Abstract
Background: Evidence suggests that increased maternal calcium intake during pregnancy may result in lower offspring blood pressure, prompting calls for more robust data in this field, particularly in settings of habitually low calcium intake.
Objective: The objective was to investigate the effect of maternal calcium supplementation on blood pressure in offspring by recruiting children born after a randomized, double-blind, placebo-controlled trial of calcium supplementation during pregnancy.
Design: Children (n = 389) from a rural area of The Gambia (mean age: 7.4 ± 1.2 y; range: 5–10 y), whose mothers received a calcium supplement (1500 mg Ca/d from 20 wk of gestation until delivery) or placebo, were followed up in West Africa. Blood pressure was assessed under standardized conditions with use of the Omron 705IT automated oscillometric device (Morton Medical Ltd, London, United Kingdom), and anthropometric and body composition (bioelectrical impedance) measurements were also made.
Results: The analysis was restricted to 350 children born at term, which represented 64% of original trial births. There was no difference in systolic (adjusted mean difference: −0.04 mm Hg; 95% CI: −1.78, 1.69 mm Hg) or diastolic (adjusted mean difference: 0.25 mm Hg; 95% CI: −1.27, 1.77 mm Hg) blood pressure between children whose mothers had received calcium and those who received placebo. No interaction between childhood body mass index (in kg/m2; mean: 14.0) and maternal calcium supplementation was observed in this study.
Conclusion: Calcium supplementation in the second half of pregnancy in Gambian women with very low habitual calcium intakes may not result in lower offspring blood pressure at 5–10 y of age.
INTRODUCTION
A large number of studies have reported an inverse association between birth weight and blood pressure in later life. These have been extensively reviewed (1, 2) and are interpreted as revealing the importance of fetal nutrition for the ‘programming’ of blood pressure. Calcium is considered to be a key factor in the regulation of blood pressure throughout life (3) and particularly during pregnancy, when calcium supplementation in high risk women and/or those with low calcium intakes may reduce the risk of pregnancy-induced hypertension (4). Mechanisms linking calcium intake in pregnancy to offspring blood pressure are not yet determined but may involve the setting of calcium regulating hormones such as parathyroid hypertensive factor (5).
There is some evidence from observational studies that increased calcium intake during pregnancy, either from food (6) or from supplements (7), may be related to lower blood pressure in the offspring, although this association has not been universally observed (8). To date only 3 randomized controlled trials of maternal calcium supplementation during pregnancy have published follow-up data on blood pressure among the offspring (9–11). One of these showed an overall effect of maternal calcium supplementation on reduced offspring blood pressure at 2 y of age in the United States (9). Although there was no effect on mean offspring blood pressure, an Argentinean trial of maternal calcium supplementation reported a decreased risk of having high blood pressure [defined by age, sex, and height-specific cutoffs (12)] in the offspring at 7 y (10). In addition, there was an interaction with child body mass index (BMI); the maternal intervention was associated with lower blood pressure in offspring with higher BMI (kg/m2) (10). Most recently, an Australian trial showed no association between maternal calcium supplementation and offspring blood pressure at 4–7 y of age (11).
Calcium intake during pregnancy in rural areas of The Gambia is much lower than international recommendations, typically 300–400 mg/d (13–15), and close to the estimated biological requirement. We have conducted a large randomized, placebo-controlled trial of calcium supplementation of pregnant women from 20 wk of gestation until delivery. The primary outcome of this trial was the effect on systolic blood pressure at 36 wk of gestation. Here we present the results of a follow-up study conducted on the offspring when they were 5–10 y old. The primary objective of this study was to investigate the effect of maternal calcium supplementation on offspring blood pressure; secondarily, we investigated a possible interaction with offspring BMI (10).
SUBJECTS AND METHODS
Between November 2005 and August 2006 in The Gambia, West Africa, we undertook a follow-up study of children whose mothers had participated in a randomized, placebo-controlled trial of calcium supplementation during pregnancy. The study was part of a European Union consortium investigation of the early life nutritional determinants of disease (Framework 6: ‘Early Nutrition Programming Project’). The original supplementation trial is registered on the International Standard Randomized Controlled Trial Number Register as ISRCTN96502494 and is described briefly below. To date, outcomes in a subset of the mothers and infants studied in detail have been reported (16).
