Abstract
Optokinetic testing is a noninvasive technique, widely used for visual functional evaluation in rodents. The modulatory influence of optokinetic stimulus parameters such as contrast level and grating speed on head-tracking response in normal and retinal degenerate (RD) mice (rd10) and rats (S334ter-line-3) was evaluated using a computer-based testing apparatus. In normal (non-RD) mice and rats, specific stripe width and grating speed was found to evoke maximum optokinetic head-tracking response. In line-3 RD rats, the contrast sensitivity loss was slow and remained close to the baseline (normal control) level until very late in the disease, whereas, in rd10 mice the progression of the contrast sensitivity loss was more rapid. Observed differences between rd10 mice and line-3 RD rats in the progression of contrast sensitivity loss may not be directly related to the degree of photoreceptor loss. In young RD mice, the modulatory influence of stimulus parameters on optokinetic head-tracking response was similar to normal control animals. During later stages, slower grating speed was required to evoke the maximum optokinetic response. Grating speed had lesser apparent influence on the response properties of line-3 RD rats. Discrepancies between the two RD models in the modulatory influence of optokinetic stimulus parameters can be the manifestation of fundamental species differences and/or differences in the degeneration pattern. This study highlights the importance of careful selection of appropriate stimulus parameters for testing optokinetic head-tracking response in RD animals.
Keywords: Retinal degeneration, visual behavior, optokinetic testing, S334ter-line-3 rat, rd10 mice
Introduction
The optokinetic head-tracking (OHT) response is a compensatory eye movement that reduces movement of images across the retina [7]. Animals with normal vision exhibit this automatic reflex, tracking a moving stimulus by repeatedly turning their heads in the direction of the movement [9]. According to Cahill and Nathans[2], image stabilization, by means of head turning reflex, is mediated by two types of oculomotor responses: the optokinetic reflex or optokinetic nystagmus and the vestibulo-ocular reflex. Measurement of the length of time an animal spends tracking the moving stimulus has been shown to be a reliable method for quantifying the level of visual function. Studies performed in transgenic rat models, such as S334ter-line-3 rats, suggested that a certain level of OHT visual function is preserved in these animals until the very late stages of the disease progression [15]. Variations in head-tracking visual preservation among different RD models may depend on the population and distribution of surviving photoreceptors, remodeling status of the inner retina and/or the morphological status of higher visual areas of the brain such as the superior colliculus (SC). Most previous OHT studies in RD animals were performed using stimulus parameters designed for normal (non-degenerate) animals; hence, little information regarding the modulatory influence of stimulus parameters on optokinetic response in RD animals at various stages of the disease progression is available. Schmucker et al [12] determined the optomotor grating acuity in various mice models of rod and or cone photoreceptor degeneration by measuring the head-tracking response level at 1 month of age. In the above experiment, the grating acuity of the animals was tested at different luminance levels to determine the rod and cone sensitivity threshold level. Thuang et al. [13] used an electrically controlled optokinetic drum to evaluate visual behavior in different strains of mice, between 8 and 26 weeks of age. The head-tracking response was evaluated using a specific stripe width, grating speed and contrast sensitivity.
By changing stimulus parameters such as stripe width and grating speed, it is possible to elucidate the stimulus parameters that can evoke maximum OHT response in RD animals, especially during various stages of the disease progression. Therefore, more effective functional assessments may be possible after therapeutic interventions such as cell transplantation and gene therapy. For the present investigation, OHT responses in rats and mice were evaluated using a computer–based testing apparatus. The feasibility of using a computer-based testing apparatus for evaluating OHT responses in rats has been recently demonstrated[16]. We used a Java-based software program to create and modulate the optokinetic stimulus parameters, such as stripe width, grating speed, and direction of the stripe movement, and to compare responses between normal and retinal degenerate rats and mice.
