Abstract
OBJECTIVE
Human papillomavirus (HPV) vaccine uptake rate among young adult US women was only 23% in 2010. One way to improve this low rate is to administer the vaccine postpartum. We examined whether this population requires vaccination and whether they would be agreeable to receiving it free of charge after delivery.
STUDY DESIGN
Women 26 years of age or younger seeking prenatal care in publicly funded clinics in southeast Texas were interviewed in 2012 regarding their HPV vaccination status, barriers to vaccination, and whether they would be willing to receive this vaccine postpartum if offered free of charge. Medical charts were reviewed to extract additional information.
RESULTS
Overall, 13.0% (65 of 500) stated they had initiated and 7.6% (38 of 500) completed the 3-dose vaccine series. Ethnic differences were noted with 21.0% of non-Hispanic whites, 14.6% of blacks, and 9.3% of Hispanics (P = .002) initiating the vaccine and 13.5%, 7.8%, and 5.2% (P = .006) competing all 3 doses, respectively. Lowest initiation (4.2%) and completion (1.4%) rates were observed among recently immigrated Hispanic women. Those who had not graduated from high school and older women were less likely to have been vaccinated. Almost 83% of those who had not received any HPV doses or completed the series were willing to receive the injection free of charge in the hospital after their delivery.
CONCLUSION
HPV vaccine uptake rates are very low among women receiving prenatal care in southeast Texas. Offering this vaccine free of charge to postpartum women could be an effective strategy in this population because 5 of 6 women favored receiving it in this setting.
Keywords: correlates, HPV, human papillomavirus, postpartum, race/ethnicity, vaccine
In 2006, a virus-like particle vaccine was approved for use in the United States, which protects against 4 human papillomavirus (HPV) strains (6, 11, 16, and 18) responsible for 70% of cervical cancer cases and 90% of genital warts.1,2 To prevent these diseases, the Advisory Committee on Immunization Practices (ACIP) recommends routine HPV vaccination of adolescents 11–12 years of age with catch-up vaccination of individuals 13–26 years of age not previously vaccinated.3,4 Recent national data, however, demonstrate that vaccination rates have been far less than needed with only 14% of US women 11–17 years of age5 and 13% of those 18–26 years of age completing the 3-dose vaccination regimen (0, 1–2 months, and 6 months).6 To protect the entire community (herd immunity), however, it is necessary to vaccinate 75–94% of the eligible population.7
A committee opinion recently published by the American College of Obstetricians and Gynecologists recommended that obstetrician-gynecologists help improve this low uptake rate by “embracing immunizations as an integral part of their practice.”8 Although the HPV vaccine is contraindicated during pregnancy, the first dose could be given postpartum before the patient is discharged from the hospital. The subsequent doses would then be given as an outpatient.
Before this type of program can be recommended on a wide-scale basis, however, it is essential to determine the need of this population for HPV vaccination and whether they would be receptive to receiving it postpartum. To date, this has been assessed in only 1 study of 150 women who delivered in the Northeast.9 Thus, additional data are needed on women residing in other areas of the United States.
The objectives of this study were to estimate rates of prior HPV vaccination among patients receiving prenatal care and their willingness to receive the injection free of charge on the postpartum unit among women residing in south Texas.
Materials and Methods
Between January and April 2012, a total of 500 women 26 years of age or younger attending 1 of 5 publicly funded prenatal clinics located in southeast Texas were asked whether they were willing to participate in a brief interview, conducted in Spanish or English, on HPV vaccination. These facilities provide care to low-income women, of which nearly 80% have a family income less than $30,000/year.10 All eligible women who were approached agreed to participate in this interviewer-administered questionnaire survey.
