SUMMARY
Dorsomedial prefrontal cortex is critical for the temporal control of behavior. Dorsomedial prefrontal cortex might alter neuronal activity in areas such as motor cortex to inhibit temporally inappropriate responses. We tested this hypothesis by recording from neuronal ensembles in rodent dorsomedial prefrontal cortex during a delayed-response task. One-third of dorsomedial prefrontal neurons were significantly modulated during the delay period. The activity of many of these neurons was predictive of premature responding. We then reversibly inactivated dorsomedial prefrontal cortex while recording ensemble activity in motor cortex. Inactivation of dorsomedial prefrontal cortex reduced delay-related firing, but not response-related firing, in motor cortex. Finally, we made simultaneous recordings in dorsomedial prefrontal cortex and motor cortex and found strong delay-related temporal correlations between neurons in the two cortical areas. These data suggest that functional interactions between dorsomedial prefrontal cortex and motor cortex might serve as a top-down control signal that inhibits inappropriate responding.
Keywords: delayed response, preparation, waiting, goals, temporal inhibition, rules
INTRODUCTION
Which brain regions might instruct motor systems to wait for a stimulus? Classic frontal lesion studies described profound impairments in delayed-response performance (Jacobsen, 1936). Neurophysiological studies of primate medial prefrontal regions have reported single neurons that increase their activity while animals are waiting to respond (Kolb, 1984; Niki and Watanabe, 1976; Niki and Watanabe, 1979). Recent evidence from human functional imaging studies show that medial frontal areas are selectively activated when responses to external stimuli must be inhibited in stop-signal reaction time tasks (Li et al., 2006). Medial frontal regions might be part of higher cortical systems thought to modulate motor cortex to suppress responding until the right time or the right stimulus has occurred (Brunia, 1999; Fuster, 2000). The inhibition of temporally inappropriate responses reflects “top-down” executive functions (Miller and D'Esposito, 2005) such as working memory and response inhibition. These critical functions are impaired in a number of psychiatric disorders, including attention deficit hyperactivity disorder (Barkley, 1997; Miller and D'Esposito, 2005).
In rodents, dorsomedial prefrontal cortex (dmPFC) is comprised of the anterior cingulate and prelimbic areas (Conde et al., 1995; Gabbott et al., 2005; Groenewegen and Uylings, 2000; Heidbreder and Groenewegen, 2003; Milad et al., 2004; Sesack et al., 1989; Uylings et al., 2003; Vertes, 2004). These regions might be functionally analogous to prefrontal regions in primates (Groenewegen and Uylings, 2000; Preuss, 1995; Uylings et al., 2003). If dmPFC is lesioned (Broersen and Uylings, 1999; Risterucci et al., 2003) or inactivated (Narayanan et al., 2006), rats can no longer wait for trigger stimuli and exhibit dramatically increased premature responding. These impairments suggest a role for dmPFC in behavioral inhibition, specifically, in suppressing temporally inappropriate responses before the end of a delay period (Barkley, 1997; Kolb, 1984). Such inhibition might be related to the temporal rules of the task. One such rule suggests that when waiting to respond, subjects inhibit responses until the maximum delay duration, or ‘deadline’ (Ollman and Billington, 1972). In rats, previous work from our lab has suggested that dmPFC is critical for mediating this response rule (Narayanan et al., 2006).
Prefrontal regions are critical for controlling behavior in accordance with internal states, rules and intentions (Brunia, 1999; Fuster, 2000; Miller and D'Esposito, 2005). In the delayed lever release task used in our studies, this view suggests that dmPFC should be especially active during the delay period while animals are waiting to respond. Such delay-related neurons in dmPFC might achieve temporal control over behavior by altering the firing rates of neurons in motor cortex. Dorsomedial PFC could influence motor cortical activity in two ways. First, dmPFC neurons could provide information about the temporal rules of the task to motor cortex during the delay period. In this case, functional interactions between dmPFC and motor cortex would be specific to the delay period. Second, dmPFC might be functionally independent of motor cortex and alter motor cortical activity in a non-specific manner. For example, dmPFC could alter the activity of motor cortex through its extensive connections with the limbic system, striatum, thalamus, or “neuromodulatory” subcortical nuclei such as locus coeruleus (Brunia, 1999; Burwell, 2000; Conde et al., 1995; Divac et al., 1993; Gabbott et al., 2005; Groenewegen and Uylings, 2000; Kyuhou and Gemba, 2002; Mulder et al., 2003; Reep et al., 1987; Sesack et al., 1989). Such non-specific interactions between dmPFC and motor cortex would alter firing rates in motor cortex but would not be expected to be specific to the delay period. A non-specific modulation of motor cortical excitability by dmPFC could alter the firing properties of many motor cortical neurons, including those involved in movements (i.e., lever pressing and releasing) as well as those not engaged in the task.
To distinguish between specific and non-specific effects of dmPFC activity on the motor cortex, we performed three experiments. First, we recorded from single dmPFC neurons during performance of a delayed-response task (Fig 1). A significant fraction of dmPFC neurons were modulated during the delay period. The firing rates of many of these neurons were predictive of premature errors in the task. These results suggest that neurons in rodent dmPFC are engaged while animals are waiting to respond.
Figure 1.
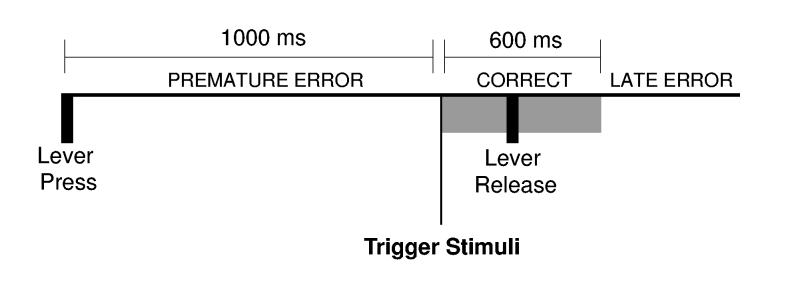
(A) Sequence of events in the delayed-response task. Rats pressed a lever for 1000 ms (delay period) and released the lever within 600 ms of the onset of an auditory trigger stimulus (Correct responses) presented at the end of the delay period. Two types of errors occurred in the task. Premature errors occurred if the lever was released before the trigger stimulus. Late errors occurred if the reaction time was greater than 600 ms.
In the second experiment, we examined the idea that dmPFC exerts specific control over motor cortex to inhibit temporally inappropriate responses. We recorded from motor cortex ensembles while reversibly inactivating rodent dmPFC with the GABA-A agonist muscimol (Lomber, 1999; Martin and Ghez, 1999; Narayanan et al., 2006). If motor cortex is influenced by a temporally specific control signal from dmPFC, then inactivation of dmPFC should alter motor cortex activity while animals are waiting to respond. On the other hand, if motor cortex is nonspecifically influenced by dmPFC, then inactivation of dmPFC should alter many aspects of motor cortex activity, including neuronal firing during motor responses, basal rates and patterns of firing by task-related and unrelated neurons, and perhaps overall levels of activation in motor cortex (Brunia, 1999). We found that dmPFC inactivation specifically altered a subpopulation of motor cortical neurons that were active during the delay period. That is, many fewer delay-related neurons were found in motor cortex with dmPFC inactivated.
Finally, in the third experiment, we examined the time-course of functional coupling between dmPFC and motor cortex by simultaneously recording from neuronal ensembles in both cortical areas. We found strong temporal correlations between dmPFC and motor cortical neurons during the delay period, but not during the motor response. These data suggest that there is a specific delay-related functional coupling of neurons in dmPFC and motor cortex.
Together, our studies suggest that temporally inappropriate responding during delayed-response performance is suppressed by specific functional interactions between dmPFC and motor cortical neurons. This functional coupling between dmPFC and motor cortex neurons might serve as a top-down control signal that is critical for the temporal control of behavior.
RESULTS
Experiment 1: dmPFC Ensemble Recording
dmPFC contains neurons that are active during delay periods
Eleven rats were trained to perform a delayed-response task, in which a lever is held down over a 1000 ms delay period and then released in response to an auditory trigger stimulus (Fig 1). Trials on which animals successfully waited for the trigger stimulus and responded within 600 ms of the onset of the trigger stimulus are referred to as correct trials (mean±SEM: 64±3% of trials in the behavioral sessions analyzed in this manuscript). Two types of errors could be made in the task. First, animals could release the lever before the end of the delay period, a premature error (17±1% of trials). Second, the reaction time could be longer than 600 ms, a late error (18±3% of trials). As inactivation of dmPFC does not lead to increased late responding (Narayanan et al. 2006), we have restricted the focus of our analysis in the present study to comparisons of correct trials and trials with premature errors.