Maternal calcium supplementation trial
Between 1995 and 2000, all pregnant women from 16 villages in the rural West Kiang region of The Gambia were invited to participate in a double-blind, randomized, placebo-controlled trial of calcium supplementation (1500 mg Ca/d provided as 3750 mg calcium carbonate or cellulose-lactose placebo) from 20 wk of pregnancy (P20) until delivery. Study participants were randomized, with use of published tables, in blocks of 4 to ensure an even distribution of participants from different seasons. Maternal antenatal care was stratified by 3 clinic blocks corresponding to areas overseen by the 3 trial midwives (16). The primary outcome of the study was maternal blood pressure at 36 wk of gestation (measured in 536 women), and 546 live singleton infants were deemed to be delivered at term.
Follow-up study
Scientific approval for the cross-sectional follow-up study was granted by the Medical Research Council (MRC) The Gambia Scientific Coordinating Committee and ethical permission was granted by the joint Gambian Government and MRC The Gambia Ethics Committee and the London School of Hygiene and Tropical Medicine Ethics Committee. Community sensitization meetings were held in relevant villages, and support was gathered from village elders and school head teachers. Fully informed consent was obtained from the parents or guardians of participating children. All field staff and investigators (except the code holder, AP) remained blinded to the treatment group of the subjects.
At the time of this follow-up study, the surviving offspring were aged between 5 and 10 y (mean age: 7.4 y, SD ± 1.2). Recruitment was limited to those children still residing in West Kiang or within an hour's drive from the MRC laboratories at the urban/peri-urban coastal areas (n = 452).
Study measurements
Measurements were conducted by fully trained fieldworkers following standard operating procedures. An automated oscillometric monitor (Omron 705IT; Morton Medical Ltd, London, United Kingdom) was used to measure blood pressure following guidelines from the American Heart Association (17). Subjects were seen in the early morning (0800–1000) before breakfast, they were seated at rest for 5 min before the first measurement, and readings were continued until 3 were within 5 mm Hg of each other. Additional readings were taken on a separate day for any children with unusually high blood pressure readings, as defined by the United States National Heart, Lung and Blood Institute guidelines (18). These subjects were referred to a physician if readings remained high; if the second series of readings was within the normal range, these values were entered in the database to be used in the analysis. The average of the 3 blood pressure readings was used to estimate systolic and diastolic blood pressure. Pulse pressure was calculated as systolic – diastolic pressure, and mean arterial pressure was calculated as diastolic + (1/3 × pulse pressure).
Anthropometric measurements included height, weight, midupper arm circumference, and triceps skinfold thickness. These were assessed using standard techniques by the same fully-trained fieldworker (98% of measurements) to minimize observer bias. Equipment was calibrated daily, weight was measured to the nearest 0.1 kg with use of digital scales (Tanita; Chasmors Ltd, London, United Kingdom), height was measured with use of a stadiometer (Seca 214; Chasmors Ltd) to the nearest 0.1 cm, and BMI was defined as weight(kg)/height(m)2.
We used the Tanita BC-418MA (Chasmors Ltd) segmental bioelectrical impedance analyzer to assess body composition. Impedance measurements were converted into an estimate of percentage fat-free mass (FFM) from prediction equations that were previously generated in this population with use of deuterium oxide dilution as the reference method. Details of this validation study (19) and of the equation used (20) have been published elsewhere. Percentage fat mass (FM) was calculated by difference (% FM = 100 − % FFM). Log-log regression analysis, described by Wells et al (21), was used to assess the relation of FM and FFM with height (Ht) and to generate FM and FFM indices that were uncorrelated with height by using the value of the slope variable from the regression equation. FM index (FMI) was calculated as FM/Ht1.6 and lean mass index (LMI) was calculated as FFM/Ht2.3. We used the Tanita analyzer's inbuilt equations for an estimate of percentage trunk fat (indicating central adiposity), because it had not been possible to create population-specific prediction equations for segmental body regions.
Statistical analysis
All statistical analysis was conducted with use of Stata 10 (Stata Corporation, College Station, TX), and all outcome variables and continuous covariates were normally distributed. An intention-to-treat analysis with use of linear regression was used to assess the relation between supplementation status of the mother (calcium compared with placebo) and blood pressure in the offspring. Variables that were potential correlates of blood pressure were assessed by simple linear regression and those that were associated were included in the adjusted regression models. The analysis was first conducted as a simple main effects model, then any interaction of the intervention effect with age, sex, maternal baseline (P20, before start of supplementation) BMI, maternal baseline blood pressure (measured with Dinamap Pro 400, Critikon Ltd, United Kingdom), and child body composition was assessed by fitting interaction terms in a sequential manner.
We investigated the impact of the intervention on the odds of having high blood pressure by using logistic regression adjusted for the same covariates. Children were defined as having high blood pressure if their systolic blood pressure was above the 95th percentile with use of age and height specific reference data (18).
