Abstract
Purpose
To estimate dry eye prevalence in the Beaver Dam Offspring Study (BOSS), including a young adult population, and investigate associated risk factors and impact on health-related quality of life.
Design
Cohort study.
Methods
The BOSS (2005–2008) is a study of aging in the adult offspring of the population-based Epidemiology of Hearing Loss Study cohort. Questionnaire data on health history, medication use, risk factors, and quality of life were available for 3275 participants. Dry eye was determined by self-report of frequency of symptoms and the intensity of those symptoms. Associations between dry eye and risk factors were analyzed using logistic regression.
Results
The prevalence of dry eye in the BOSS was 14.5%, 17.9% of women and 10.5% of men. In a multivariate model, statistically significant associations were found with female sex (Odds Ratio (OR), 1.68; 95% Confidence Interval (CI), 1.33–2.11), current contact lens use (OR, 2.01; 95%CI, 1.53–2.64), allergies (OR, 1.59; 95%CI 1.22–2.08), arthritis (OR, 1.44; 95%CI, 1.12–1.85), thyroid disease (OR, 1.43; 95%CI, 1.02–1.99), antihistamine use (OR, 1.54; 95%CI, 1.18–2.02), and steroid use (OR, 1.54; 95%CI, 1.16–2.06). Dry eye was also associated with lower scores on the Medical Outcomes Short Form-36 (β=−3.9, p<0.0001) as well as on the National Eye Institute Visual Functioning Questionnaire (NEI-VFQ-25) (β= −3.4, p<0.0001) when controlling for age, sex, and comorbid conditions.
Conclusions
The prevalence of dry eye and its associated risk factors in the BOSS were similar to previous studies. In this study, DES was associated with lower quality of life on a health-related quality of life instrument and the vision-specific NEI-VFQ-25.
INTRODUCTION
Dry eye is a multifactorial disease of the tears and ocular surface, resulting in symptoms of discomfort, visual disturbance, and tear film instability with potential damage to the ocular surface.1 Dry eye can be characterized by a dry, gritty or burning feeling in the eyes which may be accompanied by excessive tearing or sensitivity to light among other symptoms.2 Prevalence estimates of dry eye disease and severe symptoms have largely varied by study, ranging between 5 and 35 percent.3,4,5,6,7,8,9 It has been shown to affect visual functioning, including visual acuity as well as having a negative impact on some health-related quality of life measures.10,11,12 Dry eye has also been found to be correlated with anxiety and depression.13
The known association between dry eye and age has led to thorough study of the disorder in older adult populations, generally focusing on those over 50 years of age. The prevalence and associated risk factors for dry eye have not been widely investigated in younger populations. One of the few investigations that included a wider age range of adults (21–90) was conducted in a very specific group of Veterans Affairs patients.6 Further the impact of dry eye on quality of life in younger populations is relatively unknown. In one study including younger adults it was found that both health-related quality of life measures and a vision-specific measure were sensitive to severity of dry eye, though this was in a small sample recruited from eye clinics.14
The aims of this investigation in a large cohort predominately composed of middle-aged adults were to determine the prevalence of dry eye symptoms, identify independent risk factors, and quantify the their impact on quality of life.
METHODS
The Beaver Dam Offspring Study (BOSS) is an ongoing cohort study of aging in the adult children of the population-based Epidemiology of Hearing Loss Study (EHLS). Baseline examinations of BOSS participants (N=3285), aged 21–84 years, took place between 2005 and 2008. Information on symptoms of dry eye was provided by 3275 participants. The BOSS was approved by the Health Sciences Institutional Review Board of the University of Wisconsin, all participants provided written informed consent, and all study protocols were carried out in accordance with the tenets of the Declaration of Helsinki.
The BOSS examination consisted of an extensive questionnaire including demographic information, employment history, medical history, risk-behaviors, and health-related quality of life. Questionnaires were completed by in-person interviews conducted by technicians trained to a standard protocol or for those unable to come to the examination sites, by self-administration through a web-based or mailed form (n=439).
Participants were asked “How often do you have dry eyes, a dry, gritty, or burning feeling?”, “How much does the dryness in your eyes bother you?”, “Is there a season of the year when the dryness in your eyes is the worst?”, and “Are you currently using eye drops at least once a day for dry eyes?” Objective measures of dry eye, such as the tear break-up time (T-BUT) test, Schirmer test, or Rose Bengal staining were not administered and information of previous doctor diagnosis was not available. Participants who reported symptoms were present sometimes or more often and they were moderately bothersome or greater, or those who reported currently using eye drops at least once a day for dry eyes were considered to be cases.
Health conditions considered in this investigation include history of allergies and doctor-diagnosed systemic diseases including arthritis, osteoporosis, thyroid disease, and diabetes Hypertension, was present if they had a measured systolic blood pressure greater than 140mmHg or a measured diastolic blood pressure greater than 90mmHg (Dynamap Procare 120, GE Medical Systems, Milwaukee, WI) or a history of having been told by a doctor that they had high blood pressure and currently using antihypertensive medications. Information on history of head injury and loss of consciousness was also collected.
