Abstract
Activation of receptor tyrosine kinases is a key feature in receptor signaling and membrane trafficking processes. In this study, we found that the insulin receptor tyrosine kinase activity is required for fusion between early endosomes. AG1024, a receptor tyrosine kinase inhibitor, blocked the in vitro endosome fusion in a concentration-dependent manner. We observed that Rab5: wild type partially rescued the fusion reaction, whereas Rab5: Q79L mutant fully rescued it. We also observed that treatment of cells with insulin receptor kinase inhibitor HNMPA-(AM)3 blocked the formation of Rab5-positive endosomes as well as the activation of Rab5 upon addition of insulin in intact cells. HNMPA-(AM)3 inhibitor also affected the endosomal co-localization of Rab5 and insulin receptor. However, the formation of Rab5: Q79L mutant-positive endosomes were not affected by the HNMPA-(AM)3 inhibitor. In addition, HNMPA-(AM)3 inhibitor affected the association of Rin1 to membrane upon insulin stimulation. Furthermore, Rin1 did not fully support endosome fusion in the presence of the AG1024 inhibitor. These results constitute the first evidence that, at least in part, the enzymatic activity of insulin receptor is required for the fusion events via the activation of Rab5.
Keywords: Endosome fusion, Receptor tyrosine kinase, small GTPases, Kinase Inhibitors
INTRODUCTION
The early endosome is a key check-point in endocytic pathways, in which a decision is made to either be sorted to the late endosome/ lysosome compartment or to be recycled back to the plasma membrane (Doherty and McMahon, 2009; Pfeffer, 2007). Rab5 and its effectors, including EEA1, Rin1, Rabaptin-5, RAP6 and Rabex-5, together with other small GTPases (i.e., Rab 4, 7, 11, 15 and 22), are likely to tightly control the fusion and sorting of molecules that have entered the early endosome (Scita and Di Fiore, 2010; Sorkin and von Zastrow, 2009).
These homotypic and heterotypic vesicle fusions are regulated by several cytosolic and membranous factors. For example, the small GTPase Rab5 and its effectors regulate the fusion between early endosomes without affecting the fusion with late endosome or lysosomes. Thus, early endosome fusion is dependent on Rab5 proteins; it is symmetrical and selective, thereby allowing orderly modification of ligand-receptor interaction complexes and signaling in a sequential manner by altering the surroundings during receptor-ligand internalization (Barbieri et al., 1998; Barbieri et al., 1994; Barbieri et al., 2000; Brandhorst et al., 2006; Bucci et al., 1992; Li et al., 1995). In addition, several Rab5-asociated proteins are also required for endosome-endosome fusion (Christoforidis et al., 1999; Horiuchi et al., 1997; Li et al., 1995; Lippe et al., 2001; McBride et al., 1999; Nielsen et al., 2000; Simonsen et al., 1998; Tall et al., 2001).
Endocytosis of the insulin receptor is initiated by the binding of its ligand (Di Guglielmo et al., 1998; Khan et al., 1989; Liu and Roth, 1995; Maggi et al., 1998; Russell et al., 1987). The insulin receptor-ligand complex is then transported through the endocytic pathway, where it is then either recycled back to the cell surface or transported to late endosomes, and ultimately, the lysosome for degradation (Fucini et al., 1999; Siemeister et al., 1995; Waters et al., 1995). The internalization of insulin receptors has been shown to be dependent of insulin receptor autophosphorylation, followed by the downstream phosphorylation and/or activation of insulin receptor substrate (IRS) or phosphatidylinositol 3 (PI3)-kinase through the clathrin-mediated pathway (Carpentier et al., 1993; Carpentier et al., 1992; Klein et al., 1987). However, other reports have also suggested other pathways for insulin receptor internalization (Fan et al., 1982; Paccaud et al., 1992; Smith and Jarett, 1990).
We have taken advantage of the well-characterized trafficking pathway of the activated insulin receptor (Fan et al., 1982; Klein et al., 1987), in order to measure fusion of insulin–insulin receptor vesicles (internalized Biotin-insulin) with other endocytic vesicles that have been prepared by internalizing Avidin-β-galatosidase during fluid phase endocytosis in HepG2 cells. By allowing different populations of HepG2 cells to engage in receptor-mediated endocytosis of insulin linked to biotin and fluid phase endocytosis of Av-β-galatosidase, we are able to isolate donor and acceptor pools of endosomes and examine for endosome fusion as previously developed cell-free endosome fusion assays (Braell, 1987; Gorvel et al., 1991; Gruenberg and Howell, 1986; Mayorga et al., 1988; Mullock and Luzio, 1992; Rubino et al., 2000; Wessling-Resnick and Braell, 1990).
Here, we demonstrate that, at least in part, tyrosine kinase activity of the insulin-receptor is required for the formation for enlarged Rab5-positive endosomes as well as for the activation of Rab5 in intact cells. We also observed that AG1024 inhibitor blocked the endosome fusion, whose inhibitory effect is linked to the activation of Rab5, since the addition of Rin1 was required for optimal fusion activity. We have also observed that the addition of Rab5: Q79L mutant reversed the inhibitory effect, suggesting a mechanism by which the tyrosine kinase activity of the receptor modulates early endosome fusion.
MATERIAL AND METHODS
Cell culture and Materials
HepG2 cells (American Type Culture Collection) were grown to confluence in Dulbecco’s modified Eagle’s medium supplemented with 5% fetal bovine serum. NIH3T3-human insulin receptor (NIH-IR) were grown in Dulbecco’s modified Eagle’s medium supplemented with 5% fetal bovine serum containing 0.3–0.5 mg/ml G418. Kinase inhibitors were purchased from EMD Biosciences (La Jolla, CA). EEA-1, Rab5, and Rab11 antibodies were from Cell Signaling Technology (Beverly, MA). Rin1 antibodies were from Abcam Inc. Biotin-insulin and Avidin β-galactodidsase and the anti-insulin antibodies were purchased from Sigma-Aldrich (St. Louis, MO). All secondary antibodies were purchased from Jackson ImmunoResearch Laboratories (West Grove, PA). All other chemicals were obtained from Sigma unless otherwise stated.
Endocytic Probes and Fractionation
HepG2 cells were collected by centrifugation, washed three times in Hank's balanced salt solution (HBSS) with 15 mM HEPES and supplemented with 1 mg /ml BSA (HBSS-BSA, pH 7.0). Endocytosis of probes was performed with cells suspended in HBSS-BSA buffer. To prepare early endosomes loaded with Biotin-Insulin or Avidin-β-galatosidase, cells (8 × 108/ml) were allowed to internalize either 1 mg/ml Avidin-β-galatosidase or Biotin-Insulin 100 ng/ml for 5 min at 37°C in order to prepare the endosome fractions. The cells were then washed in uptake media at 800 × g, followed by two more washes, once in HBSS-BSA buffer and once in homogenization buffer (HB, 0.25 M sucrose, 20 mM HEPES, 0.5 mM EGTA, pH 7.0). Cells were then resuspended in homogenization buffer (4 × 108/ml) and passed 17 times through a stainless steel ball homogenizer. The homogenate was centrifuged at 800 × g for 10 min to generate a postnuclear supernatant (PNS). Typical homogenization yielded >80% of endocytosed marker in the PNS as determined by either radioactivity or immunoprecipitation of ligand. PNSs could then be used fresh for fractionation studies, cytosol preparation, or stored frozen in liquid nitrogen for later use (Gruenberg and Howell, 1986).
Fusion Assay
Two populations of vesicles containing Avidin-β-galatosidase and Biotin-Insulin were mixed at 4°C to a final volume of 11 µl in fusion buffer (250 mM Sucrose, 0.5 mM EGTA, 1 mM DTT, 1.5 mM MgCl2, 50 µg/ml B-BSA, 50 mM KCl, and 20 mM HEPES-KOH, pH 7.0). This buffer was supplemented with a regenerating system (1 mM ATP, 8 mM creatine phosphate, 31 units/ml creatine phosphokinase) or with a depleting system (5 mM mannose, 25 units/ml hexokinase). AG1024 inhibitor and AG9, an inactive analog, were added to the reaction at the concentration indicated in each figure. After incubation at 37°C for 45-min, the fusion reaction was stopped by cooling to 4°C (ice) for 10 min. To measure the immune complexes formed, the vesicles were solubilized by adding 150 µl of solubilization buffer (1% Triton X-100, 1 mM EDTA, 0.1% BSA, 0.15 M NaCl, 10 mM Tris-HC1, pH 7.4) containing 35 µg/ml Biotin-BSA. Biotin-BSA was used to quench any free Av-Gal due the presece of broken endosomes during the homozenization process. The solubilized samples were then added to wells that were plate coated with anti-insulin antibody. After a 60-min incubation period at 4°C, immunoprecipitates were washed twice with 200 µl of solubilization buffer and the β-galactosidase activity was measured by using 4-methylumbelliferyl β-D-galactoside substrate (Mayorga et al., 1988). There was a linear relationship between the amount of immunoprecipitate added to the enzymatic assay and the amount of product formed. Fusion-dependent immunoprecipitable enzymatic activity was expressed as a percent of the total immunoprecipitable activity in the assay. Total activity was determined by immunoprecipitating the immune complexes after mixing vesicles in the presence of detergent but in the absence of Biotin-BSA.
Electron microscopy
Colloidal gold particles of 10 and 20 nm were obtained from Sigma. Gold particles were coated with proteins (insulin and β-galactosidase) by standard techniques (Mayorga et al., 1989). Binding and uptake of gold particles coated with the ligands were carried out under the similar conditions described by Mayorga et al., 1989 for soluble ligands into two separate populations of HepG2 cells by 5-min incubated at 37°C. The homogenization of cells and the fusion reaction were performed using the same protocols described above, except that both homogenization and fusion buffers were buffered with 30 mM MES, pH: 6.6. They were centrifuged, and then the vesicles were mixed under fusogenic conditions. After the incubation, the fusion reaction was then stopped by adding 1% glutaraldehyde, which was prepared in HB buffer at room temperature. After 10 min, samples were centrifuged for 15 min at 12,000 × g, washed and the samples were processed by transmission electron microscopy (Mayorga et al., 1989).
Western Blot Analysis
Lysate Preparation, SDS-PAGE, and Western Blotting-To prepare whole cell lysates, cell were washed twice with ice-cold PBS and lysed in ice-cold lysis buffer (20mM Tris-HCl pH 7.5, 150mM NaCl, 1% Triton X-100). The lysates were clarified by centrifugation, and protein concentrations were determined using the BCA Protein Assay Reagent Kit (Pierce). Proteins were resolved by SDS-PAGE and transferred to nitrocellulose membranes, which were blocked and probed with the indicated antibodies. To determine relative protein amounts, three representative exposures for each sample were quantified using National Institutes of Health ImageJ software.
