Abstract
Intraspecific male-male aggression, important for sexual selection, is regulated by environment, experience and internal states through largely undefined molecular and cellular mechanisms. To understand the basic neural pathway underlying the modulation of this innate behavior, we established a behavioral paradigm in Drosophila melanogaster and investigated the relationship between sexual experience and aggression. In the presence of mating partners, adult male flies exhibited elevated levels of aggression, which was largely suppressed by prior exposure to females via a sexually dimorphic neural mechanism. The suppression involved the ability of male flies to detect females by contact chemosensation through the pheromone-sensing ion channel, ppk29, and was mediated by male specific GABAergic neurons acting upon GABA-a receptor RDL in target cells. Silencing or activation of this circuit led to dis-inhibition or elimination of sex-related aggression, respectively. We propose that the GABAergic inhibition represents a critical cellular mechanism that enables prior experience to modulate aggression.
Aggression is an innate behavior important for both individual survival and group fitness. While animals display a wide variety of aggressive behavior with species- and gender-specific sensory input and motor output patterns1, 2, the level of aggression is largely defined by the environment, prior experience and internal states of the animal3. These external and internal factors may influence common underlying mechanisms associated with the balance of survival and reproductive needs of individuals2 and modulate the behavioral output accordingly. To understand the central mechanism underlying the regulation of aggression, we investigated the modulation of intra-specific aggression by prior experience in Drosophila melanogaster, a useful model for genetic studies of aggression4-7.
When encountering opponents in competition for territory, food and mating partners, male fruit flies display stereotypical aggressive behavior, quantifiable by both human inspection and computer surveillance8, 9. Previous studies have demonstrated that specific sensory inputs elicit or temper aggression depending on the social context8, 9. For example, acute exposure of the male specific pheromone 11-cis-vaccenyl acetate (cVA) promotes aggression when one male encounter another male5, while chronic cVA exposure during long-term social grouping reduces aggression6. However, the central neural pathway that is responsible for the execution of the behavior, as well as the mechanism that allows experience to modify the intensity and duration of the behavior remains elusive. As one main motivation of male aggression is to win over mating partners10, the presence of females generally elicits elevated levels of male-male aggression. Indeed, we found that naïve males without prior exposure to females were more aggressive towards each other in the presence of virgin females. Remarkably, our behavioral analyses demonstrated that prior contact with females strongly modified the subsequent behavioral choice of males and drastically suppressed this sex-related male-male aggression. This new paradigm, combined with recent advances in circuit mapping techniques in Drosophila, allowed us to study the basic cellular and molecular events involved in the experience-dependent modulation of sex-related aggression, and revealed a strong inhibitory mechanism mediated by GABA signaling pathways.
RESULTS
Suppression of male aggression by prior sexual experience
To assess male-male aggression, we recorded actions of a pair of wild-type male flies for two hours and quantified intensive aggressive interactions as previously described8, 9 (Fig. 1a, b). We found very similar levels of aggression quantified based on either duration or frequency of the fighting episodes (Fig. 1c and Supplementary Fig. 2c) and the differences among groups could be readily detected within a 30-minute window (60 to 90 min after introducing the flies into the test chamber) (Fig. 1b, c and Supplementary Fig. 2c). Therefore, we used the duration of aggressive behavior displayed during this 30-minute window as a measure of aggression in the following analyses.
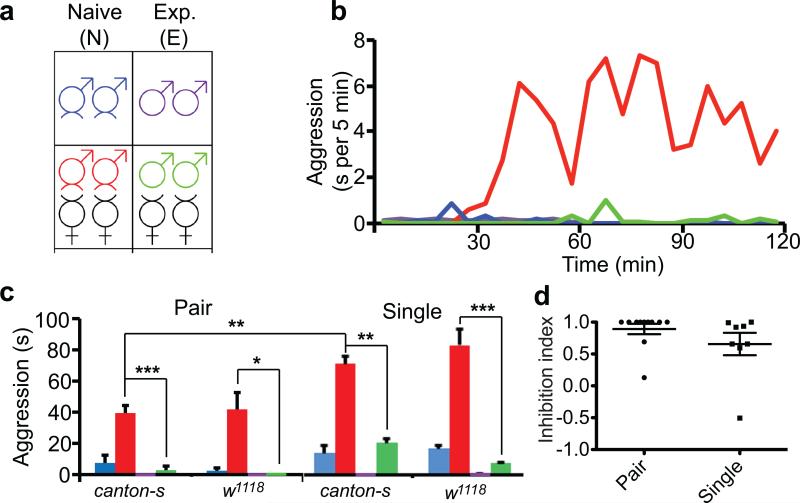
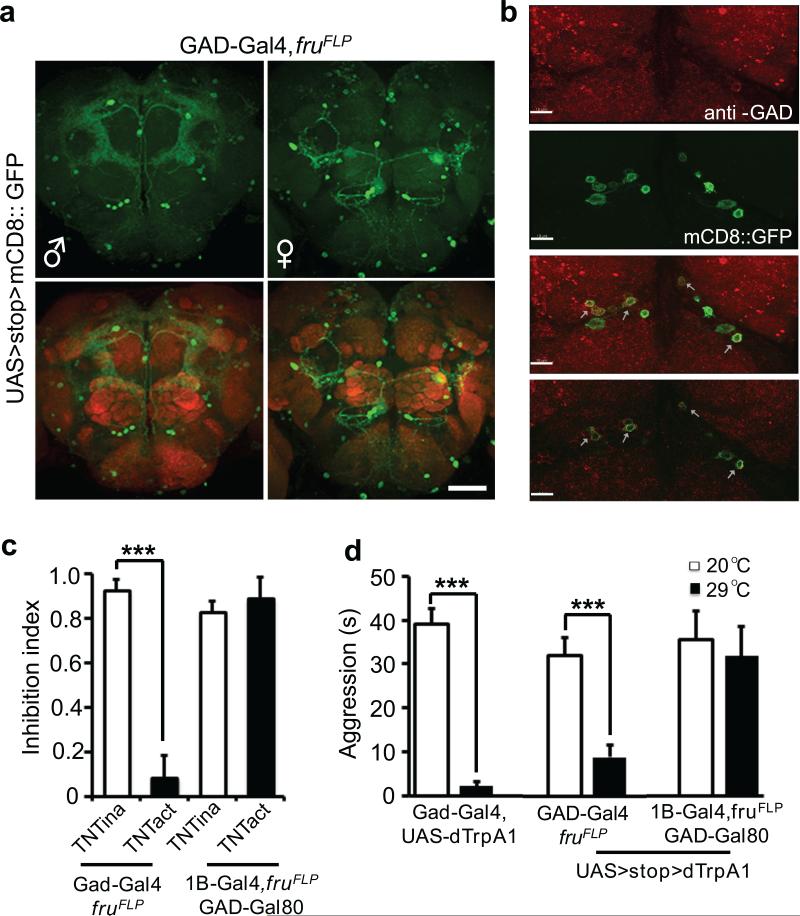
Figure 1. Prior female experience inhibits sex-related male-male aggression.
(a) Scheme for the aggression assay, four conditions are shown with color coding as naïve male only (blue), experienced male only (purple), naïve male + virgin female (red), experienced male + virgin female (green). (b) Timeline of the average aggression duration of wild-type Canton-S (cs) males within 2 hours. Each data point represents average aggression duration quantified in 5 min intervals. The lack of aggression within the first 30 min reflects courtship behavior. (n = 4 pairs for each condition). (c) Average aggression duration of cs and w1118 males under different conditions. Both genotypes showed female experience induced inhibition of aggression in pair-or single-housed settings. (n = 11 and 6 for cs and w1118 in pair-housed, 8 and 7 in single-housed). Aggression interactions 60-90 min after the onset were quantified. p <0.0001 (***), =0.019 (*), =0.0084 (**), =0.0059 (**), <0.0001 (***) . (d) The inhibition index, defined as (AggressionN – AggressionE) / AggressionN, for pair- and single-housed settings. (n = 11 and 8 for pair and single.) p = 0.1989. Student's t-test. *: p<0.05, **: p <0.01, ***: p <0.001. Error bars denote s.e.m.
