Abstract
The protein kinase mTOR (Mammalian or Mechanistic Target of Rapamycin) is an atypical serine/threonine kinase that exerts its main cellular functions by interacting with specific adaptor proteins to form two different multiprotein complexes, mTOR complex 1 (mTORC1) and mTOR complex 2 (mTORC2). mTORC1 regulates protein synthesis, cell growth and proliferation, autophagy, cell metabolism and stress responses, whereas mTORC2 appears to regulate cell survival and polarity.
The mTOR pathway plays a key regulatory function in cardiovascular physiology and pathology. However, the majority of the information available about mTOR function in the cardiovascular system is related to the role of mTORC1 in the unstressed and stressed heart. mTORC1 is required for embryonic cardiovascular development and for postnatal maintenance of cardiac structure and function. In addition, mTORC1 is necessary for cardiac adaptation to pressure overload and development of compensatory hypertrophy. However, partial and selective pharmacologic and genetic inhibition of mTORC1 was shown to extend life span in mammals, reduce pathological hypertrophy and heart failure caused by increased load or genetic cardiomyopathies, reduce myocardial damage after acute and chronic myocardial infarction and reduce cardiac derangements caused by metabolic disorders. The optimal therapeutic strategy to target mTORC1 and increase cardioprotection is under deep investigation.
This article reviews the information available regarding the effects exerted by mTOR signaling in cardiovascular physiology and pathological states.
Keywords: mTOR signaling, mTORC1, rapamycin, hypertrophy, ischemia, metabolism, heart
Introduction
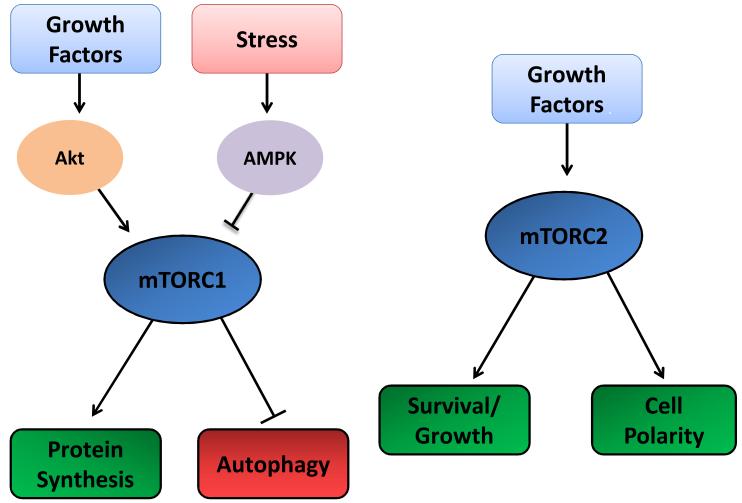
The protein kinase mTOR was purified and characterized for the first time in mammalian cells in the independent works conducted by Eric Brown1, David Sabatini2 and Candace Sabers3 published in 1994 and 1995. mTOR is an atypical serine/threonine kinase that exerts its main cellular functions by interacting with specific adaptor proteins to form two distinct multiprotein complexes, mTORC1 and mTORC24-8. mTOR signaling plays a key role in the regulation of cell homeostasis and stress responses. mTORC1 is a master regulator of protein synthesis, cell growth and proliferation, ribosomal and mitochondrial biogenesis, autophagy and metabolism. In addition, mTORC1 inhibition during stress is an adaptive response that promotes upregulation of stress-responsive mechanisms. On the other hand, mTORC2 appears to regulate cell survival and polarity4-8 (Figure 1).
Figure 1. General cellular functions of mTORC1 and mTORC2.
This figure summarizes the most well characterized functions of mTORC1 and mTORC2. mTORC1 regulates protein synthesis and autophagy in response to growth factors and stress. mTORC2 is known to regulate cell growth, survival and polarity.
Studies of animal models with loss of function of the components of the mTOR complexes have indicated that mTOR is involved in the regulation of embryonic cardiovascular development and in the control of vital cellular processes necessary for normal postnatal growth and maintenance of cardiac function. Cardiac deletion of mTOR is associated with a high rate of embryonic lethality, and cardiac disruption of the components of mTORC1 is associated with cardiac dilation, dysfunction, apoptosis, mitochondrial and metabolic derangements, heart failure and, ultimately, mortality in the postnatal stage9-12. In addition, complete genetic disruption of mTORC1 impairs the ability of the heart to respond to pressure overload and to undergo compensatory hypertrophy, resulting in the development of dilated cardiomyopathy10. However, the available evidence suggests that partial and selective inhibition of mTORC1 is cardioprotective during aging and cardiac stress. mTORC1 inhibition extends the life span of mice13-15. It also reduces cardiac hypertrophy and improves cardiac function in the presence of pressure overload16-19 and genetic cardiomyopathies20-22 and reduces ischemic injury after acute23, 24 and chronic myocardial infarction19, 25, 26. Finally, inhibition of mTORC1 reactivates cardiac autophagy, which is impaired in the presence of obesity and metabolic syndrome23. These results are very likely dependent on the fact that a partial inhibition of mTORC1 eliminates the detrimental effects of the maladaptive functions of mTORC1 during cardiac stress, while maintaining its physiological functions. In this regard, it is known that rapalogs do not inhibit all the functions of mTORC127. The degree of mTORC1 inhibition and the mTORC1 physiological functions needed to be preserved to convert the mTORC1 inhibition from detrimental into beneficial during cardiac stress are unclear. In contrast, the information available about the pathophysiological functions of mTORC2 in the heart is still scarce.
This article reviews and interprets the evidence currently available regarding the role of the mTOR signaling pathway in cardiovascular physiology and disease (Table 1).
Table 1.
Main studies that investigated the role of m TOR kinase in the regulation of cardiac physiology and response to stress in vivo.
| CARDIAC CONDITION | STUDY | TYPE OF MTOR MODULATION AND ANIMAL MODEL | EFFECT ON THE HEART |
|---|---|---|---|
| DEVELOPMENT/ PHYSIOLOGICAL CONDITIONS | Zhu Y et al (9) | Constitutive α-MHC-CRE-mediated mTOR gene deletion | High embryonic lethality due to cardiac failure. Massive cardiac dilation, dysfunction, heart failure and early mortality in the postnatal stage. Metabolic derangements. |
| Zhang D et al (10) | Tamoxifen-induced α-MHC-CRE-mediated mTOR deletion during adulthood | Massive cardiac dilation, apoptosis, autophagy, mitochondrial abnormalities, sarcomere disarray, cardiac dysfunction, heart failure and early mortality. | |
| Shende P et al (11) | Tamoxifen-induced α-MHC-CRE-mediated mTOR deletion during adulthood | Massive cardiac dilation, apoptosis, autophagy, mitochondrial abnormalities, sarcomere disarray, metabolic abnormalities, cardiac dysfunction, heart failure and early mortality. | |
| Tamai T et al (12) | Constitutive α-MHC-CRE-mediated rheb1 gene deletion | Reduction of mTORCl activity 5 days after birth. Defect in physiological cardiac growth. Cardiac dilation, dysfunction and heart failure. Mortality within 10 days after birth. | |
| AGING | Flynn JM et al (14) | Pharmacological mTOR inhibition using rapamycin | Reduced age-induced cardiac inflammation and fibrosis. Upregulation of energy metabolism. |
| Zhou et al (77) | Systemic GSK3-alpha deletion | Cardiac hypertrophy, dysfunction and sarcomere abnormalities during aging through mTOR activation and autophagy inhibition. | |
| CARDIAC HYPERTROPHY | Zhang D et al (10) | Tamoxifen-induced α-MHC-CRE-mediated mTOR deletion during adulthood and pressure overload | Inhibition of compensatory hypertrophy. Inhibition of protein synthesis. Rapid cardiac dilation, apoptosis, cardiac dysfunction and heart failure. |
| Shende P et al (11) | Tamoxifen-induced α-MHC-CRE-mediated mTOR deletion during adulthood and pressure overload | Inhibition of compensatory hypertrophy. Inhibition of protein synthesis. Rapid cardiac dilation, cardiac dysfunction and heart failure. | |
| Shioi T et al (16) | mTORC1 inhibition using rapamycin and then pressure overload | Inhibition of pathological cardiac hypertrophy and preservation of cardiac function. | |
| McMullen JR et al (17) | mTORC1 inhibition using rapamycin in mice with established TAC-induced cardiac hypertrophy | Regression of compensated and decompensated hypertrophy. Improved cardiac function in the latter. | |
| Volkers M et al (18) | AAV9-mediated PRAS40 overexpression in hearts subjected to pressure overload | Prevention of TAC-induced pathological hypertrophy and myocardial fibrosis. Maintenance of cardiac function. | |
| Wu X wt al (19) | Constitutive α-MHC-CRE-mediated heterozygous rheb1 gene deletion and then pressure overload | Reduction of TAC-induced hypertrophy and fibrosis. | |
| Marin TM et al (20) | Mouse model of LEOPARD disease treated with rapamycin | Regression of hypertrophic cardiomyopathy and cardiac disarray. | |
| Choi et al. (21); Ramos FJ et al (22) | Mouse model of cardiomyopathy caused by Lamin A/C mutation treated with pharmacological mTORC1 inhibition | Autophagy reactivation and improved cardiac function, muscle dystrophy and increased survival. | |
| Song X et al (96) | α-MHC-CRE-mediated mTOR overexpression | No increase in cardiac mass. Preservation of cardiac function and reduced myocardial inflammation through inhibition of NF-κB signaling. | |
| CARDIAC ISCHEMIA and REPERFUSION | Sciarretta S et al (23) | Pharmacological inhibition of mTORC1 during prolonged ischemia. Prolonged ischemia in mice with a-MHC-CRE-mediated Rheb overexpression | Inhibition of ischemia-induced mTORC1 downregulation and autophagy activation. Increased infarct size after ischemia that is reversed by rapamycin treatment. |
| Zhai P et al (24) | Mice with α-MHE-CRE-mediated dominant negative-GSK-3P overexpression and systemic heterozygous GSK-3ϐ deletion subjected to prolonged ischemia and I/R | GSK-3β is activated during prolonged ischemia but inhibited during reperfusion. Genetic inhibition of GSK-3β increases ischemic injury after prolonged ischemia but reduces reperfusion injury through a deregulated activation of mTORC1. | |
| Buss SJ et al (25) | mTORC1 inhibition with everolimus during chronic myocardial infarction | Reduction of infarct size, pathological growth and cardiac dilation. Improvement of cardiac function. Activation of autophagy. | |
| Volkers M et al (26) | AAV9-mediated PRAS40 cardiac overexpression and Rictor knockdown in mice subjected to chronic myocardial infarction | Overexpression of PRAS40 inhibits mTORC1, reduces ischemic injury, apoptosis and cardiac remodeling and improves cardiac function in an mT0RC2-dependent manner. Rictor knockdown causes deterioration of cardiac function and remodeling after myocardial infarction. | |
| Di R et al (109) | Rapamycin and S6K inhibitors during chronic myocardial infarction | Reduction of cardiac ischemic remodeling and cardiomyocte apoptosis. | |
| Aoyagi T et al (117) | α-MHC-CRE-mediated mTOR overexpression and I/R | Inhibition of cardiac remodeling after I/R in vivo, and reduction of necrosis and myocardial inflammation after I/R ex vivo. | |
| METABOLIC DISORDERS | Wang CY et al (122) | Rapamycin treatment during high fat diet-induced obesity | Akt and mTOR are activated in the vasculature of obese animals. Rapamycin inhibits endothelial senescence and the increased susceptibility to peripheral ischemia in obese animals. |
| Sciarretta et al (23) | Rapamycin treatment and partial inducible mTOR genetic disruption in mice with high fat diet-induced obesity and metabolic syndrome subjected to prolonged ischemia | Ischemia-induced autophagy activation is inhibited in the hearts of obese animals through deregulated activation of Rheb and mTORC1. Infarct size after ischemia is larger in obese animals. Pharmacological and partial genetic inhibition of mTOR rescue autophagy and reduce ischemic injury in obese mice. | |
| Li ZL et al (123) | Swine model of metabolic syndrome | Increased cardiac mTOR level and reduction of cardiac autophagy that is proportional to the degree of the derangements of cardiac structure and function. | |
| Guo R et al (124) | Rapamycin in mice with high fat diet-induced obesity | Increased mTOR activity and reduced autophagosome formation in the hearts of obese mice, associated with reduction of cardiac function. Cardiac function is rescued by rapamycin and deteriorated by genetic adiponectin disruption. | |
| Xu X et al (125) | Mice with systemic Akt2 knockout subjected to high fat diet | mTOR activation and disruption of autophagic flux in the hearts of obese mice. Akt2 deletion rescues autophagic flux and cardiac dysfunction in obese animals. | |
| Xu X et al (126) | Streptozotocin-induced diabetes | Cardiac autophagosome formation and flux are impaired in diabetic mice. These effects are associated with increased mTOR activity. |
Biology of the mTOR pathway
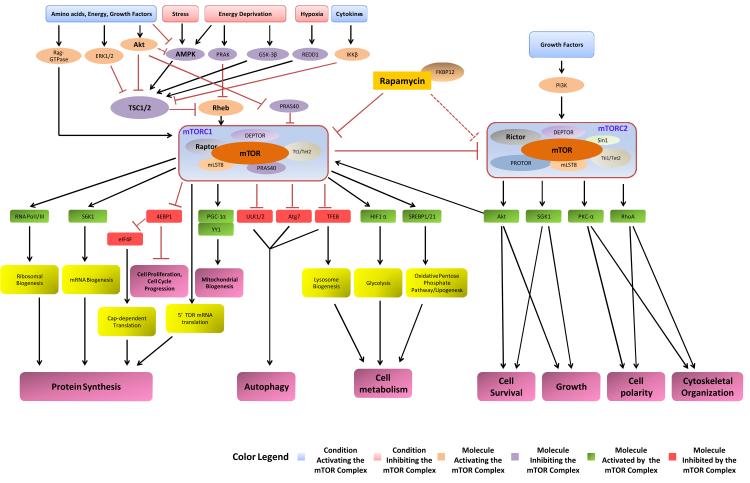
The mTOR kinase is an atypical serine/threonine kinase of 289 kDa that belongs to the family of the phosphoinositide 3-kinase related kinase. The mTOR kinase is encoded by a single gene in mammals, but it exerts its main cellular functions by forming mTORC1 and mTORC2 through assembly with specific adaptor proteins4-8. The mTORC1 and mTORC2 signaling pathways are evolutionarily conserved, and mTORC1 and mTORC2 represent the functional homologs of yeast TORC1 and TORC2. However, TOR1 and TOR2 are encoded by distinct genes in yeast, as first identified in Michael Hall’s laboratory in 199128. mTOR was isolated and cloned as a physical target of rapamycin through a cellular screening aimed at identifying the binding proteins of the FKB12-rapamycin complex1-3. Rapamycin and its analogs bind to the cytosolic protein FKBP12, thereby forming a protein complex that only targets a specific domain of the mTOR protein when it is part of mTORC1. As a consequence of rapamycin-FKBP12 binding, mTORC1 activity is strongly inhibited4, 6. Conversely, mTORC2 is relatively insensitive to rapamycin, although it has been demonstrated that prolonged treatment with rapamycin can also reduce the activity of mTORC2 by disrupting the complex29. So far, the proteins that are known to bind to mTOR in mTORC1 are Raptor30, mLST831, PRAS4032, DEPTOR33 and Tt1/Tel234. On the other hand, mTORC2 contains mTOR, the scaffold protein Rictor35, SIN136, PROTOR37, mLST831, DEPTOR33 and Tt1/Tel234. The main functions of the proteins forming the mTOR complexes are to maintain the integrity of the complexes, regulate their complex subcellular localization and control their complex interactions with substrates and regulators4-8 (Figure 2).
