Abstract
β-Secretase-1 (BACE1) is the rate-limiting enzyme for the genesis of amyloid-β (Aβ) peptides, the main constituents of the amyloid plaques in the brains of Alzheimer’s disease (AD) patients. BACE1 is being evaluated as an anti-Aβ target for AD therapy. Recent studies indicate that BACE1 elevation is associated with axonal and presynaptic pathology during plaque development. Evidence also points to a biological role for BACE1 in axonal outgrowth and synapse formation during development. Axonal, including presynaptic, pathology exists in AD as well as many other neurological disorders such as Parkinson’s disease, epilepsy, stroke, and trauma. In this review, we discuss pharmaceutical BACE1 inhibition as a therapeutic option for axonal pathogenesis, in addition to amyloid pathology. We first introduce the amyloidogenic processing of amyloid-β protein precursor and describe the normal expression pattern of the amyloidogenic proteins in the brain, with an emphasis on BACE1. We then address BACE1 elevation relative to amyloid plaque development, followed by updating recent understanding of a neurotrophic role of BACE1 in axon and synapse development. We further elaborate the occurrence of axonal pathology in some other neurological conditions. Finally, we propose pharmacological inhibition of excessive BACE1 activity as an option to mitigate early axonal pathology occurring in AD and other neurological disorders.
Keywords: aging, Alzheimer’s disease, anti-amyloid therapy, dementia, dystrophic neurites, neurodegenerative disorders, neuroplasticity, senile plaques, synaptic dysfunction
INTRODUCTION
Synaptic and axonal abnormalities are observed in many neurological conditions including Alzheimer’s disease (AD), Parkinson’s disease (PD), traumatic brain injury (TBI), temporal lobe epilepsy (TLE), and cerebral stroke. Synaptic pathology can occur in the pre- and postsynaptic components, with the latter largely involving atrophic changes of dendrites and dendritic spines. Axonal pathology may occur along the axonal shaft and at the presynaptic terminal, and is characterized microscopically by aberrant sprouting, dystrophic expansion, and accumulation of various cellular organelles and cytoskeletal/signaling proteins. Synaptic and axonal pathology impairs the integrity of neuronal circuitry and neurotransmission. Aberrant axonal sprouting may also result in abnormal synaptic connectivity and cause excitatory/inhibitory imbalance. Accumulation of certain functional proteins in axon terminals may compromise normal neuronal functions. In addition, axonal pathology may propagate and lead to retrograde neuronal degeneration in a “dying-back” manner. The cellular and molecular mechanism underlying synaptic and axonal pathology remains largely elusive, but it might include deficient axonal transport, impaired autophagy activity, and malignant regeneration.
β-Secretase-1 (BACE1) is the obligatory enzyme for the amyloidogenic processing of the amyloid β-protein precursor (AβPP). BACE1 inhibition is currently evaluated as an anti-Aβ therapy for AD. BACE1 appears to be enriched at presynaptic terminals in the normal brain, and promotes axonal outgrowth during development. BACE1 overexpression is inherent with the formation of dystrophic axonal neurites during amyloid pathogenesis. Emerging evidence also suggests a correlation of BACE1 elevation with aberrant axonal sprouting in TBI and TLE. These data converge to suggest that BACE1 inhibition may mitigate neuritic dystrophy and aberrant axonal sprouting. This review will briefly introduce and update the biochemical, anatomical, and pathological perspectives of this enzyme, and propose BACE1 inhibition as a pharmacological option against axonal pathology in AD and other neurological conditions.
SECRETASE-MEDIATED AβPP PROTEOLYSIS AND Aβ GENESIS
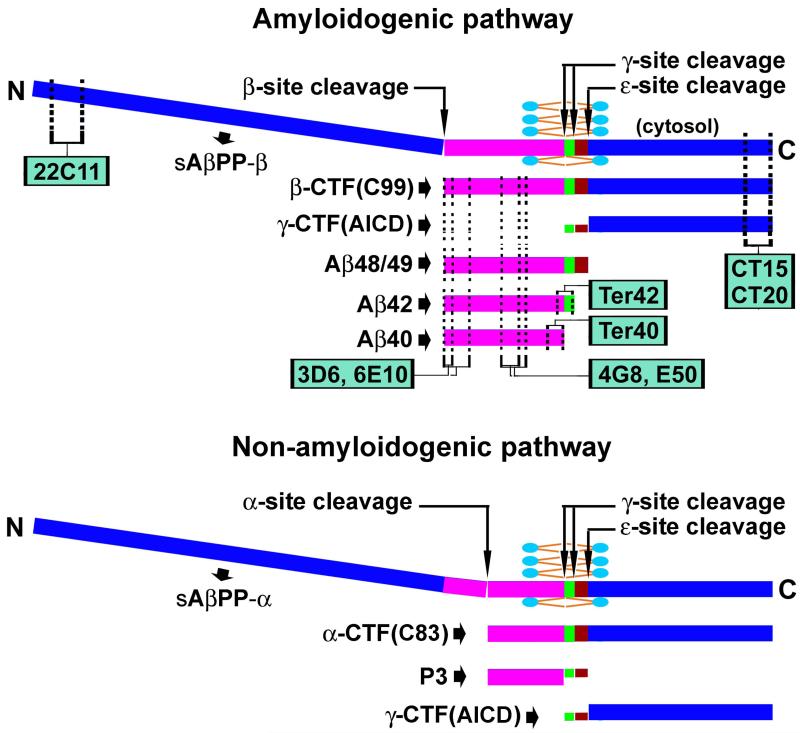
AβPP is an integral membrane protein existing in several alternative splicing isoforms, ranging from 365 to 770 amino acids in length. AβPP consists of a large N-terminal domain containing multiple extracellular loops, and a C-terminal domain that is composed of a transmembrane part and an intracellular sequence [1-6] (Fig. 1). The Aβ domain occupies the N-terminal segment of the AβPP C-terminal and is partially inserted in the bilayer lipid membrane. Aβ genesis likely involves sequential proteolytic cleavages by two enzymes named β-secretase (BACE) and γ-secretase. BACE mediates the β-site cut at the extracellular portion of AβPP near the lipid membrane. This processing releases the β-site cleaved N-terminal (β-NTF) or soluble AβPP-β fragment extracellularly, leaving the β-C-terminal fragment (β-CTF, C99) anchored to the lipid membrane. β-CTF is subsequently cleaved by γ-secretase at the γ-site inside the lipid membrane (as a part of the hydrophobic residues of AβPP), releasing γ-site cleaved AβPP CTF (γ-CTF) intracellularly. This γ-site cleavage appears to shed the Aβ peptides (mostly Aβ40 and Aβ42) extracellularly, although it is also considered that these peptides may enter the intracellular compartment. γ-Secretase may execute a novel ε-site AβPP cleavage several amino acids down to initially defined γ-site, yielding longer Aβ species such as Aβ48/49 [6-8]. AβPP is also enzymatically catabolized via a non-amyloidogenic pathway [9]. In this case, the first cleavage is carried out by α-secretase in the middle of the Aβ domain. The resulting AβPP C-terminals (α-CTF, C83) are also further cut by γ-secretase, yielding a short peptide (P3) from the Aβ sequence and γ-CTF intracellularly (Fig. 1). The various species of γ-CTFs derived from the amyloid and the non-amyloid pathways are also collectively named as the AβPP intracellular domain (AICD), which may translocate into the nucleus and regulate protein expression by controlling gene transcription [5-8].
Figure 1.
Schematic illustration of the structure of the amyloid-β protein precursor (AβPP), its enzymatic processing via the amyloidogenic (top) and non-amyloidogenic (bottom) pathways, and the expected detection of its cleavage products by representative antibodies. The N-terminal segment of AβPP is extracellular and can be cleaved by β-secretase-1 (BACE1) or α-secretase to form soluble β- or α-site cleaved fragments (sAβPPβ or sAβPPα). The AβPP C-terminal segment is divided into the transmembrane Aβ domain (purple/green/brown) and the intracellular sequence, with the later corresponding to 99 (β-CTF, C99) or 83 (α-CTF, C83) amino acid (a.a.) residue-long peptides depending on the initial BACE1 or α-secretase processing of the full-length AβPP. C99 and C83 are further cleaved by γ-secretase inside the lipid bilayer at the γ-site, releasing monomeric Aβ peptides (38-42 a.a.), or at the ε-site, releasing certain longer Aβ species such as Aβ48/49. Thus, the final γ-site cleavage yields the γ-CTFs of varying lengths in the cytosol, collectively named as AβPP intracellular domain of CTFs (ACID), which may enter the nucleus and regulate gene expression. Antibodies targeting the N-terminal (22C11) and C-terminal (CT15, CT20) of AβPP may detect the corresponding terminal fragments, and the full-length AβPP as well. Antibodies targeting the N-terminal (3D6, 6E10), middle (4G8, E50), and C-terminal (Ter40 and Ter42) regions of the Aβ domain may detect monomeric Aβ peptides and their aggregates in amyloid plaques. Theoretically, they may across-react against the same epitopes in the AβPP holoprotein and β-CTF in neuronal somata and processes.
A large number of antibodies have been developed for the detection of AβPP and its cleavage products (Fig. 1). For instances, the monoclonal antibody 22C11 is generated against the N-terminal residues of AβPP and can detect human and murine full-length AβPP. Antibodies raised against the C-terminal residues (e.g., CT15, CT20, 369) also recognize holo-AβPP and the C-terminal fragments. Numerous antibodies against amino acid residues of the Aβ domain are also available. 3D6, 6E10, and several other antibodies (e.g., 82E1, FCA3340, and FCA3542) are designed to target the N-terminal residues of Aβ, while 4G8 and E50 are raised against the middle region of Aβ. Some antibodies may be specific to the C-terminal residues of the Aβ domain, including Ter40 and Ter42. These Aβ antibodies may label Aβ monomers, oligomers, and aggregates in immunohistochemistry and western blot. Many Aβ antibodies (e.g., 3D6, 6E10, 4G8) can detect the full-length AβPP and/or β-CTFs in immunoblot by targeting the same antigen epitopes as in the Aβ domain. Such antibody cross-reactivities could occur in immunohistochemistry, and may raise interpretative difficulty especially regarding the nature of labeling inside neural cells [10-16].
EXPRESSION OF AMYLOIDOGENIC PROTEINS IN NORMAL BRAIN
AβPP expression is found in neuronal and nonneuronal components in the central nervous system and peripheral tissues, with an abundant presence in the brain. Immunohistochemical studies indicate that neuronal somata and dendrites are labeled with antibodies detecting the full-length AβPP, such as 22C11 and CT15. Electron microscopic and biochemical analyses suggest that AβPP may be concentrated in synapses. AβPP may be transported inside neurons, including from somata into axons and synaptic terminals [3-6].
