Abstract
Objectives
Pain is under recognized and under managed in older adults with dementia. Because dementia patients have a diminished capacity to communicate discomfort, untreated pain may be expressed in the form of behavioral and psychiatric symptoms. The goal of the present study was to examine the relationship between pain and behavioral and psychiatric symptoms of dementia in community residing older adults from the perspective of the family caregiver.
Methods
Dyads composed of 272 dementia patients and their family caregivers were assessed to determine dementia patient’s mental status, and family caregiver’s assessment of care recipient’s pain, functional dependence and number of behavioral symptoms, analgesic use and demographic information.
Results
Hierarchical multiple regression analysis controlling for age, marital status, race, functional disability, and analgesic use showed that pain explained a small but significant percent of variance in the number of behavioral symptoms (3%, p<.001). Pain had a stronger influence on the number of BPDS among those with severe cognitive impairment (F [1, 69] = 11.75, p < .001) compared to those with low to moderate cognitive impairment (F [1,199] = 4.543, p=.034.).
Discussion
The findings indicate that pain is a risk factor for behavioral symptoms in individuals with dementia and suggest that pain is a more significant predictor of behavior for individuals with severe dementia, compared to those with mild/moderate stage dementia. These results reinforce the importance of proper pain assessment and its management as part of dementia care planning.
Keywords: dementia, pain, behavioral and psychiatric symptoms, family caregiver
INTRODUCTION
Despite major advances in pain management, pain remains under recognized, and undertreated in many older adults, and severely undermanaged in older adults with dementia1, 2. The prevalence of pain among the older adult population is estimated to be three times higher than among the younger adult population3, 4. Studies consistently demonstrate that 25% to 50% of community-dwelling older adults have persistent pain and that 45% to 80% of nursing home residents have untreated pain5, 6. Nonetheless, analgesics are less likely to be prescribed to cognitively impaired older adults when compared to those without cognitive impairment7, 8. As individuals progress to later stages of dementia, their capacity to effectively communicate their discomfort becomes diminished9, 10. Consequently, untreated pain may be expressed in the form of behavioral and psychiatric symptoms11,12.
Behavioral and psychiatric symptoms of dementia (BPSD) refer to the full range of symptoms that occur throughout the dementia disease process. BPSD has been conceptualized as a function of the interactive effects of patient characteristics including cognitive function and disease stage, and the social and environmental context in which they occur13. The prevalence of BPSD in the population with dementia range between 64% and 83%14,15. The most common of these behaviors include aimless walking/pacing, yelling or screaming, physical agitation, sleep disturbance, delusions and socially disruptive behavior16,17. Although the etiology of BPSD remains unclear, these behavioral symptoms often precipitate a sequelae of adverse outcomes including caregiver burden, patient morbidity, and increased healthcare utilization18,19.
Previous studies on the relationship between BPSD and pain in persons with sdementia suggest that as pain increases, symptoms of disruptive behavior increase20. Yet most research on the behavioral manifestations of pain typically focuses on agitation as a single construct without considering other BPSD manifestations16 and has involved primarily nursing home residents. One exception is a study by Pelletier and Landreville12 that demonstrated a significant correlation between pain and the verbal agitation and physical aggressive subscales of the Cohen Mansfield Agitation Inventory. Still lacking are investigations into the specific symptoms of behavioral disturbances that are expressed by individuals at varying stages of dementia who are in pain and living in the community-where most individuals with dementia reside. This study extends previous research in this area by identifying specific behaviors that are associated with caregiver reports of patient pain, and evaluating whether the relationship of pain to behaviors differs for those with mild-moderate and severe cognitive impairment in a community-based sample of dementia patients from the perspective of the caregiver. We hypothesized that the frequency of BPSD would be positively related to the severity of pain. Based on previous research which suggests that the capacity to communicate pain is diminished in advanced stages of dementia21, we further hypothesized that the relationship of pain to BPSD would be stronger in those with moderate to severe dementia than in those with mild dementia. We also explored the specific behavioral symptoms demonstrated in those experiencing pain.
MATERIALS AND METHODS
Participants
The study sample consisted of 272 community residing older adults and their family caregivers who were participants in a randomized trial (Project ACT), that tested the efficacy of a multi-component, non-pharmacologic intervention to reduce the occurrence of BPSD and associated caregiver distress22. Participants in Project ACT were recruited between 2003 and 2007 through media announcements and mailings by social service agencies. Interested caregivers of individuals with dementia contacted the research office, were explained study procedures and administered a brief telephone screening test. Eligible patients had a physician diagnosis of NINCDS/ADRDA criteria for probable dementia or Mini-mental State Examination (MMSE)23 score <24, were ≥21 years; and English speaking. Eligible caregivers provided oversight or care for 8 or more hours weekly, planned to live in the area for 9 months, were not seeking nursing home placement, and reported upset managing patient behaviors. Dyads were excluded if either had terminal illness with life expectancy <6 months; active treatments for cancer, >3 acute hospitalizations in past year; or involvement in another trial concerning behaviors. They were also excluded if patients had schizophrenia or bi-polar disorder, dementia secondary to probable head trauma, or an MMSE=0 and bed-bound. Data reported for this study were derived from the baseline assessment conducted by a trained interviewer and prior to randomization and exposure to the intervention24.