To address the influence of experience on male-male aggression, male flies were subjected to social and/or sexual experiences then tested for their level of aggression in a sex-related context. The conditions we used were as the following (Fig. 1a and Supplementary Fig. 1): 1. Pair housed vs. single housed flies. Newly eclosed males were housed either in pairs or by itself for 7 days before aggression assays. The pair housing allowed long-term male-male social interactions and reduced the baseline aggression as previously reported11 (Fig. 1b, c). 2. Aggression with or without sexual context. During the aggression assay, we tested males with or without the presence of virgin females (Fig. 1a and Supplementary Fig. 1). In the absence of females, males displayed limited aggression (blue), which may reflect aggression over territory/food (Fig. 1b, c). In the presence of virgin females (red), males first exhibited courtship behavior and copulated with virgin females within 30–40 min, then displayed elevated aggressive behavior towards the other male (Fig. 1b, c), which we regarded as sex-related aggression. 3. Experienced vs. naïve flies. To introduce prior sexual experience, males were housed either in pairs or singly for 6 days and then housed with virgin females for 24 hours before aggression assays (experienced males) or had no female contact at all (naïve males). Notably, the presence of females during the aggression assay significantly elevated male-male aggression for naïve males (red vs. blue) but failed to do so for experienced males (green vs. purple) (Fig. 1b, c and Supplementary Video 1), indicative of an inhibitory effect of prior sexual experience on aggression in males.
Notably, our experiments indicated that the inhibition was specifically acting upon the sex-related aggression. We observed that naïve males showed enhanced aggression only with the female presence. There was no significant difference in the aggression levels between naïve and experienced flies when there is no female present during aggression assay (blue vs. purple) (Fig.1b, c). In addition, if the females were removed after copulation during the aggression assay, the males no longer exhibited aggressive behavior (Supplementary Fig. 5). Furthermore, virgin females were more effective than mated females in inducing aggression (Supplementary Fig. 2a). This is not due to the lack of interest of males in courting mated females, but – on the contrary – because males spent most of the time courting the unreceptive mated females, as demonstrated by their high courtship index measured at 70-75 min (Supplementary Fig. 2b), leaving them with little time for aggressive interactions. In the case of virgin females, after copulation, the males exhibited limited re-mating attempts (Supplementary Fig. 2b), coincident with the elevated engagement of aggression. These observations raise the possibility of a guarding behavior in these males after successful courtship encounter with their mating partners.
This hypothesis warrants further investigation in future studies. The inhibition was quantified via the inhibition index, defined as (AggressionN – AggressionE) / AggressionN, which was consistently close to 1 for most of the genotypes tested (Fig. 1d and 2). Thus, prior experience with females largely suppressed sex-related male-male aggression. The suppression on sex-related aggression appeared to be specific for the aggressive behavior; it is not an artifact due to the fatigue exhibited by the experienced males after sexual interactions with females for 24 hours, because our analyses of the courtship index and climbing index revealed no differences between the experienced and naive male flies (Supplementary Fig. 3a). Previous report has suggested that prior fighting experience will influence the likelihood for that male fly to initiate subsequent fights as well as the outcomes, reminiscent of the establishment of dominance in animals with social hierarchy12. However, the inhibition index was comparable for the single or pair raised flies (Fig. 1d), indicating any hierarchy established between males housed in pairs has little to do with this inhibition of sex-related male aggression. Moreover, when we brought together two male flies that were housed in pairs but not with each other (mixed group), so that they had social experiences but no established hierarchy with each other, they exhibited as much inhibition of the sex-related aggression as did males that were raised together (Supplementary Fig. 3b). Taken together, these observations reveal that previous exposure to females suppressed the sex-related aggression of males regardless of social interactions between the males.
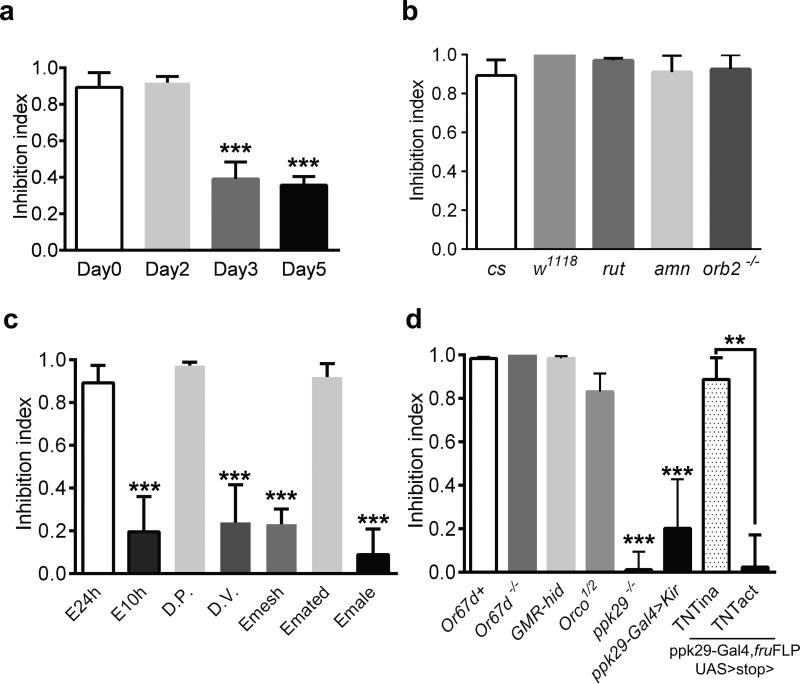
Figure 2. Prior female-contacts dependent inhibition of aggression is long term and requires chemosensation through the pheromone sensing channel ppk29.
(a) The inhibitory effect of prior female experience on aggression lasted up to two days. The experienced (E) male flies were housed with females for 24 hours and were then separated from the females and reared for 0, 2, 3 or 5 days until behavior assay. (n = 11, 6, 7 and 5 for each manipulation). (b) Memory mutants had no defects in female experience induced inhibition. Inhibition index was comparable among cs, w1118, rut, amn and Orb2k/o flies. (n = 11, 6 ,4, 4 and 4 for each genotype). (c) Inhibition index for various conditioning paradigms. (n = 11, 6, 5, 6, 5, 5 and 7). (d) Inhibition of aggression by female experience was impaired specifically in mutants lacking ppk29. Compared to genotypes with defects in pheromone sensation, vision or olfaction, only ppk29–/–, ppk29-Gal4>Kir and ppk29-Gal4, fruFLP, UAS>stop>TNTact flies showed significant reduction of inhibition index (n = 5, 4, 4, 4, 6, 5, 5 and 5 for each genotype). Genotypes tested were as indicated. TNTina and TNTact denote the inactive and active forms of TNT transgenes respectively. (a-d) One-way ANOVA followed by Bonferroni's multiple comparison test. (d, last two genotypes) Student's t-test. p = 0.0013 (**). **: p<0.01, ***: p<0.001. Error bars denote s.e.m.
Notably, the level of sex-related aggression remained suppressed for two days after the 24-hour exposure to females, and recovered in about 3-4 days (Fig. 2a and Supplementary Fig. 4a). However, this long lasting inhibition did not seem to involve previously identified machineries for learning and memory, because classical memory mutants such as rut and amn, and a courtship conditioning mutant, Orb2 knockout flies13, all had similar inhibition index comparable to that of the wild-type controls (Fig. 2b and Supplementary Fig. 4b).