Confocal microscopy
NIH3T3-human insulin receptor cells were seeded on glass coverslips at 1.0 × 105 per 3.8 cm2 well. The following day, cells were transfected using FugeneHD with either pEGFP-Rab5: wild type or pEGFP-Rab5: Q79L. Cells were starved in serum-free Dulbecco's modified Eagle's medium supplemented with 5% BSA Fraction V (MP Biomediclas, Solon, OH) for 14 hours, and treated as indicated with 100 µM AG9, 100 µM HNMPA-(AM)3 for 30 minutes at 4°C, after which they were treated with Insulin at 100 ng/ml for 1hr at 4°C and then stimulated to uptake the ligand for 5 min at 37°C. They were washed with PBS and fixed in 4% paraformaldehyde for 20 minutes. Fixed cells were probed with rabbit polyclonal insulin receptor antibodies for 1h. Secondary antibodies used were Alexa594-conjugated goat anti-rabbit IgG. Coverslips were mounted with Prolong and viewed on a Leica TCS SP2 confocal microscope.
Statistical analysis
All experiments presented were repeated a minimum of three times. The data represent the mean ± SD. Student’s t test was performed to calculate statistical significance.
RESULTS
Insulin receptor tyrosine kinase inhibitor blocks in vitro endosome fusion
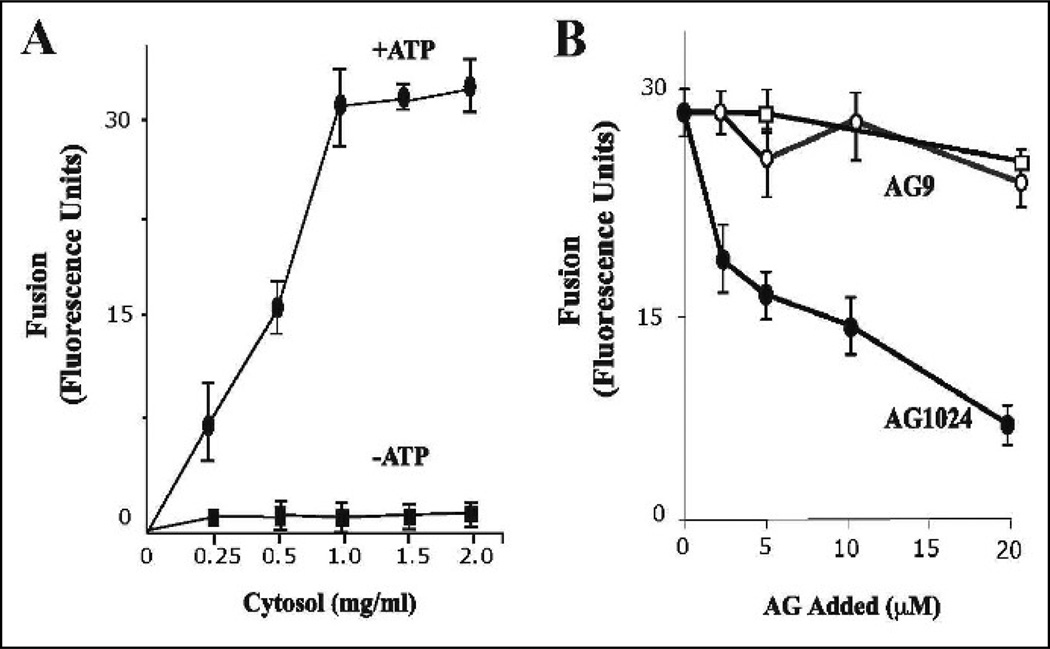
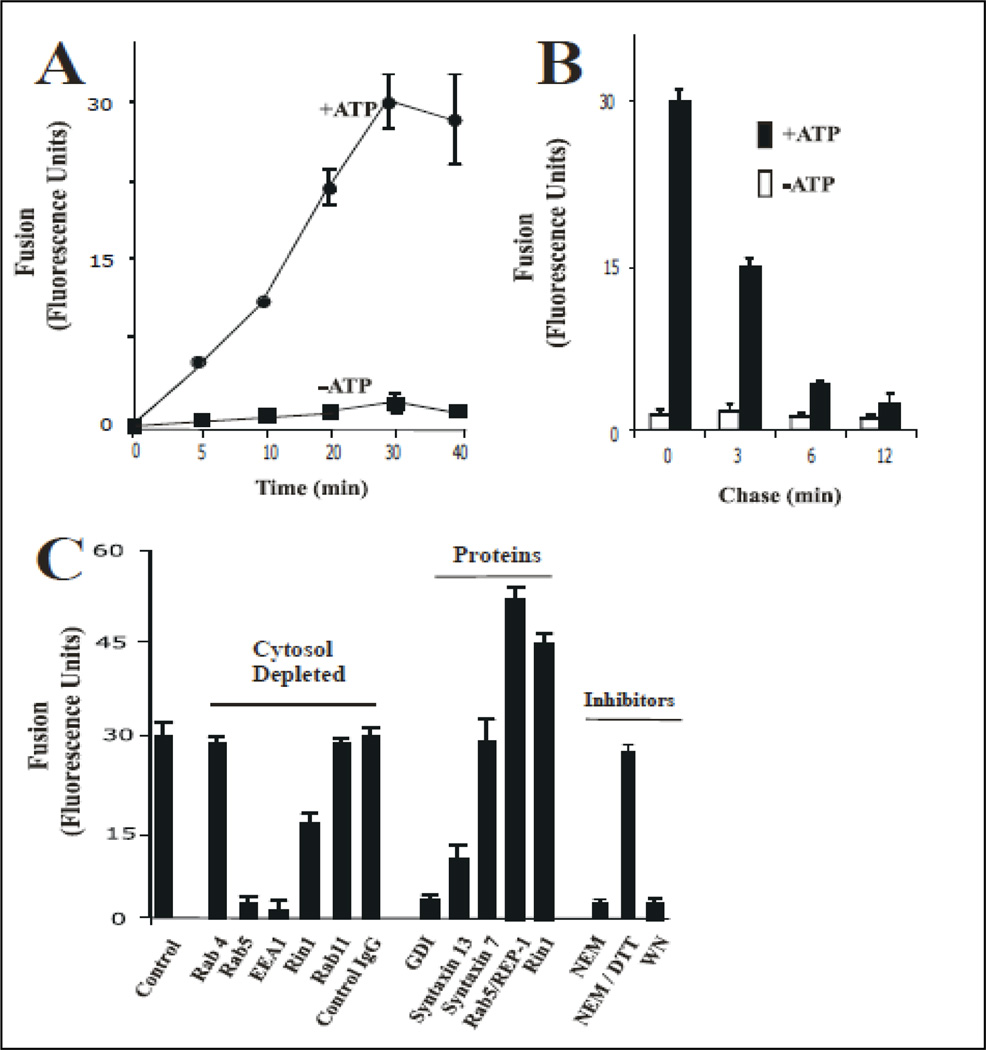
To prepare endosomes, 0.1µg /ml Biotin-insulin and 2 mg/ml Avidin-β-Galactosidase were separately internalized for 5 min at 37°C in HepG2 cells as described in Material and Methods. When the isolated vesicles containing Biotin-Insulin were mixed with vesicles containing Avidin-β-Galactosidase in an in vitro system in the presence of ATP regenerating system at 37°C, a cytosolic-dependent increase of complex formation was observed (Figure 1A). The effect of cytosol appeared to be saturable at about 1.0 mg/ml protein and no fusion activity was observed without cytosol. As expected, in the presence of ATP-depleting system, very little fusion activity was observed (Figure 1A). Furthermore, the fusion was also time-dependent (Figure 2A).
Figure 1.
Effect of insulin receptor kinase inhibitor on fusion between endosomes. (A) Effect of cytosol and energy on the endosome-endosome fusion. Five-min vesicles containing either Biotin-Insulin (B-Ins) or Avidin-β-Galactosidase (Av-GAL) were mixed in fusion buffer supplemented with different amount of cytosol containing ATP-regenerated (■) or ATP-depleted systems (●). Samples were then transferred to 37°C for the indicated times and processed as described in Material and Methods to determine the percentage forming the immune complex formation. The data are presented as means ± SD of four independent experiments. (B) Fusion assay was performed under standard conditions as described above either in the absence (□) or in the presence of different amounts of AG9 (○) and AG1024 (●) containing 1 mg/ml of cytosol. The data are presented as means ± SD of four independent experiments.
Figure 2.
Fusion between endosomes. (A) Effect of time on the endosome-endosome fusion. Two sets of vesicles prepared from cells that have been allowed to endocytose B-Ins and Av-GAL for 5 min at 37°C, respectively. Then the vesicles were mixed in fusion buffer supplemented with different amounts of cytosolic proteins /ml containing ATP-regenerated (●) or ATP-depleted systems (■). Samples were then transferred to 37°C for the indicated times and processed as described in Material and Methods to determine the percentage immune complex formation. The data are presented as means ± SD of four independent experiments. (B) Av-GAL was internalized by a 3-min uptake at 37°C and chased for 0, 3, 6, 12 min. Cells were then incubated at 4°C for 1 h with B-Ins. Endocytosis of Insulin was induced by incubation at 37°C and the amount of B-Ins associated with Av-GAL was quantitated at each time by solubilizing the cells in the presence of excess of biotin-BSA. Results are expressed as fluorescence units, which indicates the percentage of the immune complex formed after solubilization of vesicles in the absence of biotin-BSA. (C) Vesicles fractions were obtained and resuspended in homogenization and treated as follow: Antibodies: 200 ng/ml control IgG, or anti-Rab4, anti-Rab5, or anti-EEA1, or anti-Rab11, or anti-Rin1 antibodies were added to fusion reaction; GDI proteins: vesicles were incubated in the presence of 1 uM GDI, washed and then resuspended in fusion buffer; Rab5 proteins: 0.3 µg/ml Rab5-REP-1 complex was added to the fusion reaction; Rin1 proteins: 0.2 µg/ml of Rin1 was added to the fusion reaction; NEM treatment: Cytosol and vesicles were pretreated with 0.75 mM NEM at 4°C for 45 min and excess of NEM was inactivated by 2 mM DTT. Fusion was carried out in the presence of ATP-regenerating system, and the ATP-dependent fusion was expressed as a fraction of the fusion obtained with non-treated vesicles resuspended in 1.5 mg of normal cytsosol/ml for 45 min at 37°C. Background was measured by incubation for 45 min at 4°C. The data are presented as means ± SD of three independent experiments.