Measures
Dependent Variable: Behavioral and Psychiatric Symptoms
To examine BPSD, we used 21 behavioral items of which 16 were derived from the Agitated Behavior in Dementia (ABID) Scale23. The ABID has been previously shown to be psychometrically sound and corresponding to objective reports of commonly presenting symptoms (e.g. verbal aggressiveness, physical aggressiveness, screaming/crying out, behaviors harmful to self, roaming, destroying property, resisting/refusing care, arguing/irritability, socially inappropriate behavior, inappropriate sexual behavior, easily agitated/upset, restlessness, fearful/anxious, getting up at night, distressing beliefs, seeing/hearing distressing people/things). Validity of the ABID has been confirmed by correlations with related measures and lack of correlation with unrelated constructs. Overall scale reliability is acceptable (α=.78). We also included 2 items (repetitive questioning, hiding/hoarding) from the Revised Memory and Behavior Problem Checklist (RMBPC)26, and 3 items (wandering, incontinence, shadowing) from our previous research showing these behaviors as common and distressful (α=.76 for sample).27 Caregivers were asked to indicate the presence or absence of each behavior in the past month. The dependent variable was calculated as the sum of the total number of behaviors observed in the past month.
Independent Variables
Mental Status
Global cognitive function was assessed using the Mini Mental State Examination (MMSE)23. Subjects were then categorized into those with mild to moderate cognitive impairment (MMSE>10) or severe impairment (MMSE<10). These cutoff scores have demonstrated good sensitivity and specificity for staging of dementia in Alzheimer’s diesease23,27.
Pain
To obtain caregiver’s report of subject’s pain status, we used four items from the NIH REACH Battery28 (pain in the past 2 weeks, pain currently, severe pain, and pain that interferes with daily activities). For each item, caregivers were asked to rate the extent of pain (not at all, a little, quite a bit, extremely) using a Likert scale. A total pain score was calculated by summing the score for the 4 items (α=.90). Summed pain scores of 1–4 were categorized as slight (no pain to a little pain on the four items); a score of 5–11 was categorized as moderate (quite a bit of pain in at least one item); and a score of 12 or more was categorized as severe (extreme pain on 3 or more items).
Functional Status
For functional dependence, we used the activities of daily living subscale of the Caregiver Assessment of Function and Unset (CAFU) measure. The CAFU, modeled after the Functional Independence Measure,29 has been previously shown as psychometrically sound and corresponding to objective determinations of dependence and assistance required. The instrument includes items on seven activities of daily living (ADLs) including bathing, dressing upper/lower body, toileting, grooming, eating, and getting in/out of bed. For each item, caregivers indicated whether patients were completely independent (score=7), there was a safety concern, excessive time required, or assistive devices used, (score=6), patients needed supervision, set-up, or cueing but no physical help (score=5), or physical help (4=a little help, 25% assistance, 3=moderate help, 50% assistance, 2=a lot of help, 75% assistance, or 1=complete help, >75% assistance). A functional status score was derived by summing across all items with lower scores representing greater dependence (α =.92).
Background variables
The following information was also collected from the caregiver at the baseline interview: patient’s and caregiver’s date of birth, gender, ethnicity, education, and marital status. Caregivers also reported on the number of years caregiving, their relationship with patient, and whether they had paid caregiver help. Age was analyzed as a continuous variable. Race was categorized as White, African American, or Other. Marital status was coded as living alone, living together but unmarried, or married. Medications taken on a daily basis were also recorded using a brown bag review by asking caregiver to place all medications and supplements taken by the patient in a brown bag. Current analgesic use was recorded and coded as “yes” or “no”.
Statistical Analysis
Descriptive data included caregiver sociodemographic (age, sex, race, education, relationship to patient, living arrangement, years caregiving) and patient (age, sex, race, number of problem behaviors, MMSE score). Categorical variables were dummy coded. The normality assumption for the dependent measure of BPSDs was tested by examining the distribution of residuals. Data analysis was performed using STATA software, version 10 for Windows (StataCorp, College Station, Texas). Statistical significance was defined as a 2-sided p value of 0.05 or less. Missing values were not replaced since the missing item rates were low (3%). Hierarchical multiple regressions were performed to specify the relationship between pain and number of behaviors while statistically controlling for age, marital status, race, functional status, and current use of analgesics. Analyses included patient factors identified in previous research as being associated with caregiver reports of dementia patient’s pain and BPSD (age, race, functional status, analgesic use)30, 31 and significant in univariate analysis. The total pain score was introduced in Step 2. Next, to further examine the associations between specific symptoms of BPSD and pain, we used logistical regression with each BPSD symptom as a dichotomous outcome variable (present/not present) and total pain as a predictor variable, while controlling for age, marital status, race, functional status, and current use of analgesics. A modified Bonferroni test was used to account for multiple testing (α =.0051). Lastly, full regressions were conducted for each MMSE group. Regression parameters (intercept, slope) between pain and behaviors across the low mental status and high mental status groups were compared using Chow’s test32.
