Abstract
Sleep disturbances are associated with a greater risk of serious adverse health events, economic consequences, and, most importantly, increased all-cause mortality. Several studies support the associations among sleep, immune function, and inflammation. The relationship between sleep disturbances and inflammatory conditions is complex and not completely understood. Sleep deprivation can lead to increased levels of inflammatory cytokines, including interleukin (IL)-1β IL-6, tumor necrosis factor-α and C-reactive protein, which can lead to further activation of the inflammatory cascade. The relevance of sleep in inflammatory bowel disease (IBD), a chronic immune-mediated inflammatory disease of the gastrointestinal tract, has recently received more attention. Several studies have shown that patients with both inactive and active IBD have self-reported sleep disturbances. Here, we present a concise review of sleep and its association with the immune system and the process of inflammation. We discuss the studies that have evaluated sleep in patients with IBD as well as possible treatment options for those patients with sleep disturbances. An algorithm for evaluating sleep disturbances in the IBD population is also proposed. Further research is still needed to better characterize sleep disturbances in the IBD population as well as to assess the effects of various therapeutic interventions to improve sleep quality. It is possible that the diagnosis and treatment of sleep disturbances in this population may provide an opportunity to alter disease outcomes.
Keywords: Sleep, inflammation, Crohn’s disease, ulcerative colitis
The pathogenesis of inflammatory bowel disease (IBD), an immune-mediated chronic inflammatory disease of the intestinal tract, is complex and has been associated with several environmental factors besides genetic mutations and immune system defects. An association with smoking and appendectomy has been seen in the pathogenesis of IBD.1 There has been increasing interest in the roles that vitamin D levels, use of medications (eg, nonsteroidal anti-inflammatory drugs, and oral contraceptives), prior exposure to antibiotics, dietary composition, depression, psychologic stress, and geographic variation play in disease modification.1,2 In addition, a recent avenue of study has been on whether sleep disturbance is a possible environmental risk factor of IBD.
According to the Centers for Disease Control and Prevention, approximately 70 million Americans have chronic sleep problems.3 Some studies have reported that the sleep deficit has been increasing over time, as people are sleeping fewer hours than they were 25 years ago.4,5 In the modern era, increasing work demands and shifting work schedules are major causes for chronic sleep deficiency in the general population.6 The impact of shift work on health can be seen by the increase in sleep disorders, gastrointestinal (GI) diseases (eg, colitis and peptic ulcer disease), and cardiovascular diseases in the population.7 In the era of increasing prevalence of autoimmune conditions, researchers have asked important questions about how sleep quality is affecting health, especially in terms of effects on immune system regulation. Increasing sleep deprivation has been shown to increase the risk of infections and activate the immune cascade.8-10 Sleep deficiency also has been linked to an increase in all-cause mortality in young adults in addition to an increase in the risk of obesity, diabetes, and cardiovascular disease.5,11
Although lack of sleep might be a contributing factor to global health issues, researchers in the IBD community have begun to look at the effects of sleep quality on disease and outcomes. A large retrospective study looking at over 12,000 German workers found that those who worked long or irregular hours had an increased prevalence of IBD compared with those who worked regular hours.12 In published studies, at least 50% of patients with inactive IBD have reported poor sleep quality; this percentage is even greater in those patients with active disease.10,13-19 The high prevalence of sleep disturbances in these studies raises many questions about the role that sleep and sleep quality play in disease activity and management. Several studies would suggest that there is an increased risk of active disease in those with sleep impairment or an increased risk of sleep impairment in those with active disease; the direction of the relationship has not been well established.10,18,20,21
The Basics of Sleep
Humans sleep an average of 8 hours per night and have an average life expectancy of 77 years. This means people spend almost one-third of their lives sleeping.22 In the past 60 years, we have come to realize that sleep is not merely a passive state in which we just close our eyes and shut down our body functions and rest; rather, it is an active state with restorative properties. Human sleep is divided into 2 states: rapid eye movement (REM) sleep and non-REM (NREM) sleep. REM sleep accounts for approximately 20% of total sleep time in adults, and it is the stage in which memorable dreams occur. NREM sleep accounts for the other 80%, and it is broken down into 4 stages. Sleep onset occurs after a certain latency period, which varies from person to person, and initiates with stage 1 and progresses to deeper stages of sleep. After stage 4, sleep progresses into REM sleep and then back to stage 1. This cycle repeats itself every 90 minutes. Prior to awakening, the percentage of REM sleep is greater than that of NREM sleep. Most people awaken directly from REM sleep. Stages 3 and 4 are often referred to as slow-wave sleep (SWS) and are considered the most restorative stages of sleep and where the greatest impact from immune regulation happens. The effects of SWS can lead to a decrease in colon contractility, which is considered the “rest period” for the colon, so alterations in this stage of sleep can have direct effects on GI physiology, including diminished mucosal integrity.23,24 Inadequate sleep or sleep deprivation can lead to daytime tiredness, irritability, and possible decreased work productivity.
As people are sleeping less, the most significant changes are seen in the duration of SWS and an increase in sleep fragmentation.25 Recent studies have also shown that the sleep-wake cycle, also known as the circadian rhythm, is controlled by a central “clock” in the brain as well as peripheral GI clocks. Different environmental factors, such as light, food, chemicals, and genes, modulate the functions of these clocks.26,27
The quality of sleep can be evaluated by both subjective instruments and objective measurements. Several tools have been designed to evaluate sleep disturbances in humans. Buysse and colleagues developed a commonly used sleep instrument, the Pittsburgh Quality Sleep Index (PSQI), in 1989.28 The PSQI is a 19-item self-rated questionnaire used to assess sleep quality and disturbances over a 1-month period, with calculated scores ranging from 0 (good sleep quality) to 21 (poor sleep quality).28 This validated instrument has a nearly 90% sensitivity and specificity to distinguish good sleepers from poor sleepers and has broad clinical and research uses. It is widely being used in studies that look at sleep quality in patients with IBD,10,14,15,18,29 irritable bowel syndrome (IBS),15,17,30-32 gastroesophageal reflux disease (GERD),33-35 and fibromyalgia.36-38 Buysse and colleagues more recently contributed to a newer sleep assessment tool created for the Patient Reported Outcomes Measurement Information System (PROMIS) to overcome challenges with prior instruments used for self-reported sleep dysfunction.39
Objective sleep measurement involves use of a nocturnal polysomnography (PSG), which can provide more information about biophysiologic changes occurring during sleep. However, PSG is often performed in a sleep laboratory, which takes a patient out of his or her natural sleep environment. In comparison with PSG, the PSQI reflects patients’ sleep patterns over a 30-day period. Given the “first night” sleep effect seen with PSG, various home sleep monitors have been designed to provide more natural sleep assessment over a longer period of time.40 The use of less invasive sleep monitoring utilizes actigraphy technology, which monitors movement during sleep. This technology can be found in various medical sleep devices such as Actiwatch (Phillips) and several commercially available wristwatches, such as UP (Jawbone) and Flex (Fitbit). Although these devices have not been validated against medical-device actigraphy or PSG, people are able to track and analyze their sleep quality at home using this wrist-worn technology.41
Sleep quality assessment is complex and involves the evaluation of several variables, including sleep duration (number of hours spent sleeping), sleep latency (how long it takes to fall asleep), sleep efficiency (hours asleep in bed compared with time spent in bed), and number of sleep arousals (sleep fragmentation). Sleep disturbances can be characterized by chronic sleep deprivation, primary sleep disorders (insomnia, restless leg syndrome [RLS], periodic limb movement disorder, and obstructive sleep apnea [OSA]), and secondary sleep disorders (due to other medical conditions). Although not fully understood, the presence of active disease in IBD can lead to sleep deprivation, which then can lead to further immune activation, creating a vicious cycle for patients.
