECS controls different steps of spermiogenesis that is the phase of spermatogenesis consisting in the differentiation of SPT in SPZ. In particular, post-meiotic haploid round spermatids (rSPT) undergo biochemical and morphological changes becoming elongated cells (eSPT) and then SPZ. Sperm cells are differentially released from Sertoli cells by spermiation, a process characterized by species-specific features (65, 98). In mammals, SPZ undergo further transformations in the epididymis, which enables SPZ for fertilization (76, 77). These cellular modifications, and in particular some structural changes observed in SPT (i.e., acrosome development, nuclear shaping and chromatin condensation), seem to be related to ECS and in particular to CB1 activity.
A detailed immunolocalization of CB1 has been reported in rat SPT. CB1 appears in rSPT, around the nucleus, during acrosome development; the signal is retained in the head of elongating and condensing SPT, always close to the acrosome region, suggesting a role for CB1 in spermiogenesis, probably in chromatin packaging and in acrosome and/or cellular shape configuration (57, 72, 81). In agreement, several data demonstrate that CB1 regulates acrosome reaction, chromatin condensation, and nuclear size of SPZ (82, 99). Recent observations demonstrate that CB1 is involved in chromatin remodeling of SPT. In fact, during spermiogenesis, as the nucleus elongates and assumes a specie-specific shape, the chromatin condenses. It is worth noting that chromatin condensation differentially occurs, depending on the species. In mammals, chromatin condensation starts in eSPT producing condensing and then condensed SPT, which are mature elongated cells with strongly packaged chromatin (100). Many events characterize these chromatin cyto-architecture changes (101). Early during spermiogenesis, it is possible to observe the expression and storage of specific proteins involved in condensation and in DNA integrity maintenance, such as transition proteins (TNPs) and protamines (PRMs) (102). Others events concern the following: (i) displacement and degradation of the nucleosome structure; (ii) histone replacement by TNPs and then by PRMs; (iii) transcriptional silencing; (iv) DNA repair; and finally, (v) repackaging of the protaminated chromatin into toroidal structures (103, 104) [for recent review, see Ref. (94)]. These events strongly preserve DNA by damage and are involved in mechanism related to sperm maturation. Indeed, it is well known that inefficient expression or activity of TNPs/PRMs deranges histone displacement and causes production of SPZ with histone retention, incomplete chromatin condensation, and DNA damage (74, 105, 106). In both humans and rodents, abnormal levels of sperm DNA damage are associated with lower conception, implantation, and fecundity rates, and with higher miscarriage probability (95, 107, 108). In this context, Chioccarelli et al. (74) showed that Cb1 gene deletion negatively influences chromatin remodeling in SPT, by reducing either transition protein 2 (Tnp2) levels or histone displacement. Secondary effects, related to the inefficient histone displacement (i.e., histone retention, uncondensed chromatin, DNA damage, and nuclear size elongation) have been postulated (22, 74). In agreement, in vivo and in vitro experiments show that AEA is able to act locally and upregulate Tnp2 mRNA levels through CB1, via PKC/PKA pathways (17, 74). Furthermore, in caput epididymis from CB1−/− mice, the percentage of SPZ retaining histones as well as the percentage of SPZ with uncondensed chromatin or with DNA damage, is higher as compared to normal mice. Interestingly, DNA damage increased during the epididymal transit, from caput to cauda, suggesting that CB1 preserve sperm DNA integrity of SPZ during epidydimal transit (74).
Recently, it has been demonstrated that estradiol, probably via stimulatory effects on FSH secretion and/or directly via paracrine actions within the testis, preserve chromatin condensation, and DNA integrity of SPZ, likely by promoting histone displacement in SPT (99). Indeed, it has been reported that CB1−/− male mice show low levels of circulating E2, and when treated with 17β-estradiol, they rescue sperm chromatin quality by restoring histone content, chromatin packaging, DNA integrity, and nuclear length of SPZ (22, 99). These results corroborate the intriguing findings that the small nucleus of SPZ, containing chromatin that did not retain histones, appear fully condensed and able to preserve DNA from damage. On the contrary, the longer nucleus of SPZ, containing chromatin that retained histones, is uncondensed and unable to avoid DNA damage. The emerging exciting idea is that sperm nuclear dimensions can be a good marker for SPZ chromatin quality useful to select the SPZ qualitatively suitable for intracytoplasmic sperm injection (99).
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors wish to remember Prof. Maria Fosca Franzoni, who proficiently dedicated her life to the study of comparative neuroendocrinology. This work was supported by PRIN 2010/2011 to Patrizia Bovolin, Rosaria Meccariello, and Gilda Cobellis.