Other covariates were considered based on previous findings and biologic plausibility. Medications considered in these analyses included antihistamines, anti-anxiety medications, acetaminophen, benzodiazepine, anticholinergics, antidepressants, statins, steroids, diuretics, multivitamins, and among women, hormones used for birth control, infertility, or hormone replacement therapy. Contact lens use (current and past), smoking (current status, pack years smoked, number of cigarettes per day), alcohol consumption (previous year and grams of ethanol per week), and season at interview were also considered in the analyses.
The Medical Outcomes Short-Form 36 (SF-36) and the National Eye Institute-Visual Functioning Questionnaire (NEI-VFQ 25) were administered to assess quality of life. The Physical Component Summary (PCS), Mental Component Summary (MCS), General Health Perception Index (GHP), and Bodily Pain Index (BPI), were calculated for the SF-36.15,16 The composite and 12 subscales scores were calculated for the NEI-VFQ 25.17 The self-administered, Center for Epidemiologic Studies-Depression Scale (CES-D) was used to assess symptoms of depression.
Risk factor investigation consisted of chi-squared analysis, as well as age and sex controlled logistic regression models. Associations were considered statistically significant at or below p-value=0.05. Additionally, a stepwise procedure was performed to create a final model estimating independent odds of dry eye by risk factor. Risk factors with results suggestive of association (p-value<0.10) in age and sex adjusted models were considered for entry into the final model. Age and sex were retained in the model, while retention of all other variables was determined by a two-tailed p-value at or below 0.05.
Mean score differences were calculated between those with and without dry eye symptoms on both the vision specific and health-related quality of life instruments through ordinary single and multiple linear regression models. Finally, the association between dry eye and depressive symptoms (score of 16 or greater on the CES-D) was estimated using logistic regression. All statistical analyses were conducted using SAS version 9.2 (SAS Institute, Cary NC).
RESULTS
Prevalence
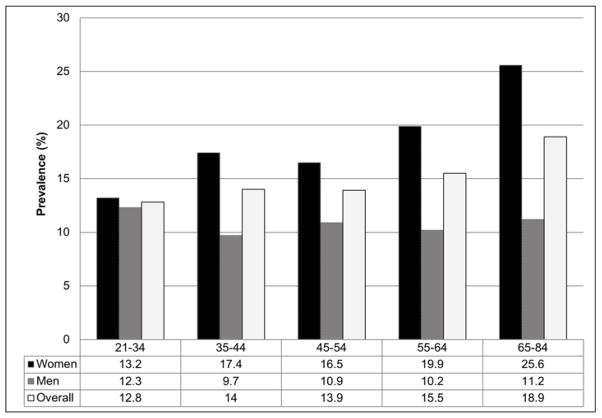
The mean age of the participants was 49 years (range 21–84 years), 1789 (54.6%) were female and 2271 (70%) had higher than a high school education (see Table 1). The prevalence of dry eye symptoms was 14.5% overall (14.1% in those aged 21–49 years, 15.2% in those 50 and older), and was significantly higher in women than men (17.9% versus 10.5%, p<0.0001). Although a slight increase in prevalence of dry eye symptoms was observed by age, this trend for the population overall did not reach statistical significance (p=0.06). When stratified by sex, the effect of age on dry eye differed between men and women. In men, estimated prevalence was similar among all age groups and there was no observed effect of age (p=0.91), while in women prevalence increased with age (p=0.02), though this interaction was not significant in multivariable models (See Figure 1).
Table 1.