Sleep and Immunity
The interaction between sleep and the immune system is complex and has been increasingly studied over the past few decades. A close link between sleep and immune function has been observed, and studies have shown that adequate sleep strengthens immune function.42 On the other hand, sleep deprivation has been shown to have negative effects on the immune function. Alterations in sleep patterns can lead to leukocytosis and an increase in natural killer cells, which can lead to increased inflammatory cytokine production.5,20,43 Interestingly, the cytokines implicated in the regulation of the sleep-wake cycle also are involved in the pathogenesis of chronic inflammatory conditions, including IBD.
Effects of Sleep Deprivation on the Immune System
Researchers have shown that sleep deprivation can lead to systemic inflammation as defined by changes in proinflammatory cytokines and inflammatory markers. In particular, elevations in interleukin (IL)-1β, IL-6, tumor necrosis factor (TNF)-α and C-reactive protein have been seen after sleep deprivation in both animal and human studies.9,44-47 IL-1β and TNF-α in particular, have been referred to as sleep regulatory cytokines, and the studies looking at their effects suggest that the relationship may be bidirectional.48 In a study of 30 healthy adults undergoing sleep deprivation, a significant elevation in proinflammatory cytokines was found. These findings suggest that response is mediated by activation of the NF-kB pathway.9 The proinflammatory effects of TNF-α have been well defined in the pathogenesis of chronic inflammation, especially in IBD, and anti—TNF-α therapy plays a central role in the treatment of IBD.49 The use of anti—TNF-α therapy in patients with rheumatoid arthritis (RA) and ankylosing spondylitis has been associated with improved sleep quality.50-52
Immune Regulation Effects on the Sleep- Wake Cycle
In animal studies, increased levels of IL-1 and TNF-α were associated with an increase in NREM sleep, in particular SWS.48,53,54 IL-1 also may regulate sleep through a serotonin receptor pathway and, at low levels, induces NREM sleep, but at higher levels, it can cause NREM suppression and sleep fragmentation.20,55-57 IL-6 plays a central role in the pathogenesis of Crohn’s disease (CD); it mediates acute-phase reactant response and has been shown to suppress REM sleep and promote wakefulness in patients with IBD.20,42,47 As will be discussed in more detail, patients with active IBD are more likely to have subjective sleep complaints than patients with inactive disease, which supports the role for inflammation in the regulation of sleep-wake cycles.
Nonimmune-mediated Factors in Sleep Regulation
In addition to cytokines, hormones have been shown to play a central role in sleep regulation. Melatonin is made by the pineal gland, and it has multiple neurohormonal functions, including circadian regulation.58,59 The highest levels of melatonin are found within the GI tract and play a central role in the regulation of GI motility, with both inhibitory and excitatory effects.60,61 Another important hormone in sleep regulation is endogenous glucocorticoids, in particular Cortisol, which is responsible for daily wakening and alertness.24 Endogenous Cortisol levels are associated with total wake time and negatively associated with REM sleep.62 The use of exogenous glucocorticoids is associated with altered sleep patterns, as observed in patients requiring corticosteroid therapy for chronic inflammation.63,64 Both endogenous and exogenous Cortisol inhibits the effects of IL-1β which regulates the sleep-wake cycle.8 Cortisol also has effects on melatonin, the secretion of which typically occurs during low Cortisol secretion.25
Sleep, Infection, and Inflammation
The Role of Infection in Sleep Disturbances
Acute infection plays an important role in sleep regulation. Studies of various pathogens (bacterial, viral, and protozoan) have been shown to result in the alteration of sleep patterns in animals, usually resulting in an increase in SWS following infection.65-67 Sleeping sickness, caused by Human African trypanosomiasis, results in fragmented sleep-wake cycles and is initially characterized by increased sleepiness that progresses to premature REM episodes. Affected patients are found to have elevated TNF-α and IL-1βlevels.68,69 Several studies have looked at the changes of sleep architecture related to viral infection, including rhinovirus, which, contrary to popular belief, is associated with decreased sleep duration.70 The sleep patterns of people with HIV infection are also found to be significantly altered, with studies showing sleep abnormalities in 73% of HIV-seropositive patients.71
Inflammatory Conditions and Sleep
Adequate SWS can attenuate the inflammatory cascade, whereas the presence of a sleep deficit can activate a proinflammatory response.20 King and colleagues demonstrated that longer sleep duration measured by actigraphy was associated with a decrease in the incidence of coronary artery calcification.72 Fifty percent to 70% of patients with RA report sleep disturbances and daytime fatigue.73 Objective evaluation of sleep disturbances in a small sample of patients with RA using PSG found improvement in sleep efficiency and decreased awakenings after initiation of anti—TNF-α therapy.51,52 Many other diseases have been associated with patient-reported poor sleep quality, including asthma, systemic lupus erythematosus, multiple sclerosis, and ankylosing spondylitis.74-78
Sleep and the Gastrointestinal Tract
Adequate sleep has important effects on GI physiology leading to inhibition of colon contractility during SWS, with the elimination of propagating contractions leading to colonic inactivity.8,79 A cross-sectional survey found a strong relationship between self-reported insomnia and the presence of bowel disorders in a Canadian population. This study found a nearly 3-fold increased risk of bowel disorders in patients with insomnia even after adjusting for sex, age, self-perceived stress, and the presence of chronic fatigue syndrome.80 Although an important observation, the causality of these findings is still not very clear. GERD is the most common GI diagnosis in the United States and has served as a good model for the bidirectional relationship between sleep and the GI tract.81 One study demonstrated that sleep deprivation increased symptom rating and decreased time to symptom generation in patients with GERD. Studies also have shown that patients with GERD symptoms have more frequent nighttime awakenings and poorer sleep quality.82,83 A study looking at the sleep disturbances in Olmsted County, Minnesota found that people were more likely to have IBS if they had subjective sleep complaints, further supporting the important relationship between sleep and the GI tract.84 Studies have shown increased subjective sleep complaints in the IBS population and increased sleep fragmentation on PSG.31,85 The effects of sleep on bowel inflammation can be seen in initial studies of the effects of sleep disturbances in the mouse dextran sodium sulfate—induced colitis model. Preuss and colleagues were the first to publish the adverse effects of circadian desynchronization on mucosal inflammation.86 They found that mice subjected to 3 months of alternating light-dark periods had more severe colonic inflammation. The same group published another study that showed that, after circadian disruption, mice had worsening colonic inflammation and delayed mucosal recovery.23
Sleep in Inflammatory Bowel Disease
The Presence of Sleep Disturbances in Inflammatory Bowel Disease