Figure 2. General overview of the mTOR signaling pathway.
The Dashed line signifies that rapamycin inhibits mTORC2 in specific cell types or after prolonged treatment.
The evidence available regarding the biological functions of mTORC1 and mTORC2 suggests that they have distinct cellular functions, substrates and regulators4-8. However, based on the current evidence, it is difficult to completely distinguish the function of one complex from the other. It has been shown that the two complexes are functionally interconnected and most of the studies that investigated the role of mTORC1 in the regulation of cellular homeostasis did not precisely rule out the involvement of mTORC2 in these mechanisms. In general, much more is known about the biology of mTORC1 than of mTORC2 (Figure 2). mTORC1 plays a crucial role in the regulation of cellular homeostasis, growth and response to stress. The main function of mTORC1 is the promotion of protein synthesis and, subsequently, cellular growth. The most studied substrates of mTORC1 are S6 kinase 1 (S6K1) and 4E (eIF4E)-binding protein 1 (4E-BP1). mTORC1 phosphorylates and activates S6K1, which in turn promotes mRNA biogenesis and activates the protein translation process. In contrast, mTORC1 inhibits 4E-BP1 and allows the formation of the eIF4F complex that triggers cap-dependent translation4-8. Additionally, mTORC1 promotes protein synthesis through translation of the 5’TOP mRNAs and the promotion of ribosomal biogenesis4-8. This mechanism explains why mTORC1 activity is often found to be increased in cancer cells4-8. mTORC1 also promotes cell proliferation by promoting the de novo synthesis of cellular membrane lipids through the SREBP1/2-dependent expression of lipogenic genes7, 38. However, mTORC1 not only activates anabolic processes but also inhibits catabolic processes. mTORC1 strongly inhibits autophagy, an evolutionarily conserved intracellular bulk degradation process responsible for the cellular degradation of proteins and organelles6, 7, 39, 40. mTORC1 appears to regulate autophagy both at a post-translational and transcriptional level. It phosphorylates the autophagic protein ULK1/2, thereby inhibiting the macrocomplex ULK1/Atg13/FIP200 that promotes autophagosome formation41. Additionally, mTORC1 activation inhibits the expression of autophagic proteins, particularly Atg723, which is crucial for the initiation of the autophagic process39. It has been shown that mTOR significantly inhibits the p73 factor and the transcription factor EB (TFEB) that can induce autophagy through the upregulation of autophagic proteins such as Atg742. 43, 44. mTORC1 also plays an important role in the regulation of cellular metabolism.
mTORC1 promotes mitochondrial biogenesis and oxidative metabolism through the PGC-1α-mediated activation of the transcription factor Ying-Yang 1 (YY1)45. mTORC1 can activate glycolysis and the oxidative pentose phosphate pathway through the activation of hypoxia-inducible factor-1α and SREBP1/2, respectively46. Finally, mTORC1 activity is inhibited by acid deprivation, energy stress, hypoxia, ER stress and genotoxic stress4-8. Under these conditions, mTORC1 inhibition allows the upregulation of stress-responsive mechanisms that limit cell death, such as reduction of protein synthesis, autophagy, cell cycle arrest and DNA repair.
The Akt and AMPK pathways represent the most well characterized regulators of mTORC1. In the presence of nutrients and growth factors such as insulin, IGF-1, PDGF and EGF, Akt is activated and, in turn, phosphorylates and activates mTOR or inhibits PRAS40, an endogenous mTORC1 inhibitor 4-8, 32, 47. On the other hand, AMPK, which is inactive in the presence of nutrients and high ATP levels, is strongly activated during energy deprivation and other types of cellular stress. AMPK activates the TSC1/TSC2 complex48, which displays a strong GTPase activity and inhibits the small GTP-binding protein, Rheb, a direct mTORC1 activator49, 50. GSK-3β can also activate the TSC1/TSC2 complex and inhibit mTORC1 during energy stress48. Alternatively, REDD1, which is upregulated during hypoxia, activates TSC2 independently of AMPK and GSK-3β51. Rheb can also be inhibited by PRAK during energy deprivation, independently of TSC252. Conversely, the Akt, ERK1/2 and IKKβ pathways inhibit the TSC1/TSC2 complex in response to growth factors and cytokines53-55. Recently, a new mechanism promoting the activation of mTORC1 has been elucidated. In the presence of amino acids, Rag GTPases are activated and mediate mTORC1 translocation to lysosome membranes, where mTORC1 is activated by Rheb56, 57. This process appears to be negatively regulated by MARK4, independently of Rheb activity58.
mTORC2 can be activated by insulin and growth factors, whereas it is relatively insensitive to nutrient deprivation5, 6. The PI3K pathway was shown to activate mTORC259, and it appears that the TSC1/2 complex does as well60. mTORC1 appears to inhibit mTORC2 through phosphorylation of Rictor61, suggesting that mTORC1 and mTORC2 are functionally interconnected. This hypothesis is also strongly supported by the fact that the best known substrate of mTORC2 is Akt, which is phosphorylated at serine 473, particularly in response to insulin62, 63. The biological importance of mTORC2-dependent phosphorylation of Akt is not yet understood, since it has been shown that mTORC2 is dispensable for Akt phosphorylation in certain cell types and conditions64. Both PKC-α65 and SGK166 have also been shown to be substrates of mTORC2, despite mTORC1 also being shown to regulate SGK167. Whether SGK1 exerts different functions in cardiomyocytes when it is activated by mTORC1 or mTORC2 is unclear. The most studied function of mTORC2 is the regulation of survival and growth, likely through the regulation of Akt and SGK1. SGK1 has been shown to promote cardiomyocyte survival while inhibiting hypertrophy68, whereas SGK1 chronic activation during heart failure is detrimental69. mTORC2 also regulates cell polarity and cytoskeletal organization through the regulation of PKC-α and RhoA65. PKC-α has also been shown to negatively regulate cardiac contractility70.
The role of mTOR signaling in the regulation of cardiac homeostasis and physiological growth
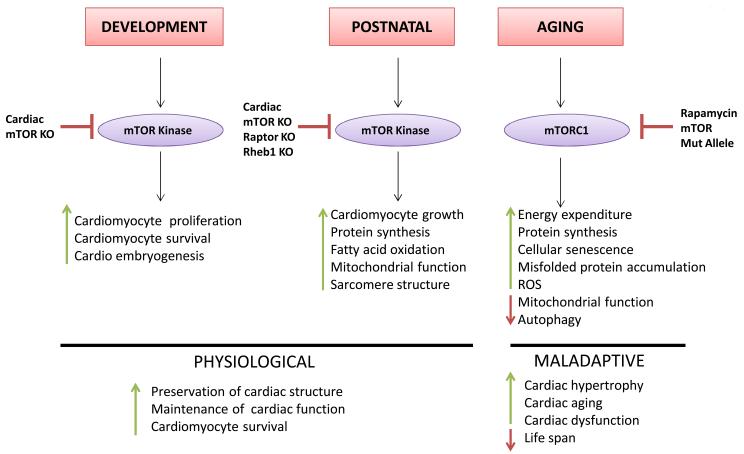
Given its myriad cellular functions, it is not surprising that the mTOR kinase is necessary for normal regulation of cardiomyocyte homeostasis and growth during both development and the postnatal period (Figure 3). Systemic mTOR and Raptor knockout embryos die early during development, right after implantation63. However, embryos with constitutive α-MHC-Cre-mediated mTOR deletion also display a dramatic mortality starting around E13.5, with only 8% of embryos surviving the developmental stage. Cardiac-specific mTOR knockout embryos present a significant reduction in cardiomyocyte proliferation and an increase in apoptosis, with a 34% reduction of the cardiomyocyte number. As a result, cardiac-specific mTOR knockout embryos present cardiac dilation and dysfunction, with signs of terminal cardiac failure9. Consistent with these results, systemic deletion of the rheb1 gene that extensively reduces mTORC1 activity is embryonically lethal, most likely due to cardiac defects such as ventricular wall thinning and cardiomyocyte apoptosis71. Global rictor gene deletion that selectively disrupts mTORC2 is also lethal in the developmental stage, and embryos with rictor deletion display significant cardiovascular abnormalities35. Thus, both mTORC1 and mTORC2 are highly important for cardiac development and embryo survival.
Figure 3. The role of mTOR in the regulation of cardiac homeostasis.
mTOR is required for cardiomyocyte growth and for the preservation of cardiac structure and function in unstressed conditions. However, partial inhibition of mTOR appears to be beneficial during the aging process. The pharmacological modulators of mTOR and the animal models with genetic modifications of the components of the mTOR signaling pathway that have been used in the studies focused on the role of mTOR in cardiac physiology are displayed.
The mTOR pathway also appears vital for the maintenance of cardiac structure and function in the postnatal period and adulthood. Mice with α-MHC-Cre-mediated cardiac mTOR disruption that do not die during gestation and are born alive die within a few weeks after birth from massive cardiac dilation, dysfunction and heart failure9. Constitutive mTOR knockout mice present with derangements in fatty acid metabolism in the heart. Inducible cardiac-specific mTOR deletion in adulthood also leads to cardiac dysfunction and heart failure, with chamber dilation and wall thinning. mTOR knockout mice display massive apoptosis, fibrosis, autophagy, mitochondrial abnormalities and dysfunction, sarcomere disarray and ultimately death within 8 weeks after tamoxifen-induced gene deletion. There is a reduction in S6K activity in the hearts of these mice, a surprising over-activation of Akt phosphorylation at serine 473, despite the inactivation of mTORC2, and a marked and progressive accumulation of 4E-BP1 protein. Concomitant ablation of the Eif4ebp1 gene partially rescues the detrimental effects of mTOR ablation in knockout mice10. These data suggest that a main detrimental effect of mTOR deletion in cardiomyocytes is the inhibition of cap-dependent protein translation. Mice with inducible cardiac-specific raptor deletion also progressively develop cardiac dilation and dysfunction associated with apoptosis, autophagy and mitochondrial abnormalities. Raptor knockout mice die a few weeks after cardiac-specific tamoxifen-induced gene deletion. A switch from fatty acid to glucose oxidation is observed in Raptor knockout mice11. Mice with constitutive α-MHC-Cre-mediated cardiac rheb1 deletion display a dramatic inhibition of the cardiac mTORC1 pathway 5 days after birth, but mTORC1 activity is maintained until at least 3 days after birth12. This suggests that Rheb regulates mTORC1 in the heart only in the postnatal period. Rheb knockout mice also rapidly develop cardiac dilation and dysfunction and die within 10 days after birth. This dramatic phenotype is accompanied by a defect in cardiomyocyte growth and sarcomere disarray. Rheb knockout mice do not show increased cardiomyocyte apoptosis and do not die during gestation, differing somewhat from the constitutive cardiac-specific mTOR knockout phenotype. Again, genetic deletion of the Eif4ebp1 gene partially rescues the phenotype of Rheb knockout mice12. Conversely, genetic disruption of atg5 does not rescue the cardiac phenotype of Rheb knockout mice, thus ruling out involvement of autophagy in these mechanisms. Of note, a significant increase in the LC3II/I ratio was observed in the hearts of Rheb knockout mice, which suggests an increase in autophagy. However, the absolute LC3II level was not found to be increased in these mice, in which Rheb deletion is constitutive (chronic). The apparent discrepancy between these mice and those with inducible (acute) mTOR or raptor gene deletion, in which the cardiac LC3II level is significantly increased, may be explained by the fact that LC3 is rapidly degraded when autophagic flux is chronically activated, making it more difficult to observe any significant LC3II accumulation72. Collectively, these data indicate that mTORC1 is required for maintenance of cardiac structure and function and regulation of cellular metabolism in the postnatal period. No evidence is available thus far regarding the specific role of mTORC2 in the heart in unstressed conditions.
