Abstract
Missense variants in the BRCA2 gene are routinely detected during clinical screening for pathogenic mutations in patients with a family history of breast and ovarian cancer. These subtle changes frequently remain of unknown clinical significance because of the lack of genetic information that may help establish a direct correlation with cancer predisposition. Therefore alternative ways of predicting the pathogenicity of these variants are urgently needed. Since BRCA2 is a protein involved in important cellular mechanisms such as DNA repair, replication and cell cycle control, functional assays have been developed that exploit these cellular activities to explore the impact of the variants on protein function. In this review we summarize assays developed and currently utilized for studying missense variants in BRCA2. We specifically depict details of each assay, including VUS analyzed, and describe a validation set of (genetically) proven pathogenic and neutral missense variants to serve as a golden standard for the validation of each assay. Guidelines are proposed to enable implementation of laboratory-based methods to assess the impact of the variant on cancer risk.
Keywords: Variants of uncertain significance, VUS, BRCA2, Genetic testing, Functional analysis, Breast cancer, Ovarian cancer
Genetic epidemiology of BRCA2
Germline mutations in the BRCA2 tumor suppressor gene (MIM# 600185) confer predisposition to breast and ovarian cancer and increase the risk for several other cancer types including male breast cancer, pancreatic cancer, and prostate cancer (van Asperen et al., 2005; Easton et al., 2007). In addition, hypomorphic mutations in BRCA2 are involved in the cancer predisposition syndrome Fanconi Anemia (FA), leading to other types of tumors such as acute myeloid leukemia (Howlett et al., 2002).
BRCA2 mutations are rare in the population and account for less than 10% of familial breast cancer cases. Inactivating mutations in this gene confer an average cumulative breast cancer and ovarian cancer risk by age 80 of 51% and 12% respectively (Antoniou et al., 2008). Individuals carrying pathogenic BRCA2 mutations can benefit from risk assessment and management strategies including enhanced cancer surveillance and/or prophylactic mastectomy and oophorectomy. In addition, mutation carriers diagnosed with breast or ovarian cancer may benefit from therapies such as platinum agents or PARP inhibitors that selectively target BRCA2 related tumors (Evers et al., 2008; Fong et al., 2009; Audeh et al., 2010; Tutt et al., 2010; Gelmon et al., 2011; Sandhu et al., 2013).
While BRCA2 mutations that result in truncation or inactivation of the protein are classified as pathogenic, genetic screening has also identified many variants of uncertain significance (VUS), i.e. missense and intronic variants or small insertions/ deletions, for which the impact on protein function and therefore the clinical significance is unknown. Results from the Breast Cancer Information Core Database (http://research.nhgri.nih.gov/bic/) (Szabo et al., 2000) and the Evidence-based Network for the Interpretation of Germline Mutant Alleles (ENIGMA)(Spurdle et al., 2012), indicate that over 1,600 unique BRCA2 missense VUS have been identified to date. The inclusion of intronic and small in-frame alterations places the number of unique variants at more than 2,000. Since it is unknown whether these variants are associated with an increased risk of cancer, the clinical management of carriers of VUS is complicated and predictive testing is not offered to family relatives.
Therefore, there is a strong demand for reliable tests to rapidly assess the clinical significance of VUS, providing carriers of these variants with the necessary information to make an informed clinical decision and refining cancer treatment by personalized therapy strategies.
Evaluation of the clinical relevance of VUS has been significantly improved by the development of a posterior probability model for BRCA1 (MIM# 113705) and BRCA2 variants introduced by an International Agency for Research on Cancer (IARC) Working Group (Plon et al., 2008). This method combines prior probabilities of causality derived from an evolutionary sequence conservation model (Align-GVGD) (Tavtigian et al., 2008) with likelihoods of causality derived from measures of association between the VUS and cancer (Goldgar et al., 2004); (Lindor et al., 2012). The latter, known as multifactorial likelihood approach, includes family-based evidence (personal and family history of cancer and co-segregation of variants with cancer in families) and individual evidence (histopathological tumor features and effects on RNA splicing). This method allows to calculate the probability that a variant in BRCA1 or BRCA2 presents the features of known pathogenic mutations (Goldgar et al., 2008; Plon et al., 2008; Tavtigian et al., 2008; Lindor et al., 2012; Vallee et al., 2012). Accordingly to this model Class 1 (posterior probability < 0.001) and Class 2 (0.001 <posterior probability < 0.049) VUS are non-pathogenic and likely non-pathogenic VUS. Class 3 (0.05 <posterior probability < 0.949) VUS remain unclassified because of lack of sufficient family information for classification. Class 4 (0.95 < posterior probability < 0.99) and Class 5 (posterior probability > 0.99) VUS are likely pathogenic and pathogenic, respectively.
The multifactorial approach is most powerful when there are sufficient numbers of families with a given variant for analysis. The ENIGMA consortium was initiated in 2009 to facilitate pooling of genetic, clinical and histopathological information from a worldwide network of laboratories and hospitals (Spurdle et al., 2012). However, because the majority of unique VUS are very rare (1/10,000 to 1/100,000; http://research.nhgri.nih.gov/bic/(Szabo et al., 2000)), family-based analyses often lack statistical power to accurately predict contribution to cancer susceptibility. As a result, the use of in vitro assays that evaluate the consequences of a given variant on the structure and function of the protein is an essential alternative approach to predict VUS pathogenicity. Such assays have been developed for BRCA1 and BRCA2 and those available for BRCA1 have recently been reviewed by Millot and authors (Millot et al., 2012). The goal of this review is primarily to describe and discuss the functional assays available for BRCA2. Furthermore, the manuscript discusses the promising steps that have been taken towards the translation of the functional outcome into a probability of pathogenicity associated with these variants.
BRCA2 architecture and interacting proteins
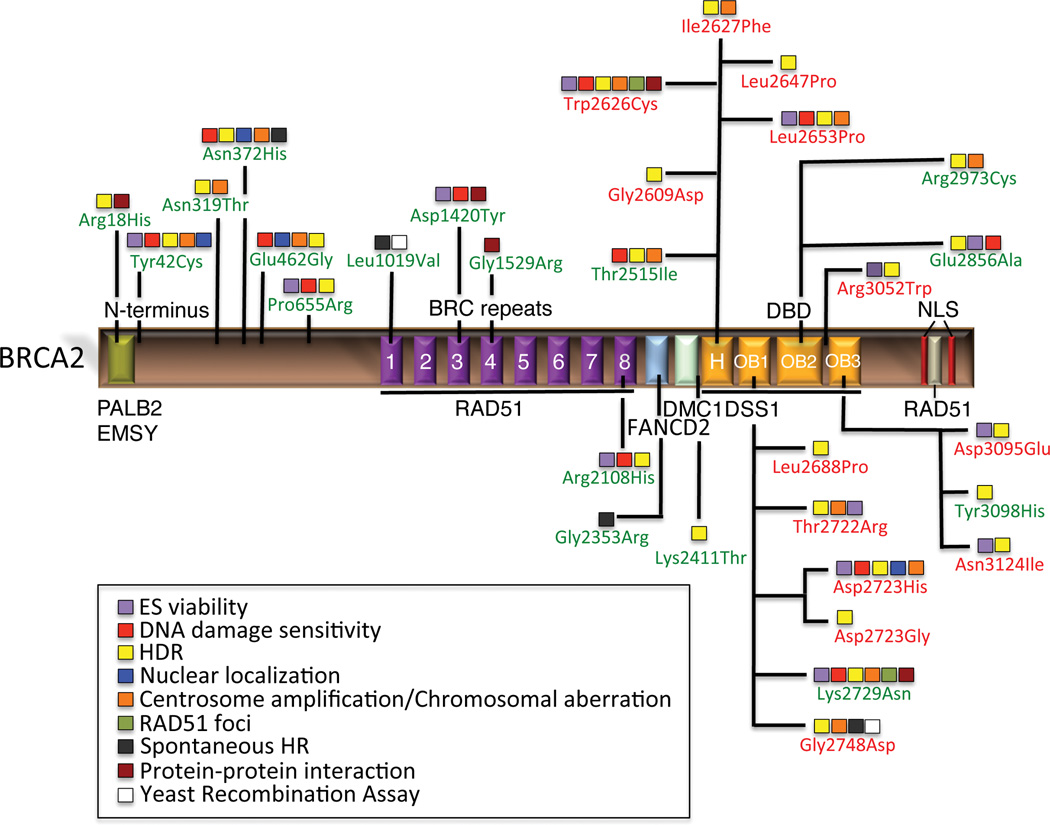
BRCA2 has 26 coding exons that encode a 3418 amino acids protein. The protein comprises a multi-domain structure (Figure 1) with limited overall sequence homology to any known protein (Lo et al., 2003). Sequencing studies identified full or partial homologous proteins to human BRCA2 in various eukaryotic organisms, from plants to fungi, but none in yeast or bacteria (Lo et al., 2003). These BRCA2-like proteins differ greatly in size but contain at least a BRC repeat, a DNA binding domain (DBD) and a nuclear localization signal (NLS). Based on these features, human BRCA2 is generally divided into three regions: the N-terminus; the BRC repeat region containing also one nuclear export signal (NES); and the C-terminal region containing the NLS, another NES and the DBD.
Figure 1.
Landscape of classified BRCA2 variants evaluated by functional assays. Schematic representation of BRCA2 protein and functional domains showing the approximate location of Class 1, 4 and 5 missense variants that have been analyzed in one or more functional assays and classified by the posterior probability model (Plon et al., 2008; Lindor et al., 2012). Class 1 or neutral variants are shown in green; Class 4/5 or pathogenic and likely pathogenic mutations are shown in red. H represents the helical domain; OB represents the oligonucleotide/oligosaccharide-binding fold; NLS depicts the nuclear localization signals.
The N-terminus of BRCA2 (residues 10–40) interacts with PALB2/FANCN (Partner and Localizer of BRCA2, Supp. Figure S1A) (MIM# 610355), that physically links BRCA1 and BRCA2 (Sy et al., 2009; Zhang et al., 2009a, 2009b) and is critical for maintenance of the DNA repair function of these proteins (Xia et al., 2006; Oliver et al., 2009; Tischkowitz and Xia, 2010; Bowman-Colin et al., 2013; Huo et al., 2013). The same region has also been implicated in binding to EMSY (MIM# 608574) and HP1 (MIM# 259900) proteins that are involved in chromatin remodeling (Hughes-Davies et al., 2003). In addition, the region encoded by exon 3 (residue 24–105) has been proposed to be implicated in transcriptional activation (Milner et al., 1997).
Residues 200–600 have been shown to interact with centrosome and midbody associated complexes CEP55-TSG101 and CEP55-ALIX that are required for completion of abscission and cytokinesis (Mondal et al., 2012).
The central region of BRCA2 (residues 900–2000) contains eight interspersed BRC repeats of about 35 residues (Bork et al., 1996), through which BRCA2 binds to RAD51 (MIM# 179617) (Supp. Figure S1B) (Chen et al., 1998), the recombinase that mediates strand invasion to process homologous recombination (HR) in humans. This series of repeats has been recently shown to work in two groups (BRC1-4 and BRC5-8) that display unique functional characteristics to ultimately facilitate loading of RAD51 onto single stranded DNA (ssDNA) at sites of DNA damage (Carreira et al., 2009; Carreira and Kowalczykowski, 2011). In the context of HR, the main function of BRCA2 is to mediate this loading (Yuan et al., 1999). Biochemical assays have shown that the purified full length BRCA2 protein binds about six molecules of RAD51, targets RAD51 loading onto ssDNA leading to displacement of RPA, and promotes the DNA repair activity of RAD51 (Jensen et al., 2010; Liu et al., 2010; Thorslund et al., 2010).
This region also harbors one NES (residues 1383–1393) necessary for centrosome localization of BRCA2 (Han et al., 2008). Downstream the BRC repeats there is a DMC1 (MIM# 602721) binding site implying an involvement of BRCA2 in meiotic recombination (Thorslund et al., 2007).
Apart from the PALB2/FANCN binding site in the N-terminus, BRCA2 has been shown to interact with two other FA proteins. FANCG (MIM# 602956) mapped in two regions: 499–994 and 2350–2545. FANCD2 (MIM# 613984) also binds this last region (Hussain et al., 2003; Hussain et al., 2004).
The C-terminal region (amino-acids 2459–3190, Supp. Figure S1C) contains a DBD. This domain is the most conserved portion of BRCA2 across metazoans, plants and fungal orthologs (Holloman, 2011). It is composed of a helical domain, three oligonucleotide binding (OB) folds that are ssDNA-binding modules and a tower domain (TD) that protrudes from OB2 and has been suggested to bind dsDNA (Supp. Figure S1C). The DBD also binds to DSS1 (MIM# 601285) (deleted in split-hand/split foot syndrome, Supp. Figure S1C), a protein involved in ubiquitin dependent protein turnover (Funakoshi et al., 2004; Sone et al., 2004) and possibly in stabilizing BRCA2 (Yang et al., 2002; Josse et al., 2006; Li et al., 2006). Recently it was shown that the nuclear localization of BRCA2 is controlled by DSS1 interaction with BRCA2 through a NES present at that position (Jeyasekharan et al., 2013). In addition, the C-terminus of BRCA2 contains another RAD51-binding site (residues 3265–3330), that is phosphorylated by cyclin dependent kinase 1 (CDK1 (MIM# 116940)). CDK1 regulates BRCA2 interaction with RAD51 filaments (Davies and Pellegrini, 2007; Esashi et al., 2007), and is important for the entry into mitosis (Ayoub et al., 2009). More recently, this phosphorylation site has been linked to inhibition of MRE11 (MIM# 600814) -dependent degradation of DNA strands at stalled DNA replication forks during S-phase (Schlacher et al., 2011). The C-terminus of BRCA2 also contains two functional NLS (residue 3263–3269 and 3381–3385) essential for the translocation of BRCA2 to the nucleus (Spain et al., 1999). Additional interacting partners of BRCA2 have been identified, supporting the notion that BRCA2 is a multifunctional protein (Gudmundsdottir and Ashworth, 2006; Roy et al., 2012).