Characteristics of participants with dry eye symptom data, the Beaver Dam Offspring Study 2005–2008
| Characteristic | N (%) Overall |
N (%) <50 years of age |
N (%) ≥50 years of age |
p-value |
|---|---|---|---|---|
| N | 3275 | 1764 | 1511 | |
| Female | 1789 (54.6) | 983 (55.7) | 806 (53.3) | 0.17 |
| Male | 1486 (45.4) | 781 (44.3) | 705 (46.7) | |
| Age | ||||
| 21–34 | 179 (5.5) | 179 (10.1) | NA | NA |
| 35–44 | 931 (28.4) | 931 (52.8) | NA | |
| 45–54 | 1227 (37.5) | 654 (37.1) | 573 (37.9) | |
| 55–64 | 710 (21.7) | NA | 710 (47.0) | |
| 65–84 | 228 (7.0) | NA | 228 (15.1) | |
| Education (Years) | ||||
| <12 | 75 (2.3) | 31 (1.8) | 42 (2.9) | <0.0001 |
| 12 | 902 (27.8) | 439 (24.9) | 463 (30.6) | |
| 13–15 | 1088 (33.5) | 597 (34.0) | 491 (32.9) | |
| 16+ | 1183 (36.4) | 688 (39.2) | 495 (33.2) | |
| Smoking History | ||||
| Never | 1751 (53.8) | 1011 (57.6) | 740 (49.3) | <0.0001 |
| Past | 927 (28.5) | 392 (22.4) | 535 (35.7) | |
| Current | 576 (17.7) | 351 (20.0) | 225 (15.0) | |
| Alcohol Consumption (grams of ethanol per week) | ||||
| 0 | 348 (10.7) | 155 (8.8) | 193 (12.9) | <0.0001 |
| 1–14 | 1323 (40.7) | 690 (39.3) | 633 (42.2) | |
| 15–74 | 766 (23.5) | 449 (25.6) | 317 (21.1) | |
| 75–140 | 392 (12.0) | 231 (13.2) | 161 (10.7) | |
| >140 | 426 (13.1) | 229 (13.1) | 197 (13.1) | |
| Body Mass Index | ||||
| <25 | 607 (21.6) | 418 (27.3) | 189 (14.8) | <0.0001 |
| 25–29.9 | 947 (33.7) | 519 (33.9) | 428 (33.5) | |
| 30+ | 1254 (44.7) | 593 (38.8) | 661 (51.7) |
Figure 1.
Dry eye symptoms by age group (years) and sex, the Beaver Dam Offspring Study 2005–2008
Risk Factors
In separate age- sex-adjusted models, arthritis, osteoporosis, allergies, thyroid disease, severe headaches or migraine in the previous three months, and history of head injury were associated with increased odds of dry eye. Use of a number of medications including antihistamines, acetaminophen, benzodiazepine, anti-depressants, and steroids, also were associated with dry eye in the overall population (See Table 2). The association of use of steroids with symptoms of dry eye, was only significant with inhaled steroid use, OR=2.04 (95% CI=1.24, 3.33), as oral steroid use did not exhibit a statistically significantly association, OR=1.47 (95% CI=0.63, 3.42). Additionally, use of multivitamins was associated with symptoms of dry eye in those under the age of 50.
Table 2.
Odds ratios and 95% confidence intervals for dry eye symptoms by risk factor, the Beaver Dam Offspring Study 2005–2008 a
| Risk factor | < 50 years of age b | ≥ 50 years of age b | Overall b |
|---|---|---|---|
| Agec (per increase in age group) | 1.07 (0.87, 1.33) | 1.23 (1.00, 1.50) | 1.01 (1.00, 1.02) |
| Sex | |||
| Male | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) |
| Female | 1.60 (1.21, 2.11) | 2.23 (1.65, 3.01) | 1.88 (1.53, 2.30) |
| Contact Lens Use | |||
| Never | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) |
| Past | 1.13 (0.79, 1.63) | 1.58 (1.13, 2.21) | 1.34 (1.05, 1.71) |
| Current | 2.39 (1.73, 3.29) | 1.58 (0.98, 2.55) | 2.14 (1.65, 2.77) |
| Chronic Conditions | |||
| Arthritis | 2.07 (1.46, 2.94) | 1.31 (0.97, 1.77) | 1.59 (1.26, 2.00) |
| Osteoporosis | 2.29 (0.70, 7.54) | 1.50 (0.85, 2.65) | 1.70 (1.02, 2.82) |
| Allergies | 1.53 (1.12, 2.10) | 2.19 (1.54, 3.10) | 1.81 (1.43, 2.28) |
| Thyroid Disease | 1.45 (0.90, 2.33) | 1.72 (1.17, 2.54) | 1.62 (1.20, 2.18) |
| Migraine Headache | 1.57 (1.15, 2.13) | 1.91 (1.31, 2.79) | 1.69 (1.33, 2.15) |
| History of Head Injury | |||
| None | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) |
| No loss of consciousness | 0.99 (0.60, 1.64) | 1.06 (0.64, 1.75) | 1.04 (0.73, 1.49) |
| Loss of consciousness | |||
| < 5 minutes | 1.16 (0.78, 1.72) | 1.63 (1.07, 2.47) | 1.34 (1.01, 1.79) |
| >=5 minutes | 2.38 (0.64, 8.85) | 0.51 (0.07, 3.91) | 1.24 (0.42, 3.62) |
| Medication Use | |||
| Antihistamines | 1.45 (1.01, 2.09) | 1.94 (1.35, 2.79) | 1.65 (1.28, 2.13) |
| Acetaminophen | 1.24 (0.90, 1.70) | 1.34 (0.95, 1.88) | 1.28 (1.02, 1.62) |
| Benzodiazepine | 0.77 (0.32, 1.83) | 2.25 (1.34, 3.78) | 1.59 (1.03, 2.45) |
| Anti-depressants | 1.27 (0.87, 1.84) | 1.68 (1.15, 2.45) | 1.44 (1.11, 1.88) |
| Steroids | 1.62 (1.07, 2.45) | 2.06 (1.43, 2.98) | 1.84 (1.40, 2.42) |
| Multi-vitamin | 1.43 (1.09, 1.88) | 1.03 (0.77, 1.37) | 1.03 (0.77, 1.37) |
| Hormones (Women) | 1.85 (1.18, 2.90) | 1.05 (0.53, 2.09) | 1.54 (1.06, 2.24) |
Listed as OR (95% CI)
Adjusted for age and sex
Age groups (years): 21–34, 35–44, 45–54, 55–64, 65–84
Among women, those who had used hormones (for birth control, infertility, or for menopausal symptoms) were more likely to report dry eye. This effect also differed by age. Among women under the age of 50 years who reported having a period in the past 12 months, use of hormones for contraception, fertility or hormone replacement therapy was associated with a 71% increase in odds of dry eye symptoms, OR=1.71 (95% CI=1.08, 2.73). There was no association between hormone use and dry eye among women 50 years of age and older
There was no association between a history of smoking or alcohol consumption and dry eye overall or stratified by age. Past and current contact lens use was found to be associated with dry eye symptoms compared to those who had never used contact lenses, OR=1.34 (95%CI=1.05, 1.71) and OR=2.14 (95%CI=1.65, 2.77) respectively. Current contact lens use displayed a stronger association, OR=2.39, in those under 50 years of age. The season at interview was not associated with self-report of dry eye symptoms.