Prior female contacts led to inhibition of aggression
Clearly, the female exposure had strong influences on male flies’ subsequent behavior choice. To define the nature of the prior female exposure or the sexual experience that induced the inhibition, we carried out a series of behavioral tests. Significant inhibition of male aggression was induced by housing the males for more than 10 hours with females, but not with males (Fig. 2c and Supplementary Fig. 5). Importantly, mating was not a necessary part of the experience, since aggression was suppressed by prior exposure to both virgin females and mated females, as well as virgin females expressing membrane-bound sex-peptide, causing them to reject the male's attempts to mate14, 15. Nor was mating sufficient to suppress aggression; if females were removed shortly after mating, the mated males behaved like naïve males and remained aggressive (Supplementary Fig. 5). Moreover, male aggression was suppressed by prior exposure with virgin females from D.pseudoobscura (D.P), but not D.virilis (D.V), which is more distantly related and possess different body hydrocarbon profiles16, even though these Drosophila melanogaster males exhibited courtship interest towards females of both species, indicating a role of chemosensation in the female experience (Fig. 2c and Supplementary Fig. 5). Lastly, no inhibition of male aggression resulted from transferring males into vials that had previously been occupied by virgin females, or housing males with females that were prevented from contacts by the males due to the placement of a nylon mesh during the 24 hour experience period. (Fig. 2c and Supplementary Fig. 5). Therefore, the prior experience with females that led to inhibition of aggression required direct physical contact between the male and female during courtship regardless whether there was copulation.
ppk29-chemosensation is required for aggression inhibition
To identify the sensory pathway for this female-contacts-induced behavioral modulation, we used genetic approaches by screening a number of mutants, including Or67d knockout flies which are unable to sense the male pheromone cVA17, 18, GMR-hid flies with compound eyes eliminated by cell death gene expression via an eye-specific promoter19, Orco knockout flies with defective olfactory function that disrupts their behavioral and electrophysiological responses to a wide range of odors20, and ppk29 knockout flies with defects in sensing female pheromones through direct physical contacts21. Remarkably, inhibition of male aggression was only reduced by mutation of the recently identified pheromone sensing sodium channel, ppk29, which is expressed in a specific group of sensory neurons innervating the sensory bristles on the legs of male flies (Fig. 2d and Supplementary Fig. 4c). Moreover, silencing the ppk29 neurons, through the expression of the potassium channel Kir2.122 also impaired the inhibition of aggression (Fig. 2d and Supplementary Fig. 4c). Furthermore, blocking synaptic transmission of the ppk29 expressing male specific sensory neurons specifically through the combination of the fruFLP allele and the active form of the Tetnus toxin (TNTact) transgene, which restricted the expression to just the ppk29+, fru+ neurons21, 22, caused a similar loss of inhibition whereas the control group that expressed the inactive form of the toxin (TNTina) exhibited no impairment of inhibition (Fig. 2d and Supplementary Fig. 4d).
Previous studies have shown that cuticular hydrocarbons act as pheromones and serves critical functions in sex and species recognition for Drosophila23. These genetic manipulations of male sensory pathways, taken together with multiple tests that illustrated the importance of direct physical contact and the female body hydrocarbon profile in inhibitory effects, suggested that sex-related male-male aggression is inhibited by prior female experience through contact dependent chemosensation.
fru+, d5-HT1B+ neurons mediates inhibition of aggression
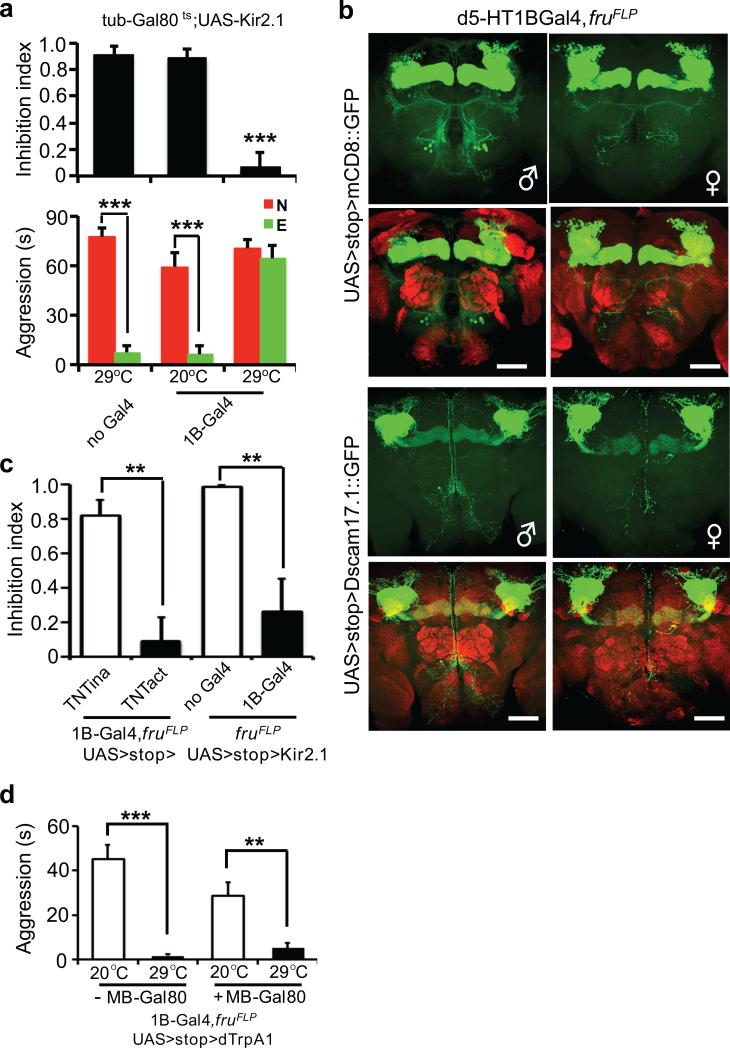
Whereas the neural circuit controlling aggression remains unidentified in Drosophila, our observation of the experience-dependent inhibition of sex-related aggression provided an entry point to look into the central control of the aggressive behavior, as the identification of the inhibitory mechanism and its targets could eventually lead to the discovery of the main components of the aggression circuit. To search for neurons in the central brain that receive inputs from the periphery and mediate the female-contacts-induced inhibition, we screened a number of enhancer driver lines with Gal4 active in distinct groups of neurons in the fly nervous system, driving the expression of the potassium channel Kir2.1 and the temperature sensitive Gal80ts, for spatially and temporally controlled neuronal silencing24. We kept the flies at 20 °C and then raised the temperature to 29 °C for 2 days prior to the assay, in order to inactivate the Gal80ts and silence the Gal4 expressing cells through the expression of Kir2.1, and looked for those Gal4 lines that produced a dis-inhibition phenotype, the loss of inhibition by prior female encounter (Supplementary Fig. 1b). Among the enhancer driver lines we tested, only one, d5-HT1B-Gal4, exhibited the dis-inhibition phenotype without affecting the courtship activity or baseline aggression (Fig. 3a and Supplementary table). This enhancer line was generated using the upstream regulatory region of Drosophila serotonin receptor 1B (d5-HT1B), and is expressed in about 3000 neurons in the fly brain25.
Figure 3. fru+, d5-HT1B+ sexually dimorphic neurons mediate female experience dependent inhibition of aggression.