BRCA2 functions
A role for the BRCA2 protein in DNA repair and genome stability maintenance was first indicated by the hypersensitivity of BRCA2-deficient cells to DNA damaging agents (Sharan and Bradley, 1997; Patel et al., 1998). Specifically, these cells showed inability to repair DNA double-strand breaks (DSBs) by HR (Moynahan et al., 2001). The HR repair mechanism uses sister chromatids as templates for the accurate repair of replication-associated DSBs during S and G2 phases of the cell cycle. While other mechanisms of DSB repair, including non-homologous-end-joining (NHEJ), are error-prone and result in translocations and deletions, the HR pathway is error-free and contributes to the maintenance of genome stability.
BRCA2 inactivation leads to micronuclei and centrosome amplification in different cell lines (Tutt et al., 1999; Kraakman-van der Zwet et al., 2002), suggesting a role for BRCA2 in the control of centrosome duplication and/or spindle-pole function (Tutt et al., 1999). Moreover, the interaction of BRCA2 with BRAF35 (MIM# 164757) and chromatin during early phases of mitotic chromosome condensation suggests involvement of BRCA2 in regulation of cell cycle progression (Marmorstein et al., 2001). In addition, BRCA2 deficient MEFs exhibit delayed cytokinesis, an abnormal number of nuclei, and defective furrow formation suggesting a regulatory role for the protein in the cytokinesis process (Daniels et al., 2004). Indeed, BRCA2 heterozygosity has been associated with delayed cytokinesis in primary human fibroblasts (Jonsdottir et al., 2009) and BRCA2 has been shown to be a component and regulator of midbody structure and function (Mondal et al., 2012).
The role of BRCA2 in the maintenance of genome integrity is also reflected by involvement in FA. FA is a rare disease characterized by the hypersensitivity to DNA cross-linking agents like Mitomycin C (MMC). Both FA and hereditary breast and/or ovarian cancer (HBOC) are inherited syndromes whose pathogeneses stem from defects in DNA repair. FA patients of the complementation group D1 carry biallelic mutations in BRCA2 (Howlett et al., 2002). At the molecular level, these disorders are fundamentally related because products of the FA and BRCA genes operate in concert to repair specific types of DNA damage, in particular, inter-strand crosslinks. Furthermore, the interaction with FA proteins and RAD51 is necessary for BRCA2 role in protecting nascent DNA strands from degradation at stalled DNA replication forks (Schlacher et al., 2011; Schlacher et al., 2012). Interestingly, both the role of BRCA2 in the protection of replication forks and regulation of midbody structure in cytokinesis appear to be independent of BRCA2 HR function.
BRCA2 behaves as a tumor suppressor gene with functional inactivation of BRCA2 associated with tumorigenesis, often in combination with loss of other DNA damage response (DDR) mediators such as TP53 (MIM# 191170). However, the evidence in support of the loss of the wild-type allele in BRCA2 related tumors is inconsistent (Clarke et al., 2006; Spearman et al., 2008; Walsh et al., 2008; Brozek et al., 2009).
BRCA2 deficient tumors show gross chromosomal rearrangements and display extensive chromosomal instability (van Beers et al., 2005; Roy et al., 2012). The absence of a functional HR pathway in these tumors has been exploited for chemotherapy treatment with platinum salts (carboplatin and cisplatin) that promote interstrand crosslinks and DNA breaks leading to lethal levels of genomic aberrations and chromosomal rearrangements. Similarly, PARP inhibitors, that induce single and double strand DNA breaks (Bryant et al., 2005; Farmer et al., 2005) and promote error prone repair of DNA breaks and crosslinks in the absence of efficient HR repair, are being evaluated as targeted therapies for BRCA1 and BRCA2 deficient tumors (Lord and Ashworth, 2012).
BRCA2 functional assays
Based on the presence of several protein domains associated with different functions of the BRCA2 protein, distinct in-vitro assays have been developed to analyze the effect of variants on BRCA2 function in a qualitative or quantitative way. As some functions attributed to BRCA2 may not necessarily influence cancer risk, the sensitivity (percentage pathogenic variants that is correctly classified) and specificity (percentage of non-pathogenic variants that is correctly classified) of each assay should be carefully evaluated against a series of pathogenic and non-pathogenic variants that have been classified using clinical genetic data (Table 1). Here we describe the characteristics of functional assays established for BRCA2.
Table 1.
Validation panel
| HGVS: protein level |
HGVS: DNA level♯ |
BIC: DNA level# |
Functional domain/interacting proteins¥ |
IARC Class* | References≠ |
|---|---|---|---|---|---|
| p.Arg18His | c.53G>A | 281G>A | PALB2 interaction | 1 | Spurdle et al., 2008 |
| p.Tyr42Cys | c.125A>G | 353A>G | PALB2 interaction | 1 | bTurner et al., 1999; Spitzer et al., 2000; bEdwards SM et al., 2003; bBergthorsson et al. 2001; Goldgar et al., 2004; Wu et al., 2005; Salazar et al., 2006; ¥Chenevix-Trench et al., 2006; Farrugia et al., 2008; Gomez-Garcia et al., 2009 ¥Spearman et al., 2008; Lindor et al., 2012 |
| p.Asn56Thr | c.167A>C | 395A>C | / | 1 | Easton et al., 2007; Lindor et al., 2012 |
| p.Ala75Pro | c 223G>C | 451G>C | / | 1 | Diez et al., 2003; Easton et al., 2007; Lindor et al., 2012 |
| pPro168Thr | c.502C>A | 730C>A | / | 1 | Easton et al., 2007; Lindor et al., 2012 |
| p.Asn319Thr | c.956A>C | 1184A>C | CEP55-TSG101 and CEP55-ALIX interaction | 1 | Farrugia al., 2008; Lindor et al., 2012 |
| p.Ser326Arg | c.978C>A | 1206C>A | CEP55-TSG101 and CEP55-ALIX interaction | 1 | ¥Spearman et al., 2008;; Edwards SM et al., 2003; Lindor et al., 2012 |
| p.Asn372His | c.1114C>A | 1342C>A | CEP55-TSG101 and CEP55-ALIX interaction | 1 | bHealey et al., 2000; bEdwards SM et al., 2003; bHadjisavvas A et al., 2003; Diez et al., 2003; Wu et al., 2005; Johnson et al., 2007; Farrugia et al., 2008; Lindor et al., 2012 |
| p.Pro375Ser | c.1123C>T | 1351C>T | CEP55-TSG101 and CEP55-ALIX interaction | 1 | Easton et al., 2007; Lindor et al., 2012 |
| p.Ser384Phe | c.1151C>T | 1379C>T | CEP55-TSG101 and CEP55-ALIX interaction | 1 | Diez et al., 2003; Wappenschmidt et al., 2005 ¥Chenevix-Trench et al., 2006; Salazar et al., 2006; ¥Spearman et al., 2008; Lindor et al., 2012 |
| p.Glu462Gly | c.1385A>G | 1613A>G | CEP55-TSG101 and CEP55-ALIX interaction | 1 | Wu et al 2005; Easton et al., 2007; Farrugia al., 2008; Mohammadi et al., 2009; Gomez-Garcia et al., 2009; Lindor et al., 2012 |
| p.Lys513Arg | c.1538A>G | 1766A>G | CEP55-TSG101 and CEP55-ALIX interaction | 1 | Easton et al., 2007; Spurdle et al., 2008; Lindor et al., 2012 |
| p.Cys554Trp | c.1662T>G | 1890T>G | CEP55-TSG101 and CEP55-ALIX interaction | 1 | Easton et al., 2007; Lindor et al., 2012 |
| p.Thr582Pro | c.1744A>C | 1972A>C | CEP55-TSG101and CEP55-ALIX interaction | 1 | Wagner et al., 1999; Easton et al., 2007; Lindor et al., 2012 |
| pGly602Arg | c.1804G>A | 2032G>A | / | 1 | Easton et al., (2007); Lindor et al., 2012 |
| p.Thr630Ile | c.1889C>T | 2117C>T | / | 1 | Easton et al., (2007); Lindor et al., 2012 |
| p.Pro655Arg | c.1964C>G | 2192C>G | / | 1 | Goldgar et al., 2004; ¥Spearman et al., 2008; Lindor et al., 2012 |
| p.Asp806His | c.2416G>C | 2644G>C | / | 1 | Easton et al., 2007; Lindor et al., 2012 |
| p.Val894Ile | c.2680G>A | 2908G>A | / | 1 | Easton et al., 2007; Lindor et al., 2012 |
| p.Leu929Ser | c.2786T>C | 3014T>C | / | 1 | ¥Spearman et al, 2008; Lindor et al., 2012 |
| p.Asp935Asn | c.2803G>A | 3031G>A | / | 1 | Wagner et al., 1999; Edwards et al., 2003; Salazar et al., 2006; Spurdle et al., 2008; Lindor et al., 2012 |
| p.Asn987Ile | c.2960A>T | 3188A>T | / | 1 | ¥Spearman et al., 2008; Lindor et al., 2012 |
| p.Leu1019Val | c.3055C>G | 3283C>G | BRC1 | 1 | Edwards et al., 2003; Easton et al., 2007; ¥Balia et al., 2011; Lindor et al., 2012 |
| p.Asn1102Tyr | c.3304A>T | 3532A>T | / | 1 | Easton et al., 2007; Lindor et al., 2012 |
| pSer1172Leu | c.3515C>T | 3743C>T | / | 1 | Easton et al., 2007; Spurdle et al., 2008; ¥Spearman et al., 2008; Lindor et al., 2012 |
| p.Arg1190Trp | c.3568C>T | 3796C>T | / | 1 | Easton et al., 2007; Lindor et al., 2012 |
| p.Gly1194Asp | c.3581G>A | 3809G>A | / | 1 | Easton et al., 2007; Lindor et al., 2012 |
| p.Asn1228Asp | c.3682A>G | 3910A>G | BRC2 | 1 | Easton et al., 2007; Lindor et al., 2012 |
| p.Cys1265Ser | c.3793T>A | 4021T>A | / | 1 | ¥Chenevix-Trench et al, 2006; Lindor et al., 2012 |
| p.Asp1280Val | c.3839A>T | 4067A>T | / | 1 | Easton et al., 2007; Lindor et al., 2012 |
| p.Val1306Ile | c.3916G>A | 4144G>A | / | 1 | Easton et al., 2007; Lindor et al., 2012 |
| p.Ile1349Thr | c.4046T>C | 4274T>C | / | 1 | Easton et al., 2007; Lindor et al., 2012 |
| p.Thr1354Met | c.4061C>T | 4289C>T | / | 1 | Easton et al., 2007; Lindor et al., 2012 |
| p.Cys1365Tyr | c.4094G>A | 4322G>A | / | 1 | Easton et al., 2007; Lindor et al., 2012 |
| p.Gln1396Arg | c.4187A>G | 4415A>G | / | 1 | Easton et al., 2007; Lindor et al., 2012 |
| p.Asp1420Tyr | c.4258G>T | 4486G>T | BRC3 | 1 | Wagner et al., 1999; Spitzer et al., 2000; Edwards SM et al., 2003; bScott et al., 2003; Deffenbaugh et al., 2004; ¥Spearman et al., 2008; Lindor et al., 2012 |