Material and Methods
Experimental Animals
Normal Copenhagen rats (n=6, Harlan, Indianapolis, IN) and pigmented S334-ter line- 3 transgenic rats (n = 6) expressing a mutated human rhodopsin protein were used. The transgenic rats were produced by Xenogen Biosciences (formerly Chrysalis DNX Transgenic Sciences, Princeton, NJ) and developed and supplied with the support of the National Eye Institute by Dr. Matthew LaVail, University of California San Francisco (http://www.ucsfeye.net/mlavailRDratmodels.shtml). The time course of photoreceptor degeneration in these rats has been previously reported [14]. Normal (C57BL/6J, n=6) and retinal degenerate (RD) (Pde6brd10/Pde6brd10, n=6) mice were obtained from the Jackson Laboratory (Bar Harbor, ME). The rd10 mouse is a relatively recently discovered retinal degeneration model [3]. The time course of photoreceptor degeneration and visual sensitivity loss in the rd10 mouse has been previously reported [3, 6]. Although the location of the genetic defect is different in the two animal models, in both models, the disease primarily affects the rod photoreceptors with secondary loss of cones and complete loss of visual function. The OHT response of RD animals was tested at various stages of disease progression. All animals were maintained in accordance with the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research. The research followed the “Principles of laboratory animal care” (NIH publication No. 86-23, revised 1996) and approved by the Animal Care and Use Committee of the University of Southern California. Testing was performed using a custom-made, computer-based apparatus consisting of four, 13-inch computer monitors arranged in a square to form an optokinetic chamber. A Java-based computer program was designed to generate the optokinetic stimuli, consisting of moving alternate black and white stripes. The stimulus was displayed on all computer monitors using a 4 port video splitter (Startech.com, model#ST124PROA). The luminance of individual stripes was measured using a 371 R Optical Power Meter (Graseby Optronics, Orlando, FL) from the level of the eyes. The contrast at a given spatial frequency was calculated using the formula (Lmax − Lmin) / (Lmax+Lmin) where Lmax is the brightness (in cd/m2) of the white stripe, and Lmin is the brightness of the black stripe (in cd/m2) [16]. The Java program enabled modulation of contrast level, stripe width, grating speed, and direction of stripe movement (clockwise vs. counterclockwise). During testing, the mice were released over a small plexiglass platform (12 cm diameter and 17 cm high) placed at the middle of the optokinetic chamber and rats were placed inside a small transparent plexiglass square container (20cm) kept over a pedestal at the center of the optokinetic chamber. The space available for the animals to move in their respective testing arenas is limited. Movements within this limited space may cause only minor variation in their viewing distance from the optokinetic stimuli displayed on the wall of the testing chamber (computer screens). Hence, no major variability can be expected in the stimulus level as a result of movement of the animals inside the chamber.
Rats and mice were tested for OHT response, once a month beginning at 30 days of age. The tests were conducted up to the age of 6 months. The OHT stimulus was not activated until 3 min after each animal was released into the test arena to allow the animal to settle. Once the animals are settled, the testing procedure is initiated by turning on the stripe movement. Stripes are moved in the clockwise direction for 1 min, then counterclockwise for 1 min. Optokinetic response of the animals was determined based on the level of head-tracking response. Animals having normal vision automatically track moving stimuli by turning their head in the direction of the movement. At a certain point, the animal will turn the head back and will start tracking over again. Such head-tracking movements can be used to establish a measure of visual function in rats [15]. The degree of head-tracking may vary between experiments and considerable individual variation is also noticed. In the present investigation, based on the level of OHT response, the visual response of each animal was scored on a scale of 0-4 (‘0’ = no apparent head-tracking, ‘1’ = minimum head tracking, ‘2’ = good head-tracking but no body movement, ‘3’ = maximum head tracking and minimum body movement, ‘4’ = maximum head tracking and considerable body movement). The rat and mice experiments were conducted by two independent observers masked as to whether the animals were RD or normal. Rat and mice (normal and RD) experiments were randomly repeated by the second observer to confirm the reproducibility of the testing method and the reliability of the scoring system. The data obtained from the two independent observations were found to be consistent. Preliminary experiments were performed to identify the optimum OHT stimulus paradigm for normal mice and rats. For this, the animals were initially tested using the optimum stripe width and grating speed that evoked the maximum head-tracking response in a previous investigation [15] using an older non-computerized mechanical instrument. To optimize the stimulus parameters for our new digital apparatus, normal rats and mice were tested using a wide range of grating frequencies (0.2 cycle/degree to 1.0 cycle/degree) and grating speeds (2.3 cm/second to 16.1 cm/second). Each animal was tested at least 3 times. To avoid possible habituation, a minimum one hour interval was provided between the consecutive tests. The animals were tested initially at the maximum contrast level (0.91for rats and 0.92 for mice). This is presumably the optimum contrast level (that evokes the best optokinetic stimuli). The contrast sensitivity threshold for each animal was determined by changing the luminance level of black and/or white stripes by approximately 25 cd/m2. The threshold was established based on the presence of definite head-tracking behavior (regardless of duration). The RD animals were tested using varying stripe speeds to determine the optimum optokinetic stimulus parameters corresponding to the various stages of the disease progression.