We also ran an “as-treated” analysis relating the number of tablets a woman consumed to her offspring's blood pressure. Two observational (nonrandomized) variables determined the number of tablets consumed: the duration of time that the woman was in the trial and her compliance, defined as a percentage (observed tablets consumed/expected tablets to consume) with treatment. The models were fitted with these 2 variables in addition to the calcium dose consumed, which was defined as the interaction among compliance, time in the study, and treatment group (calcium compared with placebo).
RESULTS
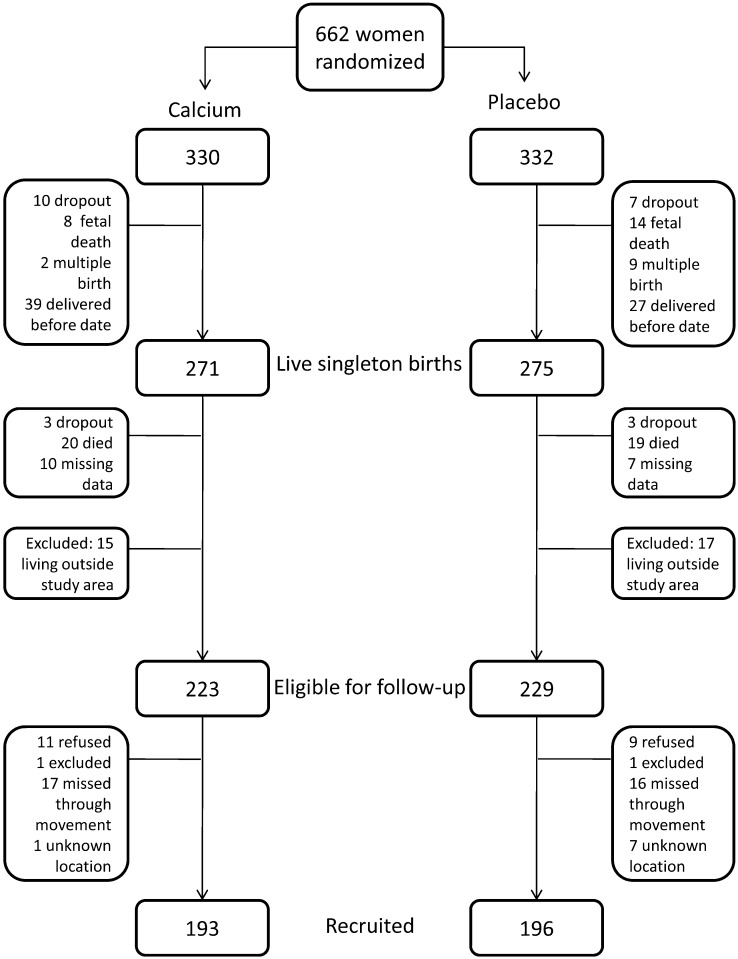
A total of 389 children (193 born to calcium-supplemented mothers and 196 born to mothers who received placebo) were enrolled into the follow-up study, representing 86% of those eligible and 71% of the original trial births (Figure 1). The main reason for nonrecruitment (7.6% of calcium supplement children and 7.0% of placebo children) was due to family movement within The Gambia, resulting in eligible subjects being unavailable during the follow-up study period.
FIGURE 1.
Flow of subjects from the original calcium supplementation trial who were recruited into the present follow-up.
Recruitment
There were minor differences in available characteristics between those recruited and those not recruited into the present follow-up (Table 1). The mothers of recruited individuals were on average 1.8 y older and had slightly lower baseline (P20) systolic blood pressure (−1.7 mm Hg) than the mothers of individuals not enrolled into the follow-up study. Children in the follow-up study were on average slightly shorter (−0.1 cm) in length at 2 wk of age compared with those lost to follow-up. Thirty-eight of the enrolled children were classified as having a gestational age <37 wk, as assessed by their Dubowitz score at delivery (22), and were excluded from the analysis. One blood pressure measurement was missing, because the child refused to cooperate, leaving a final sample of 350 (171 born to calcium-supplemented mothers and 179 to placebo mothers) for analysis, representing 64% of original trial births.
TABLE 1.