| p.Phe1524Val | c.4570T>G | 4798T>G | BRC4 | 1 | Easton et al., 2007; Lindor et al., 2012 |
| p.Gly1529Arg | c.4585G>A | 4813G>A | BRC4 | 1 | Easton et al., 2007; Lindor et al., 2012 |
| p.Lys1690Asn | c.5070A>C | 5298A>C | BRC5 | 1 | Easton et al., 2007; Lindor et al., 2012 |
| p.Ser1733Phe | c.5198C>T | 5426C>T | / | 1 | Easton et al., 2007; Lindor et al., 2012 |
| p.Gly1771Asp | c.5312G>A | 5540G>A | / | 1 | Wagner et al., 1999; Edwards SM et al., 2003; Easton et al., 2007; Lindor et al., 2012 |
| p.Pro1819Ser | c.5455C>T | 5683C>T | / | 1 | Easton et al., 2007, ¥Spearman et al., 2008; Lindor et al., 2012 |
| p.Leu1904Val | c.5710C>G | 5938C>G | / | 1 | Easton et al., 2007; ¥Spearman et al., 2008; Lindor et al., 2012 |
| p.Thr1915Met | c.5744C>T | 5972C>T | / | NA | Wagner et al. (1999); Spitzer et al. (2000); Diez et al. (2003); Johnson et al. (2007) |
| p.His1918Tyr | c.5752C>T | 5980C>T | / | 1 | Easton et al., 2007; Lindor et al., 2012 |
| pIle1929Val | c.5785A>G | 6013A>G | / | 1 | Easton et al., 2007; Lindor et al., 2012 |
| p.Arg2034Cys | c.6100C>T | 6328C>T | / | 1 | Edwards SM et al., 2003; Diez et al., 2003; Deffenbaugh et al., 2004; ¥Spearman et al., 2008; Caputo et al., 2011; Salazar et al., 2006; Johnson et al., 2007; Lindor et al., 2012 |
| p.Asn2048Ile | c.6143A>T | 6371A>T | / | 1 | Easton et al., 2007; Lindor et al., 2012 |
| p.His2074Asn | c.6220C>A | 6448C>A | BRC8 | 1 | Wagner et al., 1999; Easton et al., 2007; Lindor et al., 2012 |
| p.Arg2108His | c.6323G>A | 6551G>A | / | 1 | Diez et al., 2003; Easton et al., 2007; Lindor et al., 2012 |
| p.Asn2113Ser | c.6338A>G | 6566A>G | / | 1 | Easton et al., 2007; Lindor et al., 2012 |
| p.Thr2250Ala | c.6748A>G | 6976A>G | / | 1 | Easton et al., 2007; Lindor et al., 2012 |
| p.Ile2285Val | c.6853A>G | 7081A>G | / | 1 | Easton et al., 2007; ¥Spearman et al., 2008; Lindor et al., 2012 |
| p.Asp2312Val | c.6935A>T | 7163A>T | / | 1 | Easton et al., 2007; Lindor et al., 2012 |
| p.Gly2353Arg | c.7057G>C | 7285G>C | FANCD2 interaction | 1 | ¥Balia et al., 2011; Lindor et al., 2012 |
| p.Gln2384Lys | c.7150C>A | 7378C>A | FANCD2 interaction | 1 | Easton et al., 2007; Lindor et al., 2012 |
| p.Leu2396Phe | c.7188G>T | 7416G>T | FANCD2 interaction | 1 | Easton et al., 2007; Lindor et al., 2012 |
| p.Lys2411Thr | c.7232A>C | 7460A>C | FANCD2 interaction | 1 | Easton et al., 2007; Lindor et al., 2012 |
| p.Asn2436Ile | c.7307A>T | 7535A>T | FANCD2 interaction | 1 | Easton et al., 2007; Lindor et al., 2012 |
| p.Ala2466Val | c.7397C>T | 7625C>T | FANCD2 interaction | NA | Wagner et al., 1999; Freedman et al., 2004 |
| p. Lys2472Thr | c.7415A>C | 7643A>C | HD/FANCD2 interaction | 1 | Spurdle et al., 2008; Lindor et al., 2012 |
| p.Thr2515Ile | c.7544C>T | 7772C>T | HD | 1 | Wagner et al., 1999; Diez et al., 2003; Campos et al., 2003; Wu et al 2005; Spurdle et al., 2008; Lindor et al., 2012 |
| p.Gly2609Asp | c.7826G>A | 8054G>A | HD | 4 | Lindor et al., 2012; Guidugli et al., 2013 |
| p.Trp2626Cys | c.7878G>C | 8106G>C | HD | 5 | Easton et al., 2007; Lindor et al., 2012 |
| p.Ile2627Phe | c.7879A>T | 8107A>T | HD | 5 | Spitzer et al., 2000; Easton et al., 2007; Farrugia et al 2008 Lindor et al., 2012 |
| p.Leu2647Pro | c.7940T>C | 8168T>C | HD | 4 | Farrugia al., 2008; Lindor et al., 2012 |
| p.Leu2653Pro | c.7958T>C | 8186T>C | HD | 5 | Easton et al., 2007; Farrugia al., 2008; Lindor et al., 2012 |
| p.Leu2688Pro | c.8063T>C | 8291T>C | OB1 | 4 | Guidugli et al., 2013; Lindor et al., 2012 |
| p.Ala2717Ser | c.8149G>T | 8377G>T | OB1 | 1 | Edwards SM et al., 2003; Diez et al., 2003; Salazar et al., 2006; Spurdle et al., 2008; Lindor et al., 2012 |
| p.Thr2722Arg | c.8165C>G | 8393C>G | OB1 | 5 | Easton et al., 2007; Farrugia et al 2008; Lindor et al., 2012 |
| p.Asp2723His | c.8167G>C | 8395G>C | OB1 | 5 | Goldgar et al. 2004; Wu et al 2005; ¥Chenevix-Trench et al, 2006; Farrugia et al., 2008; Lindor et al., 2012 |
| ap.Asp2723G | c.8168A>G | 8396A>G | OB1 | 5 | Easton et al., 2007; Farrugia et al 2008; Walker et al., 2010; Lindor et al., 2012 |
| p.Lys2729Asn | c.8187G>T | 8415G>T | OB1 | 1 | Easton et al., 2007; Farrugia et al 2008; Lindor et al., 2012 |
| p.Gly2748Asp | c.8243G>A | 8471G>A | OB1 | 5 | Easton et al., 2007; Farrugia et al 2008; Lindor et al., 2012 |
| p.Arg2842His | c.8525G>A | 8753G>A | Tα | 1 | Easton et al., 2007; Lindor et al., 2012 |
| p.Glu2856Ala | c.8567A>C | 8795A>C | Tα | 1 | Spitzer et al., 2000; Edwards SM et al., 2003; ¥Chenevix-Trench et al., 2006; Johnson et al., 2007; ¥Spearman et al., 2008; Lindor et al., 2012 |
| p.Arg2888Cys | c.8662C>T | 8890C>T | Tα | 1 | Easton et al., 2007; Gomez-Garcia et al., 2009; Lindor et al., 2012 |
| p.Ala2951Thr | c.8851G>A | 9079G>A | Tα | NA | Wagner et al., 1999; Spitzer et al., 2000; Diez et al., 2003; Deffenbaugh et al., 2004; Bergthorsson et al. 2001; bHammet et al., 2007 |
| p.Val2969Met | c.8905G>A | 9133G>A | OB2 | 1 | Wagner et al., 1999; Easton et al., 2007; Lindor et al., 2012 |
| p.Arg2973Cys | c.8917C>T | 9145C>T | OB2 | 1 | Easton et al., 2007; Farrugia et al 2008; ¥Spearman et al., 2008; Lindor et al., 2012 |
| p.Arg3052Trp | c.9154C>T | 9382C>T | OB2 | 5 | Farrugia et al 2008; Gomez-Garcia et al., 2009; Mohammadi et al., 2009; Walker et al., 2010; Lindor et al., 2012 |
| p.Val3079Ile | c.9235G>A | 9463G>A | OB3 | 1 | Easton et al., 2007; Lindor et al., 2012 |
| p.Asp3095Glu | c.9285C>G | 9513C>G | OB3 | 4 | Easton et al., 2007; Farrugia et al 2008; Lindor et al., 2012 |
| p.Tyr3098His | c.9292T>C | 9520T>C | OB3 | 1 | Edwards SM et al., 2003; Easton et al., 2007; Lindor et al., 2012 |
| p.Asn3124Ile | c.9371A>T | 9599A>T | OB3 | 4 | Lindor et al., 2012; Guidugli et al., 2013 |
| p.Asp3170Gly | c.9509A>G | 9737A>G | OB3 | 1 | Easton et al., 2007; Lindor et al., 2012 |
| p.Cys3198Arg | c.9592T>C | 9820T>C | / | 1 | Easton et al., 2007; Lindor et al., 2012 |
| p.Thr3349Ala | c.10045A>G | 10273A>G | / | 1 | Diez et al., 2003; Easton et al., 2007; Lindor et al., 2012 |
GenBank Reference BRCA2 NM_000059.3
NA not applicable because the variant is a well-defined polymorphism* 1=Class 1 Not Pathogenic or “of No Clinical Significance (posterior probability<0.001); 2=Class 2 Likely Not Pathogenic or “of Little Clinical Significance” (0.001<posterior probability<0.049).4=Class 4 Likely Pathogenic (0.95<posterior probability<0.99); 5=Class 5 Definitely Pathogenic (posterior probability >0.99).
Asn2723Gly variant was shown to cause aberrant splicing (deletion of 163 bp from exon 18) of a small proportion of the mutant allele (Walker LC et al., 2010)
Report suggesting a classification of variants that is divergent from the classification based on the multifactorial approach.
Bold=classified Class 4/5 mutations.
/ no functional domains identified
LOH is improperly utilized as a measure of association of the variant with the disease.
Nucleotide numbering in “HGVS: DNA level” reflects cDNA numbering with +1 corresponding to the A of the ATG translation initiation codon in the reference sequence, (http://www.hgvs.org/mutnomen/).
Nucleotide numbering in “BIC: DNA level” refers to the original nomenclature for BRCA1 and BRCA2 before adoption of HGVS standards where BRCA1 +119 and BRCA2 +228 correspond to the A of the ATG translation codon in the reference sequences.
List of publications that have defined the pathological importance of the variants through the multifactorial and/or the posterior probability methods or through other genetic approaches including the frequency in cases and controls.
BRC=interspersed BRC repeats of about 35 residues in the central region of BRCA2; HD=helical domain within the DNA binding domain in the C-terminal region of BRCA2 (DBD); OB= oligonucleotide-binding folds within the DBD; Tα= tower domain which is an insertion within OB2.
Homology-directed repair (HDR) assay
This assay is based on gene conversion repair of an I-SceI induced DSB in a green fluorescent protein (DR-GFP) reporter construct in Brca2 deficient cells (Moynahan et al., 2001). Complementation of the DSB repair deficiency in Brca2 deficient cells by full-length human BRCA2 cDNA expression constructs results in reconstitution of a functional GFP gene. Quantitation of the proportion of GFP-positive cells by flow cytometry serves as a measure of homology directed repair activity. By using the reporter assay in V-C8 Brca2 deficient hamster lung fibroblast cells (XRCC11) it has been possible to measure and compare the HR activity of wild-type and mutant forms of BRCA2 (Wu et al., 2005; Farrugia et al., 2008; Guidugli et al., 2013) (Table 2). Most recently, the sensitivity and specificity of the assay for VUS in the BRCA2 DBD has been evaluated using a set of 13 known pathogenic and 18 known non-pathogenic mutations (defined by family-based genetic data). Sensitivity has been estimated at 100% (95% CI: 75.3%–100%), and specificity at 100% (95% CI: 81.5%–100%) (Guidugli et al., 2013). Therefore a statistical classifier for estimating the probability of pathogenicity of VUS using the functional results was developed. Through this probability model 18 variants for which the clinical significance was unknown were predicted as pathogenic and eight as non-pathogenic (Guidugli et al., 2013) (Table 2).
Table 2.