The candidate enzymes for AβPP β-site cleavage are first reported in 1999 and 2000, named BACE1 (also named as memapsin 2 and Asp2) and BACE2 [17-21]. Follow-up genetic, electrophysiological, pharmacological, and pathological studies point to BACE1 as being the principal, if not the sole, β-secretase responsible for Aβ genesis in the brain [22-26]. BACE1 may exist as a zymogen as well as various immature and mature forms that are enzymatically active [24, 27, 28]. BACE1 may undergo extensive posttranscriptional modulations, including glycosylation, acetylation, and palmitoylation and phosphorylation in Golgi apparatus and endosomes [23, 28-31], and may be trafficked into lipid rafts or non-raft membranous compartments and degraded in the lysosomal components [28, 31].
Immunohistochemical studies using different antibodies report that BACE1 is expressed in neuronal and glial cells in mammalian brain [24, 26, 32-35]. We have used a rabbit antibody against human BACE1 to explore the localization of this enzyme in the central nervous system [24, 26]. This antibody detects the 70 kd mature BACE1 by western blot, which is absent in BACE1 knockout brain. Deglycosylation of brain extracts results in a shift of detected protein products from 70 kd to lower molecular weight positions, consistent with the known property of glycosylation of mature BACE1 protein [27]. No specific immunolabeling is found in brain sections from BACE1 null mice [26, 36]. In immunohistochemistry, the hippocampal mossy fiber terminal and the olfactory bulb glomeruli exhibit heavy labeling by this antibody, whereas no somata or dendritic profiles are clearly visualized in the forebrain or subcortical structures [26, 36-38]. This brain distribution pattern is consistent with the findings by several other groups using different specific BACE1 antibodies [35, 39]. Thus, BACE1 appears to be largely expressed in fine neuronal processes in the central nervous system, and may be particularly enriched at presynaptic terminals. Indeed, a recent electron microscopic study has confirmed a preferential BACE1 localization to presynaptic axon terminals in normal rodent brain [39].
γ-Secretase is a multimeric complex consisting of several distinct proteins. The N- and C-terminals fragments derived from proteolytic processing of the inactive holoproteins of presenilins (PS1 and PS2) contribute to the active core of the enzyme complex. Nicastrin may function as the enzyme receptor, and Aph-1 and Pen-2 are important for the assembly, trafficking, and maturation of the enzyme complex (for reviews, see [40, 41]). In addition, TMP21, a member of the p24 cargo protein family, is characterized as a component of the presenilin complex and differentially regulates the γ-site but not ε-site cleavage [42]. The γ-secretase subunit proteins appear to be richly expressed in the brain during development, suggesting a role for the enzyme in neuronal morphogenesis and synaptic development [43-46].
Specific radiolabeled γ-secretase inhibitors may probe putative active sites of γ-secretase complex in situ. Overall, γ-secretase binding sites are present largely in the grey matter [46-49]. However, the binding sites are not necessarily restricted to brain areas or lamina occupied by neuronal somata, but may be densely packed in areas/lamina relatively sparse of neuronal somata, such as the molecular layer of the cerebellar cortex, the substantia nigra reticulate, the hippocampal mossy fiber field, and the olfactory glomeruli. Therefore, as with BACE1, active γ-secretase binding sites may concentrate in neuronal terminals, especially in areas with high synaptic plasticity [46].
BACE1 ELEVATION IN AMYLOID PLAQUE PATHOGENESIS
Aβ peptides and/or derivatives in the amyloid plaques may play a pathogenic role in AD [50-57]. Plaque formation in the brain is also thought to be a consequence of other pathological process. Cajal, Fischer, and Bonfiglio, the very first generation of researchers studying AD neuropathology, considered that the amyloid materials seen in senile plaques may derive from the surrounding dystrophic neurites [55]. Cajal describes senile plaque formation as beginning with the appearance of some dystrophic neurites until the final cored neuritic plaque is formed [58]. Such a concept continued to develop during the 60s to 80s of last century and even later [53-59]. Anatomically, neuritic plaques are classified into the small primitive form and the relatively large, cored form, with the latter containing a central core of highly concentrated amyloid deposits [10, 12, 55, 60, 61]. Terry and Wisniewski have proposed that primitive plaques evolve into cored plaques as the amyloid materials accumulates inside the growing cluster of the dystrophic neurites [62].
The availability of transgenic AD models and molecular markers for Aβ producing enzymes provides new tools for exploring the origin of pathologically important Aβ products and plaque development. BACE1 is the first, obligatory, and rate-limiting enzyme for Aβ genesis. Therefore, localizing abnormal BACE1 expression in the brain, if it occurs, and comparing it with plaque development, may reveal the anatomic sites of Aβ overproduction and potentially elucidate the process of extracellular amyloid plaque formation in vivo. To date, several dozen mouse and rat transgenic AD models have been developed, many of which are engineered to overexpress mutant forms of human AβPP, presenilins, and/or tau protein identified in pedigrees with early-onset dementia, as listed at the Alzheimer’s Disease Forum (http://www.alzforum.org/res/com/tra/default.asp). These animal models recapitulate, more or less, the main pathological and functional deficits observed in AD patients, including age-related cognitive impairments, cerebral amyloid deposition, tau hyperphosphorylation, and other neuropathological changes (e.g., [63-65]).
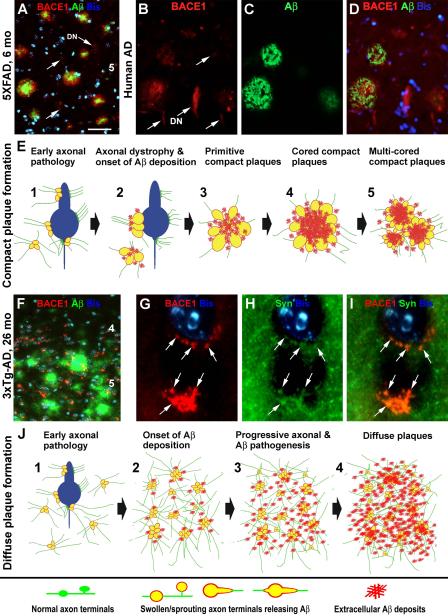
BACE1 elevation appears to occur in the brain from a fairly early age among several plaque-forming transgenic AD models examined so far, including Tg2576, 2XFAD, 5XFAD, and 3xTgAD [10,11,35-37,39,66,67]. Importantly, the onset and evolution of typical neuritic plaques in the brain and spinal cord correlate with a progressive axon terminal pathology associated with BACE1 overexpression. This pathology emerges as microscopically noticeable BACE1 immunoreactive swollen and sprouting axon terminals, often occurring perisomatically on the principal neurons expressing the mutant AβPP in the cerebral cortex, Ammon’s horn, and the ventral horn of the spinal cord. Swollen axonal spheroids are also initially detected in cortical white matter and subcortical areas such as the striatum and septum. Besides BACE1, the abnormal axonal elements can also be co-labeled by antibodies against the transgenic AβPP, Aβ monomers or aggregates, and some specific neuronal phenotype markers. Extracellular Aβ deposits are absent from the small and isolated dystrophic axon terminals, but emerge and accumulate with the growth/expansion of the dystrophic neurites, especially as these neurites become rosette-like clusters [36,37,55,59, 62, 66-70] (Fig. 2A-D). This co-evolution of axonal terminal dystrophy and extracellular Aβ deposition, together with fact that BACE1 is clearly elevated inside the dystrophic neurites, provides anatomical and biochemical evidence that neuritic pathogenesis likely precedes site-specific plaque formation (Fig. 2E).
Figure 2.
Images and schematic drawings illustrating a hypothesis that amyloidogenic axonal pathology facilitates compact (A-E) and diffuse (F-J) plaque formation in the brain. Panels (A-D) show BACE1/Aβ double immunofluorescent images taken from the temporal cortices of a 6 month-old 5XFAD transgenic mouse (A) and a perfused Alzheimer’s disease (AD) human brain (B-D). BACE1 labeling (red) localizes to isolated and clusterized dystrophic neurites (DN), mostly associated with local extracellular Aβ reactivity. However, some small and isolated dystrophic neurites (indicated by arrows) are not surrounded by Aβ deposits. Panel (E) illustrates a possible process of compact/neuritic plaque development. We hypothesize that plaque-forming Aβ products largely derive from axonal (including perisomatic presynaptic) terminals that are undergoing a progressive dystrophic pathogenesis, which is intrinsically inherent with BACE1 overexpression. This pathology results in an increased Aβ release into the extracellular spaces, causing an aggressive local amyloidosis. Panel (F) shows a double immunofluorescent image taken from the frontal cortex of an aged 3×Tg-AD mouse. The Aβ reactivity (green) appears as diffuse plaques with variable intensity and no clear borders (compare F with A-D). Small and irregularly shaped BACE1 immunoreactive elements (red) are present in the same area, often around cell bodies, as visualized by bisbenzimide nuclear stain (Bis, blue). These perisomatic BACE1 immunoreactive profiles (as indicated by arrows) colocalize with synaptophysin (SYN) (G-I), therefore likely representing abnormal presynaptic terminals. Panel (J) illustrates a potential process of diffuse plaque formation facilitated by amyloidogenic axonal pathology activated over a relatively large brain area. This axonal pathology is not as aggressive as in the case of compact plaque formation, therefore causing a lesser extent of neuritic dystrophy. It should be noted that the perisomatic BACE1 immunoreactive swollen/sprouting axon terminals characterized in the transgenic mouse models do not appear to be prominent, if they exist, in aged monkey or AD human cerebrum [77]. It is possible that axonal pathology may mainly occur in the neuropil or paravascular areas, rather than the perisomatic sites, in humans [59, 153]. Arab numbers in (A, F) indicate cortical layers. Scale bar in (A) = 50 μm applying to (B-D, F), equivalent to 5 μm for (G-I).
Diffuse plaques are defined as spreading extracellular Aβ deposition in brain parenchyma, which are conceptually differentiated from neuritic or compact plaques that are surrounded by dystrophic neurites and activated astrocytes [12, 60, 61]. It is considered that during compact plaque formation, early occurring Aβ “seeds” induce dystrophic changes in nearby neuronal processes [51]. However, this theory cannot well reconcile the fact that there are no or rare dystrophic neurites around the diffuse plaques, which contain similar Aβ products as found in compact plaques. The finding that axon terminals and synapses are the primary sites of BACE1 upregulation and hence Aβ overproduction points to a coherent explanation for the pathogenesis of compact (as described above) as well as diffuse plaques [10]. BACE1 elevation may occur in fine axon terminals and synapses over a brain area, which with time may release a sufficient amount of Aβ triggering regional amyloidosis in a diffuse pattern. In this case, there exist progressive pathophysiological changes in axons, including BACE1-mediated Aβ overproduction. However, the axonal pathology may not proceed to overt morphological dystrophy as seen in compact plaque development (Fig. 2F-J).