Statistics
Two Factor Analysis of Variance (ANOVA) with replication (Microsoft Excel, alpha=0.05 for a 95% confidence) was used to assess the effectiveness of varying stimulus conditions (stripe width and rotation speed) and disease progression (age) on OHT response.
Results
In normal mice, the maximum head-tracking score was consistently observed at a grating frequency of 0.3 cycles/degree, stripe width of 1.20 cm, and stripe speed of 11.5 cm/second. In normal rats, the maximum score was reproducibly observed at a grating frequency of 0.1 cycles/degree, stripe width of 3.60 cm, and stripe speed of 6.9 cm/second. Two Factor ANOVA demonstrates significant interaction between the stripe width and grating speed in determining the maximum head-tracking score (P<0.05).
Detailed evaluation of the contrast sensitivity threshold in RD animals as the RD condition progressed revealed considerable differences between the two animal models (line-3 RD rats and rd10 mice). Differences were observed in the progression pattern of the contrast sensitivity loss. In rd10 mice, the contrast sensitivity curve was close to linear, suggesting progressive loss in sensitivity with increasing age (Fig. 1). On the other hand, in line-3 RD rats, the sensitivity level remained close to the normal baseline (non-RD control) level during the initial phase of the disease. After 4 months of age, there was a steep decline in the contrast sensitivity, suggesting considerable loss of sensitivity at this age (Fig. 2).
Figure 1.
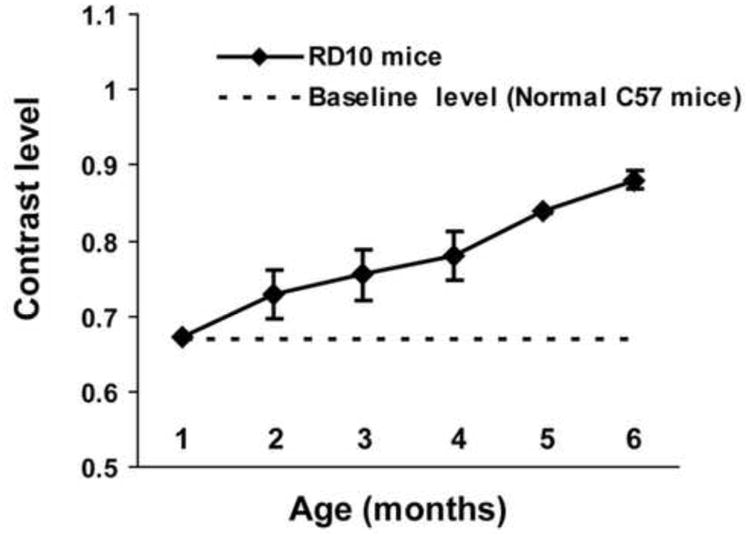
Progressive loss of contrast sensitivity in rd10 mice. The contrast sensitivity threshold for each animal was determined by changing the luminance level of the stripes by approximately 25 cd/m2. The contrast sensitivity threshold was estimated based on whether any tracking behavior was observed. The sensitivity curve was constructed by plotting contrast vs age. The linear shape of the curve suggests consistent loss of contrast sensitivity in rd10 mice throughout the course of the disease progression.