To examine the specificity of the fusion assay, different populations of vesicles were employed. First, Biotin-insulin was internalized for 3 min at 37°C. Second, Avidin-β-Galactosidase was internalized by cells for 3 min and then chased for 0, 3, 6, or 12 min to load the later compartments at 37°C (Figure 2B). Since the maximum fusion activity was observed when both probes were present at 3-min endosomes, we then examined fusion of such vesicles containing Biotininsulin. As Avidin-β-Galactosidase was chased for an extended period of time, ATP-dependent fusion activity decreased rapidly (Figure 2B). After a 12-min chase, fusion activity was reduced by 70% and no fusion activity was detected when Av-Gal was chased for 30 min (data not shown). These results indicate that fusion between Biotin-Insulin and Avidin-β-Galactosidase containing vesicles requires ATP, cytosolic proteins and is also time-dependent. The endosome reaction was affected by the ionic strength of the fusion buffer. The optimum concentration of KCl was 60 mM as is the case in reconstitution studies with intracellular vesicular transport (Mayorga et al., 1989). This in vitro endosome fusion assay showed similar requirements with other in vitro vesicle fusion systems (i.e., ATP, cytosol proteins, and salts).
We also investigated the requirements of the small Rab5 in this in vitro assay since Rab5 is a critical regulator of the early endosome fusion (Bucci et al., 1992). In Figure 2C, we show that the addition of cytosol depleted of Rab5 inhibited the endosome fusion. In contrast, the cytosol treated with control IgG or depleted of Rab11 and Rab4 did not affect the fusion reaction. Clearly our results indicate the specificity of the fusion reaction and more importantly, that this in vitro system is Rab5-dependent. We then confirmed the requirement of Rab5 in this in vitro system by observing that the addition of GDI to the in vitro system blocked the fusion reaction (Figure 2C). Thus, the requirement of Rab5 in this in vitro system raises the question whether Rab5 interacting proteins were also required in this endosome fusion system. We examined the requirement of Rin1, a Rab5-guanine exchange factor, or other effectors (i.e., EEA1) in the fusion reaction. As shown in Figure 2C the depletion of Rin1 or EEA1 from the cytosol and the subsequent fusion reaction, showed an inhibitory effect. We also found that the addition of inactive fragment of Syntaxin 13 inhibited the fusion reaction (Figure 2C). These observations further confirmed that our in vitro fusion reaction was SNARE-dependent, as previously determined for the heterotypic fusion between clathrin coated vesicle and early endosomes, and homotypic fusion between early endosomes (Rubino et al., 2000). Because PI3-kinase and NSF activities have been shown to regulate the fusion reaction, we investigated the role of these two activities in the fusion reaction by incubating endosomes in the presence of NEM (a NSF inhibitor), or Wortmannin (a PI3-kinase inhibitor). As shown in Figure 2C, the addition of 1 mM NEM and 100 nM Wortmannin strongly inhibited the fusion reaction. Taken together, these results indicate that the fusion between endosomes containing Biotin-insulin and Avidine-β-Galactosidase was mediated by a specific fusion event whose characteristics are similar to those described in other endosome-endosome fusion reactions (Braell, 1987; Gruenberg and Howell, 1986; Mayorga et al., 1988).
As described above, our data indicate that different activities (i.e., Rab5, Rin1, and PI3-kinase) are required in this novel fusion reaction between endosomes. Interestingly these activities have also been linked to the activated insulin receptor, including the formation of enlarged Rab5-positive endosomes in intact cells upon addition of insulin (Hunker et al., 2006b). Therefore, we raised the question whether the tyrosine kinase activity of the insulin receptor was also required in the fusion between endosomes. To investigate this requirement, we first examined the effect of AG1024, an insulin receptor tyrosine kinase inhibitor (Parrizas et al., 1997) on the fusion reaction. As a control, we also examined the effect of an inactive tyrosine kinase inhibitor (AG9). When the fusion reaction was conducted in the presence of AG1024, we found a strong inhibition of the fusion reaction in a concentration dependent manner (Figure 1B). However, the addition of AG9 did not affect the fusion reaction, which suggests that this inhibitory effect was specific.
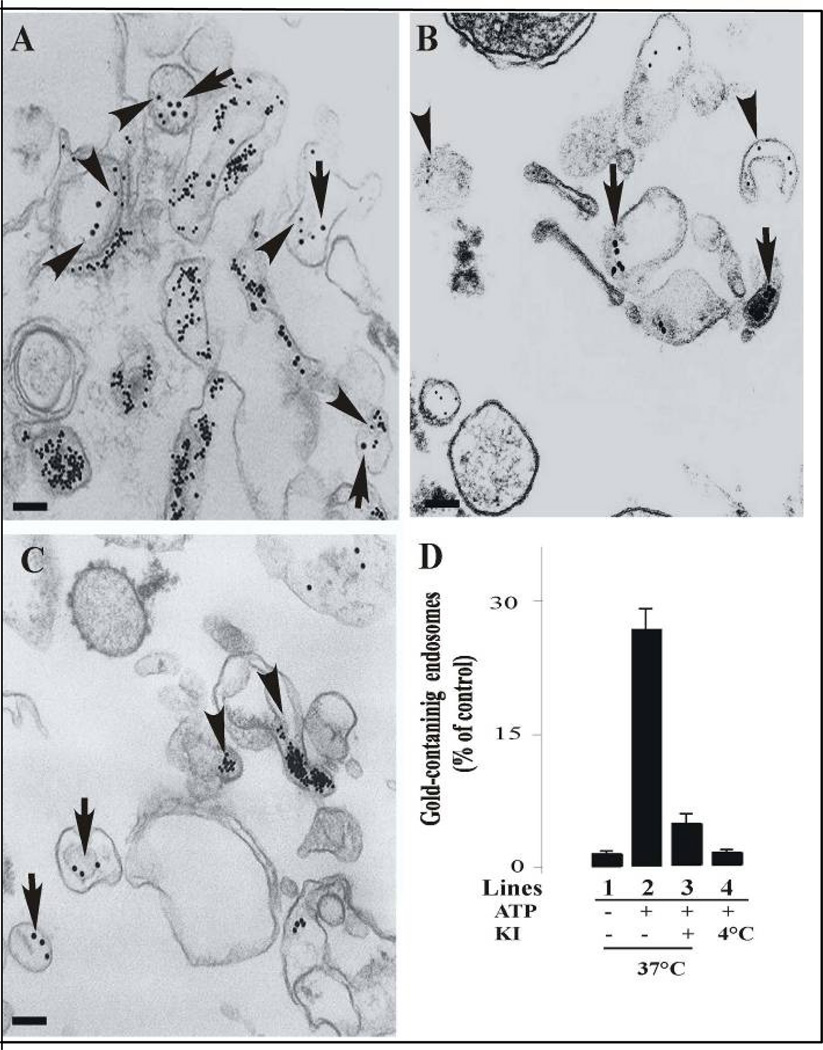
A similar protocol was followed to examine the endosome-endosome fusion morphologically. In these experiments, 20 nm colloidal gold coated with Biotin-insulin and 10 nm colloidal gold coated with Avidin-β-Galactosidase were localized in 5-min endosomes. These endosomes were incubated under standard fusogenic conditions and the vesicle pellets were then processed and observed by electron microscopy as described in Material and Methods. As shown in Figure 3A, we observed vesicle fusion in the presence of cytosol at 37°C containing an ATP-regenerating system, evidenced by the presence of varying sized of gold particles within an endosome. However, we did not observe vesicle fusion when the vesicles were incubated in the presence of cytosol either at 4°C (Figure 3B) or in the presence of 20 µM AG1024 kinase inhibitor (Figure 3C). We also determined the number of vesicles containing more than one size of gold particles within an endosome under several experimental conditions. After fusion reactions, we observed that 25 ± 4 % of the endosomes contained two different sizes of gold particles when the endosomes were incubated in the presence of cytosol supplemented with ATP (Figure 3D, line 2). However, in the absence of ATP at 37°C (Figure 3D line 1) or at 4°C (Figure 3D, line 4), or the addition of AG1024, we observed a strong inhibition of formation of endosomes containing two different sizes of gold particles (Figure 3D line 3).
Figure 3. Morphology of in vitro fusion between endosomes.
Sub-cellular fractions containing colloidal gold of 10 nm (B-Ins) and 20 nm (Av-GAL) loaded in 5-min early endosomes were incubated in vitro for 45 min in fusion buffer in the presence of 1.2 mg of cytosolic protein/ml at 37°C (A) and at 4°C (B). (C) Fuison was also supplemented by 20 µM AG1024 kinase inhibitor in the presence of 1.2 mg of cytosolic protein/ml at 37°C. After the fusion, the samples were fixed in suspension, pelleted, and analyzed for transmission electron microscopy. Bars: 100 nm. (D) For each time of the fusion reaction, the presence of either 10 or 20 nm gold particles was assessed for at least 2,000 endosomes under four different experimental conditions (−ATP/37°C, lane 1; +ATP/37°C, lane 2; +ATP/37°C in the presence of 20 µM AG1024 (KI), lane 3; +ATP/4°C, lane 4). Results are expressed as a percentage of the total endosomes that contained 10 nm and 20 nm gold. Values are means ± SD, n = 3.