If axonal pathogenesis does drive amyloid plaque formation, inducing axonal pathology should trigger early plaque development in a region-specific manner. Consistent with this speculation, BACE1 upregulation occurs concomitantly with limbic axonal sprouting in an experimental model of chronic TLE in CD1 mice [38]. In epileptic 3×TgAD mice, plaque pathology is accelerated and enhanced in the hippocampal formation, limbic cortex, and amygdala relative to age-matched counterparts, regionally correlated with increased axonal sprouting and dystrophy [70]. Also in 3×TgAD mice, sciatic nerve axotomy facilitates neuritic dystrophy in parallel with increased plaque formation in the ventral horn of the lumber spinal segments, suggesting a retrograde modulation by the axonal injury on neuritic plaque pathogenesis around the somata of traumatized motor neurons [66].
Increased BACE1 expression and activity are reported in the brain of sporadic AD subjects [71-78]. In order to understand the spatiotemporal relevance of BACE1 elevation to plaque formation, cerebral tissues from diagnosed AD subjects and aged nonhuman primates are comparatively examined [77]. In both AD human and aged monkey cortices, increased BACE1 labeling occurs in isolated and rosette-like dystrophic neurites (Fig. 2B-D). There is a better anatomical and densitometric correlation between BACE1 and Aβ labeled profiles in AD cases with mild, relative to those with advanced, cerebral amyloid pathology. In end-stage AD cases, amyloid plaques may be associated with a few or no BACE1-labeled dystrophic neurites [77]. Two factors might explain why the amount of BACE1-labeled dystrophic neurites does not match to the extent of amyloid deposition in the advanced AD brains: 1) accumulation of insoluble Aβ products with time or disease course, and 2) degeneration of the dystrophic neurites (“burn-out” effect). It should be noted that BACE1-labeled neurites in the monkey and human cerebrum localize in proximity to cerebral vasculature, [77], a distribution pattern also featured by parenchyma amyloid plaques in AD [79-81].
BIOLOGICAL ROLE OF BACE1 IN AXON AND SYNAPSE DEVELOPMENT
BACE1-null mice exhibit multiple neurological phenotypes, including growth retardation, high mortality, memory dysfunction, central and peripheral hypomyelination, seizure vulnerability, and schizophrenia-like behaviors, suggesting that BACE1 may play complex biological roles [26, 28, 82-90]. An intriguing recent finding involves a “neurotrophic” effect of this enzyme on axon outgrowth and synapse formation during development. In particular, BACE1 knockout causes malformation of the olfactory bulb glomeruli and mis-targeting of the hippocampal mossy fibers [91-93]. It has also been shown that increased BACE1 expression accelerates neurite outgrowth yet reduces the overall number of filopodia-like extensions in vitro [94]. Besides AβPP, BACE1 may proteolytically process a diverse array of substrates, many of which appear to play a critical role in intercellular communication, axonal guidance, and myelination [82-85, 87-89, 93, 95, 96]. The rich presence of BACE1 in presynaptic terminals allows this enzyme to execute an active role in synaptic development and plasticity, presumably via its proteolytic modulation to AβPP and other substrates [11, 26, 36-39, 91, 92].
Other data suggest that BACE1 may play a role in neuronal stress response and normal neuroplasticity. BACE1 is upregulated under stressful conditions, including ischemia, hypoxia, and traumatic injury [27, 97-103]. Oxidative stress and/or mitochondrial bioenergetic deficiency upregulate BACE1 expression in vitro and in vivo. Pharmacological studies in vitro and in vivo indicate that neuronal activity potentiates synaptic Aβ release, possibly via BACE1 upregulation [104, 105]. In the olfactory system, blocking physiological activity by naris-occlusion enhances BACE1 mRNA and protein expression in neuronal somata and axonal terminals [37, 92, 106]. This suggests a role for BACE1 in modulating synaptoplasticity during adulthood, given that the primary olfactory pathway undergoes constant structural modulation regulated by experience [106].
SYNAPTIC AND AXONAL PATHOLOGY IN NEUROLOGICAL DISORDERS
Synaptic and axonal lesions may contribute to pathogenesis and functional decline in many other neurological conditions in addition to AD [107]. TBI and TLE are probably the best studied disorders with regards to the extent of axonal pathology [108-120]. TBI is associated with early and broad axonal pathology that can be anatomically detected by AβPP and Aβ antibodies [115-117]. BACE1 elevation has been also reported in dystrophic neurites in human TBI [68, 69]. Axonal pathology is a pathological feature of TLE, mostly evidenced by the hippocampal mossy fiber sprouting [111-113]. Both TBI and TLE may be associated with brain amyloid pathology [68-70, 116-118]. Neuritic changes are a part of the neuropathology seen in PD and Lewy body dementia. Axonal spheroids and dystrophic neurites containing α-synuclein and other protein aggregates are found in the cerebral cortex, hippocampal formation, and subcortical structures of PD brains [121-124]. As typical AD (plaques and tangles) and PD (Lewy body and neurites) pathologies may coexist in clinically diagnosed AD or PD patients (or aged individuals) [125], the possibility of α-synuclein colocalization with AβPP or BACE1 in dystrophic neurites is worth further investigation. For additional examples, evidence suggests that axonal or neuritic pathology is associated with ischemic cerebral stroke [112, 113] and diabetic neuropathy [126].
Much work is needed to answer why axonal pathology occurs in various neurological disorders. Since there is loss of synaptic function in neurological diseases, this pathology may represent a part of neurodegenerative changes [107]. However, the swelling/sprouting of axonal processes and presynaptic terminals may also implicate an aberrant regenerative phenomenon [53, 55-57]. Axonal and synaptic pathology could be linked to neuroplasticity, a fundamental feature of the brain in response to internal and environmental stimuli. Early regenerative axonal and synaptic responses may serve a compensatory role to restore neuronal function, whereas persistent aberrant neuroplasticity could contribute to or exacerbate disease progression and functional loss [56, 57, 108-110, 118].
The molecular underpinning of axonal pathology is not clear to date. Deficient axonal transport owing to dysfunctional protein trafficking and deregulation of the autophagy machinery may cause neuritic dystrophy and accumulation of intracellular organelles [59, 127-130]. Notably, neuritic dystrophy can occur early or predominantly at the presynaptic sites without concurrently involving the axonal tract regions, at least in some cases [10, 36-38, 66]. This may be consistent with the notion that neuritic dystrophy may occur as a part of regenerative cellular attempts [53-57, 131, 132]. Thus, extensive investigations are warranted to identify the molecular substrates and signaling pathways responsible for axonal dystrophy, which may lead to the discovery of novel pharmaceutical targets for this pathology.
BACE1 INHIBITION AS A THERAPEUTIC OPTION FOR AXONAL PATHOLOGY
Many previous reviews have discussed BACE1 inhibition as a promising anti-Aβ therapy for AD, which is supported by genetic and pharmacological data from animal and human studies (e.g., [5, 6, 24, 28, 133]). A number of orally bioactive BACE1 inhibitors have been developed and show efficacy of lowering central Aβ levels and reducing cerebral amyloidosis in animal studies [25, 134-136]. Several potential BACE1 inhibitors are currently in phases I to III clinical trials, such as CTS-21166 (CoMentis), AZD3293 (AstraZeneca), RG7129 (Roche), LY2886721 (Lilly), E2609 (Eisai), and MK-8931 (Merck) (http://www.alzforum.org/new/detail.asp?id=3222). Scholarly reports about the effect of these BACE1 inhibitors on cerebrospinal fluid and plasma Aβ levels, brain Aβ deposition, and ultimately cognitive performance, might become available in the near future. To avoid redundancy with previous reviews, we do not discuss this expected efficacy of BACE1 inhibition here. As the best known function of BACE1 so far is its metabolic control over the amyloidogenic pathway, we propose that BACE1 inhibition to downregulate this pathway can influence neuritic and synaptic pathology, through Aβ-dependent and Aβ-independent mechanisms.
In vitro and in vivo studies suggest that at a certain level Aβ42 or amyloid fibrils may stimulate BACE1 expression, thereby self-enforce cerebral amyloid pathogenesis by a feed-forward mechanism [137-141]. Aβ products are considered to induce synaptic dysfunction and neuritic dystrophy [45, 50-53]. Lowering Aβ production via BACE1 inhibition will block the vicious pathogenic cycling induced by toxic Aβ products, thereby protecting synapses and neuronal terminals. BACE1 inhibition might mitigate axonal and presynaptic pathology via an Aβ independent mechanism. In particular, genetic and pharmacological studies suggest that the immediate BACE1 product, AβPP β-CTF, may be neurotoxic. Thus, β-CTF may accumulate early in neurons, especially in presynaptic terminals and dystrophic axons, in transgenic AD animal models and human AD subjects, which may worsen following administration of γ-secretase inhibitors [10-13, 66, 77, 78, 142-149]. Therefore, BACE1 inhibition would offer a unique protective effect on synapses and neuronal circuitry by preventing intraneuronal β-CTF accumulation.
With regard to other BACE1 substrates, chances are that BACE1 inhibition may cause either beneficial or mechanism-based side-effects to axons and synapses, pending more data from additional investigations into the molecular interaction between BACE1 and its novel substrates. As BACE1 plays a role in axonal outgrowth and synaptic development [91-94], BACE1 inhibition might interrupt normal synaptoplasticity in the adult brain. However, since axonal pathology is associated with excessive BACE1 activity, it is also possible that normalizing BACE1 to physiological levels might not necessarily cause dramatic adverse effects as seen in the neuronal systems wherein BACE1 levels are genetically inhibited to well below physiological levels.
To promote BACE1 inhibition as a strategy to antagonize axonal pathology in neurological diseases, much basic and translational research will be needed. Experimental studies using BACE1 deficient mice will help understand more about the biological functions and biochemical interaction and modulation of the enzyme in vivo. However, assessing pathological and behavioral outcomes following experimental manipulations on genetically modified systems may also be complicated [22, 150, 151], since both deficient and compensatory molecular responses may co-exist in the testing background. Potent and bioavailable BACE1 inhibitors will be very useful, and could provide a great opportunity to evaluate the utility and efficacy of BACE1 inhibition on wild-type animal models of neurological diseases with axonal pathology.