In the multivariable model, dry eye symptoms were associated with age, sex, current contact lens use, arthritis, allergies, thyroid disease, migraine headache, antihistamine use, and steroid use in the population overall (See Table 3). Results were similar when current contact lens wearers were excluded from the analysis (data not shown). In analysis limited to those younger than 50 years, female sex, current contact lens use (compared to those who had never used contact lenses), arthritis, allergies, and multivitamin use were all significantly associated with dry eye symptoms (See Table 3). In those 50 years of age or older, age, female sex, allergies, migraine headache, and use of anti-histamines and benzodiazepine, were associated with dry eye symptoms.
Table 3.
Odds ratios and 95% confidence intervals for dry eye symptoms by risk factor stratified by age, the Beaver Dam Offspring Study 2005–2008 a,b
| Risk factor | < 50 years of age | ≥50 years of age | Overall |
|---|---|---|---|
| Agec (per increase in age group) | 1.08 (0.85, 1.37) | 1.31 (1.03, 1.65) | 1.12 (1.00, 1.27) |
| Sex | |||
| Male | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) |
| Female | 1.41 (1.02, 1.95) | 1.76 (1.25, 2.47) | 1.45 (1.14, 1.85) |
| Contact Lens Use | |||
| Past | 0.92 (0.61, 1.38) | 1.09 (0.83, 1.43) | |
| Current | 2.38 (1.67, 3.37) | 2.09 (1.56, 2.79) | |
| Chronic Conditions | |||
| Arthritis | 2.14 (1.46, 3.13) | 1.41 (1.09, 1.82) | |
| Allergies | 1.49 (1.05, 2.10) | 2.06 (1.37, 3.10) | 1.54 (1.18, 2.01) |
| Thyroid Disease | 1.40 (1.00, 1.97) | ||
| Migraine Headache | 1.71 (1.10, 2.65) | 1.44 (1.10, 1.90) | |
| Medication Use | |||
| Antihistamines | 1.80 (1.23, 2.63) | 1.41 (1.07, 1.86) | |
| Steroids | 1.47 (1.10, 1.97) | ||
| Benzodiazepine | 2.08 (1.21, 3.56) | ||
| Multivitamin Use | 1.44 (1.06, 1.94) | ||
Listed as OR (95% CI)
Multivariable final models generated by stepwise regression. The model for each category includes the risk factors with a listed estimate.
Age groups (years): 21–34, 35–44, 45–54, 55–64, 65–84
Health Related Quality of Life
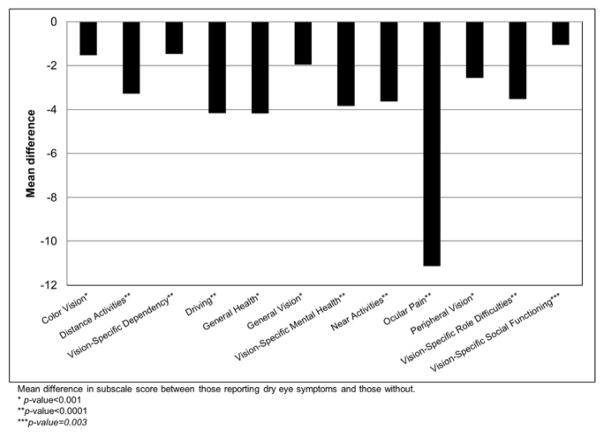
Participants with dry eye symptoms scored lower on the SF-36 and on the vision specific NEI-VFQ when controlling for age, sex, and comorbid conditions (See Table 4). In the SF-36 the largest differences were in the Bodily Pain and General Health Indices. Those with dry eye symptoms scored lower on all 12 of the NEI-VFQ subscales; with the largest difference appearing in the ocular pain score (See Figure 2). In analysis controlling for age, sex, education, and a number of comorbid conditions, those who reported dry eye symptoms were also 64% more likely to report depressive symptoms (score greater than or equal to 16) as measured by the CES-D (See Table 4). Stratification by age showed similar results.