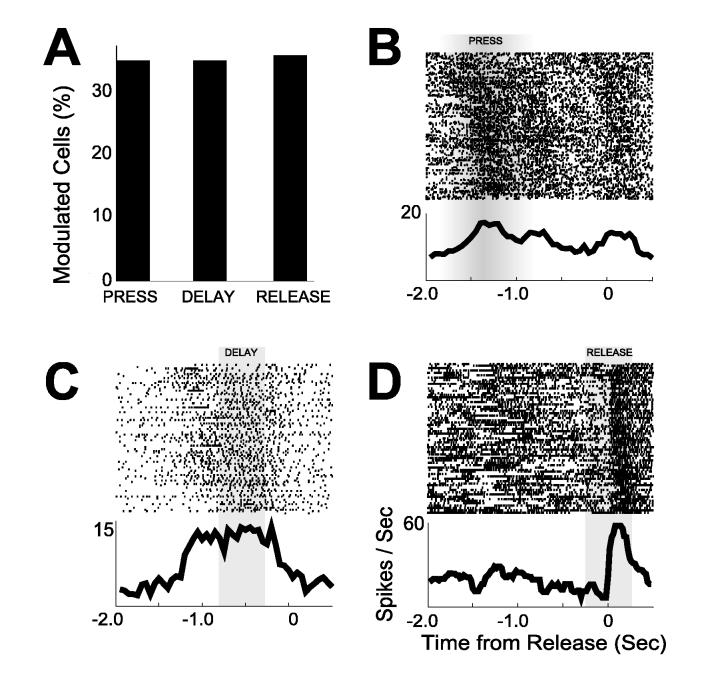
Arrays of microwire electrodes (16 50-μm stainless steel wires, insulated with Teflon, arranged in 3×3×2, 2×8, or 4×4 configurations with spacing between wires of approximately 200 μm; impedances in the range of 200-300 kΩ, as measured in vitro at 1kHz) were implanted in the dmPFC (AP: +3.2, ML ± 1.4, DV −3.6 @ 12° in the frontal plane; see Fig S1) of well-trained animals. A total of 212 single neurons (on 264 microwires, 0.8 units per wire; see Fig S2 for details on unit isolation) were recorded during the delayed-response task. Of these, 122 neurons (58%) were modulated by behavior; that is, firing rates during the epoch from 2000 ms before to 500 ms after lever release were higher (or lower) than that obtained in a matching set of epochs around pseudo-randomly chosen event times during the behavioral session. Of the task-modulated neurons, 71 neurons (34% of the total) were modulated around lever press (250 ms before to 250 ms after lever press), 72 neurons (34%) were modulated during the delay period while animals were waiting to respond (800 ms before to 300 ms before lever release), and 74 neurons (35%) were modulated around lever release (250 ms before to 250 ms after lever release) (Fig 2). Each animal (N=11) had at least 3 neurons modulated during the delay period (average: 6.5±1.5 delay neurons per animal or 36%±5%; range: 10%-47%). Of the neurons modulated during the delay period, 17 were modulated exclusively during the delay. Other neurons were modulated in conjunction with release (18 neurons), press (9 neurons), or press and release (28 neurons).
Figure 2.
(A) Percentage of neurons with significant press, delay, and release activity among neuronal ensembles recorded from dmPFC. Example of single neurons modulated around (B) press, (C) delay, and (D) release. Rasters aligned to lever release. Gray regions indicate temporal epochs used to assess the statistical significance of event-related modulation of firing rate. In (B), modulation was assessed using rasters aligned to lever press. Rasters are sorted by lever press duration with long lever presses at the top.
Activity of dmPFC neurons predicts premature errors
If neurons in dmPFC are involved in actively inhibiting responding during the delay period, the firing rates of the neurons should be correlated with task performance. To quantify these relationships, we trained statistical classifiers, using the Regularized Discriminant Analysis of Friedman (1989), to discriminate between neuronal activity from correct trials and premature error trials (Laubach, 2004). Classifier performance was assessed using the AUC (Area Under the Curve derived from ROC analysis) and predictive mutual information (IAB) (see supplementary methods). Neuronal activity only around lever release (from 250 ms before to 100 ms after release) was examined; outside this epoch, premature trials are difficult to compare with correct trials due to differences in movements (Laubach et al., 2000).
Many neurons in dmPFC (61 of 212 or 29%, 5.5±1.3 predictive cells per animal or 28%±4%; range: 10%-50%) discriminated between correct and error trials significantly better than could expected by chance (AUC > 0.5; see supplementary methods) with an average AUC of 0.63±0.01 (IAB: 0.10±0.01 bits). Twenty-one of these neurons (53%) were modulated around lever press, 20 neurons (51%) were modulated during the delay period while animals were waiting to respond, 18 neurons (40%) were modulated around lever release, and 9 neurons (23%) were not significantly modulated by any task events but fired differently on correct and premature error trials.
To determine if neuronal activity on error trials was associated with random or structured patterns of neuronal activity, we trained classifiers with features defined only by neuronal activity on error trials (see supplementary methods for details). The rationale for this analysis was that if dmPFC fired in noisy or apparently random patterns on error trials, then classifiers trained with features from error trials would not discriminate between correct and error trials. By contrast, if dmPFC fired in consistent patterns that were distinct on correct and error trials, then classifiers could be successfully trained with features from both correct trials (described above) and error trials.
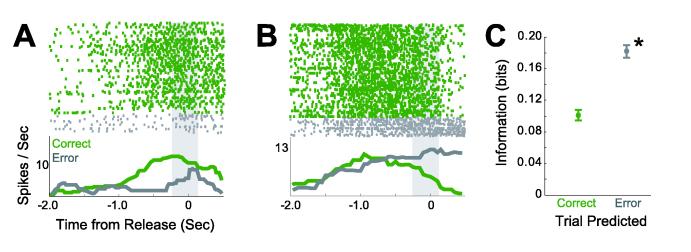
We found that activity on error trials from 33% of neurons in dmPFC (70 of 212) could be used to train classifiers to successfully discriminate between correct and error trials (Fig 3A-B). Ten (of 11) animals had at least 3 neurons whose activity that predicted premature errors from error trials (average: 6.36±1.2 delay neurons per animal or 32%±5%; range: 19-50%). On average, these neurons had an AUC of 0.66±0.01 (IAB: 0.18±0.01 bits). Furthermore, dmPFC neuronal activity predicted premature errors significantly better than correct trials (paired T(1, 211) = 7.74, p < 10−12; Fig 3C). These results are convergent with earlier work in primates (Niki and Watanabe, 1976; Niki and Watanabe, 1979) and suggest that dmPFC neurons fire in consistent and distinct patterns on trials with correct and premature error responses in the delayed-response task.
Figure 3.
Examples of neurons that fired differently on correct trials (green) and on premature error trials (gray). (A) Neuron that predicted correct trials (IAB = 0.21 bits) better than premature errors (IAB = 0.16 bits). (B) Neuron that predicted premature errors (IAB = 0.24 bits) better than correct trials (IAB = 0.10 bits). Gray regions indicate temporal epochs analyzed with statistical classifiers. (C) Neurons in dmPFC predict premature errors (mean ± SEM) significantly better than correct trials. Asterisk indicates significance (p < 0.005).
We also examined raw firing rates around lever release (250 ms before to 100 ms after lever release) on correct vs. premature trials. Twenty-one percent of neurons (44 of 212) fired at significantly different rates (p <0.05 via a Wilcoxon sign test comparing firing rates on correct vs. premature trials) on correct and premature trials. Of these, 71% (31 of 44 neurons) had greater firing rates on premature trials relative to correct trials, and 29% (13 of 44) had greater firing rates on correct trials relative to premature trials. Activity from half of these neurons (50%; 22 of 44) resulted in significant discriminations between correct and error trials by statistical classifiers, as described above.
Experiment 2: Reversible dmPFC Inactivation and Motor Cortex Ensemble Recordings
Inactivation of dmPFC increases premature responding
Thus far, we have demonstrated that dmPFC neurons are modulated while animals are waiting to respond. These data, in combination with previous studies, (Broersen and Uylings, 1999; Narayanan et al., 2006; Risterucci et al., 2003), suggest that dmPFC encodes a temporal response rule that controls activity in the motor system.
To test this hypothesis, we reversibly inactivated dmPFC while recording neuronal ensemble activity in motor cortex during the delayed-response task (Fig 1). Six animals were implanted with bilateral cannulae in dmPFC (coordinates identical to Experiment 1; Fig S3) and microwire arrays (identical to Experiment 1) in the motor cortex contralateral to the limb used to press the lever (AP: −0.5, ML: ± 2.5-3.5, DV: −1.5 @ −25° in the sagittal plane; see Fig S3) (Donoghue and Wise, 1982; Neafsey et al., 1986). Animals were tested with trigger stimuli on 50% of trials, so that we could dissociate effects of dmPFC on stimulus-evoked and response-related firing in motor cortex.
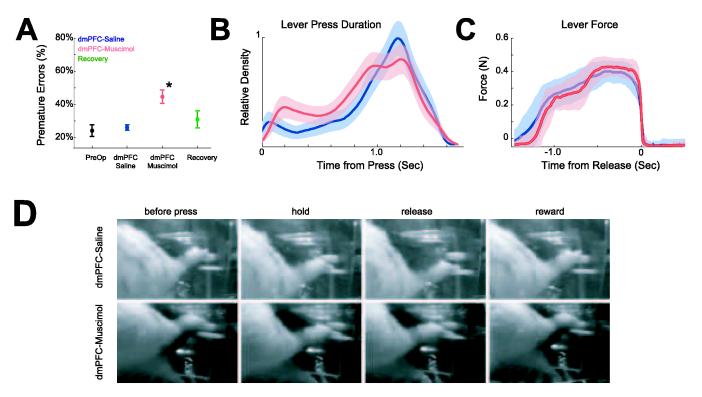
As we reported in a previous study (Narayanan et al. 2006), no significant changes in behavior were observed following surgery (preoperative premature errors: 24±3%; postoperative premature errors, 26±2%; paired T(1, 5) = 0.54, p < 0.61). In sessions with dmPFC inactivated, premature errors increased significantly to 45±4% (paired T(1, 5) = 5.02, p < 0.004). An important point for our neurophysiological analysis was that an equivalent number of trials were performed in control sessions and in sessions with dmPFC inactivated (234±17 trials in control sessions vs 244±27 trials in sessions with dmPFC inactivated; paired T(1, 5) = 1.43, p < 0.21). Animals recovered within 24 hours (31±5% premature errors in recovery sessions; paired T(1, 5) = 0.84, p < 0.44 for sessions with saline infused into dmPFC and the recovery sessions; Fig 4A). Inactivation of dmPFC also shortened average lever press durations over all trials (1134±20 ms in control session vs 1011±37ms in sessions with dmPFC inactivated; paired T(1, 5) = 3.5, p < 0.02) (Fig 4B) but did not change the percentage of late responses (16±3% of trials in control sessions vs 17±4% of trials with dmPFC inactivated; paired T(1, 5) = 0.52, p < 0.6). These effects were observed in all six animals.