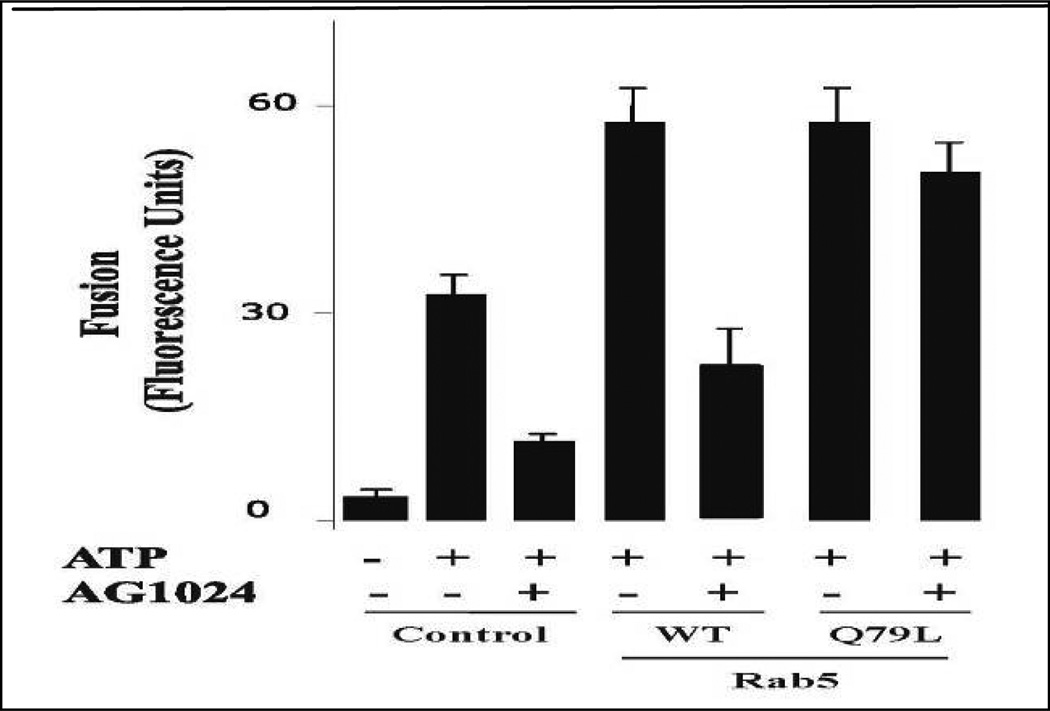
Because the fusion reaction required Rab5 and was also inhibited by AG1024, we tested whether AG1024 inhibitor would affect the endosome fusion stimulated by Rab5. Early endosome and cytosol preparations were pre-incubated with 20 µM of AG1024 for 15 min prior to the initiation of the fusion reaction at 4°C. It was seen that AG1024 partially inhibited early endosome fusion in the presence of Rab5: wild type. However, addition of Rab5: Q79L reversed the inhibitory effect of AG1024 inhibitor and stimulated endosome fusion (Figure 4). This result made it unlikely that the inhibition by AG1024 was due to nonspecific damaging of endosomal membranes. Altogether, these observations may establish a potential role of the insulin receptor tyrosine kinase activity on the activation of the fusion reaction that is mediated by Rab5.
Figure 4.
Selective effect of insulin receptor kinase inhibitor on the fusion reaction. The fusion assay was performed under standard conditions as described in Figure 1A, either in the absence or in the presence of Rab5:WT or Rab5:Q79L mutant, supplemented with either 20 µM AG1024 or ATP in the presence of 0.5 mg/ml cytosol. The data are presented as means ± SD of four independent experiments.
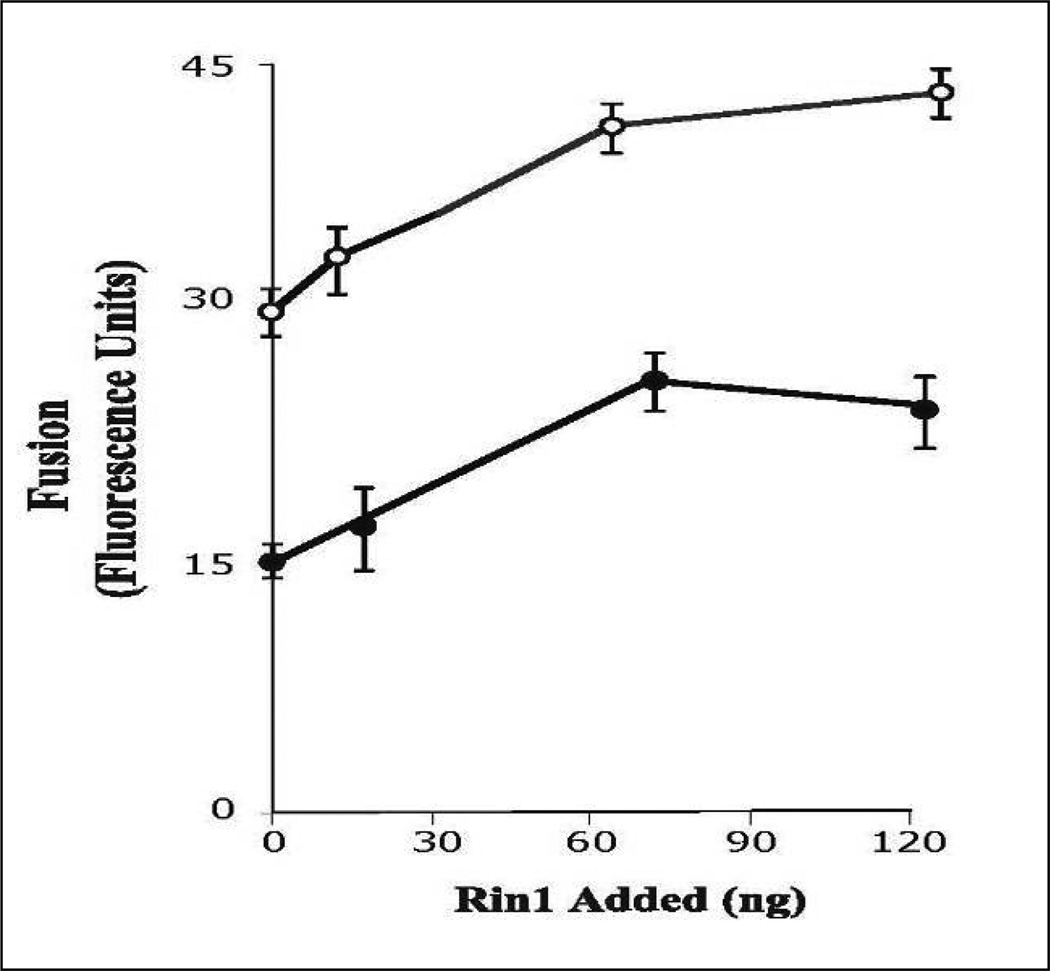
Based on our observations that Rab5: wild type partially restored the fusion reaction affected by AG1024 and the fact that Rin1 stimulated endosome fusion, we further examined the role of AG1024 inhibitor on the fusion reaction by investigating whether AG1024 inhibitor affects the endosome fusion stimulated by Rin1. In Figure 5, we showed that Rin1 stimulated endosome fusion in a concentration-dependent manner. However, Rin1 partially restored the inhibitory of AG1024. Taken together, these observations indicate that tyrosine kinase activity of insulin receptor plays, at least in part, an important role in Rin1-mediated endosome fusion.
Figure 5.
Insulin receptor kinase inhibitor blocks endosome fusion stimulated by Rin1. The fusion assay was performed under standard conditions as described in Figure 1A, either in the absence or in the presence of different concentrations of Rin1 supplemented with several concentrations of AG1024 (○: no addition, ●: 20 µM) inhibitor in the presence of 0.5 mg/ml cytosol. The data are presented as means ± SD of four independent experiments.
Tyrosine kinase inhibitors block fusion between endosomes in intact cells
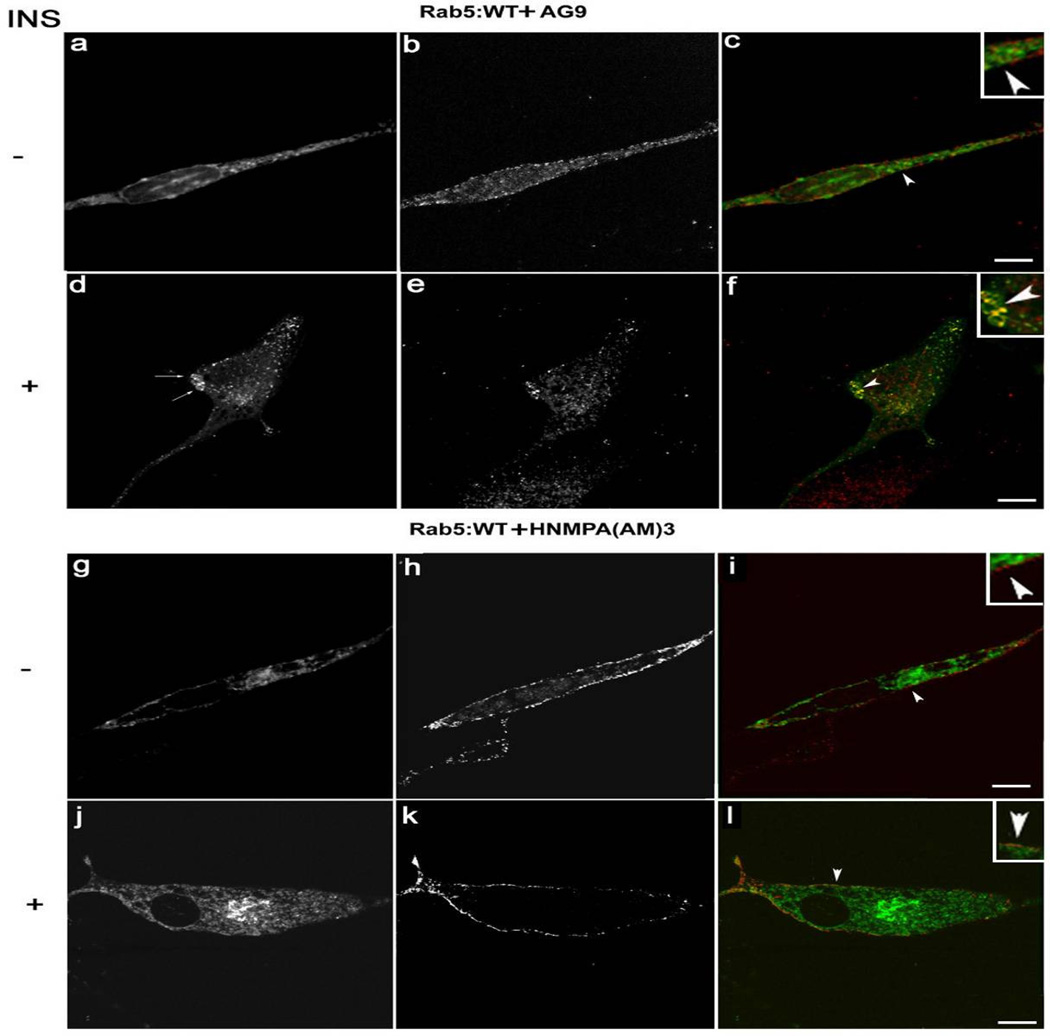
Given the effect of AG1024 inhibitor on the in vitro Rab5-dependent fusion between endosomes, we examined whether the addition of insulin receptor tyrosine kinase inhibitor HNMPA-(AM)3 affects the changes in the Rab5 distribution and/or localization upon insulin stimulation in intact cells. HNMPA-(AM)3 is a cell-permeable and selective insulin receptor tyrosine kinase inhibitor analog of HNMPA (Saperstein et al., 1989). For this purpose, we prepared cell lines expressing Rab5: wild type and Rab5: Q79L mutant, respectively. These cell lines were then treated 10 min with 100 ng/ml insulin in the absence or presence of 100 µM HNMPA-(AM)3 inhibitor.