However, while complete deletion of the mTOR pathway in the heart is not compatible with life, both pharmacological and partial genetic disruption of mTORC1 exert beneficial effects during the aging process and appear to increase cardiomyocyte resistance to aging stress. mTORC1 inhibition extends life span in lower organisms4-8, and pharmacological and partial genetic inhibition of mTOR extend life span in mammals13-15. Rapamycin treatment significantly extends life span in mice, regardless of whether it is started early or late in life13. Mice with hypomorphic mTOR alleles also live longer, with a significant reduction in the age-dependent functional decline of some organs15. S6K1 genetic disruption increases life span in female, though not in male, mice73. These beneficial effects may be due to a reduction in energy expenditure over time, inhibition of protein synthesis with reduced cellular senescence and misfolded protein accumulation, renewal of the endogenous stem cell pool, improvement of mitochondrial function and reduction of ROS and activation of autophagy5, 8. mTOR inhibition might also be beneficial during aging, particularly in the presence of obesity, through an increase in skeletal muscle insulin sensitivity due to interruption of the negative feedback on IRS-1 by mTORC174, 75. However, it has been demonstrated that chronic rapamycin treatment causes a diabetes-like syndrome due to a loss of pancreatic β-cells75, 76. Pharmacological mTOR inhibition has been shown to reduce age-related cardiac abnormalities, such as cardiac hypertrophy and systolic dysfunction. Rapamycin treatment reduced age-induced cardiac inflammation and fibrosis and upregulated genes involved in metabolic function and energy metabolism14, in line with the rapamycin-induced increase in mitochondrial function4-8. Accordingly, caloric restriction, which also increases life span in lower organisms and mammals, was shown to improve diastolic function and reduce cellular senescence in the aged heart, and these effects were associated with a reduction in mTORC1/S6K pathway signaling5, 8, 77.
Chronic mTOR activation appears to accelerate the cardiac aging process. Obesity and metabolic syndrome, which are associated with chronic cardiac activation of mTOR, accelerate cardiac aging23, 78, 79. Mice with systemic GSK-3α deletion present with cardiac hypertrophy, dysfunction and sarcomere abnormalities during aging due to deregulated activation of mTORC1 and inhibition of autophagy80. This indicates that GSK-3α is an important negative regulator of mTORC1 function during aging. Chronic Akt1 activation, which activates mTORC1, was shown to worsen aging-induced cardiac hypertrophy and myocardial contractile dysfunction through inhibition of autophagy81. This further suggests a potential role of autophagy in the beneficial effects of mTORC1 inhibition during aging. A recent study confirmed that rapamycin extends life span but failed to demonstrate that it prevents senescence in the cardiovascular system82. Therefore, additional studies are needed to elucidate the actual impact of mTORC1 inhibition on age-related cardiac abnormalities.
The role of mTOR signaling in the regulation of cardiac hypertrophy
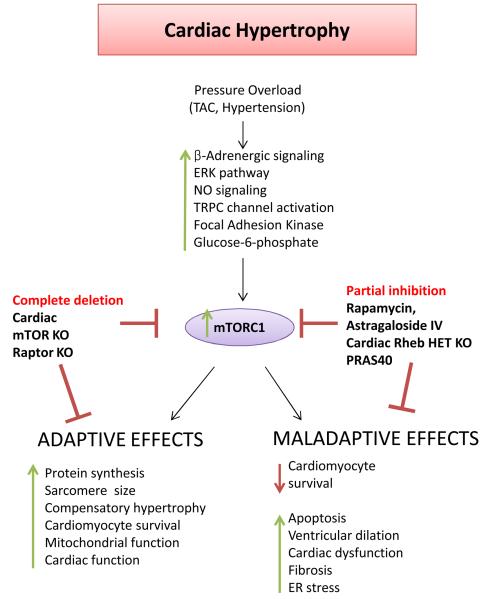
The mTOR pathway appears to play a key role in the development of cardiac hypertrophy (Figure 4). This is not particularly surprising if we consider that cardiac hypertrophy is a process that requires a marked increase in the synthesis of sarcomeric proteins and that the mTOR pathway is a master promoter of protein synthesis. mTORC1 activity is increased during the cardiomyocyte hypertrophic response to β-adrenergic stimulation83, angiotensin-II84 and IGF-185, and inhibition of mTORC1 inhibits hypertrophy development. mTORC1 is activated during physiological hypertrophy induced by physical exercise and during pathological hypertrophy induced by transverse aortic constriction (TAC) and spontaneous hypertension. However, there is evidence that mTORC1 is later inactivated when cardiac function deteriorates and heart failure develops10, 11, 86. The PI3K/Akt pathway contributes significantly to the activation of mTORC1 during the development of cardiac hypertrophy, particularly in response to physical exercise18, 87, 88. However, β-adrenergic signaling, the ERK pathway and nitric oxide signaling are also involved in the activation of mTORC1 during the development of cardiac hypertrophy83, 89, 90. Furthermore, biomechanical activation of TRPC channels and focal adhesion kinase promote mTORC1 activation during pressure overload91, 92, and glucose-6-phosphate accumulation contributes to mTORC1 activation in the overloaded heart as well93. Thus, a complex network of mechanical, biochemical and metabolic signals are sensed by mTORC1 signaling during cardiac pressure overload.
Figure 4. The role of mTOR in cardiac hypertrophy.
mTOR activation promotes pathological hypertrophy during pressure overload. However, mTOR kinase is also required for physiological mechanisms that are necessary for cardiac adaptation to cardiac overload. The pharmacological modulators of mTOR and the animal models with genetic modifications of the components of the mTOR signaling pathway that have been used in the studies focused on the role of mTOR in cardiac hypertrophy are displayed.
mTOR inhibition significantly reduces cardiac hypertrophy. Mice with inducible cardiac-specific mTOR or raptor deletion do not develop compensatory hypertrophy in response to pressure overload and rapidly develop ventricular dilation and cardiac dysfunction associated with apoptosis, autophagy and mitochondrial derangements10, 11. Protein synthesis in these animals is significantly reduced. These observations indicate that mTOR is necessary for the development of compensatory cardiomyocyte growth and for cardiac adaptation to pressure overload. Total disruption of mTOR signaling not only abrogates hypertrophy but also impairs the capacity of the heart to adapt to stress.
In contrast, partial genetic and pharmacological inhibition of mTORC1 appears to inhibit pathological cardiac hypertrophy while still maintaining the ability of the heart to adapt to increased load (Figure 4). Rapamycin pretreatment blunts cardiac hypertrophy development in response to pressure overload16. Rapamycin administration also regresses both established compensated and decompensated cardiac hypertrophy induced by TAC and improves cardiac function in mice with decompensated hypertrophy17. Rapamycin activates Akt, promotes protein ubiquitination and inhibits apoptosis in the pressure-overloaded rat myocardium94, and it reduces cardiac hypertrophy and fibrosis in spontaneously hypertensive rats95. Mice with heterozygous cardiac-specific rheb1 deletion show reduced activation of mTORC1 during pressure overload, and reduced cardiac hypertrophy and fibrosis19. The mTORC1 inhibitor astragaloside IV also reduces hypertrophy and fibrosis during pressure overload19. An interesting study recently showed that mTORC1 is activated during pressure overload through Akt-dependent inactivation of PRAS40. Cardiac overexpression of PRAS40 inhibited mTORC1 signaling, prevented cardiac hypertrophy development during TAC, and even reduced established TAC-induced hypertrophy. Importantly, PRAS40 overexpression preserved cardiac function during long-term pressure overload and reduced fibrosis18. A constitutively active form of PRAS40 was also able to reduce the development of physiological hypertrophy in response to physical exercise18. This is consistent with the evidence that PI3K inhibition and Akt1 disruption blunt the establishment of physiological hypertrophy in response to exercise87, 96. However, whether this effect is dependent on mTORC1 activation still needs to be addressed. In fact, it has been shown that cardiomyocyte-restricted overexpression of a dominant-negative form of mTOR that efficiently inhibits mTORC1 signaling does not affect the development of either exercise-induced or isoproterenol-induced hypertrophy97. Additional studies of the mTORC1 components using loss-of-function animal models would be useful to further investigate this issue. Furthermore, it will be important to elucidate the downstream signals that mediate the pro-hypertrophic effects of mTORC1 in the future. Of note, existing evidence indicates that S6Ks are not involved in the regulation of either physiological or pathological hypertrophy98, whereas it is likely that 4E-BPs play a role10.
While a considerable amount of evidence indicates that mTORC1 activation is absolutely required for the development of pathological cardiac hypertrophy, it seems that mTORC1 activation alone is not sufficient to induce cardiac hypertrophy. In fact, neither constitutively active nor wild-type mTOR overexpression in the mouse heart induces a significant increase in cardiac mass97, 99. These data must be reconciled with the evidence that Akt overexpression100, cardiomyocyte-restricted LKB1 deletion101 and cardiac TSC1 deletion102 are all associated with extensive development of cardiac hypertrophy that can be reversed by rapamycin treatment. The most reasonable explanation for the lack of induction of hypertrophy by mTOR overexpression is the existence of multiple signaling pathways that are modulated and contribute in an integrated manner to the synthesis of sarcomeric myofibrils and increase the cardiomyocyte volume during the establishment of pathological hypertrophy. Each of these pathways is strictly required for inducing hypertrophy, but if they are activated individually, particularly in the absence of a mechanical load, they cannot induce hypertrophy. Of note, Song et al. showed that cardiac overexpression of wild-type mTOR preserves cardiac function during pressure overload through inhibition of NF-κB signaling and myocardial inflammation, in apparent disagreement with the beneficial effects of mTORC1 inhibition discussed above. mTOR overexpression did not significantly increase the mTORC1 signaling during TAC in this study; instead, it actually decreased phosphorylation of S6K99. Therefore, it is possible that, in the specific transgenic mouse model described in this paper, overexpressed mTOR preferentially modulates signaling pathways that are cardioprotective without activating maladaptive signals. Activation of mTORC2 by mTOR overexpression may also contribute to the preservation of cardiac function during TAC. Unfortunately, the information available on the specific role of mTORC2 in the regulation of cardiomyocyte growth and cardiac adaptation to pressure overload is scarce and no studies on cardiac-specific mTORC2 loss-of-function animal models have been published.
Therefore, mTORC1 appears to be a potentially highly therapeutic target for treating human diseases associated with pathological cardiac hypertrophy and cardiomyopathy. In mouse models of LEOPARD disease, which is caused by a mutation of the PTPN11 gene that abolishes the catalytic activity of the SHP2 protein, cardiac ERK/MAPK signaling is inactivated and mTOR activity is increased in a deregulated manner. LEOPARD disease is characterized by the presence of hypertrophic cardiomyopathy, myocardial disarray, fibrosis, conduction defects and cardiac dysfunction. mTORC1 inhibition by rapamycin completely rescues the cardiac phenotype of these animals20. In a model of hypertrophic cardiomyopathy caused by a mutation of the TRIM63 gene encoding for the MuRF1 protein, cardiac mTOR was also found to be activated103. Cardiac mTOR activation and autophagy inhibition were observed even in mouse models of cardiomyopathy caused by Lamin A/C gene mutation, through ERK1/2-dependent activation of DUSP4. Pharmacological mTORC1 inhibition reactivated autophagy and significantly improved cardiac function, muscle dystrophy and survival of these animals21, 22. These effects were associated with a reduction of abnormal desmin accumulation21. Overall, these results indicate that deregulated cardiac mTORC1 activation is the pathophysiological mediator of cardiac hypertrophy and dysfunction in different types of cardiomyopathy. The exception to this general observation is represented by doxorubicin-induced cardiomyopathy, in which mTOR signaling inhibition seems to contribute to a reduction of cardiac mass and development of cardiac dysfunction independently of cardiomyocyte apoptosis104. Thus, it appears that, in this condition, the combination of doxorubicin toxicity and mTOR inhibition affects cardiomyocytes detrimentally by promoting cardiac atrophy. Whether an exaggerated activation of autophagy plays a role in the detrimental effects induced by doxorubicin is unclear.
The role of mTOR signaling in ischemic injury
Accumulating lines of evidence indicate that mTOR regulates the cardiomyocyte response to energy deprivation and ischemia (Figure 5). In lower organisms and mammalian cell lines, mTORC1 is inhibited during energy deprivation4-8. mTORC1 inhibition preserves the energy status through the reduction of cellular energy expenditure and activation of autophagy and, thus, promotes survival. Through its activation of autophagy, mTORC1 inhibition is required for postnatal survival before lactation begins105 and preserves skeletal muscle integrity and function106. Similarly, rapamycin promotes survival of nutrient-deprived cardiomyocytes through autophagy activation107. We recently demonstrated that mTORC1 is inhibited during cardiomyocyte energy deprivation and ischemia through the inhibition of Rheb23. Forced reactivation of Rheb/mTORC1 signaling inhibited autophagy activation in energy-deprived cardiomyocytes through Atg7 inhibition and promoted cardiomyocyte death both in vitro and in vivo. This effect was associated with depletion of ATP levels and misfolded protein accumulation. On the other hand, inhibition of the Rheb/mTORC1 signaling pathway limited cardiomyocyte death during energy stress. These results indicate that Rheb is a main regulator of mTORC1 during cardiomyocyte energy stress and that Rheb/mTORC1 inhibition is an adaptive cellular response that promotes survival through activation of autophagy. In fact, when autophagy was restored in Rheb-overexpressing cardiomyocytes, cell survival was rescued during energy stress23. Autophagy is also regulated during energy deprivation through mTORC1-independent mechanisms, such as AMPK-dependent Ulk1 phosphorylation41, phosphorylation/activation of TIP60 by GSK-3β, which in turn activates Ulk1108, and production of ROS by Nox4 in the endoplasmic reticulum109. However, Rheb/mTORC1 inhibition is a required signaling event to promote autophagy activation in energy-deprived cardiomyocytes in tight coordination with the other pathways regulating the autophagic machinery. Of note, we previously observed that, during prolonged myocardial ischemia without reperfusion, inhibition of GSK-3β activation was associated with autophagy inhibition and increased ischemic injury through mTORC1 reactivation, which was rescued by rapamycin treatment24. Inhibition of AMPK activation in the ischemic heart also led to decreased autophagy and increased ischemic injury110. Therefore, based on our findings, we propose that Rheb integrates the signals from AMPK and GSK-3β in ischemic cardiomyocytes, thereby mediating mTORC1 inhibition and autophagy activation.