References
- 1.Elphick MR, Egertová M. The phylogenetic distribution and evolutionary origins of endocannabinoid signalling. Handb Exp Pharmacol (2005) 168:283–97 10.1007/3-540-26573-2_9 [DOI] [PubMed] [Google Scholar]
- 2.Elphick MR. The evolution and comparative neurobiology of endocannabinoid signaling. Philos Trans R Soc B Biol Sci (2012) 367:3201–15 10.1098/rstb.2011.0394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mackie K. Distribution of cannabinoid receptors in the central and peripheral nervous system. Handb Exp Pharmacol (2005) 168:299–325 10.1007/3-540-26573-2_10 [DOI] [PubMed] [Google Scholar]
- 4.Cottone E, Salio C, Conrath M, Franzoni MF. Xenopus laevis CB1 cannabinoid receptor: molecular cloning and mRNA distribution in the central nervous system. J Comp Neurol (2003) 464:487–96 10.1002/cne.10808 [DOI] [PubMed] [Google Scholar]
- 5.Cottone E, Forno S, Campantico E, Guastalla A, Viltono L, Mackie K, et al. Expression and distribution of CB1 cannabinoid receptors in the central nervous system of the African cichlid fish Pelvicachromis pulcher. J Comp Neurol (2005) 485:293–303 10.1002/cne.20502 [DOI] [PubMed] [Google Scholar]
- 6.Van Sickle MD, Duncan M, Kingsley PJ, Mouihate A, Urbani P, Mackie K, et al. Identification and functional characterization of brainstem cannabinoid CB2 receptors. Science (2005) 310(5746):329–32 10.1126/science.1115740 [DOI] [PubMed] [Google Scholar]
- 7.Atwood BK, Mackie K. CB2: a cannabinoid receptor with an identity crisis. Br J Pharmacol (2010) 160:467–79 10.1111/j.1476-5381.2010.00729.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cottone E, Pomatto V, Cerri F, Campantico E, Mackie K, Delpero M, et al. Cannabinoid receptors are widely expressed in goldfish: molecular cloning of a CB2-like receptor and evaluation of CB1 and CB2 mRNA expression profiles in different organs. Fish Physiol Biochem (2013) 39:1287–96 10.1007/s10695-013-9783-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Di Marzo V, De Petrocellis L. Endocannabinoids as regulators of transient receptor potential (TRP) channels: a further opportunity to develop new endocannabinoid-based therapeutic drugs. Curr Med Chem (2010) 17:1430–49 10.2174/092986710790980078 [DOI] [PubMed] [Google Scholar]
- 10.Castillo PE, Younts TJ, Chávez AE, Hashimotodani Y. Endocannabinoid signaling and synaptic function. Neuron (2012) 76(1):70–81 10.1016/j.neuron.2012.09.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ahn K, McKinney MK, Cravatt BF. Enzymatic pathways that regulate endocannabinoid signaling in the nervous system. Chem Rev (2008) 108:1687–707 10.1021/cr0782067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Di Marzo V. The endocannabinoid system: its general strategy of action, tools for its pharmacological manipulation and potential therapeutic exploitation. Pharmacol Res (2009) 60:77–84 10.1016/j.phrs.2009.02.010 [DOI] [PubMed] [Google Scholar]
- 13.Pertwee RG, Howlett AC, Abood ME, Alexander SP, Di Marzo V, Elphick MR, et al. International union of basic and clinical pharmacology. LXXIX. Cannabinoid receptors and their ligands: beyond CB1 and CB2. Pharmacol Rev (2010) 62:588–631 10.1124/pr.110.003004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Piomelli D. The molecular logic of endocannabinoid signalling. Nat Rev Neurosci (2003) 4:873–84 10.1038/nrn1247 [DOI] [PubMed] [Google Scholar]
- 15.Meccariello R, Battista N, Bradshaw HB, Wang H. Updates in reproduction coming from the endocannabinoid system. Int J Endocrinol (2014) 2014:412354. 10.1155/2014/412354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cottone E, Pomatto V, Bovolin P. Role of the endocannabinoid system in the central regulation of nonmammalian vertebrate reproduction. Int J Endocrinol (2013) 2013:941237. 10.1155/2013/941237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Battista N, Meccariello R, Cobellis G, Fasano S, Di Tommaso M, Piazzi V, et al. The role of endocannabinoids in gonadal function and fertility along the evolutionary axis. Mol Cell Endocrinol (2012) 355:1–14 10.1016/j.mce.2012.01.014 [DOI] [PubMed] [Google Scholar]