Increasing evidence shows that there is a relationship between sleep, GI physiology, and inflammation as described above. Researchers have now started to ask important questions about the impact of sleep disturbances in IBD. The Table summarizes the studies to date that examine sleep quality in IBD. Zimmerman was the first to publish data that examined sleep disturbances in the IBD patient population.17 This was a small observational study of age-matched male patients with IBS and IBD. It assessed the relationship between intestinal and extraintestinal symptoms, including sleep disturbances. Compared with healthy controls, higher sleep disturbances, as measured on a 5-point Likert scale, were discovered. In this study, the presence of diarrhea and acid reflux predicted the presence of sleep disturbances. The exclusion of female patients and the use of age-matched controls excluded gender and age effects. The lack of control for other potential confounders and the absence of standardized sleep measurement tools were some of the other limitations of this study. A few years later, another study looked at sleep in the pediatric population and found that 54% (21/41) of pediatric patients with IBD had moderate to severe sleep disturbances.19 The most common disturbances were morning fatigue on awakening (75%) and sleep fragmentation (53.6%). The study did not find any association between the presence of sleep disturbance and disease activity or current treatment regimen. Although the study was prospective in nature and used a validated mini-sleep questionnaire, its small sample size and lack of information regarding disease activity measurement tools and confounders, such as corticosteroid use and the presence of depression and anxiety, limit the interpretation of the results. Keefer and colleagues were the first to perform a small prospective study that examined subjective (using PSQI) and objective (using PSG) sleep disturbances in adult controls, patients with IBS, and patients with IBD.15,87 The study found increasing subjective sleep complaints among patients with IBS or IBD and that abnormal sleep affected quality-of-life measures. Using PSG, the researchers found that 13% of patients with IBD had OSA. This was the first study to further classify sleep disturbances using objective measures. The same group further evaluated the effects of sleep disturbances and quality of life in a larger sample of patients with inactive IBD using a mail-in survey.10 The subjects with IBD reported significantly prolonged sleep latency, frequent sleep fragmentation, a higher rate of using sleeping pills, decreased daytime energy, increased tiredness, and poor overall sleep quality compared with healthy controls. Similar to many other previous studies, this study lacked the objective evaluation of sleep and included other confounding factors. Reliance on self-reported retrospective assessment by patients and their sleep quality was another major limitation.
Table.
Studies of Sleep Disruption in Inflammatory Bowel Disease
| Author (Year) | Study Population(s) | Sleep Measurement(s) | Main Findings |
|---|---|---|---|
| Zimmerman (2003)17 | Healthy control IBS Inactive IBD | MSI | Increased subjective sleep complaints (IBD/IBS) vs healthy controls |
| Nachmias et al (2006)19 (pediatric) | Inactive IBD | Mini-sleep questionnaire | Moderate to severe sleep disturbances, early morning fatigue |
| Keefer et al(2006)15 | Healthy control IBS Inactive IBD | PSQI PSG | Increased subjective sleep complaints (IBD/IBS) vs healthy controls Sleep disturbances affect quality of life |
| Ranjbaran et al (2007)10 | Healthy control IBS Inactive IBD | PSQI | Poor sleep quality and effect on quality of life in patients with IBD |
| Burgess et al (2010)88 | Inactive IBD | Actigraphy | Decreased sleep efficiency |
| Pirinen et al (2010)16 (pediatric) | Inactive IBD Active IBD | Multiple questionnaires | Severe IBD symptoms associated with sleep disturbance |
| Graff et al(2011)14 | Inactive IBD Active IBD | Multidimensional Fatigue Inventory PSQI ESS | Active disease increased subjective sleep complaints |
| Ali et al(2013)91 | Inactive IBD Active IBD | PSQI | Sleep impairment predictive for subclinical inflammation |
| Graffetal(2013)89 | Inactive IBD Active IBD | Multidimensional Fatigue Inventory PSQI | Poor sleep quality independently associated with increased fatigue |
| Benhayon et al(2013)90 (pediatric) | Inactive/active CD | PSQI | Poor sleep quality in pediatric depressed CD patients Increased PSQI scores associated with disease activity |
| Ananthakrishnan et al (2013)13 | Inactive IBD Active IBD | PROMIS online survey | Sleep impairment associated with disease activity 2-fold increase in active disease at 6 months (CD) |
| Ali et al(2013)29 | Inactive IBD Active IBD | PSQI | Sleep impairment associated with disease activity 3-fold increase in active disease at 6 months (IBD) |
CD, Crohn’s disease; ESS, Epworth Sleepiness Scale; IBD, inflammatory bowel disease; IBS, irritable bowel syndrome; MSI, multisystem inventory; PROMIS, Patient Reported Outcomes Measurement Information System; PSG, nocturnal polysomnography; PSQI, Pittsburgh Sleep Quality Index.
Focusing on the objective assessment of sleep, a small study analyzed wrist-worn actigraphy in 4 patients with inactive IBD.88 The patients were found to have increased sleep-onset latency and decreased sleep efficiency compared with healthy matched controls. The researchers also evaluated melatonin secretion profiles in this sample and showed circadian rhythms similar to those of controls. Given the small sample size, the findings were observational but an important addition to previous studies.
Sleep Disturbances and Disease Activity
The presence of sleep disturbances in the majority of patients with IBD prompted researchers to begin looking at the correlation between sleep and disease activity. In the pediatric IBD population, a study showed that patients with self-reported severely active IBD were more likely to have sleep disturbances than children with mild or inactive disease. Although patient perceptions of their disease activity are important, the use of a visual analog scale in this study to assess disease activity possibly biased the results, as a clinical or endoscopic assessment of disease severity would be more accurate, given that some symptoms are not always driven by inflammation. Similar findings were seen in an adult population with active IBD in a large study performed by Graff and colleagues that used multiple patient-reported outcomes.14 This study showed that a greater percentage of patients with active IBD (82%) had poor sleep quality compared with patients with inactive IBD (51%). In this study, disease activity was defined by clinical indices but not endoscopic evaluation, although the C-reactive protein levels between the inactive and active groups were the same despite differences in clinical indices.
A more recent study published by Graff and colleagues evaluated fatigue in adults with IBD.89 After controlling for disease activity, disease type, gender, and age, the researchers found that poor sleep quality was independently associated with increasing fatigue over time. Similarly, in a small cohort of pediatric patients with depression, significantly greater subjective sleep disturbances were seen in patients with CD compared with controls.90 This study was confounded by the fact that all of the patients screened positive for depression, which alone is linked to abnormal sleep patterns, and that the use of the PSQI in children and adolescents has not been validated.
Our group recently published a study confirming the association between poor sleep quality and active disease.91 In this study, all patients with clinically active disease had an abnormal PSQI. Of the patients in clinical remission with abnormal PSQI, 61% were found to have histologic inflammation on colonoscopy. Patients with an abnormal PSQI score had a positive predictive value of 83% for underlying mucosal inflammation. This was the first study to look at endoscopic and histologic disease activity and sleep quality and, unlike previous studies, controlled for confounding factors such as depression, fatigue, corticosteroid use, and over-the-counter medication use.