Figure 4.
(A) Percentage of premature errors in six rats during preoperative behavior, in control sessions (with saline infused into dmPFC; blue), in sessions with dmPFC inactivated via muscimol (red), and in recovery sessions (green). Asterisk indicates significance (p < 0.005). (B) Kernel density estimates of the distribution of lever press duration (from press to release) for all responses in control sessions (blue) and in sessions with dmPFC inactivated (red). (C) Lever force data revealed that rats exerted similar levels of force on the lever with and without dmPFC inactivated. (D) Video data suggests that dmPFC inactivation does not change motor behavior (See supplementary videos).
Inactivation of dmPFC does not change motor control
Dorsomedial PFC inactivation might alter the ability of rats to make controlled lever presses (Whishaw et al., 1992); however, we found that this was not the case in our previous study (Narayanan et al. 2006). To ensure this issue did not affect our neurophysiological analysis, we examined lever force data, lever position data, and behavioral video recordings in control and in dmPFC-inactivation sessions. Response force was equivalent between correct trials in control sessions and in sessions with dmPFC inactivated (T(1, 276) = 0.78, p < 0.44; Fig 4C). Video recordings showed no qualitative differences in the way rats pressed the lever following infusions of saline or muscimol into dmPFC (Fig 4D; Fig S4; supplementary videos 1 and 2).
Inactivation of dmPFC does not change basic motor cortex activity
Dorsomedial PFC inactivation might have nonspecific effects on motor cortex (Brunia 1999), including changes in the basal firing properties of motor cortical neurons. However, we found no evidence for such an effect in our studies. Activity from 90 neurons (on 96 microwires; 0.9 units isolated per wire) was recorded in control sessions. The same number of neurons was also recorded in sessions with dmPFC inactivated. Single units recorded in control and dmPFC-inactivation sessions had equivalent median interspike intervals (T(1, 178) = 0.65, p < 0.52) and average firing rates (8.9±0.8 Hz in control sessions vs 9.7±0.9 Hz with dmPFC inactivated; T(1,178) = 0.55, p < 0.58). The neurons also fired with the same level of bursting (Legendy and Salcman, 1985) in the control and dmPFC-inactivation sessions (percentage of spikes in bursts: T(1, 178) = 0.24, p < 0.81; average surprise entropy of bursts: T(1, 178) = 0.15, p < 0.88). In addition, dmPFC inactivation did not change the overall level of neuronal firing as measured with recordings of multiunit activity (i.e., thresholded but unsorted waveforms) and local field potentials (<200 Hz) (see supplementary results; Fig S5). These data do not provide any evidence that dmPFC inactivation changes the basic firing properties of motor cortex.
Inactivation of dmPFC decreases motor cortex activity during delay periods
If dmPFC exerts specific control over motor cortex, we would expect to find alterations in delay-related firing in motor cortex with dmPFC inactivated. On the other hand, if dmPFC nonspecifically influenced motor cortex activity, we would expect to find alterations in event-related firing across task events (lever press, delay and lever release). To distinguish between these possibilities, we compared the percentage of task-modulated neurons as a function of dmPFC inactivation around lever press (250 ms before to 250 ms after lever press), during the delay period (800 ms before to 300 ms before release), and around lever release (250 ms before to 250 ms after lever release). Only correct trials were included in this analysis, as on premature errors short lever presses (<750 ms) interfered with attempts to assign modulations to a particular event.
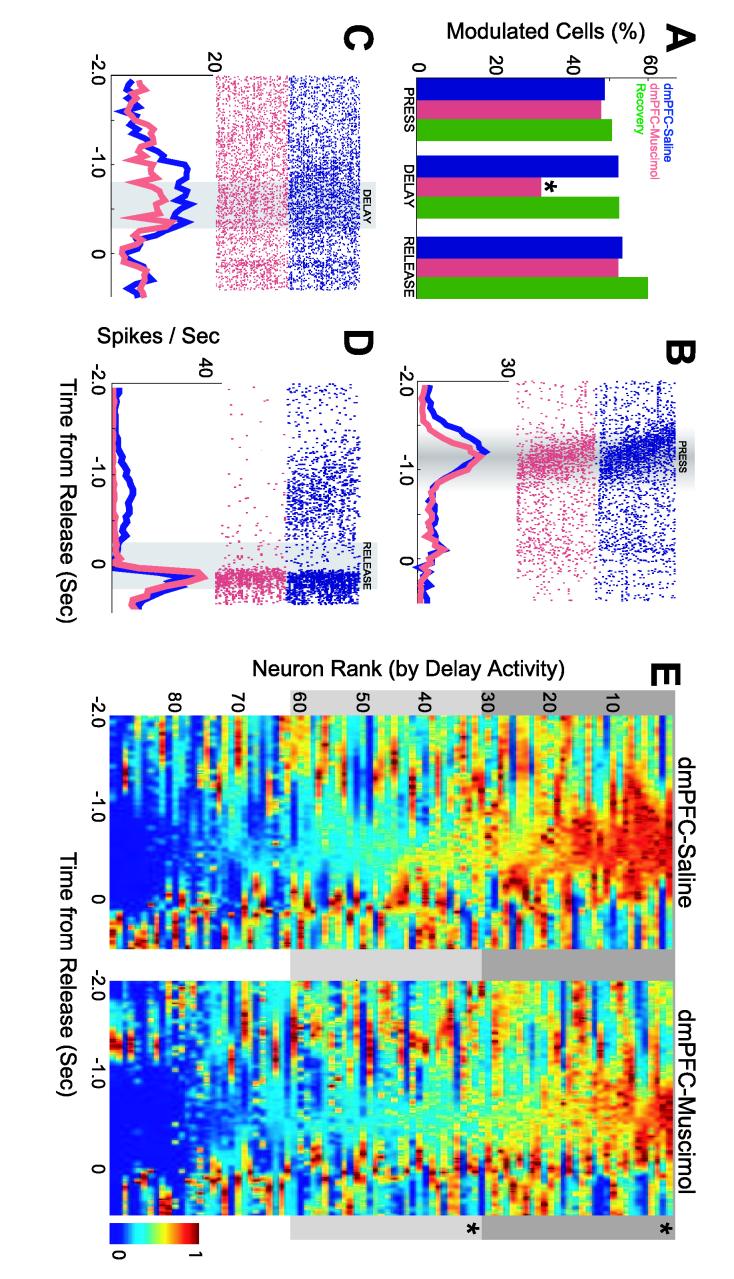
Inactivation of dmPFC specifically reduced the extent of delay-related modulation in the motor cortex. Significantly more neurons were modulated during the delay period in control sessions (47 neurons, 52%) as compared to sessions with dmPFC inactivated (29 neurons; 32%; χ2=7.37, p < 0.004). In five animals (one animal had only 2 neurons and was excluded from within-subjects analyses), an average of 7.8±1.6 delay-modulated neurons (52±2%; range 44-60%) were recorded during control sessions, significantly decreasing to 4.83±1.9 delay-modulated neurons (38±14%; range 7%-54%; repeated measures ANOVA using a Generalized Linear Model: F(1, 5) = 9.42, p < 0.02) during sessions with dmPFC inactivated. By contrast, similar numbers of neurons were modulated around lever press in control sessions (44 of 90 neurons, 49%) compared to sessions with dmPFC inactivated (43 neurons; 48%; χ2=0.022, p < 1.0). Similar numbers of neurons were also modulated around lever release in sessions with dmPFC inactivated (48 neurons, 53%) compared to control sessions (47 neurons; 52%; χ2=0.022, p < 1.0; Fig 5A). Importantly, response properties returned to control levels in recovery sessions (24 hr after dmPFC inactivation): 46 neurons of 90 neurons were modulated by press (51%; χ2=0.089, p < 1.0 comparing control and recovery sessions), 48 neurons were modulated by delay (53%; χ2=0.022, p < 1.0), and 54 neurons were modulated by release (60%; χ2=1.11 p < 0.22).
Figure 5.