CONCLUSION
Strong genetic, molecular, and pathological data support a link of the amyloidogenic proteins to AD pathogenesis and neuronal/synaptic dysfunction. However, several anti-Aβ approaches, including Aβ immunization, anti-Aβ aggregation, and γ-secretase inhibition, are yet to demonstrate the expected neurological benefits in clinical trials [152]. Based on experimental data showing a close association of BACE1 elevation with axonal and presynaptic dystrophy, we propose that BACE1 inhibition may provide a unique pharmacological revenue against axonal pathology and synaptic dysfunction, especially by preventing intraneuronal β-CTF accumulation. Future studies should explore the spectrum of BACE1 elevation in axonal pathology under various neurological conditions. Pharmacological inhibition of BACE1 in wild-type animal models may be particular informative in translational medicine.
ACKNOWLEDGMENTS
This work is supported by the Natural Science Foundation of China (81171091 to XXY, 81171160 to XGL), the Intramural research program of National Institute on Aging (to HC), and the Xiangya-PUMC Human Brain Banking Consortium (to CM, XXY, XGL).
Footnotes
Authors’ disclosures available online (http://www.j-alz.com/disclosures/view.php?id=1899).
REFERENCES
- 1.Hynes TR, Randal M, Kennedy LA, Eigenbrot C, Kossiakoff AA. X-ray crystal structure of the protease inhibitor domain of Alzheimer's amyloid beta-protein precursor. Biochemistry. 1990;29:10018–10022. doi: 10.1021/bi00495a002. [DOI] [PubMed] [Google Scholar]
- 2.Beyreuther K, Dyrks T, Hilbich C, Mönning U, König G, Multhaup G, Pollwein P, Masters CL. Amyloid precursor protein (APP) and beta A4 amyloid in Alzheimer's disease and Down syndrome. Prog Clin Biol Res. 1992;379:159–182. [PubMed] [Google Scholar]
- 3.Hartmann T, Bieger SC, Brühl B, Tienari PJ, Ida N, Allsop D, Roberts GW, Masters CL, Dotti CG, Unsicker K, Beyreuther K. Distinct sites of intracellular production for Alzheimer's disease A beta40/42 amyloid peptides. Nat Med. 1997;3:1016–1020. doi: 10.1038/nm0997-1016. [DOI] [PubMed] [Google Scholar]
- 4.Turner PR, O'Connor K, Tate WP, Abraham WC. Roles of amyloid precursor protein and its fragments in regulating neural activity, plasticity and memory. Prog Neurobiol. 2003;70:1–32. doi: 10.1016/s0301-0082(03)00089-3. [DOI] [PubMed] [Google Scholar]
- 5.O'Brien RJ, Wong PC. Amyloid precursor protein processing and Alzheimer's disease. Annu Rev Neurosci. 2011;34:185–204. doi: 10.1146/annurev-neuro-061010-113613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang H, Ma Q, Zhang YW, Xu H. Proteolytic processing of Alzheimer's β-amyloid precursor protein. J Neurochem. 2012;120:9–21. doi: 10.1111/j.1471-4159.2011.07519.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Passer B, Pellegrini L, Russo C, Siegel RM, Lenardio MJ, Schettini G, Bachmann M, Tabaton M, D’Adamio L. Generation of an apoptotic intracellular peptide by γ-secretase cleavage of Alzheimer’s amyloid β protein precursor. J Alzheimers Dis. 2000;2:289–301. doi: 10.3233/jad-2000-23-408. [DOI] [PubMed] [Google Scholar]
- 8.Pardossi-Piquard R, Checler F. The physiology of the β-amyloid precursor protein intracellular domain AICD. J Neurochem. 2012;120:109–124. doi: 10.1111/j.1471-4159.2011.07475.x. [DOI] [PubMed] [Google Scholar]
- 9.Vingtdeux V, Marambaud P. Identification and biology of α-secretase. J Neurochem. 2012;120:34–45. doi: 10.1111/j.1471-4159.2011.07477.x. [DOI] [PubMed] [Google Scholar]
- 10.Cai Y, Zhang XM, Macklin LN, Cai H, Luo XG, Oddo S, Laferla FM, Struble RG, Rose GM, Patrylo PR, Yan XX. BACE1 elevation is involved in amyloid plaque development in the triple transgenic model of Alzheimer's disease: differential Aβ antibody labeling of early-onset axon terminal pathology. Neurotox Res. 2012;21:160–174. doi: 10.1007/s12640-011-9256-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cai Y, Xue ZQ, Zhang XM, Li MB, Wang H, Luo XG, Cai H, Yan XX. An age-related axon terminal pathology around the first olfactory relay that involves amyloidogenic protein overexpression without plaque formation. Neuroscience. 2012;215:160–173. doi: 10.1016/j.neuroscience.2012.04.043. [DOI] [PubMed] [Google Scholar]
- 12.Duyckaerts C, Delatour B, Potier MC. Classification and basic pathology of Alzheimer disease. Acta Neuropathol. 2009;118:5–36. doi: 10.1007/s00401-009-0532-1. [DOI] [PubMed] [Google Scholar]
- 13.Winton MJ, Lee EB, Sun E, Wong MM, Leight S, Zhang B, Trojanowski JQ, Lee VM. Intraneuronal APP, not free Aβ peptides in 3×Tg-AD mice: implications for tau versus Aβ-mediated Alzheimer neurodegeneration. J Neurosci. 2011;31:7691–7699. doi: 10.1523/JNEUROSCI.6637-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 14.Wegiel J, Kuchna I, Nowicki K, Frackowiak J, Mazur-Kolecka B, Imaki H, Wegiel J, Mehta PD, Silverman WP, Reisberg B, Deleon M, Wisniewski T, Pirttilla T, Frey H, Lehtimäki T, Kivimäki T, Visser FE, Kamphorst W, Potempska A, Bolton D, Currie JR, Miller DL. Intraneuronal Abeta immunoreactivity is not a predictor of brain amyloidosis-beta or neurofibrillary degeneration. Acta Neuropathol. 2007;113:389–402. doi: 10.1007/s00401-006-0191-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Aho L, Pikkarainen M, Hiltunen M, Leinonen V, Alafuzoff I. Immunohistochemical visualization of amyloid-beta protein precursor and amyloid-beta in extra- and intracellular compartments in the human brain. J Alzheimers Dis. 2010;20:1015–1028. doi: 10.3233/JAD-2010-091681. [DOI] [PubMed] [Google Scholar]
- 16.Barelli H, Lebeau A, Vizzavona J, Delaere P, Chevallier N, Drouot C, Marambaud P, Ancolio K, Buxbaum JD, Khorkova O, Heroux J, Sahasrabudhe S, Martinez J, Warter JM, Mohr M, Checler F. Characterization of new polyclonal antibodies specific for 40 and 42 amino acid-long amyloid beta peptides: their use to examine the cell biology of presenilins and the immunohistochemistry of sporadic Alzheimer's disease and cerebral amyloid angiopathy cases. Mol Med. 1997;3:695–707. [PMC free article] [PubMed] [Google Scholar]
- 17.Hussain I, Powell D, Howlett DR, Tew DG, Meek TD, Chapman C, Gloger IS, Murphy KE, Southan CD, Ryan DM, Smith TS, Simmons DL, Walsh FS, Dingwall C, Christie G. Identification of a novel aspartic protease (Asp 2) as beta-secretase. Mol Cell Neurosci. 1999;14:419–427. doi: 10.1006/mcne.1999.0811. [DOI] [PubMed] [Google Scholar]
- 18.Sinha S, Anderson JP, Barbour R, Basi GS, Caccavello R, Davis D, Doan M, Dovey HF, Frigon N, Hong J, Jacobson-Croak K, Jewett N, Keim P, Knops J, Lieberburg I, Power M, Tan H, Tatsuno G, Tung J, Schenk D, Seubert P, Suomensaari SM, Wang S, Walker D, Zhao J, McConlogue L, John V. Purification and cloning of amyloid precursor protein beta-secretase from human brain. Nature. 1999;402:537–540. doi: 10.1038/990114. [DOI] [PubMed] [Google Scholar]
- 19.Vassar R, Bennett BD, Babu-Khan S, Kahn S, Mendiaz EA, Denis P, Teplow DB, Ross S, Amarante P, Loeloff R, Luo Y, Fisher S, Fuller J, Edenson S, Lile J, Jarosinski MA, Biere AL, Curran E, Burgess T, Louis JC, Collins F, Treanor J, Rogers G, Citron M. Beta-secretase cleavage of Alzheimer's amyloid precursor protein by the transmembrane aspartic protease BACE. Science. 1999;286:735–741. doi: 10.1126/science.286.5440.735. [DOI] [PubMed] [Google Scholar]
- 20.Yan R, Bienkowski MJ, Shuck ME, Miao H, Tory MC, Pauley AM, Brashier JR, Stratman NC, Mathews WR, Buhl AE, Carter DB, Tomasselli AG, Parodi LA, Heinrikson RL, Gurney ME. Membrane-anchored aspartyl protease with Alzheimer's disease beta-secretase activity. Nature. 1999;402:533–537. doi: 10.1038/990107. [DOI] [PubMed] [Google Scholar]
- 21.Lin X, Koelsch G, Wu S, Downs D, Dashti A, Tang J. Human aspartic protease memapsin 2 cleaves the beta-secretase site of beta-amyloid precursor protein. Proc Natl Acad Sci U S A. 2000;97:1456–1460. doi: 10.1073/pnas.97.4.1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Luo Y, Bolon B, Kahn S, Bennett BD, Babu-Khan S, Denis P, Fan W, Kha H, Zhang J, Gong Y, Martin L, Louis JC, Yan Q, Richards WG, Citron M, Vassar R. Mice deficient in BACE1, the Alzheimer's beta-secretase, have normal phenotype and abolished beta-amyloid generation. Nat Neurosci. 2001;4:231–232. doi: 10.1038/85059. [DOI] [PubMed] [Google Scholar]
- 23.Yan R, Han P, Miao H, Greengard P, Xu H. The transmembrane domain of the Alzheimer's beta-secretase (BACE1) determines its late Golgi localization and access to beta -amyloid precursor protein (APP) substrate. J Biol Chem. 2001;276:36788–36796. doi: 10.1074/jbc.M104350200. [DOI] [PubMed] [Google Scholar]
- 24.Cai H, Wang Y, McCarthy D, Wen H, Borchelt DR, Price DL, Wong PC. BACE1 is the major beta-secretase for generation of Abeta peptides by neurons. Nat Neurosci. 2001;4:233–234. doi: 10.1038/85064. [DOI] [PubMed] [Google Scholar]
- 25.Hussain I, Hawkins J, Harrison D, Hille C, Wayne G, Cutler L, Buck T, Walter D, Demont E, Howes C, Naylor A, Jeffrey P, Gonzalez MI, Dingwall C, Michel A, Redshaw S, Davis JB. Oral administration of a potent and selective non-peptidic BACE-1 inhibitor decreases beta-cleavage of amyloid precursor protein and amyloid-beta production in vivo. J Neurochem. 2007;100:802–809. doi: 10.1111/j.1471-4159.2006.04260.x. [DOI] [PubMed] [Google Scholar]