Sleep Disturbances Increase the Risk of Relapse
A large online survey completed by over 3100 patients in the Crohn’s and Colitis Foundation of America Partners IBD cohort found that patients with CD in clinical remission and subjective sleep disturbances (using the PROMIS questionnaire) had a 2-fold increased risk of active disease at 6 months.13 Patients with ulcerative colitis (UC) were not found to have this same association. The study also showed an association between disease activity, depression, female sex, and the use of corticosteroids or narcotics with abnormal subjective sleep complaints. The study was limited by the self-reported information on the disease phenotype, treatment, and disease activity. Our group presented the preliminary results of an ongoing prospective study at Digestive Disease Week 2013, looking at patients with IBD (both CD and UC), and found a 3-fold increased risk of disease relapse at 6 months in patients with clinical remission and poor sleep.29 Patients with an abnormal PSQI had a relapse rate of 47% at 3 months and 67% at 6 months compared with 0% at 3 and 6 months in patients with a normal PSQI. Patients with known depression, anxiety, corticosteroid use, and use of over-the-counter medications known to affect sleep were excluded from this analysis. These studies add important information to the current knowledge about sleep and IBD. There is a need for larger prospective studies to confirm the association between poor sleep quality and risk of disease relapse. One such study is ongoing at our center.
Therapeutic Implications of Sleep Quality in Inflammatory Bowel Disease
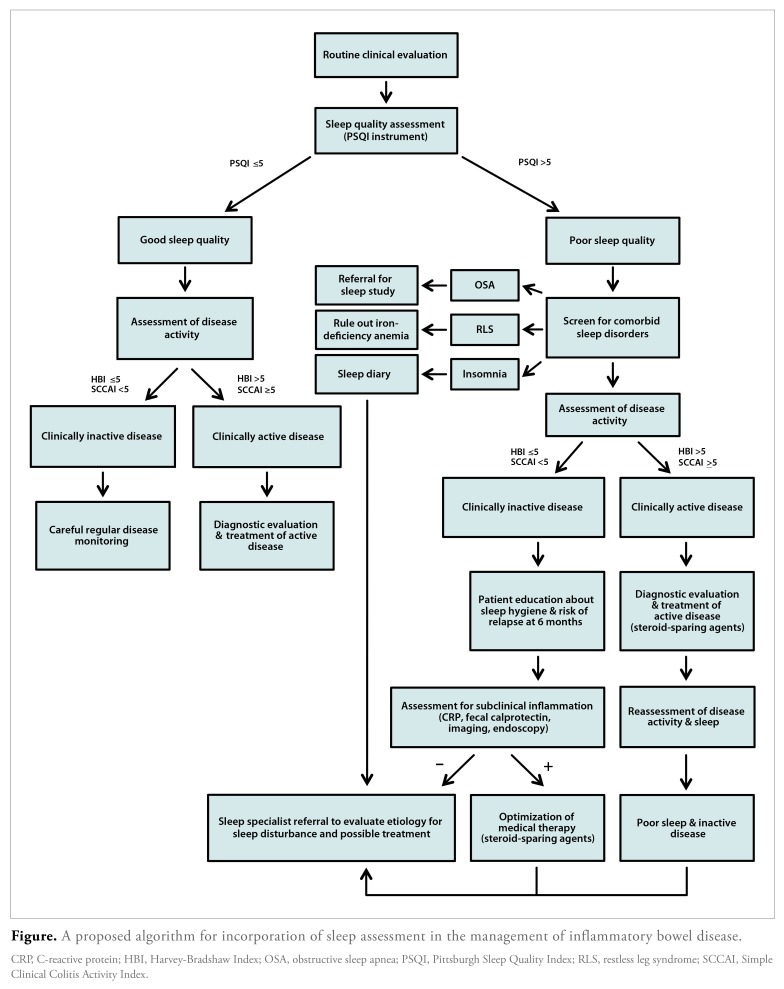
The above studies highlight the significant impact of sleep on the IBD population and also raise a few important questions: How can we improve sleep quality in patients? Can current IBD therapies improve sleep quality? Does improving sleep quality improve disease outcomes? Studies in patients with insomnia support the use of cognitive behavioral therapy (CBT) to improve sleep quality,92 but the effectiveness of CBT has not been assessed in the IBD population. If studies looking at behavioral interventions in patients with IBD were able to show improved sleep quality and decreased disease activity, it could be a less invasive and inexpensive means to improve patients’ overall quality of life; however, how to incorporate sleep assessment into a clinical practice has yet to be decided. We propose an algorithm for incorporating sleep assessment in the evaluation and management of patients with IBD (Figure).
Figure.
A proposed algorithm for incorporation of sleep assessment in the management of inflammatory bowel disease.
CRP, C-reactive protein; HBI, Harvey-Bradshaw Index; OSA, obstructive sleep apnea; PSQI, Pittsburgh Sleep Quality Index; RLS, restless leg syndrome; SCCAI, Simple Clinical Colitis Activity Index.
Studies in the RA population have shown improvement in sleep quality after initiation of therapy with anti—TNF-α drugs.51,52 However, it can be argued that the effects seen could be due to improved disease control resulting in fewer symptoms (eg, arthralgia) that drive sleep disturbances. There also has been increasing interest in the use of melatonin, given its important regulatory role in the sleep-wake cycle, effects on the GI immune system, and anti-inflammatory effects. Although there have been no clinical trials on the use of melatonin in IBD, researchers have studied the effects of melatonin in similar models, including patients with IBS, showing that the use of melatonin resulted in symptom control and improvement in the quality of life.93-95 There have been more than a dozen studies looking at the use of melatonin in animal models showing anti-inflammatory benefits, including a reduction in mucosal inflammation severity.96,97 Anecdotal evidence suggests that melatonin may have an impact on IBD; a published case report described a patient with UC who had resolution of symptoms after use of melatonin for jet lag.98 Melatonin may be useful as adjuvant therapy in patients with IBD and sleep disturbances, although more studies need to be performed in the IBD population to assess the true benefit. Other important considerations are the evaluation and treatment of sleep disorders in evaluating comorbid conditions that affect sleep, including anxiety and depression, use of corticosteroids, and other inflammatory conditions. One study showed the importance of treating both depression and sleep disturbances in the general population and found that those who received CBT for sleep had greater remission rates for depression.99 The approach to patients with IBD and sleep disorders will need to involve a multi-disciplinary team that includes sleep specialists, psychologists, and IBD experts to begin to adequately address sleep disturbances in this patient population.
Summary and Future Directions
There is no question that patients with IBD report poor sleep quality. Currently, 1.4 million Americans have received a diagnosis of IBD, and studies would suggest that 750,000 to 1 million of these patients are affected by sleep disturbances.98 At the present time, the evaluation of sleep disturbances in this population is not standard clinical practice. With studies suggesting an association of subjective sleep disturbances with increased disease activity and even subclinical inflammation, this problem deserves more attention. Clinicians should consider screening patients with IBD for sleep disturbances using available validated instruments such as PSQI or PROMIS questionnaires. Further research is needed to develop better and more user-friendly sleep measurement tools to establish the causality and direction of the association between sleep disturbances and disease activity. Evaluation of sleep disturbances could be considered another noninvasive approach to the evaluation of subclinical inflammation, given that the presence of abnormal sleep is associated with underlying inflammation and increased risk of relapse.13,18,29 Patients with reported poor sleep quality should be evaluated for comorbid sleep conditions, including OSA, RLS, and primary insomnia. If present, referral for further diagnosis and treatment should be considered. Patients with poor sleep quality who are in clinical remission warrant further investigation for subclinical inflammation as well as sleep hygiene assessment and education to reduce the risk of relapse. Patients with poor sleep quality and active disease should first undergo optimization of medical therapy and then further assessment of both disease activity and sleep quality.