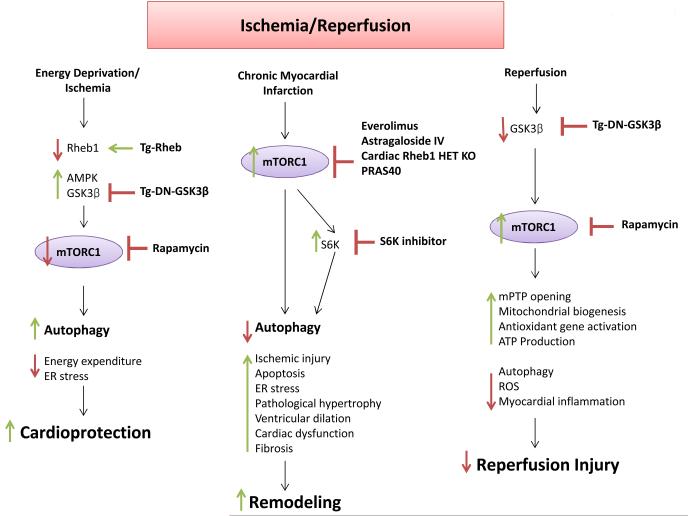
Figure 5. The role of mTORC1 in ischemia-reperfusion.
mTORC1 inhibition is protective during ischemia through the upregulation of adaptive mechanisms. On the other hand, mTOR is reactivated during reperfusion and takes part in the regulation of physiological processes. The pharmacological modulators of mTOR and the animal models with genetic modifications of the components of the mTOR signaling pathway that have been used in the studies focused on the role of mTOR in ischemia-reperfusion are displayed.
mTORC1 inhibition also appears to be beneficial during chronic ischemic injury (Figure 5). mTORC1 is activated in the remote myocardium during chronic myocardial infarction as a consequence of increased load and contributes to ventricular remodeling25, 26. Pharmacological mTORC1 inhibition with everolimus reduces cardiac dilation and infarct size and improves cardiac function during chronic myocardial infarction. These effects are associated with activation of autophagy and inhibition of proteosome activity25. Mice with partial cardiac Rheb deletion display better cardiac function after experimental myocardial infarction and a reduction of infarct size and cardiac dilation as compared to control mice, thus corroborating our evidence of a beneficial effect of Rheb inhibition during acute ischemia19. Rapamycin and S6K inhibitors reduce cardiac ischemic remodeling and cardiomyocyte apoptosis through PDK1-dependent activation of the Akt pathway111. Völkers et al. have recently provided compelling evidence that the balance between mTORC1 and mTORC2 activity is important for the regulation of ischemic damage and cardiac remodeling after myocardial infarction26. They demonstrated that cardiac overexpression of PRAS40 inhibits mTORC1, reduces ischemic injury, apoptosis and cardiac remodeling and improves cardiac function through the preservation of SERCA2a function during chronic myocardial infarction. The protective effects of PRAS40 were mediated by mTORC2 and by activation of the Akt pathway. Conversely, mTORC2 inhibition by in vivo AAV9-mediated cardiac Rictor knockdown caused deterioration of cardiac function and remodeling after myocardial infarction26. Therefore, while this study confirms the beneficial effects of mTORC1 inhibition during ischemic injury, it also demonstrates that mTORC2 promotes survival under ischemic conditions and highlights the importance of developing new selective mTORC1 inhibitors that do not affect or possibly even increase mTORC2 activity. PRAS40 or Rheb inhibitors could be potential candidates. Additional studies should also be conducted to elucidate the substrates mediating the protective effects of mTORC2 in the ischemic heart. Akt1 is certainly one of these. Previous studies demonstrated that, in some cases, genetic inhibition of PI3K or Akt1 can confer beneficial effects to the ischemic heart112, 113. Therefore, it is likely that mTORC2 also protects the ischemic heart through other Akt1-independent mechanisms. In this regard, in a recent elegant work, mTOR overexpression was found to partially reduce cardiomyocyte death during hypoxia in vitro through mTOR-dependent direct activation of NF-κB and inhibition of Bnip3 expression114. It will be interesting to evaluate the relative contribution of mTORC2 activation by mTOR overexpression with respect to mTORC1 in regulating these mechanisms. This intriguing hypothesis would also be consistent with the evidence that mTORC2 activates Akt1 and that Akt1 is a positive regulator of NF-κB114.
The role of mTOR signaling in reperfusion injury is still controversial (Figure 5). mTORC1 is rapidly activated in the heart during reperfusion. Rapamycin reduces infarct size in ex vivo and in vivo ischemia-reperfusion models through activation of the JAK2/STAT3 signaling pathway when administered before ischemia115. Simvastatin reduces ischemia-reperfusion injury through inhibition of mTOR and activation of mitophagy116. On the other hand, rapamycin was not cardioprotective during ischemia-reperfusion when administered before the reperfusion phase24. We previously observed that inhibition of GSK-3β in transgenic mice with cardiac-specific overexpression of dominant-negative GSK-3β reduces reperfusion injury through mTORC1 hyper-activation24. These results suggest that mTORC1 may exert some protective effects during the reperfusion phase. We found that mTORC1 activation by GSK-3β inhibition reduces reperfusion injury by limiting exaggerated activation of autophagy, which is maladaptive110. Alternatively, mTORC1 may regulate mPTP opening, it promotes mitochondrial biogenesis, which may favor cardiac recovery after ischemia, and it may promote upregulation of antioxidant genes through the activation of PGC-1α24, 45, 117. Previous reports indicated that rapamycin abolishes the cardioprotective effects of ischemic preconditioning, indicating that ROS-induced mTORC1 activation mediates the protection associated with ischemic preconditioning118. Consistent with the idea that mTORC1 exerts a protective effect during reperfusion damage, a recent study found that cardiac-specific mTOR overexpression reduces chronic cardiac remodeling after in vivo ischemia-reperfusion. Although the effects of mTOR overexpression on acute ischemic injury after ischemia-reperfusion were not evaluated in vivo, mTOR overexpression was found to reduce necrosis, as evaluated by Evans blue dye perfusion, and myocardial inflammation in an ex vivo model of ischemia-reperfusion119. It is also possible that, in this study, the protective effects mediated by mTOR overexpression are dependent upon mTORC2 activation, which is required for cardiomyocyte survival during ischemia and limitation of chronic ischemic remodeling26. Collectively, these data indicate that mTORC1 inhibition is protective during ischemia through the activation of autophagy, the reduction of protein synthesis and the subsequent activation of mTORC2. On the other hand, mTORC1 appears to potentiate physiological mechanisms during reperfusion. Ideally, mTORC1 should be inhibited before an ischemic episode and reactivated at the time of reperfusion in patients suffering an acute myocardial infarction. However, in the clinical setting, patients with acute myocardial infarction usually experience prolonged periods of ischemia (hours) before coronary perfusion can be reestablished. Furthermore, in certain cases, coronary flow is not restored or coronary reperfusion is not indicated. Ischemia is a major determinant of myocardial damage in patients with acute coronary syndrome120. Therefore, it is very likely that, in patients with acute myocardial ischemia, the beneficial effects of mTORC1 inhibition largely overcome the potential harmful effects during reperfusion. Additional studies are needed to better investigate this issue.
The role of mTOR in the regulation of cardiac metabolism
mTOR signaling appears to be deeply involved in the regulation of cardiac metabolism. In mice with cardiac mTOR disruption induced in adulthood, fatty acid oxidation was significantly decreased, whereas glucose oxidation was increased121. Expression of fatty acid metabolism genes such as fatty acid-binding protein 3, medium-chain acyl-CoA dehydrogenase and hydroxyacyl-CoA dehydrogenase/3-ketoacyl-CoA thiolase/enoyl-CoA hydratase (trifunctional protein)-α and -β was reduced, and carnitine palmitoyl transferase-1 and -2 enzymatic activities were also decreased. These metabolic abnormalities were not associated with a reduction in the abundance of PGC-1α, a master regulator of fatty acid oxidation genes121. In contrast, in cardiac-specific Raptor knockout mice, significant downregulation of ERRα, PGC-1α and PPAR-α was observed11. Again, fatty acid oxidation was reduced and glucose utilization was increased in the hearts of Raptor knockout mice. This was accompanied by a reduction in carnitine palmitoyl transferase-1β and malonyl-CoA decarboxylase-1 expression levels. Importantly, all these changes were observed in mTOR and Raptor knockout mice while cardiac function was still preserved. In Raptor knockout mice, a reduction of mitochondrial content was also seen after TAC, which is consistent with a reduction of PGC-1α, since PGC-1α promotes mitochondrial biogenesis and function11. Of note, recent evidence suggests that mTOR can also promote cardiomyocyte mitochondrial function in response to insulin through the regulation of NF-κB122.
However, mTOR not only regulates metabolism but is also affected by metabolic alterations. It is now well-established that tissue mTORC1 activity is increased in the presence of nutritional excess, obesity and metabolic syndrome (Figure 6)4-8, 75. Deregulated mTORC1 activation further deteriorates the cellular metabolic status, promotes cellular senescence and ultimately leads to organ dysfunction4-8, 75, 123. AMPK is usually inhibited under these conditions, suggesting that it is involved in a main intracellular mechanism leading to mTORC1 activation. It is possible that Rag GTPases also contribute to organ mTORC1 activation in obesity and metabolic syndrome. In the presence of metabolic alterations, increased energy status, high cardiac and circulating levels of lipids and amino acids, hyperinsulinemia and increased serum levels of cytokines and adipokines would all likely lead to activation of mTORC1 signaling4-8, 75, 123.
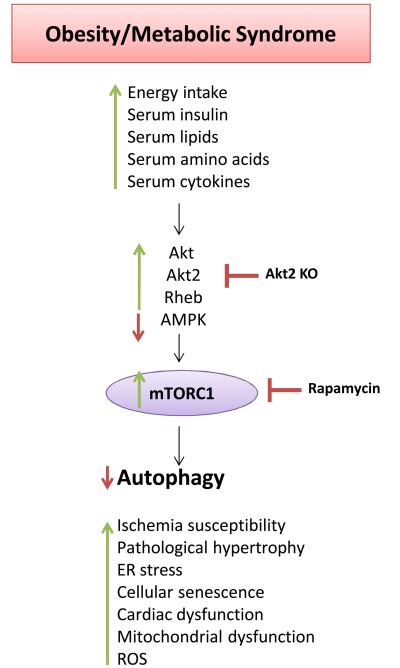
Figure 6. Cardiac mTORC1 activation in metabolic disorders.
Cardiac mTORC1 activation contributes to cardiac abnormalities in obesity, metabolic syndrome and diabetes.
In dietary and genetic models of obesity and metabolic syndrome, basal mTORC1 activity was found to be increased in the liver, where it promotes insulin resistance, contributes to dyslipidemia and may predispose to cancer development, in adipose tissue, where it promotes fat deposition, in the kidney, where it causes autophagy inhibition and podocyte loss, in skeletal muscle, where it promotes insulin resistance and fat deposition, and, ultimately, in the vasculature and in the heart4-8, 75, 123. High fat diet-induced obesity leads to an increased activation of the Akt/mTOR pathway in the vasculature that causes endothelial senescence and increases the susceptibility to peripheral ischemia. These effects are rescued by rapamycin124. We recently found that, in a model of dietary obesity and metabolic syndrome, autophagy is suppressed in the heart through deregulated activation of Rheb and mTORC1 activity. This suggests that Rheb/mTORC1 activation contributes to pathological cardiac growth in obesity and metabolic disorders125. We found that the activity of Rheb and mTORC1 remains higher during ischemia, which, in contrast, is associated with Rheb/mTORC1 inhibition in the hearts of lean animals23. Accordingly, autophagy was significantly inhibited in the hearts of obese mice, and this was associated with increased susceptibility to ischemic injury. Rapamycin administration or partial mTOR deletion significantly reduced infarct size following ischemia through the restoration of autophagy23. Therefore, our results provide a mechanistic explanation for the reduction in ischemic tolerance associated with metabolic abnormalities and suggest that mTORC1 inhibition is a valid therapeutic option to reduce ischemic injury in subjects with acute coronary syndromes, particularly those with metabolic syndrome. Subsequent studies have confirmed that either autophagosome formation or autophagic flux is impaired in the hearts of obese and diabetic animals, and these effects were found to be associated with increased mTORC1 activity and the development of cardiac abnormalities. In a swine model of metabolic syndrome, a reduction in autophagosome formation was associated with mTOR activation, increased apoptosis, reduced mitochondrial function and derangements of cardiac structure and function126. In a model of high fat diet-induced obesity, cardiac autophagosome formation was reduced, mTOR activity was increased and cardiac function was decreased. These effects were rescued by rapamycin and worsened by genetic adiponectin disruption127. Interestingly, obesity and metabolic syndrome not only affect autophagosome formation but also autophagic flux. Deregulated activation of cardiac Akt2 is involved in the activation of the mTOR pathway and in the disruption of autophagic flux in the hearts of obese mice. These abnormalities are rescued by Akt2 genetic disruption128. In a model of streptozotocin-induced diabetes, autophagosome formation and flux were impaired in the heart, and these effects were associated with increased mTOR activity129. However, whether autophagy inhibition in the diabetic heart is maladaptive or adaptive at baseline is still unclear129, 130. Obesity was also found to be associated with inhibition of autophagosome formation in the kidney through mTORC1 activation and in the liver in mice131, 132. This suggests that mTORC1 inhibition in subjects with obesity could be beneficial not only to the heart but also to other organs, specifically through autophagy reactivation79. Further studies are encouraged to investigate this issue.