- 26.Laird FM, Cai H, Savonenko AV, Farah MH, He K, Melnikova T, Wen H, Chiang HC, Xu G, Koliatsos VE, Borchelt DR, Price DL, Lee HK, Wong PC. BACE1, a major determinant of selective vulnerability of the brain to amyloid-beta amyloidogenesis, is essential for cognitive, emotional, and synaptic functions. J Neurosci. 2005;25:11693–11709. doi: 10.1523/JNEUROSCI.2766-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xiong K, Cai H, Luo XG, Struble RG, Clough RW, Yan XX. Mitochondrial respiratory inhibition and oxidative stress elevate beta-secretase (BACE1) proteins and activity in vivo in the rat retina. Exp. Brain Res. 2007;181:435–446. doi: 10.1007/s00221-007-0943-y. [DOI] [PubMed] [Google Scholar]
- 28.Kandalepas PC, Vassar R. Identification and biology of β-secretase. J Neurochem. 2012;120:55–61. doi: 10.1111/j.1471-4159.2011.07512.x. [DOI] [PubMed] [Google Scholar]
- 29.Benjannet S, Elagoz A, Wickham L, Mamarbachi M, Munzer JS, Basak A, Lazure C, Cromlish JA, Sisodia S, Checler F, Chrétien M, Seidah NG. Post-translational processing of beta-secretase (beta-amyloid-converting enzyme) and its ectodomain shedding. The pro- and transmembrane/cytosolic domains affect its cellular activity and amyloid-beta production. J Biol Chem. 2001;276:10879–10887. doi: 10.1074/jbc.M009899200. [DOI] [PubMed] [Google Scholar]
- 30.Tamagno E, Guglielmotto M, Monteleone D, Vercelli A, Tabaton M. Transcriptional and post-transcriptional regulation of β-secretase. IUBMB Life. 2012;64:943–950. doi: 10.1002/iub.1099. [DOI] [PubMed] [Google Scholar]
- 31.Motoki K, Kume H, Oda A, Tamaoka A, Hosaka A, Kametani F, Araki W. Neuronal β-amyloid generation is independent of lipid raft association of β-secretase BACE1: analysis with palmitoylation-deficient mutant. Brain Behav. 2012;2:270–282. doi: 10.1002/brb3.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rossner S, Lange-Dohna C, Zeitschel U, Perez-Polo JR. Alzheimer's disease beta-secretase BACE1 is not a neuron-specific enzyme. J Neurochem. 2005;92:226–234. doi: 10.1111/j.1471-4159.2004.02857.x. [DOI] [PubMed] [Google Scholar]
- 33.Fukumoto H, Cheung BS, Hyman BT, Irizarry MC. Beta-secretase protein and activity are increased in the neocortex in Alzheimer disease. Arch Neurol. 2002;59:1381–1389. doi: 10.1001/archneur.59.9.1381. [DOI] [PubMed] [Google Scholar]
- 34.Leuba G, Wernli G, Vernay A, Kraftsik R, Mohajeri MH, Saini KD. Neuronal and nonneuronal quantitative BACE immunocytochemical expression in the entorhinohippocampal and frontal regions in Alzheimer's disease. Dement Geriatr Cogn Disord. 2005;19:171–183. doi: 10.1159/000083496. [DOI] [PubMed] [Google Scholar]
- 35.Zhao J, Fu Y, Yasvoina M, Shao P, Hitt B, O'Connor T, Logan S, Maus E, Citron M, Berry R, Binder L, Vassar R. Beta-site amyloid precursor protein cleaving enzyme 1 levels become elevated in neurons around amyloid plaques: implications for Alzheimer's disease pathogenesis. J Neurosci. 2007;27:3639–3649. doi: 10.1523/JNEUROSCI.4396-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang XM, Cai Y, Xiong K, Cai H, Luo XG, Feng JC, Clough RW, Struble RG, Patrylo PR, Yan XX. Beta-secretase-1 elevation in transgenic mouse models of Alzheimer's disease is associated with synaptic/axonal pathology and amyloidogenesis: implications for neuritic plaque development. Eur J Neurosci. 2009;30:2271–2283. doi: 10.1111/j.1460-9568.2009.07017.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang XM, Xiong K, Cai Y, Cai H, Luo XG, Feng JC, Clough RW, Patrylo PR, Struble RG, Yan XX. Functional deprivation promotes amyloid plaque pathogenesis in Tg2576 mouse olfactory bulb and piriform cortex. Eur J Neurosci. 2010;31:710–721. doi: 10.1111/j.1460-9568.2010.07103.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yan XX, Cai Y, Zhang XM, Luo XG, Cai H, Rose GM, Patrylo PR. BACE1 elevation is associated with aberrant limbic axonal sprouting in epileptic CD1 mice. Exp Neurol. 2012;235:228–237. doi: 10.1016/j.expneurol.2012.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kandalepas PC, Sadleir KR, Eimer WA, Zhao J, Nicholson DA, Vassar R. The Alzheimer's β-secretase BACE1 localizes to normal presynaptic terminals and to dystrophic presynaptic terminals surrounding amyloid plaques. Acta Neuropathol. 2013;126:329–352. doi: 10.1007/s00401-013-1152-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kaether C, Haass C, Steiner H. Assembly, trafficking and function of gamma-secretase. Neurodegener Dis. 2006;3:275–283. doi: 10.1159/000095267. [DOI] [PubMed] [Google Scholar]
- 41.Chan SL, Furukawa K, Mattson MP. Presenilins and APP in neuritic and synaptic plasticity: implications for the pathogenesis of Alzheimer's disease. Neuromolecular Med. 2002;2:167–196. doi: 10.1385/NMM:2:2:167. [DOI] [PubMed] [Google Scholar]
- 42.Chen F, Hasegawa H, Schmitt-Ulms G, Kawarai T, Bohm C, Katayama T, Gu Y, Sanjo N, Glista M, Rogaeva E, Wakutani Y, Pardossi-Piquard R, Ruan X, Tandon A, Checler F, Marambaud P, Hansen K, Westaway D, St George-Hyslop P, Fraser P. TMP21 is a presenilin complex component that modulates gamma-secretase but not epsilon-secretase activity. Nature. 2006;440:1208–1212. doi: 10.1038/nature04667. [DOI] [PubMed] [Google Scholar]
- 43.Kodam A, Vetrivel KS, Thinakaran G, Kar S. Cellular distribution of gamma-secretase subunit nicastrin in the developing and adult rat brains. Neurobiol Aging. 2008;29:724–238. doi: 10.1016/j.neurobiolaging.2006.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Uchihara T, Sanjo N, Nakamura A, Han K, Song SY, St George-Hyslop P, Fraser PE. Transient abundance of presenilin 1 fragments/nicastrin complex associated with synaptogenesis during development in rat cerebellum. Neurobiol Aging. 2006;27:88–97. doi: 10.1016/j.neurobiolaging.2004.12.011. [DOI] [PubMed] [Google Scholar]
- 45.Wasling P, Daborg J, Riebe I, Andersson M, Portelius E, Blennow K, Hanse E, Zetterberg H. Synaptic retrogenesis and amyloid-beta in Alzheimer's disease. J Alzheimers Dis. 2009;16:1–14. doi: 10.3233/JAD-2009-0918. [DOI] [PubMed] [Google Scholar]
- 46.Yan XX, Li T, Rominger CM, Prakash SR, Wong PC, Olson RE, Zaczek R, Li YW. Binding sites of gamma-secretase inhibitors in rodent brain: distribution, postnatal development, and effect of deafferentation. J Neurosci. 2004;24:2942–2952. doi: 10.1523/JNEUROSCI.0092-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Patel S, O'Malley S, Connolly B, Liu W, Hargreaves R, Sur C, Gibson RE. In vitro characterization of a gamma-secretase radiotracer in mammalian brain. J Neurochem. 2006;96:171–178. doi: 10.1111/j.1471-4159.2005.03525.x. [DOI] [PubMed] [Google Scholar]
- 48.Xiong K, Clough RW, Luo XG, Struble RG, Li YM, Yan XX. [3H]-L-685,458 as a radiotracer that maps gamma-secretase complex in the rat brain: Relevance to Abeta production and presence of active presenilin-1 components. Brain Res. 2007;1157:81–91. doi: 10.1016/j.brainres.2007.04.068. [DOI] [PubMed] [Google Scholar]
- 49.Liu F, Xue ZQ, Deng SH, Kun X, Luo XG, Patrylo PR, Rose GM, Cai H, Struble RG, Cai Y, Yan XX. γ-Secretase binding sites in aged and Alzheimer's disease human cerebrum: the choroid plexus as a putative origin of CSF Aβ. Eur J Neurosci. 2013;37:714–725. doi: 10.1111/ejn.12159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Struble RG, Ala T, Patrylo PR, Brewer GJ, Yan XX. Is brain amyloid production a cause or a result of dementia of the Alzheimer's type? J Alzheimers Dis. 2010;22:393–399. doi: 10.3233/JAD-2010-100846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Walker LC, Diamond MI, Duff KE, Hyman BT. Mechanisms of protein seeding in neurodegenerative diseases. JAMA Neurol. 2013;70:304–310. doi: 10.1001/jamaneurol.2013.1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pimplikar SW. Reassessing the amyloid cascade hypothesis of Alzheimer's disease. Int J Biochem Cell Biol. 2009;41:1261–1268. doi: 10.1016/j.biocel.2008.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Arendt T. Alzheimer's disease as a disorder of mechanisms underlying structural brain self-organization. Neuroscience. 2001;102:723–765. doi: 10.1016/s0306-4522(00)00516-9. [DOI] [PubMed] [Google Scholar]
- 54.Gouras GK, Almeida CG, Takahashi RH. Intraneuronal Abeta accumulation and origin of plaques in Alzheimer’s disease. Neurobiol Aging. 2005;26:1235–1244. doi: 10.1016/j.neurobiolaging.2005.05.022. [DOI] [PubMed] [Google Scholar]
- 55.Fiala JC. Mechanisms of amyloid plaque pathogenesis. Acta Neuropathol. 2007;114:551–571. doi: 10.1007/s00401-007-0284-8. [DOI] [PubMed] [Google Scholar]
- 56.Geddes JW, Cotman CW. Plasticity, pathology, and Alzheimer's disease. Neurobiol Aging. 1989;10:571–573. doi: 10.1016/0197-4580(89)90131-0. discussion 588-590. [DOI] [PubMed] [Google Scholar]
- 57.Hashimoto M, Masliah E. Cycles of aberrant synaptic sprouting and neurodegeneration in Alzheimer's and dementia with Lewy bodies. Neurochem Res. 2003;28:1743–1756. doi: 10.1023/a:1026073324672. [DOI] [PubMed] [Google Scholar]