- 18.Murphy LL, Steger RW, Smith MS, Bartke A. Effects of delta-9-tetrahydrocannabinol, cannabinol and cannabidiol, alone and in combinations, on luteinizing hormone and prolactin release and on hypothalamic neurotransmitters in the male rat. Neuroendocrinology (1990) 52(4):316–21 10.1159/000125604 [DOI] [PubMed] [Google Scholar]
- 19.Murphy LL, Muñoz RM, Adrian BA, Villanúa MA. Function of cannabinoid receptors in the neuroendocrine regulation of hormone secretion. Neurobiol Dis (1998) 5:432–46 10.1006/nbdi.1998.0224 [DOI] [PubMed] [Google Scholar]
- 20.Scorticati C, Fernandez-Solari J, De Laurentiis A, Mohn C, Prestifilippo JP, Lasaga M, et al. The inhibitory effect of anandamide on luteinizing hormone releasing hormone secretion is reversed by estrogen. Proc Natl Acad Sci U S A (2004) 32:11891–6 10.1073/pnas.0404366101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wenger T, Ledent C, Csernus V, Gerendai I. The central cannabinoid receptor inactivation suppresses endocrine reproductive functions. Biochem Biophys Res Commun (2001) 284:363–8 10.1006/bbrc.2001.4977 [DOI] [PubMed] [Google Scholar]
- 22.Cacciola G, Chioccarelli T, Altucci L, Ledent C, Mason JI, Fasano S, et al. Low 17beta-estradiol levels in Cnr1 knock-out mice affect spermatid chromatin remodeling by interfering with chromatin reorganization. Biol Reprod (2013) 88:152. 10.1095/biolreprod.112.105726 [DOI] [PubMed] [Google Scholar]
- 23.Tsou K, Brown S, Sanudo-Pena MC, Mackie K, Walker JM. Immunohistochemical distribution of cannabinoid CB1 receptors in the rat central nervous system. Neuroscience (1998) 83:393–411 10.1016/S0306-4522(97)00436-3 [DOI] [PubMed] [Google Scholar]
- 24.Cota D, Marsicano G, Tschöp M, Grübler Y, Flachskamm C, Schubert M, et al. The endogenous cannabinoid system affects energy balance via central orexigenic drive and peripheral lipogenesis. J Clin Invest (2003) 112(3):423–31 10.1172/JCI200317725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Marsicano G, Lutz B. Expression of the cannabinoid receptor CB1 in distinct neuronal subpopulations in the adult mouse forebrain. Eur J Neurosci (1999) 11(12):4213–25 10.1046/j.1460-9568.1999.00847.x [DOI] [PubMed] [Google Scholar]
- 26.Matsuda LA, Bonner TI, Lolait SJ. Localization of cannabinoid receptor mRNA in rat brain. J Comp Neurol (1993) 327:535–50 10.1002/cne.903270406 [DOI] [PubMed] [Google Scholar]
- 27.Wittmann G, Deli L, Kalló I, Hrabovszky E, Watanabe M, Liposits Z, et al. Distribution of type 1 cannabinoid receptor (CB1)-immunoreactive axons in the mouse hypothalamus. J Comp Neurol (2007) 503(2):270–9 10.1002/cne.21383 [DOI] [PubMed] [Google Scholar]
- 28.Meccariello R, Franzoni MF, Chianese R, Cottone E, Scarpa D, Donna D, et al. Interplay between the endocannabinoid system and GnRH-I in the forebrain of the anuran amphibian Rana esculenta. Endocrinology (2008) 149:2149–58 10.1210/en.2007-1357 [DOI] [PubMed] [Google Scholar]
- 29.Valenti M, Cottone E, Martinez R, De Pedro N, Rubio M, Viveros MP, et al. The endocannabinoid system in the brain of Carassius auratus and its possible role in the control of food intake. J Neurochem (2005) 95(3):662–72 10.1111/j.1471-4159.2005.03406.x [DOI] [PubMed] [Google Scholar]
- 30.Lam CS, Rastegar S, Strähle U. Distribution of cannabinoid receptor 1 in the CNS of zebrafish. Neuroscience (2006) 138(1):83–95 10.1016/j.neuroscience.2005.10.069 [DOI] [PubMed] [Google Scholar]
- 31.Migliarini B, Marucci G, Ghelfi F, Carnevali O. Endocannabinoid system in Xenopus laevis development: CB1 receptor dynamics. FEBS Lett (2006) 580(8):1941–5 10.1016/j.febslet.2006.02.057 [DOI] [PubMed] [Google Scholar]
- 32.Meccariello R, Chianese R, Cacciola G, Cobellis G, Pierantoni R, Fasano S. Type-1 cannabinoid receptor expression in the frog, Rana esculenta, tissues: a possible involvement in the regulation of testicular activity. Mol Reprod Dev (2006) 73:551–8 10.1002/mrd.20434 [DOI] [PubMed] [Google Scholar]