Analysis of VUS; Results from functional assays
| HDR assay¥ | Embryonic stem cell (ESC)-based survival assay# | Centrosome- amplification assay* |
Spontaneous
HR assay** |
Yeast recombination assay*** |
MMC survival assay± | SyVal model† | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Viability | DNA- damaging agents |
HDR | RAD51 foci |
Chromosomal aberration |
RAD51 foci |
DNA- damaging agents |
||||||||||||||
| p.Pro9Leu | + | p.Lys2729Asn | + | p.Gly25Arg | + | +/− | +/− | / | + | p.Tyr42Cys | + | p.Gly173Val | + | p.Ser286Pro | + | p.Tyr42Cys | + | p.Tyr3308Tyr | + | + |
| p.Phe12Val | + | p.Gly2748Asp | − | p.Trp31Arg♯ | − | NA | NA | NA | NA | p.Asn319Thr | + | p.Asp191Val | − | p.Met927Val | + | p.Asn372His | + | p.Pro3292Leu | + | + |
| p.Arg18His | + | p.Arg2784Gln | − | p.Trp31Cys | − | NA | NA | NA | NA | p.Asn372His | + | p.Ser286Pro | + | p.Thr1011Arg | − | p.Glu462Gly | + | p.Pro3280His | + | + |
| p.Gly25Arg | − | p.Arg2784Trp | − | p.Tyr42Cys | + | + | / | / | / | p.Glu462Gly | + | p.Asn372His | + | p.Leu1019Val | + | p.Thr1302del | − | p.Ser3291Glu | − | − |
| p.Ile27Val | + | p.Arg2787His | + | p.Pro655Arg | + | + | + | / | / | p.Thr1302del | − | p.Met927Val | + | p.Asn1878Lys | + | p.Glu1382del | − | p.Ser3291Ala | + | + |
| p.Trp31Arg | − | p.Leu2792Pro | − | p.Asn991Asp | + | + | / | / | / | p.Glu1382del | + | p.Thr1011Arg | + | p.Thr1915Met | + | p.Thr2515Ile | − | |||
| p.Trp31Cys | − | p.Gly2793Glu | − | p.Asp1420Tyr | + | + | / | / | / | p.Thr2515Ile | +/− | p.Leu1019Val | + | p.Ser2006Arg | − | p.Asp2723His | − | |||
| p.Tyr42Cys | + | p.Gly2793Arg | − | p.Arg2108His | + | + | + | / | / | p.Arg2520Gln | + | p.Asn1878Lys | − | p.Arg2108Cys | + | p.Val2908Gly | + | |||
| p.Asn319Thr | + | p.Pro2800Ser | +/− | p.Phe2406Leu | + | + | + | / | / | p.Ile2627Phe | − | p.Met1915Thr | + | p.Val3091Ile | + | |||||
| p.Asn372His | + | p.Pro2800Arg | +/− | p.Ile2490Thr | + | + | + | + | + | p.Ala2643Gly | + | p.Ser2006Arg | − | p.Gly2748Asp | − | |||||
| p.Glu462Gly | + | p.Ser2807Leu | + | p.Leu2510Pro | +/− | − | − | − | − | p.Thr2722Arg | − | p.Arg2108Cys | − | p.Ala2951Thr | + | |||||
| p.Thr1302del | + | p.Gly2812Glu | +/− | p.Trp2626Cys | +/− | − | − | − | − | p.Lys2729Asn | + | p.Gly2353Arg | − | |||||||
| p.Glu1382del | − | p.Gly2813Glu | + | p.Leu2653Pro | +/− | − | − | / | − | p.Gly2748Asp | − | p.Gly2748Asp | − | |||||||
| p.Lys2411Thr | + | p.Arg2842Leu | +/− | p.Ser2695Leu | + | + | + | / | / | p.Arg2784Trp | + | p.Ala2951Thr | + | |||||||
| p.Ser2414Leu | + | p.Arg2842Cys | − | p.Thr2722Arg | − | NA | NA | NA | NA | p.Leu2865Val | + | p.Val3091Ile | − | |||||||
| p.Leu2510Pro | − | p.Glu2856Ala | + | p.Asp2723His | − | NA | NA | NA | NA | p.Val2908Gly | + | |||||||||
| p.Thr2515Ile | +/− | p.Leu2865Val | + | p.Lys2729Asn | + | + | + | + | + | p.Arg2973Cys | + | |||||||||
| p.Arg2520Gln | + | p.Gly2901Asp | + | p.Val2728Ile | + | + | / | / | / | |||||||||||
| p.Gly2585Arg | − | p.Val2908Gly | + | p.Glu2856Ala | + | + | / | / | / | |||||||||||
| p.Pro2589His | + | p.Ala2951Thr | + | p.Gly2901Asp | + | + | / | / | / | |||||||||||
| p.Gly2609Asp | − | p.Asp2965His | + | p.Ile2944Phe | + | + | + | / | / | |||||||||||
| p.Trp2626Cys | − | p.Arg2973Cys | + | p.Ser2988Gly | + | + | / | / | / | |||||||||||
| p.Ile2627Phe | − | p.Glu3002Lys | − | p.Glu3002Lys | − | NA | NA | NA | NA | |||||||||||
| p.Ala2643Gly | + | p.Glu3002Asp | +/− | p.Arg3052Trp | − | NA | NA | NA | NA | |||||||||||
| p.Leu2647Pro | − | p.Thr3013Ile | + | p.Arg3052Gln | + | +/− | / | / | / | |||||||||||
| p.Leu2653Pro | − | p.Ser2030Cys | + | p.Asp3095Glu | − | NA | NA | NA | NA | |||||||||||
| p.Leu2654Pro | − | p.Thr3033Ile | +/− | p.Asn3124Ile | − | NA | NA | NA | NA | |||||||||||
| p.Arg2659Gly | − | p.Arg3052Trp | − | |||||||||||||||||
| p.Tyr2660Asp | − | p.Pro3063Ser | + | |||||||||||||||||
| p.Asp2665Gly | + | p.Asp3073Gly | − | |||||||||||||||||
| p.Met2676Thr | + | p.Gly3076Val | − | |||||||||||||||||
| p.Asp2679Gly | + | p.Gly3076Glu | − | |||||||||||||||||
| p.Asp2679Tyr | + | p.Tyr3092Cys | + | |||||||||||||||||
| p.Leu2688Pro | − | p.Tyr3092Ser | +/− | |||||||||||||||||
| p.Thr2722Arg | - | p.Asp3095Glu | − | |||||||||||||||||
| p.Asp2723Ala | − | p.Tyr3098His | + | |||||||||||||||||
| p.Asp2723Gly | − | p.Asn3124Ile | − | |||||||||||||||||
| p.Asp2723His | − | |||||||||||||||||||
| p.Tyr2726Cys | − | |||||||||||||||||||
Kuznetsov et al., 2008; Biswas et al., 2011; Biswas et al. et al., 2012 (this assay is also applicable for the analysis of intronic variants but these are not listed here).
This missense change causes skipping of the exon 3 of BRCA2 that in turn generates an in-frame deletion of 83 amino acids essential for BRCA2 interaction with PALB2 (Biswas et al., 2012; Xia et al., 2006). Loss of exon 3 has been associated with increased breast/ovarian cancer (Nordling et al., 1998).
NA=not applicable; /=not done; (+) denotes the missense variant has no effect on BRCA2 function; (-) denotes the missense variant disrupts BRCA2 function.
Italics=variants classified as Class 1with the posterior probability method (Plon et al., 2008) or well-defined polymorphisms
Bold=variants classified as Class 4/5 with the posterior probability method (Plon et al., 2008)
Interestingly, several variants have displayed intermediate HR activity in this assay. It is not known whether intermediate function equates to intermediate risk or if the reduction in activity is sufficient to confer a high-risk of breast and ovarian cancer (see Discussion).
Homologous recombination assay in human cells
Recently, Balia and authors (Balia et al., 2011) developed an assay that determines the effect of the transient overexpression of BRCA2 VUS on restoration of a recombination reporter substrate. It utilizes a HeLa G1 cell line containing a recombination substrate with two differentially mutated hygromycin resistance (HygR) genes. An intra-chromosomal recombination event leads to the restoration of the wild-type form of HygR and cell survival in the presence of hygromycin. The frequency of intra-chromosomal recombination events is calculated as the total number of viable cells after selection with hygromycin. Wild-type BRCA2 overexpression only marginally enhances the number of viable cells after hygromycin selection. However, when a loss-of function BRCA2 variant is overexpressed, an increase in the number of viable cells is observed, indicating enhanced intra-chromosomal recombination events that result in HygR gene restoration and cell survival. Albeit the nature of the specific recombination event was not examined by the authors, Larminat et al. have previously reported that loss of BRCA2 in V-C8 cells increases intra-chromosomal recombination most likely by SSA (Larminat et al., 2002). Similarly, in the HeLa G1 cells, the expression of a loss-of function BRCA2 variant may, in a dominant-negative manner, result in an increase of recombination, thereby restoring the HygR gene. The authors selected 15 BRCA2 variants that localize in different regions of the BRCA2 protein (Table 2). The assay was calibrated using three neutral variants and one pathogenic mutation. The latter increased recombination by 3.5 fold relative to the wild-type. Eleven variants that had not previously been described or classified were also tested. Five variants did not enhance recombination and were classified as non-pathogenic. Six other variants increased recombination as much as the pathogenic mutation and were classified as “probably pathogenic”. However, the p.Gly2353Arg variant that had the strongest effect on recombination activity and was therefore classified as “probably pathogenic” has recently been shown to be a neutral variant (Class 1) (Lindor et al., 2012). This latter data suggest a reduced specificity of the assay. Importantly, this method does not directly measure a known BRCA2 function but it rather measures error-prone repair mechanisms such as SSA in the absence of functional BRCA2. Consequently, the application of this assay to evaluate the functional effect of VUS is a matter of debate.
Yeast recombination assay
This assay involves expression of human full-length BRCA2 wild-type protein in the diploid strain of Saccharomyces cerevisiae, RS112, which contains two substrates to measure HR (Spugnesi et al., 2013). The intra-chromosomal HR substrate consists of two HIS3 alleles, one with a deletion at the 3’ end and the other with a deletion at the 5’ end, sharing 400 bp of homology. An intra-chromosomal recombination event leads to HIS3 reversion. The diploid RS112 strain also contains the two alleles, ade2–40 and ade2–101, located in two homologous chromosomes that allow the measurement of inter-chromosomal recombination events. Expression of wild-type BRCA2 increased both intra- and inter-chromosomal HR in yeast and enhanced resistance to methyl methanesulfonate without affecting the number of RAD51 nuclear foci, suggesting that BRCA2 interferes with yeast DNA repair. The influence of the expression of three neutral BRCA2 variants (i.e. p.Leu1019Val, p.Thr1915Met and p.Ala2951Thr) and the known pathogenic variant (p.Gly2748Asp) on HR was evaluated. The neutral variants induced a significant increase in HR whereas p.Gly2748Asp did not. In addition, seven VUS have been tested with this assay. Five out of seven BRCA2 variants induced at least one recombination event in yeast (Table 2). These results suggest that BRCA2 missense variants that increase HR in the yeast assay are not pathogenic because they confer the same phenotype as the BRCA2 wild-type. On the other hand, variants that do not stimulate HR may be associated with an increased risk of cancer.
Centrosome-amplification assay
Based on the observation that BRCA2 inactivation leads to centrosome amplification, a cell-based functional assay that measures the influence of BRCA2 variants on centrosome number has been developed (Wu et al., 2005; Farrugia et al., 2008). Briefly, centrioles and centrosomes were visualized by immunofluorescence and enumerated in 293T or HeLa cells 72 h after transfection with GFP-tagged full-length BRCA2 cDNA (wild-type or mutant forms of BRCA2) (Wu et al., 2005). Wild-type BRCA2 and neutral variants did not influence centrosome number, whereas the c.5946delT and p.Asp2723His pathogenic mutations induced a significantly increased number of centrosomes (Wu et al., 2005).
The influence of BRCA2 on centrosome numbers was also assessed using a reconstitution approach in BRCA2 deficient V-C8 cells (Wu et al., 2005). Wild-type BRCA2 reduced the number of amplified centrosomes whereas the pathogenic p.Asp2723His mutation did not change the level of centrosome amplification (Wu et al., 2005). The sensitivity and specificity of the centrosome assay was assessed using eight classified pathogenic mutations and eight neutral variants (Farrugia et al., 2008). The assay showed a sensitivity of 85% (95% CI 47–100) since the pathogenic p.Arg3052Trp did not induce centrosome amplification. Specificity was 100% (95% CI, 63–100).
Mitomycin C (MMC) survival assay
BRCA2-null cells exhibit hypersensitivity to DNA damaging agents (Connor et al., 1997; Sharan et al., 1997; Morimatsu et al., 1998; Patel et al., 1998) and the re-introduction of a BRCA2 functional gene significantly reduces this sensitivity (Kraakman-van der Zwet et al., 2002). Based on this observation, BRCA2-deficient V-C8 cells were complemented with a series of known neutral and pathogenic BRCA2 missense changes and evaluated for survival by clonogenic survival assay following exposure to MMC (Wu et al., 2005). Wild-type BRCA2 and five neutral variants displayed 5-fold enhanced survival and 12 to 14-fold improved viability relative to control V-C8 cells, while the mutations c.5946delT and the p.Asp2723His could not rescue the sensitivity to MMC (Table 2). The influence of other DNA damaging agents such as ionizing radiation, cisplatin or ultraviolet radiation (UV) can also be tested using this approach.
Embryonic stem cell (ESC)-based functional assay
A versatile system for assessing the clinical relevance of BRCA2 VUS is represented by the mouse Embryonic Stem (ES) cell assay. The mouse ES cells contain one defective mouse Brca2 allele (mBrca2) and one functional mBrca2 allele that can be conditionally switched off. The assay is based on the observation that functional BRCA2 is essential for ES cell viability. Transfection of a BAC clone containing a wild-type human BRCA2 (hBRCA2) allele will rescue mouse Brca2 deficient ES cells (Sharan et al., 1997; Kuznetsov et al., 2008). The advantage of this system is that ES cells are not inherently prone to genomic instability and therefore represent a more stable system for studying BRCA2 function with respect to the majority of mammalian cancer cell lines. Furthermore, any type of mutation, including regulatory mutations and intronic alterations, can be introduced and subsequently assessed for influence on ES cell survival. Variants that fail to rescue the lethality of the mBrca2 deficient ES are pathogenic, variants that (partially) rescue the lethality of the mBrca2 deficient ES cells, are analyzed by a series of biological assays (e.g. drug sensitivity, HR, chromosomal instability). Briefly, assays that measure the sensitivity to chemical agents or radiation (e.g. UV and ionizing radiation) are performed using a cell viability assay such as the XTT-assay (Scudiero et al., 1988). Drug sensitivity is expressed as the percentage of surviving cells compared to untreated cells. An HR assay is performed using a DR-GFP reporter construct stably integrated in the ES cells as for the HDR assay described above, or using two gene-targeting vectors (Kuznetsov et al., 2008). In addition, the RAD51 foci assay is used to assess the ability of the cells to perform HR after induction of DSB by ionizing radiation (Kuznetsov et al., 2008). Chromosomal aberrations are scored by karyotype analysis of metaphase ES cells (Kuznetsov et al., 2008). Variants that are deficient in any of the functional assays are scored as pathogenic. The ES survival assay has been used to successfully discriminate between six known pathogenic and seven known neutral BRCA2 missense changes (Table 2 and Figure 1). In addition, 14 missense variants that map to the N-terminal PALB2-binding and the C-terminal DBD have been analyzed using this approach (Table 2) (Kuznetsov et al., 2008; Biswas et al., 2011; Biswas et al., 2012), including variants that have been described to induce RNA splice aberrations in human (Claes et al., 2004; Thomassen et al., 2006; Farrugia et al., 2008). Using this assay, aberrant splice transcripts similar to those observed in carriers were identified, supporting the value of this approach for the analysis of variants that may affect RNA splicing.