(a) Temperature shift induced silencing of d5-HT1B+ neurons resulted in dis-inhibition of aggression, without affecting baseline aggression. (n = 5, 5 and 6 for each genotype). (b) fru+, d5-HT1B+ neurons in the adult brain labeled by mCD8::GFP and Dscam17.1::GFP, including γ-neurons of the mushroom bodies and a cluster with soma located between antennal lobes and the SOG region. The sexual dimorphism was evident with more cells and more elaborated arbors in male brains as compared to female brains. nc82 co-staining (red) showed the neuropil. Scale bar = 40 μm. (c) Blocking chemical transmission by a TNT transgene or electrically silencing by a Kir2.1 transgene expression in the fru+, d5-HT1B+ neurons led to strong dis-inhibition of aggression. (n = 6, 6, 5 and 7 for each genotype). p = 0.0012 (**), =0.0099 (**). (d) Inducible activation of fru+, d5-HT1B+ neurons by a heat-activated channel dTrpA1 led to reduced baseline aggression at 29°C as compared to the 20°C control. This reduction of baseline aggression persisted when the mushroom body neurons were excluded using MB-Gal80. The genotype for the manipulation is w+; UAS>stop>dTrpA1; fruFLP/d5-HT1B-Gal4, without or with MB-Gal80. (n = 5 for each genotype). p <0.0001 (***), =0.0049 (**). (b upper) One-way ANOVA followed by Bonferroni's multiple comparison test. (b lower, d, e) Student's t-test. *: p<0.05, **: p<0.01, ***: p<0.001. Error bars denote s.e.m.
To identify the relevant cells within this large group of neurons expressing d5-HT1B-Gal4, we tested the hypothesis that the male aggression circuit is sexually dimorphic and its cellular components are genetically defined by the sex-determinant-gene, fruitless (fru). Previous studies have identified fru+ neurons that vary in number and arborization patterns in the brain of adult male and female flies22, 26. fruFLP, an allele generated by targeting a flippase transgene into the fru locus, is an effective genetic tool to label and manipulate fru+ neurons22. Using the fruFLP allele in combination with d5-HT1B-Gal4, we were able to identify a small number of fru+, d5-HT1B+ neurons in male flies, including γ-neurons of mushroom bodies, a cluster of neurons located between antennal lobes and the subesophageal ganglion (SOG) region (~20), and a single neuron in the ventral nerve cord (VNC) (Fig. 3b, Supplementary Fig. 6a, c). A comparison of tissues collected from male and female flies 7 days after eclosion revealed that there were fewer fru+, d5-HT1B+ neurons in the female brain and VNC, and these sexually dimorphic neurons in female brains had much less elaborate arbors, labeled with mCD8::GFP or Dscam17.1::GFP fusion protein22, in female brains as compared to those in male brains (Fig. 3b and Supplementary Fig. 6c). Importantly, when we silenced fru+, d5-HT1B+ neurons in males by expressing TNT or Kir2.1, it caused a strong dis-inhibition phenotype, suggesting that these neurons are responsible for the experience-induced inhibition of aggression (Fig. 3c, Supplementary Fig. 7a, Supplementary Video 2 and 3). To further test this hypothesis, we asked whether activation of these fru+, d5-HT1B+ neurons could eliminate aggression. Indeed, expression of the heat-activated ion channel dTrpA122, 27 in the fru+, d5-HT1B+ neurons of males greatly reduced the baseline sex-related aggression at 29°C, as compared to the 20°C control conditions (Fig. 3d, Supplementary Video 4 and 5). These observations indicate that fru+, d5-HT1B+ neurons in male brains are critical for female experience-induced inhibition of male-male aggression; silencing these neurons led to dis-inhibition while their activation suppressed aggression.
Which subsets of the fru+, d5-HT1B+ neurons may mediate female-contacts-induced inhibition of aggression? Could it be the γ-neurons of mushroom bodies? Mushroom bodies have been implicated in sensory integration, learning and memory, and regulation of behaviors such as locomotion and sleep13. Using a Gal80 transgene expressed under the mushroom body specific enhancer (MB-Gal80)28, we tested if it could block the inhibitory effect caused by activation of fru+, d5-HT1B+ neurons. MB-Gal80 effectively inhibited mushroom body neurons from expressing transgenes such as mCD8::GFP (Supplementary Fig. 6b). However, at 29°C, the flies’ baseline aggression was inhibited to the same extent with either dTrpA1 activation of all fru+, d5-HT1B+ neurons, or dTrpA1 activation of those fru+, d5-HT1B+ neurons that are not in mushroom bodies (Fig. 3d). It thus appears the cluster of neurons above the SOG region in the brain and possibly the lone neuron in the VNC, but not the mushroom body neurons, are critical for female-contacts-induced inhibition of aggression.
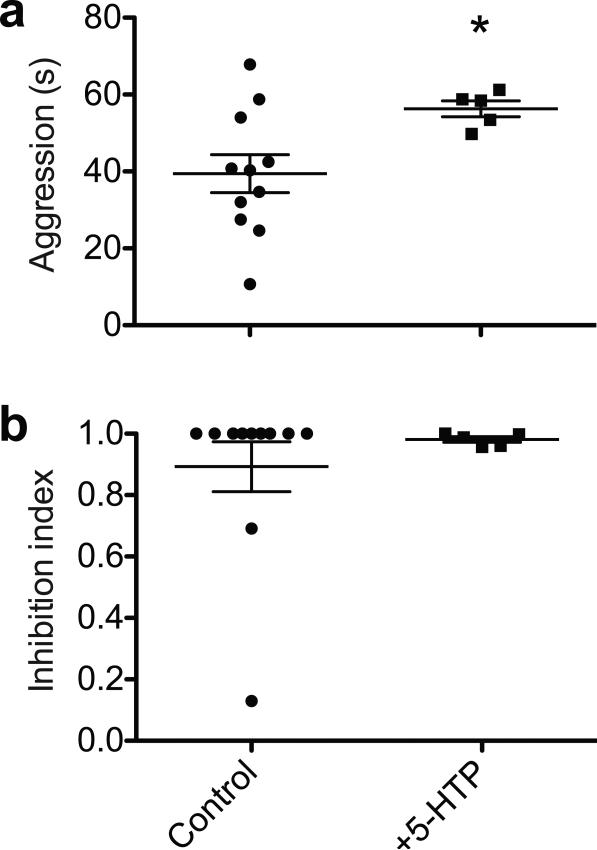
Since the fru+, d5-HT1B+ neurons express a subtype of serotonin receptor, and serotonin has been linked to aggressive behaviors in both mammals and Drosophila29, 30, we wondered if the serotonergic mechanism is involved in the female-contacts-induced inhibition of aggression. We first administered the serotonin synthesis precursor 5-hydroxy-tryptophan (5-HTP), which elevates the serotonin level in flies and leads to increased baseline aggression30. We found that using our paradigm, 5-HTP treatments also enhanced baseline sex-related aggression but did not alter the inhibition index significantly (Fig. 4a, b). Moreover, the inhibition of male aggression was not altered by d5-HT1B receptor overexpression (UAS-d5-HT1B) or knockdown (UAS-d5-HT1B-RNAi) using the pan-neuronal driver elav-Gal4 (data not shown). It thus appears that the serotonergic system is not involved in the female-contacts-induced inhibition of aggression.
Figure. 4. Ectopic activation of the serotoninergic circuit elevates baseline aggression without affecting female-contacts dependent inhibition of aggression.
5-HTP treatment elevated baseline sex-related aggression without affecting the female-contacts dependent inhibition of aggression. (n = 11 and 5 for each condition). *: p = 0.0424. Student's t-test. Error bars denote s.e.m.
GABAergic neurotransmission mediates aggression inhibition
We further tested other neurochemical systems including dopaminergic, octopaminergic, peptidergic and GABAergic systems by using both behavioral and anatomical approaches (Supplementary Table and Fig. 5d), through which we identified the GABAergic system as the candidate for regulating aggression in flies. Aggression studies using mammalian models as well as observations in human patients have implicated the GABAergic system in the modulation of aggression3. However, there is no consensus on the role of GABA in aggression.