(A) Percentage of neurons with significant press, delay, and release activity in control sessions (blue), in sessions with dmPFC inactivated (red), and in recovery sessions (green). Example of single neurons modulated by (B) press, (C) delay, and (D) release in control sessions (blue) and in sessions with dmPFC inactivated. Rasters aligned to lever release. Gray regions indicate temporal epochs used to assess the statistical significance of event-related modulation of firing rate. In (B), modulation was assessed using rasters aligned to lever press. (E) Normalized peri-event histograms for all neurons recorded in control sessions (left panel) and sessions with dmPFC inactivated (right panel). The order of neurons is sorted by firing rate during the delay period. The top two-thirds of motor cortical neurons fired at reduced rates in sessions with dmPFC inactivated as compared to control sessions. Asterisk indicates significance (p <0.005).
In some cases (20 of 90 neurons; 22%), we recorded from the same single neurons in control and dmPFC-inactivation sessions (This assessment was based on waveform shape, firing rate distributions, and interspike intervals). Examples of the effects of dmPFC inactivation on these putative single neurons with press, delay, and release activity are shown in Fig 5B-D. In these neurons, dmPFC inactivation specifically attenuated delay-related activity while having no effect on movement-related activity related to pressing and releasing the lever.
The reduction in delay-related neurons was also apparent in comparisons of firing rates before lever release in control and dmPFC-inactivation sessions. In Figure 5E, the average responses of neurons in control and dmPFC-inactivation sessions are shown and sorted by firing rates during the delay period (800 to 300 ms prior to lever release). The top two-thirds of neurons, as ranked by delay activity, fired at higher rates in control sessions than in dmPFC inactivation sessions (T(1, 58) = 4.19, p <10−4; for the middle 1/3 neurons: T(1, 58) = 2.95, p < 0.005; bottom 1/3: T(1, 58) = 0.9, p < 0.33) (Fig 5E).
To investigate if motor cortex activity was influenced by the trigger stimuli, we examined firing rates between 1000 and 1100 ms after lever press (when the trigger stimulus would occur on 50% of trials) on trials with and without trigger stimuli. We found no evidence for differences in motor cortex firing rates during this epoch in control sessions (paired T(1, 89)=1.08, p< 0.28). We also found no difference in the fraction of neurons that were modulated on trials with and without trigger stimuli (χ =0.91, p < 0.26). Furthermore, we found no differences in motor cortex firing rates or modulation on trials with and without trigger stimuli in sessions with dmPFC inactivated (Firing rate: paired T(1,89) = 1.24, p < 0.21; Modulation: χ2=0.03, p <1). These data suggest that activity in motor cortex is not altered by the trigger stimulus itself. This result is not surprising as our task has a fixed delay and animals could use a timing strategy to initiate responding (Narayanan et al., 2006).
Together, these results indicate that dmPFC inactivation specifically alters motor cortex neurons with delay activity. This finding is consistent with the view that dmPFC has a top-down role in controlling motor cortical activity during the delayed-response task.
Inactivation of dmPFC alters motor cortex prediction of premature errors
Previous studies have revealed that neuronal activity in rodent motor cortex is correlated with successful waiting behavior (Laubach et al., 2000; Narayanan et al., 2005). To examine if inactivation of dmPFC altered this relationship, we trained statistical classifiers to discriminate between trials with correct responses and premature errors. Experiment 1 revealed that dmPFC neurons strongly predicted premature errors. This result suggests that dmPFC inactivation should preferentially alter prediction of premature error trials by motor cortical neurons (i.e., using task-related features from premature error trials to predict correct or premature error trials).
To test this hypothesis, we made comparisons between predictions of premature errors (i.e., using task-related features from premature errors) in control and dmPFC-inactivation sessions from motor cortex activity. In control sessions, activity from 36% of neurons (32 of 90) predicted premature errors with an AUC of 0.68±0.02 (IAB: 0.09±0.01 bits). In each animal (of 6 in this experiment), activity from an average of 5.5±1.6 neurons (31±7%; range 0-44%) predicted premature errors. In sessions with dmPFC inactivated, activity from 27% of neurons (26 of 90) predicted premature errors with an AUC of 0.63±0.01 (IAB: 0.03±0.01 bits). Predictions of premature errors were significantly decreased by dmPFC inactivation (T(1, 178) = 4.4, p < 10−4).
We also made comparisons between predictions of correct trials (i.e., using task-related features from correct trials) in control and dmPFC-inactivation sessions from motor cortex activity. In control sessions, activity from 37% of neurons (33 of 90) predicted correct trials with an AUC of 0.65±0.02 (IAB: 0.08±0.01 bits). In each animal (of 6), activity from an average of 5.8±1.6 neurons (34±7%; range 0-48%) predicted correct trials. In sessions with dmPFC inactivated, activity from 39% of neurons (35 of 90) predicted correct trials with an AUC of 0.74±0.01 (IAB: 0.12±0.01 bits). Predictions of correct trials were not significantly altered by dmPFC inactivation (T(1, 178) = 1.8, p < 0.07). Finally, unlike neuronal activity in dmPFC, neuronal activity in motor cortex predicted correct and premature error trials equivalently in control sessions (paired T(1, 178) = 0.48, p < 0.63).
To see if dmPFC influenced relationships between motor cortex and reaction time, we also examined the correlation between firing rates of motor cortical neurons and reaction times in control and dmPFC-inactivation sessions. In control sessions, correlation coefficients between firing rates of motor cortical neurons at the time of the response (from 0 to 500 ms after the end of the delay period) and reaction times had an average absolute value of 0.23±0.2 (range: −0.67 to 0.80). In sessions with dmPFC inactivated, correlation coefficients between firing rates of motor cortical neurons and reaction times had an average absolute value of 0.20±0.2 (range: −0.71 to 0.75). We observed no change in the relationship between motor cortex neurons and reaction time as a function of dmPFC inactivation (T(1, 178)=1.01, p < 0.31).
Our results suggest that dmPFC inactivation specifically diminishes predictions of premature errors that can be made by using spiking activity from motor cortical neurons, while predictions of correct trials and correlations with reaction times are unchanged.
Experiment 3: Simultaneous recordings from dmPFC and Motor Cortex
Pairs of dmPFC and motor cortex neurons reveal delay-related correlations
In order to further test the hypothesis that dmPFC exerts specific control on motor cortex, we recorded simultaneously from ensembles of neurons in dmPFC and motor cortex. If dmPFC specifically controls delay-related activity in motor cortex, we expect to find delay-related correlations between dmPFC and motor cortex.
Six animals trained to perform the delayed-response task were implanted with microwire arrays in both dmPFC and motor cortex (data from arrays in dmPFC in these animals were also reported above in Experiment 1; dmPFC was implanted unilaterally and ipsilateral to the motor cortex array). On average, in each rat 17.1±3.1 neurons were recorded from dmPFC while 6.5±2.7 neurons were recorded from motor cortex. As in Experiments 1 and 2, neurons in dmPFC and motor cortex were significantly modulated while animals were waiting to respond (dmPFC: 30 / 107 neurons, 28%; Motor cortex: 17 / 39 neurons, 44%).
To quantify functional coupling between dmPFC and motor cortical neurons, we used the joint peri-stimulus time histogram (JPSTH) technique (Aertsen et al., 1989). This method normalizes the cross-correlation function between a pair of neurons by accounting for bin-by-bin fluctuations in the neurons' firing rates. Peaks in the JPSTH represent an increased probability of temporal relationships between spikes from one neuron given spikes of another neuron.
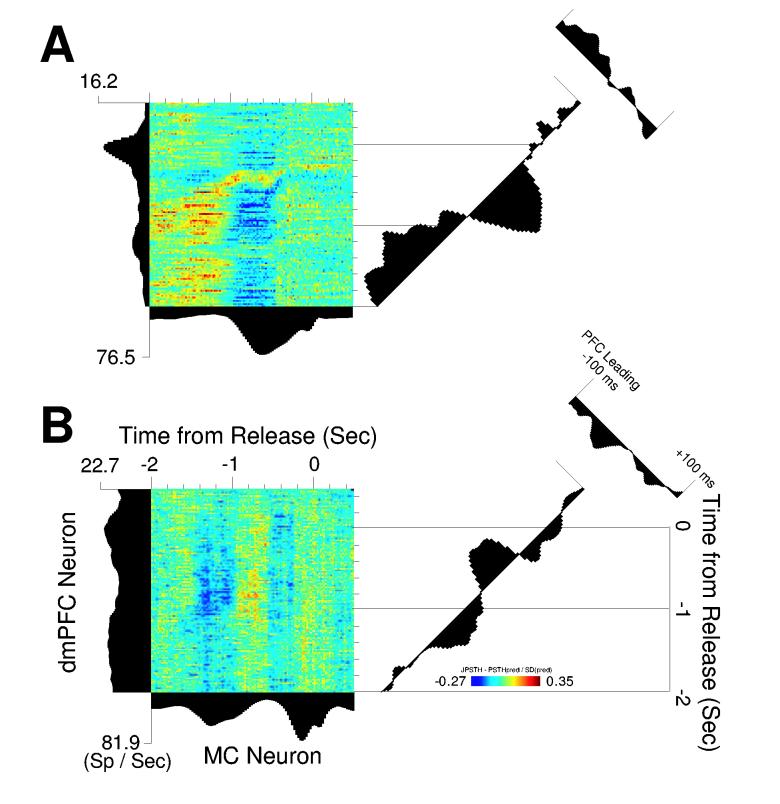
We found many interesting functional interactions between neurons in dmPFC and motor cortex. For instance, one pair of neurons recorded simultaneously in dmPFC and motor cortex had prominent positive correlations during the initial delay period (Fig 6A). Later, the neurons became anti-correlated; the motor cortical neuron increased its firing rate as the dmPFC neuron fired at a reduced rate. We also found interactions that were independent of task-related modulations (Vaadia et al., 1995). For example (Fig 6B), a dmPFC neuron that lacked task-modulated activity nevertheless had prominent delay-related positive correlations with a task-modulated motor cortex neuron. In both cases (Fig 6A-B), the normal cross-correlation function (time-averaged correlation over an epoch of ±100 ms) showed that activity of dmPFC neurons led activity of motor cortical neurons (Fig 6A-B, right most panels).