The assessment of sleep disturbances in patients with IBD can play an important role in optimizing medical management and keeping disease in remission, and it may even alter disease outcomes. Further studies are warranted to evaluate the impact of sleep evaluation and subsequent therapeutic intervention on the disease outcomes of patients with IBD.
Footnotes
The authors have no conflicts of interest to disclose.
References
- 1.Ng SC, Bernstein CN, Vatn MH, et al. Geographical variability and environmental risk factors in inflammatory bowel disease. Gut. 2013;62(4):630–649. doi: 10.1136/gutjnl-2012-303661. [DOI] [PubMed] [Google Scholar]
- 2.Ananthakrishnan AN. Environmental triggers for inflammatory bowel disease. Curr Gastroenterol Rep. 2013;15(1):302. doi: 10.1007/s11894-012-0302-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention. Sleep and sleep disorders. [October 7, 2013]. http://www.cdc.gov/sleep/about_us.htm
- 4.Jean-Louis G, Kripke DF, Ancoli-Israel S, Klauber MR, Sepulveda RS. Sleep duration, illumination, and activity patterns in a population sample: effects of gender and ethnicity. Biol Psychiatry. 2000;47(10):921–927. doi: 10.1016/s0006-3223(99)00169-9. [DOI] [PubMed] [Google Scholar]
- 5.Van Cauter E, Spiegel K, Tasali E, Leproult R. Metabolic consequences of sleep and sleep loss. Sleep Med. 2008;9(Suppl 1):S23–S28. doi: 10.1016/S1389-9457(08)70013-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rajaratnam SM, Arendt J. Health in a 24-hsociety. Lancet. 2001;358(9286):999–1005. doi: 10.1016/S0140-6736(01)06108-6. [DOI] [PubMed] [Google Scholar]
- 7.Costa G. The impact of shift and night work on health. Appl Ergon. 1996;27(1):9–16. doi: 10.1016/0003-6870(95)00047-x. [DOI] [PubMed] [Google Scholar]
- 8.Bryant PA, Trinder J, Curtis N. Sick and tired: does sleep have a vital role in the immune system? Nat Rev Immunol. 2004;4(6):457–467. doi: 10.1038/nri1369. [DOI] [PubMed] [Google Scholar]
- 9.Irwin MR, Wang M, Campomayor CO, Collado-Hidalgo A, Cole S. Sleep deprivation and activation of morning levels of cellular and genomic markers of inflammation. Arch Intern Med. 2006;166(16):1756–1762. doi: 10.1001/archinte.166.16.1756. [DOI] [PubMed] [Google Scholar]
- 10.Ranjbaran Z, Keefer L, Farhadi A, Stepanski E, Sedghi S, Keshavarzian A. Impact of sleep disturbances in inflammatory bowel disease. J Gastroenterol Hepatol. 2007;22(11):1748–1753. doi: 10.1111/j.1440-1746.2006.04820.x. [DOI] [PubMed] [Google Scholar]
- 11.Buysse DJ, Angst J, Gamrna A, Ajdacic V, Eich D, Rossler W. Prevalence, course, and comorbidity of insomnia and depression in young adults. Sleep. 2008;31(4):473–480. doi: 10.1093/sleep/31.4.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sonnenberg A. Occupational distribution of inflammatory bowel disease among German employees. Gut. 1990;31(9):1037–1040. doi: 10.1136/gut.31.9.1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ananthakrishnan AN, Long MD, Martin CF, Sandler RS, Kappelman MD. Sleep disturbance and risk of active disease in patients with Crohn’s disease and ulcerative colitis. Clin Gastroenterol Hepatol. 2013;11(8):965–971. doi: 10.1016/j.cgh.2013.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Graff LA, Vincent N, Walker JR, et al. A population-based study of fatigue and sleep difficulties in inflammatory bowel disease. Inflamm Bowel Dis. 2011;17(9):1882–1889. doi: 10.1002/ibd.21580. [DOI] [PubMed] [Google Scholar]
- 15.Keefer L, Stepanski EJ, Ranjbaran Z, Benson LM, Keshavarzian A. An initial report of sleep disturbance in inactive inflammatory bowel disease. J Clin Sleep Med. 2006;2(4):409–4l6. [PubMed] [Google Scholar]
- 16.Pirinen T, Kolho KL, Simola P, Ashorn M, Aronen ET. Parent and self-report of sleep-problems and daytime tiredness among adolescents with inflammatory bowel disease and their population-based controls. Sleep. 2010;33(11):1487–1493. doi: 10.1093/sleep/33.11.1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zimmerman J. Extraintestinal symptoms in irritable bowel syndrome and inflammatory bowel diseases: nature, severity, and relationship to gastrointestinal symptoms. Dig Dis Sci. 2003;48(4):743–749. doi: 10.1023/a:1022840910283. [DOI] [PubMed] [Google Scholar]
- 18.Ali T, Ahmad S, Madhoun M, Orr W, Rubin DT. Assessment of the relationship between quality of sleep and disease activity in inflammatory bowel disease (IBD) patients. Poster presented at: American College of Gastroenterology meeting; October 19-24, 2012; Las Vegas, NV. Abstract P1624.