Table 4.
Dry eye symptoms and health-related quality of life in the Beaver Dam Offspring Study, 2005–2008a, b
| Scale | <50 years | ≥50 Years | Overall | |||
|---|---|---|---|---|---|---|
|
| ||||||
| β | p-value | β | p-value | β | p-value | |
| VFQ Composite | −2.79 | <0.0001 | −3.82 | <0.0001 | −3.27 | <0.0001 |
| SF-36 GHPI | −4.66 | 0.0001 | −2.98 | 0.03 | −3.92 | <0.0001 |
| SF-36 MCS | −1.65 | 0.006 | −1.63 | 0.01 | −1.67 | 0.0001 |
| SF-36 PCS | −1.32 | 0.01 | −1.73 | 0.01 | −1.50 | 0.0005 |
| CES-D 16+ | ||||||
| No symptoms | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) | |||
| Dry eye symptoms | 1.53 (1.03, 2.29) | 1.81 (1.19, 2.77) | 1.64 (1.23, 2.19) | |||
VFQ= National Eye Institute Visual Functioning Questionnaire, GHPI= General Health Perception Index, MCS= Mental Component Score, PCS= Physical Component Score, CES-D= Center for Epidemiologic Studies-Depression Scale
Presented as mean difference in score (dry eye symptom positive-dry eye symptom negative), and p-value; except for CES-D presented as odds of a score greater than or equal to 16 (indicating depression) for those with dry eye symptoms versus those without and 95% Confidence Interval.
All estimates controlling for age, sex, education, CVD, diabetes, severe headaches or migraines, arthritis, cancer, hypertension, asthma, thyroid disease, diabetic retinopathy, age-related macular degeneration, retinal detachment, cataract, and glaucoma.
Figure 2.
Dry eye symptoms and the National Eye Institute-Visual Functioning Questionnaire subscales, the Beaver Dam Offspring Study 2005–2008*
DISCUSSION
Few studies have investigated the prevalence of and risk factors for dry eye among adults younger than 50 years of age. In the BOSS cohort, which includes young adults, dry eye symptoms were relatively common and had a significant impact on health-related quality of life. Prevalence in the BOSS was 14.1% among those aged 21–49 years, 15.2% among those 50 years of age and older, and 14.5% overall. The overall estimate falls in the range of the prevalence rates reported in previous studies.3, 9
The small difference in prevalence by age goes against some of the previous findings that age is a strong risk factor for dry eye. In this population, a few notable differences in risk factors may help to explain the similarity in prevalence. First, among the younger age group, current contact lens use is more strongly associated with dry eye. As a larger proportion of the younger group uses contact lenses to correct vision, the prevalence of dry eye may become more similar to the older group. The observed effect of multivitamins may be explained in the same way. A final risk-factor that showed an increased risk in the younger population is arthritis, potentially indicating that common chronic inflammatory processes may begin to occur at younger ages or that earlier onset of arthritis may also increase comorbidity.
Even in this population including those under 50, dry eye symptoms were associated significantly with lower scores on health-related quality of life as measured by the SF-36 and the NEI-VFQ. This association remained even when controlling for factors and diseases more commonly thought to impact these scores. In previous studies the relationship between dry eye symptoms and health-related quality of life has varied with some showing no correlation,18 while others have shown an association between general health-related quality of life10, vision specific quality of life12, and activities of daily living.19 In the BOSS, dry eye was inversely associated with the physical and mental component score of the SF-36. Similarly, dry eye was inversely associated with multiple subscales in the NEI-VFQ in this cohort. In a previous study focusing on vision specific quality of life, only two subscales, ocular pain and mental health, were related to dry eye symptoms.12 However, in the current study associations were found between dry eye and all of the 12 NEI-VFQ subscales though the effect was greatest with ocular pain. The lower scores on other subscales, such as the near vision scale, show that dry eye may be associated with disruptions in daily function in addition to discomfort or pain. Additionally, the impact of dry eye on health-related quality of life in this population was similar across age groups. In the case of the SF-36 General Health Perception Index, those under 50 years of age with dry eye symptoms scored lower than those over 50 respective to the participants without dry eye in their age group. While it is unclear if this is due to a lower frequency of other major health conditions at younger ages, or is a reflection of the intensity of dryness symptoms experienced by younger participants is unclear. The effect of dry eye at all ages in this study suggests that the impact of dry eye on an individual’s perception of their health is substantial and of importance as a public health problem.
