G&H Why are new topical hemostatic agents needed?
AB Bleeding in the upper gastrointestinal (GI) tract, particularly bleeding induced by ulcers or other nonvariceal lesions, is usually managed effectively via thermal methods, clips, or a combination of these modalities with or without injection. The current management of lower GI tract bleeding is less clear-cut, however, because of the difficulty of accessing and identifying bleeding sites in the acute setting with endoscopic approaches that have unclear benefits. A significant proportion of these patients have a high risk of rebleeding, and it is particularly difficult to manage them if they are very sick. Therefore, in addition to the 3 principal hemostatic methods used for nonvariceal upper GI tract bleeding, other modalities such as angiography (ie, embolization) have been examined as complementary methods to endoscopic treatment. We are eagerly anticipating the results of an ongoing randomized trial looking at the use of prophylactic angiographic embolization with endoscopic treatment to further decrease the risk of rebleeding in patients at greatest risk of ulcer rebleeding. However, angiography is quite invasive and associated with risks (although they are low in expert hands using contemporary techniques). Despite the current management approach, the risk of nonvariceal upper GI bleeding remains around 10% to 15% in community-based registries, with resulting morbidity and mortality. Therefore, additional hemostatic approaches are needed.
G&H How do these powders work?
AB It is unclear exactly how these hemostatic agents work. They appear to be adhesives to active sites of bleeding; thus, if a site is not actively bleeding, hemostatic powders will not attach to it. In addition to being adhesive, the powders dehydrate tissue by absorbing the local moisture of water molecules, swelling up significantly, which completes the postulated hemostatic effect. Conceptually, this process is similar to the effect of a bandage being applied to a bleeding site coupled with a local tamponade effect. Hemostatic powders may also have a role to play regarding other facilitatory mechanisms, possibly including platelet aggregation and activation of clotting factors, but these additional effects remain unproven.
G&H What animal model and clinical data are available thus far on these new agents?
AB Of the 3 new hemostatic powders, only TC-325 has undergone animal evaluations in a laparoscopic or endoscopic setting, although even these studies have been limited. This agent has been assessed in an animal model of bleeding, in which it appeared to be successful in terms of immediate hemostasis and subsequent reduction of the risk of rebleeding. The animal model that was developed did not allow for the assessment of rebleeding. Several uncontrolled case series assessing Ankaferd Blood Stopper and others assessing TC-325 suggested that the agents led to decreased rebleeding. Importantly, no embolization has been reported with these agents in animal or clinical experience, although the manufacturer has suggested that TC-325 not be used in variceal bleeding because of this theoretical risk. Similarly, no bowel obstruction has been noted with the doses used clinically.
G&H How safe do these hemostatic powders appear to be thus far?
AB As with every new modality, physicians should be careful because there are a limited amount of follow-up data available to date; however, based on the reports and use of Ankaferd Blood Stopper and TC-325 in hundreds of patients worldwide to date, these products appear to be safe. Indeed, to the best of my knowledge, there have been no reports of systemic or vascular embolization or bowel obstruction, as previously mentioned.
G&H How difficult are these hemostatic powders to use?
AB I have not used Ankaferd Blood Stopper or EndoClot, but our group does have quite a bit of experience applying TC-325 in the upper and lower GI tracts. Although the delivery system is in its second generation and has been improved, it is probably still not optimal. Indeed, the endoscopist must be careful when delivering the agent and should avoid being too close to the tissue. If the endoscopist is not careful, the delivery catheter may clog; in addition, if the endoscopist is too close to the lesion, the resulting backspray of powder may impair adequate visualization until the dust settles. It is important to let the powder settle because any attempt to aspirate will draw in powder and humidity, resulting in the blocking of the operative channel or delivery catheter (particularly the 7 French catheter).
G&H Thus far, what appear to be the indications for these new hemostatic powders?