Figure 5. Female experience acts through GABAergic neurotransmission in fru+, GABA+ and d5-HT1B+ neurons to inhibit aggression.
(a) fru+, GABA+ neurons display sexual dimorphism in the adult brain, especially in the region above the SOG, that show difference both in the cell number and the projection pattern in female and male brains. nc82 co-staining (red) showed the neuropil. Scale bar = 40 μm. (b) A subpopulation of the fru+, d5-HT1B+ neurons (green) above the SOG region is labeled with anti-GAD antibody (red), indicated by arrows, suggesting these neurons are GABAergic. Top three panels are representative max projection images and the bottom panel is an image from a single confocal optical section. Scale bar = 10 μm. (c) Blocking neurotransmission by TNT in GABA+, fru+ neurons led to dis-inhibition of aggression. Whereas inhibition of fru+, d5-HT1B+ neurons by TNT resulted in dis-inhibition (see Fig. 3c), adding Gad-Gal80 abolished this effect, suggesting that the GABAergic fru+, d5-HT1B+ neurons are responsible for the dis-inhibition of aggression. (n = 5 for each genotype). (d) Activation of GABA+ neurons or GABA+, fru+ neurons by dTrpA1 at 29°C suppressed the baseline aggression. Whereas activation of fru+, d5-HT1B+ neurons by dTrpA1 suppressed the baseline aggression (see Fig. 3d), adding Gad-Gal80 abolished this reduction, suggesting that the GABAergic fru+, d5-HT1B+ neurons are responsible for the reduction of baseline aggression. (n = 5, 5, 5, 6, 7 and 4 for each genetype). ***: p<0.001. Student's t-test. Error bars denote s.e.m.
In Drosophila, GABAergic interneurons are involved in the modulation of olfactory perception31, learning and memory32, and sleep33. To test if GABA is also involved in the modulation of aggression in flies, we used a Gal4 line driven by the Gad (Glutamic acid decarboxylase 1) enhancer to identify and manipulate the potential GABAergic neurons. We also used the fruFLP allele to specifically label the subset of fru+ neurons that are GABAergic. Similar to fru+, d5-HT1B+ neurons, fru+, GABA+ neurons showed sexual dimorphisms in both the number and projection patterns, with a wide distribution in the adult brain and VNC, including clusters of neurons localized below antennal lobes and above the SOG region (Fig. 5a and Supplementary Fig. 8). Immunohistochemical studies using anti-GAD antibody showed that some of the fru+, d5-HT1B+ neurons in the brain were GAD positive (Fig. 5b, arrows), but not the lone fru+, d5-HT1B+ neuron in the VNC (Supplementary Fig. 6c), raising the possibility that the clusters of fru+, GABA+ neurons above the SOG region may correspond to those fru+, d5-HT1B+ neurons implicated in mediating experience-induced inhibition. Indeed, behavioral analyses of the prior female-contacts-induced inhibition of aggression demonstrated that blocking chemical transmission in fru+, GABA+ neurons led to dis-inhibition (Fig. 5c and Supplementary Fig. 7b), while activating these neurons suppressed baseline aggression (Fig. 5d). We also tested a Gal80 transgene driven by the Gad enhancer (Gad-Gal80) in combination with d5-HT1B-Gal4 and fruFLP. Gad-Gal80 effectively eliminated behavioral effects generated by either silencing (Fig. 5c and Supplementary Fig. 7b) or activating fru+, d5-HT1B+ neurons (Fig. 5d). Thus, the cluster of GABAergic fru+, d5-HT1B+ neurons located above the SOG (approximately 8–10 neurons per hemisphere) are likely to be responsible for the experience-induced inhibition of aggression.
RDL as a target for regulation of aggression by experience
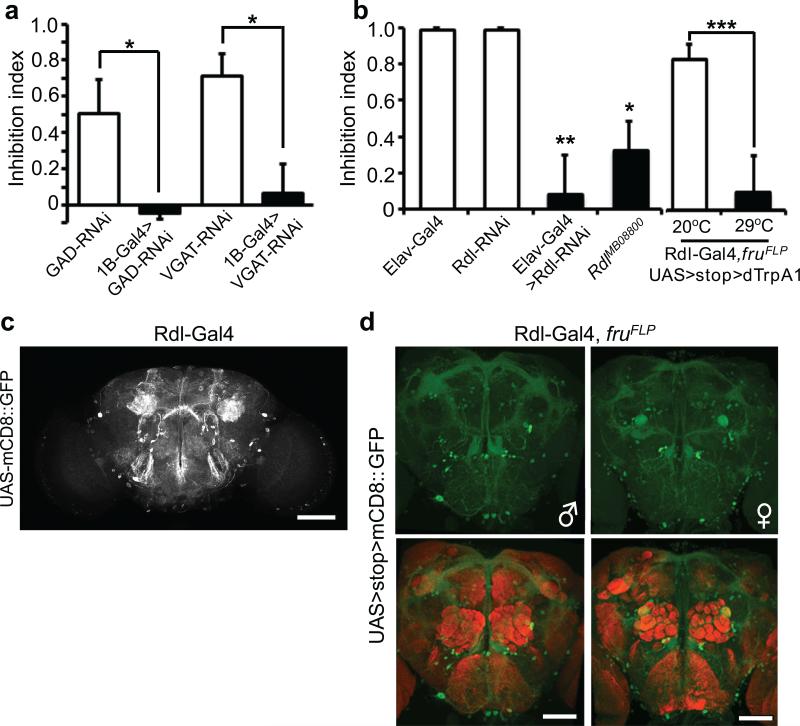
Finally, we confirmed the involvement of GABA neurotransmission in the GABAergic fru+, d5-HT1B+ neurons in the inhibition of aggression, by the genetic manipulation of GABA level in the d5-HT1B+ neurons. Knocking down the expression of either the GABA synthesis enzyme GAD, or the vesicular GABA transporter for GABA reuptake, VGAT, specifically in d5-HT1B+ neurons, produced the disinhibition phenotype, similar to the effects observed through silencing the d5-HT1B+ neurons, suggesting the release of GABA from d5-HT1B+ neurons is important for the inhibition of male aggression (Fig. 6a and Supplementary Fig. 9a).
Figure 6. GABA neurotransmission, the GABA-a receptor RDL and Rdl+, fru+ sexually dimorphic neurons mediate female experience dependent inhibition of aggression.
(a) Blocking GABA neurotransmission in d5-HT1B+ neurons using RNAi against GAD or VGAT led to dis-inhibition of aggression. (n = 5, 4, 5 and 4 for each genotype). p = 0.0359 (*), =0.0139 (*). (b) Inhibition of aggression was reduced in flies with Rdl knockdown pan-neuronally (elav-Gal4>UAS-Rdl RNAi), or with the hypomorphic allele RdlMB08800 caused by P-element insertion. (n = 4, 4, 5 and 5 for each genotype). Activation of fru+, Rdl+ neurons by dTrpA1 at 29°C resulted in dis-inhibition of male-male aggression induced by female contacts. (n = 8 and 6). (c) Neurons labeled by Rdl-Gal4 are widely distributed in the brain. Scale bar = 40 μm. (d) Sexual dimorphisms of fru+, Rdl+ neurons in the fly brain. Representative images of the male and female brains are shown. nc82 co-staining (red) showed the neuropil. Scale bar = 40 μm. (a, b, last two genotypes) Student's t-test, (b) One-way ANOVA followed by Bonferroni's multiple comparison test. *: p<0.05, **: p<0.01, ***: p<0.001. Error bars denote s.e.m.