Differences between recruited individuals and those lost to follow-up1
| Recruited |
||||
| Placebo (n = 196) | Supplement (n = 193) | Lost to follow-up (n = 157) | P value2 | |
| Maternal age (y) | 27.6 ± 7.2 [127] | 28.3 ± 6.7 [122] | 26.1 ± 6.7 [93] | 0.03 |
| Maternal baseline weight (kg)3 | 55.0 ± 6.9 [195] | 54.9 ± 6.7 [192] | 55.3 ± 8.1 [156] | 0.4 |
| Maternal height (cm) | 1.60 ± 5.5 | 159.9 ± 5.2 | 159.7 ± 5.6 | 0.6 |
| Maternal baseline systolic BP (mm Hg)3 | 101.9 ± 9.1 | 101.0 ± 8.2 | 103.2 ± 10.0 | 0.04 |
| Maternal baseline diastolic BP (mm Hg)3 | 55.8 ± 7.8 | 54.7 ± 7.3 | 56.5 ± 6.9 | 0.09 |
| Child weight at 2 wk (kg)4 | 3.35 ± 0.49 [194] | 3.33 ± 0.50 [191] | 3.35 ± 0.56 [136] | 0.1 |
| Child length at 2 wk (cm)4 | 50.9 ± 2.0 [194] | 50.5 ± 2.0 [191] | 50.8 ± 2.4 [136] | 0.08 |
Values are means ± SDs; placebo and supplement refer to maternal intervention; lost to follow-up defined as all live, singleton children born during original trial who were not recruited into follow-up study (ie, includes those ineligible for current follow-up). Numbers in brackets reflect subject numbers that differ from those indicated in the column headings. BP, blood pressure.
P values are derived from independent t tests to assess the difference in mean values between those recruited (as a total) and those lost to follow-up.
Baseline for original supplementation trial was 20 wk of gestation (P20) before commencement of supplementation.
Anthropometric measurements at 2 wk of age were used as a proxy measure for birth weight due to a large number of missing data at the earlier age point.
Intention-to-treat analysis
Mean systolic blood pressure was 97.6 (SD ± 8.1) mm Hg for boys and 98.6 (SD ± 8.8) mm Hg for girls (Table 2). Mean diastolic blood pressure was 57.2 (SD ± 7.3) mm Hg for boys and 58.7 (SD ± 7.5) mm Hg for girls. The reliability of the blood pressure measurement was 97% for the systolic and 86% for the diastolic measurement. There was no difference in blood pressure between children born to women who received calcium or placebo during pregnancy (Table 3). The fully adjusted mean difference in systolic blood pressure between the groups was 0.29 mm Hg (95% CI: −1.41, 1.98; P = 0.74) and for diastolic blood pressure was 0.38 mm Hg (95% CI: −1.13, 1.90; P = 0.62). Only 4% of children had a systolic blood pressure that was above the 95th percentile for their height and age; the odds of having high blood pressure was not related to the maternal calcium intervention (unadjusted odds ratio: 1.1; 95% CI: 0.4, 3.1; P = 0.86).
TABLE 2.
Characteristics of children enrolled into the follow-up study whose mothers participated in a trial of calcium supplementation during pregnancy1
| Placebo |
Supplement |
|||
| Males (n = 88) | Females (n = 83) | Males (n = 87) | Females (n = 93) | |
| Age (y) | 7.4 ± 1.2 | 7.3 ± 1.2 | 7.5 ± 1.2 | 7.3 ± 1.2 |
| Systolic BP (mm Hg) | 97.0 ± 8.1 | 99.3 ± 9.0 | 98.1 ± 8.1 | 97.9 ± 8.7 [92] |
| Diastolic BP (mm Hg) | 57.1 ± 7.2 | 58.7 ± 7.1 | 57.3 ± 7.5 | 58.6 ± 7.8 [92] |
| Pulse pressure (mm Hg)2 | 39.9 ± 6.4 | 40.6 ± 6.1 | 40.9 ± 6.8 | 39.3 ± 5.8 [92] |
| MAP (mm Hg)3 | 70.4 ± 6.9 | 72.2 ± 7.3 | 70.9 ± 7.0 | 71.7 ± 7.6 [92] |
| Height (cm) | 118.3 ± 7.8 | 118.8 ± 8.7 | 118.4 ± 7.4 | 117.3 ± 7.9 |
| HAZ4 | −1.13 ± 0.77 | −0.81 ± 1.02 | −1.18 ± 0.84 | −1.12 ± 0.75 |
| Weight (kg) | 19.6 ± 2.8 | 20.0 ± 4.1 | 20.1 ± 3.4 | 19.1 ± 3.2 |
| BMI (kg/m2) | 14.0 ± 0.9 | 14.0 ± 1.2 | 14.3 ± 1.1 | 13.8 ± 1.2 |
| FMI (kg/m1.6) | 1.88 ± 0.38 [85] | 2.51 ± 0.62 [81] | 2.01 ± 0.49 [86] | 2.39 ± 0.55 [92] |
| LMI (kg/m2.3)3 | 11.59 ± 0.77 [85] | 11.12 ± 0.73 [81] | 11.76 ± 0.80 [86] | 11.02 ± 0.82 [92] |
| Trunk fat (%)5 | 13.4 ± 2.5 [85] | 14.4 ± 3.0 [81] | 13.8 ± 2.9 [86] | 14.1 ± 2.7 [92] |
Values are means ± SDs; placebo and supplement refer to maternal intervention. Numbers in brackets reflect subject numbers that differ from those indicated in the column headings. MAP, mean arterial pressure; HAZ, height-for-age z score; FMI, fat mass index; LMI, lean mass index; BP, blood pressure.