After the incubation, cells were processed for immunofluorescence microscopy as described in Materials and Methods. As expected, in the absence of insulin, Rab5: wild type appears in diffuse and typically punctuate endosomal structures (Figure 6A–C). However, upon addition of insulin, Rab5: wild type was found in enlarged vesicles (Figure 6D–F). The sizes of these enlarged Rab5-positive endosomes were comparable to the endosomal size observed in cells expressing Rab5: Q79L mutant (compare Figure 6D–F with Figure 7M–X). Interestingly, in cells stimulated with insulin and treated with HNMPA-(AM)3 inhibitor, we observed a significant decrease in size of Rab5-positve endosomes in cells expressing Rab5: wild type (Figure 6J–L), but not in cells expressing Rab5: Q79L mutants (Figure 7V–X). These observations are consistent with our in vitro endosome fusion data, in which the addition of Rab5: wild type, but not Rab5: Q79L mutant, partially supported the fusion reaction in the presence of insulin receptor tyrosine kinase inhibitor (Figure 4).
Figure 6.
Confocal immunofluorescence analysis of cells co-expressing Insulin receptor, Rab5: wild-type in the presence of insulin receptor tyrosine kinase inhibitor. NIH-IR cells were transfected with plasmids encoding GFP-Rab5: wild type (A–L) in the absence (A–C; G–I) or in the presence of 100 ng/ml insulin (D–F; J–L). Cells were also supplemented with 100 µM HNMPA-(AM)3 inhibitors (G–L) and inactive analog (AG9) (A–F). 100 ng/ml insulin was bound to the cells at 4°C for 60 min. The cells were then washed with ice-cold PBS and then incubated at 37°C for 8 min. Subsequently, the cells were washed three times with ice-cold PBS and fixed with 4% paraformaldehyde and then were permeabilized with 0.1% Triton X-I00 prior to incubation with antibodies. The primary antibodies used were rabbit anti-insulin receptor. The secondary antibodies used were Alexa564-labelled donkey anti-rabbit IgG antibodies. Yellow color indicates areas of co-localization between the internalized insulin receptor and the overexpressed GFP-Rab5 proteins (C, F, I and L). An inactive analog (AG9) was used as control. The optical sections viewed are 0.4 µm. Size bars, 10 µm.
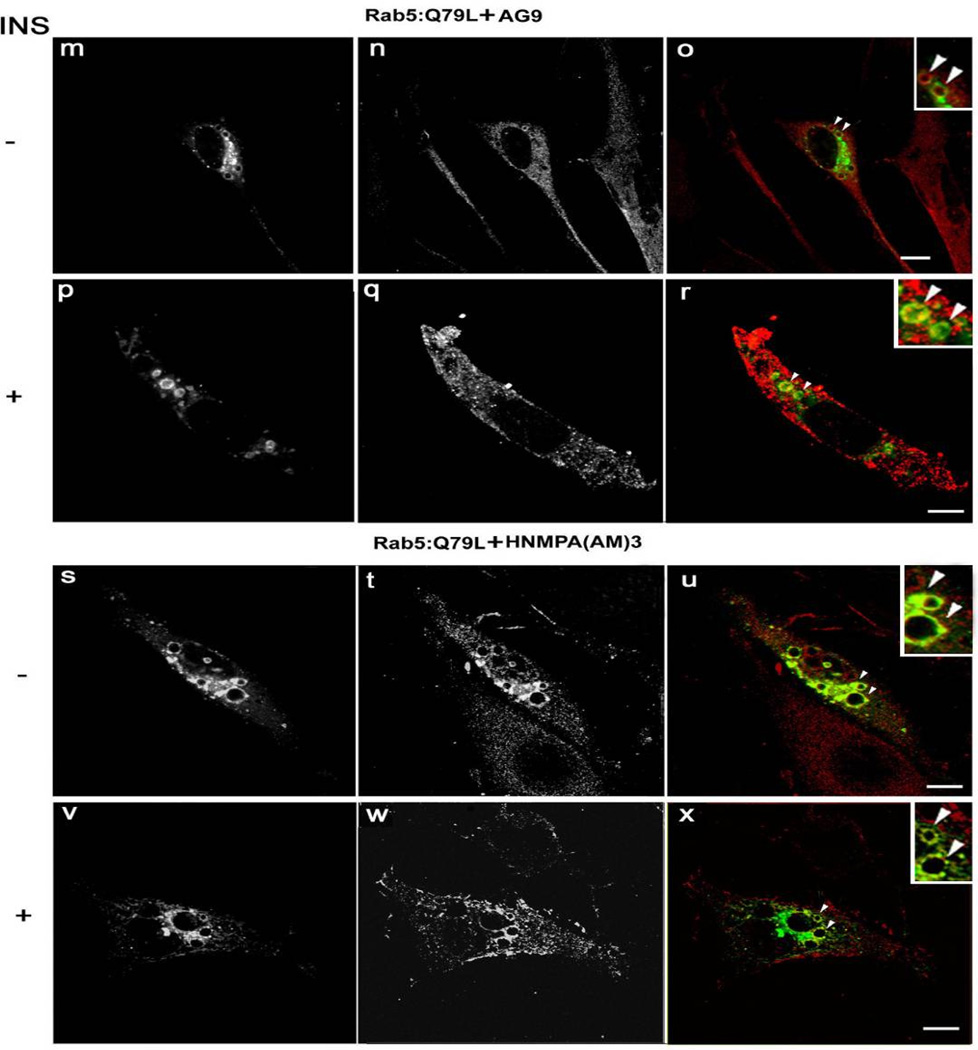
Figure 7.
Confocal immunofluorescence analysis of cells co-expressing Insulin receptor, Rab5: Q79L mutant in the presence of insulin receptor tyrosine kinase inhibitor. NIH-IR cells were transfected with plasmids encoding GFP-Rab5: Q79L (A–L) and in the absence (A–C and G–I) or in the presence of insulin (D–F and J–L). Cells were also supplemented with 100 µM HNMPA-(AM)3 inhibitors (G–L) and inactive analog (AG9) (A–F). 100 ng/ml insulin was bound to cells at 4°C for 60 min. Cells were washed with ice-cold PBS and then incubated at 37°C for 8 min. Subsequently, the cells were washed three times with ice-cold PBS and fixed with 4% paraformaldehyde and then permeabilized with 0.1% Triton X-I00 prior to incubation with antibodies. The primary antibodies used were rabbit anti-insulin receptor. The secondary antibodies used were Alexa564-labelled donkey anti-rabbit IgG antibodies. Yellow color indicates areas of co-localization between the internalized insulin receptor and the overexpressed GFP-Rab5 proteins (C, F, I and L). An inactive analog (AG9) was used as control. The optical sections viewed are 0.4 µm. Size bars, 10 µm.
We also observed that insulin receptor co-localizes with Rab5 on endosomes when cells were stimulated with insulin in the absence of HNMPA-(AM)3 inhibitor (Figure 6D–F), but not in the presence of the inhibitor (compare Figure 6D–F and 6J–L). Furthermore, analysis of Rab5: wild type in cells stimulated with insulin in the presence of inhibitor showed a more diffuse (primary cytosolic) pattern, that is comparable with the distinct distribution of Rab5: wild type in the absence of insulin (compare Figure 6A–C and 6J–L). In contrast, in cells expressing Rab5: Q79L mutant, the addition of HNMPA-(AM)3 inhibitor failed to affect the formation of significantly enlarged Rab5:Q79L mutant-positive endosomes (compare Figure 7P–R and 7V–Y). Moreover, we observed that the internalized insulin receptor also co-localized with Rab5: Q79L mutant-positive endosomes, when cells were treated with HNMPA-(AM)3 inhibitor (Figure 7P–R and 7V–Y). These results suggest that the formation of these enlarged Rab5: Q79L-positive endosomes as well as the localization of insulin receptor were independent of the activation of the insulin receptor.