To search for the downstream cellular and molecular targets of GABA-mediated inhibition of male-male aggression by prior encounters with females, we screened through all GABA receptors using the pan-neuronal RNAi approach. Resistant to dieldrin (Rdl) loss of function showed the most severe defects of inhibition of aggression, while GABA-b-R1/R2/R3 and Lcch3 showed mild to no disinhibition (Fig. 6b and data not shown). Therefore, we focused our analyses on RDL, a GABA-a receptor for the GABAergic inhibition underlying olfactory learning and sleep regulation31-33. Flies with reduced levels of RDL, due to either pan-neuronal RNAi knockdown driven by the elav-Gal4 or the hypomorphic allele arising from a P-element insertion, RdlMB08800, showed the dis-inhibition phenotype (Fig. 6b, Supplementary Fig. 9b and Supplementary Fig. 9c). We further carried out anatomical and behavioral studies using a line with Gal4 expression driven by the upstream regulatory region of Rdl (Rdl-Gal4)34 in combination with fruFLP. Because silencing the large number of fru+, Rdl+ neurons (Fig. 6c, d) via TNT or Kir2.1 caused lethality or severe courtship defects, it was difficult to assess female-contacts-induced inhibition of aggression of these flies. Nonetheless, dTrpA1 expression in these neurons led to dis-inhibition of aggression at 29°C, suggesting that activation of fru+, Rdl+ neurons could overcome the GABAergic inhibition of aggression by the prior female encounter (Fig. 6b and Supplementary Fig. 9d). We have also attempted to search for a subgroup of fru+, Rdl+ neurons as the target of the GABAergic inhibition, by studying the relationship between the dendrite branching pattern of fru+, Rdl+ neurons and the GABA distribution in the male fly brain and VNC (Supplementary Fig. 8b). However, these attempts were unsuccessful owing to the wide distribution of GABA system in the fly nervous system. Future studies that employ more sophisticated genetic manipulations combined with the behavioral analyses may help in further delineating the circuitry.
DISCUSSION
Aggression is a complex behavior regulated by various internal and external stimuli. However, studies thus far remain largely focused on sensory pathways involved in regulating the baseline aggression, with much less understanding of the central components of the underlying neural pathway. Moreover, the close relationship between sex and aggression has been a fascinating topic in biology as well as literature, but their intertwined nature and the underlying neurobiological basis have remained elusive. In this study, using a behavioral genetics approach, we report the identification of a novel neural pathway underlying the modulation of sex-related male-male aggression in Drosophila by prior contacts with females.
Our study suggests that prior female encounter through direct physical contacts activates the pheromone sensing ppk29 neurons, and causes inhibition of the central aggression circuit through GABAergic mechanisms involving the RDL GABA-a receptor, thereby suppressing the behavioral output for male-male aggression (Fig. 9e). The three levels of the neural pathway involved in this experience-dependent behavior modification all exhibit sexual dimorphism, consistent with the notion that morphological differences in male and female brains correlate with their distinct behavioral needs. We were able to modify the aggressive behavior output by manipulating the circuit at each of these three steps, which possibly represented the sequence of the information relay involved in the native behaviors, the sensory input, the information processing, and the execution of the behavior. However, we do recognize that these circuit components elucidated in our studies are clearly only a part of the machinery responsible for aggression modulation. In addition, by identifying RDL as a molecular target for aggression regulation, our study has provided an entry point for characterizing the missing link of aggression studies, namely the central neurons that respond to experience-dependent modulation and mediate the execution of aggressive behaviors. Thus, our work provides new insights regarding the intricate interactions between sexual experience and aggression, and delineates the underlying mechanisms to inform potential means to suppress excessive aggression.
The female-contacts-dependent suppression of male aggression may also be viewed as a form of learning induced plasticity. The learning paradigm in this case requires extended physical interactions between the male and the female (over 10 hours), which could consist of repeated sessions of male courtship attempts and female rejection. Since we did not observe obvious defects in aggression suppression in a group of genetic mutants with deficits in courtship conditioning35, such as homer, eag, Shaker and orb2 mutants (Fig. 2b and data not shown), this experience-induced suppression of aggression is likely different from the conventional courtship conditioning. Another interesting feature of this suppression is that it is long term, yet reversible, lasting up to two days after the female encounter. However it also differs from the well-studied long-term memory formation (LTM), as no defect was observed in amn mutants, which is required for LTM36. Our results implicate the fru+ d5-HT1B+ and GABA+ cluster of neurons in the central brain as the regulator of this suppression, but it remains to be determined whether these neurons are involved in the initiation, acquisition, execution or consolidation phase(s) of this behavior, and what takes form as the underlying “memory trace”, whether plasticity is manifested at the level of the number of neurons activated, neurite arborization, neuronal activity, or some other aspects of neuronal signaling.
Notwithstanding the emergence of Drosophila as a successful genetic model for aggression studies2 and the extensive characterization of its stereotypical motor display of aggression10, 37, the strong influence of genetic background over baseline aggression and locomotor activity often complicates Drosophila aggression studies. Our paradigm avoids such difficulties by consistently eliciting aggression in naïve male flies in the presence of females, and by inducing a strong suppression of aggression in males with prior female encounter. The small variations among different genetic backgrounds in our behavioral paradigm have made it possible to identify critical cellular and molecular components involved in the regulation of aggression by experience.
One purpose of studying aggression regulation in animal models is to eventually understand the basis of human violence and establish venues to reduce or prevent it. Psychophysiological studies suggest that the failure to maintain an appropriate level of aggression in humans is associated with impaired executive cognitive processes or emotion registration2. As an innate behavior built largely on predetermined neural pathways, aggression in Drosophila males can be modulated by prior exposure to females through GABAergic inhibition. Our study raises the possibility that an ancient and basic machinery of the central neuronal circuitry, GABAergic inhibition could be part of a conserved mechanism to modulate the level of aggression in males and ensure proper balance between reproductive competition and individual survival.
ONLINE METHODS
Fly stocks and rearing conditions
Fly stocks are maintained in the standard medium in a circadian and humidity controlled 25°C incubator, unless otherwise noted. Wild type, mutant and transgenic fly lines used are from the following sources: Canton-S was from R. Greenspan; GMR-hid was from J. Blau; MB-Gal80 was from S. Waddell; Gad1-Gal4 and UAS-Rdl-RNAi were from L. Griffith; ΔOrb2 was from K. Keleman; Orco1/2 was from L. Voshall; ppk29k/o and ppk29-Gal4 were from K. Scott; Or67d+, Or67dk/o, fru-Gal4, fruFLP, UAS>stop>mCD8::GFP, UAS>stop>Dscam17.1::GFP, UAS>stop>TNTactive, UAS>stop>TNTinactive, UAS>stop>Kir2.1 and UAS>stop>dTrpA1 were from B. Dickson; Gad1-Gal80 was from T. Kitamoto; Rdl-Gal4 was from J. Simpson; UAS-mSP was from T. Aigaki; Trh-Gal4 was from E. Kravitz; w1118, rut, amn, UAS-Kir2.1, UAS-Gal80ts,UAS-dTrpA1, elav-Gal4, RdlMB08800, Tdc2-Gal4, TH-Gal4, npf-Gal4, Ddc-Gal4, Cha-Gal4, V-Glut-Gal4, dim-Gal4 and d5-HT1B-Gal4 were from Bloomington stock center; UAS-GAD-RNAi, UAS-VGAT-RNAi, UAS-GABA-b-R1-RNAi, UAS-GABA-b-R2-RNAi, UAS-GABA-b-R3-RNAi and UAS-Lcch3-RNAi were from VDRC stock center; Drosophila pseudoobscura and Drosophila virilis were from DGRC stock center.