Research coordinators asked patients about their prior HPV vaccinations, with the following question: “Have you taken the first HPV shot?” Responses included the following: yes, no, and “I have not taken any HPV shots.” Two additional questions were asked for second and third doses with similar wording and responses. Barriers to vaccination were explored using the question: “If you have not had an HPV shot yet, why not?” Responses included the following: “I have never heard of it”; “I don’t know enough about it”; “I don’t know where to get it”; “I can’t afford to get it”; “I am worried about side effects”; “I don’t think I need it”; “I do not get any vaccines”; and other.
Willingness to receive the HPV vaccine in the hospital after the delivery was assessed by asking, “Would you be willing to get an HPV shot in the hospital after your baby was born, if it were free of charge?” Responses included yes and no. A chart review of their medical record was then performed to obtain additional information on demographic characteristics, sexual history, and other clinical variables of interest.
Bivariate comparisons were performed using χ2 tests or Fisher exact test as appropriate. Multivariable logistic regression was used to examine the correlates of HPV vaccine initiation and completion of the series. Variables were screened, and candidate variables with P ≤ .20 with any of the dependent variables (vaccine initiation and completion) were included in the initial multivariable models, whereas variables with P ≥ .20 were excluded from the final models. The Hosmer-Lemeshow test11 and area under the receiver-operating characteristics (ROC) curve were used to assess the fit and predictive ability of the final models. Analyses were performed using STATA 12 (StataCorp, College Station, TX). All procedures were approved by the institutional review board of the University of Texas Medical Branch.
Results
A total of 500 women completed the survey questionnaire. Of them, 53.8% (n = 269) were Hispanic, 23.8% (n = 119) white, 20.6% (n = 103) African American, and 1.8% (n = 9) from other racial/ethnic groups (Table 1). Seventy-two women (14.4%) moved to the United States within the last 5 years. The mean age of the sample was 21.8 years (SD, 2.8, range, 14–26 years). Most of the women were high school graduates (58.9%; n = 294), worked in the home (61.0%; n = 305), and were multi-gravidas (61.4%; n = 307). The majority had sexual intercourse for the first time at 16 years of age or older (62.2; n = 311) and had obtained a Papanicolaou test in the past (73.2%; n = 366). Nearly half had 3 or more lifetime sexual partners, whereas 42 (8.4%) had a history of an abnormal Papanicolaou test.
TABLE 1.
Summary statistics based on questionnaire survey (n = 500)
| Survey question | Response | n (%) or mean (±SD) |
|---|---|---|
| What is your race? | Non-Hispanic white | 119 (23.8) |
| Non-Hispanic black | 103 (20.6) | |
| Hispanic or Latino | 269 (53.8) | |
| Othersa | 9 (1.8) | |
| What is your current age? | Age in years | 21.8 (2.8) |
| Have you taken the first HPV shot? | Yes | 65 (13.0) |
| No | 435 (87.0) | |
| Did you take the second shot? | Yes | 43 (8.6) |
| No | 22 (4.4) | |
| I have not taken any HPV shot | 435 (87.0) | |
| Did you take the third shot? | Yes | 38 (7.6) |
| No | 5 (1.0) | |
| I have not taken any HPV shot | 435 (87.0) | |
| If you have not had an HPV shot yet, why not?b | I have never heard of it | 225 (51.7) |
| I don’t know enough about it | 272 (62.5) | |
| I don’t know where to go and get it | 71 (16.3) | |
| I can’t afford to get it | 99 (22.8) | |
| I am worried about side effects | 47 (10.8) | |
| I don’t think I need it | 38 (8.7) | |
| I do not get any vaccines | 8 (1.8) | |
| Are you pregnant? | Yes | 500 (100.0) |
| No | 0 (0.0) | |
| Where do you plan to deliver your baby? | UTMB | 392 (78.4) |
| Other clinics | 108 (21.6) | |
| Would you be willing to get an HPV shot in the hospital after your baby was born, if it were free of charge?c | Yes | 382 (82.7) |
| No | 80 (17.3) |
HPV, human papillomavirus; UTMB, University of Texas Medical Branch at Galveston, TX.