Calculated as systolic – diastolic blood pressure.
MAP calculated as diastolic + (1/3 × pulse pressure).
Estimated from UK 1990 reference data (26).
Estimated by the Tanita inbuilt equations, not from population-specific equations.
TABLE 3.
Effect of maternal calcium supplementation on offspring blood pressure: intention-to-treat analysis1
| Model 1 (n = 350) |
Model 2 (n = 348) |
Model 3 (n = 341) |
Model 4 (n = 341) |
|||||
| (95% CI) | P value | (95% CI) | P value | (95% CI) | P value | (95% CI) | P value | |
| Systolic pressure (mm Hg) | −0.10 (−1.89, 1.68) | 0.91 | −0.04 (−1.78, 1.69) | 0.96 | 0.37 (−1.32, 2.06) | 0.67 | 0.29 (−1.41, 1.98) | 0.74 |
| Diastolic pressure (mm Hg) | 0.10 (−1.46, 1.67) | 0.90 | 0.25 (−1.27, 1.77) | 0.75 | 0.48 (−1.03, 2.00) | 0.53 | 0.38 (−1.13, 1.90) | 0.62 |
| Pulse pressure (mm Hg) | −0.20 (−1.53, 1.12) | 0.76 | −0.29 (−1.60, 1.01) | 0.66 | −0.12 (−1.42, 1.18) | 0.86 | −0.11 (−1.41, 1.20) | 0.87 |
| Mean arterial pressure (mm Hg) | 0.03 (−1.49, 1.55) | 0.97 | 0.14 (−1.33, 1.62) | 0.85 | 0.44 (−1.01, 1.89) | 0.55 | 0.34 (−1.11, 1.80) | 0.64 |
Results are the difference in mean blood pressure (95% CI) for individuals born to women receiving calcium supplements compared with placebo during pregnancy derived from linear regression analysis. Model 1: unadjusted. Model 2: adjusted for age, sex, antenatal clinic, maternal baseline [P20 (20 wk of gestation)] BMI, and maternal baseline blood pressure. Model 3: adjusted as in model 2 but additionally adjusted for child height, lean mass index, and fat mass index. Model 4: adjusted as in model 3 but additionally adjusted for maternal compliance (observed tablet consumption/expected tablet consumption) and length of supplementation.
Interactions with maternal calcium supplementation
The possibility of the intervention effect being modified by covariates was assessed by fitting interaction terms into the linear regression models. There was a significant interaction between maternal baseline blood pressure and the effect of the intervention on childhood systolic blood pressure (interaction coefficient, β = 0.26 mm Hg/mm Hg; 95% CI: 0.06, 0.46; P = 0.01). For women with low baseline systolic blood pressure, the calcium intervention was associated with lower child systolic blood pressure compared with placebo, whereas for women with high blood pressure this association was reversed.
No other maternal variables (BMI or antenatal clinic attended) were found to modify the effect of the intervention (data not shown). Neither age nor sex of the offspring were shown to be effect modifiers (data not shown), but there was a significant interaction between the maternal intervention and child height at follow-up on offspring systolic blood pressure (interaction coefficient, β = −0.23 mm Hg/cm; 95% CI: −0.44, −0.02; P = 0.03). For shorter children, the maternal calcium intervention was associated with higher blood pressure compared with placebo, whereas for taller children it was associated with lower blood pressure. There was no interaction between the maternal intervention and any of the remaining anthropometric measurements (weight, BMI) or body composition (FMI, LMI, trunk fat) variables on child blood pressure (data not shown).