AB My colleagues and I recently published an article in Gastrointestinal Endoscopy to try to identify some of the key areas where these agents might be useful in different patient populations. The first indication would probably be patients who have massive, active bleeding, although it might be difficult to access the bleeding site effectively in such clinical situations. Preliminary data suggest that these products wash off quite quickly, so it is likely— although not certain—that they do not stop rebleeding beyond 24 hours of application.
Hemostatic powders have been around for some time and have been applied to many areas of the body, but only within the past 3 years or so have they been adapted to delivery systems that can be used in the upper and lower GI tracts. I am aware of only 3 hemostatic powders currently in use in the GI tract (although none are approved for use yet in the United States): TC-325 (Hemospray, Cook Medical), which is the product that has undergone evaluation most recently and is currently marketed in many countries, including Canada (Figure); Ankaferd Blood Stopper (Ankaferd Ilaç Kozmetik AŞ), which is a traditional herbal mixture that was approved by the Turkish Food and Drug Administration several years ago; and EndoClot (EndoClot Plus, Inc), which is a mineral-based powder currently available in several countries, although it has been discussed in little published literature in the peer-review domain to date. The hope is that these new hemostatic powders may help further lower the risk of rebleeding by achieving enhanced immediate hemostasis and decreasing the subsequent risk of recurrent hemorrhage.
A number of small case series have suggested that the powders may be able to play a role in different conditions. Results of a recent large cohort study examining TC-325 were presented 2 years ago at Digestive Disease Week, but they have not yet been published in full. This multinational cohort study assessed how the agent might work and in which context. However, it is important to note that such series have adopted purely observational study methodologies and, thus, are limited with regard to any possible comparisons with other hemostatic approaches. Small case series have assessed Ankaferd Blood Stopper and TC-325 in the upper and lower GI tracts in different patient groups (including patients on antiplatelet agents, patients bleeding from GI malignancies, and patients with variceal bleeding), with good results—but again, in an uncontrolled or poorly controlled fashion.
In a study of TC-325, our group reported an episode of transient obstruction of a sphincterotomy opening of the biliary tree in a patient who experienced postsphincterotomy bleeding, although this blockage spontaneously resolved during the procedure without further manipulation. This highlights the importance of using the powder carefully when near a very small orifice such as a pancreatic or biliary opening. Having said this, other researchers have used TC-325 with success in the context of postsphincterotomy bleeding, with a stent in place in the biliary tree. Both Ankaferd Blood Stopper and TC-325 have been used in the lower GI tract, including in the cecum, and in the setting of diverticular bleeding, with good success and no complications. Nonetheless, caution should be exercised during delivery of the powder, particularly in the case of TC-325, which is delivered by a carbon dioxide delivery system that is under pressure. Overall, based on preliminary and published data available thus far, these agents may be the most efficacious modalities currently available for providing immediate hemostasis in the case of active bleeding, with minimal risks.
A technical challenge associated with all 3 of the hemostatic powders is that by applying them early on in the procedure, the endoscopist may lose his or her landmarks and, thus, not be able to perform additional treatment. Therefore, combination treatment using a hemostatic powder may not be easy to perform unless the powder is used last. However, a powder can be used first by endoscopists who are experienced with its use if they do not apply a thick layer.
It should also be noted that en-face positioning of the endoscope, endoscope tip, or catheter tip is not necessary when using these agents. This makes this class of products easier to use by endoscopists who have less expertise and may facilitate a role for these products as a bridge to more specialized endoscopic hemostasis after appropriate transfer and/or second-look endoscopy.
The second indication would probably be that of rescue treatment. If a patient is continuing to bleed despite receiving conventional hemostatic treatment, I think it is very reasonable to attempt to use a hemostatic powder, although there are actually very few data on this indication at this time. On the other hand, these powders can probably be used as sole hemostatic modalities as well. If the rebleeding risk does not extend past 24 hours, the powders would probably work well alone, and severe postpolypectomy bleeding is probably an ideal indication.