Syngeneic human cancer BRCA2 knockout cell line model (SyVal model)
Targeted introduction of variants into the p53 deficient human epithelial colorectal cancer cell line DLD1 has been described as a method for characterizing BRCA2 missense variants (Hucl et al., 2008). Targeted disruption of BRCA2 exon 11 (BRCA2 Δex11/wt) yields a hemizygous human cancer cell that was further modified to contain nucleotide changes resulting in four unclassified missense variants and one synonymous change in exon 27 of BRCA2 (Hucl et al., 2008). A clone bearing one functional copy of BRCA2 (BRCA2 wt/Δex11) was established as a wild-type control and clones containing the pathogenic BRCA2 p.Tyr3308X mutation and a biallelic disruption of the gene (BRCA2 Δex11/Δex11) were established as negative controls. RAD51 foci formation and sensitivity to MMC or etoposide was evaluated in each clone (Table 2). The BRCA2 wt/Δex11 clone, the BRCA2 synonymous mutant, and three clones with VUS, induced RAD51 foci and did not show hypersensitivity to MMC or etoposide indicating that they did not affect BRCA2 function. The BRCA2-null clone, the pathogenic mutant and VUS p.Ser3291Glu showed no induction of RAD51 foci upon DNA damage and enhanced sensitivity to MMC or etoposide, indicating a severe defect in BRCA2 function. This model provides the first human syngeneic BRCA2 model and therefore seems promising for the characterization of BRCA2 VUS.
Nuclear localization assay
The BRCA2 protein is predominantly localized within the nucleus (Spain et al., 1999) (Yano et al., 2000), but can relocalize to certain structures, such as centrosomes, during S and early M phases of the cell cycle (Nakanishi et al., 2007). To examine whether variants alter the subcellular localization of BRCA2, which might be associated with altered protein function, the influence of variants on localization of GFP-tagged BRCA2 was assessed (Wu et al., 2005). Studies in BRCA2-deficient (V-C8) and proficient cells (293T and HeLa) showed that BRCA2 protein encoded by non-pathogenic variants was localized in the nucleus. By contrast, BRCA2 protein encoded by pathogenic missense variants localized fully or partially in the cytoplasm (Wu et al., 2005). Variants in the N-terminal region of BRCA2 that showed impaired HR activity (p.Gly25Arg, p.Trp31Arg, and p.Trp31Cys) displayed proficient nuclear localization (Xia et al., 2006; Biswas et al., 2012). The mechanism by which some variants are mislocalized remains to be elucidated. As these missense changes do not disrupt the known C-terminal NLS, the mutant proteins may be retained in the cytoplasm due to protein misfolding. The development of this method as a quantitative functional assay is complicated as the amount of protein overexpression, which is variable, would affect the protein mislocalization and misfolding.
BRCA2 protein-protein interaction-based assays
As described above, the functions of BRCA2 rely on interaction with other proteins. Differences in binding affinity due to the presence of a variant in one of the binding domains of BRCA2 can be tested directly by various biochemical methods such as pull downs, co-immunoprecipitation, or yeast two-hybrid strategies. In addition, these variants can be further analyzed by other assays to pinpoint the function affected. Several studies have assessed the effect of BRCA2 variants on the interaction with PALB2 (Xia et al., 2006; Biswas et al., 2011), APRIN (Brough et al., 2012), or with midbody components (Mondal et al., 2012). A similar rationale could be used to interrogate mutants located in the DBD of BRCA2 using EMSA (Electrophoretic Mobility Shift Assay) with the purified mutant protein as described for the wild type BRCA2 (Yang et al., 2002; Jensen et al., 2010; Biswas et al., 2012). While there is strong evidence that VUS influencing the interaction with PALB2 and ssDNA are cancer-associated BRCA2 mutations (Xia et al., 2006; Biswas et al., 2012), the significance of disruption of other interactions remains to be established.
Analysis of variants that affect RNA splicing
There is strong evidence that a number of variants influence BRCA2 function and are associated with increased breast cancer risk due to induction of aberrant splicing of BRCA2. This subject is extensively addressed in a number of recent papers published by the ENIGMA splicing working group (Houdayer et al., 2012; Spurdle et al., 2012; Thomassen et al., 2012). Characterization of these variants relies on minigene-based splicing assays or analysis of RNA derived from blood or tissue samples from patients. Importantly, there are examples showing that RNA analysis is not sufficient to elucidate the clinical relevance of a subset of variants influencing consensus splice sites or forming de novo splice sites. In some cases, the absence of exonic SNPs in the patient will render RNA analysis uninformative since the allelic contribution of aberrant and normal transcripts cannot be distinguished. In other cases, albeit the nature of the aberrant transcript might be elucidated, the effect on protein function might still be unclear (e.g. if there is a stable transcript with an in-frame deletion of a whole exon or an in-frame retention of a few intronic bases). Furthermore, some missense variants may cause incomplete aberrant splicing and also influence BRCA2 function associated with the non-spliced mutant transcript (Farrugia et al., 2008; Walker et al., 2010). In these cases, assays based on genomic DNA (such as the ES cell assay) are required to assess the functional effect of the aberrant RNA splicing (Kuznetsov et al., 2008; Biswas et al., 2011; Biswas et al., 2012).
Phenotype in heterozygous carriers
Several studies have described approaches to look for differences in the DNA damage response between cells from BRCA2 heterozygous mutation carriers and healthy controls. These assays have been conducted using lymphoblastoid cells, non-immortalized lymphocytes, skin fibroblasts and breast tumor cells. Although these studies have yielded conflicting results and show large inter-individual differences, there are indications that cells derived from heterozygous BRCA2 carriers might respond differently than controls at the gene expression level (Kote-Jarai et al., 2006; Waddell et al., 2008) or show different level of chromosomal damage following exposure to ionizing radiation (Buchholz et al., 2002; Baeyens et al., 2004; Ernestos et al., 2010; Becker et al., 2012). If robust assays can be developed with high specificity and sensitivity, this approach might also be applicable for interpreting VUS pathogenicity.
Discussion
An important obstacle to improved risk assessment for breast and ovarian cancers is due to the presence of a large number of BRCA2 variants for which cancer association has not been determined. Here we provided detailed background about specific functional assays that have been developed to determine whether these variants may be associated with increased cancer risk. Although functional assays are very useful, they are technically demanding and their use is currently restricted to research laboratories. In addition it is important to highlight that there are two main caveats for the use of functional assays as a way of inferring the disease risk associated with BRCA2 variants. The first caveat resides in the multifunctional role of BRCA2, its interaction with different proteins and the lack of a comprehensive understanding of every single biological function of BRCA2. A variant with no impact on one function may lead to severe impact on another function. Therefore, a combination of functional approaches for characterizing variants is necessary. The second caveat relates to the fact that distinct BRCA2 functions may contribute differently to the tumor suppressor activity of the protein and variants that disrupts specific BRCA2 functions may or may not be relevant in terms of cancer risk. For this reason it is essential to determine the sensitivity and specificity of each assay.
Validation panel
A functional assay should be validated against a panel of variants for which the clinical significance has been determined with the posterior probability approach using direct genetic evidence (e.g. co-segregation analysis, family history, co-occurrence). This validation set, consisting of Class 4/5 (pathogenic) and Class 1 (neutral) variants will allow determination of the sensitivity and specificity of an assay (Table 1). A low-sensitivity test will identify a large number of false negatives (pathogenic variants misclassified as non-pathogenic) while a low specificity test will identify a large number of false positives (non-pathogenic variants misclassified as pathogenic). While validation using a panel of known pathogenic and neutral variants is essential, the nature of the validation panel will vary depending on whether the assay is being applied to a specific domain or to the entire protein. Assays for specific domains should be validated with known pathogenic and neutral variants in the same domain. In contrast, because the function of BRCA2 is still not completely elucidated, and depends on interactions interspersed throughout the sequence, any assay being applied to VUS throughout the protein should be validated using classified variants across the entire gene. However, the lack of known pathogenic mutations, defined by the multifactorial likelihood model, in the BRC repeats and the N-terminus of BRCA2 suggests that this approach may currently not be possible. Up to date, the HDR assay is the only functional approach that has been validated using a sufficient number of variants to establish the sensitivity and specificity of the assay. Other assays first have to be extensively validated before they can be used for clinical purpose.
Technical and practical considerations
A series of important considerations apply for almost all assays. Some of these issues were already addressed in a recent review that describes the functional assays available for variants in BRCA1 (Millot et al., 2012) and are only briefly mentioned here.
-
-
The use of proper controls in each experiment (Millot et al., 2012). At least one neutral variant (or polymorphism) and one pathogenic mutation should be included as controls in each independent experiment. These controls should map in the BRCA2 domain interrogated by the specific functional assay
-
-
Replicates of each sample (i.e. construct carrying the variant) should be included within each independent experiment and each independent experiment should be repeated at least twice (Millot et al., 2012).
-
-
Whenever assays are performed using transient transfection approaches, the transfection efficiency associated with each variant must be measured and used to normalize BRCA2 activity. In addition, since in transient transfection experiments protein levels are more variable compared to stable transfection experiments, it is essential to examine whether proteins are expressed at a similar level preferably by western blots. Ideally one should assess protein expression in the same sample being used for functional analysis (Millot et al., 2012).
-
-
When performing transient transfection experiments, plasmids should be prepared and assayed in batches to control variability in the assay caused by differences in DNA quality (Millot et al., 2012).
-
-
The use of partial BRCA2 proteins may lead to misinterpretation of the clinical relevance of certain BRCA2 variants. For instance p.Tyr42Cys encoded by exon 3 was first shown to influence transcription (Milner et al., 1997) and a fragment containing this variant has been shown to disrupt the BRCA2 interaction with RPA (Wong et al., 2003). However it was later clearly shown by genetic evidence (Goldgar et al., 2004) and the ES cell assay (Kuznetsov et al., 2008) that p.Tyr42Cys is a neutral allele with no influence on BRCA2 function. Therefore there is a clear preference for the use full-length BRCA2 proteins in assays, despite the associated problems with expression and detection of the 384 kDa protein.
-
-
Assay results should be quantified and thresholds of activity that separate pathogenic and neutral variants should be established (Millot et al., 2012). Only the HDR assay has been developed in this manner (Farrugia et al., 2008; Guidugli et al., 2013). Highly quantitative assays will be necessary to discriminate between variants that totally inactivate or only partially inactivate protein function.
-
-
A panel of different assays representative for different BRCA2 functions should be used to evaluate variants in order to minimize the risk that a specific functional effect of the protein will be overlooked. Conversely, in-vitro assays can suggest an impact of a variant on a certain function that does not necessarily translate into a defect in vivo, especially when the specific effect is compensated by other factors and/or functions.
Development and improvement of functional assays
While mouse ES cells and the BRCA2-deficient V-C8 hamster lung fibroblasts have been widely utilized for BRCA2 functional assays, the lack of an easy to manipulate, stable BRCA2 deficient human cell has hampered the development of assays in human cells. It is to be expected that improved methods for genetic manipulation of human cells, such as TAL endonucleases or RNA-guided engineering (Ding et al., 2013; Mali et al., 2013) will facilitate the study of VUS. In fact the introduction of variants into the endogenous gene as opposed to transient transfection will likely improve the reliability of functional assays. Similarly, inducible methods for expression of mutant forms of BRCA2 in BRCA2-deficient cells may improve functional assays. Additional assays might be used in the future for the functional evaluation of variants in BRCA2. For example immortalized MEFs containing one allele with an exon 27 deletion (lex1) and one allele with a deletion of part of exon 26 and whole 27 (lex2) (brca2lex1/lex2) have been developed (Donoho et al., 2003). Brca2lex1/lex2 MEFs showed mild sensitivity and hypersensitivity to MMC and γ-radiation, respectively. The influence of hypomorphic missense alterations on BRCA2 function could also be analyzed by this approach. Also, the work described by Schlacher and colleagues (Schlacher et al., 2011), elucidating the role of BRCA2 in the protection of DNA at stalled replication forks, could form the basis of an assay for evaluation of HR independent activity of BRCA2. The single-molecule fiber analysis method is in principle suitable for testing variants located at the C-terminal domain of BRCA2. Whether the method is applicable to variants in other domains of BRCA2 remains to be determined.
A complementary approach to evaluate the role of variants in tumorigenesis is to study them in genetically engineered mouse models as has recently been described for BRCA1 (Drost et al., 2011; Shakya et al., 2011). These models are invaluable for studying the effect of different BRCA2 variants on BRCA2 function, impact on tumorigenesis and therapy response. Although conditional Brca2/Trp53 mice are available (Jonkers et al., 2001; Evers and Jonkers, 2006), the effect of Brca2 variants has only rarely been studied in mouse models (Xia et al., 2006; Kuznetsov et al., 2008; Biswas et al., 2012).
The challenge of risk prediction: Intermediate function, intermediate risk?
Functional analysis may also identify variants with intermediate function that may have intermediate or moderate effects on cancer risk (Monteiro and Couch, 2006; Lovelock et al., 2007; Kuznetsov et al., 2008; Guidugli et al., 2013). These variants do not fully complement the deficient phenotype or show discordant results using different functional assays. It is difficult to classify such missense changes using the standard multifactorial likelihood approach that is based on comparing data for a particular variant under the hypothesis that the variant is a fully penetrant pathogenic mutation against the hypothesis that the variant is neutral or of no clinical significance with respect to risk. As shown for the p.Arg1699Gln variant in BRCA1, the standard co-segregation analysis yielded odds of only 5:1 in favor of the variant being pathogenic compared to the >6000:1 odds when a lower penetrance was allowed (Spurdle et al., 2012). To determine whether intermediate function translates to intermediate cancer risk, family history and clinical information of families carrying intermediate function variants need to be collected to estimate age related and/or cumulative risk of breast/ovarian cancer associated with these missense changes.