Figure 6.
(A) Functional coupling during the delay period is apparent in simultaneously recorded pairs of neurons in dmPFC (vertical axis) and motor cortex (horizontal axis). This pair is initially positively correlated and then becomes negatively correlated as the rat waits to respond (diagonal axis). Spikes from the dmPFC neuron led those from the motor cortical neuron by −50 ms. (B) Another pair of neurons in dmPFC and motor cortex with delay-related functional coupling. This pair is negatively correlated early in the delay period and then becomes positively correlated. Importantly, in this example functional coupling exists despite the weak modulation of the dmPFC neuron during the trial.
To estimate the frequency of task-related functional coupling between dmPFC and motor cortical neurons, we made comparisons between temporal correlations during three trial epochs: lever press (250 ms before to 250 ms after lever press), during the delay period when animals were waiting to respond (800 ms before to 300 ms before release), and around lever release (250 ms before to 250 ms after lever release). These comparisons revealed that significant temporal correlations (significance was determined by destroying correlations via trial-shuffling and revealed that a JPSTH value of 0.225 corresponded to a p value of 0.005; see supplementary methods) between dmPFC and motor cortical neurons were most common during the delay period (36 of 581 pairs, 6.2 %; more than could be expected by chance: χ2= 24.46, p < 10−6; average: 9±4 pairs per animal; range: 0-21 interactions; significant interactions found in 4 of 6 animals). Around lever press, 3.8% of pairs (22 of 581; more than chance: χ2=10.96, p < 0.001) had significant interactions. Around lever release, few pairs (12 of 581, 2%; not more than chance: χ2= 2.92, p < 0.1) showed significant interactions (Fig 7). Pairs of dmPFC and motor cortex neurons revealed more significant interactions during delay than during release (paired T(1, 581)=6.20, p < 10−9). Significant peaks in time-averaged cross-correlation (i.e., standard cross-correlation analysis) were not found at timescales less than 20 ms. This result suggests that the functional couplings between dmPFC and motor cortical neurons, described above, were not due to direct, synaptic interactions between the neurons.
Figure 7.
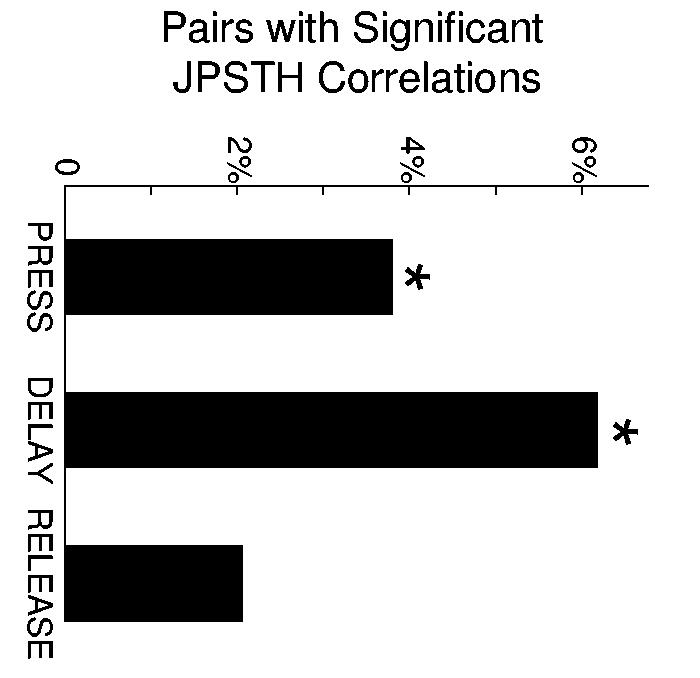
Percentage of neuronal pairs with significant functional coupling around press, delay, and release epochs. Asterisk indicates that more neuronal pairs with significant JPSTH correlations were found than expected by chance (p < 0.005).
Our paired recording experiments indicate that neurons in dmPFC and motor cortex are functionally coupled, particularly during the delay period. These results are convergent with those from Experiments 1 and 2. Together, our experiments suggest that dmPFC has a specific influence of neuronal activity in motor cortex as animals wait for trigger stimuli.
DISCUSSION
We tested the hypothesis that dmPFC specifically controls neurons in motor cortex during delay periods in a delayed-response task. In Experiment 1, we found that about one-third of neurons in dmPFC were significantly modulated during the delay period. By analyzing these neurons with statistical classifiers, we found that many of these neurons were predictive of premature errors in the task. In Experiment 2, we reversibly inactivated dmPFC while recording from motor cortex ensembles during delayed-response performance. Consistent with previous results (Narayanan et al., 2006), we found that dmPFC inactivation increased premature errors. Critically, we found that dmPFC inactivation specifically decreased delay-related firing of motor cortex neurons but did not alter movement-related firing (i.e., lever press and release). In Experiment 3, we simultaneously recorded pairs of neurons in dmPFC and in motor cortex during delayed-response performance and found that dmPFC and motor cortex neurons fired in a correlated manner during the delay period but not during the motor response (lever release). These data provide novel evidence that temporal control over behavior is achieved by a top-down signal from prefrontal cortex that acts on motor cortical neurons during the delay period.
Prefrontal cortex and top-down control
The prefrontal cortex is thought to exert top-down control on other brain systems to guide behavior according to internal states or intentions (Brunia, 1999; Fuster, 2000; Miller and D'Esposito, 2005; Miller and Cohen, 2001). While few studies have investigated how prefrontal and motor regions interact, several studies have investigated top-down influences of prefrontal cortex on other cortical areas. Fuster et al., (1985) and Chafee and Goldman-Rakic, (2000) found that inactivating primate prefrontal regions attenuates the activity of parietal neurons to behaviorally relevant cues. Tomita et al., (1999) recorded from IT neurons during a visual recall task, and found that IT neurons could receive information about stimulus identity from prefrontal regions in the absence of bottom-up activity. Moore and colleagues (Moore and Armstrong, 2003; Moore and Fallah, 2004) found that stimulation of the frontal eye fields altered the receptive field structure of neurons in V4. Finally, Winkowski and Knudsen (2006) report that microstimulation of neurons in the avian forebrain (analogous to mammalian prefrontal regions) shifted spatial tuning of neurons on the optic tectum. Each of these studies investigates the role of frontal brain regions on modulating sensory systems in accordance with behaviorally relevant goals.
The present study extends this body of work to the motor cortex. Our data suggest that dmPFC activity controls delay period firing by neurons in motor cortex (Fig 8), which, in turn, directs the execution of movement. These data are convergent with a study (Rowe et al., 2005), which reports that prefrontal and motor regions increase their correlation in the BOLD signal during a task in which subjects freely select actions but not objects. Importantly, our results do not shed light onto how responses are inhibited by the motor cortex. Rather than having an inhibitory effect on motor cortex that suppresses a response, the neurons we recorded in rat dmPFC seem instead to supply motor cortex with information about the expected timing of the trigger stimulus. We therefore speculate that the actual inhibition of temporally inappropriate responses is achieved by activity in motor cortex itself or in a downstream component of the motor system.
Figure 8.
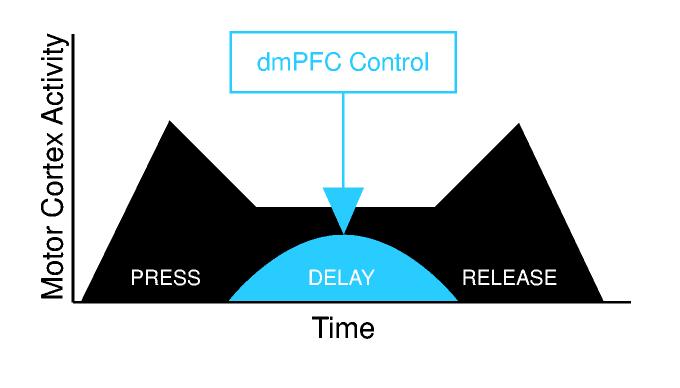
During delayed-response performance, activity in motor cortex (blue) is prominently modulated during lever press, delay, and lever release. While animals are holding down the lever and waiting to respond, delay-related activity in motor cortex is influenced by top-down control from dmPFC (red) in order to inhibit temporally inappropriate responses.
Prefrontal cortex and rule-based behavior
The prefrontal cortex has been hypothesized to be involved in hierarchically coordinating and planning motor actions (Fuster, 1997; Fuster, 2000). Many studies have suggested that prefrontal neurons encode the “rules of the game” (Miller and Cohen, 2001). In a delayed-response task, these rules would include which response to make to a given stimulus and when to make the response. Several recent studies suggest that prefrontal regions are involved in temporal processing. A recent study of primate dorsolateral PFC found neurons that were sensitive to the timing of trigger stimuli in a delayed-response task (Genovesio et al., 2005). Recent neuroimaging and lesion studies implicated prefrontal regions in temporal processing (Li et al., 2006; Naito et al., 2000; Stuss et al., 2005). These studies, together with the results reported here, suggest that dmPFC is involved in encoding temporal information that is used to guide behavior, an idea first proposed by Fuster (2000).