- 33.Chianese R, Ciaramella V, Fasano S, Pierantoni R, Meccariello R. Anandamide modulates the expression of GnRH-II and GnRHRs in frog, Rana esculenta, diencephalon. Gen Comp Endocrinol (2011) 173:389–95 10.1016/j.ygcen.2011.07.001 [DOI] [PubMed] [Google Scholar]
- 34.Todman MG, Han SK, Herbison AE. Profiling neurotransmitter receptor expression in mouse gonadotropin-releasing hormone neurons using green fluorescent protein-promoter transgenics and microarrays. Neuroscience (2005) 132:703–12 10.1016/j.neuroscience.2005.01.035 [DOI] [PubMed] [Google Scholar]
- 35.Cristino L, De Petrocellis L, Pryce G, Baker D, Guglielmotti V, Di Marzo V. Immunohistochemical localization of cannabinoid type 1 and vanilloid transient receptor potential vanilloid type 1 receptors in the mouse brain. Neuroscience (2006) 139:1405–15 10.1016/j.neuroscience.2006.02.074 [DOI] [PubMed] [Google Scholar]
- 36.Gammon CM, Freeman GM, Jr, Xie W, Petersen SL, Wetsel WC. Regulation of gonadotropin-releasing hormone secretion by cannabinoids. Endocrinology (2005) 146(10):4491–9 10.1210/en.2004-1672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Farkas I, Kallo’ I, Deli L, Vida B, Hrabovszky E, Fekete C, et al. Retrograde endocannabinoid signaling reduces GABA-ergic synaptic transmission to gonadotropin-releasing hormone neurons. Endocrinology (2010) 151:5818–29 10.1210/en.2010-0638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cottone E, Campantico E, Guastalla A, Aramu S, Polzonetti-Magni A, Franzoni MF. Are the cannabinoids involved in fish reproduction? Ann NY Acad Sci (2005) 1040:1–4 10.1196/annals.1327.041 [DOI] [PubMed] [Google Scholar]
- 39.Cottone E, Guastalla A, Mackie K, Franzoni MF. Endocannabinoids affect the reproductive functions in teleosts and amphibians. Mol Cell Endocrinol (2008) 286:S41–5 10.1016/j.mce.2008.01.025 [DOI] [PubMed] [Google Scholar]
- 40.Cardinaletti G, Franzoni MF, Palermo FA, et al. Environmental and neuroendocrine control of fish reproduction. In: Garcia-Ayala A, Penalver JM, Chavez-Pozo E. editors. Recent Advances in Fish Reproduction Biology. Kerala: Research Signpost; (2010). p. 65–87 [Google Scholar]
- 41.Donna D, Cottone E, Aramu S, Campantico E, Guastalla A, Franzoni MF. Endocannabinoids and amphibian reproduction: an immunohistochemical study in the green frog. Accad Sci Torino Atti Sci Fis (2006) 140:37–45 [Google Scholar]
- 42.Herbison AE, Moenter SM. Depolarising and hyperpolarising actions of GABA(A) receptor activation on gonadotrophin-releasing hormone neurones: towards an emerging consensus. Neuroendocrinol (2011) 23(7):557–69 10.1111/j.1365-2826.2011.02145.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hrabovszky E, Liposits Z. Afferent neuronal control of type-I gonadotropin releasing hormone neurons in the human. Front Endocrinol (2013) 4:130. 10.3389/fendo.2013.00130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.González S, Manzanares J, Berrendero F, Wenger T, Corchero J, Bisogno T, et al. Identification of endocannabinoids and cannabinoid CB(1) receptor mRNA in the pituitary gland. Neuroendocrinology (1999) 70:137–45 10.1159/000054468 [DOI] [PubMed] [Google Scholar]
- 45.Wenger T, Rettori V, Snyder GD, Dalterio S, McCann SM. Effects of delta-9-tetrahydrocannabinol on the hypothalamic-pituitary control of luteinizing hormone and follicle-stimulating hormone secretion in adult male rats. Neuroendocrinology (1987) 46(6):488–93 10.1159/000124870 [DOI] [PubMed] [Google Scholar]
- 46.González S, Mauriello-Romanazzi G, Berrendero F, Ramos JA, Franzoni MF, Fernández-Ruiz J. Decreased cannabinoid CB1 receptor mRNA levels and immunoreactivity in pituitary hyperplasia induced by prolonged exposure to estrogens. Pituitary (2000) 3(4):221–6 10.1023/A:1012874029689 [DOI] [PubMed] [Google Scholar]
- 47.Pagotto U, Marsicano G, Fezza F, Theodoropoulou M, Grübler Y, Stalla J, et al. Normal human pituitary gland and pituitary adenomas express cannabinoid receptor type 1 and synthesize endogenous cannabinoids: first evidence for a direct role of cannabinoids on hormone modulation at the human pituitary level. J Clin Endocrinol Metab (2001) 86(6):2687–96 10.1210/jc.86.6.2687 [DOI] [PubMed] [Google Scholar]