Perspectives
Many aspects of the pathophysiology of mTOR signaling still remain unclear. First of all, it will be important to study the specific function of mTORC2 at baseline and during stress. This can be achieved through the characterization of cardiac-specific Rictor knockout mice. Much effort still needs to be made to discover the substrates of mTORC1 and mTORC2 that mediate their specific effects and the mechanisms that regulate them in response to growth factors, nutrients and stress. Not much is known about the cross-talk between mTORC1 and mTORC2, but it is very likely that the complexes tightly regulate one another in specific contexts and share some functions. In addition, the specific functions of the different adaptor proteins of mTORC1 and mTORC2 in different cardiomyocyte cellular processes need to be addressed. Finally, the subcellular localization of mTORC1 and mTORC2 in cardiomyocytes at baseline and during stress should be investigated.
mTORC1 activation is maladaptive during aging, cardiac hypertrophy development, myocardial ischemia and in the presence of obesity and metabolic syndrome. Therefore, it is important to find the optimal mTORC1 inhibitor that would be most beneficial under these conditions. Ideally, this inhibitor should selectively inhibit the maladaptive functions of mTORC1 without affecting its physiological effects. It is known, for example, that prolonged treatment with rapamycin disrupts mTORC2 and can cause insulin resistance4-8. To succeed in this difficult task, it will be important to study both the direct regulators of mTORC1 involved in its maladaptive functions, such as Rheb, PRAS40 or astrin133, and its direct substrates in these mechanisms. Additional studies of different components of mTOR signaling with heterozygous loss-of-function models are also encouraged. It will also be interesting to investigate whether there is a structural advantage to having the mTORC1 protein present, even if it is inhibited. Of note, previous studies indicated a beneficial effect of mTORC1 inhibition in preserving the stem cell pool, reducing stem cell exhaustion and increasing stem cell function4-8. Cardiac stem cells have been shown to be involved in the regulation of cardiomyocyte turnover, but the cardiac stem cell pool decreases during aging and disease134. It would be interesting to evaluate whether mTORC1 inhibition can preserve the cardiac stem cell pool during stress and, if so, whether this effect contributes to the protective effects of mTORC1 inhibition in cardiac diseases.
Finally, the information available about the physiological role of mTOR signaling in the vasculature is scarce. There is some evidence indicating that prolonged rapamycin treatment reduces endothelial cell viability and function and promotes monocyte recruitment, vascular inflammation and susceptibility to thrombosis135, 136. Conversely, S6K inhibition reduces tissue factor release and vascular inflammation135. mTORC2 was found to promote survival and proliferation of pulmonary artery vascular smooth muscle cell during pulmonary hypertension137. Studies of mTOR components via vascular-specific loss-of-function models are required to understand the involvement of mTOR signaling in vascular cellular processes.
Supplementary Material
Acknowledgements
We apologize if we have not cited all the relevant papers due to space limitations. The authors wish to thank Daniela Zablocki, Christopher D. Brady and Narayani Nagarajan for critical reading of the manuscript.
Sources of funding
This work was supported in part by U.S. Public Health Service Grants HL102738, HL67724, HL69020, HL91469, AG23039, and AG27211. This work was also supported by the Foundation Leducq Transatlantic Networks of Excellence. SS received support from a Postdoctoral Fellowship from the Founders Affiliate, American Heart Association and a grant from the Italian Society of Hypertension and from the Italian Society of Cardiology.
Nonstandard Abbreviations and Acronyms
- FKB12
FK506-binding protein of 12 kDa
- Raptor
regulatory-associated protein of mTOR
- mLST8
mammalian lethal with sec-13 protein 8
- PRAS40
proline-rich Akt substrate 40 kDa
- DEPTOR
DEP domain-containing mTOR-interacting protein
- Rictor
rapamycin-insensitive companion of mTOR
- SIN1
stress-activated map kinase-interacting protein 1
- PROTOR
protein observed with rictor
- SREBP1/2
sterol regulatory element-binding protein 1/2
- ULK1/2
unc-51-like kinase 1/2
- FIP200
focal adhesion kinase family interacting protein of 200 kDa
- Atg
autophagy-related gene
- YWHA
tyrosine 3-monooxygenase/tryptophan 5-monooxygenase activation proteins
- PPAR-α
peroxisome proliferator-activated receptor α
- PPAR-β
peroxisome proliferator-activated receptor β
- PGC-1α
PPAR-β coactivator 1α
- AMPK
adenosine monophosphate-activated protein kinase
- IGF-1
insulin growth factor 1
- PDGF
platelet-derived growth factor
- EGF
Epidermal growth factor
- TSC1/2
tuberous sclerosis protein 1/2
- Rheb
Ras homolog enriched in brain
- GSK-3
Glycogen synthase kinase 3
- REDD1
regulated in development and DNA damage response 1
- PRAK
p38-regulated/activated kinase
- ERK1/2
extracellular-signal-regulated kinase 1/2
- NF-κB
nuclear factor κB
- IKKβ
inhibitor of nuclear factor κB kinase β
- MARK4
microtubule-associated protein/microtubule affinity-regulating kinase 4
- PI3K
phosphoinositide 3 kinase
- SGK1
serum- and glucocorticoid-induced protein kinase 1
- PKC-α
protein kinase C-α
- RhoA
Ras homolog gene family, member A
- LKB1
liver kinase B1
- LEOPARD
Lentigines, ECG conduction abnormalities, Ocular hypertelorism, Pulmonic stenosis, Abnormal genitalia, Retardation of growth and sensorineural Deafness
- MAPK
mitogen-activated protein kinase
- MuRF1
Muscle RING-finger protein-1
- DUSP4
Dual specificity protein phosphatase 4
- PDK1
phosphoinositide-dependent kinase-1
- SERCA2a
sarco/endoplasmic reticulum Ca2+−ATPase 2a
- AAV9
adeno-associated virus 9
- Bnip3
BCL2/adenovirus E1B 19 kDa protein-interacting protein 3
- JAK2
Janus kinase 2
- STAT3
signal transducer and activator of transcription 3
- mPTP
mitochondrial permeability transition pore
- TRPC
transient receptor potential canonical
- SHP2
SH2 domain-containing protein tyrosine phosphatase-2
Footnotes
Disclosures
None.
References
- 1.Brown EJ, Albers MW, Shin TB, Ichikawa K, Keith CT, Lane WS, Schreiber SL. A mammalian protein targeted by g1-arresting rapamycin-receptor complex. Nature. 1994;369:756–758. doi: 10.1038/369756a0. [DOI] [PubMed] [Google Scholar]
- 2.Sabatini DM, Erdjument-Bromage H, Lui M, Tempst P, Snyder SH. Raft1: A mammalian protein that binds to fkbp12 in a rapamycin-dependent fashion and is homologous to yeast tors. Cell. 1994;78:35–43. doi: 10.1016/0092-8674(94)90570-3. [DOI] [PubMed] [Google Scholar]
- 3.Sabers CJ, Martin MM, Brunn GJ, Williams JM, Dumont FJ, Wiederrecht G, Abraham RT. Isolation of a protein target of the fkbp12-rapamycin complex in mammalian cells. J Biol Chem. 1995;270:815–822. doi: 10.1074/jbc.270.2.815. [DOI] [PubMed] [Google Scholar]
- 4.Wullschleger S, Loewith R, Hall MN. Tor signaling in growth and metabolism. Cell. 2006;124:471–484. doi: 10.1016/j.cell.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 5.Kapahi P, Chen D, Rogers AN, Katewa SD, Li PW, Thomas EL, Kockel L. With tor, less is more: A key role for the conserved nutrient-sensing tor pathway in aging. Cell Metab. 2010;11:453–465. doi: 10.1016/j.cmet.2010.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Laplante M, Sabatini DM. Mtor signaling in growth control and disease. Cell. 2012;149:274–293. doi: 10.1016/j.cell.2012.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Laplante M, Sabatini DM. Regulation of mtorc1 and its impact on gene expression at a glance. J Cell Sci. 2013;126:1713–1719. doi: 10.1242/jcs.125773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Johnson SC, Rabinovitch PS, Kaeberlein M. Mtor is a key modulator of ageing and age-related disease. Nature. 2013;493:338–345. doi: 10.1038/nature11861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhu Y, Pires KM, Whitehead KJ, Olsen CD, Wayment B, Zhang YC, Bugger H, Ilkun O, Litwin SE, Thomas G, Kozma SC, Abel ED. Mechanistic target of rapamycin (mtor) is essential for murine embryonic heart development and growth. PLoS One. 2013;8:e54221. doi: 10.1371/journal.pone.0054221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang D, Contu R, Latronico MV, Zhang JL, Rizzi R, Catalucci D, Miyamoto S, Huang K, Ceci M, Gu Y, Dalton ND, Peterson KL, Guan KL, Brown JH, Chen J, Sonenberg N, Condorelli G. Mtorc1 regulates cardiac function and myocyte survival through 4e-bp1 inhibition in mice. J Clin Invest. 2010;120:2805–2816. doi: 10.1172/JCI43008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shende P, Plaisance I, Morandi C, Pellieux C, Berthonneche C, Zorzato F, Krishnan J, Lerch R, Hall MN, Ruegg MA, Pedrazzini T, Brink M. Cardiac raptor ablation impairs adaptive hypertrophy, alters metabolic gene expression, and causes heart failure in mice. Circulation. 2011;123:1073–1082. doi: 10.1161/CIRCULATIONAHA.110.977066. [DOI] [PubMed] [Google Scholar]
- 12.Tamai T, Yamaguchi O, Hikoso S, Takeda T, Taneike M, Oka T, Oyabu J, Murakawa T, Nakayama H, Uno Y, Horie K, Nishida K, Sonenberg N, Shah AM, Takeda J, Komuro I, Otsu K. Rheb (ras homologue enriched in brain)-dependent mammalian target of rapamycin complex 1 (mtorc1) activation becomes indispensable for cardiac hypertrophic growth after early postnatal period. J Biol Chem. 2013;288:10176–10187. doi: 10.1074/jbc.M112.423640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Harrison DE, Strong R, Sharp ZD, Nelson JF, Astle CM, Flurkey K, Nadon NL, Wilkinson JE, Frenkel K, Carter CS, Pahor M, Javors MA, Fernandez E, Miller RA. Rapamycin fed late in life extends lifespan in genetically heterogeneous mice. Nature. 2009;460:392–395. doi: 10.1038/nature08221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Flynn JM, O'Leary MN, Zambataro CA, Academia EC, Presley MP, Garrett BJ, Zykovich A, Mooney SD, Strong R, Rosen CJ, Kapahi P, Nelson MD, Kennedy BK, Melov S. Late-life rapamycin treatment reverses age-related heart dysfunction. Aging Cell. 2013;12:851–862. doi: 10.1111/acel.12109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wu JJ, Liu J, Chen EB, Wang JJ, Cao L, Narayan N, Fergusson MM, Rovira II, Allen M, Springer DA, Lago CU, Zhang S, Dubois W, Ward T, Decabo R, Gavrilova O, Mock B, Finkel T. Increased mammalian lifespan and a segmental and tissue-specific slowing of aging after genetic reduction of mtor expression. Cell reports. 2013;4:913–920. doi: 10.1016/j.celrep.2013.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shioi T, McMullen JR, Tarnavski O, Converso K, Sherwood MC, Manning WJ, Izumo S. Rapamycin attenuates load-induced cardiac hypertrophy in mice. Circulation. 2003;107:1664–1670. doi: 10.1161/01.CIR.0000057979.36322.88. [DOI] [PubMed] [Google Scholar]
- 17.McMullen JR, Sherwood MC, Tarnavski O, Zhang L, Dorfman AL, Shioi T, Izumo S. Inhibition of mtor signaling with rapamycin regresses established cardiac hypertrophy induced by pressure overload. Circulation. 2004;109:3050–3055. doi: 10.1161/01.CIR.0000130641.08705.45. [DOI] [PubMed] [Google Scholar]
- 18.Volkers M, Toko H, Doroudgar S, Din S, Quijada P, Joyo AY, Ornelas L, Joyo E, Thuerauf DJ, Konstandin MH, Gude N, Glembotski CC, Sussman MA. Pathological hypertrophy amelioration by pras40-mediated inhibition of mtorc1. Proc Natl Acad Sci U S A. 2013;110:12661–12666. doi: 10.1073/pnas.1301455110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wu X, Cao Y, Nie J, Liu H, Lu S, Hu X, Zhu J, Zhao X, Chen J, Chen X, Yang Z, Li X. Genetic and pharmacological inhibition of rheb1-mtorc1 signaling exerts cardioprotection against adverse cardiac remodeling in mice. Am J Pathol. 2013;182:2005–2014. doi: 10.1016/j.ajpath.2013.02.012. [DOI] [PubMed] [Google Scholar]