RESULTS
Caregivers were primarily female (82.0%), white (69.9%), spouses (51.0%), with high education (66.9%>high school) and lived with their care recipient (90%). They had provided care an average of 3.7 years (SD = 3.0) and were on average 66.4 years old (SD = 12.2). Few (<10%) reported the use of paid help. Dementia patients were primarily male (52.7%), white (69.9%) and older (M=82.1 years, SD = 8.4) with an average MMSE of 13.0 (SD=8.1; range=0.0–24.0). On average, caregivers reported 9.6 (SD = 3.9) behaviors occurring an average of 3.2 (SD = 3.2) times weekly in the month prior to study entry (Table 1). Most patients were taking medications with 38.6% on anti-depressants, 33.5% on medications for behaviors, 71.7% on memory enhancement medications, and 36% on analgesics (25% acetaminophen, 8% nonsteroidal anti-inflammatory drugs [NSAIDs]; 3% opiate/opioid).
Table 1.
Demographic Characteristics of the Sample (N = 272)
| Characteristic | % | Mean | SD |
|---|---|---|---|
| Caregiver | |||
| Age | 66.4 | 12.2 | |
| Gender | |||
| Female | 82.0 | ||
| Male | 18.0 | ||
| Race (%) | |||
| White | 69.9 | ||
| African American | 27.2 | ||
| Other | 2.9 | ||
| Relation to Individual with Dementia | |||
| Spouse | 51.0 | ||
| Non-spouse | 49.0 | ||
| Education | |||
| < High School | 7.5 | ||
| High School | 25.8 | ||
| ➢ High School | 67.2 | ||
| Years Caregiving | 3.7 | 3.1 | |
| Individual with dementia | |||
| Age | 82.1 | 8.4 | |
| Gender | |||
| Male | 52.7 | ||
| Female | 47.3 | ||
| Race | |||
| White | 69.9 | ||
| African American | 27.6 | ||
| Other | 2.5 | ||
| Number of Behaviors (range 0–21) | 9.6 | 4.0 | |
| Functional Status | 4.5 | 2.3 | |
| MMSE | 13.0 | 8.1 |
Relationship of Pain to BPSD
Only 10% of caregivers reported that the dementia patient had no pain, 35% reported patient had slight or low pain, 40% reported that the patient had moderate levels of pain and 15% reported patient had severe pain.
Results of the hierarchical regression (Table 2) show variance explained by demographic characteristics (R2) at Step 1, incremental variance (Change R2) as a result of entering pain at Step 2, and total variance. The estimated B coefficients indicate the relative contribution of each independent variable in the prediction of the dependent variable (total behavioral frequency). As shown, caregiver reports of patient’s pain contributed weakly but significantly to the prediction of variation of behavioral frequency, beyond the characteristics that were statistically controlled in the regression equation.
Table 2.
Summary of hierarchical multiple regression analysis for variables predicting overall number of behaviors
| Variable | B | SE B | B | p | |
|---|---|---|---|---|---|
| Step 1 | Age | −.072 | .030 | −.160 | .017 |
| Marital status | −1.42 | .532 | −.181 | −.177 | |
| Race | .472 | .458 | .063 | .304 | |
| Functional Status | −.262 | .134 | −.118 | .050 | |
| On pain medications (yes/no) | .211 | .520 | .027 | .685 | |
| Step 2 | Total Pain Score | .176 | .060 | .191 | .004 |
R2 for Step 1 = 0.08, F = 3.73 (p=.001)
R2 for Step 2 = 0.11 Change R2 − .029, F = 8.67 (p=.000)
The results of the association between the presence of specific behavioral symptoms and total pain score are summarized in Table 3. Caregivers perceived more severe pain when they also perceived more agitation, anxiety, delusions, argumentative, and restlessness.
Table 3.
Summary of logistic regressions for presence of specific behaviors predicted by total pain score*
| Outcome Variable | B | SE | Wald | P** |
|---|---|---|---|---|
| Anxious | .098 | .026 | 14.51 | .000 |
| Argumentative | .128 | .040 | 10.40 | .001 |
| Agitated | .074 | .024 | 9.34 | .002 |
| Delusional | .074 | .025 | 8.88 | .003 |
| Restless | .058 | .025 | 5.12 | .020 |
LR models controlled for age, marital status, race, functional status, and current use of analgesics.