- 48.Cesa R, Guastalla A, Cottone E, Mackie K, Beltramo M, Franzoni MF. Relationships between CB1 cannabinoid receptors and pituitary endocrine cells in Xenopus laevis: an immunohistochemical study. Gen Comp Endocrinol (2002) 125:17–24 10.1006/gcen.2001.7720 [DOI] [PubMed] [Google Scholar]
- 49.Yasuo S, Koch M, Schmidt H, Ziebell S, Bojunga J, Geisslinger G, et al. An endocannabinoid system is localized to the hypophysial pars tuberalis of Syrian hamsters and responds to photoperiodic changes. Cell Tissue Res (2010) 340:127–36 10.1007/s00441-010-0930-7 [DOI] [PubMed] [Google Scholar]
- 50.Schuel H, Burkman LJ, Lippes J, Crickard K, Forester E, Piomelli D, et al. N-Acylethanolamines in human reproductive fluids. Chem Phys Lipids (2002) 121:211–27 10.1016/S0009-3084(02)00158-5 [DOI] [PubMed] [Google Scholar]
- 51.El-Talatini MR, Taylor AH, Konje JC. Fluctuation in anandamide levels from ovulation to early pregnancy in in-vitro fertilization-embryo transfer women, and its hormonal regulation. Hum Reprod (2009) 24:1989–98 10.1093/humrep/dep065 [DOI] [PubMed] [Google Scholar]
- 52.Gervasi MG, Marczylo TH, Lam PM, Rana S, Franchi AM, Konje JC, et al. Anandamide levels fluctuate in the bovine oviduct during the oestrous cycle. PLoS One (2013) 8:e72521. 10.1371/journal.pone.0072521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shire D, Carillon C, Kaghad M, Calandra B, Rinaldi-Carmona M, Le Fur G, et al. An ammino-terminal variant of the central cannabinoid receptor resulting from alternative splicing. J Biol Chem (1995) 270:3726–31 10.1074/jbc.270.8.3726 [DOI] [PubMed] [Google Scholar]
- 54.Ryberg E, Vu HK, Larsson N, Groblewski T, Hjorth S, Elebring T, et al. Identification and characterization of a novel splice variant of the human CB1 receptor. FEBS Lett (2005) 579:259–64 10.1016/j.febslet.2004.11.085 [DOI] [PubMed] [Google Scholar]
- 55.Liu QR, Pan CH, Hishimoto A, Li CY, Xi ZX, Llorente-Berzal A, et al. Species differences in cannabinoid receptor 2 (CNR2 gene): identification of novel human and rodent CB2 isoforms, differential tissue expression and regulation by cannabinoid receptor ligands. Genes Brain Behav (2009) 8:519–30 10.1111/j.1601-183X.2009.00498.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wang H, Dey SK, Maccarrone M. Jekyll and Hyde: two faces of cannabinoid signaling in male and female fertility. Endocr Rev (2006) 27:427–48 10.1210/er.2006-0006 [DOI] [PubMed] [Google Scholar]
- 57.Cacciola G, Chioccarelli T, Ricci G, Meccariello R, Fasano S, Pierantoni R, et al. The endocannabinoid system in vertebrate male reproduction: a comparative overview. Mol Cell Endocrinol (2008) 286:S24–30 10.1016/j.mce.2008.01.004 [DOI] [PubMed] [Google Scholar]
- 58.Pierantoni R, Cobellis G, Meccariello R, Cacciola G, Chianese R, Chioccarelli T, et al. CB1 activity in male reproduction: mammalian and nonmammalian animal models. Vitam Horm (2009) 81:367–87 10.1016/S0083-6729(09)81014-5 [DOI] [PubMed] [Google Scholar]
- 59.Pierantoni R, Cobellis G, Meccariello R, Cacciola G, Chianese R, Chioccarelli T, et al. Testicular gonadotropin-releasing hormone activity, progression of spermatogenesis, and sperm transport in vertebrates. Ann N Y Acad Sci (2009) 1163:279–91 10.1111/j.1749-6632.2008.03617.x [DOI] [PubMed] [Google Scholar]
- 60.Acone G, Trabucco E, Colacurci N, Cobellis L, Mackie K, Meccariello R, et al. Low type I cannabinoid receptor levels characterize placental villous in labouring delivery. Placenta (2009) 30:203–5 10.1016/j.placenta.2008.11.018 [DOI] [PubMed] [Google Scholar]
- 61.Trabucco E, Acone G, Marenna A, Pierantoni R, Cacciola G, Chioccarelli T, et al. Endocannabinoid system in first trimester placenta: low FAAH and high CB1 expression characterize spontaneous miscarriage. Placenta (2009) 30:516–22 10.1016/j.placenta.2009.03.015 [DOI] [PubMed] [Google Scholar]
- 62.Cacciola G, Chianese R, Chioccarelli T, Ciaramella V, Fasano S, Pierantoni R, et al. Cannabinoids and reproduction: a lasting and intriguing history. Pharmaceuticals (2010) 3:3275–323 10.3390/ph3103275 [DOI] [Google Scholar]