- 58.García-Marín V, García-López P, Freire M. Cajal's contributions to the study of Alzheimer's disease. J Alzheimers Dis. 2007;12:161–174. doi: 10.3233/jad-2007-12206. [DOI] [PubMed] [Google Scholar]
- 59.Stokin GB, Lillo C, Falzone TL, Brusch RG, Rockenstein E, Mount SL, Raman R, Davies P, Masliah E, Williams DS, Goldstein LS. Axonopathy and transport deficits early in the pathogenesis of Alzheimer’s disease. Science. 2005;307:1282–1288. doi: 10.1126/science.1105681. [DOI] [PubMed] [Google Scholar]
- 60.Dickson TC, Vickers JC. The morphological phenotype of beta-amyloid plaques and associated neuritic changes in Alzheimer’s disease. Neuroscience. 2001;105:99–107. doi: 10.1016/s0306-4522(01)00169-5. [DOI] [PubMed] [Google Scholar]
- 61.Thal DR, Capetillo-Zarate E, Del Tredici K, Braak H. The development of amyloid beta protein deposits in the aged brain. Sci Aging Knowledge Environ. 2006;6 doi: 10.1126/sageke.2006.6.re1. re1. [DOI] [PubMed] [Google Scholar]
- 62.Terry RD, Wisniewski HM. The ultrastructure of the neurobrillary tangle and the senile plaque. In: Wolstenholme GEW, O’Connor M, editors. In Alzheimer’s Disease and Related Conditions. J & A Churchill; London: 1971. pp. 145–168. [Google Scholar]
- 63.Elder GA, Gama Sosa MA, De Gasperi R, Dickstein DL, Hof PR. Presenilin transgenic mice as models of Alzheimer's disease. Brain Struct Funct. 2010;214:127–143. doi: 10.1007/s00429-009-0227-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hall AM, Roberson ED. Mouse models of Alzheimer's disease. Brain Res Bull. 2012;88:3–12. doi: 10.1016/j.brainresbull.2011.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lee JE, Han PL. An update of animal models of Alzheimer disease with a reevaluation of plaque depositions. Exp Neurobiol. 2012;22:84–95. doi: 10.5607/en.2013.22.2.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Li JM, Xue ZQ, Deng SH, Luo XG, Cai H, Cai Y, Yan XX. Amyloid plaque pathogenesis in 5XFAD mouse spinal cord: Retrograde transneuronal modulation after peripheral nerve injury. Neurotox Res. 2013;24:1–14. doi: 10.1007/s12640-012-9355-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Crowe SE, Ellis-Davies GC. In vivo characterization of a bigenic fluorescent mouse model of Alzheimer's disease with neurodegeneration. J Comp Neurol. 2013;521:2181–2194. doi: 10.1002/cne.23306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chen XH, Siman R, Iwata A, Meaney DF, Trojanowski JQ, Smith DH. Long-term accumulation of amyloid-beta, beta-secretase, presenilin-1, and caspase-3 in damaged axons following brain trauma. Am J Pathol. 2004;165:357–371. doi: 10.1016/s0002-9440(10)63303-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Chen XH, Johnson VE, Uryu K, Trojanowski JQ, Smith DH. A lack of amyloid beta plaques despite persistent accumulation of amyloid beta in axons of long-term survivors of traumatic brain injury. Brain Pathol. 2009;19:214–223. doi: 10.1111/j.1750-3639.2008.00176.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yan XX, Cai Y, Shelton J, Deng SH, Luo XG, Oddo S, LaFerla FM, Cai H, Rose GM, Patrylo PR. Chronic temporal lobe epilepsy is associated with enhanced Alzheimer-like neuropathology in 3×Tg-AD mice. PLoS-ONE. 2012;7:e48782. doi: 10.1371/journal.pone.0048782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Fukumoto H, Cheung BS, Hyman BT, Irizarry MC. Beta-secretase protein and activity are increased in the neocortex in Alzheimer disease. Arch Neurol. 2002;59:1381–1389. doi: 10.1001/archneur.59.9.1381. [DOI] [PubMed] [Google Scholar]
- 72.Fukumoto H, Rosene DL, Moss MB, Raju S, Hyman BT, Irizarry MC. Beta-secretase activity increases with aging in human, monkey, and mouse brain. Am J Pathol. 2004;164:719–725. doi: 10.1016/s0002-9440(10)63159-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Harada H, Tamaoka A, Ishii K, Shoji S, Kametaka S, Kametani F, Saito Y, Murayama S. Beta-site APP cleaving enzyme 1 (BACE1) is increased in remaining neurons in Alzheimer's disease brains. Neurosci Res. 2006;54:24–29. doi: 10.1016/j.neures.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 74.Holsinger RM, McLean CA, Beyreuther K, Masters CL, Evin G. Increased expression of the amyloid precursor beta-secretase in Alzheimer’s disease. Ann Neurol. 2002;51:783–786. doi: 10.1002/ana.10208. [DOI] [PubMed] [Google Scholar]
- 75.Tyler SJ, Dawbarn D, Wilcock GK, Allen SJ. Alpha- and beta-secretase: profound changes in Alzheimer's disease. Biochem Biophys Res Commun. 2002;299:373–376. doi: 10.1016/s0006-291x(02)02635-9. [DOI] [PubMed] [Google Scholar]
- 76.Li R, Lindholm K, Yang LB, Yue X, Citron M, Yan R, Beach T, Sue L, Sabbagh M, Cai H, Wong P, Price D, Shen Y. Amyloid beta peptide load is correlated with increased beta-secretase activity in sporadic Alzheimer's disease patients. Proc Natl Acad Sci U S A. 2004;101:3632–3637. doi: 10.1073/pnas.0205689101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Cai Y, Xiong K, Zhang XM, Cai H, Luo XG, Feng JC, Clough RW, Struble RG, Patrylo PR, Chu Y, Kordower JH, Yan XX. β-Secretase-1 elevation in aged monkey and Alzheimer's disease human cerebral cortex occurs around the vasculature in partnership with multisystem axon terminal pathogenesis and β-amyloid accumulation. Eur J Neurosci. 2010;32:1223–1238. doi: 10.1111/j.1460-9568.2010.07376.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Miners JS, van Helmond Z, Kehoe PG, Love S. Changes with age in the activities of beta-secretase and the Abeta-degrading enzymes neprilysin, insulin-degrading enzyme and angiotensin-converting enzyme. Brain Pathol. 2010;20:794–802. doi: 10.1111/j.1750-3639.2010.00375.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kawai M, Kalaria RN, Harik SI, Perry G. The relationship of amyloid plaques to cerebral capillaries in Alzheimer’s disease. Am J Pathol. 1990;137:1435–1446. [PMC free article] [PubMed] [Google Scholar]
- 80.Kawai M, Cras P, Perry G. Serial reconstruction of beta-protein amyloid plaques: relationship to microvessels and size distribution. Brain Res. 1992;592:278–282. doi: 10.1016/0006-8993(92)91686-9. [DOI] [PubMed] [Google Scholar]
- 81.Miyakawa T, Shimoji A, Kuramoto R, Higuchi Y. The relationship between senile plaques and cerebral blood vessels in Alzheimer’s disease and senile dementia. Morphological mechanism of senile plaque production. Virchows Arch. B Cell Pathol Mol Pathol. 1982;40:121–129. doi: 10.1007/BF02932857. [DOI] [PubMed] [Google Scholar]
- 82.Dominguez D, Tournoy J, Hartmann D, Huth T, Cryns K, Deforce S, Serneels L, Camacho IE, Marjaux E, Craessaerts K, Roebroek AJ, Schwake M, D’Hooge R, Bach P, Kalinke U, Moechars D, Alzheimer C, Reiss K, Saftig P, De Strooper B. Phenotypic and biochemical analyses of BACE1- and BACE2-deficient mice. J Biol Chem. 2005;280:30797–30806. doi: 10.1074/jbc.M505249200. [DOI] [PubMed] [Google Scholar]
- 83.Savonenko AV, Melnikova T, Laird FM, Stewart KA, Price DL, Wong PC. Alteration of BACE1-dependent NRG1/ErbB4 signaling and schizophrenia-like phenotypes in BACE1-null mice. Proc Natl Acad Sci U S A. 2008;105:5585–5590. doi: 10.1073/pnas.0710373105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Farah MH, Pan BH, Hoffman PN, Ferraris D, Tsukamoto T, Nguyen T, Wong PC, Price DL, Slusher BS, Griffin JW. Reduced BACE1 activity enhances clearance of myelin debris and regeneration of axons in the injured peripheral nervous system. J Neurosci. 2011;31:5744–5754. doi: 10.1523/JNEUROSCI.6810-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wong HK, Sakurai T, Oyama F, Kaneko K, Wada K, Miyazaki H, Kurosawa M, De Strooper B, Saftig P, Nukina N. beta Subunits of voltage-gated sodium channels are novel substrates of beta-site amyloid precursor protein-cleaving enzyme (BACE1) and gamma-secretase. J Biol Chem. 2005;280:23009–230017. doi: 10.1074/jbc.M414648200. [DOI] [PubMed] [Google Scholar]
- 86.Wang H, Song L, Laird F, Wong PC, Lee HK. BACE1 knock-outs display deficits in activity-dependent potentiation of synaptic transmission at mossy fiber to CA3 synapses in the hippocampus. J Neurosci. 2008;28:8677–8681. doi: 10.1523/JNEUROSCI.2440-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Willem M, Garratt AN, Novak B, Citron M, Kaufmann S, Rittger A, De Strooper B, Saftig P, Birchmeier C, Haass C. Control of peripheral nerve myelination by the beta-secretase BACE1. Science. 2006;314:664–666. doi: 10.1126/science.1132341. [DOI] [PubMed] [Google Scholar]
- 88.Hitt BD, Jaramillo TC, Chetkovich DM, Vassar R. BACE1−/− mice exhibit seizure activity that does not correlate with sodium channel level or axonal localization. Mol Neurodegener. 2010;5:31–44. doi: 10.1186/1750-1326-5-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hu X, Hicks CW, He W, Wong P, Macklin WB, Trapp BD, Yan R. Bace1 modulates myelination in the central and peripheral nervous system. Nat Neurosci. 2006;9:1520–1525. doi: 10.1038/nn1797. [DOI] [PubMed] [Google Scholar]