Modified Bonferoni results reported (α = .0051)
Pain and BPSD for Severe and Mild/moderate Cognitive Impairment
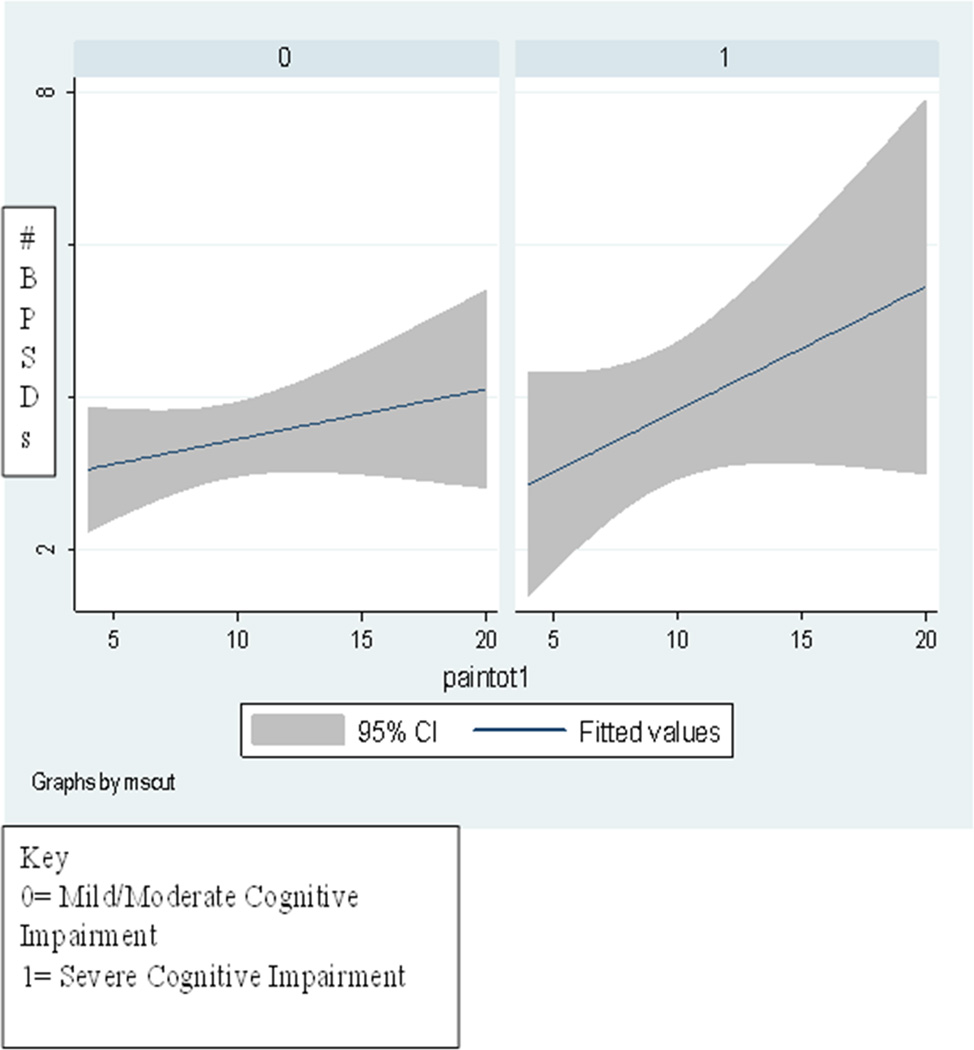
Figure 1 depicts the relationship between total pain scores and BPSD for those with mild/moderate cognitive impairment and those with severe levels of impairment. A comparison of the two slopes suggests that pain exerts a stronger influence on behavioral symptoms for individuals with severe dementia, compared to those with mild/moderate stage dementia.
Figure 1.
Association between total pain and BPDS in Mild/Moderate Stage Cognitive Impairment group (0) versus Impairment groups(1).
The significant differences in intercept and slope between those with severe dementia and those with mild/moderate dementia are reported in Table 4. Parameter estimates for the severely impaired group versus the mild/moderately impaired group suggest that pain is a more important predictor of behaviors for those with severe cognitive impairment (F [1, 69] = 11.75, p < .001) than those in the low to moderate cognitive impairment (F [1,199] = 4.543, p=.034.)
Table 4.
Chow’s Tests for differences in intercept and slope between the number of behavior symptoms and pain score in Mild/Moderate Stage Cognitive Impairment group (1) versus Severe Impairment group (2).