- 63.Chan HW, McKirdy NC, Peiris HN, Rice GE, Mitchell MD. The role of endocannabinoids in pregnancy. Reproduction (2013) 146:R101–9 10.1530/REP-12-0508 [DOI] [PubMed] [Google Scholar]
- 64.Ruggeri B, Soverchia L, Mosconi G, Franzoni MF, Cottone E, Polzonetti-Magni AM. Changes of gonadal CB1 cannabinoid receptor mRNA in the gilthead seabream, Sparus aurata, during sex reversal. Gen Comp Endocrinol (2007) 150:263–9 10.1016/j.ygcen.2006.09.001 [DOI] [PubMed] [Google Scholar]
- 65.Cobellis G, Cacciola G, Scarpa D, Meccariello R, Chianese R, Franzoni MF, et al. Endocannabinoid system in frog and rodent testis: type-1 cannabinoid receptor and fatty acid amide hydrolase activity in male germ cells. Biol Reprod (2006) 75:82–9 10.1095/biolreprod.106.051730 [DOI] [PubMed] [Google Scholar]
- 66.Meccariello R, Chianese R, Cobellis G, Pierantoni R, Fasano S. Cloning of type 1 cannabinoid receptor in Rana esculenta reveals differences between genomic sequence and cDNA. FEBS J (2007) 274:2909–20 10.1111/j.1742-4658.2007.05824.x [DOI] [PubMed] [Google Scholar]
- 67.Chianese R, Cobellis G, Pierantoni R, Fasano S, Meccariello R. Non-mammalian vertebrate models and the endocannabinoid system: relationships with gonadotropin-releasing hormone. Mol Cell Endocrinol (2008) 286:S46–51 10.1016/j.mce.2008.01.009 [DOI] [PubMed] [Google Scholar]
- 68.Chianese R, Ciaramella V, Scarpa D, Fasano S, Pierantoni R. Meccariello anandamide regulates the expression of GnRH1, GnRH2, and GnRH-Rs in frog testis. Am J Physiol Endocrinol Metab (2012) 303:E475–87 10.1152/ajpendo.00086.2012 [DOI] [PubMed] [Google Scholar]
- 69.Gye MC, Kang HH, Kang HJ. Expression of cannabinoid receptor 1 in mouse testes. Arch Androl (2005) 51:247–55 10.1080/014850190898845 [DOI] [PubMed] [Google Scholar]
- 70.Maccarrone M, Cecconi S, Rossi G, Battista N, Pauselli R, Finazzi-Agrò A. Anandamide activity and degradation are regulated by early postnatal aging and follicle-stimulating hormone in mouse Sertoli cells. Endocrinology (2003) 144:20–8 10.1210/en.2002-220544 [DOI] [PubMed] [Google Scholar]
- 71.Rossi G, Gasperi V, Paro R, Barsacchi D, Cecconi S, Maccarrone M. Follicle-stimulating hormone activates fatty acid amide hydrolase by protein kinase A and aromatase-dependent pathways in mouse primary Sertoli cells. Endocrinology (2007) 148:1431–9 10.1210/en.2006-0969 [DOI] [PubMed] [Google Scholar]
- 72.Cacciola G, Chioccarelli T, Mackie K, Meccariello R, Ledent C, Fasano S, et al. Expression of type-1 cannabinoid receptor during rat postnatal testicular development: possible involvement in adult leydig cell differentiation. Biol Reprod (2008) 79:758–65 10.1095/biolreprod.108.070128 [DOI] [PubMed] [Google Scholar]
- 73.Grimaldi P, Orlando P, Di Siena S, Lolicato F, Petrosino S, Bisogno T, et al. The endocannabinoid system and pivotal role of the CB2 receptor in mouse spermatogenesis. Proc Natl Acad Sci U S A (2009) 106:11131–6 10.1073/pnas.0812789106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Chioccarelli T, Cacciola G, Altucci L, Lewis SE, Simon L, Ricci G, et al. Cannabinoid receptor 1 influences chromatin remodeling in mouse spermatids by affecting content of transition protein 2 mRNA and histone displacement. Endocrinology (2010) 151:5017–29 10.1210/en.2010-0133 [DOI] [PubMed] [Google Scholar]
- 75.Schuel H, Goldstein E, Mechoulam R, Zimmerman AM, Zimmerman S. Anandamide (arachidonylethanolamide), a brain cannabinoid receptor agonist, reduces sperm fertilizing capacity in sea urchins by inhibiting the acrosome reaction. Proc Natl Acad Sci U S A (1994) 91:7678–82 10.1073/pnas.91.16.7678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ricci G, Cacciola G, Altucci L, Meccariello R, Pierantoni R, Fasano S, et al. Endocannabinoid control of sperm motility: the role of epididymus. Gen Comp Endocrinol (2007) 153:320–2 10.1016/j.ygcen.2007.02.003 [DOI] [PubMed] [Google Scholar]