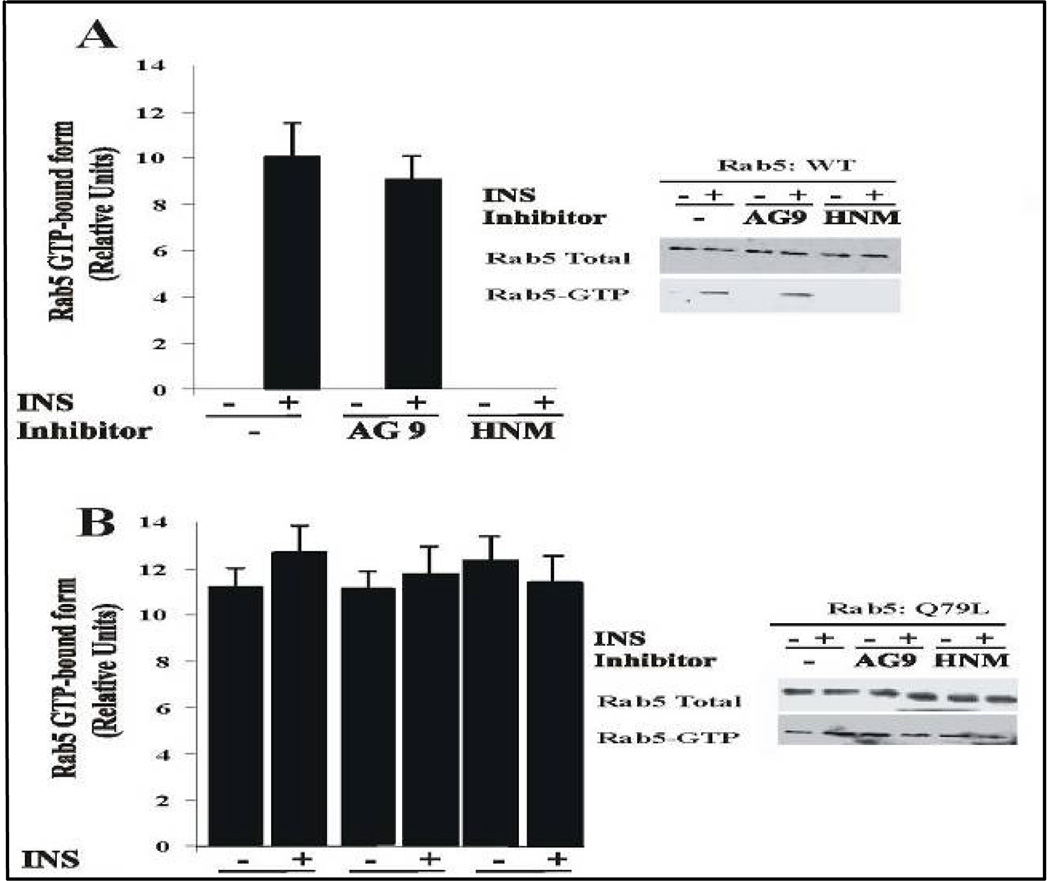
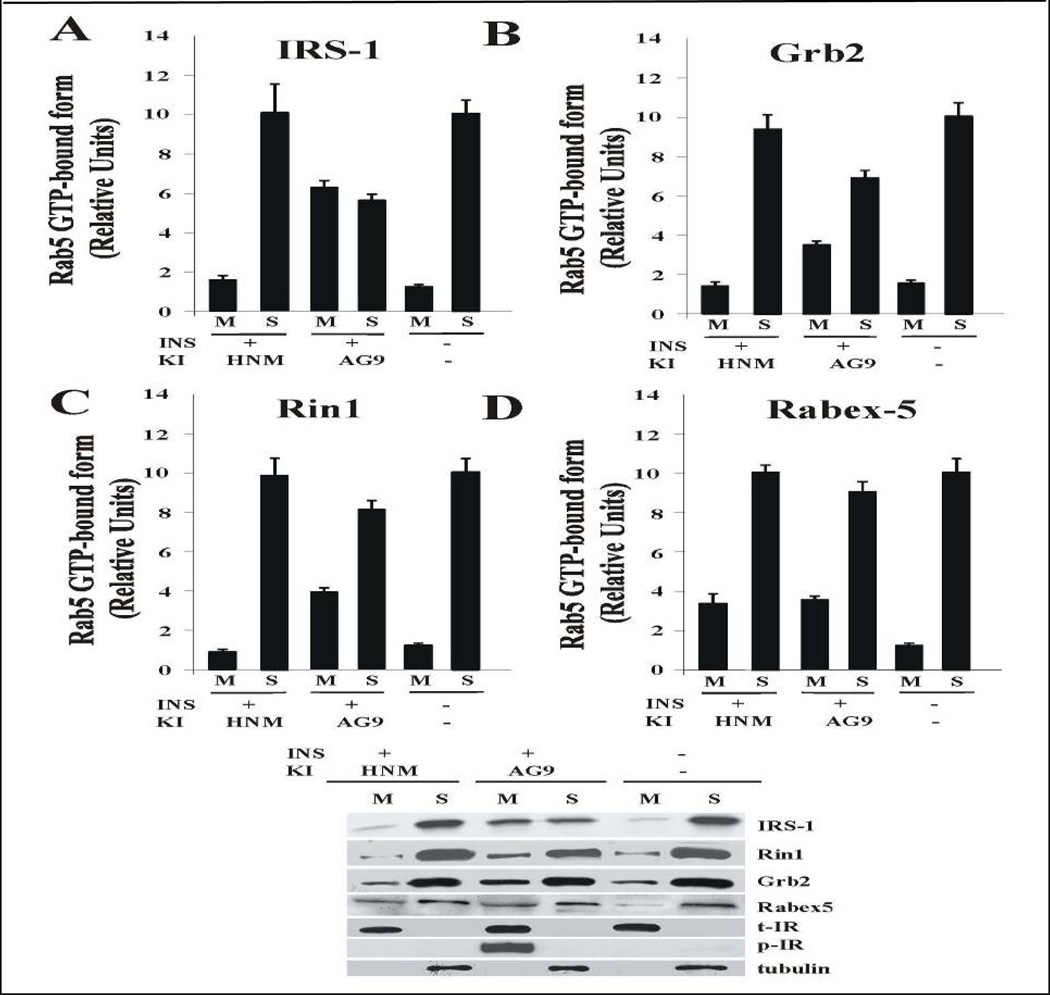
HNMPA-(AM)3 inhibitor blocks Rab5 activation and targeting of Rin1 to membrane upon insulin stimulation
To examine the role of insulin receptor on the activation of Rab5 upon stimulation of insulin, cells expressing either Rab5: wild type or Rab5: Q79L mutant were incubated with or without HNMPA-(AM)3 inhibitor in the absence or presence of insulin. After incubation, Rab5 GTP-bound form was precipitated with GST-EEA1/N domain, as described in the Materials and Methods. As shown in Figure 8A, the addition of HNMPA-(AM)3 inhibitor significantly diminished the amount of Rab5-GTP bound form when cells expressing Rab5: wild type were stimulated with insulin. However, in cells expressing Rab5: Q79L, the addition of HNMPA-(AM)3 inhibitor did not exhibit any effect at all (Figure 8B).
Figure 8.
Insulin receptor tyrosine kinase inhibitor blocks the activation of Rab5 in intact cells. NIH-IR were transfected with plasmids encoding GFP-Rab5: wild type (A) and GFP-Rab5: Q79L mutant (B) in the absence or in the presence of 100 ng/ml insulin containing either 100 µM AG9 or 100 µM HNMPA-(AM)3 (HNM) inhibitors as indicated in the Figure. Insulin was bound to the cells at 4°C for 60 min. The cells were washed and then incubated at 37°C for 8 min. Subsequently, the cells were washed three times with ice-cold PBS, lysed and incubated with gluthathione beads either in the presence of GST alone or GST-EEA1 at 4°C for 60 min. After incubation, the beads were washed and the presence of activated Rab5 was analyzed by Western blotting using anti-Rab5 antibodies. The data are presented as means ± SD of four independent experiments.
Insulin receptor’s activation leads to autophosphorylation, which is followed by the downstream phosphorylation of IRS (Fan et al., 1982; Russell et al., 1987) and association of molecules such as Grb2 (Liu and Roth, 1995) and Rin1 (Hunker et al., 2006a) through the clathrin-mediated pathway. To further examine whether the insulin receptor kinase activity is linked to the membrane targeting to Rin1, the effect of HNMPA-(AM)3 inhibitor was evaluated to determine whether this nucleotide exchange factor for Rab5 was targeted to membranes upon insulin stimulation. In Figure 9, we show that insulin receptor was tyrosine phosphorylated in the presence, but not in the absence, of insulin. Furthermore, insulin receptor was found associated to membranes, which indicates that the integrity of the isolated membrane fraction. IRS-1 and Grb2 were associated with membranes upon insulin stimulation. However, the addition of HNMPA-(AM)3 inhibitor partially blocked the membrane association of these molecules. Interestingly, Rin1 was also associated with membrane upon insulin stimulation, but not in the presence of HNMPA-(AM)3 inhibitor.
Figure 9.
Effect of HNMPA-(AM)3 inhibitor on the tyrosine phosphorylation of Insulin receptor and recruitment of Rin1. Cells were incubated in the absence or in the presence of 100 ng/ml insulin either 100 µM AG9 or 100 µM HNMPA-(AM)3 inhibitor as described in the Figure. After treatment, cells were washed with ice cold PBS and incubated at 37°C for 8 minutes. Cells were then washed again using ice-cold PBS, homogenized and membrane fractions were prepared as described in Materials and Methods. Membrane (M) and cytosol (S) fractions were treated with sample buffer and proteins were subject to SDS-PAGE, blotted to a nitrocellulose membrane, and antibodies specific to IRS-1 (A), Grb2 (B), Rin1 (B), Rabex-5 (C), tubulin, phospo-insulin receptor (p)-IR and total (t)-IR, and tubulin were used to visualize these proteins by Western blot analysis. A representative experiment is shown (E). This experiment was repeated at least three times, and the results were reproducible. The data are presented as means ± SD of three independent experiments.
We also examined the effect of HNMPA-(AM)3 inhibitor on the distribution of Rabex-5, another Rab5-GEF. As shown in Figure 9, Rabex-5 was recruited to the membrane upon stimulation with insulin. However, the addition of HNMPA-(AM)3 upon stimulation with insulin had no effect on the recruitment of Rabex-5 to the membrane. Taken together, these results are consistent with the observation that HNMPA-(AM)3 blocked the activation of Rab5 via Rin1 in intact cells.
DISCUSSION
We have shown that the addition of HNMPA-(AM)3 tyrosine kinase inhibitor blocked the formation of enlarged Rab5-positive endosomes upon insulin stimulation in intact cells expressing Rab5:wild type, but not Rab5:Q79L mutant. Consistent with this observation, the receptor tyrosine kinase inhibitors (i.e., AG1024) specifically block the fusion between early endosomes. Interestingly, the inhibitory effect was reversed by the addition of Rab5: Q79L mutant. These data suggest a positive role of receptor tyrosine kinase activity on endosome fusion that is mediated by the activation of Rab5, which is consistent with increase in endosome size observed upon ligand stimulation.
The insulin receptor is a well-characterized system, which involves the internalization of the receptor from the plasma membrane to endosome upon ligand stimulation. In our experimental conditions, the binding of Biotin-insulin was saturated and nonspecific binding, measured in the presence of 300 fold of unlabeled Insulin, was less than 3.2 % of the total counts/min in the pellet. By Scatchard analysis, we determined dissociation constants for Biotin-insulin (Kd=0.33 nM) and for unmodified insulin (Kd=0.29 nM) in HepG2 cells and these values were comparable to values reported for unmodified insulin in the order of the nM (Backer et al., 1991; Scholz et al., 1992).
When these two endocytic vesicles containing Biotin-insulin and Avidin-β-Galactosidse are brought together upon vesicle fusion, the resulting enzymatic activity of β-galactosidase in the complex, points to the specificity of the fusion reaction (Figures 1 and 2). The results reported here show that the fusion reaction is time, ATP and cytosol-dependent with an optimal temperature of 37°C. Morphological analysis of the fusion events clearly shows that in the presence of cytosol the formation of endosome containing different size of gold particles. Consistent with these observations, fluorescence microscope analysis revealed that the accumulation of insulin receptor in early endosomes is dependent on tyrosine kinase activity since the insulin receptor is localized on the plasma membrane by the addition of the HNMPA-(AM)3 inhibitor (Figure 6). Interestingly, we also observed that the presence of HNMPA-(AM)3 inhibitor blocked the formation of enlarged Rab5-positve endosomes (Figure 6) as well as the activation of Rab5 in intact cells (Figure8). However, the addition of HNMPA-(AM)3 inhibitor neither blocked the intracellular localization of insulin receptor nor the formation of enlarged Rab5-positve endosomes in cells expressing Rab5: Q79L mutant. Interestingly, we also observed that the presence of AG1024 inhibitor blocked the activation of Rab5: wild type, but not the activation of Rab5: Q79L mutant, in intact cells (Figures 7 and 8). Thus, these data establish that tyrosine kinase activity of the insulin receptor is, in part, responsible for the formation of enlarged Rab5-positive endosomes, which is linked to the activation of Rab5.