- 20.Marin TM, Keith K, Davies B, Conner DA, Guha P, Kalaitzidis D, Wu X, Lauriol J, Wang B, Bauer M, Bronson R, Franchini KG, Neel BG, Kontaridis MI. Rapamycin reverses hypertrophic cardiomyopathy in a mouse model of leopard syndrome-associated ptpn11 mutation. J Clin Invest. 2011;121:1026–1043. doi: 10.1172/JCI44972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ramos FJ, Chen SC, Garelick MG, Dai DF, Liao CY, Schreiber KH, MacKay VL, An EH, Strong R, Ladiges WC, Rabinovitch PS, Kaeberlein M, Kennedy BK. Rapamycin reverses elevated mtorc1 signaling in lamin a/c-deficient mice, rescues cardiac and skeletal muscle function, and extends survival. Sci Transl Med. 2012;4:144ra103. doi: 10.1126/scitranslmed.3003802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Choi JC, Wu W, Muchir A, Iwata S, Homma S, Worman HJ. Dual specificity phosphatase 4 mediates cardiomyopathy caused by lamin a/c (lmna) gene mutation. J Biol Chem. 2012;287:40513–40524. doi: 10.1074/jbc.M112.404541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sciarretta S, Zhai P, Shao D, Maejima Y, Robbins J, Volpe M, Condorelli G, Sadoshima J. Rheb is a critical regulator of autophagy during myocardial ischemia: Pathophysiological implications in obesity and metabolic syndrome. Circulation. 2012;125:1134–1146. doi: 10.1161/CIRCULATIONAHA.111.078212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhai P, Sciarretta S, Galeotti J, Volpe M, Sadoshima J. Differential roles of gsk-3{beta} during myocardial ischemia and ischemia/reperfusion. Circ Res. 2011;109:502–511. doi: 10.1161/CIRCRESAHA.111.249532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Buss SJ, Muenz S, Riffel JH, Malekar P, Hagenmueller M, Weiss CS, Bea F, Bekeredjian R, Schinke-Braun M, Izumo S, Katus HA, Hardt SE. Beneficial effects of mammalian target of rapamycin inhibition on left ventricular remodeling after myocardial infarction. J Am Coll Cardiol. 2009;54:2435–2446. doi: 10.1016/j.jacc.2009.08.031. [DOI] [PubMed] [Google Scholar]
- 26.Volkers M, Konstandin MH, Doroudgar S, Toko H, Quijada P, Din S, Joyo A, Ornelas L, Samse K, Thuerauf DJ, Gude N, Glembotski CC, Sussman MA. Mtorc2 protects the heart from ischemic damage. Circulation. 2013 doi: 10.1161/CIRCULATIONAHA.113.003638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Thoreen CC, Sabatini DM. Rapamycin inhibits mtorc1, but not completely. Autophagy. 2009;5:725–726. doi: 10.4161/auto.5.5.8504. [DOI] [PubMed] [Google Scholar]
- 28.Heitman J, Movva NR, Hall MN. Targets for cell cycle arrest by the immunosuppressant rapamycin in yeast. Science. 1991;253:905–909. doi: 10.1126/science.1715094. [DOI] [PubMed] [Google Scholar]
- 29.Sarbassov DD, Ali SM, Sengupta S, Sheen JH, Hsu PP, Bagley AF, Markhard AL, Sabatini DM. Prolonged rapamycin treatment inhibits mtorc2 assembly and akt/pkb. Mol Cell. 2006;22:159–168. doi: 10.1016/j.molcel.2006.03.029. [DOI] [PubMed] [Google Scholar]
- 30.Kim DH, Sarbassov DD, Ali SM, King JE, Latek RR, Erdjument-Bromage H, Tempst P, Sabatini DM. Mtor interacts with raptor to form a nutrient-sensitive complex that signals to the cell growth machinery. Cell. 2002;110:163–175. doi: 10.1016/s0092-8674(02)00808-5. [DOI] [PubMed] [Google Scholar]
- 31.Kim DH, Sarbassov DD, Ali SM, Latek RR, Guntur KV, Erdjument-Bromage H, Tempst P, Sabatini DM. Gbetal, a positive regulator of the rapamycin-sensitive pathway required for the nutrient-sensitive interaction between raptor and mtor. Mol Cell. 2003;11:895–904. doi: 10.1016/s1097-2765(03)00114-x. [DOI] [PubMed] [Google Scholar]
- 32.Sancak Y, Thoreen CC, Peterson TR, Lindquist RA, Kang SA, Spooner E, Carr SA, Sabatini DM. Pras40 is an insulin-regulated inhibitor of the mtorc1 protein kinase. Mol Cell. 2007;25:903–915. doi: 10.1016/j.molcel.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 33.Peterson TR, Laplante M, Thoreen CC, Sancak Y, Kang SA, Kuehl WM, Gray NS, Sabatini DM. Deptor is an mtor inhibitor frequently overexpressed in multiple myeloma cells and required for their survival. Cell. 2009;137:873–886. doi: 10.1016/j.cell.2009.03.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kaizuka T, Hara T, Oshiro N, Kikkawa U, Yonezawa K, Takehana K, Iemura S, Natsume T, Mizushima N. Tti1 and tel2 are critical factors in mammalian target of rapamycin complex assembly. J Biol Chem. 2010;285:20109–20116. doi: 10.1074/jbc.M110.121699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sarbassov DD, Ali SM, Kim DH, Guertin DA, Latek RR, Erdjument-Bromage H, Tempst P, Sabatini DM. Rictor, a novel binding partner of mtor, defines a rapamycin-insensitive and raptor-independent pathway that regulates the cytoskeleton. Curr Biol. 2004;14:1296–1302. doi: 10.1016/j.cub.2004.06.054. [DOI] [PubMed] [Google Scholar]
- 36.Jacinto E, Facchinetti V, Liu D, Soto N, Wei S, Jung SY, Huang Q, Qin J, Su B. Sin1/mip1 maintains rictor-mtor complex integrity and regulates akt phosphorylation and substrate specificity. Cell. 2006;127:125–137. doi: 10.1016/j.cell.2006.08.033. [DOI] [PubMed] [Google Scholar]
- 37.Pearce LR, Huang X, Boudeau J, Pawlowski R, Wullschleger S, Deak M, Ibrahim AF, Gourlay R, Magnuson MA, Alessi DR. Identification of protor as a novel rictor-binding component of mtor complex-2. Biochem J. 2007;405:513–522. doi: 10.1042/BJ20070540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Laplante M, Sabatini DM. An emerging role of mtor in lipid biosynthesis. Curr Biol. 2009;19:R1046–1052. doi: 10.1016/j.cub.2009.09.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Levine B, Kroemer G. Autophagy in the pathogenesis of disease. Cell. 2008;132:27–42. doi: 10.1016/j.cell.2007.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sciarretta S, Zhai P, Volpe M, Sadoshima J. Pharmacological modulation of autophagy during cardiac stress. J Cardiovasc Pharmacol. 2012;60:235–241. doi: 10.1097/FJC.0b013e3182575f61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Egan DF, Shackelford DB, Mihaylova MM, Gelino S, Kohnz RA, Mair W, Vasquez DS, Joshi A, Gwinn DM, Taylor R, Asara JM, Fitzpatrick J, Dillin A, Viollet B, Kundu M, Hansen M, Shaw RJ. Phosphorylation of ulk1 (hatg1) by amp-activated protein kinase connects energy sensing to mitophagy. Science. 2011;331:456–461. doi: 10.1126/science.1196371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rosenbluth JM, Mays DJ, Pino MF, Tang LJ, Pietenpol JA. A gene signature-based approach identifies mtor as a regulator of p73. Mol Cell Biol. 2008;28:5951–5964. doi: 10.1128/MCB.00305-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Martina JA, Chen Y, Gucek M, Puertollano R. Mtorc1 functions as a transcriptional regulator of autophagy by preventing nuclear transport of tfeb. Autophagy. 2012;8:903–914. doi: 10.4161/auto.19653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Settembre C, Di Malta C, Polito VA, Garcia Arencibia M, Vetrini F, Erdin S, Erdin SU, Huynh T, Medina D, Colella P, Sardiello M, Rubinsztein DC, Ballabio A. Tfeb links autophagy to lysosomal biogenesis. Science. 2011;332:1429–1433. doi: 10.1126/science.1204592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cunningham JT, Rodgers JT, Arlow DH, Vazquez F, Mootha VK, Puigserver P. Mtor controls mitochondrial oxidative function through a yy1-pgc-1alpha transcriptional complex. Nature. 2007;450:736–740. doi: 10.1038/nature06322. [DOI] [PubMed] [Google Scholar]
- 46.Duvel K, Yecies JL, Menon S, Raman P, Lipovsky AI, Souza AL, Triantafellow E, Ma Q, Gorski R, Cleaver S, Vander Heiden MG, MacKeigan JP, Finan PM, Clish CB, Murphy LO, Manning BD. Activation of a metabolic gene regulatory network downstream of mtor complex 1. Mol Cell. 2010;39:171–183. doi: 10.1016/j.molcel.2010.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sekulic A, Hudson CC, Homme JL, Yin P, Otterness DM, Karnitz LM, Abraham RT. A direct linkage between the phosphoinositide 3-kinase-akt signaling pathway and the mammalian target of rapamycin in mitogen-stimulated and transformed cells. Cancer Res. 2000;60:3504–3513. [PubMed] [Google Scholar]
- 48.Inoki K, Ouyang H, Zhu T, Lindvall C, Wang Y, Zhang X, Yang Q, Bennett C, Harada Y, Stankunas K, Wang CY, He X, MacDougald OA, You M, Williams BO, Guan KL. Tsc2 integrates wnt and energy signals via a coordinated phosphorylation by ampk and gsk3 to regulate cell growth. Cell. 2006;126:955–968. doi: 10.1016/j.cell.2006.06.055. [DOI] [PubMed] [Google Scholar]
- 49.Inoki K, Li Y, Xu T, Guan KL. Rheb gtpase is a direct target of tsc2 gap activity and regulates mtor signaling. Genes Dev. 2003;17:1829–1834. doi: 10.1101/gad.1110003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Inoki K, Zhu T, Guan KL. Tsc2 mediates cellular energy response to control cell growth and survival. Cell. 2003;115:577–590. doi: 10.1016/s0092-8674(03)00929-2. [DOI] [PubMed] [Google Scholar]
- 51.Brugarolas J, Lei K, Hurley RL, Manning BD, Reiling JH, Hafen E, Witters LA, Ellisen LW, Kaelin WG., Jr Regulation of mtor function in response to hypoxia by redd1 and the tsc1/tsc2 tumor suppressor complex. Genes Dev. 2004;18:2893–2904. doi: 10.1101/gad.1256804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zheng M, Wang YH, Wu XN, Wu SQ, Lu BJ, Dong MQ, Zhang H, Sun P, Lin SC, Guan KL, Han J. Inactivation of rheb by prak-mediated phosphorylation is essential for energy-depletion-induced suppression of mtorc1. Nat Cell Biol. 2011;13:263–272. doi: 10.1038/ncb2168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Inoki K, Li Y, Zhu T, Wu J, Guan KL. Tsc2 is phosphorylated and inhibited by akt and suppresses mtor signalling. Nat Cell Biol. 2002;4:648–657. doi: 10.1038/ncb839. [DOI] [PubMed] [Google Scholar]
- 54.Ma L, Chen Z, Erdjument-Bromage H, Tempst P, Pandolfi PP. Phosphorylation and functional inactivation of tsc2 by erk implications for tuberous sclerosis and cancer pathogenesis. Cell. 2005;121:179–193. doi: 10.1016/j.cell.2005.02.031. [DOI] [PubMed] [Google Scholar]
- 55.Lee DF, Kuo HP, Chen CT, Hsu JM, Chou CK, Wei Y, Sun HL, Li LY, Ping B, Huang WC, He X, Hung JY, Lai CC, Ding Q, Su JL, Yang JY, Sahin AA, Hortobagyi GN, Tsai FJ, Tsai CH, Hung MC. Ikk beta suppression of tsc1 links inflammation and tumor angiogenesis via the mtor pathway. Cell. 2007;130:440–455. doi: 10.1016/j.cell.2007.05.058. [DOI] [PubMed] [Google Scholar]
- 56.Kim E, Goraksha-Hicks P, Li L, Neufeld TP, Guan KL. Regulation of torc1 by rag gtpases in nutrient response. Nat Cell Biol. 2008;10:935–945. doi: 10.1038/ncb1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sancak Y, Bar-Peled L, Zoncu R, Markhard AL, Nada S, Sabatini DM. Ragulator-rag complex targets mtorc1 to the lysosomal surface and is necessary for its activation by amino acids. Cell. 2010;141:290–303. doi: 10.1016/j.cell.2010.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Li L, Guan KL. Microtubule-associated protein/microtubule affinity-regulating kinase 4 (mark4) is a negative regulator of the mammalian target of rapamycin complex 1 (mtorc1) J Biol Chem. 2013;288:703–708. doi: 10.1074/jbc.C112.396903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zinzalla V, Stracka D, Oppliger W, Hall MN. Activation of mtorc2 by association with the ribosome. Cell. 2011;144:757–768. doi: 10.1016/j.cell.2011.02.014. [DOI] [PubMed] [Google Scholar]
- 60.Huang J, Dibble CC, Matsuzaki M, Manning BD. The tsc1-tsc2 complex is required for proper activation of mtor complex 2. Mol Cell Biol. 2008;28:4104–4115. doi: 10.1128/MCB.00289-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Dibble CC, Asara JM, Manning BD. Characterization of rictor phosphorylation sites reveals direct regulation of mtor complex 2 by s6k1. Mol Cell Biol. 2009;29:5657–5670. doi: 10.1128/MCB.00735-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sarbassov DD, Guertin DA, Ali SM, Sabatini DM. Phosphorylation and regulation of akt/pkb by the rictor-mtor complex. Science. 2005;307:1098–1101. doi: 10.1126/science.1106148. [DOI] [PubMed] [Google Scholar]