Despite the quality of life impact shown in the BOSS, only a few participants reported a history of being diagnosed by their doctor as having Sjogren’s syndrome, a common cause of dry eye symptoms, and 157 (33%) of the 477 participants with dry eye symptoms reported using eye drops daily for dry eye specifically. The impact of treatment on quality of life could not be investigated as information on type of eye drops and frequency of administration was not available. Similarly, information on cost of eye drops or loss of productivity could not be quantified and may also have an impact on quality of life estimates. Even so, among young-middle aged adults, most people with dry eye symptoms had not been diagnosed and many had not been treated in the BOSS. While this lack of treatment may indicate that knowledge of dry eye is low in the population or that the symptoms of dryness among the BOSS participants were not severe enough to seek care, it is important to note that the impact on the health-related quality of life measures remains. In either case knowledge about the risk factors of dry eye would benefit care seeking behavior in similar populations.
In women in the BOSS, prevalence of dry eye symptoms increased with age, while in men the prevalence was relatively stable across all age groups. Previous studies of older adults showed an increased prevalence of dry eye for men and women as age increased.3,9 One possible explanation for this differences may deal with patterns of hormone use among women.
As in some previous studies, hormone use in the BOSS was found to increase odds of dry eye symptoms among women. Previous findings deal almost exclusively with hormone replacement therapy however.20 In this population any use of hormones for contraception or infertility was found to be significantly associated with dry eye, even among premenopausal women younger than 50, suggesting that these hormones may also contribute to dry eye symptoms. This relationship remains unclear and warrants further investigation including incidence of dry eye symptoms with use of specific hormones and mode of administration.
Also in the BOSS, a number of medications, such as antihistamines and steroids, some commonly used, were found to be associated with dry eye. The associations between many of these medications and dry eye symptoms have been found in previous studies.3,4 Although causal inferences cannot be determined due to the cross sectional nature of this study, use of alternative medications, when possible, should be considered for patients who complain of dry eye symptoms.
Many of the systemic diseases found previously to be associated with dry eye in older adults also showed significantly increased odds of comorbidity in this younger population. As previously noted arthritis was associated with dry eye. Similarly, thyroid disease was associated with increased odds of dry eye, indicating that hormone dysfunction may play a role in early onset of symptoms.
Also, an association was found between report of migraine headaches and dry eye symptoms. A previous study also found this relationship, though the causal direction of the relationship is unclear.21 It is possible that dry eye may trigger or cause migraines, though it is also possible that dry eye and migraines stem from a common inflammatory process. This association should be investigated further.
In previous studies, behavioral risk factors, like smoking and alcohol consumption, were found to be associated with dry eye signs and symptoms.3,22 These did not prove to be significant factors in the BOSS population overall or by age group. The data on alcohol consumption in this study deals with previous year use only, and as suggested by a previous study, past heavy drinking, may be associated with dry eye.3 This relationship warrants further investigation. Different smoking patterns or current number of cigarettes smoked per day did not display an association with dry eye. Whether this is due to changes in social norms or settings where smoking takes place is unknown. The season at interview was also not found to be a significant factor in reporting dry eye, which lowers concern that examinations conducted during heating season could show increased reporting of symptoms.
The strengths of this investigation include the large sample size, including young adults; something that has been missing in previous studies of dry eye. The large amount of data collected in the BOSS allows for adequate control of possible confounders. Also, the BOSS cohort is not clinic based, which limits the potential biases that come from access to care and care seeking behavior, which can be found in previous dry eye studies.
A limitation of this study is that dry eye symptom data were collected by self-report and no objective measurements were conducted. Symptom-based assessment of dry eye has been used and validated in numerous studies and is commonly used in diagnoses of dry eye clinically often in conjunction with objective measures.23,24,25,26 Further, the Schimer test, rose bengal staining, and tear break-up time (TBUT) test, have been shown to have poor correlation with dry eye symptoms and to have poor repeatability.27,28,29 Clinician assessment has also been show to underestimate severity of dry eye when compared to patients self-reported symptoms.30 However, it is possible that symptoms in the young may not be caused by Meibomian gland and corneal surface problems that are commonly found in the old. Physical examination to better characterize the cause of the symptoms are needed. One further limitation of this study is the cross-sectional design and as such causal inferences cannot be made.
In conclusion, prevalence of dry eye symptoms was relatively high in this study of a U.S. population including young adults. Few studies have investigated dry eye in this younger age range. Dry eye symptoms also proved to have a significant impact on health related quality of life and symptoms of depression, independent of other chronic diseases. Its high prevalence and its impact on quality of life in young and middle aged adults make this an important public health problem. Many of the chronic diseases, medications, and other risk factors previously found to be associated with dry eye were also found in this study and some, such as hormone use warrant further investigation. Further longitudinal study of dry eye is necessary to measure incidence, establish the temporal relationship between exposures and onset of symptoms, and to measure the impact of long term symptoms on quality of life.