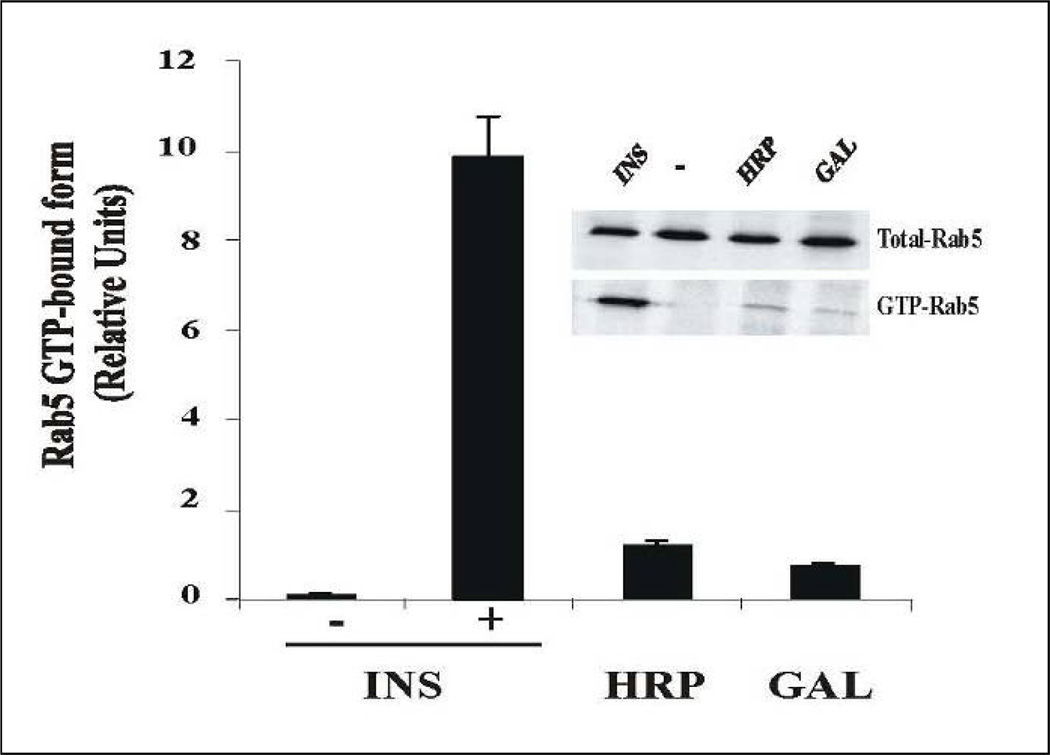
The potential mechanism of this inhibitory effect may be associated with Rin1, since it is associated with the insulin receptor upon ligand stimulation (Hunker et al., 2006a), and because it is also required for the fusion assay, whose fusogeneic activity is partially reversed by AG1024 inhibitor. These observations raise the possibility that the tyrosine kinase enzymatic activity of the insulin receptor may be required during the fusion assay. This inhibitory effect was concentration-dependent and specific since AG9 (an inactive analog) did not affect the fusion reaction. More importantly, the presence of this inhibitory effect may be associated with an effect on the interaction between Rin1 and activated insulin receptor. This is in strong agreement with the fact that Rin1 was found significantly less associated with membrane fraction in the presence of HNMPA-(AM)3 upon insulin stimulation (Figure 9). However, the association of Rabex-5 (another Rab5 exchange factor) with membranes was not affected in the presence of inhibitor. These observations indicate that this inhibition by HNMPA-(AM)3 may be selectively associated with Rin1, but not with Rabex-5. Because stimulation of the fusion reaction by Rin1 and Rab5, but not Rab5: Q79L mutant, were also partially blocked by AG1024 raises the possibility that once Rab5 is already activated by its exchange factor, part of this in vitro endosome fusion may be independent of AG1024 inhibitor. Alternatively, it is also possible that AG1024 does not completely block insulin receptor tyrosine kinase enzymatic activity in our in vitro system, which, in part, may also contribute the inability of the AG1024 to fully block the fusion reaction. Nevertheless, our in vitro and in vivo data support that the tyrosine kinase enzymatic activity of the insulin-receptor is required during the endosome fusion assay. Another view of the fusion reaction between endosomes containing Biotin-insulin and Avidin-β–tosidase is that AG1024 inhibitor may affect the activation of Rab5 in one set of endosomes containing insulin receptor (i.e., Biotin-insulin endosomes) but not in the other set of endosomes containing Avidin-β-Galactosidase, a fluid phase marker. Therefore, this model predicts that Rab5 should be present and active in both sets of endosomes during the fusion reaction. Our results are in strong agreement with previous observations that Rab5 was required for the fusion in both endosomes (Barbieri et al., 1998). However, we have found that Rab5 is activated during the enodocytosis of several fluid phase markers (i.e., HRP and β-Galactosidase). Specifically, we observed that activation of Rab5 during the uptake of fluid phase marker is less robust (~10%) as compared with the activation of Rab5 during insulin stimulation (Figure 10), which may help to explain, in part, the fact that this endosome fusion is not completly inhibited in the presence of insulin receptor kinase inhibitor (Figure 1). These observations are consistent with previous observations where Rab5 was localized in endosomes containing HRP as a fluid phase marker (Gruenberg and Howell, 1986). Interestingly, it has been recently demonstrated that active Rab5 was found on macropinosomes (Feliciano, et al., 2011). Thus, it is possible that several mechanisms of activation of Rab5 are taking place during receptor-mediated endocytosis and endosome fusion. Clearly more work needs to be done in order to investigate how is Rab5 activated during fluid phase endocytosis.
Figure 10.
Activation of Rab5 during fluid phase and receptor-mediated endocytosis. NIH-IR cells were transfected with plasmids encoding GFP-Rab5: wild type in the absence or in the presence of 100 ng/ml insulin (INS), 2 mg/ml HRP and 1 ug/ml β-Galactosidase (GAL) as indicated in the Figure. Each ligand was incubated with cells at 4°C for 60 min. Cells were then incubated at 37°C for 8 min. Subsequently, the cells were washed three times with ice-cold PBS, lysed and incubated with glutathione beads either in the presence of GST-EEA1 at 4°C for 60 min. After incubation, the beads were washed and the presence of activated Rab5 was analyzed by Western blotting using anti-Rab5 antibodies. The data are presented as means ± SD of two independent experiments.
Given the importance of tyrosine phosphorylation in signaling and endocytosis by hormones and growth factors, specific and selective inhibitors of tyrosine kinase activity are important means for investigating receptor tyrosine kinases biological functions. Thus, the utilization of these inhibitors may be critical for elucidation of the role of tyrosine kinase activity for both in vitro and in vivo setting. Furthermore, the activation of intrinsic or associated tyrosine kinases during the internalization of growth factor receptor is a key feature of endocytosis and signaling processes, in which several small GTP-ases have been implicated, including Rab5.
In conclusion, we have used in vivo and in vitro approaches to investigate the role of insulin tyrosine phosphorylation during the fusion reaction. We demonstrated that tyrosine kinase inhibitors affect in vitro endosome fusion, the formation of enlarged Rab5-positive endosomes and the activation of Rab5 in intact cells. This data suggest that the insulin receptor kinase activity may provide, at least in part, a link between endosome fusion and the insulin receptor COOH terminus via Rin1, which in turn, will activate the small GTPase Rab5 and also increases the fusion activity.
ACKNOWLEDGMENTS
The authors gratefully acknowledge Miguel Seabra for the generous gifts of experimental materials. This work was supported by the National Institutes of Health grant SC1DK084343 (to MAB). We also thank the Florida International University Foundation.
REFERENCES
- Backer JM, Shoelson SE, Haring E, White MF. Insulin receptors internalize by a rapid, saturable pathway requiring receptor autophosphorylation and an intact juxtamembrane region. Journal of Cell Biology. 1991;115:1535–1545. doi: 10.1083/jcb.115.6.1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbieri MA, Hoffenberg S, Roberts R, Mukhopadhyay A, Pomrehn A, Dickey BF, Stahl PD. Evidence for a symmetrical requirement for Rab5-GTP in in vitro endosome-endosome fusion. Journal of Biological Chemistry. 1998;273:25850–25855. doi: 10.1074/jbc.273.40.25850. [DOI] [PubMed] [Google Scholar]
- Barbieri MA, Li G, Colombo MI, Stahl PD. Rab5, an early acting endosomal GTPase, supports in vitro endosome fusion without GTP hydrolysis. Journal of Biological Chemistry. 1994;269:18720–18722. [PubMed] [Google Scholar]
- Barbieri MA, Roberts RL, Gumusboga A, Highfield H, Alvarez-Dominguez C, Wells A, Stahl PD. Epidermal growth factor and membrane trafficking. EGF receptor activation of endocytosis requires Rab5a. Journal of Cell Biology. 2000;151:539–550. doi: 10.1083/jcb.151.3.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braell WA. Fusion between endocytic vesicles in a cell-free system. Proceedings of the National Academy of Sciences U S A. 1987;84:1137–1141. doi: 10.1073/pnas.84.5.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandhorst D, Zwilling D, Rizzoli SO, Lippert U, Lang T, Jahn R. Homotypic fusion of early endosomes: SNAREs do not determine fusion specificity. Proceedings of the National Academy of Sciences U S A. 2006;103:2701–2706. doi: 10.1073/pnas.0511138103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bucci C, Parton RG, Mather IH, Stunnenberg H, Simons K, Hoflack B, Zerial M. The small GTPase rab5 functions as a regulatory factor in the early endocytic pathway. Cell. 1992;70:715–728. doi: 10.1016/0092-8674(92)90306-w. [DOI] [PubMed] [Google Scholar]
- Carpentier JL, Paccaud JP, Backer J, Gilbert A, Orci L, Kahn CR, Baecker J. Two steps of insulin receptor internalization depend on different domains of the beta-subunit. Journal of Cell Biology. 1993;122:1243–1252. doi: 10.1083/jcb.122.6.1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpentier JL, Paccaud JP, Gorden P, Rutter WJ, Orci L. Insulin-induced surface redistribution regulates internalization of the insulin receptor and requires its autophosphorylation. Proceedings of the National Academy of Sciences U S A. 1992;89:162–166. doi: 10.1073/pnas.89.1.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christoforidis S, Miaczynska M, Ashman K, Wilm M, Zhao L, Yip SC, Waterfield MD, Backer JM, Zerial M. Phosphatidylinositol-3-OH kinases are Rab5 effectors. Nature Cell Biology. 1999;1:249–252. doi: 10.1038/12075. [DOI] [PubMed] [Google Scholar]
- Di Guglielmo GM, Drake PG, Baass PC, Authier F, Posner BI, Bergeron JJ. Insulin receptor internalization and signalling. Molecular and Cell Biochemistry. 1998;182:59–63. [PubMed] [Google Scholar]
- Doherty GJ, McMahon HT. Mechanisms of endocytosis. Annual Review of Biochemistry. 2009;78:857–902. doi: 10.1146/annurev.biochem.78.081307.110540. [DOI] [PubMed] [Google Scholar]