- 63.Guertin DA, Stevens DM, Thoreen CC, Burds AA, Kalaany NY, Moffat J, Brown M, Fitzgerald KJ, Sabatini DM. Ablation in mice of the mtorc components raptor, rictor, or mlst8 reveals that mtorc2 is required for signaling to akt-foxo and pkcalpha, but not s6k1. Dev Cell. 2006;11:859–871. doi: 10.1016/j.devcel.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 64.Xie X, Zhang D, Zhao B, Lu MK, You M, Condorelli G, Wang CY, Guan KL. Ikappab kinase epsilon and tank-binding kinase 1 activate akt by direct phosphorylation. Proc Natl Acad Sci U S A. 2011;108:6474–6479. doi: 10.1073/pnas.1016132108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Jacinto E, Loewith R, Schmidt A, Lin S, Ruegg MA, Hall A, Hall MN. Mammalian tor complex 2 controls the actin cytoskeleton and is rapamycin insensitive. Nat Cell Biol. 2004;6:1122–1128. doi: 10.1038/ncb1183. [DOI] [PubMed] [Google Scholar]
- 66.Garcia-Martinez JM, Alessi DR. Mtor complex 2 (mtorc2) controls hydrophobic motif phosphorylation and activation of serum- and glucocorticoid-induced protein kinase 1 (sgk1) Biochem J. 2008;416:375–385. doi: 10.1042/BJ20081668. [DOI] [PubMed] [Google Scholar]
- 67.Hong F, Larrea MD, Doughty C, Kwiatkowski DJ, Squillace R, Slingerland JM. Mtor-raptor binds and activates sgk1 to regulate p27 phosphorylation. Mol Cell. 2008;30:701–711. doi: 10.1016/j.molcel.2008.04.027. [DOI] [PubMed] [Google Scholar]
- 68.Aoyama T, Matsui T, Novikov M, Park J, Hemmings B, Rosenzweig A. Serum and glucocorticoid-responsive kinase-1 regulates cardiomyocyte survival and hypertrophic response. Circulation. 2005;111:1652–1659. doi: 10.1161/01.CIR.0000160352.58142.06. [DOI] [PubMed] [Google Scholar]
- 69.Das S, Aiba T, Rosenberg M, Hessler K, Xiao C, Quintero PA, Ottaviano FG, Knight AC, Graham EL, Bostrom P, Morissette MR, del Monte F, Begley MJ, Cantley LC, Ellinor PT, Tomaselli GF, Rosenzweig A. Pathological role of serum- and glucocorticoid-regulated kinase 1 in adverse ventricular remodeling. Circulation. 2012;126:2208–2219. doi: 10.1161/CIRCULATIONAHA.112.115592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Braz JC, Gregory K, Pathak A, Zhao W, Sahin B, Klevitsky R, Kimball TF, Lorenz JN, Nairn AC, Liggett SB, Bodi I, Wang S, Schwartz A, Lakatta EG, DePaoli-Roach AA, Robbins J, Hewett TE, Bibb JA, Westfall MV, Kranias EG, Molkentin JD. Pkc-alpha regulates cardiac contractility and propensity toward heart failure. Nat Med. 2004;10:248–254. doi: 10.1038/nm1000. [DOI] [PubMed] [Google Scholar]
- 71.Goorden SM, Hoogeveen-Westerveld M, Cheng C, van Woerden GM, Mozaffari M, Post L, Duckers HJ, Nellist M, Elgersma Y. Rheb is essential for murine development. Mol Cell Biol. 2011;31:1672–1678. doi: 10.1128/MCB.00985-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Mizushima N, Yoshimori T, Levine B. Methods in mammalian autophagy research. Cell. 2010;140:313–326. doi: 10.1016/j.cell.2010.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Selman C, Tullet JM, Wieser D, Irvine E, Lingard SJ, Choudhury AI, Claret M, Al-Qassab H, Carmignac D, Ramadani F, Woods A, Robinson IC, Schuster E, Batterham RL, Kozma SC, Thomas G, Carling D, Okkenhaug K, Thornton JM, Partridge L, Gems D, Withers DJ. Ribosomal protein s6 kinase 1 signaling regulates mammalian life span. Science. 2009;326:140–144. doi: 10.1126/science.1177221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Um SH, Frigerio F, Watanabe M, Picard F, Joaquin M, Sticker M, Fumagalli S, Allegrini PR, Kozma SC, Auwerx J, Thomas G. Absence of s6k1 protects against age- and diet-induced obesity while enhancing insulin sensitivity. Nature. 2004;431:200–205. doi: 10.1038/nature02866. [DOI] [PubMed] [Google Scholar]
- 75.Um SH, D'Alessio D, Thomas G. Nutrient overload, insulin resistance, and ribosomal protein s6 kinase 1, s6k1. Cell Metab. 2006;3:393–402. doi: 10.1016/j.cmet.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 76.Lamming DW, Ye L, Katajisto P, Goncalves MD, Saitoh M, Stevens DM, Davis JG, Salmon AB, Richardson A, Ahima RS, Guertin DA, Sabatini DM, Baur JA. Rapamycin-induced insulin resistance is mediated by mtorc2 loss and uncoupled from longevity. Science. 2012;335:1638–1643. doi: 10.1126/science.1215135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Shinmura K, Tamaki K, Sano M, Murata M, Yamakawa H, Ishida H, Fukuda K. Impact of long-term caloric restriction on cardiac senescence: Caloric restriction ameliorates cardiac diastolic dysfunction associated with aging. J Mol Cell Cardiol. 2011;50:117–127. doi: 10.1016/j.yjmcc.2010.10.018. [DOI] [PubMed] [Google Scholar]
- 78.Niemann B, Chen Y, Teschner M, Li L, Silber RE, Rohrbach S. Obesity induces signs of premature cardiac aging in younger patients: The role of mitochondria. J Am Coll Cardiol. 2011;57:577–585. doi: 10.1016/j.jacc.2010.09.040. [DOI] [PubMed] [Google Scholar]
- 79.Sciarretta S, Volpe M, Sadoshima J. Is reactivation of autophagy a possible therapeutic solution for obesity and metabolic syndrome? Autophagy. 2012;8:1252–1254. doi: 10.4161/auto.20670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zhou J, Freeman TA, Ahmad F, Shang X, Mangano E, Gao E, Farber J, Wang Y, Ma XL, Woodgett J, Vagnozzi RJ, Lal H, Force T. Gsk-3alpha is a central regulator of age-related pathologies in mice. J Clin Invest. 2013;123:1821–1832. doi: 10.1172/JCI64398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hua Y, Zhang Y, Ceylan-Isik AF, Wold LE, Nunn JM, Ren J. Chronic akt activation accentuates aging-induced cardiac hypertrophy and myocardial contractile dysfunction: Role of autophagy. Basic Res Cardiol. 2011;106:1173–1191. doi: 10.1007/s00395-011-0222-8. [DOI] [PubMed] [Google Scholar]
- 82.Neff F, Flores-Dominguez D, Ryan DP, Horsch M, Schroder S, Adler T, Afonso LC, Aguilar-Pimentel JA, Becker L, Garrett L, Hans W, Hettich MM, Holtmeier R, Holter SM, Moreth K, Prehn C, Puk O, Racz I, Rathkolb B, Rozman J, Naton B, Ordemann R, Adamski J, Beckers J, Bekeredjian R, Busch DH, Ehninger G, Graw J, Hofler H, Klingenspor M, Klopstock T, Ollert M, Stypmann J, Wolf E, Wurst W, Zimmer A, Fuchs H, Gailus-Durner V, Hrabe de Angelis M, Ehninger D. Rapamycin extends murine lifespan but has limited effects on aging. J Clin Invest. 2013;123:3272–3291. doi: 10.1172/JCI67674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Simm A, Schluter K, Diez C, Piper HM, Hoppe J. Activation of p70(s6) kinase by beta-adrenoceptor agonists on adult cardiomyocytes. J Mol Cell Cardiol. 1998;30:2059–2067. doi: 10.1006/jmcc.1998.0768. [DOI] [PubMed] [Google Scholar]
- 84.Sadoshima J, Izumo S. Rapamycin selectively inhibits angiotensin ii-induced increase in protein synthesis in cardiac myocytes in vitro. Potential role of 70-kd s6 kinase in angiotensin ii-induced cardiac hypertrophy. Circ Res. 1995;77:1040–1052. doi: 10.1161/01.res.77.6.1040. [DOI] [PubMed] [Google Scholar]
- 85.Lavandero S, Foncea R, Perez V, Sapag-Hagar M. Effect of inhibitors of signal transduction on igf-1-induced protein synthesis associated with hypertrophy in cultured neonatal rat ventricular myocytes. FEBS Lett. 1998;422:193–196. doi: 10.1016/s0014-5793(98)00008-8. [DOI] [PubMed] [Google Scholar]
- 86.Kemi OJ, Ceci M, Wisloff U, Grimaldi S, Gallo P, Smith GL, Condorelli G, Ellingsen O. Activation or inactivation of cardiac akt/mtor signaling diverges physiological from pathological hypertrophy. J Cell Physiol. 2008;214:316–321. doi: 10.1002/jcp.21197. [DOI] [PubMed] [Google Scholar]
- 87.McMullen JR, Shioi T, Zhang L, Tarnavski O, Sherwood MC, Kang PM, Izumo S. Phosphoinositide 3-kinase(p110alpha) plays a critical role for the induction of physiological, but not pathological, cardiac hypertrophy. Proc Natl Acad Sci U S A. 2003;100:12355–12360. doi: 10.1073/pnas.1934654100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Proud CG. Ras, pi3-kinase and mtor signaling in cardiac hypertrophy. Cardiovasc Res. 2004;63:403–413. doi: 10.1016/j.cardiores.2004.02.003. [DOI] [PubMed] [Google Scholar]
- 89.Wang L, Gout I, Proud CG. Cross-talk between the erk and p70 s6 kinase (s6k) signaling pathways. Mek-dependent activation of s6k2 in cardiomyocytes. J Biol Chem. 2001;276:32670–32677. doi: 10.1074/jbc.M102776200. [DOI] [PubMed] [Google Scholar]
- 90.Zhang P, Xu X, Hu X, van Deel ED, Zhu G, Chen Y. Inducible nitric oxide synthase deficiency protects the heart from systolic overload-induced ventricular hypertrophy and congestive heart failure. Circ Res. 2007;100:1089–1098. doi: 10.1161/01.RES.0000264081.78659.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Seth M, Zhang ZS, Mao L, Graham V, Burch J, Stiber J, Tsiokas L, Winn M, Abramowitz J, Rockman HA, Birnbaumer L, Rosenberg P. Trpc1 channels are critical for hypertrophic signaling in the heart. Circ Res. 2009;105:1023–1030. doi: 10.1161/CIRCRESAHA.109.206581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Clemente CF, Tornatore TF, Theizen TH, Deckmann AC, Pereira TC, Lopes-Cendes I, Souza JR, Franchini KG. Targeting focal adhesion kinase with small interfering rna prevents and reverses load-induced cardiac hypertrophy in mice. Circ Res. 2007;101:1339–1348. doi: 10.1161/CIRCRESAHA.107.160978. [DOI] [PubMed] [Google Scholar]
- 93.Sen S, Kundu BK, Wu HC, Hashmi SS, Guthrie P, Locke LW, Roy RJ, Matherne GP, Berr SS, Terwelp M, Scott B, Carranza S, Frazier OH, Glover DK, Dillmann WH, Gambello MJ, Entman ML, Taegtmeyer H. Glucose regulation of load-induced mtor signaling and er stress in mammalian heart. Journal of the American Heart Association. 2013;2:e004796. doi: 10.1161/JAHA.113.004796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Harston RK, McKillop JC, Moschella PC, Van Laer A, Quinones LS, Baicu CF, Balasubramanian S, Zile MR, Kuppuswamy D. Rapamycin treatment augments both protein ubiquitination and akt activation in pressure-overloaded rat myocardium. Am J Physiol Heart Circ Physiol. 2011;300:H1696–1706. doi: 10.1152/ajpheart.00545.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Soesanto W, Lin HY, Hu E, Lefler S, Litwin SE, Sena S, Abel ED, Symons JD, Jalili T. Mammalian target of rapamycin is a critical regulator of cardiac hypertrophy in spontaneously hypertensive rats. Hypertension. 2009;54:1321–1327. doi: 10.1161/HYPERTENSIONAHA.109.138818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.DeBosch B, Treskov I, Lupu TS, Weinheimer C, Kovacs A, Courtois M, Muslin AJ. Akt1 is required for physiological cardiac growth. Circulation. 2006;113:2097–2104. doi: 10.1161/CIRCULATIONAHA.105.595231. [DOI] [PubMed] [Google Scholar]