| Unstandardized Coefficients |
Standardized Coefficients |
|||||
|---|---|---|---|---|---|---|
| Model | B | Std. Error | Beta | t | Sig. | |
| 1 | (Constant) | 5.218 | 1.108 | 4.710 | .000 | |
| pain | .360 | .105 | .381 | 3.427 | .001 | |
| 2 | (Constant) | 8.500 | .651 | 13.055 | .000 | |
| pain | .063 | .149 | 2.131 | .034 | .034 | |
| Model | R | R Square |
Adjusted R Square |
Std. Error of the Estimate |
Change Statistics | ||||
|---|---|---|---|---|---|---|---|---|---|
| R Square Change |
F Change |
df1 | df2 | Sig. F Change | |||||
| 1 | .171 | .029 | .022 | 3.77858 | .029 | 4.038 | 2 | 269 | .019 |
| 2 | .279 | .078 | .064 | 3.69691 | .048 | 7.008 | 2 | 267 | .001 |
DISCUSSION
This study investigated the relationship between pain and behavioral symptoms in community-based older adults with dementia and considered whether the relationship differed for those with severe or mild to moderate cognitive impairment. Noteworthy is that 90% of caregivers reported presence of pain with 55% indicating pain was significant (moderate to severe). As hypothesized, our results show a positive and significant relationship between the degree of pain and the frequency of BPSD, especially in those with more severe cognitive impairment. In addition, patients with severe pain had more symptoms such as agitation, delusions, anxiety, restlessness and argumentativeness compared to those patients with milder pain. The findings are consistent with previous research showing that the majority of persons with dementia deal with painful stimuli7,8,31. Of importance is that pain appears to manifest in certain behaviors and not in others. The manifestation of behaviors such as physical and verbal aggression and crying out did not differ among those with varying levels of pain. This confirms results obtained previously by Bradford37, Cipher16, and Tosato11 and colleagues and provides further evidence that the relationship between pain and behaviors varies by specific behavioral symptoms.
Why is pain associated with behavioral manifestations? Individuals with dementia may have difficulty understanding the meaning of the sensation of pain and placing the sensation of pain in context. This could potentially explain the atypical behavioral responses observed in cognitively impaired individuals with painful conditions. The behavioral expression of pain in the presence of caregivers may also be explained by a basic sociobiological perspective. The sociobiological framework postulates a physiological basis for the behavioral dimension of pain and suggests that dementia patients contend with difficulty communicating the negative appraisal associated with pain's presence33. This perspective further suggests that the expression of pain is a form of meaningful communication with the caregiver, and it may involve select behavioral components.
The concept of illness behavior is another useful framework for understanding the expression of behavior in the presence of pain. The concept draws on psychological theories of perception, cognition and meaning and on theories of social relationships34. The framework contents that the expression of pain depends on any number of interpersonal factors including individuals past experiences will illness, personality and coping styles, familial and cultural norms and current interpersonal interactions. These factors, in turn, affect the nature and extent of help-seeking behavior35.
A broader interpersonal model may be required to explore how behaviors expressed by persons with dementia shape the caregivers’ perception of the pain experience36. Craig suggests that the non-verbal expression of pain is a social process in which motor behaviors that are modified by social code. In individuals with dementia who are unable to process socially acceptable behaviors, the expression of pain would be expected to be altered37. It is unclear whether the caregivers in our study took certain behaviors into account when making their judgments regarding pain. Additional research is needed to understand the factors that may shape behavioral pain expression in individuals with dementia and that may influence caregiver reports of pain.
Individuals with advanced dementia may also have progressively lower thresholds to process internal stimuli. The behavioral magnitude of expressing pain may be explained as the consequence of an internal stimulus representing the perceived threat associated with the pain. Kovachs and colleagues support this assertion in the Serial Trial Intervention. Their application of a stepwise protocol for the assessment and management of internal stimuli such as discomfort has shown a positive effect on reducing BPSD38.
The findings are limited in that the model explained a low proportion (11%) of the variance of BPSDs and pain explained only 3% of the variance in the model. These results suggest that although pain is a small but significant, and modifiable, contributor to behavior there are other potentially modifiable factors that need to be explored.
Our study relied on caregiver perceptions of dementia patient’s pain rather than patient self assessment. As such, the study is lacking an index of the patient’s actual pain experience. While self assessment of pain is considered the gold standard it may be problematic even in persons with mild dementia due to language and higher order processing deficits that accompany dementia39. We therefore relied on caregiver’s proxy reports and acknowledge this was a limitation in our study.
Other limitations of the current study include the cross-sectional retrospective approach which cannot confirm causality, and the observation that participants may not be fully representative of the community-residing dementia population. Another limitation is the reliance on caregiver report of behavioral occurrences. It may be that as dementia progresses, burden of care increases, and caregivers' perception and memory of symptoms becomes more extreme (a negative response shift39. However, we used psychometrically valid instruments and use of collateral information remains the primary data source of behaviors and pain in individuals with dementia for both research and clinical evaluations. Sample size prevented our ability to control for potentially important confounders. For example, we were unable to control for type of dementia or source and type of pain as these are important factors that may influence the behaviors observed. We were also unable to explore the relationship between caregiver distress in the face of pain and its effects on reports of behavior. Future research is needed to explore how caregivers decide if their care recipient with dementia is in pain.