Figure 2.
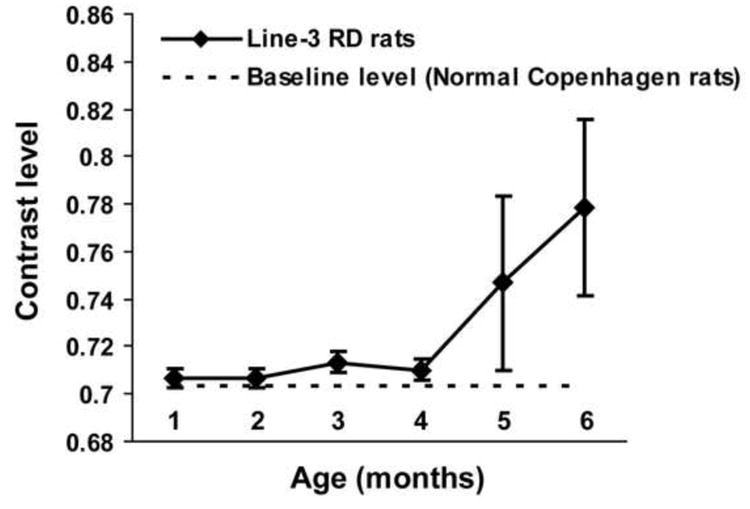
Progressive changes in the contrast sensitivity in SS334ter-line-3 RD rats due to the progress of the RD disease. The contrast sensitivity threshold for each animal was determined by changing the luminance level of the stripes by approximately 25 cd/m2. The contrast sensitivity threshold was estimated based on whether any tracking behavior was observed. The sensitivity was plotted using contrast vs age. In line-3 RD rats, the sensitivity remained close to the normal baseline (non-RD control) level during the initial phase of the disease. After 4 months of age, the contrast sensitivity plot showed a vertical deflection suggesting considerable loss of sensitivity at this age.
Progressive changes in visual head-tracking responses were compared between the two RD models (rd10 mice and line-3 RD rats) by varying the grating speed during testing. In both models (mice and rats), when tested at a younger age (30-35 days), the head-tracking score was close to the responses recorded in normal control animals (Figs. 3,4). As the disease progressed, considerable loss of head-tracking response was observed in both RD models. The grating speed that evoked the maximum head-tracking response also changed during various stages of the disease. A significant influence of grating speed on visual head-tracking response is demonstrated by Two Factor ANOVA (P<0.05). In young rd10 mice (up to 2 months of age), the optokinetic score was maximum when the grating speed was similar to the optimum speed identified for normal control animals (Fig. 3). As the disease progressed, a slower grating speed was required to evoke the maximum head-tracking response. Compared to the results obtained with the RD mice, the influence of grating speed on OHT response was less apparent in line-3 RD rats (Fig. 4, Two Factor ANOVA, P>0.05).
Figure 3.
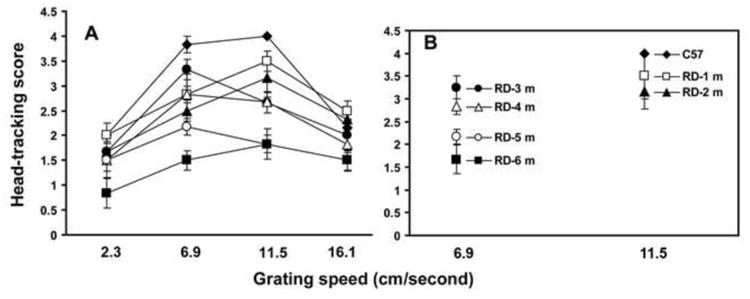
Progressive changes in the OHT response in rd10 mice evaluated by testing under varying grating speeds (mean±SE). Based on the level of head-tracking, the responses were scored on a scale of 0-4 (‘0’ = no response; ‘4’ = best response). RD mice were tested for head-tracking response once a month from 30 days to 6 months of age. (A) At younger ages (30-35 days), the optokinetic score was close to the normal control level; as the disease progressed, considerable loss of head-tracking score was observed. The grating speed that evoked the maximum head-tracking score changed during the progression of the disease.(B) Scatter plot demonstrating that in rd10 mice at early stages of degeneration (1 and 2 months of age), the maximum head-tracking score was observed at a grating speed that evoked the maximum response in normal control animals. As the disease further progressed (3, 4, and 5 months of age), maximum head-tracking score was apparent at a slower grating speed. The interaction between age and grating speed in determining the maximum head-tracking score is found to be statistically significant (Two Factor ANOVA, P<0.05).