Acknowledgments
The project was supported by Award Number R01AG021917 from the National Institute on Aging, the National Eye Institute and the National Institute on Deafness and Communicative Disorders and an unrestricted grant from Research to Prevent Blindness (RPB). The content is solely the responsibility of the authors and does not necessarily represent the official views of the aforementioned supporting institutions.
Footnotes
All authors have completed and submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. The authors have no proprietary or commercial interest in any materials discussed in this article.
Contributions of authors: Design of the study (KJC), Analysis and Interpretation (AJP, KJC, MEF, GH, BEK, RK, DSD), Writing the article (AJP, KJC), Critical revision of the article (AJP, KJC, MEF, GH, BEK, RK, DSD), Final approval of the article (AJP, KJC, MEF, GH, BEK, RK, DSD).
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.The definition and classification of dry eye disease: report of the Definition and Classification Subcommittee of the International Dry Eye Workshop (2007) Ocul Surf. 2007;5(2):75–92. doi: 10.1016/s1542-0124(12)70081-2. [DOI] [PubMed] [Google Scholar]
- 2.Begley CG, Caffery B, Chalmers RL, Mitchell GL. Use of the dry eye questionnaire to measure symptoms of ocular irritation in patients with aqueous tear deficient dry eye. Cornea. 2002;21(7):664–670. doi: 10.1097/00003226-200210000-00007. [DOI] [PubMed] [Google Scholar]
- 3.Moss SE, Klein R, Klein BE. Prevalence of and risk factors for dry eye syndrome. Arch Ophthalmol. 2000;118(9):1264–1268. doi: 10.1001/archopht.118.9.1264. [DOI] [PubMed] [Google Scholar]
- 4.Chia EM, Mitchell P, Rochtchina E, Lee AJ, Maroun R, Wang JJ. Prevalence and associations of dry eye syndrome in an older population: the Blue Mountains Eye Study. Clin Experiment Ophthalmol. 2003;31(3):229–232. doi: 10.1046/j.1442-9071.2003.00634.x. [DOI] [PubMed] [Google Scholar]
- 5.Guo B, Lu P, Chen X, Zhang W, Chen R. Prevalence of dry eye disease in Mongolians at high altitude in China: The Henan eye study. Ophthalmic Epidemiol. 2010;17(4):234–241. doi: 10.3109/09286586.2010.498659. [DOI] [PubMed] [Google Scholar]
- 6.Galor A, Feuer W, Lee DJ, et al. Prevalence and risk factors of dry eye syndrome in a United States veterans affairs population. Am J Ophthalmol. 2011;152(3):377–384. doi: 10.1016/j.ajo.2011.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Uchino M, Nishiwaki Y, Michikawa T, et al. Prevalence and risk factors of dry eye disease in Japan: Koumi study. Ophthalmology. 2011;118(12):2361–2367. doi: 10.1016/j.ophtha.2011.05.029. [DOI] [PubMed] [Google Scholar]
- 8.Lekhanont K, Rojanaporn D, Chuck RS, Vongthongsri A. Prevalence of dry eye in Bangkok, Thailand. Cornea. 2006;25(10):1162–1167. doi: 10.1097/01.ico.0000244875.92879.1a. [DOI] [PubMed] [Google Scholar]
- 9.Viso E, Rodriguez-Ares MT, Gude F. Prevalence of and associated factors for dry eye in a Spanish adult population (The Salnes Eye Study) Ophthalmic Epidemiol. 2009;16(1):15–21. doi: 10.1080/09286580802228509. [DOI] [PubMed] [Google Scholar]
- 10.Schiffman RM, Walt JG, Jacobsen G, Doyle JJ, Lebovics G, Sumner W. Utility assessment among patients with dry eye disease. Ophthalmology. 2003;110(7):1412–1419. doi: 10.1016/S0161-6420(03)00462-7. [DOI] [PubMed] [Google Scholar]
- 11.Vitale S, Goodman LA, Reed GF, Smith JA. Comparison of the NEI-VFQ and OSDI questionnaires in patients with Sjögren’s syndrome-related dry eye. Health Qual Life Outcomes. 2004;2:44. doi: 10.1186/1477-7525-2-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Le Q, Zhou X, Ge L, Wu L, Hong J, Xu J. Impact of dry eye syndrome on vision-related quality of life in non-clinic-based general population. BMC Ophthalmol. 2012;12:22. doi: 10.1186/1471-2415-12-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li M, Gong L, Sun X, Chapin WJ. Anxiety and depression in patients with dry eye syndrome. Curr Eye Res. 2011;36(1):1–7. doi: 10.3109/02713683.2010.519850. [DOI] [PubMed] [Google Scholar]