Despite these limitations our findings add incrementally to an understanding of the relationship between pain and BPSD. We show that caregiver reports of dementia patient’s pain is associated with some behaviors but not others and that this relationship is stronger for individuals with severe cognitive impairment compared to those with mild to moderate impairment. Still unclear is the pathway through which pain affects certain behavioral symptoms in dementia. Nevertheless, given the high percentage of individuals with dementia in this study for whom caregivers reported some to a lot of pain (75%), individualized pain assessment and management should be considered standard care. Furthermore, in line with other recent findings11, 16, 41 pain appears to have an atypical presentation such that certain behaviors suggestive of active resistance (e.g., argumentativeness, restlessness) may serve as potential indicators of pain. Families could benefit from understanding the relationship of pain and behavioral manifestations so that they can alert physicians and assist in pain management42. Accurate assessment of pain in patients with dementia also depends on the ability of health professions to register verbal and nonverbal expressions of pain. It is important to note that clinicians often misinterpret BPSDs as signaling the need for psychotropic medication rather than whether there is undetected pain. Thus many older adults may be suffering from unrecognized pain.
The expression of certain behavioral symptoms in the presence of pain can be used to guide clinical management of common BPSDs. Although several promising observational instruments based on behavioral manifestations of pain have been developed, the psychometric properties of these measures have not been established43. Existing observational measures of pain in individuals with dementia were developed for individuals with advanced stage illness in the hospital or institutional setting and include observational indicators such as grimacing, moaning, restlessness. Our sample differed in important ways from those typically included in studies of pain assessment in persons with dementia and builds on recent findings of atypical presentations of pain in persons with dementia44–46. Future research is needed to establish a gold standard of observed pain in individuals with dementia in the community residing population and with consideration of the disruptive behaviors, such as active resistance, that were observed in these studies.
Acknowledgment
Research reported was supported in part by funds from the National Institute on Aging and the National Institute on Nursing Research (RO1 AG22254)
Footnotes
Conflict of Interests and Financial Statement: NONE
Contributor Information
Nancy Hodgson, Johns Hopkins University.
Laura N. Gitlin, Johns Hopkins University
Laraine Winter, Philadelphia VA Medical Center
Walter W. Hauck, Sycamore Consulting LLC
REFERENCES
- 1.Sengupta M, Bercovitz A, Harris-Kojetin LD. Prevalence and management of pain, by race and dementia among nursing home residents: United States, 2004. NCHS Data Brief. 2010;30:1–8. [PubMed] [Google Scholar]
- 2.Shega J, Emanuel L, Vargish L, Levine SK, Bursch H, Herr K, Karp JF, Weiner DF. Pain in persons with dementia: Complex, common, and challenging. J Pain. 2007;8(5):373–378. doi: 10.1016/j.jpain.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 3.Mobily PR, Herr KA, Clark MK, Wallace RB. An epidemiological analysis of pain in the elderly: the Iowa 65+ rural health study. J Aging Health. 1994;64:139–154. [Google Scholar]
- 4.AGS Panel on Persistent Pain in Older Persons. The Management of Persistent Pain in Older Persons. J Am Geriatr Soc. 2002;6(Suppl):S205–S224. doi: 10.1046/j.1532-5415.50.6s.1.x. [DOI] [PubMed] [Google Scholar]
- 5.Hutt E, Pepper GA, Vojir C, Fink R, Jones KR. Assessing the appropriateness of pain medication prescribing practices in nursing homes. J Am Geriatr Soc. 2006;54(2):231–239. doi: 10.1111/j.1532-5415.2005.00582.x. [DOI] [PubMed] [Google Scholar]
- 6.Helme RD, Gibson SJ. The epidemiology of pain in elderly people. Clin Geriatr Med. 2001;2(17):417–431. doi: 10.1016/s0749-0690(05)70078-1. [DOI] [PubMed] [Google Scholar]
- 7.Pickering G, Jourdan D, Dubray C. Acute versus chronic pain treatment in Alzheimer’s disease. Eur J Pain. 2006;10:379–384. doi: 10.1016/j.ejpain.2005.06.010. [DOI] [PubMed] [Google Scholar]
- 8.Malloy DC, Hadjistavropoulos T. The problem of pain management among persons with dementia, personhood, and the ontology of relationships. Nurs Phil. 2004;5:147–159. doi: 10.1111/j.1466-769X.2004.00174.x. [DOI] [PubMed] [Google Scholar]