Asian, American Indian/Alaskan native, Native Hawaiian, or other Pacific Islander;
Denominators are based on women who did not receive any dose of the HPV vaccine (n = 435);
Denominator is based on women who had not received any HPV vaccine doses or completed the series (n = 462).
Overall awareness about the HPV vaccine was low because only 55.0% of women had ever heard about the vaccine. Awareness was lowest among Hispanics who had moved to the United States within the last 5 years (37.5%), followed by those Hispanics who were born in the United States or had moved to the United States more than 5 years ago (46.7%). Awareness was higher among white (72.3%) and black women (63.1%) than Hispanic women (P < .001).
Overall, 13.0% of women interviewed (65 of 500) stated they had received at least 1 dose of the vaccine and 7.6% (38 of 500) had completed all 3 doses. Slightly more than half of those who had initiated the series received all 3 doses (38 of 65).
Ethnic differences were noted, with 21% of non-Hispanic whites, 14.6% of blacks, and 9.3% of Hispanics (P = .002) having received the first dose, whereas 13.5%, 7.8%, and 5.2% (P = .006) completed all 3 doses, respectively. Among Hispanics, those who had moved to the United States within the past 5 years (n = 72) had the lowest initiation (4.2%) and completion (1.4%) rates.
Vaccine series completion among the initiators did not differ by race/ethnicity. Bivariate analyses showed that being white, having at least a high school diploma, and having a gravidity less than 2 were associated with higher HPV vaccine initiation and completion of the series. Moreover, having 3 or more lifetime sexual partners was associated with higher HPV vaccine initiation (Table 2).
TABLE 2.
HPV initiation and completion rates by different characteristics among 14–26 year old women
| Variable | Totala
|
Received ≥1 doses
|
Completed 3-dose vaccine
series |
||
|---|---|---|---|---|---|
| n = 500 | % (95% CI) | P value | % (95% CI) | P value | |
| Race/ethnicity | .009 | .033 | |||
|
| |||||
| White | 119 | 21.0 (13.6–28.4) | 13.5 (7.2–20.0) | ||
|
| |||||
| Black | 103 | 14.6 (7.6–21.5) | 7.8 (2.5–13.0) | ||
|
| |||||
| Hispanic (born in the US/moved >5 years ago) | 197 | 11.2 (6.7–15.6) | 6.6 (3.1–10.1) | ||
|
| |||||
| Hispanic (moved to the US ≤5 years ago) | 72 | 4.2 (0.5–8.9) | 1.4 (less than 1.0 to 4.2) | ||
|
| |||||
| Othersb | 9 | 0 | 0 | ||
|
| |||||
| Education, n (%) | < .001 | < .001 | |||
|
| |||||
| Did not graduate high school | 205 | 6.3 (3.0–9.7) | 3.9 (1.2–6.6) | ||
|
| |||||
| High school graduate | 294 | 17.4 (13.0–21.7) | 10.2 (6.7–13.7) | ||
|
| |||||
| Employment status, n (%) | .075 | .210 | |||
|
| |||||
| Homemaker | 305 | 10.8 (7.3–14.3) | 6.6 (3.8–9.6) | ||
|
| |||||
| Part/full-time student | 61 | 21.3 (10.7–31.9) | 13.1 (4.4–21.8) | ||
|
| |||||
| Part/full-time worker | 134 | 14.2 (8.2–20.2) | 7.5 (3.0–12.0) | ||
|
| |||||
| Gravidity | < .001 | .001 | |||
|
| |||||
| <2 | 193 | 21.2 (15.4–27.1) | 12.4 (7.7–17.1) | ||
|
| |||||
| ≥2 | 307 | 7.8 (4.8–10.8) | 4.6 (2.2–6.9) | ||
|
| |||||
| Age at first sexual intercourse, n (%) | .061 | .399 | |||
|
| |||||
| Younger than 16 years | 169 | 16.6 (10.9–22.2) | 8.9 (4.5–13.2) | ||
|
| |||||
| 16 years or older | 311 | 10.6 (7.2–14.0) | 6.8 (3.9–9.6) | ||