- 19.Nachmias V, Sheinberg A, Weiss B, Fradkin A, Bujanover Y. Sleep disturbances among young patients with IBD in Israel. J Pediatr Gastroenterol Nutr. 2006;43(suppl 2):S48. [Google Scholar]
- 20.Ranjbaran Z, Keefer L, Stepanski E, Farhadi A, Keshavarzian A. The relevance of sleep abnormalities to chronic inflammatory conditions. Inflamm Res. 2007;56(2):51–57. doi: 10.1007/s00011-006-6067-1. [DOI] [PubMed] [Google Scholar]
- 21.Swanson GR, Burgess HJ, Keshavarzian A. Sleep disturbances and inflammatory bowel disease: a potential trigger for disease flare? Expert Rev Clin Immunol. 2011;7(1):29–36. doi: 10.1586/eci.10.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Centers for Disease Control and Prevention. Life expectancy. [June 27, 2013]. http://www.cdc.gov/nchs/fastats/lifexpec.htm
- 23.Tang Y, Preuss F, Turek FW, Jakate S, Keshavarzian A. Sleep deprivation worsens inflammation and delays recovery in a mouse model of colitis. Sleep Med. 2009;10(6):597–603. doi: 10.1016/j.sleep.2008.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zisapel N. Sleep and sleep disturbances: biological basis and clinical implications. Cell Mol Life Sci. 2007;64(10):1174–1186. doi: 10.1007/s00018-007-6529-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Van Cauter E, Leproult R, Plat L. Age-related changes in slow wave sleep and REM sleep and relationship with growth hormone and Cortisol levels in healthy men. JAMA. 2000;284(7):861–868. doi: 10.1001/jama.284.7.861. [DOI] [PubMed] [Google Scholar]
- 26.Peuhkuri K, Sihvola N, Korpela R. Diet promotes sleep duration and quality. Nutr Res. 2012;32(5):309–319. doi: 10.1016/j.nutres.2012.03.009. [DOI] [PubMed] [Google Scholar]
- 27.Konturek PC, Brzozowski T, Konturek SJ. Gut clock: implication of circadian rhythms in the gastrointestinal tract. J Physiol Pharmacol. 2011;62(2):139–150. [PubMed] [Google Scholar]
- 28.Buysse DJ, Reynolds CF III, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28(2):193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 29.Ali T, Madhoun M, Crosby A, Orr WC, Rubin DT. Poor sleep quality predicts disease relapse in patients with inflammatory bowel disease. Gastroenterology. 2013;144(5 suppl 1):S12. [Google Scholar]
- 30.Bellini M, Gemignani A, Gambaccini D, et al. Evaluation of latent links between irritable bowel syndrome and sleep quality. World J Gastroenterol. 2011;17(46):5089–5096. doi: 10.3748/wjg.v17.i46.5089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Elsenbruch S, Harnish MJ, Orr WC. Subjective and objective sleep quality in irritable bowel syndrome. Am J Gastroenterol. 1999;94(9):2447–2452. doi: 10.1111/j.1572-0241.1999.01374.x. [DOI] [PubMed] [Google Scholar]
- 32.Robert JJ, Orr WC, Elsenbruch S. Modulation of sleep quality and autonomic functioning by symptoms of depression in women with irritable bowel syndrome. Dig Dis Sci. 2004;49(7-8):1250–1258. doi: 10.1023/b:ddas.0000037820.54069.6c. [DOI] [PubMed] [Google Scholar]
- 33.Dickman R, Green C, Fass SS, et al. Relationships between sleep quality and pH monitoring findings in persons with gastroesophageal reflux disease. J Clin Sleep Med. 2007;3(5):505–513. [PMC free article] [PubMed] [Google Scholar]
- 34.Farup C, Kleinman L, Sloan S, et al. The impact of nocturnal symptoms associated with gastroesophageal reflux disease on health-related quality of life. Arch Intern Med. 2001;161(1):45–52. doi: 10.1001/archinte.161.1.45. [DOI] [PubMed] [Google Scholar]
- 35.Schey R, Dickman R, Parthasarathy S, et al. Sleep deprivation is hyperalgesic in patients with gastroesophageal reflux disease. Gastroenterology. 2007;133(6):1787–1795. doi: 10.1053/j.gastro.2007.09.039. [DOI] [PubMed] [Google Scholar]
- 36.Orlandi AC, Ventura C, Gallinaro AL, Costa RA, Lage LV. Improvement in pain, fatigue, and subjective sleep quality through sleep hygiene tips in patients with fibromyalgia. Rev Bras Reumatol. 2012;52(5):666–678. [PubMed] [Google Scholar]
- 37.Osorio CD, Gallinaro AL, Lorenzi-Filho G, Lage LV. Sleep quality in patients with fibromyalgia using the Pittsburgh Sleep Quality Index. J Rheumatol. 2006;33(9):1863–1865. [PubMed] [Google Scholar]
- 38.Ulus Y, Akyol Y, Tander B, Durmus D, Bilgici A, Kuru O. Sleep quality in fibromyalgia and rheumatoid arthritis: associations with pain, fatigue, depression, and disease activity. Clin Exp Rheumatol. 2011;29(6 Suppl 69):S92–S96. [PubMed] [Google Scholar]
- 39.Buysse DJ, Yu L, Moul DE, et al. Development and validation of patient-reported outcome measures for sleep disturbance and sleep-related impairments. Sleep. 2010;33(6):781–792. doi: 10.1093/sleep/33.6.781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kelly JM, Strecker RE, Bianchi MT. Recent developments in home sleep-monitoring devices. ISRN Neurol. 2012;2012:768–794. doi: 10.5402/2012/768794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ancoli-Israel S, Cole R, Alessi C, Chambers M, Moorcroft W, Pollak CP. The role of actigraphy in the study of sleep and circadian rhythms. Sleep. 2003;26(3):342–392. doi: 10.1093/sleep/26.3.342. [DOI] [PubMed] [Google Scholar]
- 42.Gamaldo CE, Shaikh AK, McArthur JC. The sleep-immunity relationship. Neurol Clin. 2012;30(4):1313–1343. doi: 10.1016/j.ncl.2012.08.007. [DOI] [PubMed] [Google Scholar]
- 43.Sookoian S, Gemma C, Fernandez Gianotti T, et al. Effects of rotating shift work on biomarkers of metabolic syndrome and inflammation. J Intern Med. 2007;261(3):285–292. doi: 10.1111/j.1365-2796.2007.01766.x. [DOI] [PubMed] [Google Scholar]
- 44.Born J, Lange T, Hansen K, Molle M, Fehm HL. Effects of sleep and circadian rhythm on human circulating immune cells. J Immunol. 1997;158(9):4454–4464. [PubMed] [Google Scholar]
- 45.Haack M, Sanchez E, Mullington JM. Elevated inflammatory markers in response to prolonged sleep restriction are associated with increased pain experience in healthy volunteers. Sleep. 2007;30(9):1145–1152. doi: 10.1093/sleep/30.9.1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Uthgenannt D, Schoolmann D, Pietrowsky R, Fehm HL, Born J. Effects of sleep on the production of cytokines in humans. Psychosom Med. 1995;57(2):97–104. doi: 10.1097/00006842-199503000-00001. [DOI] [PubMed] [Google Scholar]