As-treated analysis
The mean number of calcium tablets consumed by women in the intervention arm was 422 (range: 267–561) and in the placebo arm it was 415 (range: 285–588). For both intervention and control women, mean compliance was 98% (range: 75–100%), and there was no difference in mean compliance between the calcium and placebo arms of the trial (calcium: 98%, placebo: 98%, t test P = 0.43). The as-treated analysis (Table 4) suggests that women who demonstrated greater compliance had offspring with higher systolic blood pressure (regression coefficient: 0.30 mm Hg/%, 95% CI: 0.01, 0.59, P = 0.04). However, there was no association between the amount of calcium consumed (modeled by the dose consumed term) and offspring blood pressure, suggesting that the compliance term was reflecting an association with an unmeasured characteristic that correlated with compliance. Offspring diastolic blood pressure was also not associated with the maternal calcium dose (Table 4).
TABLE 4.
Maternal calcium intake and offspring blood pressure: as-treated analysis1
| Systolic blood pressure (n = 350) | P value | Diastolic blood pressure (n = 350) | P value | |
| mm Hg | mm Hg | |||
| Maternal compliance2 | 0.30 (0.01, 0.59) | 0.04 | 1.89 (−0.06, 0.44) | 0.14 |
| Duration of supplementation | 0.52 (−1.00, 2.05) | 0.50 | 1.07 (−0.26, 2.41) | 0.12 |
| Maternal calcium dose3 | −0.00 (−0.004, 0.004) | 0.83 | 0.00 (−0.004, 0.004) | 0.94 |
Results are the effect (95% CI) on child's blood pressure of a 1-unit increase in exposure variable (compliance, duration, or dose), derived from unadjusted linear regression analysis.
Compliance calculated as a percentage: (observed tablet consumption/expected tablet consumption).
Calcium dose fitted as an interaction term among compliance, time in study, and treatment allocation (supplement vs placebo).
DISCUSSION
This study found no evidence that maternal calcium supplementation (1500 mg Ca/d) of rural Gambian women during the second half of pregnancy was associated with offspring blood pressure at 5–10 y of age. This is consistent with a recent study from Australia (11) and is one of the first reports from a region of habitually very low dietary calcium intake.
A recent systematic review of the literature relating maternal calcium intake (from both observational studies and randomized controlled trials) to offspring blood pressure found that methodological flaws and small study sizes prohibited strong conclusions (23). In the present study we were able to recruit 86% of eligible children with only minor differences, in the available characteristics, between those recruited and those lost to follow-up. In contrast, the only trial to report an overall association between maternal calcium supplementation and offspring blood pressure suffered losses to follow-up of over 90% (9). In addition to the intention-to-treat analysis, our as-treated analysis provided no evidence that the amount of calcium consumed by the study women was associated with offspring blood pressure, despite an association with compliance irrespective of treatment arm.
A comparable trial in Argentina reported that the intervention was associated with a reduced risk of the offspring having high blood pressure at 5–9 y (10), an effect that has not been replicated in our study. The Argentinean follow-up also reported an interaction between the intervention and childhood BMI; for individuals with a BMI above the mean (15.7), maternal calcium supplementation was associated with lower systolic blood pressure (10). In contrast to these findings, in the current study we did not find that the effect of the maternal intervention was modified by childhood BMI or by FMIs and LMIs of body composition. This inconsistency in findings may be explained by differences in the range of BMI; only 7% of subjects in our study would be classified as having a BMI in the top 2 quartiles of the Argentinean study. In the current study we observed an interaction with child height, with the intervention tending toward lower blood pressure in taller individuals, and we also observed that maternal baseline blood pressure modified the interaction effect. We are cautious of overinterpretation of these interaction analyses, however, which could reflect chance findings due to multiple testing.
There are a number of limitations to the current study that should be raised. First, although we were able to recruit 86% of eligible children, the analysis involved only 66% of children born to mothers receiving calcium and 62% of those born to mothers receiving the placebo. Loss to follow-up is a well-recognized issue for long-term follow-up studies within this research field, and these rates of loss are relatively low for a study with a 5–10 y follow-up time scale (24). Recommendations have highlighted the importance of providing clear study details, which we have done by providing information on subject recruitment rates and characteristics associated with loss to follow-up (24). In this study loss to follow-up was associated with maternal baseline blood pressure; recruited children had been born to women with slightly lower blood pressure. This represents a potential selection bias but one we were able to adjust for in our analysis of the effect of the maternal intervention.
A related issue is the reduced sample size as a consequence of subject attrition, which affects study power. In this study the precision estimate for the effect of maternal calcium supplementation on offspring systolic blood pressure ranged from −1.41 to 1.98 and effects within this range therefore cannot be discounted. However, it is arguable whether effect sizes of this magnitude would be meaningful from a public health standpoint.