- 9.Shega JW, Hougham GW, Stocking CB, Cox-Hayley D, Sachs GA. Factors associated with self- and caregiver report of pain among community-dwelling persons with dementia. J Palliat Med. 2005;8(3):567–575. doi: 10.1089/jpm.2005.8.567. [DOI] [PubMed] [Google Scholar]
- 10.Yeaman PA, Ford JL, Kim YY. Providing quality palliative care in end-stage Alzheimer’s Disease. Am J Hosp Palliat Care. 2012;8:1–4. doi: 10.1177/1049909112453644. [DOI] [PubMed] [Google Scholar]
- 11.Tosato M, Lukas A, van der Roest HG, Danese P, Antocicco M, Finne-Soveri H, Nikolaus T, Landi F, Bernabei R, Onder G. Association of pain with behavioral and psychiatric symptoms among nursing home residents with cognitive impairment: results from the SHELTER study. Pain. 2012;53(2):305–310. doi: 10.1016/j.pain.2011.10.007. [DOI] [PubMed] [Google Scholar]
- 12.Pelletier IC, Landreville P. Discomfort and agitation in older adults with dementia. BMC Geriatr 22. 2007;7:27. doi: 10.1186/1471-2318-7-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Corbett A, Husebo B, Malcangio M, Staniland A, Cohen-Mansfield J, Aarsland D, Ballard C. Assessment and treatment of pain in people with dementia. Nat Rev Neurol. 10. 2012;8(5):264–274. doi: 10.1038/nrneurol.2012.53. [DOI] [PubMed] [Google Scholar]
- 14.Ballard C, Lowery K, Powell L, et al. Impact of behavioral and psychological symptoms of dementia on caregivers. Int Psychogeriatr. 2000;12:93–105. [Google Scholar]
- 15.Finkel SI, Burns A. Behavioral and psychological symptoms of dementia (BPSD): a clinical and research update. Int Psychogeriatr. 2000;12:9–18. [Google Scholar]
- 16.Cipher DJ, Clifford A, Roper K. Behavioral manifestations of pain in the demented elderly. JAMDA. 2005:355–365. doi: 10.1016/j.jamda.2005.11.012. [DOI] [PubMed] [Google Scholar]
- 17.Lyketsos CG, Sheppard JME, Steinberg M, et al. Neuropsychiatric disturbance in Alzheimer’s disease clusters into three groups the Cache County study. Int J Geriatr Psychiatry. 2001;16:1043–1053. doi: 10.1002/gps.448. [DOI] [PubMed] [Google Scholar]
- 18.Sink KM, Covinsky KE, Barnes DE, Newcomer RJ, Yaffe K. Caregiver characteristics are associated with neuropsychiatric symptoms of dementia. J Am Geriatr Soc. 2006;54(5):796–803. doi: 10.1111/j.1532-5415.2006.00697.x. [DOI] [PubMed] [Google Scholar]
- 19.Sink KM, Holder KF, Yaffe K. Pharmacological treatment of neuropsychiatric symptoms of dementia: A review of the evidence. JAMA. 2005;293:596–608. doi: 10.1001/jama.293.5.596. [DOI] [PubMed] [Google Scholar]
- 20.Benedetti F, Arduino C, Vighetti S, Asteggiano G, Tarenzi L, Rainero I. Pain reactivity in Alzheimer patients with different degrees of cognitive impairment and brain electrical activity deterioration. Pain. 2004;111(1–2):22–29. doi: 10.1016/j.pain.2004.05.015. [DOI] [PubMed] [Google Scholar]
- 21.Rainero I, Vighetti S, Bergamasco B, Pinessi L, Benedetti F. Autonomic responses and pain perception in Alzheimer's disease. Eur J Pain. 2000;4:267–274. doi: 10.1053/eujp.2000.0185. [DOI] [PubMed] [Google Scholar]
- 22.Gitlin LN, Winter L, Dennis MP, Hauck WW. A non-pharmacological intervention to manage behavioral and psychological symptoms of dementia and reduce caregiver distress: Design and methods of project ACT. Clin Int Aging. 2007;2(4):695–703. doi: 10.2147/cia.s1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tombaugh TM, McIntyre NJ. The Mini-Mental State Examination: a comprehensive review. J Am Geriatr Soc. 1992;40:922–935. doi: 10.1111/j.1532-5415.1992.tb01992.x. [DOI] [PubMed] [Google Scholar]
- 24.Gitlin LN, Winter L, Dennis MP, Hodgson N, Hauck WW. Targeting and managing behavioral symptoms in individuals with dementia: a randomized trial of a nonpharmacological intervention. J Am Geriatr Soc. 2010;58(8):1465–1474. doi: 10.1111/j.1532-5415.2010.02971.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logsdon RG, Teri L, Weiner MF, et al. Assessment of agitation in Alzheimer’s disease: The agitated behavior in dementia scale. JAGS. 1999;47:1354–1358. doi: 10.1111/j.1532-5415.1999.tb07439.x. [DOI] [PubMed] [Google Scholar]
- 26.Teri L, Truax P, Logsdon R, et al. Assessment of behavioral problems in dementia: The Revised Memory and Behavior Problems Checklist (RMBPC) Psychol Aging. 1992;7:622–631. doi: 10.1037//0882-7974.7.4.622. [DOI] [PubMed] [Google Scholar]
- 27.Perneczky R, Wagenpfeil S, Komossa K, Grimmer T, Diehl J, Kurl A. Mapping scores onto stages: Mini-Mental State Examination and Clinical Dementia Rating. Amer J Geriatr Psychiatry. 2006;14(2):139–144. doi: 10.1097/01.JGP.0000192478.82189.a8. [DOI] [PubMed] [Google Scholar]