|
| |||||
| Number of lifetime sexual partners | .007 | .146 | |||
|
| |||||
| <3 | 234 | 8.1 (4.6–11.6) | 6.4 (3.2–9.6) | ||
|
| |||||
| 3–5 | 136 | 18.4 (11.8–25.0) | 6.6 (2.4–10.8) | ||
|
| |||||
| ≥6 | 106 | 17.0 (9.7–24.2) | 12.3 (5.9–18.6) | ||
|
| |||||
| Had at least 1 Papanicolaou test | .169 | .146 | |||
|
| |||||
| No | 134 | 16.4 (10.1–22.8) | 10.4 (5.2–15.7) | ||
|
| |||||
| Yes | 366 | 11.8 (8.4–15.1) | 6.6 (4.0–9.1) | ||
|
| |||||
| Had abnormal Papanicolaou test result | .471 | .091 | |||
|
| |||||
| Noc | 455 | 12.7 (9.7–15.8) | 7.0 (4.7–9.4) | ||
|
| |||||
| Yes | 42 | 16.7 (4.9–28.4) | 14.3 (3.2–25.3) | ||
CI, confidence interval; HPV, human papillomavirus; US, United States.
Numbers do not add up because of missing values;
Asian, American Indian, or Alaskan;
Includes participants without a history of Papanicolaou testing.
Adjusted multivariable logistic regression models showed that younger women, high school graduates, and women with gravidity less than 2 were more likely to initiate and complete all 3 doses (Table 3). Compared with white women, Hispanics who were born in the United States or moved to the United States more than 5 years ago (P = .030) and Hispanics who moved to the United States during the last 5 years (P = .026) were less likely to initiate the HPV vaccine.
TABLE 3.
ORs of 14–26 year old women’s HPV initiation and completion by their characteristics
| Variable | Received 1 dose OR (95% CI) | P value | Completed 3-dose series OR (95% CI) | P value |
|---|---|---|---|---|
| Age, y | 0.84 (0.75–0.95) | .005 | 0.80 (0.68–0.93) | .005 |
| Race/ethnicity | ||||
| White | Referent | Referent | ||
| Black | 0.49 (0.23–1.05) | .067 | 0.41 (0.16–1.06) | .066 |
| Hispanic (born in the US or moved >5 years ago) | 0.48 (0.25–0.93) | .030 | 0.46 (0.21–1.04) | .063 |
| Hispanic (moved to the US ≤5 years ago) | 0.23 (0.07–0.84) | .026 | 0.14 (0.02–1.09) | .060 |
| Education | ||||
| Did not graduate high school | Referent | Referent | ||
| High school graduate | 3.37 (1.68–6.77) | .001 | 3.03 (1.26–7.30) | .013 |
| Gravidity | ||||
| <2 | Referent | Referent | ||
| ≥2 | 0.42 (0.23–0.78) | .006 | 0.44 (0.19–0.99) | .047 |
| Had abnormal Papanicolaou test result | — | — | ||
| Noa | — | — | Referent | |
| Yes | — | — | 4.08 (1.40–11.95) | .010 |
Data are based on multivariable logistic regression analysis. Two logistic regression models were used. Dependent variables included the following: “Received 1 dose or not (yes vs no)” and completed 3-dose series or not (yes vs no). Independent variables include the following: age in years (continuous variable), race/ethnicity (categorical), education (categorical), gravidity (categorical), and abnormal Papanicolaou test (categorical). Because the response “had abnormal Papanicolaou test result” is dependent on the response “had at least 1 Papanicolaou test,” we used the response “had abnormal Papanicolaou test result” in both multivariable models. Area under the receiver-operating characteristics curve includes 0.76 and 0.79 for the vaccine initiation and completion logistic regression models, respectively. The P value for Hosmer-Lemeshow goodness of fit test = .547 and .964 for the vaccine initiation and completion models, respectively.