Figure 4.
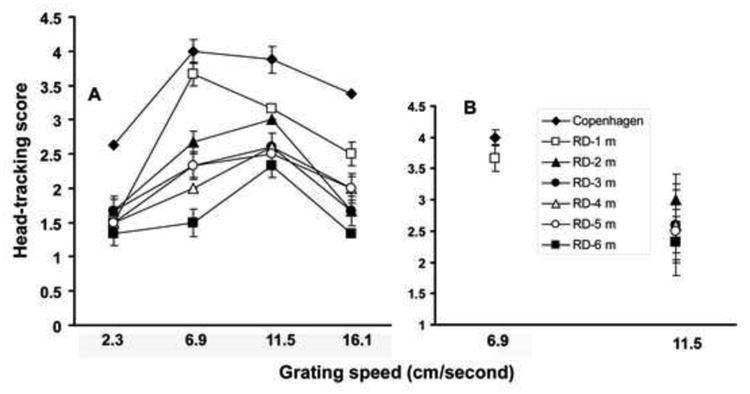
Progressive changes in optokinetic response in SS334ter-line-3 RD rats evaluated at varying grating speeds (mean±SE). Based on the level of the head-tracking, the responses were scored on a scale of 0-4 (‘0’ = no response; ‘4’ = best response). Rats were tested for head-tracking response once a month from 30 days to 6 months of age. (A) optokinetic score at different grating speed plotted for normal Copenhagen rats (filled diamond) and S334ter-line-3 RD rats at 2- 6 months of age. (B) In RD rats no significant pattern is apparent to demonstrate a positive interaction between age and grating speed in determining the maximum head-tracking score (Two Factor ANOVA, P>0.05).
Discussion
Detailed investigation of the stimulus parameters revealed that the optimum grating speed and stripe width required to evoke the maximum optokinetic response differed between rats and mice. In normal mice, a narrow stripe (width =1.20 cm) and a faster grating speed (11.5 cm/second) evoked the maximum head-tracking response, whereas, in normal rats, a wider stripe (width = 3.60 cm) and slower grating speed (6.9cm/second) was preferred. Previously, Prusky et al [10] tested visual acuity in different species of rodents using a visual water task and suggested that visual acuity differences may be based on strain differences. Variations in visual acuity among different rodent models may be explained in terms of differences in the eye size, receptive field size, and central visual processing mechanisms.
The progressive pattern of visual sensitivity loss in two RD animal models was compared. In line-3 RD rats, the OHT based contrast sensitivity was well preserved (almost to the level of normal, non-degenerate control animals) until approximately 6 months of age. In rd10 mice on the other hand, considerable loss of contrast sensitivity was apparent at a younger age (2 months). Potential explanations for the differences between the two models in the progressive loss of contrast sensitivity include differences in the level of photoreceptor (rod and/or cone) loss or variability in the changes affecting the inner retinal neural circuitries secondary to photoreceptor loss. Previous morphological data, however, have suggested that the patterns of photoreceptor loss in rd10 mice [3] and line-3 RD rats [1, 8, 11] are similar. In both models, at the age of 1 month, only a single layer of photoreceptors remains in the retina. Electrophysiological measurements at about this age in rd10 mice [3] and line-3 RD rats [1, 5] suggested apparent loss of ERG waveforms. These findings suggest that the dichotomy observed in the progression of optokinetic contrast sensitivity loss in these RD models may not be related to photoreceptor loss, but rather may be due to differential alterations in the inner retina or upstream visual pathways.