- 47.Vgontzas AN, Papanicolaou DA, Bixler EO, Kales A, Tyson K, Chrousos GP. Elevation of plasma cytokines in disorders of excessive daytime sleepiness: role of sleep disturbance and obesity. J Clin Endocrinol Metab. 1997;82(5):1313–1316. doi: 10.1210/jcem.82.5.3950. [DOI] [PubMed] [Google Scholar]
- 48.Opp MR. Cytokines and sleep. Sleep Med Rev. 2005;9(5):355–364. doi: 10.1016/j.smrv.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 49.Roberts-Thomson IC, Fon J, Uylaki W, Cummins AG, Barry S. Cells, cytokines and inflammatory bowel disease: a clinical perspective. Expert Rev Gastroenterol Hepatol. 2011;5(6):703–716. doi: 10.1586/egh.11.74. [DOI] [PubMed] [Google Scholar]
- 50.Karadag O, Nakas D, Kalyoncu U, Akdogan A, Kiraz S, Ertenli I. Effect of anti-TNF treatment on sleep problems in ankylosing spondylitis. Rheumatol Int. 2012;32(7):1909–1913. doi: 10.1007/s00296-011-1907-x. [DOI] [PubMed] [Google Scholar]
- 51.Taylor-Gjevre RM, Gjevre JA, Nair BV, Skomro RP, Lim HJ. Improved sleep efficiency after anti-tumor necrosis factor α therapy in rheumatoid arthritis patients. Ther Adv Musculoskelet Dis. 2011;3(5):227–233. doi: 10.1177/1759720X11416862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wells G, Li T, Maxwell L, Maclean R, Tugwell P. Responsiveness of patient reported outcomes including fatigue, sleep quality, activity limitation, and quality of life following treatment with abatacept for rheumatoid arthritis. Ann Rheum Dis. 2008;67(2):260–265. doi: 10.1136/ard.2007.069690. [DOI] [PubMed] [Google Scholar]
- 53.Dickstein JB, Moldofsky H. Sleep, cytokines and immune function. Sleep Med Rev. 1999;3(3):219–228. doi: 10.1016/s1087-0792(99)90003-5. [DOI] [PubMed] [Google Scholar]
- 54.Shoham S, Davenne D, Cady AB, Dinarello CA, Krueger JM. Recombinant tumor necrosis factor and interleukin 1 enhance slow-wave sleep. Am J Physiol. 1987;253(1 Pt 2):R142–R149. doi: 10.1152/ajpregu.1987.253.1.R142. [DOI] [PubMed] [Google Scholar]
- 55.Olivadoti MD, Opp MR. Effects of i.c.v. administration of interleukin-1 on sleep and body temperature of interleukin-6-deficient mice. Neuroscience. 2008;153(1):338–348. doi: 10.1016/j.neuroscience.2008.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Opp MR, Toth LA. Neural-immune interactions in the regulation of sleep. Front Biosci. 2003;8:d768–d779. doi: 10.2741/1061. [DOI] [PubMed] [Google Scholar]
- 57.Shearer WT, Reuben JM, Mullington JM, et al. Soluble TNF-alpha receptor 1 and IL-6 plasma levels in humans subjected to the sleep deprivation model of spaceflight. J Allergy Clin Immunol. 2001;107(1):165–170. doi: 10.1067/mai.2001.112270. [DOI] [PubMed] [Google Scholar]
- 58.Macchi MM, Bruce JN. Human pineal physiology and functional significance of melatonin. Front Neuroendocrinal. 2004;25(3-4):177–195. doi: 10.1016/j.yfrne.2004.08.001. [DOI] [PubMed] [Google Scholar]
- 59.Mallo C, Zaidan R, Galy G, et al. Pharmacokinetics of melatonin in man after intravenous infusion and bolus injection. Eur J Clin Pharmacol. 1990;38(3):297–301. doi: 10.1007/BF00315035. [DOI] [PubMed] [Google Scholar]
- 60.Bubenik GA. Gastrointestinal melatonin: localization, function, and clinical relevance. Dig Dis Sci. 2002;47(10):2336–2348. doi: 10.1023/a:1020107915919. [DOI] [PubMed] [Google Scholar]
- 61.Terry PD, Villinger F, Bubenik GA, Sitaraman SV. Melatonin and ulcerative colitis: evidence, biological mechanisms, and future research. Inflamm Bowel Dis. 2009;15(1):134–140. doi: 10.1002/ibd.20527. [DOI] [PubMed] [Google Scholar]
- 62.Bell-McGinty S, Habeck C, Hilton HJ, et al. Identification and differential vulnerability of a neural network in sleep deprivation. Cereb Cortex. 2004;14(5):496–502. doi: 10.1093/cercor/bhh011. [DOI] [PubMed] [Google Scholar]
- 63.Gillin JC, Jacobs LS, Fram DH, Snyder F. Acute effect of a glucocorticoid on normal human sleep. Nature. 1972;237(5355):398–399. doi: 10.1038/237398a0. [DOI] [PubMed] [Google Scholar]
- 64.Vgontzas AN, Chrousos GP. Sleep, the hypothalamic-pituitary-adrenal axis, and cytokines: multiple interactions and disturbances in sleep disorders. Endocrinol Metab Clin North Am. 2002;31(1):15–36. doi: 10.1016/s0889-8529(01)00005-6. [DOI] [PubMed] [Google Scholar]
- 65.Toth LA. Sleep, sleep deprivation and infectious disease: studies in animals. Adv Neuroimmunol. 1995;5(1):79–92. doi: 10.1016/0960-5428(94)00045-p. [DOI] [PubMed] [Google Scholar]
- 66.Toth LA, Krueger JM. Alteration of sleep in rabbits by Staphylococcus aureus infection. Infect Immun. 1988;56(7):1785–1791. doi: 10.1128/iai.56.7.1785-1791.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Toth LA, Rehg JE. Effects of sleep deprivation and other stressors on the immune and inflammatory responses of influenza-infected mice. Life Sci. 1998;63(8):701–709. doi: 10.1016/s0024-3205(98)00321-x. [DOI] [PubMed] [Google Scholar]
- 68.Buguet A, Bert J, Tapie P, et al. Sleep-wake cycle in human African trypanosomiasis. J Clin Neurophysiol. 1993;10(2):190–196. doi: 10.1097/00004691-199304000-00006. [DOI] [PubMed] [Google Scholar]
- 69.Rodgers J. Trypanosomiasis and the brain. Parasitology. 2010;137(14):1995–2006. doi: 10.1017/S0031182009991806. [DOI] [PubMed] [Google Scholar]
- 70.Drake CL, Roehrs TA, Royer H, Koshorek G, Turner RB, Roth T. Effects of an experimentally induced rhinovirus cold on sleep, performance, and daytime alertness. Physiol Behav. 2000;71(l-2):75–81. doi: 10.1016/S0031-9384(00)00322-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Salahuddin N, Barroso J, Leserman J, Harmon JL, Pence BW. Daytime sleepiness, nighttime sleep quality, stressful life events, and HIV-related fatigue. J Assoc Nurses AIDS Care. 2009;20(1):6–13. doi: 10.1016/j.jana.2008.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.King CR, Knutson KL, Rathouz PJ, Sidney S, Liu K, Lauderdale DS. Short sleep duration and incident coronary artery calcification. JAMA. 2008;300(24):2859–2866. doi: 10.1001/jama.2008.867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Drewes AM, Svendsen L, Taagholt SJ, Bjerregard K, Nielsen KD, Hansen B. Sleep in rheumatoid arthritis: a comparison with healthy subjects and studies of sleep/wake interactions. Br J Rheumatol. 1998;37(1):71–81. doi: 10.1093/rheumatology/37.1.71. [DOI] [PubMed] [Google Scholar]
- 74.Brass SD, Duquette P, Proulx-Therrien J, Auerbach S. Sleep disorders in patients with multiple sclerosis. Sleep Med Rev. 2010;14(2):121–129. doi: 10.1016/j.smrv.2009.07.005. [DOI] [PubMed] [Google Scholar]