CI, confidence interval; HPV, human papillomavirus; OR, odds ratio; US, United States.
Includes participants without a history of Papanicolaou testing.
Older women were less likely to be vaccinated; each 1 year increase in age resulted in a 16% decrease in the odds of initiation and a 20% decrease in completion of the vaccine series. Women who had a history of an abnormal Papanicolaou test were more likely to complete the vaccine series compared with their counterparts. The final logistic models for the vaccine initiation yielded a P value for the Hosmer-Lemeshow test of .547, and the estimated area under the ROC curve was 0.76. The respective figures were 0.964 and 0.79 for the final model, with vaccine series completion as a dependent variable. These features indicate a good fit of the models.
When women were asked why they had not been vaccinated, 62.5% of those who had not received any injections (272 of 435) stated the primary reason was that they did not know enough about it. Overall, Hispanic women were more likely to agree with the statements, “I don’t know enough about it” (P < .001) and “I have never heard of it” (P < .001), compared with their counterparts.
In addition, black women were more likely to state that they could not afford the vaccine (P = .004) and that they were afraid of its side effects (P = .001) (Figure). Hispanic women who moved to the United States within the last 5 years were more likely to report “I don’t know where to go and get it” (P = .012). Almost 83% of women who had not received any HPV vaccine doses or completed the series (382 of 462) reported they were willing to receive the first vaccine dose in the hospital after delivery of their infant.
FIGURE.
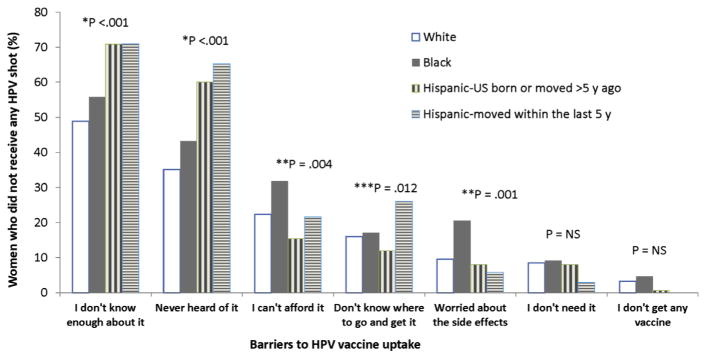
Barriers to HPV vaccine uptake by race/ethnicity among young women 14–26 years of age
The asterisk indicates Hispanics moved to the United States within the last 5 years vs others. Double asterisks indicate black vs others. Triple asterisks indicate all Hispanics vs others.
HPV, human papillomavirus.
Comment
When possible, the HPV vaccine should be administered at 11–12 years of age when the risk of having been previously infected by the virus is lowest. However, many women do not receive it at a young age. The vaccine is still recommended up to age 26 years, even if the patient has already initiated sexual activity because it still provides protection against infections and precancerous lesions caused by vaccine-type HPV among adolescents and young women who are naïve to those strains.12–17
In this study, 83% women who had not started or completed the series stated that they would be willing to receive the HPV vaccine in the hospital if it were offered free of charge after delivery. This finding is consistent with previous studies that have observed high uptake rates of the Tdap (96%) and hepatitis B (86%) vaccines when offered post-partum.18,19 One recent study also observed a very high acceptance rate (95%) of the first dose of the HPV vaccine among women who gave birth in New York City.9 Of those who obtained the vaccine postpartum, only 31% went on to complete the entire series. Thus, our study confirms this prior report that postpartum vaccination could be an effective way to initiate HPV vaccine uptake among vulnerable populations. However, additional studies are still needed to determine how completion rates could be improved among these women.