- 14.Rajagopalan K, Abetz L, Mertzanis P, et al. Comparing the discriminative validity of two generic and one disease-specific health-related quality of life measures in a sample of patients with dry eye. Value Health. 2005;8(2):168–174. doi: 10.1111/j.1524-4733.2005.03074.x. [DOI] [PubMed] [Google Scholar]
- 15.Ware JE, Snow KK, Kosinski M, Gandek B. SF-36 Health Survey Manual and Interpretation Guide. 1–6. Vol. 6. Boston: The Health Institute, New England Medical Center; 1993. Chapter 6: Scoring the SF-36; p. 22. [Google Scholar]
- 16.Ware JE, Kosinski M, Keller SD. SF-36 Physical and Mental Health Summary Scales: A User’s Manual. 1–7. Vol. 7. Boston: The Health Assessment Lab, New England Medical Center; 1994. Chapter 7: Interpretation: Content- and Criterion-Based; p. 32. [Google Scholar]
- 17.Mangione CM, Lee PP, Gutierrez PR, Spritzer K, Berry S, Hays RD. Development of the 25-item National Eye Institute Visual Function Questionnaire. Arch Opththalmol. 2001;119(7):1050–1058. doi: 10.1001/archopht.119.7.1050. [DOI] [PubMed] [Google Scholar]
- 18.Mizuno Y, Yamada M, Miyake Y. Association between clinical diagnostic tests and health-related quality of life surveys in patients with dry eye syndrome. Jpn J Ophthalmol. 2010;54(4):259–265. doi: 10.1007/s10384-010-0812-2. [DOI] [PubMed] [Google Scholar]
- 19.Miljanović B, Dana R, Sullivan DA, Schaumberg DA. Impact of dry eye syndrome on vision-related quality of life. Am J Ophthalmol. 2007;143(3):409–415. doi: 10.1016/j.ajo.2006.11.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schaumberg DA, Buring JE, Sullivan DA, Dana MR. Hormone replacement therapy and dry eye syndrome. JAMA. 2001;286(17):2114–2119. doi: 10.1001/jama.286.17.2114. [DOI] [PubMed] [Google Scholar]
- 21.Koktekir BE, Celik G, Karalezli A, Kal A. Dry eyes and migraines: is there really a correlation? Cornea. 2012;31(12):1414–1416. doi: 10.1097/ICO.0b013e318247ec2a. [DOI] [PubMed] [Google Scholar]
- 22.Moss SE, Klein R, Klein BE. Incidence of dry eye in an older population. Arch Ophthalmol. 2004;122(3):369–373. doi: 10.1001/archopht.122.3.369. [DOI] [PubMed] [Google Scholar]
- 23.Schaumberg DA, Sullivan DA, Buring JE, Dana MR. Prevalence of dry eye syndrome among US women. Am J Opthalmol. 2003;136(2):318–326. doi: 10.1016/s0002-9394(03)00218-6. [DOI] [PubMed] [Google Scholar]
- 24.Bandeen-Roche K, Muñoz B, Tielsch JM, West SK, Schein OD. Self-reported assessment of dry eye in a population-based setting. Invest Ophthalmol Vis Sci. 1997;38(12):2469–2475. [PubMed] [Google Scholar]
- 25.Nichols KK, Mitchell GL, Zadnik K. The repeatability of clinical measurements of dry eye. Cornea. 2004;23(3):272–285. doi: 10.1097/00003226-200404000-00010. [DOI] [PubMed] [Google Scholar]
- 26.Begley CG, Chalmers RL, Abetz L, et al. The relationship between habitual patient-reported symptoms and clinical signs among patients with dry eye of varying severity. Invest Ophthalmol Vis Sci. 2003;44(11):4753–4761. doi: 10.1167/iovs.03-0270. [DOI] [PubMed] [Google Scholar]
- 27.Nichols JJ, Mitchell GL, Nichols KK. An assessment of self-reported disease classification in epidemiological studies of dry eye. Invest Ophthalmol Vis Sci. 2004;45(10):3453–3457. doi: 10.1167/iovs.04-0468. [DOI] [PubMed] [Google Scholar]
- 28.Hay EM, Thomas E, Pal B, Hajeer A, Chambers H, Silman AJ. Weak association between subjective symptoms of and objective testing for dry eyes and dry mouth: results from a population based study. Ann Rheum Dis. 1998;57(1):20–24. doi: 10.1136/ard.57.1.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cardona G, Marcellán C, Fornieles A, Vilaseca M, Quevedo L. Temporal stability in the perception of dry eye ocular discomfort symptoms. Optom Vis Sci. 2010;87(12):1023–1029. doi: 10.1097/OPX.0b013e3181ff99ab. [DOI] [PubMed] [Google Scholar]
- 30.Chalmers RL, Begley CG, Edrington T, et al. The agreement between self-assessment and clinician assessment of dry eye severity. Cornea. 2005;24(7):804–810. doi: 10.1097/01.ico.0000154410.99691.3c. [DOI] [PubMed] [Google Scholar]