Experimental design
Detailed experimental schemes are illustrated in Supplementary Figure 1. Newly eclosed males were collected and reared in pairs for 7 days before the behavioral assays. Virgin females were collected shortly after eclosion and reared at ~20 females per vial for 7 days before assays. The day before aggression assay, males were either housed together with females (1:1 ratio) as the experienced (E) group, or not, as the naïve (N) group. During the aggression assay, both groups were divided and tested in two configurations, males only and two males with two wild type virgin females. The flies were anesthetized with CO2 briefly and loaded in a chamber with grape juice agar medium similar to that described previously14. Their activities were videotaped for 2 hours. Aggression duration and frequency were quantified by visual inspection and scoring of aggressive male-male interactions including shoving, lunging, boxing, tussling and head-butting. To score the aggression phenotypes without confounding locomotion or courtship defects, and to ensure the two males had the same courtship experiences, we included only those cases in which both males copulated with both virgin females. For most of the analyses, aggression quantification was performed in the 60-90 min time window, given that aggression was most consistent in this time period and copulation was usually finished within the first 40 min. Summed duration and frequency were presented in bar graphs. Inhibition index was defined as (AggressionN – AggressionE) / AggressionN: the aggression duration of naïve males minus that of experienced males and normalized by the duration of naïve males. If the value is 1, it means that the aggression is completely inhibited in the experienced group; if the value is 0, it means that the experienced group showed the same level of aggression, thus dis-inhibition. Courtship index quantification was performed in the 70-75 min time window for experiments in Supplementary Fig. 2b, and the one-minute period right before copulation for experiments in Supplementary Fig. 3a. In all experiments involving genetic manipulations, we compared genotypes in the same genetic background.
Behavioral assays were performed in behavior chambers made from cuvettes filled with silicone with a thin layer of grape juice agar medium on top. Chambers were covered by an adhesive film after flies were loaded, then placed in a light-controlled incubator at the desired temperature during the video recording. In the experiment shown in Fig. 4, male flies were fed with 50 mM 5-HTP, which was mixed into food30, after eclosion untill assay30. The aggression duration for every 5 min of scoring were plotted in Fig. 1b and Supplementary Fig. 2a.
For the experiment shown in Fig. 2a, the males were housed with females at various time points (on day 6, day 4, day 3 or day 1) for 24 hours and were then separated from the females and reared for the number of days (0, 2, 3 or 5 days) until behavior testing.
For the experiment shown in Supplementary Fig. 2a, males were also tested with mated females. Under this configuration, males spent most of their time attempting to mate with the mated females and displayed low levels of aggression.
For the experiments with temporally inhibiting neuronal activity with Kir2.1 shown in Fig. 3, the males with the corresponding genotypes were raised at 20°C for five days and were switched to the non-permissive temperature 29°C for two days until assay.
For the experiments with temporally activating neurons with dTrpA1 shown in Fig. 3, Fig. 5 and Fig. 6, the males with the corresponding genotypes were raised at 20°C until the behavioral testing at 29°C.
Climbing assay was performed as previously38 with the following modifications: briefly, ten flies raised in pairs at 25°C for 7 days after eclosion were placed in a 25 mL pipet that was sealed at the top with cotton (n = 30 animals per group). The flies were gently knocked to the bottom of the pipet, which was then turned and stood straight on a rack. The percentage of the flies to cross the 20 cm height after 10 seconds was recorded. Five trials were completed for each group and the results were averaged for statistical analysis using Student's t-test. Data are presented as mean ± s.e.m.
All behavioral experiments were done on the morning of the experimental day.
Immunohistochemistry
The procedures for dissection, fixation, immunochemistry on adult brains were as described previously39. Primary antibodies used were rat anti-CD8 (Caltag Laboratories, MCD0800, 1:200), mouse anti-GFP (Invitrogen, A11120, 1:200), mouse anti-nc82 (DSHB, 1:100) and rabbit anti-GAD (F. Jackson, 1:200). For the representative images shown, each experiment has been successfully reproduced at least three times and was performed on multiple days.
Confocal imaging
Confocal images were taken on a Leica SP5 Confocal Microscope. Serial optic sections of 0.5 or 1 μm thickness (depending on the required resolution) were obtained from fixed whole-mount adult brain samples.
Semi-quantitative RT-PCR
Semi-quantitative RT-PCR was done for Rdl and α-tubulin according to the manufacturer's protocols. The primer sequences were as follows: two regions of the Rdl transcripts were amplified with primers Rdl 339-512 5′-ttggaccgatcctcgtttag-3′ and 5′-cgatccagaatgatgcacac-3′; Rdl 914-1073 5′-caaccgtgttgacaatgacc-3′ and 5′-attcgttttgccatgtagcc-3′; α-tubulin was amplified with 5-’acaacgaggctatctacgaca-3’ and 5’-ttttcagtgttgcagtgaattt-3’.
Statistical analysis
No statistical methods were used to pre-determine sample sizes but our sample sizes are similar to those reported in previous publications4-7. Two-tailed unpaired Student's t-test was performed for comparison between two groups of samples, and One-way ANOVA followed by Bonferroni's multiple comparison test was performed for comparisons among three or more groups of samples. The data meet the assumptions of the tests. The variance has been tested in each group of the data and the variance is similar among genotypes. Data distribution was assumed to be normal but this was not formally tested. Data collection and analysis were not performed blind to the conditions of the experiments. The data were collected and processed randomly. Statistical significance was assigned, *: p<0.05, **: p<0.01, ***: p<0.001.
Supplementary Material
Acknowledgements
We thank R. Greenspan, J. Blau, S. Waddell, L. Griffith, K. Keleman, L. Voshall, K. Scott, B. Dickson, T. Kitamoto, J. Simpson, T. Aigaki, E. Kravitz and F. Jackson for reagents and fly lines; K. Ori-McKenney and S. Rumpf and Jan lab members for helpful discussions, and Caixia Long and Paula Haynes for technical assistance Q.Y. was supported by an Autism Speaks postdoctoral fellowship. This work is supported by NIH grant 2R37NS040929 to Y.N.J. Also, Q.Y. and Y.S. are postdoctoral associates and L.Y.J. and Y.N.J. are investigators of the Howard Hughes Medical Institute.
Footnotes
Author Contributions
Q.Y. and Y.S. carried out all the experiments and performed the data analysis. C.Y. contributed to the behavioral paradigm, Q.Y., Y.S., L.Y.J. and Y.N.J. together conceived the research and wrote the manuscript.
Competing Finalcial Interests
The authors declare no competing financial interests.