In this study, we observed even lower rates in our region than the national average among women presenting for prenatal care in south Texas (22.7% vs 13.0% for initiation; 12.7% vs 7.6% for completion).7 Moreover, we observed that Hispanic women were less likely than whites to have initiated the vaccine series. Those who had moved to the United States during the last 5 years were at greatest risk of not having received any doses. The lower likelihood of completing the vaccine series among this population is especially concerning because the incidence of cervical cancer is higher among Hispanic women than whites.20 Thus, based on data in south Texas, the population that has the greatest need for protection against HPV is the least likely to be vaccinated.
We also observed that older women were less likely to have initiated and completed the vaccine series. This finding may be due to the recent availability of this vaccine. The quadrivalent vaccine was approved for use in late 2006, approximately 6 years before the survey was conducted.
The primary ages targeted for vaccination are adolescents 11–12 years of age. Because women 21 years old would already have been at least 15 years old when the HPV vaccine was first introduced, they may have missed being vaccinated. This problem will be partially corrected as time passes. However, many women do not seek out medical care as young adolescents and will still need to receive the vaccine in later adolescence. This is especially true for recent immigrants and hard-to-reach populations. For these women, post-partum vaccination may be a good opportunity to initiate this vaccine series.
The primary barrier to vaccination reported by all women was lack of awareness and knowledge. This was most evident among Hispanic women. Thus, interventions promoting HPV awareness among pregnant women are needed. These could range from distribution of materials developed by the Centers for Disease Control and Prevention, which are available free of charge in English and Spanish, to more intensive interventions.
Concern about the cost and side effects was a barrier among black women, even though safety has not been demonstrated to be an issue in scientific studies.21 Thus, additional educational efforts are also needed to inform women 18 years old or younger about programs that provide the vaccine at a reduced cost or free of charge, such as the Vaccine for Children (VFC) program, and the safety of this vaccine.
Public support for this 3-dose series would also likely increase vaccine uptake among low-income women. Moreover, mandating school-entry administration of the HPV vaccine could be an important propeller to increase vaccine uptake as observed in Australia.22,23 However, there are widespread controversies around this issue in the United States because only Virginia and the District of Columbia were successful in getting a mandate for sixth graders to be immunized against HPV among 42 states (including the District of Columbia) that attempted to pass this legislation.24 Furthermore, Virginia later repealed this law, demonstrating that a universal school mandate in the United States in the near future is unlikely.
This study has several limitations. First, our survey data may be subject to recall bias because they are self-reported. Second, significant correlates of vaccine completion observed in this study should be interpreted with caution because of the small number who reported completion of all 3 doses. Third, we could not evaluate whether the 3 doses were completed within the ACIP recommended time frame because of a lack of data on vaccination dates. Fourth, vaccinating women who are already infected by the vaccine-type HPV strains may not be effective. However, a nationally representative study of women 14–59 years old found that less than 1% had evidence of both HPV 16 and 18.25 Thus, it appears that approximately 99% of this age group could have benefitted from this vaccination. Finally, intent to vaccinate is not the same as actual vaccination.9 As a result, we cannot determine from this study how many women would actually accept the vaccine if offered.
In conclusion, we found that many of the barriers to HPV vaccination could be addressed by administering the first dose of this vaccine postpartum. Women who are not aware of the HPV vaccine or have concerns about its safety could be educated as part of their prenatal or postpartum care.
The burden imposed by its high cost would also be reduced if administered to women postpartum because low-income women who are no longer eligible for VFC funding would likely have Medicaid coverage for their pregnancy. This would allow them to initiate the vaccine series while they have federal coverage for health care. Because the second HPV injection can be given as early as 4 weeks after the first dose (dose 2 is recommended 1–2 months after dose 1) and Medicaid coverage is active for 8 weeks after delivery, federal funding would likely be available for the first 2 doses. Currently financing for the third dose could be problematic because it is not due until after Medicaid benefits expire. However, the Affordable Care Act will likely provide coverage for all 3 injections in the near future for most women.
Acknowledgments
Federal support for this study was provided by the Eunice Kennedy Shriver National Institute of Child Health and Human Development grant K24 HD043659 (A.B.B.).