Finally, malignant bleeding is another area where, hopefully, the powders may have a role to play. Patients who have malignant causes of bleeding do not have healthy bleeding tissues; thus, every attempt at direct tissue manipulation results in more bleeding. Therefore, having a true nontraumatic, noncontact method of hemostasis may be quite helpful and best adapted for treating a malignant bleeding site. In addition, these powders can be sprayed over a large surface area, which is convenient for treating malignant bleeding tissue, as the affected surface area is usually quite large with multiple bleeding points. Although, again, there exist very limited data on the use of hemostatic powders in malignant bleeding, preliminary uncontrolled data from a number of groups, including our own, appear to be promising and warrant a comparative trial, which will start soon. At the very least, the use of powders in malignant bleeding may provide immediate hemostasis, allowing subsequent additional treatment when the patient is more stable. However, these assumptions require prospective, controlled data for confirmation.
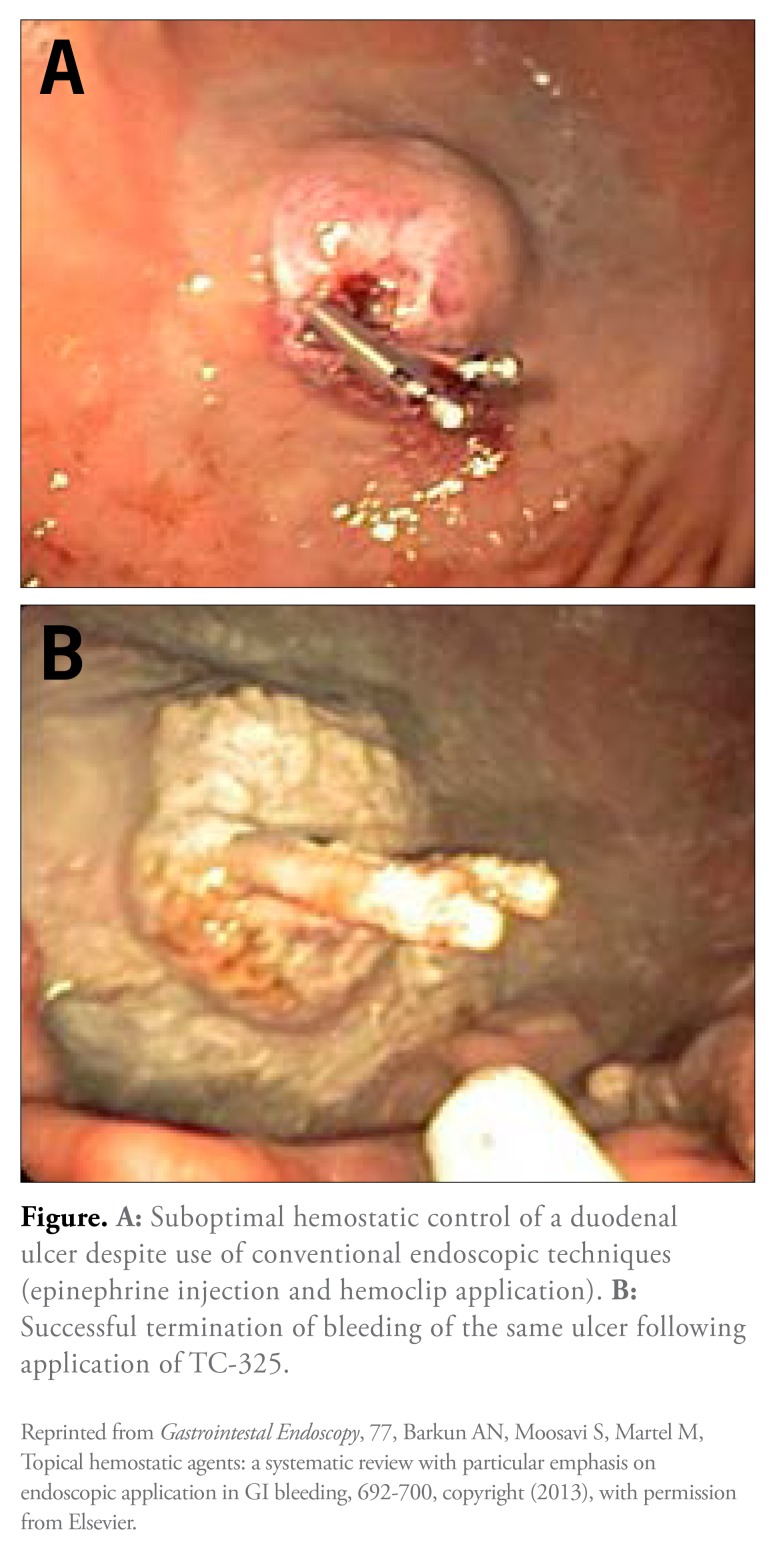
Figure 1.