- 75.Fleming WE, Pollak CP. Sleep disorders in multiple sclerosis. Semin Neurol. 2005;25(1):64–68. doi: 10.1055/s-2005-867075. [DOI] [PubMed] [Google Scholar]
- 76.Janson C, De Backer W, Gislason T, et al. Increased prevalence of sleep disturbances and daytime sleepiness in subjects with bronchial asthma: a population study of young adults in three European countries. Eur Respir J. 1996;9(10):2132–2138. doi: 10.1183/09031936.96.09102132. [DOI] [PubMed] [Google Scholar]
- 77.Li Y, Zhang S, Zhu J, Du X, Huang F. Sleep disturbances are associated with increased pain, disease activity, depression, and anxiety in ankylosing spondylitis: a case-control study. Arthritis Res Ther. 2012;14(5):R215. doi: 10.1186/ar4054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Valencia-Flores M, Resendiz M, Castano VA, et al. Objective and subjective sleep disturbances in patients with systemic lupus erythematosus. Arthritis Rheum. 1999;42(10):2189–2193. doi: 10.1002/1529-0131(199910)42:10<2189::AID-ANR21>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 79.Steadman CJ, Phillips SF, Camilleri M, Haddad AC, Hanson RB. Variation of muscle tone in the human colon. Gastroenterology. 1991;101(2):373–381. doi: 10.1016/0016-5085(91)90014-c. [DOI] [PubMed] [Google Scholar]
- 80.Hon CY, Nicol AM. Examining the association between insomnia and bowel disorders in Canada: is there a trend? Univ Br Columbia Med J. 2012;2(1):11–15. [Google Scholar]
- 81.Fujiwara Y, Arakawa T, Fass R. Gastroesophageal reflux disease and sleep disturbances. J Gastroenterol. 2012;47(7):760–769. doi: 10.1007/s00535-012-0601-4. [DOI] [PubMed] [Google Scholar]
- 82.Fass R. Effect of gastroesophageal reflux disease on sleep. J Gastroenterol Hepatol. 2010;25(Suppl 1):S41–S44. doi: 10.1111/j.1440-1746.2009.06210.x. [DOI] [PubMed] [Google Scholar]
- 83.Poh CH, Allen L, Gasiorowska A, et al. Conscious awakenings are commonly associated with acid reflux events in patients with gastroesophageal reflux disease. Clin Gastroenterol Hepatol. 2010;8(10):851–857. doi: 10.1016/j.cgh.2010.05.022. [DOI] [PubMed] [Google Scholar]
- 84.Vege SS, Locke GR, III, Weaver AL, Farmer SA, Melton LJ, III, Talley NJ. Functional gastrointestinal disorders among people with sleep disturbances: a population-based study. Mayo Clin Proc. 2004;79(12):1501–1506. doi: 10.4065/79.12.1501. [DOI] [PubMed] [Google Scholar]
- 85.Rotem AY, Sperber AD, Krugliak P, Freidman B, Tal A, Tarasiuk A. Polysomnographic and actigraphic evidence of sleep fragmentation in patients with irritable bowel syndrome. Sleep. 2003;26(6):747–752. doi: 10.1093/sleep/26.6.747. [DOI] [PubMed] [Google Scholar]
- 86.Preuss F, Tang Y, Laposky AD, Arble D, Keshavarzian A, Turek FW. Adverse effects of chronic circadian desynchronization in animals in a “challenging” environment. Am J Physiol Regul Integr Comp Physiol. 2008;295(6):R2034–R2040. doi: 10.1152/ajpregu.00118.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Young T, Peppard PE, Gottlieb DJ. Epidemiology of obstructive sleep apnea: a population health perspective. Am J Respir Crit Care Med. 2002;165(9):1217–1239. doi: 10.1164/rccm.2109080. [DOI] [PubMed] [Google Scholar]
- 88.Burgess HJ, Swanson GR, Keshavarzian A. Endogenous melatonin profiles in asymptomatic inflammatory bowel disease. Scand J Gastroenterol. 2010;45(6):759–761. doi: 10.3109/00365521003749818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Graff LA, Clara I, Walker JR, et al. Changes in fatigue over 2 years are associated with activity of inflammatory bowel disease and psychological factors. Clin Gastroenterol Hepatol. 2013;11(9):1140–1146. doi: 10.1016/j.cgh.2013.03.031. [DOI] [PubMed] [Google Scholar]
- 90.Benhayon D, Youk A, McCarthy FN, et al. Characterization of relations among sleep, inflammation, and psychiatric dysfunction in depressed youth with Crohn disease. J Pediatr Gastroenterol Nutr. 2013;57(3):335–342. doi: 10.1097/MPG.0b013e31829641df. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ali T, Madhoun MF, Orr WC, Rubin DT. Assessment of the relationship between quality of sleep and disease activity in inflammatory bowel disease patients. Inflamm Bowel Dis. 2013;19(11):2440–2443. doi: 10.1097/MIB.0b013e3182a0ea54. [DOI] [PubMed] [Google Scholar]
- 92.Vincent N, Lewycky S. Logging on for better sleep: RCT of the effectiveness of online treatment for insomnia. Sleep. 2009;32(6):807–815. doi: 10.1093/sleep/32.6.807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lu WZ, Gwee KA, Moochhalla S, Ho KY. Melatonin improves bowel symptoms in female patients with irritable bowel syndrome: a double-blind placebo-controlled study. Aliment Pharmacol Ther. 2005;22(10):927–934. doi: 10.1111/j.1365-2036.2005.02673.x. [DOI] [PubMed] [Google Scholar]
- 94.Saha L, Malhotra S, Rana S, Bhasin D. Pandhi P A preliminary study of melatonin in irritable bowel syndrome. J Clin Gastroenterol. 2007;41(1):29–32. doi: 10.1097/MCG.0b013e31802df84c. [DOI] [PubMed] [Google Scholar]
- 95.Song GH, Leng PH, Gwee KA, Moochhala SM, Ho KY. Melatonin improves abdominal pain in irritable bowel syndrome patients who have sleep disturbances: a randomised, double blind, placebo controlled study. Gut. 2005;54(10):1402–1407. doi: 10.1136/gut.2004.062034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Esposito E, Mazzon E, Riccardi L, Caminiti R, Meli R, Cuzzocrea S. Matrix metalloproteinase-9 and metalloproteinase-2 activity and expression is reduced by melatonin during experimental colitis. J Pineal Res. 2008;45(2):166–173. doi: 10.1111/j.1600-079X.2008.00572.x. [DOI] [PubMed] [Google Scholar]
- 97.Mazzon E, Esposito E, Crisafulli C, et al. Melatonin modulates signal transduction pathways and apoptosis in experimental colitis. J Pineal Res. 2006;41(4):363–373. doi: 10.1111/j.1600-079X.2006.00378.x. [DOI] [PubMed] [Google Scholar]
- 98.Mann S. Melatonin for ulcerative colitis? Am J Gastroenterol. 2003;98(1):232–233. doi: 10.1111/j.1572-0241.2003.07190.x. [DOI] [PubMed] [Google Scholar]
- 99.Manber R, Edinger JD, Gress JL, San Pedro-Salcedo MG, Kuo TF, Kalista T. Cognitive behavioral therapy for insomnia enhances depression outcome in patients with comorbid major depressive disorder and insomnia. Sleep. 2008;31(4):489–495. doi: 10.1093/sleep/31.4.489. [DOI] [PMC free article] [PubMed] [Google Scholar]