The overall lack of an association between maternal calcium supplementation and offspring blood pressure in this study may reflect that additional calcium was not transferred to the offspring in utero (14, 15). In an analysis of a subset of mothers and their infants, we found that maternal calcium supplementation had no effect on fetal growth, bone mineral accretion of infants in their first year, or breast milk calcium concentration (16). This suggests that even when habitual calcium intake is extremely low, physiologic adaptations may operate that override any effects of supplementation and provide both the mother and developing fetus with their calcium requirements (16). Alternatively, it may be that in this setting of general undernutrition any potential benefits of calcium supplementation are not seen.
Another explanation for the lack of association may relate to the age of the children. Studies that have reported lower offspring blood pressure in relation to maternal calcium intake during pregnancy (either trial or observational data) have mainly included children aged 2 y and under (6, 7, 9), whereas 3 studies reporting no association were in children aged 3 (25), 4–7 (11), and 7–10 y (8). McGarvey et al (6) reported an inverse association between maternal self-reported calcium intake (from food and personal supplements) and offspring systolic blood pressure at 1 mo of age, which was no longer apparent by 12 mo, although recruitment was greatly reduced at the second follow-up. Similarly, maternal calcium supplement use in Project Viva was associated with reduced offspring blood pressure at 6 mo (7), but there was no longer an association at 3 y (25). It may be that any influence of maternal calcium intake is short-lived and/or does not track into later childhood. In addition, the background calcium intakes of the 3 published intervention studies are very different from the intakes of Gambian women involved in this study; in the United States, Hatton et al (9) reported the average baseline calcium intake of women enrolled in their trial was over 1000 mg/d compared with 300–400 mg/d in The Gambia (16). These discrepancies may help to explain the differential effects of the maternal intervention in these settings.
In conclusion, we found no evidence that maternal calcium supplementation of 1500 mg/d from 20 wk of gestation until delivery influences offspring blood pressure measured at 5–10 y of age in a rural area of The Gambia.
Acknowledgments
We thank the mothers who took part in the original trial and their children for so enthusiastically taking part in the present study. We are extremely grateful to Meaghan Kall and Marijke Prins for their help with running the study at specific times. We also acknowledge the tireless and enthusiastic commitment of fieldworkers Kabiru Ceesay, Morikebba Sanyang, Saul Jarjou, and Sheriff Kolley as well as Kalilu Sanneh, the study nurse. Finally, we are indebted to all of the staff at MRC Keneba whose help ensured the smooth running of this study.
The authors’ responsibilities were as follows—SH, YS, and SEM (principal investigators): study design and data collection of the EU Sixth Framework-funded work; SH: conducted the data analysis with advice and input from AJCF, SEM, and AP. SEM, AP, and GRG: conceived the study; AP and SEM: gaining funding for these analyses; LMAJ and AP: original trial design and data collection; and SH: drafted the manuscript. All authors critically reviewed and edited the final article. None of the authors had a financial or personal conflict of interest to report.
REFERENCES
- 1.Law CM, Shiell AW. Is blood pressure inversely related to birth weight? The strength of evidence from a systematic review of the literature. J Hypertens 1996;14:935–41 [PubMed] [Google Scholar]
- 2.Huxley RR, Shiell AW, Law CM. The role of size at birth and postnatal catch-up growth in determining systolic blood pressure: a systematic review of the literature. J Hypertens 2000;18:815–31 [DOI] [PubMed] [Google Scholar]
- 3.van Mierlo LA, Arends LR, Streppel MT, et al. Blood pressure response to calcium supplementation: a meta-analysis of randomized controlled trials. J Hum Hypertens 2006;20:571–80 [DOI] [PubMed] [Google Scholar]
- 4.Hofmeyr GJ, Duley L, Atallah A. Dietary calcium supplementation for prevention of pre-eclampsia and related problems: a systematic review and commentary. BJOG 2007;114:933–43 [DOI] [PubMed] [Google Scholar]
- 5.Bergel E, Belizan JM. A deficient maternal calcium intake during pregnancy increases blood pressure of the offspring in adult rats. BJOG 2002;109:540–5 [PubMed] [Google Scholar]