- 77.Cobellis G, Ricci G, Cacciola G, Orlando P, Petrosino S, Cascio MG, et al. A gradient of 2-arachidonoylglycerol regulates mouse epididymal sperm cell start-up. Biol Reprod (2010) 82:451–8 10.1095/biolreprod.109.079210 [DOI] [PubMed] [Google Scholar]
- 78.Catanzaro G, Battista N, Rossi G, Di Tommaso M, Pucci M, Pirazzi V, et al. Effect of capacitation on the endocannabinoid system of mouse sperm. Mol Cell Endocrinol (2011) 343:88–92 10.1016/j.mce.2011.01.022 [DOI] [PubMed] [Google Scholar]
- 79.Banerjee A, Singh A, Srivastava P, Turner H, Krishna A. Effects of chronic bhang (cannabis) administration on the reproductive system of male mice. Birth Defects Res B Dev Reprod Toxicol (2011) 92:195–205 10.1002/bdrb.20295 [DOI] [PubMed] [Google Scholar]
- 80.Gervasi MG, Osycka-Salut C, Caballero J, Vazquez-Levin M, Pereyra E, Billi S, et al. Anandamide capacitates bull spermatozoa through CB1 and TRPV1 activation. PLoS One (2011) 6:e16993. 10.1371/journal.pone.0016993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Maccarrone M, Barboni B, Paradisi A, Bernabò N, Gasperi V, Pistilli MG, et al. Characterization of the endocannabinoid system in boar spermatozoa and implications for sperm capacitation and acrosome reaction. J Cell Sci (2005) 118:4393–404 10.1242/jcs.02536 [DOI] [PubMed] [Google Scholar]
- 82.Schuel H, Burkman LJ, Lippes J, Crickard K, Mahony MC, Giuffrida A, et al. Evidence that anandamide-signalling regulates human sperm functions required for fertilization. Mol Reprod Dev (2002) 63:376–87 10.1002/mrd.90021 [DOI] [PubMed] [Google Scholar]
- 83.Rossato M, Ion Popa F, Ferigo M, Clari G, Foresta C. Human sperm express cannabinoid receptor Cb1, the activation of which inhibits motility, acrosome reaction, and mitochondrial function. J Clin Endocrinol Metab (2005) 90:984–91 10.1210/jc.2004-1287 [DOI] [PubMed] [Google Scholar]
- 84.Francavilla F, Battista N, Barbonetti A, Vassallo MR, Rapino C, Antonangelo C, et al. Characterization of the endocannabinoid system in human spermatozoa and involvement of transient receptor potential vanilloid 1 receptor in their fertilizing ability. Endocrinology (2009) 150:4692–700 10.1210/en.2009-0057 [DOI] [PubMed] [Google Scholar]
- 85.Amoako AA, Marczylo TH, Marczylo EL, Elson J, Willets JM, Taylor AH, et al. Anandamide modulates human sperm motility: implications for men with asthenozoospermia and oligoasthenoteratozoospermia. Hum Reprod (2013) 28:2058–66 10.1093/humrep/det232 [DOI] [PubMed] [Google Scholar]
- 86.Darling JR, Doody DR, Sun X, Trabert BL, Weiss NS, Chen C, et al. Association of marijuana use and the incidence of testicular germ cell tumors. Cancer (2009) 115:1215–23 10.1002/cncr.24159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Trabert B, Sigurdson AJ, Sweeney AM, Strom SS, McGlynn KA. Marijuana use and testicular germ cell tumors. Cancer (2011) 117:848–53 10.1002/cncr.25499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lacson JC, Carroll JD, Tuazon E, Castelao EJ, Bernstein L, Cortessis VK. Population-based case-control study of recreational drug use and testis cancer risk confirms an association between marijuana use and nonseminoma risk. Cancer (2012) 118:5374–83 10.1002/cncr.27554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ayakannu T, Taylor AH, Marczylo TH, Willets JM, Konje JC. The endocannabinoid system and sex steroid hormone-dependent cancers. Int J Endocrinol (2013) 2013:259676. 10.1155/2013/259676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lewis SE, Paro R, Borriello L, Simon L, Robinson L, Dincer Z, et al. Long-term use of HU210 adversely affects spermatogenesis in rats by modulating the endocannabinoid system. Int J Androl (2012) 35:731–40 10.1111/j.1365-2605.2012.01259.x [DOI] [PubMed] [Google Scholar]
- 91.Lewis SE, Rapino C, Di Tommaso M, Pucci M, Battista N, Paro R, et al. Differences in the endocannabinoid system of sperm from fertile and infertile men. PLoS One (2012) 7:e47704. 10.1371/journal.pone.0047704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Sun X, Wang H, Okabe M, Mackie K, Kingsley PJ, Marnett LJ, et al. Genetic loss of Faah compromises male fertility in mice. Biol Reprod (2009) 80:235–42 10.1095/biolreprod.108.072736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Chianese R, Ciaramella V, Fasano S, Pierantoni R, Meccariello R. Hypothalamus–pituitary axis: an obligatory target for endocannabinoids to inhibit steroidogenesis in frog testis. Gen Comp Endocrinol (2014). 10.1016/j.ygcen.2014.02.010 [DOI] [PubMed] [Google Scholar]