Translating functional effects to cancer risk; the use of functional test results in the multifactorial likelihood model
Genetic studies based on family history, co-segregation or co-occurrence, have established a clear impact of certain variants on cancer risk. Nevertheless, functional assays to interpret the VUS pathogenicity are essential since many individual variants are unique in the population and there is not enough genetic information for classification purposes. However, impairment of a specific function does not translate directly to cancer predisposition. While a variant that completely knocks out gene function in an assay could be regarded as a “pathogenic mutation”, a genetic counselor would like to see additional evidence of pathogenicity from the clinical presentation of the patient and from family history before taking a definitive decision.
The use of quantitative measurements for assays that have been extensively validated is the first step towards developing a model for predicting the VUS pathogenicity (Guidugli et al., 2013). The model developed by Guidugli and authors for BRCA2 VUS analyzed with the HDR assay derives a probability of pathogenicity for each variant using estimates of the mean and the variances of the distribution of the HDR results for the known pathogenic and the non-pathogenic mutations. Such probabilities can be converted into likelihood ratios (LR) for inclusion in a Bayesian integrative analysis, in which data from other sources (e.g. family history, co-segregation, in silico protein prediction analysis) can be combined with functional data to generate an overall likelihood that a given variant is pathogenic or neutral (Iversen et al., 2011).
In time, functional assay data will form part of a multivariate model for the evaluation of VUS carriers and in this way will be a valuable and indispensable tool for the assessment of the clinical relevance of variants of uncertain significance.
Supplementary Material
Acknowledgments
Grant support
This work was supported by the Dutch Cancer Society grant UL 2012-5649 (MPGV); National Institutes of Health grant CA116167; a Mayo Clinic specialized program of research excellence (SPORE) in Breast Cancer (P50 CA116201); a fellowship from the Komen Foundation for the cure (LG); the ATIP-AVENIR CNRS/INSERM Young Investigator grant and EC-Marie Curie Career Integration Grant (AC); Association d’Aide à la Recherche Cancérologique de Saint-Cloud (ARCS) and the Ligue against Cancer Haut-de-Seine 92, French National Cancer Institute (INCa) (SC); Teggers Foundation, Sweden (AE), Miles for Moffitt award and a Phi Beta Psi Sorority grant (ANM). SLN is the Morris and Horowitz Families Endowed Professor.
References
- Antoniou AC, Cunningham AP, Peto J, Evans DG, Lalloo F, Narod SA, Risch HA, Eyfjord JE, Hopper JL, Southey MC, Olsson H, Johannsson O, et al. The BOADICEA model of genetic susceptibility to breast and ovarian cancers: updates and extensions. Br J Cancer. 2008;98(8):1457–1466. doi: 10.1038/sj.bjc.6604305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Audeh MW, Carmichael J, Penson RT, Friedlander M, Powell B, Bell-McGuinn KM, Scott C, Weitzel JN, Oaknin A, Loman N, Lu K, Schmutzler RK, et al. Oral poly(ADP-ribose) polymerase inhibitor olaparib in patients with BRCA1 or BRCA2 mutations and recurrent ovarian cancer: a proof-of-concept trial. Lancet. 2010;376(9737):245–251. doi: 10.1016/S0140-6736(10)60893-8. [DOI] [PubMed] [Google Scholar]
- Ayoub N, Rajendra E, Su X, Jeyasekharan AD, Mahen R, Venkitaraman AR. The carboxyl terminus of Brca2 links the disassembly of Rad51 complexes to mitotic entry. Curr Biol. 2009;19(13):1075–1085. doi: 10.1016/j.cub.2009.05.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baeyens A, Thierens H, Claes K, Poppe B, de Ridder L, Vral A. Chromosomal radiosensitivity in BRCA1 and BRCA2 mutation carriers. Int J Radiat Biol. 2004;80(10):745–756. doi: 10.1080/09553000400017937. [DOI] [PubMed] [Google Scholar]
- Balia C, Galli A, Caligo MA. Effect of the overexpression of BRCA2 unclassified missense variants on spontaneous homologous recombination in human cells. Breast Cancer Res Treat. 2011;129(3):1001–1009. doi: 10.1007/s10549-011-1607-y. [DOI] [PubMed] [Google Scholar]
- Becker AA, Graeser MK, Landwehr C, Hilger T, Baus W, Wappenschmidt B, Meindl A, Weber RG, Schmutzler RK. A 24-color metaphase-based radiation assay discriminates heterozygous BRCA2 mutation carriers from controls by chromosomal radiosensitivity. Breast Cancer Res Treat. 2012;135(1):167–175. doi: 10.1007/s10549-012-2119-0. [DOI] [PubMed] [Google Scholar]
- Biswas K, Das R, Alter BP, Kuznetsov SG, Stauffer S, North SL, Burkett S, Brody LC, Meyer S, Byrd RA, Sharan SK. A comprehensive functional characterization of BRCA2 variants associated with Fanconi anemia using mouse ES cell-based assay. Blood. 2011;118(9):2430–2442. doi: 10.1182/blood-2010-12-324541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswas K, Das R, Eggington JM, Qiao H, North SL, Stauffer S, Burkett SS, Martin BK, Southon E, Sizemore SC, Pruss D, Bowles KR, et al. Functional evaluation of BRCA2 variants mapping to the PALB2-binding and C-terminal DNA-binding domains using a mouse ES cell-based assay. Hum Mol Genet. 2012;21(18):3993–4006. doi: 10.1093/hmg/dds222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bork P, Blomberg N, Nilges M. Internal repeats in the BRCA2 protein sequence. Nat Genet. 1996;13(1):22–23. doi: 10.1038/ng0596-22. [DOI] [PubMed] [Google Scholar]
- Bowman-Colin C, Xia B, Bunting S, Klijn C, Drost R, Bouwman P, Fineman L, Chen X, Culhane AC, Cai H, Rodig SJ, Bronson RT, et al. Palb2 synergizes with Trp53 to suppress mammary tumor formation in a model of inherited breast cancer. Proc Natl Acad Sci U S A. 2013;110(21):8632–8637. doi: 10.1073/pnas.1305362110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brough R, Bajrami I, Vatcheva R, Natrajan R, Reis-Filho JS, Lord CJ, Ashworth A. APRIN is a cell cycle specific BRCA2-interacting protein required for genome integrity and a predictor of outcome after chemotherapy in breast cancer. Embo J. 2012;31(5):1160–1176. doi: 10.1038/emboj.2011.490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brozek I, Ochman K, Debniak J, Morzuch L, Ratajska M, Stepnowska M, Stukan M, Emerich J, Limon J. Loss of heterozygosity at BRCA1/2 loci in hereditary and sporadic ovarian cancers. J Appl Genet. 2009;50(4):379–384. doi: 10.1007/BF03195697. [DOI] [PubMed] [Google Scholar]
- Bryant HE, Schultz N, Thomas HD, Parker KM, Flower D, Lopez E, Kyle S, Meuth M, Curtin NJ, Helleday T. Specific killing of BRCA2-deficient tumours with inhibitors of poly(ADP-ribose) polymerase. Nature. 2005;434(7035):913–917. doi: 10.1038/nature03443. [DOI] [PubMed] [Google Scholar]
- Buchholz TA, Wu X, Hussain A, Tucker SL, Mills GB, Haffty B, Bergh S, Story M, Geara FB, Brock WA. Evidence of haplotype insufficiency in human cells containing a germline mutation in BRCA1 or BRCA2. Int J Cancer. 2002;97(5):557–561. doi: 10.1002/ijc.10109. [DOI] [PubMed] [Google Scholar]
- Carreira A, Hilario J, Amitani I, Baskin RJ, Shivji MK, Venkitaraman AR, Kowalczykowski SC. The BRC repeats of BRCA2 modulate the DNA-binding selectivity of RAD51. Cell. 2009;136(6):1032–1043. doi: 10.1016/j.cell.2009.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carreira A, Kowalczykowski SC. Two classes of BRC repeats in BRCA2 promote RAD51 nucleoprotein filament function by distinct mechanisms. Proc Natl Acad Sci U S A. 2011;108(26):10448–10453. doi: 10.1073/pnas.1106971108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen PL, Chen CF, Chen Y, Xiao J, Sharp ZD, Lee WH. The BRC repeats in BRCA2 are critical for RAD51 binding and resistance to methyl methanesulfonate treatment. Proc Natl Acad Sci U S A. 1998;95(9):5287–5292. doi: 10.1073/pnas.95.9.5287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claes K, Poppe B, Coene I, Paepe AD, Messiaen L. BRCA1 and BRCA2 germline mutation spectrum and frequencies in Belgian breast/ovarian cancer families. British journal of cancer. 2004;90(6):1244–1251. doi: 10.1038/sj.bjc.6601656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke CL, Sandle J, Jones AA, Sofronis A, Patani NR, Lakhani SR. Mapping loss of heterozygosity in normal human breast cells from BRCA1/2 carriers. British journal of cancer. 2006;95(4):515–519. doi: 10.1038/sj.bjc.6603298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connor F, Bertwistle D, Mee PJ, Ross GM, Swift S, Grigorieva E, Tybulewicz VL, Ashworth A. Tumorigenesis and a DNA repair defect in mice with a truncating Brca2 mutation. Nat Genet. 1997;17(4):423–430. doi: 10.1038/ng1297-423. [DOI] [PubMed] [Google Scholar]
- Daniels MJ, Wang Y, Lee M, Venkitaraman AR. Abnormal cytokinesis in cells deficient in the breast cancer susceptibility protein BRCA2. Science. 2004;306(5697):876–879. doi: 10.1126/science.1102574. [DOI] [PubMed] [Google Scholar]
- Davies OR, Pellegrini L. Interaction with the BRCA2 C terminus protects RAD51-DNA filaments from disassembly by BRC repeats. Nat Struct Mol Biol. 2007;14(6):475–483. doi: 10.1038/nsmb1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding Q, Lee YK, Schaefer EA, Peters DT, Veres A, Kim K, Kuperwasser N, Motola DL, Meissner TB, Hendriks WT, Trevisan M, Gupta RM, et al. A TALEN genome-editing system for generating human stem cell-based disease models. Cell Stem Cell. 2013;12(2):238–251. doi: 10.1016/j.stem.2012.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donoho G, Brenneman MA, Cui TX, Donoviel D, Vogel H, Goodwin EH, Chen DJ, Hasty P. Deletion of Brca2 exon 27 causes hypersensitivity to DNA crosslinks, chromosomal instability, and reduced life span in mice. Genes Chromosomes Cancer. 2003;36(4):317–331. doi: 10.1002/gcc.10148. [DOI] [PubMed] [Google Scholar]
- Drost R, Bouwman P, Rottenberg S, Boon U, Schut E, Klarenbeek S, Klijn C, van der Heijden I, van der Gulden H, Wientjens E, Pieterse M, Catteau A, et al. BRCA1 RING function is essential for tumor suppression but dispensable for therapy resistance. Cancer Cell. 2011;20(6):797–809. doi: 10.1016/j.ccr.2011.11.014. [DOI] [PubMed] [Google Scholar]
- Easton DF, Deffenbaugh AM, Pruss D, Frye C, Wenstrup RJ, Allen-Brady K, Tavtigian SV, Monteiro AN, Iversen ES, Couch FJ, Goldgar DE. A systematic genetic assessment of 1,433 sequence variants of unknown clinical significance in the BRCA1 and BRCA2 breast cancer-predisposition genes. Am J Hum Genet. 2007;81(5):873–883. doi: 10.1086/521032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernestos B, Nikolaos P, Koulis G, Eleni R, Konstantinos B, Alexandra G, Michael K. Increased chromosomal radiosensitivity in women carrying BRCA1/BRCA2 mutations assessed with the G2 assay. Int J Radiat Oncol Biol Phys. 2010;76(4):1199–1205. doi: 10.1016/j.ijrobp.2009.10.020. [DOI] [PubMed] [Google Scholar]
- Esashi F, Galkin VE, Yu X, Egelman EH, West SC. Stabilization of RAD51 nucleoprotein filaments by the C-terminal region of BRCA2. Nat Struct Mol Biol. 2007;14(6):468–474. doi: 10.1038/nsmb1245. [DOI] [PubMed] [Google Scholar]
- Evers B, Jonkers J. Mouse models of BRCA1 and BRCA2 deficiency: past lessons, current understanding and future prospects. Oncogene. 2006;25(43):5885–5897. doi: 10.1038/sj.onc.1209871. [DOI] [PubMed] [Google Scholar]
- Evers B, Drost R, Schut E, de Bruin M, van der Burg E, Derksen PW, Holstege H, Liu X, van Drunen E, Beverloo HB, Smith GC, Martin NM, et al. Selective inhibition of BRCA2-deficient mammary tumor cell growth by AZD2281 and cisplatin. Clin Cancer Res. 2008;14(12):3916–3925. doi: 10.1158/1078-0432.CCR-07-4953. [DOI] [PubMed] [Google Scholar]