A further piece of evidence that dmPFC is involved in rule-based processing is the strong error predictions of dmPFC neurons. Neurons in dmPFC that predict errors strongly often have increased delay-related firing rates around the time of lever response that on correct trials would have returned to baseline (Fig 3B). These profiles of neural activity in dmPFC, combined with sustained delay-related increases firing, are very similar to those described by Niki and Watanabe (1976; 1979) in the primate dmPFC in a similar task. Our results extend this classic work, as we are able to show for the first time that the brain area containing such delay-related neurons is directly responsible for inhibiting responding during the delay period and alters the delay-related activity of motor cortex.
Previous studies have interpreted the influence of prefrontal regions on other areas as related to attention (Moore and Armstrong, 2003; Moore and Fallah, 2004) or working memory (Chafee and Goldman-Rakic, 2000). However, in the present study, we believe these processes were not altered by dmPFC inactivation. If rodent dmPFC were to mediate attention to the expected time of the trigger stimulus, then dmPFC inactivation should affect attention-related processing in motor cortex. In Experiment 2, we found no differences in motor cortex activity as a function of stimulus presentation in control sessions or in sessions with dmPFC inactivation. Moreover, our previous study found equivalent effects of dmPFC inactivation on premature responding in sessions with single or multiple delay periods. If the effects of dmPFC inactivation were due to effects on attending to the stimulus, we would have found increased premature responding in sessions with multiple delay period, as increasing the number of delay periods increases the uncertainty about the occurrence of the trigger stimuli (Narayanan et al., 2006). Taken together, these data suggest that dmPFC inactivation did not alter attention to the trigger stimulus.
If the role of rodent dmPFC in the task used in the present study is related to working memory, then there should be increased premature responding following inactivation of dmPFC as the length of the delay period is increased. It is possible that suppressing the tendency to respond during the delay period is similar to suppressing responses to distracting stimuli in a working memory task and that this function involves a form of working memory (Chelazzi et al., 1993; Moran and Desimone, 1985). However, our previous study found equivalent effects of dmPFC inactivation on premature responding in sessions with relatively short and long delay periods (500-1000 and 900-1400 ms) (Narayanan et al., 2006). As working memory processing should be sensitive to delay length, it is unlikely to account for the interactions between dmPFC and motor cortex described in our study. However, as our study lacks an overt mnemonic component, future studies which use tasks that explicitly engage working memory will test the role of rodent dmPFC in maintaining information online.
If dmPFC does not mediate working memory or attention, what is its functional basis of top-down control over motor cortex? A classic account of temporal processing in tasks with predictable temporal properties (the task used in this study has a fixed delay of 1000 ms) is that subjects employ a task strategy based on the expected timing of the trigger stimulus (Janssen and Shadlen, 2005; Los et al., 2001; Ollman and Billington, 1972). These expectancies lead to rules about how to perform the task, e.g., wait for 1 sec and respond whether or not the trigger stimulus has occurred. This kind of rule has been called the “deadline” for initiating a response in a simple RT task (Narayanan et al., 2006; Ollman and Billington, 1972). The profile of correlations between dmPFC and MC described in our study might represent functional interactions relevant for encoding this response deadline.
An alternative hypothesis suggests that effects of dmPFC inactivation are exhibited by motor cortical neurons only because motor cortex is part of the final common pathway of behavior. This would be true irrespective of direct influences of dmPFC on the motor cortex. According to this hypothesis, the system would be functionally independent and we would expect to find the greatest influence of dmPFC on motor cortex during movements such as lever release, when motor regions are involved in generating a response. Contrary to this idea, we found that the inactivation of dmPFC did not alter movement-related firing by motor cortical neurons (during lever presses and releases), did not change measures of motor performance (force on the lever, Figure 4C and the Supplement), and did not alter the relationship between response-related firing in motor cortex and the reaction time.
Moreover, temporal correlations between dmPFC and motor cortical neurons were greatest during the delay period and minimal at the times of response (lever release; press-related correlations may be related to the beginning of temporal control). This is despite the fact that neurons in both cortical areas were modulated around the times of the lever press, the delay period, and the lever release. These results suggest that dmPFC exerts specific, delay-related control on motor cortex.
It is also important to point out that our data do not provide evidence that prefrontal regions are sufficient to encode elapsed time. While there have been no studies in rodents on this topic, work in non-human primates suggest that neurons in prefrontal (Genovesio et al., 2006) and parietal regions (Janssen and Shadlen, 2005; Leon and Shadlen, 2003; Maimon and Assad, 2006) encode the timing of task events. It is possible that the neurons we report in dmPFC are also involved in such computations. However, the experiments carried out in the present study are not suitable for making this distinction. Instead, we would need to use a task with multiple stimulus times, as in the Janssen and Shadlen (2005) study. As such, we are at present unable to comment on whether neurons in dmPFC are sensitive to the temporal probabilities of stimulus delivery in our delayed-response task.
Connections between dmPFC and the motor system
We found no evidence for direct, monosynaptic connections between dmPFC and motor cortical neurons. This result is to be expected from tract-tracing studies that have found no evidence for direct projections from dmPFC to the motor cortex (Conde et al., 1995; Sesack et al., 1989). As shown in Figure 6, the time-averaged cross-correlation (small plots in the upper right of each panel) between neurons in these cortical areas was observed on a broad time scale and did not contain the central, short timescale peaks usually associated with common inputs. This result suggests that the functional coupling between dmPFC and motor cortex occurs at the network level.
There are several pathways through which dmPFC and MC might share network connections. There are connections between dmPFC areas and the medial agranular cortex (Van Eden et al., 1992), which contains the rostral forelimb area (Neafsey and Sievert, 1982), as well as the agranular insular cortex, the posterior region of which innervates the rostral and caudal forelimb areas (Kyuhou and Gemba, 2002). The subthalamic nucleus receives input from dmPFC (Ryan and Clark, 1991). Inactivation of this region also increased premature errors (Baunez and Robbins, 1997); however, these effects might be a result of errors in motor performance (Wichmann et al., 1994) rather than waiting behavior. Dorsomedial PFC also could control motor cortical firing through the thalamus, dorsal striatum, and monoaminergic nuclei in the brainstem. Indeed, dmPFC projects to the dorsal raphe nucleus and locus coeruleus (Lee et al., 2005; Sesack et al., 1989), which heavily innervate motor cortex (Loughlin et al., 1982; Waterhouse et al., 1986), and to the pedunculopontine nucleus (Sesack et al., 1989). Of these brain regions, the rostral forelimb area is considered to be a premotor area (Neafsey et al., 1986) and is a likely candidate for mediating functional interactions between dmPFC and motor cortical neurons. This area receives projections from dmPFC (Reep et al. 1987) and projects heavily to the motor cortex and spinal cord (Rouiller et al., 1993). Elucidating the complex network connecting dmPFC to motor cortex will require functional anatomy followed by inactivation and neurophysiological studies in behaving animals.
EXPERIMENTAL PROCEDURES
Subjects
In Experiment 1, eleven male Long-Evans rats (aged 3-4 months) were trained to perform a delayed-response task and had microwire arrays implanted (5 bilaterally, 6 unilaterally) in dmPFC. In Experiment 2, eight male Long-Evans rats (aged 3-4 months) were identically trained and had cannulae implanted in dmPFC and microwire arrays implanted in motor cortex. Two other animals did not perform enough trials with dmPFC inactivated (22 and 49 trials versus 244±27 trials in the other animals) and were excluded from further analysis. In Experiment 3, six male Long-Evans rats included in Experiment 1 also had microwire arrays implanted into motor cortex as well as unilaterally (ipsilateral to the motor cortex array) into dmPFC. The Animal Care and Use Committee at the John B. Pierce Laboratory approved all procedures.
Delayed-response task
Rats were trained to perform a delayed-response task (i.e., a simple reaction time task with a fixed foreperiod) using procedures described in detail in Narayanan et al., (2006) and in the supplementary methods.
Surgery
Arrays of microwire electrodes (NB Labs, Dennison, TX or Neurolinc, New York, NY) or twenty-six gauge guide cannulae (Plastics One, Roanoke, VA) were lowered into dmPFC (Fig S1; Fig S3) or into motor cortex (Fig S3) according to methods described in detail previously (Laubach et al., 2000; Narayanan et al., 2005; Narayanan et al., 2006) and in the supplementary methods.
Electrophysiological recordings
Neuronal ensemble recordings were performed according to methods described in detail previously (Laubach et al., 2000; Narayanan et al., 2005) and in the supplementary methods. Analysis of task-modulations in firing rates and analysis of interactions between dmPFC and motor cortex are described in detail in the supplementary methods.
Reversible inactivation of dmPFC
Reversible inactivation studies of dmPFC (Experiment 2) were carried out according to methods described in detail previously (Narayanan et al., 2006) and in the supplementary methods.
Predicting correct and premature error trials
Neurons with delay- and response-related firing were analyzed using a statistical pattern recognition approach according to methods described in detail previously (Laubach et al., 2000; Narayanan et al., 2005) and in the supplementary methods.