- 28.Schulz R, Mendelsohn AB, Haley WE, Mahoney D, Allen RS, Zhang S, Thompson L, Belle SH. Resources for Enhancing Alzheimer's Caregiver Health Investigators. End-of-life care and the effects of bereavement on family caregivers of persons with dementia. N Engl J Med 13. 2003;349(20):1936–1942. doi: 10.1056/NEJMsa035373. [DOI] [PubMed] [Google Scholar]
- 29.Gitlin LN, Roth DL, Burgio LD, et al. Appraisals of functional dependence in individuals with dementia and associated caregiver upset: psychometric properties of a new scale and response patterns by caregiver and care recipient characteristics. J Aging Health. 2005;17(2):148–171. doi: 10.1177/0898264304274184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Proctor W, Hirdes J. Pain and cognitive status among nursing home residents. Pain Res Manag. 2001;6:119–125. doi: 10.1155/2001/978130. [DOI] [PubMed] [Google Scholar]
- 31.Scherder E, Oosterman J, Swaab D, et al. Recent developments in pain and dementia. BMJ. 2005;330:461–463. doi: 10.1136/bmj.330.7489.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chow G. Tests of Equality Between Sets of Coefficients in Two Linear Regressions Econometrica. 1960;28(3):591–605. [Google Scholar]
- 33.Benedetti F, Arduino C, Costa S, Vighetti S, Tarenzi L, Rainero I, et al. Loss of expectation-related mechanisms in Alzheimer's disease makes analgesic therapies less effective. Pain. 2006;121(1–2):133–144. doi: 10.1016/j.pain.2005.12.016. [DOI] [PubMed] [Google Scholar]
- 34.Norton MJ, Allen RS, Snow AL, Hardin JM, Burgio LD. Predictors of need-driven behaviors in nursing home residents with dementia and associated certified nursing assistant burden. Aging Ment Health. 2010;14(3):303–309. doi: 10.1080/13607860903167879. [DOI] [PubMed] [Google Scholar]
- 35.Kolanowski AM. An overview of the needs driven dementia compromised behavior model. J Gerontol Nurs. 1999;25:7–9. doi: 10.3928/0098-9134-19990901-05. [DOI] [PubMed] [Google Scholar]
- 36.Craig KD, Versloot J, Goubert L, Vervoort T, Crombez G. Perceiving pain in others: automatic and controlled mechanisms. J Pain. 2010;11(2):101–108. doi: 10.1016/j.jpain.2009.08.008. [DOI] [PubMed] [Google Scholar]
- 37.Hadjistavropoulos T, Craig KD. A theoretical framework for understanding self report and observational measures for pain: a communications model. Behav Res Ther. 2002;40:551–570. doi: 10.1016/s0005-7967(01)00072-9. [DOI] [PubMed] [Google Scholar]
- 38.Kovach CD, Logan BR, Noonan PE, Schlidt AM, Smerz J, Simpson M, Wells T. Effects of the serial trial intervention on discomfort and behavior of nursing home residents with dementia. Am J Alz Dis and related dementias. 2006;21(3):147–155. doi: 10.1177/1533317506288949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Horgas AL, Elliott AF, Marsiske M. Pain Assessment in Persons with Dementia: Relationship Between Self-Report and Behavioral Observation. JAGS. 2009;57(1):126–132. doi: 10.1111/j.1532-5415.2008.02071.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sprangers MA, Schwartz CE. Integrating response shift into health-related quality of life research: a theoretical model. Social Science & Medicine. 1999;48(11):1507–1515. doi: 10.1016/s0277-9536(99)00045-3. [DOI] [PubMed] [Google Scholar]
- 41.Bradford A, Shrestha S, Snow AL, Stanley MA, Wilson N, Hersch G, Kunik M. Managing pain to prevent aggression in people with dementia: a nonpharmacologic intervention. Am J Alzheimers Dis Other Demen 27. 2012;2:41–47. doi: 10.1177/1533317512439795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Herr K, Bursch H, Ersek M, Miller LL, Swafford KJ. Use of pain-behavioral assessment tools in the nursing home: expert consensus recommendations for practice. Gerontol Nurs. 2010;36(3):18–29. doi: 10.3928/00989134-20100108-04. [DOI] [PubMed] [Google Scholar]
- 43.Zwakhalen SM, Hamers JP, Abu-Saad HH, Berger MP. Pain in elderly people with severe dementia: a systematic review of behavioral pain assessment tools. BMC Geriatr. 2006;6:3. doi: 10.1186/1471-2318-6-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ahn H, Horgas A. The relationship between pain and disruptive behaviors in nursing home resident with dementia. BMC Geriatr. 2013;13(14):1471–1476. doi: 10.1186/1471-2318-13-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ersek M, Polissar N, Neradilek MB. Development of a composite pain measure for persons with advanced dementia: exploratory analyses in self-reporting nursing home residents. J Pain Symptom Manage. 2011;43(3):566–579. doi: 10.1016/j.jpainsymman.2010.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Morgan RO, Sail KR, Snow AL, Davila JA, Fouladi NN, Kunik ME. Modeling Causes of Aggressive Behavior in Patients With Dementia. Pain Med. 2010;11(3):395–404. doi: 10.1093/geront/gns129. [DOI] [PubMed] [Google Scholar]