Footnotes
The views expressed herein are solely those of the authors and do not necessarily represent the official views of the Eunice Kennedy Shriver National Institute of Child Health and Human Development or the National Institutes of Health.
The authors report no conflict of interest.
Presented at the 16th Annual Conference on Vaccine Research, Baltimore, MD, April 22–24, 2013.
References
- 1.Bosch FX, Burchell AN, Schiffman M, et al. Epidemiology and natural history of human papillomavirus infections and type-specific implications in cervical neoplasia. Vaccine. 2008;26:1–16. doi: 10.1016/j.vaccine.2008.05.064. [DOI] [PubMed] [Google Scholar]
- 2.Garland SM, Steben M, Sings HL, et al. Natural history of genital warts: analysis of placebo arm of 2 randomized phase III trials of quadrivalent human papillomavirus (types 6, 11, 16, and 18) vaccine. J Infect Dis. 2009;199:805–14. doi: 10.1086/597071. [DOI] [PubMed] [Google Scholar]
- 3.Markowitz LE, Dunne EF, Saraiya M, Lawson HW, Chesson H, Unger ER. Quadrivalent human papillomavirus vaccine: recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR Recomm Rep. 2007;56:1–24. [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention. FDA licensure of bivalent human papillomavirus vaccine (HPV2, Cervarix) for use in females and updated HPV vaccination recommendation from the Advisory Committee on Immunization Practices (ACIP) MMWR Morb Mortal Wkly Rep. 2010;59:626–9. [PubMed] [Google Scholar]
- 5.Laz TH, Rahman M, Berenson AB. An update on human papillomavirus vaccine uptake among 11–17 year old girls in the United States: National Health Interview Survey, 2010. Vaccine. 2012;30:3534–40. doi: 10.1016/j.vaccine.2012.03.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Laz TH, Rahman M, Berenson AB. Human papillomavirus vaccine uptake among 18- to 26-year-old women in the United States: National Health Interview Survey, 2010. Cancer. 2013;119:1386–92. doi: 10.1002/cncr.27894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Merriil RM. Introduction to epidemiology. Sudbury, MA: Jones and Bartletts Publishers; 2010. pp. 68–9. [Google Scholar]
- 8.American College of Obstetricians and Gynecologists. Integrating immunizations into practice. ACOG Committee Opinion no 558. Obstet Gynecol. 2013;121:897–903. doi: 10.1097/01.AOG.0000428788.74725.90. [DOI] [PubMed] [Google Scholar]
- 9.Wright JD, Govindappagari S, Pawar N, et al. Acceptance and compliance with postpartum human papillomavirus vaccination. Obstet Gynecol. 2012;120:771–82. doi: 10.1097/AOG.0b013e31826afb56. [DOI] [PubMed] [Google Scholar]
- 10.Berenson AB, Rahman M. Prevalence and correlates of prescription drug misuse among young, low-income women receiving public healthcare. J Addict Dis. 2011;30:203–15. doi: 10.1080/10550887.2011.581984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hosmer DW, Jr, Lemeshow S. Applied logistic regression. 2. New York: John Wiley & Sons, Inc; 2000. [Google Scholar]
- 12.Villa LL, Costa RL, Petta CA, et al. Prophy-lactic quadrivalent human papillomavirus (types 6, 11, 16, and 18) L1 virus-like particle vaccine in young women: a randomised double-blind placebo-controlled multicentre phase II efficacy trial. Lancet Oncol. 2005;6:271. doi: 10.1016/S1470-2045(05)70101-7. [DOI] [PubMed] [Google Scholar]