It should be noted that in the majority of the previous OHT studies, the responses were evaluated using stimulus parameters designed for normal (non-degenerate) control animals, and evaluations were performed only at certain stages of degeneration [4, 12, 13]. Our computer-based apparatus enabled extensive modulation of the stimulus parameters. This helped us to identify the optimal stimulus paradigm for each stage of degeneration.
Our study demonstrates that, compared to the rd10 mouse model, the modulatory influence of stimulus parameters (grating speed) on optokinetic response properties is less apparent in the line-3 RD rat. In RD mice, specific grating speeds evoke the maximum head-tracking score at specific stages of the disease progression. In these animals, up to 2 months of age, the grating speed that evoked the maximum head-tracking response was similar to the optimum grating speed computed for normal control animals. However, when these mice were tested again (after 1 month), the maximum head-tracking score was observed at a faster grating speed, suggesting that additional changes to the visual sensory system may have occurred. Two Factor ANOVA demonstrates that the grating speed required for evoking the maximum optokinetic score varies significantly during the course of disease progression (age). Hence, in rd10 mice it is possible to identify the stimulus parameters required to evoke the maximum head-tracking response at specific stages of disease progression. The above information may be useful when designing functional evaluation protocols for RD animals, especially following various therapeutic interventions. The discrepancies between the two RD models (rd10 mice and line-3 RD rats) with respect to the influence of stimulus parameters on OHT score could be due to several reasons. Generally, the mice are considered to better respond to optokinetic stimuli compared with rats [4], and this appears consistent with our current investigation. The fundamental differences between the two species in the OHT response may be magnified during retinal degenerative conditions. It may also be noted that the loss of visual contrast sensitivity in rd10 mice progresses more rapidly compared to the line-3 RD rats. The rapid loss of contrast sensitivity in rd10 mice may adversely affect the OHT stimulus requirements.
In summary, our findings underscore the importance of using appropriate stimulus conditions to measure the OHT response in RD models at various stages of disease progression. The above approach may enable better assessment of visual functional changes in RD animals, especially following various therapeutic interventions.
Acknowledgments
Supported by: Foundation Fighting Blindness; NIH EY03040; Research to Prevent Blindness, Fletcher Jones Foundation. Leo Kim is a Heed Fellow and receives support from the Heed Ophthalmic Foundation.The authors want to thank Zhenhai Chen for technical assistance and Susan Clarke for manuscript editing.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.An GJ, Asayama N, Humayun MS, Weiland J, Cao J, Kim SY, Grebe R, de Juan E, Jr, Sadda S. Ganglion cell responses to retinal light stimulation in the absence of photoreceptor outer segments from retinal degenerate rodents. Curr Eye Res. 2002;24:26–32. doi: 10.1076/ceyr.24.1.26.5432. [DOI] [PubMed] [Google Scholar]
- 2.Cahill H, Nathans J. The optokinetic reflex as a tool for quantitative analyses of nervous system function in mice: application to genetic and drug-induced variation. PLoS ONE. 2008;3:e2055. doi: 10.1371/journal.pone.0002055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chang B, Hawes NL, Hurd RE, Davisson MT, Nusinowitz S, Heckenlively JR. Retinal degeneration mutants in the mouse. Vision Res. 2002;42:517–525. doi: 10.1016/s0042-6989(01)00146-8. [DOI] [PubMed] [Google Scholar]