- Farmer H, McCabe N, Lord CJ, Tutt AN, Johnson DA, Richardson TB, Santarosa M, Dillon KJ, Hickson I, Knights C, Martin NM, Jackson SP, et al. Targeting the DNA repair defect in BRCA mutant cells as a therapeutic strategy. Nature. 2005;434(7035):917–921. doi: 10.1038/nature03445. [DOI] [PubMed] [Google Scholar]
- Farrugia DJ, Agarwal MK, Pankratz VS, Deffenbaugh AM, Pruss D, Frye C, Wadum L, Johnson K, Mentlick J, Tavtigian SV, Goldgar DE, Couch FJ. Functional assays for classification of BRCA2 variants of uncertain significance. Cancer Res. 2008;68(9):3523–3531. doi: 10.1158/0008-5472.CAN-07-1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fong PC, Boss DS, Yap TA, Tutt A, Wu P, Mergui-Roelvink M, Mortimer P, Swaisland H, Lau A, O'Connor MJ, Ashworth A, Carmichael J, et al. Inhibition of poly(ADP-ribose) polymerase in tumors from BRCA mutation carriers. The New England journal of medicine. 2009;361(2):123–134. doi: 10.1056/NEJMoa0900212. [DOI] [PubMed] [Google Scholar]
- Funakoshi M, Li X, Velichutina I, Hochstrasser M, Kobayashi H. Sem1, the yeast ortholog of a human BRCA2-binding protein, is a component of the proteasome regulatory particle that enhances proteasome stability. J Cell Sci. 2004;117(Pt 26):6447–6454. doi: 10.1242/jcs.01575. [DOI] [PubMed] [Google Scholar]
- Gelmon KA, Tischkowitz M, Mackay H, Swenerton K, Robidoux A, Tonkin K, Hirte H, Huntsman D, Clemons M, Gilks B, Yerushalmi R, Macpherson E, et al. Olaparib in patients with recurrent high-grade serous or poorly differentiated ovarian carcinoma or triple-negative breast cancer: a phase 2, multicentre, open-label, non-randomised study. Lancet Oncol. 2011;12(9):852–861. doi: 10.1016/S1470-2045(11)70214-5. [DOI] [PubMed] [Google Scholar]
- Goldgar DE, Easton DF, Deffenbaugh AM, Monteiro AN, Tavtigian SV, Couch FJ. Integrated evaluation of DNA sequence variants of unknown clinical significance: application to BRCA1 and BRCA2. Am J Hum Genet. 2004;75(4):535–544. doi: 10.1086/424388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldgar DE, Easton DF, Byrnes GB, Spurdle AB, Iversen ES, Greenblatt MS. Genetic evidence and integration of various data sources for classifying uncertain variants into a single model. Hum Mutat. 2008;29(11):1265–1272. doi: 10.1002/humu.20897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gudmundsdottir K, Ashworth A. The roles of BRCA1 and BRCA2 and associated proteins in the maintenance of genomic stability. Oncogene. 2006;25(43):5864–5874. doi: 10.1038/sj.onc.1209874. [DOI] [PubMed] [Google Scholar]
- Guidugli L, Pankratz VS, Singh N, Thompson J, Erding CA, Engel C, Schmutzler R, Domchek S, Nathanson K, Radice P, Singer C, Tonin PN, et al. A classification model for BRCA2 DNA binding domain missense variants based on homology-directed repair activity. Cancer Res. 2013;73(1):265–275. doi: 10.1158/0008-5472.CAN-12-2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han X, Saito H, Miki Y, Nakanishi A. A CRM1-mediated nuclear export signal governs cytoplasmic localization of BRCA2 and is essential for centrosomal localization of BRCA2. Oncogene. 2008;27(21):2969–2977. doi: 10.1038/sj.onc.1210968. [DOI] [PubMed] [Google Scholar]
- Holloman WK. Unraveling the mechanism of BRCA2 in homologous recombination. Nat Struct Mol Biol. 2011;18(7):748–754. doi: 10.1038/nsmb.2096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houdayer C, Caux-Moncoutier V, Krieger S, Barrois M, Bonnet F, Bourdon V, Bronner M, Buisson M, Coulet F, Gaildrat P, Lefol C, Leone M, et al. Guidelines for splicing analysis in molecular diagnosis derived from a set of 327 combined in silico/in vitro studies on BRCA1 and BRCA2 variants. Hum Mutat. 2012;33(8):1228–1238. doi: 10.1002/humu.22101. [DOI] [PubMed] [Google Scholar]
- Howlett NG, Taniguchi T, Olson S, Cox B, Waisfisz Q, De Die-Smulders C, Persky N, Grompe M, Joenje H, Pals G, Ikeda H, Fox EA, et al. Biallelic inactivation of BRCA2 in Fanconi anemia. Science. 2002;297(5581):606–609. doi: 10.1126/science.1073834. [DOI] [PubMed] [Google Scholar]
- Hucl T, Rago C, Gallmeier E, Brody JR, Gorospe M, Kern SE. A syngeneic variance library for functional annotation of human variation: application to BRCA2. Cancer Res. 2008;68(13):5023–5030. doi: 10.1158/0008-5472.CAN-07-6189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes-Davies L, Huntsman D, Ruas M, Fuks F, Bye J, Chin SF, Milner J, Brown LA, Hsu F, Gilks B, Nielsen T, Schulzer M, et al. EMSY links the BRCA2 pathway to sporadic breast and ovarian cancer. Cell. 2003;115(5):523–535. doi: 10.1016/s0092-8674(03)00930-9. [DOI] [PubMed] [Google Scholar]
- Huo Y, Cai H, Teplova I, Bowman-Colin C, Chen G, Price S, Barnard N, Ganesan S, Karantza V, White E, Xia B. Autophagy opposes p53-mediated tumor barrier to facilitate tumorigenesis in a model of PALB2-associated hereditary breast cancer. Cancer Discov. 2013;3(8):894–907. doi: 10.1158/2159-8290.CD-13-0011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussain S, Witt E, Huber PA, Medhurst AL, Ashworth A, Mathew CG. Direct interaction of the Fanconi anaemia protein FANCG with BRCA2/FANCD1. Hum Mol Genet. 2003;12(19):2503–2510. doi: 10.1093/hmg/ddg266. [DOI] [PubMed] [Google Scholar]
- Hussain S, Wilson JB, Medhurst AL, Hejna J, Witt E, Ananth S, Davies A, Masson JY, Moses R, West SC, de Winter JP, Ashworth A, et al. Direct interaction of FANCD2 with BRCA2 in DNA damage response pathways. Hum Mol Genet. 2004;13(12):1241–1248. doi: 10.1093/hmg/ddh135. [DOI] [PubMed] [Google Scholar]
- Iversen ES, Jr, Couch FJ, Goldgar DE, Tavtigian SV, Monteiro AN. A computational method to classify variants of uncertain significance using functional assay data with application to BRCA1. Cancer Epidemiol Biomarkers Prev. 2011;20(6):1078–1088. doi: 10.1158/1055-9965.EPI-10-1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen RB, Carreira A, Kowalczykowski SC. Purified human BRCA2 stimulates RAD51-mediated recombination. Nature. 2010;467(7316):678–683. doi: 10.1038/nature09399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeyasekharan AD, Liu Y, Hattori H, Pisupati V, Jonsdottir AB, Rajendra E, Lee M, Sundaramoorthy E, Schlachter S, Kaminski CF, Ofir-Rosenfeld Y, Sato K, et al. A cancer-associated BRCA2 mutation reveals masked nuclear export signals controlling localization. Nat Struct Mol Biol. 2013;20(10):1191–1198. doi: 10.1038/nsmb.2666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonkers J, Meuwissen R, van der Gulden H, Peterse H, van der Valk M, Berns A. Synergistic tumor suppressor activity of BRCA2 and p53 in a conditional mouse model for breast cancer. Nat Genet. 2001;29(4):418–425. doi: 10.1038/ng747. [DOI] [PubMed] [Google Scholar]
- Jonsdottir AB, Vreeswijk MP, Wolterbeek R, Devilee P, Tanke HJ, Eyfjord JE, Szuhai K. BRCA2 heterozygosity delays cytokinesis in primary human fibroblasts. Cell Oncol. 2009;31(3):191–201. doi: 10.3233/CLO-2009-0465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Josse L, Harley ME, Pires IM, Hughes DA. Fission yeast Dss1 associates with the proteasome and is required for efficient ubiquitin-dependent proteolysis. Biochem J. 2006;393(Pt 1):303–309. doi: 10.1042/BJ20051238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kote-Jarai Z, Matthews L, Osorio A, Shanley S, Giddings I, Moreews F, Locke I, Evans DG, Eccles D, Williams RD, Girolami M, Campbell C, et al. Accurate prediction of BRCA1 and BRCA2 heterozygous genotype using expression profiling after induced DNA damage. Clin Cancer Res. 2006;12(13):3896–3901. doi: 10.1158/1078-0432.CCR-05-2805. [DOI] [PubMed] [Google Scholar]
- Kraakman-van der Zwet M, Overkamp WJ, van Lange RE, Essers J, van Duijn-Goedhart A, Wiggers I, Swaminathan S, van Buul PP, Errami A, Tan RT, Jaspers NG, Sharan SK, et al. Brca2 (XRCC11) deficiency results in radioresistant DNA synthesis and a higher frequency of spontaneous deletions. Molecular and cellular biology. 2002;22(2):669–679. doi: 10.1128/MCB.22.2.669-679.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuznetsov SG, Liu P, Sharan SK. Mouse embryonic stem cell-based functional assay to evaluate mutations in BRCA2. Nature medicine. 2008;14(8):875–881. doi: 10.1038/nm.1719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larminat F, Germanier M, Papouli E, Defais M. Deficiency in BRCA2 leads to increase in non-conservative homologous recombination. Oncogene. 2002;21(33):5188–5192. doi: 10.1038/sj.onc.1205659. [DOI] [PubMed] [Google Scholar]
- Li J, Zou C, Bai Y, Wazer DE, Band V, Gao Q. DSS1 is required for the stability of BRCA2. Oncogene. 2006;25(8):1186–1194. doi: 10.1038/sj.onc.1209153. [DOI] [PubMed] [Google Scholar]
- Lindor NM, Guidugli L, Wang X, Vallee MP, Monteiro AN, Tavtigian S, Goldgar DE, Couch FJ. A review of a multifactorial probability-based model for classification of BRCA1 and BRCA2 variants of uncertain significance (VUS) Hum Mutat. 2012;33(1):8–21. doi: 10.1002/humu.21627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Doty T, Gibson B, Heyer WD. Human BRCA2 protein promotes RAD51 filament formation on RPA-covered single-stranded DNA. Nat Struct Mol Biol. 2010;17(10):1260–1262. doi: 10.1038/nsmb.1904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo T, Pellegrini L, Venkitaraman AR, Blundell TL. Sequence fingerprints in BRCA2 and RAD51: implications for DNA repair and cancer. DNA Repair (Amst) 2003;2(9):1015–1028. doi: 10.1016/s1568-7864(03)00097-1. [DOI] [PubMed] [Google Scholar]
- Lord CJ, Ashworth A. The DNA damage response and cancer therapy. Nature. 2012;481(7381):287–294. doi: 10.1038/nature10760. [DOI] [PubMed] [Google Scholar]
- Lovelock PK, Spurdle AB, Mok MT, Farrugia DJ, Lakhani SR, Healey S, Arnold S, Buchanan D, Couch FJ, Henderson BR, Goldgar DE, Tavtigian SV, et al. Identification of BRCA1 missense substitutions that confer partial functional activity: potential moderate risk variants? Breast Cancer Res. 2007;9(6):R82. doi: 10.1186/bcr1826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mali P, Yang L, Esvelt KM, Aach J, Guell M, DiCarlo JE, Norville JE, Church GM. RNA-guided human genome engineering via Cas9. Science. 2013;339(6121):823–826. doi: 10.1126/science.1232033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marmorstein LY, Kinev AV, Chan GK, Bochar DA, Beniya H, Epstein JA, Yen TJ, Shiekhattar R. A human BRCA2 complex containing a structural DNA binding component influences cell cycle progression. Cell. 2001;104(2):247–257. doi: 10.1016/s0092-8674(01)00209-4. [DOI] [PubMed] [Google Scholar]
- Millot GA, Carvalho MA, Caputo SM, Vreeswijk MP, Brown MA, Webb M, Rouleau E, Neuhausen SL, Hansen T, Galli A, Brandao RD, Blok MJ, et al. A guide for functional analysis of BRCA1 variants of uncertain significance. Hum Mutat. 2012;33(11):1526–1537. doi: 10.1002/humu.22150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milner J, Ponder B, Hughes-Davies L, Seltmann M, Kouzarides T. Transcriptional activation functions in BRCA2. Nature. 1997;386(6627):772–773. doi: 10.1038/386772a0. [DOI] [PubMed] [Google Scholar]
- Mondal G, Rowley M, Guidugli L, Wu J, Pankratz VS, Couch FJ. BRCA2 localization to the midbody by filamin A regulates cep55 signaling and completion of cytokinesis. Dev Cell. 2012;23(1):137–152. doi: 10.1016/j.devcel.2012.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monteiro AN, Couch FJ. Cancer risk assessment at the atomic level. Cancer Res. 2006;66(4):1897–1899. doi: 10.1158/0008-5472.CAN-05-3034. [DOI] [PubMed] [Google Scholar]
- Morimatsu M, Donoho G, Hasty P. Cells deleted for Brca2 COOH terminus exhibit hypersensitivity to gamma-radiation and premature senescence. Cancer Res. 1998;58(15):3441–3447. [PubMed] [Google Scholar]
- Moynahan ME, Pierce AJ, Jasin M. BRCA2 is required for homology-directed repair of chromosomal breaks. Mol Cell. 2001;7(2):263–272. doi: 10.1016/s1097-2765(01)00174-5. [DOI] [PubMed] [Google Scholar]