A: Suboptimal hemostatic control of a duodenal ulcer despite use of conventional endoscopic techniques (epinephrine injection and hemoclip application). B: Successful termination of bleeding of the same ulcer following application of TC-325.
Reprinted from Gastrointestal Endoscopy, 77, Barkun AN, Moosavi S, Martel M, Topical hemostatic agents: a systematic review with particular emphasis on endoscopic application in GI bleeding, 692-700, copyright (2013), with permission from Elsevier.
Biography

Footnotes
Dr Barkun is a consultant for Cook Medical.
Suggested Reading
- Barkun AN, Moosavi S, Martel M. Topical hemostatic agents: a systematic review with particular emphasis on endoscopic application in GI bleeding. Gastrointest Endosc. 2013;77(5):692–700. doi: 10.1016/j.gie.2013.01.020. [DOI] [PubMed] [Google Scholar]
- Chen YI, Barkun AN, Soulellis C, Mayrand S, Ghali P. Use of the endoscopically applied hemostatic powder TC-325 in cancer-related upper GI hemorrhage: preliminary experience (with video) Gastrointest Endosc. 2012;75(6):1278–1281. doi: 10.1016/j.gie.2012.02.009. [DOI] [PubMed] [Google Scholar]
- Holster IL, Kuipers EJ. Tjwa ET Hemospray in the treatment of upper gastrointestinal hemorrhage in patients on antithrombotic therapy. Endoscopy. 2013;45(1):63–66. doi: 10.1055/s-0032-1325793. [DOI] [PubMed] [Google Scholar]
- Holster IL, Poley JW, Kuipers EJ, Tjwa ET. Controlling gastric variceal bleeding with endoscopically applied hemostatic powder (Hemospray) J Hepatol. 2012;57(6):1397–1398. doi: 10.1016/j.jhep.2012.07.024. [DOI] [PubMed] [Google Scholar]
- Leblanc S, Vienne A, Dhooge M, Coriat R, Chaussade S, Prat F. Early experience with a novel hemostatic powder used to treat upper GI bleeding related to malignancies or after therapeutic interventions (with videos) Gastrointest Endosc. 2013;78(1):169–175. doi: 10.1016/j.gie.2013.03.006. [DOI] [PubMed] [Google Scholar]
- Soulellis CA, Carpentier S, Chen YI, Fallone CA, Barkun AN. Lower GI hemorrhage controlled with endoscopically applied TC-325 (with videos) Gastrointest Endosc. 2013;77(3):504–507. doi: 10.1016/j.gie.2012.10.014. [DOI] [PubMed] [Google Scholar]
- Sung JJ, Luo D, Wu JC, et al. Early clinical experience of the safety and effectiveness of Hemospray in achieving hemostasis in patients with acute peptic ulcer bleeding. Endoscopy. 2011;43(4):291–295. doi: 10.1055/s-0030-1256311. [DOI] [PubMed] [Google Scholar]