- 90.Hu X, Zhou X, He W, Yang J, Xiong W, Wong P, Wilson CG, Yan R. BACE1 deficiency causes altered neuronal activity and neurodegeneration. J Neurosci. 2010;30:8819–8829. doi: 10.1523/JNEUROSCI.1334-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Rajapaksha TW, Eimer WA, Bozza TC, Vassar R. The Alzheimer's β-secretase enzyme BACE1 is required for accurate axon guidance of olfactory sensory neurons and normal glomerulus formation in the olfactory bulb. Mol Neurodegener. 2011;6:88. doi: 10.1186/1750-1326-6-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Cao L, Rickenbacher GT, Rodriguez S, Moulia TW, Albers MW. The precision of axon targeting of mouse olfactory sensory neurons requires the BACE1 protease. Sci Rep. 2012;2:231. doi: 10.1038/srep00231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Hitt B, Riordan S, Kukreja L, Eimer W, Rajapaksha T, Vassar R. β-site amyloid precursor protein (APP) cleaving enzyme 1 (BACE1) deficient mice exhibit a close homolog of L1 (CHL1) loss-of-function phenotype involving axon guidance defects. J Biol Chem. 2012;287:38408–38425. doi: 10.1074/jbc.M112.415505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Miyazaki H, Oyama F, Wong HK, Kaneko K, Sakurai T, Tamaoka A, Nukina N. BACE1 modulates filopodia-like protrusions induced by sodium channel beta4 subunit. Biochem Biophys Res Commun. 2007;361:43–48. doi: 10.1016/j.bbrc.2007.06.170. [DOI] [PubMed] [Google Scholar]
- 95.Hemming ML, Elias JE, Gygi SP, Selkoe DJ. Identification of beta-secretase (BACE1) substrates using quantitative proteomics. PLoS One. 2009;4:e8477. doi: 10.1371/journal.pone.0008477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kuhn PH, Koroniak K, Hogl S, Colombo A, Zeitschel U, Willem M, Volbracht C, Schepers U, Imhof A, Hoffmeister A, Haass C, Roßner S, Bräse S, Lichtenthaler SF. Secretome protein enrichment identifies physiological BACE1 protease substrates in neurons. EMBO J. 2012;31:3157–3168. doi: 10.1038/emboj.2012.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Blasko I, Beer R, Bigl M, Apelt J, Franz G, Rudzki D, Ransmayr G, Kampfl A, Schliebs R. Experimental traumatic brain injury in rats stimulates the expression, production and activity of Alzheimer’s disease beta-secretase (BACE-1) J Neural Transm. 2004;111:523–536. doi: 10.1007/s00702-003-0095-6. [DOI] [PubMed] [Google Scholar]
- 98.Wen Y, Onyewuchi O, Yang S, Liu R, Simpkins JW. Increased beta-secretase activity and expression in rats following transient cerebral ischemia. Brain Res. 2004;1009:1–8. doi: 10.1016/j.brainres.2003.09.086. [DOI] [PubMed] [Google Scholar]
- 99.Sun X, He G, Qing H, Zhou W, Dobie F, Cai F, Staufenbiel M, Huang LE, Song W. Hypoxia facilitates Alzheimer's disease pathogenesis by up-regulating BACE1 gene expression. Proc Natl Acad Sci U S A. 2006;103:18727–18732. doi: 10.1073/pnas.0606298103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Tong Y, Zhou W, Fung V, Christensen MA, Qing H, Sun X, Song W. Oxidative stress potentiates BACE1 gene expression and Abeta generation. J Neural Transm. 2005;112:455–469. doi: 10.1007/s00702-004-0255-3. [DOI] [PubMed] [Google Scholar]
- 101.Velliquette RA, O'Connor T, Vassar R. Energy inhibition elevates beta-secretase levels and activity and is potentially amyloidogenic in APP transgenic mice: possible early events in Alzheimer's disease pathogenesis. J Neurosci. 2005;25:10874–10883. doi: 10.1523/JNEUROSCI.2350-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Chami L, Checler F. BACE1 is at the crossroad of a toxic vicious cycle involving cellular stress and β-amyloid production in Alzheimer's disease. Mol Neurodegener. 2012:7–52. doi: 10.1186/1750-1326-7-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Tamagno E, Guglielmotto M, Monteleone D, Tabaton M. Amyloid-β production: major link between oxidative stress and BACE1. Neurotox Res. 2012;22:208–219. doi: 10.1007/s12640-011-9283-6. [DOI] [PubMed] [Google Scholar]
- 104.Kamenetz F, Tomita T, Hsieh H, Seabrook G, Borchelt D, Iwatsubo T, Sisodia S, Malinow R. APP processing and synaptic function. Neuron. 2003;37:925–937. doi: 10.1016/s0896-6273(03)00124-7. [DOI] [PubMed] [Google Scholar]
- 105.Cirrito JR, Yamada KA, Finn MB, Sloviter RS, Bales KR, May PC, Schoepp DD, Paul SM, Mennerick S, Holtzman DM. Synaptic activity regulates interstitial fluid amyloid-beta levels in vivo. Neuron. 2005;48:913–922. doi: 10.1016/j.neuron.2005.10.028. [DOI] [PubMed] [Google Scholar]
- 106.Yan XX, Xiong K, Luo XG, Struble RG, Clough RW. beta-Secretase expression in normal and functionally deprived rat olfactory bulbs: inverse correlation with oxidative metabolic activity. J Comp Neurol. 2007;501:52–69. doi: 10.1002/cne.21239. [DOI] [PubMed] [Google Scholar]
- 107.Adalbert R, Coleman MP. Axon pathology in age-related neurodegenerative disorders. Neuropathol Appl Neurobiol. 2012 doi: 10.1111/j.1365-2990.2012.01308.x. doi: 10.1111/j.1365-2990.2012.01308.x. [DOI] [PubMed] [Google Scholar]
- 108.Dancause N, Barbay S, Frost SB, Plautz EJ, Chen D, Zoubina EV, Stowe AM, Nudo RJ. Extensive cortical rewiring after brain injury. J Neurosci. 2005;25:10167–10179. doi: 10.1523/JNEUROSCI.3256-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Blizzard CA, Chuckowree JA, King AE, Hosie KA, McCormack GH, Chapman JA, Vickers JC, Dickson TC. Focal damage to the adult rat neocortex induce wound healing accompanied by axonal sprouting and dendritic structural plasticity. Cereb Cortex. 2011;21:281–291. doi: 10.1093/cercor/bhq091. [DOI] [PubMed] [Google Scholar]
- 110.Deller T, Haas CA, Freiman TM, Phinney A, Jucker M, Frotscher M. Lesion-induced axonal sprouting in the central nervous system. Adv Exp Med Biol. 2006;557:101–121. doi: 10.1007/0-387-30128-3_6. [DOI] [PubMed] [Google Scholar]
- 111.Scheid R, Preul C, Gruber O, Wiggins C, von Cramon DY. Diffuse axonal injury associated with chronic traumatic brain injury: evidence from T2*-weighted gradient-echo imaging at 3 T. Am J Neuroradiol. 2003;24:1049–1056. [PMC free article] [PubMed] [Google Scholar]
- 112.Li S, Carmichael ST. Growth-associated gene and protein expression in the region of axonal sprouting in the aged brain after stroke. Neurobiol Dis. 2006;23:362–373. doi: 10.1016/j.nbd.2006.03.011. [DOI] [PubMed] [Google Scholar]
- 113.Li S, Overman JJ, Katsman D, Kozlov SV, Donnelly CJ, Twiss JL, Giger RJ, Coppola G, Geschwind DH, Carmichael ST. An age-related sprouting transcriptome provides molecular control of axonal sprouting after stroke. Nat Neurosci. 2010;13:1496–504. doi: 10.1038/nn.2674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Edlow BL, Haynes RL, Takahashi E, Klein JP, Cummings P, Benner T, Greer DM, Greenberg SM, Wu O, Kinney HC, Folkerth RD. Disconnection of the ascending arousal system in traumatic coma. J Neuropathol Exp Neurol. 2013;72:505–23. doi: 10.1097/NEN.0b013e3182945bf6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Gentleman SM, Nash MJ, Sweeting CJ, Graham DI, Roberts GW. Beta-amyloid precursor protein (beta APP) as a marker for axonal injury after head injury. Neurosci Lett. 1993;160:139–144. doi: 10.1016/0304-3940(93)90398-5. [DOI] [PubMed] [Google Scholar]
- 116.Ikonomovic MD, Uryu K, Abrahamson EE, Ciallella JR, Trojanowski JQ, Lee VM, Clark RS, Marion DW, Wisniewski SR, DeKosky ST. Alzheimer's pathology in human temporal cortex surgically excised after severe brain injury. Exp Neurol. 2004;190:192–203. doi: 10.1016/j.expneurol.2004.06.011. [DOI] [PubMed] [Google Scholar]
- 117.Johnson MW, Stoll L, Rubio A, Troncoso J, Pletnikova O, Fowler DR, Li L. Axonal injury in young pediatric head trauma: a comparison study of β-amyloid precursor protein (β-APP) immunohistochemical staining in traumatic and nontraumatic deaths. J Forensic Sci. 2011;56:1198–205. doi: 10.1111/j.1556-4029.2011.01814.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Ben-Ari Y. Epilepsies and neuronal plasticity: for better or for worse? Dialogues Clin Neurosci. 2008;10:17–27. doi: 10.31887/DCNS.2008.10.1/ybenari. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Ribak CE, Seress L, Weber P, Epstein CM, Henry TR, Bakay RA. Alumina gel injections into the temporal lobe of rhesus monkeys cause complex partial seizures and morphological changes found in human temporal lobe epilepsy. J Comp Neurol. 1998;401:266–290. [PubMed] [Google Scholar]
- 120.Sutula T, Cascino G, Cavazos J, Parada I, Ramirez L. Mossy fiber synaptic reorganization in the epileptic human temporal lobe. Ann Neurol. 1989;26:321–330. doi: 10.1002/ana.410260303. [DOI] [PubMed] [Google Scholar]
- 121.Irizarry MC, Growdon W, Gomez-Isla T, Newell K, George JM, Clayton DF, Hyman BT. Nigral and cortical Lewy bodies and dystrophic nigral neurites in Parkinson's disease and cortical Lewy body disease contain alpha-synuclein immunoreactivity. J Neuropathol Exp Neurol. 1998;57:334–337. doi: 10.1097/00005072-199804000-00005. [DOI] [PubMed] [Google Scholar]
- 122.Gai WP, Blessing WW, Blumbergs PC. Ubiquitin-positive degenerating neurites in the brainstem in Parkinson's disease. Brain. 1995;118:1447–1459. doi: 10.1093/brain/118.6.1447. [DOI] [PubMed] [Google Scholar]
- 123.Gai WP, Blumbergs PC, Blessing WW. Microtubule-associated protein 5 is a component of Lewy bodies and Lewy neurites in the brainstem and forebrain regions affected in Parkinson's disease. Acta Neuropathol. 1996;91:78–81. doi: 10.1007/s004010050395. [DOI] [PubMed] [Google Scholar]