Histology
Once experiments were complete, rats were anesthetized with 100 mg/kg sodium pentobarbital and then transcardially perfused with either 10% formalin or 4% paraformaldehyde. Brains were sectioned on a freezing microtome, mounted on gelatin subbed slides, and Nissl stained with thionin. Electrode locations were visualized using custom written 3-dimensional reconstruction software (Eyal Kimchi, Laubach Lab) based on an atlas of coronal sections by Swanson (1999).
Supplementary Material
ACKNOWLEDGEMENTS
This work was supported by funds from the Tourette Syndrome Association and the John B. Pierce Laboratory for ML and from an NIH training grant to the Yale Medical Scientist Training Program and Army Research Office for NSN. We thank Eyal Kimchi, Nicole Horst, Sander Los, Chris Olivers, Ray Li, James Mazer, and Geoff Schoenbaum for helpful comments on this manuscript, Loretta Di Pietro for statistical consultation, the Instruments Shop at the John B. Pierce Laboratory for outstanding technical support, and David Krupa for advice on reversible inactivation methods.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Aertsen AM, Gerstein GL, Habib MK, Palm G. Dynamics of neuronal firing correlation: modulation of “effective connectivity”. J Neurophysiol. 1989;61:900–917. doi: 10.1152/jn.1989.61.5.900. [DOI] [PubMed] [Google Scholar]
- Barkley R. ADHD and the Nature of Self-control. The Guilford Press; New York, NY: 1997. [Google Scholar]
- Baunez C, Robbins TW. Bilateral lesions of the subthalamic nucleus induce multiple deficits in an attentional task in rats. Eur J Neurosci. 1997;9:2086–2099. doi: 10.1111/j.1460-9568.1997.tb01376.x. [DOI] [PubMed] [Google Scholar]
- Broersen LM, Uylings HB. Visual attention task performance in Wistar and Lister hooded rats: response inhibition deficits after medial prefrontal cortex lesions. Neuroscience. 1999;94:47–57. doi: 10.1016/s0306-4522(99)00312-7. [DOI] [PubMed] [Google Scholar]
- Brunia CH. Neural aspects of anticipatory behavior. Acta Psychol (Amst) 1999;101:213–242. doi: 10.1016/s0001-6918(99)00006-2. [DOI] [PubMed] [Google Scholar]
- Burwell RD. The parahippocampal region: corticocortical connectivity. Ann N Y Acad Sci. 2000;911:25–42. doi: 10.1111/j.1749-6632.2000.tb06717.x. [DOI] [PubMed] [Google Scholar]
- Chafee MV, Goldman-Rakic PS. Inactivation of parietal and prefrontal cortex reveals interdependence of neural activity during memory-guided saccades. J Neurophysiol. 2000;83:1550–1566. doi: 10.1152/jn.2000.83.3.1550. [DOI] [PubMed] [Google Scholar]
- Chelazzi L, Miller EK, Duncan J, Desimone R. A neural basis for visual search in inferior temporal cortex. Nature. 1993;363:345–347. doi: 10.1038/363345a0. [DOI] [PubMed] [Google Scholar]
- Conde F, Maire-Lepoivre E, Audinat E, Crepel F. Afferent connections of the medial frontal cortex of the rat. II. Cortical and subcortical afferents. J Comp Neurol. 1995;352:567–593. doi: 10.1002/cne.903520407. [DOI] [PubMed] [Google Scholar]
- Divac I, Mogensen J, Petrovic-Minic B, Zilles K, Regidor J. Cortical projections of the thalamic mediodorsal nucleus in the rat. Definition of the prefrontal cortex. Acta Neurobiol Exp (Wars) 1993;53:425–429. [PubMed] [Google Scholar]
- Donoghue JP, Wise SP. The motor cortex of the rat: cytoarchitecture and microstimulation mapping. J Comp Neurol. 1982;212:76–88. doi: 10.1002/cne.902120106. [DOI] [PubMed] [Google Scholar]
- Fuster JM. The Prefrontal Cortex. Raven Press; New York, NY: 1997. [Google Scholar]
- Fuster JM. Prefrontal neurons in networks of executive memory. Brain Res Bull. 2000;52:331–336. doi: 10.1016/s0361-9230(99)00258-0. [DOI] [PubMed] [Google Scholar]
- Fuster JM, Bauer RH, Jervey JP. Functional interactions between inferotemporal and prefrontal cortex in a cognitive task. Brain Res. 1985;330:299–307. doi: 10.1016/0006-8993(85)90689-4. [DOI] [PubMed] [Google Scholar]
- Gabbott PL, Warner TA, Jays PR, Salway P, Busby SJ. Prefrontal cortex in the rat: Projections to subcortical autonomic, motor, and limbic centers. J Comp Neurol. 2005;492:145–177. doi: 10.1002/cne.20738. [DOI] [PubMed] [Google Scholar]
- Genovesio A, Brasted PJ, Mitz AR, Wise SP. Prefrontal cortex activity related to abstract response strategies. Neuron. 2005;47:307–320. doi: 10.1016/j.neuron.2005.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genovesio A, Tsujimoto S, Wise SP. Neuronal activity related to elapsed time in prefrontal cortex. J Neurophysiol. 2006;95:3281–3285. doi: 10.1152/jn.01011.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groenewegen HJ, Uylings HB. The prefrontal cortex and the integration of sensory, limbic and autonomic information. Prog Brain Res. 2000;126:3–28. doi: 10.1016/S0079-6123(00)26003-2. [DOI] [PubMed] [Google Scholar]
- Heidbreder CA, Groenewegen HJ. The medial prefrontal cortex in the rat: evidence for a dorso-ventral distinction based upon functional and anatomical characteristics. Neurosci Biobehav Rev. 2003;27:555–579. doi: 10.1016/j.neubiorev.2003.09.003. [DOI] [PubMed] [Google Scholar]
- Hikosaka O, Wurtz RH. Modification of saccadic eye movements by GABA-related substances. I. Effect of muscimol and bicuculline in monkey superior colliculus. J Neurophysiol. 1985;53:266–291. doi: 10.1152/jn.1985.53.1.266. [DOI] [PubMed] [Google Scholar]
- Jacobsen C. I. The functions of the frontal association areas in monkeys. Comparative Psychology Monographs. 1936;13:1–60. [Google Scholar]
- Janssen P, Shadlen MN. A representation of the hazard rate of elapsed time in macaque area LIP. Nat Neurosci. 2005;8:234–241. doi: 10.1038/nn1386. [DOI] [PubMed] [Google Scholar]
- Kolb B. Functions of the frontal cortex of the rat: a comparative review. Brain Res. 1984;320:65–98. doi: 10.1016/0165-0173(84)90018-3. [DOI] [PubMed] [Google Scholar]
- Krupa DJ, Ghazanfar AA, Nicolelis MA. Immediate thalamic sensory plasticity depends on corticothalamic feedback. Proc Natl Acad Sci U S A. 1999;96:8200–8205. doi: 10.1073/pnas.96.14.8200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyuhou S, Gemba H. Projection from the perirhinal cortex to the frontal motor cortex in the rat. Brain Res. 2002;929:101–104. doi: 10.1016/s0006-8993(01)03383-2. [DOI] [PubMed] [Google Scholar]
- Laubach M. Wavelet-based processing of neuronal spike trains prior to discriminant analysis. J Neurosci Methods. 2004;134:159–168. doi: 10.1016/j.jneumeth.2003.11.007. [DOI] [PubMed] [Google Scholar]
- Laubach M, Wessberg J, Nicolelis MA. Cortical ensemble activity increasingly predicts behaviour outcomes during learning of a motor task. Nature. 2000;405:567–571. doi: 10.1038/35014604. [DOI] [PubMed] [Google Scholar]
- Lee HS, Kim MA, Waterhouse BD. Retrograde double-labeling study of common afferent projections to the dorsal raphe and the nuclear core of the locus coeruleus in the rat. J Comp Neurol. 2005;481:179–193. doi: 10.1002/cne.20365. [DOI] [PubMed] [Google Scholar]
- Legendy CR, Salcman M. Bursts and recurrences of bursts in the spike trains of spontaneously active striate cortex neurons. J Neurophysiol. 1985;53:926–939. doi: 10.1152/jn.1985.53.4.926. [DOI] [PubMed] [Google Scholar]
- Leon MI, Shadlen MN. Representation of time by neurons in the posterior parietal cortex of the macaque. Neuron. 2003;38:317–327. doi: 10.1016/s0896-6273(03)00185-5. [DOI] [PubMed] [Google Scholar]
- Li CS, Huang C, Constable RT, Sinha R. Imaging response inhibition in a stop-signal task: neural correlates independent of signal monitoring and post-response processing. J Neurosci. 2006;26:186–192. doi: 10.1523/JNEUROSCI.3741-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lomber SG. The advantages and limitations of permanent or reversible deactivation techniques in the assessment of neural function. J Neurosci Methods. 1999;86:109–117. doi: 10.1016/s0165-0270(98)00160-5. [DOI] [PubMed] [Google Scholar]
- Los SA, Knol DL, Boers RM. The foreperiod effect revisited: conditioning as a basis for nonspecific preparation. Acta Psychol (Amst) 2001;106:121–145. doi: 10.1016/s0001-6918(00)00029-9. [DOI] [PubMed] [Google Scholar]