- 4.Douglas RM, Alam NM, Silver BD, McGill TJ, Tschetter WW, Prusky GT. Independent visual threshold measurements in the two eyes of freely moving rats and mice using a virtual-reality optokinetic system. Vis Neurosci. 2005;22:677–684. doi: 10.1017/S0952523805225166. [DOI] [PubMed] [Google Scholar]
- 5.Gamm DM, Wang S, Lu B, Girman S, Holmes T, Bischoff N, Shearer RL, Sauve Y, Capowski E, Svendsen CN, Lund RD. Protection of visual functions by human neural progenitors in a rat model of retinal disease. PLoS ONE. 2007;2:e338. doi: 10.1371/journal.pone.0000338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gargini C, Terzibasi E, Mazzoni F, Strettoi E. Retinal organization in the retinal degeneration 10 (rd10) mutant mouse: a morphological and ERG study. J Comp Neurol. 2007;500:222–238. doi: 10.1002/cne.21144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Harvey RJ, De'Sperati C, Strata P. The early phase of horizontal optokinetic responses in the pigmented rat and the effects of lesions of the visual cortex. Vision Res. 1997;37:1615–1625. doi: 10.1016/s0042-6989(96)00292-1. [DOI] [PubMed] [Google Scholar]
- 8.Liu C, Li Y, Peng M, Laties AM, Wen R. Activation of caspase-3 in the retina of transgenic rats with the rhodopsin mutation s334ter during photoreceptor degeneration. J Neurosci. 1999;19:4778–4785. doi: 10.1523/JNEUROSCI.19-12-04778.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lund RD, Kwan AS, Keegan DJ, Sauve Y, Coffey PJ, Lawrence JM. Cell transplantation as a treatment for retinal disease. Prog Retin Eye Res. 2001;20:415–449. doi: 10.1016/s1350-9462(01)00003-9. [DOI] [PubMed] [Google Scholar]
- 10.Prusky GT, West PW, Douglas RM. Behavioral assessment of visual acuity in mice and rats. Vision Res. 2000;40:2201–2209. doi: 10.1016/s0042-6989(00)00081-x. [DOI] [PubMed] [Google Scholar]
- 11.Sagdullaev BT, Aramant RB, Seiler MJ, Woch G, McCall MA. Retinal transplantation-induced recovery of retinotectal visual function in a rodent model of retinitis pigmentosa. Invest Ophthalmol Vis Sci. 2003;44:1686–1695. doi: 10.1167/iovs.02-0615. [DOI] [PubMed] [Google Scholar]
- 12.Schmucker C, Seeliger M, Humphries P, Biel M, Schaeffel F. Grating acuity at different luminances in wild-type mice and in mice lacking rod or cone function. Invest Ophthalmol Vis Sci. 2005;46:398–407. doi: 10.1167/iovs.04-0959. [DOI] [PubMed] [Google Scholar]
- 13.Thaung C, Arnold K, Jackson IJ, Coffey PJ. Presence of visual head tracking differentiates normal sighted from retinal degenerate mice. Neurosci Lett. 2002;325:21–24. doi: 10.1016/s0304-3940(02)00223-9. [DOI] [PubMed] [Google Scholar]
- 14.Thomas BB, Seiler MJ, Aramant RB, Samant D, Qiu G, Vyas N, Arai S, Chen Z, Sadda SR. Visual functional effects of constant blue light in a retinal degenerate rat model. Photochem Photobiol. 2007;83:759–765. doi: 10.1562/2006-09-19-RA-1044. [DOI] [PubMed] [Google Scholar]
- 15.Thomas BB, Seiler MJ, Sadda SR, Coffey PJ, Aramant RB. Optokinetic test to evaluate visual acuity of each eye independently. J Neurosci Methods. 2004;138:7–13. doi: 10.1016/j.jneumeth.2004.03.007. [DOI] [PubMed] [Google Scholar]
- 16.Tomita H, Sugano E, Fukazawa Y, Isago H, Sugiyama Y, Hiroi T, Ishizuka T, Mushiake H, Kato M, Hirabayashi M, Shigemoto R, Yawo H, Tamai M. Visual properties of transgenic rats harboring the channelrhodopsin-2 gene regulated by the thy-1.2 promoter. PLoS One. 2009;4:e7679. doi: 10.1371/journal.pone.0007679. [DOI] [PMC free article] [PubMed] [Google Scholar]