- Nakanishi A, Han X, Saito H, Taguchi K, Ohta Y, Imajoh-Ohmi S, Miki Y. Interference with BRCA2, which localizes to the centrosome during S and early M phase, leads to abnormal nuclear division. Biochem Biophys Res Commun. 2007;355(1):34–40. doi: 10.1016/j.bbrc.2007.01.100. [DOI] [PubMed] [Google Scholar]
- Oliver AW, Swift S, Lord CJ, Ashworth A, Pearl LH. Structural basis for recruitment of BRCA2 by PALB2. EMBO Rep. 2009;10(9):990–996. doi: 10.1038/embor.2009.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel KJ, Yu VP, Lee H, Corcoran A, Thistlethwaite FC, Evans MJ, Colledge WH, Friedman LS, Ponder BA, Venkitaraman AR. Involvement of Brca2 in DNA repair. Mol Cell. 1998;1(3):347–357. doi: 10.1016/s1097-2765(00)80035-0. [DOI] [PubMed] [Google Scholar]
- Plon SE, Eccles DM, Easton D, Foulkes WD, Genuardi M, Greenblatt MS, Hogervorst FB, Hoogerbrugge N, Spurdle AB, Tavtigian SV. Sequence variant classification and reporting: recommendations for improving the interpretation of cancer susceptibility genetic test results. Hum Mutat. 2008;29(11):1282–1291. doi: 10.1002/humu.20880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy R, Chun J, Powell SN. BRCA1 and BRCA2: different roles in a common pathway of genome protection. Nature reviews. Cancer. 2012;12(1):68–78. doi: 10.1038/nrc3181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandhu SK, Schelman WR, Wilding G, Moreno V, Baird RD, Miranda S, Hylands L, Riisnaes R, Forster M, Omlin A, Kreischer N, Thway K, et al. The poly(ADP-ribose) polymerase inhibitor niraparib (MK4827) in BRCA mutation carriers and patients with sporadic cancer: a phase 1 dose-escalation trial. Lancet Oncol. 2013;14(9):882–892. doi: 10.1016/S1470-2045(13)70240-7. [DOI] [PubMed] [Google Scholar]
- Schlacher K, Christ N, Siaud N, Egashira A, Wu H, Jasin M. Double-strand break repair-independent role for BRCA2 in blocking stalled replication fork degradation by MRE11. Cell. 2011;145(4):529–542. doi: 10.1016/j.cell.2011.03.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlacher K, Wu H, Jasin M. A distinct replication fork protection pathway connects Fanconi anemia tumor suppressors to RAD51-BRCA1/2. Cancer Cell. 2012;22(1):106–116. doi: 10.1016/j.ccr.2012.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scudiero DA, Shoemaker RH, Paull KD, Monks A, Tierney S, Nofziger TH, Currens MJ, Seniff D, Boyd MR. Evaluation of a soluble tetrazolium/formazan assay for cell growth and drug sensitivity in culture using human and other tumor cell lines. Cancer Res. 1988;48(17):4827–4833. [PubMed] [Google Scholar]
- Shakya R, Reid LJ, Reczek CR, Cole F, Egli D, Lin CS, deRooij DG, Hirsch S, Ravi K, Hicks JB, Szabolcs M, Jasin M, et al. BRCA1 tumor suppression depends on BRCT phosphoprotein binding, but not its E3 ligase activity. Science. 2011;334(6055):525–528. doi: 10.1126/science.1209909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharan SK, Bradley A. Murine Brca2: sequence, map position, and expression pattern. Genomics. 1997;40(2):234–241. doi: 10.1006/geno.1996.4573. [DOI] [PubMed] [Google Scholar]
- Sharan SK, Morimatsu M, Albrecht U, Lim DS, Regel E, Dinh C, Sands A, Eichele G, Hasty P, Bradley A. Embryonic lethality and radiation hypersensitivity mediated by Rad51 in mice lacking Brca2. Nature. 1997;386(6627):804–810. doi: 10.1038/386804a0. [DOI] [PubMed] [Google Scholar]
- Sone T, Saeki Y, Toh-e A, Yokosawa H. Sem1p is a novel subunit of the 26 S proteasome from Saccharomyces cerevisiae. J Biol Chem. 2004;279(27):28807–28816. doi: 10.1074/jbc.M403165200. [DOI] [PubMed] [Google Scholar]
- Spain BH, Larson CJ, Shihabuddin LS, Gage FH, Verma IM. Truncated BRCA2 is cytoplasmic: implications for cancer-linked mutations. Proc Natl Acad Sci U S A. 1999;96(24):13920–13925. doi: 10.1073/pnas.96.24.13920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spearman AD, Sweet K, Zhou XP, McLennan J, Couch FJ, Toland AE. Clinically applicable models to characterize BRCA1 and BRCA2 variants of uncertain significance. J Clin Oncol. 2008;26(33):5393–5400. doi: 10.1200/JCO.2008.17.8228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spugnesi L, Balia C, Collavoli A, Falaschi E, Quercioli V, Caligo MA, Galli A. Effect of the expression of BRCA2 on spontaneous homologous recombination and DNA damage-induced nuclear foci in Saccharomyces cerevisiae. Mutagenesis. 2013;28(2):187–195. doi: 10.1093/mutage/ges069. [DOI] [PubMed] [Google Scholar]
- Spurdle AB, Healey S, Devereau A, Hogervorst FB, Monteiro AN, Nathanson KL, Radice P, Stoppa-Lyonnet D, Tavtigian S, Wappenschmidt B, Couch FJ, Goldgar DE. ENIGMA--evidence-based network for the interpretation of germline mutant alleles: an international initiative to evaluate risk and clinical significance associated with sequence variation in BRCA1 and BRCA2 genes. Hum Mutat. 2012;33(1):2–7. doi: 10.1002/humu.21628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sy SM, Huen MS, Chen J. PALB2 is an integral component of the BRCA complex required for homologous recombination repair. Proc Natl Acad Sci U S A. 2009;106(17):7155–7160. doi: 10.1073/pnas.0811159106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabo C, Masiello A, Ryan JF, Brody LC. The breast cancer information core: database design, structure, and scope. Hum Mutat. 2000;16(2):123–131. doi: 10.1002/1098-1004(200008)16:2<123::AID-HUMU4>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- Tavtigian SV, Byrnes GB, Goldgar DE, Thomas A. Classification of rare missense substitutions, using risk surfaces, with genetic- and molecular-epidemiology applications. Hum Mutat. 2008;29(11):1342–1354. doi: 10.1002/humu.20896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomassen M, Kruse TA, Jensen PK, Gerdes AM. A missense mutation in exon 13 in BRCA2, c.7235G>A, results in skipping of exon 13. Genet Test. 2006;10(2):116–120. doi: 10.1089/gte.2006.10.116. [DOI] [PubMed] [Google Scholar]
- Thomassen M, Blanco A, Montagna M, Hansen TV, Pedersen IS, Gutierrez-Enriquez S, Menendez M, Fachal L, Santamarina M, Steffensen AY, Jonson L, Agata S, et al. Characterization of BRCA1 and BRCA2 splicing variants: a collaborative report by ENIGMA consortium members. Breast Cancer Res Treat. 2012;132(3):1009–1023. doi: 10.1007/s10549-011-1674-0. [DOI] [PubMed] [Google Scholar]
- Thorslund T, Esashi F, West SC. Interactions between human BRCA2 protein and the meiosis-specific recombinase DMC1. Embo J. 2007;26(12):2915–2922. doi: 10.1038/sj.emboj.7601739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorslund T, McIlwraith MJ, Compton SA, Lekomtsev S, Petronczki M, Griffith JD, West SC. The breast cancer tumor suppressor BRCA2 promotes the specific targeting of RAD51 to single-stranded DNA. Nat Struct Mol Biol. 2010;17(10):1263–1265. doi: 10.1038/nsmb.1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tischkowitz M, Xia B. PALB2/FANCN: recombining cancer and Fanconi anemia. Cancer Res. 2010;70(19):7353–7359. doi: 10.1158/0008-5472.CAN-10-1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tutt A, Gabriel A, Bertwistle D, Connor F, Paterson H, Peacock J, Ross G, Ashworth A. Absence of Brca2 causes genome instability by chromosome breakage and loss associated with centrosome amplification. Curr Biol. 1999;9(19):1107–1110. doi: 10.1016/s0960-9822(99)80479-5. [DOI] [PubMed] [Google Scholar]
- Tutt A, Robson M, Garber JE, Domchek SM, Audeh MW, Weitzel JN, Friedlander M, Arun B, Loman N, Schmutzler RK, Wardley A, Mitchell G, et al. Oral poly(ADP-ribose) polymerase inhibitor olaparib in patients with BRCA1 or BRCA2 mutations and advanced breast cancer: a proof-of-concept trial. Lancet. 2010;376(9737):235–244. doi: 10.1016/S0140-6736(10)60892-6. [DOI] [PubMed] [Google Scholar]
- Vallee MP, Francy TC, Judkins MK, Babikyan D, Lesueur F, Gammon A, Goldgar DE, Couch FJ, Tavtigian SV. Classification of missense substitutions in the BRCA genes: a database dedicated to Ex-UVs. Hum Mutat. 2012;33(1):22–28. doi: 10.1002/humu.21629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Asperen CJ, Brohet RM, Meijers-Heijboer EJ, Hoogerbrugge N, Verhoef S, Vasen HF, Ausems MG, Menko FH, Gomez Garcia EB, Klijn JG, Hogervorst FB, van Houwelingen JC, et al. Cancer risks in BRCA2 families: estimates for sites other than breast and ovary. J Med Genet. 2005;42(9):711–719. doi: 10.1136/jmg.2004.028829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Beers EH, van Welsem T, Wessels LF, Li Y, Oldenburg RA, Devilee P, Cornelisse CJ, Verhoef S, Hogervorst FB, van't Veer LJ, Nederlof PM. Comparative genomic hybridization profiles in human BRCA1 and BRCA2 breast tumors highlight differential sets of genomic aberrations. Cancer Res. 2005;65(3):822–827. [PubMed] [Google Scholar]
- Waddell N, Ten Haaf A, Marsh A, Johnson J, Walker LC, Gongora M, Brown M, Grover P, Girolami M, Grimmond S, Chenevix-Trench G, Spurdle AB. BRCA1 and BRCA2 missense variants of high and low clinical significance influence lymphoblastoid cell line post-irradiation gene expression. PLoS Genet. 2008;4(5):e1000080. doi: 10.1371/journal.pgen.1000080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker LC, Whiley PJ, Couch FJ, Farrugia DJ, Healey S, Eccles DM, Lin F, Butler SA, Goff SA, Thompson BA, Lakhani SR, Da Silva LM, et al. Detection of splicing aberrations caused by BRCA1 and BRCA2 sequence variants encoding missense substitutions: implications for prediction of pathogenicity. Hum Mutat. 2010;31(6):E1484–E1505. doi: 10.1002/humu.21267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh CS, Ogawa S, Scoles DR, Miller CW, Kawamata N, Narod SA, Koeffler HP, Karlan BY. Genome-wide loss of heterozygosity and uniparental disomy in BRCA1/2-associated ovarian carcinomas. Clin Cancer Res. 2008;14(23):7645–7651. doi: 10.1158/1078-0432.CCR-08-1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong JM, Ionescu D, Ingles CJ. Interaction between BRCA2 and replication protein A is compromised by a cancer-predisposing mutation in BRCA2. Oncogene. 2003;22(1):28–33. doi: 10.1038/sj.onc.1206071. [DOI] [PubMed] [Google Scholar]
- Wu K, Hinson SR, Ohashi A, Farrugia D, Wendt P, Tavtigian SV, Deffenbaugh A, Goldgar D, Couch FJ. Functional evaluation and cancer risk assessment of BRCA2 unclassified variants. Cancer Res. 2005;65(2):417–426. [PubMed] [Google Scholar]
- Xia B, Sheng Q, Nakanishi K, Ohashi A, Wu J, Christ N, Liu X, Jasin M, Couch FJ, Livingston DM. Control of BRCA2 cellular and clinical functions by a nuclear partner, PALB2. Mol Cell. 2006;22(6):719–729. doi: 10.1016/j.molcel.2006.05.022. [DOI] [PubMed] [Google Scholar]
- Yang H, Jeffrey PD, Miller J, Kinnucan E, Sun Y, Thoma NH, Zheng N, Chen PL, Lee WH, Pavletich NP. BRCA2 function in DNA binding and recombination from a BRCA2-DSS1-ssDNA structure. Science. 2002;297(5588):1837–1848. doi: 10.1126/science.297.5588.1837. [DOI] [PubMed] [Google Scholar]
- Yano K, Morotomi K, Saito H, Kato M, Matsuo F, Miki Y. Nuclear localization signals of the BRCA2 protein. Biochem Biophys Res Commun. 2000;270(1):171–175. doi: 10.1006/bbrc.2000.2392. [DOI] [PubMed] [Google Scholar]
- Yuan SS, Lee SY, Chen G, Song M, Tomlinson GE, Lee EY. BRCA2 is required for ionizing radiation-induced assembly of Rad51 complex in vivo. Cancer Res. 1999;59(15):3547–3551. [PubMed] [Google Scholar]
- Zhang F, Fan Q, Ren K, Andreassen PR, et al. PALB2 functionally connects the breast cancer susceptibility proteins BRCA1 and BRCA2. Mol Cancer Res. 2009a;7(7):1110–1118. doi: 10.1158/1541-7786.MCR-09-0123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang F, Ma J, Wu J, Ye L, Cai H, Xia B, Yu X. PALB2 links BRCA1 and BRCA2 in the DNA-damage response. Curr Biol. 2009b;19(6):524–529. doi: 10.1016/j.cub.2009.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.