- 97.Shen WH, Chen Z, Shi S, Chen H, Zhu W, Penner A, Bu G, Li W, Boyle DW, Rubart M, Field LJ, Abraham R, Liechty EA, Shou W. Cardiac restricted overexpression of kinase-dead mammalian target of rapamycin (mtor) mutant impairs the mtor-mediated signaling and cardiac function. J Biol Chem. 2008;283:13842–13849. doi: 10.1074/jbc.M801510200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.McMullen JR, Shioi T, Zhang L, Tarnavski O, Sherwood MC, Dorfman AL, Longnus S, Pende M, Martin KA, Blenis J, Thomas G, Izumo S. Deletion of ribosomal s6 kinases does not attenuate pathological, physiological, or insulin-like growth factor 1 receptor-phosphoinositide 3-kinase-induced cardiac hypertrophy. Mol Cell Biol. 2004;24:6231–6240. doi: 10.1128/MCB.24.14.6231-6240.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Song X, Kusakari Y, Xiao CY, Kinsella SD, Rosenberg MA, Scherrer-Crosbie M, Hara K, Rosenzweig A, Matsui T. Mtor attenuates the inflammatory response in cardiomyocytes and prevents cardiac dysfunction in pathological hypertrophy. Am J Physiol Cell Physiol. 2010;299:C1256–1266. doi: 10.1152/ajpcell.00338.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Shiojima I, Sato K, Izumiya Y, Schiekofer S, Ito M, Liao R, Colucci WS, Walsh K. Disruption of coordinated cardiac hypertrophy and angiogenesis contributes to the transition to heart failure. J Clin Invest. 2005;115:2108–2118. doi: 10.1172/JCI24682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ikeda Y, Sato K, Pimentel DR, Sam F, Shaw RJ, Dyck JR, Walsh K. Cardiac-specific deletion of lkb1 leads to hypertrophy and dysfunction. J Biol Chem. 2009;284:35839–35849. doi: 10.1074/jbc.M109.057273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Malhowski AJ, Hira H, Bashiruddin S, Warburton R, Goto J, Robert B, Kwiatkowski DJ, Finlay GA. Smooth muscle protein-22-mediated deletion of tsc1 results in cardiac hypertrophy that is mtorc1-mediated and reversed by rapamycin. Hum Mol Genet. 2011;20:1290–1305. doi: 10.1093/hmg/ddq570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Chen SN, Czernuszewicz G, Tan Y, Lombardi R, Jin J, Willerson JT, Marian AJ. Human molecular genetic and functional studies identify trim63, encoding muscle ring finger protein 1, as a novel gene for human hypertrophic cardiomyopathy. Circ Res. 2012;111:907–919. doi: 10.1161/CIRCRESAHA.112.270207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Zhu W, Soonpaa MH, Chen H, Shen W, Payne RM, Liechty EA, Caldwell RL, Shou W, Field LJ. Acute doxorubicin cardiotoxicity is associated with p53-induced inhibition of the mammalian target of rapamycin pathway. Circulation. 2009;119:99–106. doi: 10.1161/CIRCULATIONAHA.108.799700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Efeyan A, Zoncu R, Chang S, Gumper I, Snitkin H, Wolfson RL, Kirak O, Sabatini DD, Sabatini DM. Regulation of mtorc1 by the rag gtpases is necessary for neonatal autophagy and survival. Nature. 2013;493:679–683. doi: 10.1038/nature11745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Castets P, Lin S, Rion N, Di Fulvio S, Romanino K, Guridi M, Frank S, Tintignac LA, Sinnreich M, Ruegg MA. Sustained activation of mtorc1 in skeletal muscle inhibits constitutive and starvation-induced autophagy and causes a severe, late-onset myopathy. Cell Metab. 2013;17:731–744. doi: 10.1016/j.cmet.2013.03.015. [DOI] [PubMed] [Google Scholar]
- 107.Takemura G, Maruyama R, Goto K, Kanamori H, Tsujimoto A, Minatoguchi S, Fujiwara H. Fate of isolated adult cardiomyocytes undergoing starvation-induced autophagic degeneration. Autophagy. 2009;5:90–92. doi: 10.4161/auto.5.1.7206. [DOI] [PubMed] [Google Scholar]
- 108.Lin SY, Li TY, Liu Q, Zhang C, Li X, Chen Y, Zhang SM, Lian G, Liu Q, Ruan K, Wang Z, Zhang CS, Chien KY, Wu J, Li Q, Han J, Lin SC. Gsk3-tip60-ulk1 signaling pathway links growth factor deprivation to autophagy. Science. 2012;336:477–481. doi: 10.1126/science.1217032. [DOI] [PubMed] [Google Scholar]
- 109.Sciarretta S, Zhai P, Shao D, Zablocki D, Nagarajan N, Terada LS, Volpe M, Sadoshima J. Activation of nox4 in the endoplasmic reticulum promotes cardiomyocyte autophagy and survival during energy stress through the perk/eif-2alpha/atf4 pathway. Circ Res. 2013 doi: 10.1161/CIRCRESAHA.113.301787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Matsui Y, Takagi H, Qu X, Abdellatif M, Sakoda H, Asano T, Levine B, Sadoshima J. Distinct roles of autophagy in the heart during ischemia and reperfusion: Roles of amp-activated protein kinase and beclin 1 in mediating autophagy. Circ Res. 2007;100:914–922. doi: 10.1161/01.RES.0000261924.76669.36. [DOI] [PubMed] [Google Scholar]
- 111.Di R, Wu X, Chang Z, Zhao X, Feng Q, Lu S, Luan Q, Hemmings BA, Li X, Yang Z. S6k inhibition renders cardiac protection against myocardial infarction through pdk1 phosphorylation of akt. Biochem J. 2012;441:199–207. doi: 10.1042/BJ20110033. [DOI] [PubMed] [Google Scholar]
- 112.Kerr BA, Ma L, West XZ, Ding L, Malinin NL, Weber ME, Tischenko M, Goc A, Somanath PR, Penn MS, Podrez EA, Byzova TV. Interference with akt signaling protects against myocardial infarction and death by limiting the consequences of oxidative stress. Sci Signal. 2013;6:ra67. doi: 10.1126/scisignal.2003948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.McLean BA, Kienesberger PC, Wang W, Masson G, Zhabyeyev P, Dyck JR, Oudit GY. Enhanced recovery from ischemia-reperfusion injury in pi3kalpha dominant negative hearts: Investigating the role of alternate pi3k isoforms, increased glucose oxidation and mapk signaling. J Mol Cell Cardiol. 2013;54:9–18. doi: 10.1016/j.yjmcc.2012.10.015. [DOI] [PubMed] [Google Scholar]
- 114.Dhingra R, Gang H, Wang Y, Biala AK, Aviv Y, Margulets V, Tee A, Kirshenbaum LA. Bidirectional regulation of nuclear factor-kappab and mammalian target of rapamycin signaling functionally links bnip3 gene repression and cell survival of ventricular myocytes. Circ Heart Fail. 2013;6:335–343. doi: 10.1161/CIRCHEARTFAILURE.112.000061. [DOI] [PubMed] [Google Scholar]
- 115.Das A, Salloum FN, Durrant D, Ockaili R, Kukreja RC. Rapamycin protects against myocardial ischemia-reperfusion injury through jak2-stat3 signaling pathway. J Mol Cell Cardiol. 2012;53:858–869. doi: 10.1016/j.yjmcc.2012.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Andres AM, Hernandez G, Lee P, Huang C, Ratliff EP, Sin J, Thornton CA, Damasco MV, Gottlieb RA. Mitophagy is required for acute cardioprotection by simvastatin. Antioxid Redox Signal. 2013 doi: 10.1089/ars.2013.5416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Lu Z, Xu X, Hu X, Fassett J, Zhu G, Tao Y, Li J, Huang Y, Zhang P, Zhao B, Chen Y. Pgc-1 alpha regulates expression of myocardial mitochondrial antioxidants and myocardial oxidative stress after chronic systolic overload. Antioxid Redox Signal. 2010;13:1011–1022. doi: 10.1089/ars.2009.2940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Hausenloy DJ, Mocanu MM, Yellon DM. Cross-talk between the survival kinases during early reperfusion: Its contribution to ischemic preconditioning. Cardiovasc Res. 2004;63:305–312. doi: 10.1016/j.cardiores.2004.04.011. [DOI] [PubMed] [Google Scholar]
- 119.Aoyagi T, Kusakari Y, Xiao CY, Inouye BT, Takahashi M, Scherrer-Crosbie M, Rosenzweig A, Hara K, Matsui T. Cardiac mtor protects the heart against ischemia-reperfusion injury. Am J Physiol Heart Circ Physiol. 2012;303:H75–85. doi: 10.1152/ajpheart.00241.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Maroko PR, Braunwald E. Effects of metabolic and pharmacologic interventions on myocardial infarct size following coronary occlusion. Circulation. 1976;53:I162–168. [PubMed] [Google Scholar]
- 121.Zhu Y, Soto J, Anderson B, Riehle C, Zhang YC, Wende AR, Jones D, McClain DA, Abel ED. Regulation of fatty acid metabolism by mtor in adult murine hearts occurs independently of changes in pgc-1alpha. Am J Physiol Heart Circ Physiol. 2013;305:H41–51. doi: 10.1152/ajpheart.00877.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Parra V, Verdejo HE, Iglewski M, Campo AD, Troncoso R, Jones D, Zhu Y, Kuzmicic J, Pennanen C, Lopez-Crisosto C, Jana F, Ferreira J, Noguera E, Chiong M, Bernlohr DA, Klip A, Hill JA, Rothermel BA, Abel ED, Zorzano A, Lavandero S. Insulin stimulates mitochondrial fusion and function in cardiomyocytes via the akt-mtor-nfkappab-opa-1 signaling pathway. Diabetes. 2013 doi: 10.2337/db13-0340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Yang Z, Ming XF. Mtor signalling: The molecular interface connecting metabolic stress, aging and cardiovascular diseases. Obesity reviews : an official journal of the International Association for the Study of Obesity. 2012;13(Suppl 2):58–68. doi: 10.1111/j.1467-789X.2012.01038.x. [DOI] [PubMed] [Google Scholar]
- 124.Wang CY, Kim HH, Hiroi Y, Sawada N, Salomone S, Benjamin LE, Walsh K, Moskowitz MA, Liao JK. Obesity increases vascular senescence and susceptibility to ischemic injury through chronic activation of akt and mtor. Sci Signal. 2009;2:ra11. doi: 10.1126/scisignal.2000143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Sciarretta S, Ferrucci A, Ciavarella GM, De Paolis P, Venturelli V, Tocci G, De Biase L, Rubattu S, Volpe M. Markers of inflammation and fibrosis are related to cardiovascular damage in hypertensive patients with metabolic syndrome. Am J Hypertens. 2007;20:784–791. doi: 10.1016/j.amjhyper.2007.01.023. [DOI] [PubMed] [Google Scholar]
- 126.Li ZL, Woollard JR, Ebrahimi B, Crane JA, Jordan KL, Lerman A, Wang SM, Lerman LO. Transition from obesity to metabolic syndrome is associated with altered myocardial autophagy and apoptosis. Arterioscler Thromb Vasc Biol. 2012;32:1132–1141. doi: 10.1161/ATVBAHA.111.244061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Guo R, Zhang Y, Turdi S, Ren J. Adiponectin knockout accentuates high fat diet-induced obesity and cardiac dysfunction: Role of autophagy. Biochim Biophys Acta. 2013;1832:1136–1148. doi: 10.1016/j.bbadis.2013.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Xu X, Hua Y, Sreejayan N, Zhang Y, Ren J. Akt2 knockout preserves cardiac function in high-fat diet-induced obesity by rescuing cardiac autophagosome maturation. Journal of molecular cell biology. 2013;5:61–63. doi: 10.1093/jmcb/mjs055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Xu X, Kobayashi S, Chen K, Timm D, Volden P, Huang Y, Gulick J, Yue Z, Robbins J, Epstein PN, Liang Q. Diminished autophagy limits cardiac injury in mouse models of type 1 diabetes. J Biol Chem. 2013;288:18077–18092. doi: 10.1074/jbc.M113.474650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.He C, Zhu H, Li H, Zou MH, Xie Z. Dissociation of bcl-2-beclin1 complex by activated ampk enhances cardiac autophagy and protects against cardiomyocyte apoptosis in diabetes. Diabetes. 2013;62:1270–1281. doi: 10.2337/db12-0533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Yamahara K, Kume S, Koya D, Tanaka Y, Morita Y, Chin-Kanasaki M, Araki H, Isshiki K, Araki SI, Haneda M, Matsusaka T, Kashiwagi A, Maegawa H, Uzu T. Obesity-mediated autophagy insufficiency exacerbates proteinuria-induced tubulointerstitial lesions. J Am Soc Nephrol. 2013 doi: 10.1681/ASN.2012111080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Yang L, Li P, Fu S, Calay ES, Hotamisligil GS. Defective hepatic autophagy in obesity promotes er stress and causes insulin resistance. Cell Metab. 2010;11:467–478. doi: 10.1016/j.cmet.2010.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Thedieck K, Holzwarth B, Prentzell MT, Boehlke C, Klasener K, Ruf S, Sonntag AG, Maerz L, Grellscheid SN, Kremmer E, Nitschke R, Kuehn EW, Jonker JW, Groen AK, Reth M, Hall MN, Baumeister R. Inhibition of mtorc1 by astrin and stress granules prevents apoptosis in cancer cells. Cell. 2013;154:859–874. doi: 10.1016/j.cell.2013.07.031. [DOI] [PubMed] [Google Scholar]
- 134.Anversa P, Kajstura J, Rota M, Leri A. Regenerating new heart with stem cells. J Clin Invest. 2013;123:62–70. doi: 10.1172/JCI63068. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 135.Ming XF, Rajapakse AG, Carvas JM, Ruffieux J, Yang Z. Opposing and uncoupling effects of mtor and s6k1 in the regulation of endothelial tissue factor expression. FEBS Lett. 2010;584:135–140. doi: 10.1016/j.febslet.2009.11.030. [DOI] [PubMed] [Google Scholar]
- 136.Barilli A, Visigalli R, Sala R, Gazzola GC, Parolari A, Tremoli E, Bonomini S, Simon A, Closs EI, Dall'Asta V, Bussolati O. In human endothelial cells rapamycin causes mtorc2 inhibition and impairs cell viability and function. Cardiovasc Res. 2008;78:563–571. doi: 10.1093/cvr/cvn024. [DOI] [PubMed] [Google Scholar]
- 137.Goncharov DA, Kudryashova TV, Ziai H, Ihida-Stansbury K, Delisser H, Krymskaya VP, Tuder RM, Kawut SM, Goncharova EA. Mtorc2 coordinates pulmonary artery smooth muscle cell metabolism, proliferation and survival in pulmonary arterial hypertension. Circulation. 2013 doi: 10.1161/CIRCULATIONAHA.113.004581. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.