REFERENCE
- 1.Kravitz EA, Huber R. Aggression in invertebrates. Current opinion in neurobiology. 2003;13:736–743. doi: 10.1016/j.conb.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 2.Anholt RR, Mackay TF. Genetics of aggression. Annual review of genetics. 2012;46:145–164. doi: 10.1146/annurev-genet-110711-155514. [DOI] [PubMed] [Google Scholar]
- 3.Nelson RJ, Trainor BC. Neural mechanisms of aggression. Nature reviews. Neuroscience. 2007;8:536–546. doi: 10.1038/nrn2174. [DOI] [PubMed] [Google Scholar]
- 4.Dierick HA, Greenspan RJ. Molecular analysis of flies selected for aggressive behavior. Nature genetics. 2006;38:1023–1031. doi: 10.1038/ng1864. [DOI] [PubMed] [Google Scholar]
- 5.Wang L, Anderson DJ. Identification of an aggression-promoting pheromone and its receptor neurons in Drosophila. Nature. 2010;463:227–231. doi: 10.1038/nature08678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu W, et al. Social regulation of aggression by pheromonal activation of Or65a olfactory neurons in Drosophila. Nature neuroscience. 2011;14:896–902. doi: 10.1038/nn.2836. [DOI] [PubMed] [Google Scholar]
- 7.Zhou C, Rao Y. A subset of octopaminergic neurons are important for Drosophila aggression. Nature neuroscience. 2008;11:1059–1067. doi: 10.1038/nn.2164. [DOI] [PubMed] [Google Scholar]
- 8.Dankert H, Wang L, Hoopfer ED, Anderson DJ, Perona P. Automated monitoring and analysis of social behavior in Drosophila. Nature methods. 2009;6:297–303. doi: 10.1038/nmeth.1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Certel SJ, Kravitz EA. Scoring and analyzing aggression in Drosophila. Cold Spring Harbor protocols. 2012;2012:319–325. doi: 10.1101/pdb.prot068130. [DOI] [PubMed] [Google Scholar]
- 10.Sturtevant AH. Experiments on sex recognition and the problem of sexual selection in Drosophila. Journal of Animal Behavior. 1915;5:351–366. [Google Scholar]
- 11.Hoffmann AA. The Influence of Age and Experience with Conspecifics on Territorial Behavior in Drosophila-Melanogaster. J Insect Behav. 1990;3:1–12. [Google Scholar]
- 12.Yurkovic A, Wang O, Basu AC, Kravitz EA. Learning and memory associated with aggression in Drosophila melanogaster. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:17519–17524. doi: 10.1073/pnas.0608211103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Keene AC, Waddell S. Drosophila olfactory memory: single genes to complex neural circuits. Nature reviews. Neuroscience. 2007;8:341–354. doi: 10.1038/nrn2098. [DOI] [PubMed] [Google Scholar]
- 14.Yang CH, et al. Control of the postmating behavioral switch in Drosophila females by internal sensory neurons. Neuron. 2009;61:519–526. doi: 10.1016/j.neuron.2008.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hasemeyer M, Yapici N, Heberlein U, Dickson BJ. Sensory neurons in the Drosophila genital tract regulate female reproductive behavior. Neuron. 2009;61:511–518. doi: 10.1016/j.neuron.2009.01.009. [DOI] [PubMed] [Google Scholar]
- 16.Blomguist G, Bagneres A. Insect Hydrocarbons: Biology, Biochemistry, and Chemical Ecology. Cambridge University Press; 2010. [Google Scholar]
- 17.Datta SR, et al. The Drosophila pheromone cVA activates a sexually dimorphic neural circuit. Nature. 2008;452:473–477. doi: 10.1038/nature06808. [DOI] [PubMed] [Google Scholar]
- 18.Ha TS, Smith DP. A pheromone receptor mediates 11-cis-vaccenyl acetate-induced responses in Drosophila. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2006;26:8727–8733. doi: 10.1523/JNEUROSCI.0876-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grether ME, Abrams JM, Agapite J, White K, Steller H. The head involution defective gene of Drosophila melanogaster functions in programmed cell death. Genes & development. 1995;9:1694–1708. doi: 10.1101/gad.9.14.1694. [DOI] [PubMed] [Google Scholar]
- 20.Larsson MC, et al. Or83b encodes a broadly expressed odorant receptor essential for Drosophila olfaction. Neuron. 2004;43:703–714. doi: 10.1016/j.neuron.2004.08.019. [DOI] [PubMed] [Google Scholar]
- 21.Thistle R, Cameron P, Ghorayshi A, Dennison L, Scott K. Contact chemoreceptors mediate male-male repulsion and male-female attraction during Drosophila courtship. Cell. 2012;149:1140–1151. doi: 10.1016/j.cell.2012.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yu JY, Kanai MI, Demir E, Jefferis GS, Dickson BJ. Cellular organization of the neural circuit that drives Drosophila courtship behavior. Current biology : CB. 2010;20:1602–1614. doi: 10.1016/j.cub.2010.08.025. [DOI] [PubMed] [Google Scholar]
- 23.Fernandez MP, et al. Pheromonal and behavioral cues trigger male-to-female aggression in Drosophila. PLoS biology. 2010;8:e1000541. doi: 10.1371/journal.pbio.1000541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Baines RA, Uhler JP, Thompson A, Sweeney ST, Bate M. Altered electrical properties in Drosophila neurons developing without synaptic transmission. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2001;21:1523–1531. doi: 10.1523/JNEUROSCI.21-05-01523.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yuan Q, Lin F, Zheng X, Sehgal A. Serotonin modulates circadian entrainment in Drosophila. Neuron. 2005;47:115–127. doi: 10.1016/j.neuron.2005.05.027. [DOI] [PubMed] [Google Scholar]
- 26.Cachero S, Ostrovsky AD, Yu JY, Dickson BJ, Jefferis GS. Sexual dimorphism in the fly brain. Current biology : CB. 2010;20:1589–1601. doi: 10.1016/j.cub.2010.07.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rosenzweig M, et al. The Drosophila ortholog of vertebrate TRPA1 regulates thermotaxis. Genes & development. 2005;19:419–424. doi: 10.1101/gad.1278205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Krashes MJ, Keene AC, Leung B, Armstrong JD, Waddell S. Sequential use of mushroom body neuron subsets during drosophila odor memory processing. Neuron. 2007;53:103–115. doi: 10.1016/j.neuron.2006.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chiavegatto S, et al. Brain serotonin dysfunction accounts for aggression in male mice lacking neuronal nitric oxide synthase. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:1277–1281. doi: 10.1073/pnas.031487198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dierick HA, Greenspan RJ. Serotonin and neuropeptide F have opposite modulatory effects on fly aggression. Nature genetics. 2007;39:678–682. doi: 10.1038/ng2029. [DOI] [PubMed] [Google Scholar]
- 31.Wilson RI, Laurent G. Role of GABAergic inhibition in shaping odor-evoked spatiotemporal patterns in the Drosophila antennal lobe. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2005;25:9069–9079. doi: 10.1523/JNEUROSCI.2070-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu X, Krause WC, Davis RL. GABAA receptor RDL inhibits Drosophila olfactory associative learning. Neuron. 2007;56:1090–1102. doi: 10.1016/j.neuron.2007.10.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Agosto J, et al. Modulation of GABAA receptor desensitization uncouples sleep onset and maintenance in Drosophila. Nature neuroscience. 2008;11:354–359. doi: 10.1038/nn2046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kolodziejczyk A, Sun X, Meinertzhagen IA, Nassel DR. Glutamate, GABA and acetylcholine signaling components in the lamina of the Drosophila visual system. PloS one. 2008;3:e2110. doi: 10.1371/journal.pone.0002110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Siegel RW, Hall JC. Conditioned responses in courtship behavior of normal and mutant Drosophila. Proceedings of the National Academy of Sciences of the United States of America. 1979;76:3430–3434. doi: 10.1073/pnas.76.7.3430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yu D, Akalal DB, Davis RL. Drosophila alpha/beta mushroom body neurons form a branch-specific, long-term cellular memory trace after spaced olfactory conditioning. Neuron. 2006;52:845–855. doi: 10.1016/j.neuron.2006.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dow MA, von Schilcher F. Aggression and mating success in Drosophila melanogaster. Nature. 1975;254:511–512. doi: 10.1038/254511a0. [DOI] [PubMed] [Google Scholar]
- 38.Palladino MJ, Hadley TJ, Ganetzky B. Temperature-sensitive paralytic mutants are enriched for those causing neurodegeneration in Drosophila. Genetics. 2002;161:1197–1208. doi: 10.1093/genetics/161.3.1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wu JS, Luo L. A protocol for dissecting Drosophila melanogaster brains for live imaging or immunostaining. Nature protocols. 2006;1:2110–2115. doi: 10.1038/nprot.2006.336. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.