- 124.Galvin JE, Uryu K, Lee VM, Trojanowski JQ. Axon pathology in Parkinson's disease and Lewy body dementia hippocampus contains alpha-, beta-, and gamma-synuclein. Proc Natl Acad Sci U S A. 1999;96:13450–13455. doi: 10.1073/pnas.96.23.13450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Boyle PA, Wilson RS, Yu L, Barr AM, Honer WG, Schneider JA, Bennett DA. Much of late life cognitive decline is not due to common neurodegenerative pathologies. Ann Neurol. 2013 doi: 10.1002/ana.23964. doi: 10.1002/ana.23964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Zhao B, Pan BS, Shen SW, Sun X, Hou ZZ, Yan R, Sun FY. Diabetes-induced central neuritic dystrophy and cognitive deficits are associated with the formation of oligomeric reticulon-3 via oxidative stress. J Biol Chem. 2013;288:15590–15599. doi: 10.1074/jbc.M112.440784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Morfini GA, Burns M, Binder LI, Kanaan NM, LaPointe N, Bosco DA, Brown RH Jr, Brown H, Tiwari A, Hayward L, Edgar J, Nave KA, Garberrn J, Atagi Y, Song Y, Pigino G, Brady ST. Axonal transport defects in neurodegenerative diseases. J Neurosci. 2009;29:12776–12786. doi: 10.1523/JNEUROSCI.3463-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Millecamps S, Julien JP. Axonal transport deficits and neurodegenerative diseases. Nat Rev Neurosci. 2013;14:161–176. doi: 10.1038/nrn3380. [DOI] [PubMed] [Google Scholar]
- 129.Lee S, Sato Y, Nixon RA. Primary lysosomal dysfunction causes cargo-specific deficits of axonal transport leading to Alzheimer-like neuritic dystrophy. Autophagy. 2011;7:1562–1563. doi: 10.4161/auto.7.12.17956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Wang QJ, Ding Y, Kohtz DS, Mizushima N, Cristea IM, Rout MP, Chait BT, Zhong Y, Heintz N, Yue Z. Induction of autophagy in axonal dystrophy and degeneration. J Neurosci. 2006;26:8057–8068. doi: 10.1523/JNEUROSCI.2261-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Hilliard MA. Axonal degeneration and regeneration: a mechanistic tug-of-war. J Neurochem. 2009;108:23–32. doi: 10.1111/j.1471-4159.2008.05754.x. [DOI] [PubMed] [Google Scholar]
- 132.Newman EL, Shay CF, Hasselmo ME. Malignant synaptic growth and Alzheimer's disease. Future Neurol. 2012;7:557–571. doi: 10.2217/fnl.12.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Klaver DW, Wilce MC, Cui H, Hung AC, Gasperini R, Foa L, Small DH. Is BACE1 a suitable therapeutic target for the treatment of Alzheimer's disease? Current strategies and future directions. Biol Chem. 2010;391:849–589. doi: 10.1515/BC.2010.089. [DOI] [PubMed] [Google Scholar]
- 134.May PC, Dean RA, Lowe SL, Martenyi F, Sheehan SM, Boggs LN, Monk SA, Mathes BM, Mergott DJ, Watson BM, Stout SL, Timm DE, Smith Labell E, Gonzales CR, Nakano M, Jhee SS, Yen M, Ereshefsky L, Lindstrom TD, Calligaro DO, Cocke PJ, Greg Hall D, Friedrich S, Citron M, Audia JE. Robust central reduction of amyloid-β in humans with an orally available, non-peptidic β-secretase inhibitor. J Neurosci. 2011;31:16507–16516. doi: 10.1523/JNEUROSCI.3647-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Jeppsson F, Eketjäll S, Janson J, Karlström S, Gustavsson S, Olsson LL, Radesäter AC, Ploeger B, Cebers G, Kolmodin K, Swahn BM, von Berg S, Bueters T, Fälting J. Discovery of AZD3839, a potent and selective BACE1 inhibitor clinical candidate for the treatment of Alzheimer disease. J Biol Chem. 2012;287:41245–41257. doi: 10.1074/jbc.M112.409110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Eketjäll S, Janson J, Jeppsson F, Svanhagen A, Kolmodin K, Gustavsson S, Radesäter AC, Eliason K, Briem S, Appelkvist P, Niva C, Berg AL, Karlström S, Swahn BM, Fälting J. AZ-4217: A high potency BACE inhibitor displaying acute central efficacy in different in vivo models and reduced amyloid deposition in Tg2576 mice. J Neurosci. 2013;33:10075–10084. doi: 10.1523/JNEUROSCI.1165-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Tamagno E, Bardini P, Guglielmotto M, Danni O, Tabaton M. The various aggregation states of beta-amyloid 1-42 mediate different effects on oxidative stress, neurodegeneration, and BACE-1 expression. Free Radic Biol Med. 2006;41:202–212. doi: 10.1016/j.freeradbiomed.2006.01.021. [DOI] [PubMed] [Google Scholar]
- 138.Buggia-Prevot V, Sevalle J, Rossner S, Checler F. NFkappaB-dependent control of BACE1 promoter transactivation by Abeta42. J Biol Chem. 2008;283:10037–10047. doi: 10.1074/jbc.M706579200. [DOI] [PubMed] [Google Scholar]
- 139.Guglielmotto M, Monteleone D, Giliberto L, Fornaro M, Borghi R, Tamagno E, Tabaton M. Amyloid-β42 activates the expression of BACE1 through the JNK pathway. J Alzheimers Dis. 2011;27:871–883. doi: 10.3233/JAD-2011-110884. [DOI] [PubMed] [Google Scholar]
- 140.Piccini A, Borghi R, Guglielmotto M, Tamagno E, Cirmena G, Garuti A, Pollero V, Cammarata S, Fornaro M, Messa M, Colombo L, Salmona M, Perry G, Tabaton M. β-amyloid 1-42 induces physiological transcriptional regulation of BACE1. J Neurochem. 2012;122:1023–1031. doi: 10.1111/j.1471-4159.2012.07834.x. [DOI] [PubMed] [Google Scholar]
- 141.Chami L, Buggia-Prévot V, Duplan E, Delprete D, Chami M, Peyron JF, Checler F. Nuclear factor-κB regulates βAPP and β- and γ-secretases differently at physiological and supraphysiological Aβ concentrations. J Biol Chem. 2012;287:24573–24584. doi: 10.1074/jbc.M111.333054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Oster-Granite ML, McPhie DL, Greenan J, Neve RL. Age-dependent neuronal and synaptic degeneration in mice transgenic for the C terminus of the amyloid precursor protein. J Neurosci. 1996;16:6732–6741. doi: 10.1523/JNEUROSCI.16-21-06732.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Nalbantoglu J, Tirado-Santiago G, Lahsaïni A, Poirier J, Goncalves O, Verge G, Momoli F, Welner SA, Massicotte G, Julien JP, Shapiro ML. Impaired learning and LTP in mice expressing the carboxy terminus of the Alzheimer amyloid precursor protein. Nature. 1997;387:500–505. doi: 10.1038/387500a0. [DOI] [PubMed] [Google Scholar]
- 144.Kim HS, Park CH, Cha SH, Lee JH, Lee S, Kim Y, Rah JC, Jeong SJ, Suh YH. Carboxyl-terminal fragment of Alzheimer's APP destabilizes calcium homeostasis and renders neuronal cells vulnerable to excitotoxicity. FASEB J. 2000;14:1508–1517. doi: 10.1096/fj.14.11.1508. [DOI] [PubMed] [Google Scholar]
- 145.Bittner T, Fuhrmann M, Burgold S, Jung CK, Volbracht C, Steiner H, Mitteregger G, Kretzschmar HA, Haass C, Herms J. Gamma-secretase inhibition reduces spine density in vivo via an amyloid precursor protein-dependent pathway. J Neurosci. 2009;29:10405–10409. doi: 10.1523/JNEUROSCI.2288-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Mitani Y, Yarimizu J, Saita K, Uchino H, Akashiba H, Shitaka Y, Ni K, Matsuoka N. Differential effects between γ-secretase inhibitors and modulators on cognitive function in amyloid precursor protein-transgenic and nontransgenic mice. J Neurosci. 2012;32:2037–2050. doi: 10.1523/JNEUROSCI.4264-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Saganich MJ, Schroeder BE, Galvan V, Bredesen DE, Koo EH, Heinemann SF. Deficits in synaptic transmission and learning in amyloid precursor protein (APP) transgenic mice require C-terminal cleavage of APP. J Neurosci. 2006;26:13428–13436. doi: 10.1523/JNEUROSCI.4180-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Lauritzen I, Pardossi-Piquard R, Bauer C, Brigham E, Abraham JD, Ranaldi S, Fraser P, St-George-Hyslop P, Le Thuc O, Espin V, Chami L, Dunys J, Checler F. The β-secretase-derived C-terminal fragment of βAPP, C99, but not Aβ, is a key contributor to early intraneuronal lesions in triple-transgenic mouse hippocampus. J Neurosci. 2012;32:16243–16255. doi: 10.1523/JNEUROSCI.2775-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Tamayev R, D'Adamio L. Inhibition of γ-secretase worsens memory deficits in a genetically congruous mouse model of Danish dementia. Mol Neurodegener. 2012;7:19. doi: 10.1186/1750-1326-7-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Loane DJ, Pocivavsek A, Moussa CE, Thompson R, Matsuoka Y, Faden AI, Rebeck GW, Burns MP. Amyloid precursor protein secretases as therapeutic targets for traumatic brain injury. Nat Med. 2009;15:377–379. doi: 10.1038/nm.1940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Mannix RC, Zhang J, Park J, Lee C, Whalen MJ. Detrimental effect of genetic inhibition of β-site APP-cleaving enzyme 1 on functional outcome after controlled cortical impact in young adult mice. J Neurotrauma. 2011;28:1855–1861. doi: 10.1089/neu.2011.1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Mullane K, Williams M. Alzheimer's therapeutics: continued clinical failures question the validity of the amyloid hypothesis-but what lies beyond? Biochem Pharmacol. 2013;85:289–305. doi: 10.1016/j.bcp.2012.11.014. [DOI] [PubMed] [Google Scholar]
- 153.Braak H, Del Tredici K. Amyloid-β may be released from non-junctional varicosities of axons generated from abnormal tau-containing brainstem nuclei in sporadic Alzheimer's disease: a hypothesis. Acta Neuropathol. 2013;126:303–306. doi: 10.1007/s00401-013-1153-2. [DOI] [PubMed] [Google Scholar]