- Fan JY, Carpentier JL, Gorden P, Van Obberghen E, Blackett NM, Grunfeld C, Orci L. Receptor-mediated endocytosis of insulin: role of microvilli, coated pits, and coated vesicles. Proceedings of the National Academy of Sciences U S A. 1982;79:7788–7791. doi: 10.1073/pnas.79.24.7788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feliciano WD, Yoshida S, Straight SW, Swanson JA. Coordination of the Rab5 Cycle on Macropinosomes. Traffic. 2011;12:1911–1922. doi: 10.1111/j.1600-0854.2011.01280.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fucini RV, Okada S, Pessin JE. Insulin-induced desensitization of extracellular signal-regulated kinase activation results from an inhibition of Raf activity independent of Ras activation and dissociation of the Grb2-SOS complex. Journal of Biological Chemistry. 1999;274:18651–18658. doi: 10.1074/jbc.274.26.18651. [DOI] [PubMed] [Google Scholar]
- Gorvel JP, Chavrier P, Zerial M, Gruenberg J. rab5 controls early endosome fusion in vitro. Cell. 1991;64:915–925. doi: 10.1016/0092-8674(91)90316-q. [DOI] [PubMed] [Google Scholar]
- Gruenberg JE, Howell KE. Reconstitution of vesicle fusions occurring in endocytosis with a cell-free system. Embo Journal. 1986;5:3091–3101. doi: 10.1002/j.1460-2075.1986.tb04615.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horiuchi H, Lippe R, McBride HM, Rubino M, Woodman P, Stenmark H, Rybin V, Wilm M, Ashman K, Mann M, Zerial M. A novel Rab5 GDP/GTP exchange factor complexed to Rabaptin-5 links nucleotide exchange to effector recruitment and function. Cell. 1997;90:1149–1159. doi: 10.1016/s0092-8674(00)80380-3. [DOI] [PubMed] [Google Scholar]
- Hunker CM, Giambini H, Galvis A, Hall J, Kruk I, Veisaga ML, Barbieri MA. Rin1 regulates insulin receptor signal transduction pathways. Experimental Cell Research. 2006a;312:1106–1118. doi: 10.1016/j.yexcr.2005.12.021. [DOI] [PubMed] [Google Scholar]
- Hunker CM, Kruk I, Hall J, Giambini H, Veisaga ML, Barbieri MA. Role of Rab5 in insulin receptor-mediated endocytosis and signaling. Archives of Biochemistry and Biophysics. 2006b;449:130–142. doi: 10.1016/j.abb.2006.01.020. [DOI] [PubMed] [Google Scholar]
- Khan MN, Baquiran G, Brule C, Burgess J, Foster B, Bergeron JJ, Posner BI. Internalization and activation of the rat liver insulin receptor kinase in vivo. Journal of Biological Chemistry. 1989;264:12931–12940. [PubMed] [Google Scholar]
- Klein HH, Freidenberg GR, Matthaei S, Olefsky JM. Insulin receptor kinase following internalization in isolated rat adipocytes. Journal of Biological Chemistry. 1987;262:10557–10564. [PubMed] [Google Scholar]
- Li G, D'Souza-Schorey C, Barbieri MA, Roberts RL, Klippel A, Williams LT, Stahl PD. Evidence for phosphatidylinositol 3-kinase as a regulator of endocytosis via activation of Rab5. Proceedings of the National Academy of Sciences U S A. 1995;92:10207–10211. doi: 10.1073/pnas.92.22.10207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lippe R, Miaczynska M, Rybin V, Runge A, Zerial M. Functional synergy between Rab5 effector Rabaptin-5 and exchange factor Rabex-5 when physically associated in a complex. Molecular Biology of the Cell. 2001;12:2219–2228. doi: 10.1091/mbc.12.7.2219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu F, Roth RA. Grb-IR: a SH2-domain-containing protein that binds to the insulin receptor and inhibits its function. Proceedings of the National Academy of Sciences U S A. 1995;92:10287–10291. doi: 10.1073/pnas.92.22.10287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maggi D, Andraghetti G, Carpentier JL, Cordera R. Cys860 in the extracellular domain of insulin receptor beta-subunit is critical for internalization and signal transduction. Endocrinology. 1998;139:496–504. doi: 10.1210/endo.139.2.5744. [DOI] [PubMed] [Google Scholar]
- Mayorga LS, Diaz R, Colombo MI, Stahl PD. GTP gamma S stimulation of endosome fusion suggests a role for a GTP-binding protein in the priming of vesicles before fusion. Cellular Regulation. 1989;1:113–124. doi: 10.1091/mbc.1.1.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayorga LS, Diaz R, Stahl PD. Plasma membrane-derived vesicles containing receptor-ligand complexes are fusogenic with early endosomes in a cell-free system. Journal of Biological Chemistry. 1988;263:17213–17216. [PubMed] [Google Scholar]
- McBride HM, Rybin V, Murphy C, Giner A, Teasdale R, Zerial M. Oligomeric complexes link Rab5 effectors with NSF and drive membrane fusion via interactions between EEA1 and syntaxin 13. Cell. 1999;98:377–386. doi: 10.1016/s0092-8674(00)81966-2. [DOI] [PubMed] [Google Scholar]
- Mullock BM, Luzio JP. Reconstitution of rat liver endosome-lysosome fusion in vitro. Methods Enzymol. 1992;219:52–60. doi: 10.1016/0076-6879(92)19009-u. [DOI] [PubMed] [Google Scholar]
- Nielsen E, Christoforidis S, Uttenweiler-Joseph S, Miaczynska M, Dewitte F, Wilm M, Hoflack B, Zerial M. Rabenosyn-5, a novel Rab5 effector, is complexed with hVPS45 and recruited to endosomes through a FYVE finger domain. Journal of Cell Biology. 2000;151:601–612. doi: 10.1083/jcb.151.3.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paccaud JP, Siddle K, Carpentier JL. Internalization of the human insulin receptor. The insulin-independent pathway. Journal of Biological Chemistry. 1992;267:13101–13106. [PubMed] [Google Scholar]
- Parrizas M, Gazit A, Levitzki A, Wertheimer E, LeRoith D. Specific inhibition of insulin-like growth factor-1 and insulin receptor tyrosine kinase activity and biological function by tyrphostins. Endocrinology. 1997;138:1427–1433. doi: 10.1210/endo.138.4.5092. [DOI] [PubMed] [Google Scholar]
- Pfeffer SR. Unsolved mysteries in membrane traffic. Annual Review of Biochemistry. 2007;76:629–645. doi: 10.1146/annurev.biochem.76.061705.130002. [DOI] [PubMed] [Google Scholar]
- Rubino M, Miaczynska M, Lippe R, Zerial M. Selective membrane recruitment of EEA1 suggests a role in directional transport of clathrin-coated vesicles to early endosomes. Journal of Biological Chemistry. 2000;275:3745–3748. doi: 10.1074/jbc.275.6.3745. [DOI] [PubMed] [Google Scholar]
- Russell DS, Gherzi R, Johnson EL, Chou CK, Rosen OM. The protein-tyrosine kinase activity of the insulin receptor is necessary for insulin-mediated receptor down-regulation. Journal of Biological Chemistry. 1987;262:11833–11840. [PubMed] [Google Scholar]
- Saperstein R, Vicario PP, Strout HV, Brady E, Slater EE, Greenlee WJ, Ondeyka DL, Patchett AA, Hangauer DG. Design of a selective insulin receptor tyrosine kinase inhibitor and its effect on glucose uptake and metabolism in intact cells. Biochemistry. 1989;28:5694–5701. doi: 10.1021/bi00439a053. [DOI] [PubMed] [Google Scholar]
- Scholz H, Baier W, Ratcliffe P, Eckardt K, Zapf J, Kurtz A, Bauer C. Insulin-like growth factors decrease oxygen-regulated erythropoietin production by human hepatoma cells (Hep G2) American Journal of Physiology. 1992;263:C474–C479. doi: 10.1152/ajpcell.1992.263.2.C474. [DOI] [PubMed] [Google Scholar]
- Scita G, Di Fiore PP. The endocytic matrix. Nature. 2010;463:464–473. doi: 10.1038/nature08910. [DOI] [PubMed] [Google Scholar]
- Siemeister G, al-Hasani H, Klein HW, Kellner S, Streicher R, Krone W, Muller-Wieland D. Recombinant human insulin receptor substrate-1 protein. Tyrosine phosphorylation and in vitro binding of insulin receptor kinase. Journal of Biological Chemistry. 1995;270:4870–4874. doi: 10.1074/jbc.270.9.4870. [DOI] [PubMed] [Google Scholar]
- Simonsen A, Lippe R, Christoforidis S, Gaullier JM, Brech A, Callaghan J, Toh BH, Murphy C, Zerial M, Stenmark H. EEA1 links PI(3)K function to Rab5 regulation of endosome fusion. Nature. 1998;394:494–498. doi: 10.1038/28879. [DOI] [PubMed] [Google Scholar]
- Smith RM, Jarett L. Differences in adenosine triphosphate dependency of receptor-mediated endocytosis of alpha 2-macroglobulin and insulin correlate with separate routes of ligand-receptor complex internalization. Endocrinology. 1990;126:1551–1560. doi: 10.1210/endo-126-3-1551. [DOI] [PubMed] [Google Scholar]
- Sorkin A, von Zastrow M. Endocytosis and signalling: intertwining molecular networks. Nature Reviews Molecular Cell Biology. 2009;10:609–622. doi: 10.1038/nrm2748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tall GG, Barbieri MA, Stahl PD, Horazdovsky BF. Ras-activated endocytosis is mediated by the Rab5 guanine nucleotide exchange activity of RIN1. Developmental Cell. 2001;1:73–82. doi: 10.1016/s1534-5807(01)00008-9. [DOI] [PubMed] [Google Scholar]
- Waters SB, Yamauchi K, Pessin JE. Insulin-stimulated disassociation of the SOS-Grb2 complex. Molecular and Cell Biochemistry. 1995;15:2791–2799. doi: 10.1128/mcb.15.5.2791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wessling-Resnick M, Braell WA. The sorting and segregation mechanism of the endocytic pathway is functional in a cell-free system. Journal of Biological Chemistry. 1990;265:690–699. PublishingGenomicsWeb. http://academysciencesociety.com/articles/11/6/16/1. Accessed 16 April 2009. [PubMed] [Google Scholar]