- 94.Cacciola G, Chioccarelli T, Fasano S, Pierantoni R, Cobellis G. Estrogens and spermiogenesis: new insights from type 1 cannabinoid receptor knockout mice. Int J Endocrinol (2013) 2013:501350. 10.1155/2013/501350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Grimaldi P, Pucci M, Di Siena S, Di Giacomo D, Pirazzi V, Geremia R, et al. The faah gene is the first direct target of estrogen in the testis: role of histone demethylase LSD1. Cell Mol Life Sci (2012) 69:4177–90 10.1007/s00018-012-1074-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Mizrak SC, van Dissel-Emiliani FM. Transient receptor potential vanilloid receptor-1 confers heat resistance to male germ cells. Fertil Steril (2008) 90:1290–3 10.1016/j.fertnstert.2007.10.081 [DOI] [PubMed] [Google Scholar]
- 97.Chianese R, Ciaramella V, Scarpa D, Fasano S, Pierantoni R, Meccariello R. Endocannabinoids and endovanilloids: a possible balance in the regulation of the testicular GnRH signalling. Int J Endocrinol (2013) 2013:904748. 10.1155/2013/904748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Cobellis G, Lombardi M, Scarpa D, Izzo G, Fienga G, Meccariello R, et al. Fra-1 activity in the frog, Rana esculenta, testis. Ann N Y Acad Sci (2005) 1040:264–8 10.1196/annals.1327.039 [DOI] [PubMed] [Google Scholar]
- 99.Cacciola G, Chioccarelli T, Altucci L, Viaggiano A, Fasano S, Pierantoni R, et al. Nuclear size as estrogen-responsive chromatin quality parameter of mouse spermatozoa. Gen Comp Endocrinol (2013) 193:201–9 10.1016/j.ygcen.2013.07.018 [DOI] [PubMed] [Google Scholar]
- 100.Zhao M, Shirley CR, Hayashi S, Marcon L, Mohapatra B, Suganuma R, et al. Transition nuclear proteins are required for normal chromatin condensation and functional sperm development. Genesis (2004) 38:200–13 10.1002/gene.20019 [DOI] [PubMed] [Google Scholar]
- 101.Marcon L, Boissonneault G. Transient DNA strand breaks during mouse and human spermiogenesis new insights in stage specificity and link to chromatin remodeling. Biol Reprod (2004) 70:910–8 10.1095/biolreprod.103.022541 [DOI] [PubMed] [Google Scholar]
- 102.Oliva R, Dixon GH. Vertebrate protamine genes and histone-to-protamine replacement reaction. Prog Nucleic Acid Res Mol Biol (1991) 40:25–94 10.1016/S0079-6603(08)60839-9 [DOI] [PubMed] [Google Scholar]
- 103.Rousseaux S, Caron C, Govin J, Lestrat C, Faure AK, Khochbin S. Establishment of male-specific epigenetic information. Gene (2005) 345:139–53 10.1016/j.gene.2004.12.004 [DOI] [PubMed] [Google Scholar]
- 104.Oliva R, Castillo J. Proteomics and the genetics of sperm chromatin condensation. Asian J Androl (2011) 13:24–30 10.1038/aja.2010.65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Shirley CR, Hayashi S, Mounsey S, Yanagimachi R, Meistrich ML. Abnormalities and reduced reproductive potential of sperm from Tnp1- and Tnp2-null double mutant mice. Biol Reprod (2004) 71:1220–9 10.1095/biolreprod.104.029363 [DOI] [PubMed] [Google Scholar]
- 106.Zhao M, Shirley CR, Yu YE, Mohapatra B, Zhang Y, Unni E, et al. Targeted disruption of the transition protein 2 gene affects sperm chromatin structure and reduces fertility in mice. Mol Cell Biol (2001) 21:7243–55 10.1128/MCB.21.21.7243-7255.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Lewis SE, Agbaje IM. Using the alkaline comet assay in prognostic tests for male infertility and assisted reproductive technology outcomes. Mutagenesis (2008) 23:163–70 10.1093/mutage/gem052 [DOI] [PubMed] [Google Scholar]
- 108.Hogeveen KN, Sassone-Corsi P. Regulation of gene expression in postmeiotic male germ cells: CREM-signalling pathways and male fertility. Hum Fertil (2006) 9:73–9 10.1080/14647270500463400 [DOI] [PubMed] [Google Scholar]