- 6.McGarvey ST, Zinner SH, Willett WC, Rosner B. Maternal prenatal dietary potassium, calcium, magnesium, and infant blood pressure. Hypertension 1991;17:218–24 [DOI] [PubMed] [Google Scholar]
- 7.Gillman MW, Rifas-Shiman SL, Kleinman KP, Rich-Edwards JW, Lipshultz SE. Maternal calcium intake and offspring blood pressure. Circulation 2004;110:1990–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Morley R, Carlin JB, Dwyer T. Maternal calcium supplementation and cardiovascular risk factors in twin offspring. Int J Epidemiol 2004;33:1304–9 [DOI] [PubMed] [Google Scholar]
- 9.Hatton DC, Harrison-Hohner J, Coste S, Reller M, McCarron D. Gestational calcium supplementation and blood pressure in the offspring. Am J Hypertens 2003;16:801–5 [DOI] [PubMed] [Google Scholar]
- 10.Belizan JM, Villar J, Bergel E, et al. Long-term effect of calcium supplementation during pregnancy on the blood pressure of offspring: follow up of a randomised controlled trial. BMJ 1997;315:281–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hiller JE, Crowther CA, Moore VA, Willson K, Robinson JS. Calcium supplementation in pregnancy and its impact on blood pressure in children and women: follow up of a randomised controlled trial. Aust N Z J Obstet Gynaecol 2007;47:115–21 [DOI] [PubMed] [Google Scholar]
- 12.US National Institutes of Health; National Heart, Lung and Blood Institute. National High Blood Pressure Education program. Working group on high blood pressure in children and adolescents. Pediatrics 1996;98:649–58 [PubMed] [Google Scholar]
- 13.Prentice A, Laskey MA, Shaw J, et al. The calcium and phosphorus intakes of rural Gambian women during pregnancy and lactation. Br J Nutr 1993;69:885–96 [DOI] [PubMed] [Google Scholar]
- 14.Prentice A. Calcium intakes and bone densities of lactating women and breast-fed infants in The Gambia. Adv Exp Med Biol 1994;352:243–55 [DOI] [PubMed] [Google Scholar]
- 15.Prentice A. Maternal calcium metabolism and bone mineral status. Am J Clin Nutr 2000;71:S1312–6 [DOI] [PubMed] [Google Scholar]
- 16.Jarjou LM, Prentice A, Sawo Y, et al. Randomized, placebo-controlled, calcium supplementation study in pregnant Gambian women: effects on breast-milk calcium concentrations and infant birth weight, growth, and bone mineral accretion in the first year of life. Am J Clin Nutr 2006;83:657–66 [DOI] [PubMed] [Google Scholar]
- 17.Pickering TG, Hall JE, Appel LJ, et al. Recommendations for blood pressure measurement in humans and experimental animals: part 1: blood pressure measurement in humans: a statement for professionals from the Subcommittee of Professional and Public Education of the American Heart Association Council on High Blood Pressure Research. Circulation 2005;111:697–716 [DOI] [PubMed] [Google Scholar]
- 18.National High Blood Pressure Education Program. Update on the 1987 Task Force Report on High Blood Pressure in Children and Adolescents: a working group report from the National High Blood Pressure Education Program. National High Blood Pressure Education Program Working Group on Hypertension Control in Children and Adolescents. Pediatrics 1996;98:649–58 [PubMed] [Google Scholar]
- 19.Prins M, Hawkesworth S, Wright A, et al. Use of bioelectrical impedance analysis to assess body composition in rural Gambian children. Eur J Clin Nutr 2008;62:1065–74 [DOI] [PubMed] [Google Scholar]
- 20.Hawkesworth S, Prentice AM, Fulford AJ, Moore SE. Dietary supplementation of rural Gambian women during pregnancy does not affect body composition in offspring at 11-17 years of age. J Nutr 2008;138:2468–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wells JC, Cole TJ. Adjustment of fat-free mass and fat mass for height in children aged 8 y. Int J Obes Relat Metab Disord 2002;26:947–52 [DOI] [PubMed] [Google Scholar]
- 22.Dubowitz LM, Dubowitz V, Goldberg C. Clinical assessment of gestational age in the newborn infant. J Pediatr 1970;77:1–10 [DOI] [PubMed] [Google Scholar]
- 23.Bergel E, Barros AJ. Effect of maternal calcium intake during pregnancy on children blood pressure: a systematic review of the literature. BMC Pediatr 2007;7:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fewtrell MS, Kennedy K, Singhal A, et al. How much loss to follow-up is acceptable in long-term randomised trials and prospective studies? Arch Dis Child 2008;93:458–61 [DOI] [PubMed] [Google Scholar]
- 25.Bakker R, Rifas-Shiman SL, Kleinman KP, Lipshultz SE, Gillman MW. Maternal calcium intake during pregnancy and blood pressure in the offspring at age 3 years: a follow-up analysis of the Project Viva cohort. Am J Epidemiol 2008;168:1374–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cole TJ. The LMS method for constructing normalized growth standards. Eur J Clin Nutr 1990;44:45–60 [PubMed] [Google Scholar]