- 13.FUTURE II Study Group. Quadrivalent vaccine against human papillomavirus to prevent high-grade cervical lesions. N Engl J Med. 2007;356:1915–27. doi: 10.1056/NEJMoa061741. [DOI] [PubMed] [Google Scholar]
- 14.Joura EA, Leodolter S, Hernandez-Avila M, et al. Efficacy of a quadrivalent prophylactic human papillomavirus (types 6, 11, 16, and 18) L1 virus-like-particle vaccine against high-grade vulval and vaginal lesions: a combined analysis of three randomised clinical trials. Lancet. 2007;369:1693–702. doi: 10.1016/S0140-6736(07)60777-6. [DOI] [PubMed] [Google Scholar]
- 15.Garland SM, Hernandez-Avila M, Wheeler CM, et al. Quadrivalent vaccine against human papillomavirus to prevent anogenital diseases. N Engl J Med. 2007;356:1928–43. doi: 10.1056/NEJMoa061760. [DOI] [PubMed] [Google Scholar]
- 16.Romanowski B, de Borba PC, Naud PS, et al. Sustained efficacy and immunogenicity of the human papillomavirus (HPV)-16/18 ASO4-adjuvanted vaccine: analysis of a randomized placebo-controlled trial up to 6. 4 years. Lancet. 2009;374:1975–85. doi: 10.1016/S0140-6736(09)61567-1. [DOI] [PubMed] [Google Scholar]
- 17.Paavonen J, Naud P, Salmeron J, et al. Efficacy of human papillomavirus (HPV)-16/18 ASO4-adjuvanted vaccine against cervical infection and precancer caused by oncogenic HPV types (PATRICIA): final analysis of a double-blind, randomised study in young women. Lancet. 2009;374:301–14. doi: 10.1016/S0140-6736(09)61248-4. [DOI] [PubMed] [Google Scholar]
- 18.Healy CM, Rench MA, Castagnini LA, Baker CJ. Pertussis immunization in a high-risk postpartum population. Vaccine. 2009;27:5599–602. doi: 10.1016/j.vaccine.2009.07.030. [DOI] [PubMed] [Google Scholar]
- 19.Stringer M, Ratcliffe SJ, Gross R. Acceptance of hepatitis B vaccination by pregnant adolescents. MCN Am J Matern Child Nurs. 2006;31:54–60. doi: 10.1097/00005721-200601000-00012. [DOI] [PubMed] [Google Scholar]
- 20.Watson M, Saraiya M, Benard V, et al. Burden of cervical cancer in the United States, 1998–2003. Cancer. 2008;113:2855–64. doi: 10.1002/cncr.23756. [DOI] [PubMed] [Google Scholar]
- 21.Macartney KK, Chiu C, Georgousakis M, Brotherton JM. Safety of human papilloma-virus vaccines: a review. Drug Saf. 2013;36:393–412. doi: 10.1007/s40264-013-0039-5. [DOI] [PubMed] [Google Scholar]
- 22.Donovan B, Franklin N, Guy R, et al. Quadrivalent human papillomavirus vaccination and trends in genital warts in Australia: analysis of national sentinel surveillance data. Lancet Infect Dis. 2011;11:39–44. doi: 10.1016/S1473-3099(10)70225-5. [DOI] [PubMed] [Google Scholar]
- 23.Brotherton JM, Fridman M, May CL, Chappell G, Saville AM, Gertig DM. Early effect of the HPV vaccination programme on cervical abnormalities in Victoria, Australia: an ecological study. Lancet. 2011;377:2085–92. doi: 10.1016/S0140-6736(11)60551-5. [DOI] [PubMed] [Google Scholar]
- 24.Osazuwa-Peters N. Human papillomavirus (HPV), HPV-associated oropharyngeal cancer, and HPV vaccine in the United States—do we need a broader vaccine policy? Vaccine. 2013;31:5500–5. doi: 10.1016/j.vaccine.2013.09.031. [DOI] [PubMed] [Google Scholar]
- 25.Dunne EF, Unger ER, Sternberg M, et al. Prevalence of HPV infection among females in the United States. JAMA. 2007;297:813–9. doi: 10.1001/jama.297.8.813. [DOI] [PubMed] [Google Scholar]