- Loughlin SE, Foote SL, Fallon JH. Locus coeruleus projections to cortex: topography, morphology and collateralization. Brain Res Bull. 1982;9:287–294. doi: 10.1016/0361-9230(82)90142-3. [DOI] [PubMed] [Google Scholar]
- Maimon G, Assad JA. A cognitive signal for the proactive timing of action in macaque LIP. Nat Neurosci. 2006;9:948–955. doi: 10.1038/nn1716. [DOI] [PubMed] [Google Scholar]
- Martin JH, Ghez C. Pharmacological inactivation in the analysis of the central control of movement. J Neurosci Methods. 1999;86:145–159. doi: 10.1016/s0165-0270(98)00163-0. [DOI] [PubMed] [Google Scholar]
- Milad MR, Vidal-Gonzalez I, Quirk GJ. Electrical stimulation of medial prefrontal cortex reduces conditioned fear in a temporally specific manner. Behav Neurosci. 2004;118:389–394. doi: 10.1037/0735-7044.118.2.389. [DOI] [PubMed] [Google Scholar]
- Miller BT, D'Esposito M. Searching for “the top” in top-down control. Neuron. 2005;48:535–538. doi: 10.1016/j.neuron.2005.11.002. [DOI] [PubMed] [Google Scholar]
- Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annu Rev Neurosci. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- Moore T, Armstrong KM. Selective gating of visual signals by microstimulation of frontal cortex. Nature. 2003;421:370–373. doi: 10.1038/nature01341. [DOI] [PubMed] [Google Scholar]
- Moore T, Fallah M. Microstimulation of the frontal eye field and its effects on covert spatial attention. J Neurophysiol. 2004;91:152–162. doi: 10.1152/jn.00741.2002. [DOI] [PubMed] [Google Scholar]
- Moran J, Desimone R. Selective attention gates visual processing in the extrastriate cortex. Science. 1985;229:782–784. doi: 10.1126/science.4023713. [DOI] [PubMed] [Google Scholar]
- Mulder AB, Nordquist RE, Orgut O, Pennartz CM. Learning-related changes in response patterns of prefrontal neurons during instrumental conditioning. Behav Brain Res. 2003;146:77–88. doi: 10.1016/j.bbr.2003.09.016. [DOI] [PubMed] [Google Scholar]
- Naito E, Kinomura S, Geyer S, Kawashima R, Roland PE, Zilles K. Fast reaction to different sensory modalities activates common fields in the motor areas, but the anterior cingulate cortex is involved in the speed of reaction. J Neurophysiol. 2000;83:1701–1709. doi: 10.1152/jn.2000.83.3.1701. [DOI] [PubMed] [Google Scholar]
- Narayanan NS, Horst NK, Laubach M. Reversible inactivations of rat medial prefrontal cortex impair the ability to wait for a stimulus. Neuroscience. 2006 doi: 10.1016/j.neuroscience.2005.11.072. [DOI] [PubMed] [Google Scholar]
- Narayanan NS, Kimchi EY, Laubach M. Redundancy and synergy of neuronal ensembles in motor cortex. J Neurosci. 2005;25:4207–4216. doi: 10.1523/JNEUROSCI.4697-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neafsey EJ, Bold EL, Haas G, Hurley-Gius KM, Quirk G, Sievert CF, Terreberry RR. The organization of the rat motor cortex: a microstimulation mapping study. Brain Res. 1986;396:77–96. doi: 10.1016/s0006-8993(86)80191-3. [DOI] [PubMed] [Google Scholar]
- Neafsey EJ, Sievert C. A second forelimb motor area exists in rat frontal cortex. Brain Res. 1982;232:151–156. doi: 10.1016/0006-8993(82)90617-5. [DOI] [PubMed] [Google Scholar]
- Niki H, Watanabe M. Cingulate unit activity and delayed response. Brain Res. 1976;110:381–386. doi: 10.1016/0006-8993(76)90412-1. [DOI] [PubMed] [Google Scholar]
- Niki H, Watanabe M. Prefrontal and cingulate unit activity during timing behavior in the monkey. Brain Res. 1979;171:213–224. doi: 10.1016/0006-8993(79)90328-7. [DOI] [PubMed] [Google Scholar]
- Ollman RT, Billington MJ. The Deadline Model for Simple Reaction Times. Cognitive Psychology. 1972;3:311–336. [Google Scholar]
- Preuss T. Do rats have prefrontal cortex? The Rose-Woolsey-Akert program reconsidered. Journal of Cognitive Neuroscience. 1995;7:1–24. doi: 10.1162/jocn.1995.7.1.1. [DOI] [PubMed] [Google Scholar]
- Reep RL, Corwin JV, Hashimoto A, Watson RT. Efferent connections of the rostral portion of medial agranular cortex in rats. Brain Res Bull. 1987;19:203–221. doi: 10.1016/0361-9230(87)90086-4. [DOI] [PubMed] [Google Scholar]
- Risterucci C, Terramorsi D, Nieoullon A, Amalric M. Excitotoxic lesions of the prelimbic-infralimbic areas of the rodent prefrontal cortex disrupt motor preparatory processes. Eur J Neurosci. 2003;17:1498–1508. doi: 10.1046/j.1460-9568.2003.02541.x. [DOI] [PubMed] [Google Scholar]
- Rouiller EM, Moret V, Liang F. Comparison of the connectional properties of the two forelimb areas of the rat sensorimotor cortex: support for the presence of a premotor or supplementary motor cortical area. Somatosens Mot Res. 1993;10:269–289. doi: 10.3109/08990229309028837. [DOI] [PubMed] [Google Scholar]
- Rowe JB, Stephan KE, Friston K, Frackowiak RS, Passingham RE. The prefrontal cortex shows context-specific changes in effective connectivity to motor or visual cortex during the selection of action or colour. Cereb Cortex. 2005;15:85–95. doi: 10.1093/cercor/bhh111. [DOI] [PubMed] [Google Scholar]
- Ryan LJ, Clark KB. The role of the subthalamic nucleus in the response of globus pallidus neurons to stimulation of the prelimbic and agranular frontal cortices in rats. Exp Brain Res. 1991;86:641–651. doi: 10.1007/BF00230538. [DOI] [PubMed] [Google Scholar]
- Sesack SR, Deutch AY, Roth RH, Bunney BS. Topographical organization of the efferent projections of the medial prefrontal cortex in the rat: an anterograde tract-tracing study with Phaseolus vulgaris leucoagglutinin. J Comp Neurol. 1989;290:213–242. doi: 10.1002/cne.902900205. [DOI] [PubMed] [Google Scholar]
- Stuss DT, Alexander MP, Shallice T, Picton TW, Binns MA, Macdonald R, Borowiec A, Katz DI. Multiple frontal systems controlling response speed. Neuropsychologia. 2005;43:396–417. doi: 10.1016/j.neuropsychologia.2004.06.010. [DOI] [PubMed] [Google Scholar]
- Swanson LW. Brain Maps: Structure of the Rat Brain : A Laboratory Guide With Printed and Electronic Templates for Data, Models, and Schematics. Elsevier; New York, NY: 1999. [Google Scholar]
- Tomita H, Ohbayashi M, Nakahara K, Hasegawa I, Miyashita Y. Top-down signal from prefrontal cortex in executive control of memory retrieval. Nature. 1999;401:699–703. doi: 10.1038/44372. [DOI] [PubMed] [Google Scholar]
- Uylings HB, Groenewegen HJ, Kolb B. Do rats have a prefrontal cortex? Behav Brain Res. 2003;146:3–17. doi: 10.1016/j.bbr.2003.09.028. [DOI] [PubMed] [Google Scholar]
- Vaadia E, Aertsen A, Nelken I. 'Dynamics of neuronal interactions' cannot be explained by ‘neuronal transients’. Proc Biol Sci. 1995;261:407–410. doi: 10.1098/rspb.1995.0167. [DOI] [PubMed] [Google Scholar]
- Van Eden CG, Lamme VA, Uylings HB. Heterotopic Cortical Afferents to the Medial Prefrontal Cortex in the Rat. A Combined Retrograde and Anterograde Tracer Study. Eur J Neurosci. 1992;4:77–97. doi: 10.1111/j.1460-9568.1992.tb00111.x. [DOI] [PubMed] [Google Scholar]
- Vertes RP. Differential projections of the infralimbic and prelimbic cortex in the rat. Synapse. 2004;51:32–58. doi: 10.1002/syn.10279. [DOI] [PubMed] [Google Scholar]
- Waterhouse BD, Mihailoff GA, Baack JC, Woodward DJ. Topographical distribution of dorsal and median raphe neurons projecting to motor, sensorimotor, and visual cortical areas in the rat. J Comp Neurol. 1986;249:460–476. doi: 10.1002/cne.902490403. 478-481. [DOI] [PubMed] [Google Scholar]
- Whishaw IQ, Pellis SM, Gorny BP. Medial frontal cortex lesions impair the aiming component of rat reaching. Behav Brain Res. 1992;50:93–104. doi: 10.1016/s0166-4328(05)80291-8. [DOI] [PubMed] [Google Scholar]
- Wichmann T, Bergman H, DeLong MR. The primate subthalamic nucleus. III. Changes in motor behavior and neuronal activity in the internal pallidum induced by subthalamic inactivation in the MPTP model of parkinsonism. J Neurophysiol. 1994;72:521–530. doi: 10.1152/jn.1994.72.2.521. [DOI] [PubMed] [Google Scholar]
- Winkowski DE, Knudsen EI. Top-down gain control of the auditory space map by gaze control circuitry in the barn owl. Nature. 2006;439:336–339. doi: 10.1038/nature04411. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.