Abstract
Objective: Clinical follow-up of implant survival in 11 patients comparing two different methods for mesenchymal stem cell (MSC) isolation (Ficoll and bone marrow aspirate concentrate [BMAC]) applied in maxillary sinus augmentation.
Methods: Mononuclear cells, including MSCs, were concentrated with either Ficoll (control group, n=6 sinus) or BMAC (test group, n=12 sinus) and transplanted in combination with bovine bone mineral. A total of 50 implants were placed in a second surgical intervention (17 Ficoll/33 BMAC) and loaded after 4 months. Overall implant survival was assessed with a Kaplan-Meier model using package survival under R.
Results: Implant survival of the Ficoll group was 100% compared with the BMAC group, which had 93.4% survival (95% confidence interval, 0.849–1). The difference between the groups was not significant (p=0.381).
Conclusion: The BMAC system is an effective and suitable “chair-side” method for clinical application in hard tissue regeneration.
Key words: : biomaterials, cell culture, tissue engineering
Introduction
Aprevalent modality to increase the amount of available bone prior to implantation is grafting of the maxillary sinus.1 Over the last decade the application of concentrated mononuclear cells, including mesenchymal stem cells (MSCs), has emerged as a promising alternative for bone regeneration in the field of oral and cranio-maxillofacial surgery.2,3 However, conventional open cell-concentration systems are restricted to good manufacturing practice conditions and thus clinically impractical.4,5
The present study evaluates implant survival in a clinical follow-up of 11 patients who were involved in the study of Sauerbier et al.3 on new bone formation in the maxillary sinus. Patients requiring dental implant placement in the posterior maxilla with a maximum of 3 mm of residual bone received sinus augmentation with bovine bone mineral (BBM) particles and MSCs, isolated with either the Ficoll method or the closed, chair-side bone marrow aspirate concentrate (BMAC) method. Successful tissue regeneration was assumed on the basis of implant survival after up to 2.5 years of loading.
Materials and Methods
The study protocol and sinus augmentation procedure is described in detail by Sauerbier et al.3 In brief, bone marrow was obtained with a bone marrow biopsy needle from the pelvic bone latero-caudal from the superior posterior iliac spine. Aspirates were either given to the laboratory to separate the mononucleated cells with the Ficoll-method or directly processed in the operating room using the BMAC method according to manufacturer's instructions.
Sinuses were augmented with BBM enriched with mononucleated cells in thrombin, and covered with a collagen membrane. After a 3-month healing time, a total of 50 implants (17 Ficoll, 33 BMAC) were placed in a second-stage procedure and allowed to osseointegrate for an additional 4 months before prosthetic loading. Implant survival was clinically evaluated, according to the modified parameters described by Buser et al.,6 in the patient follow-up, up to 2.5 years.
Implant survival was analyzed by a Kaplan-Meier model using package survival under R.7
Results
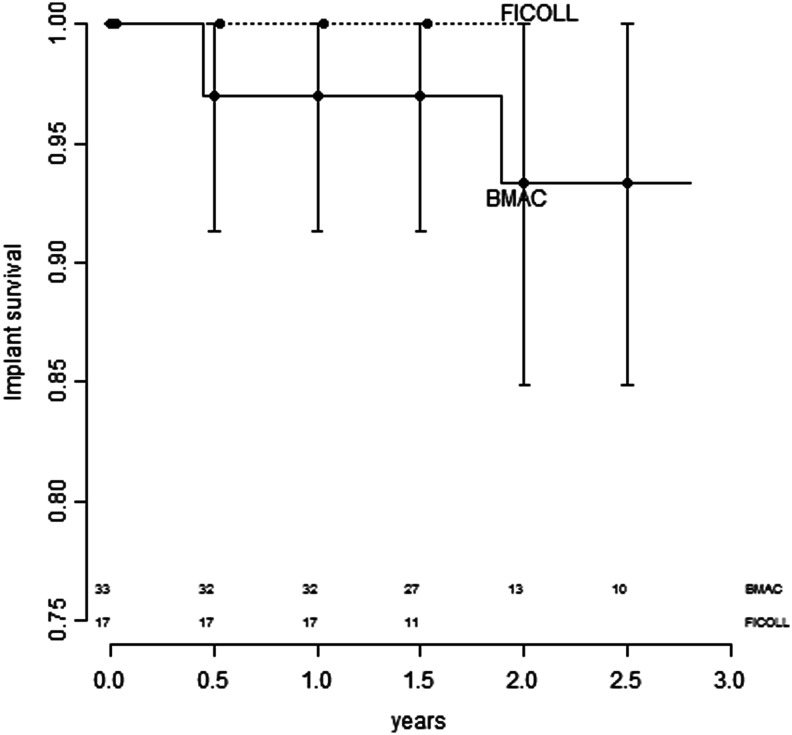
In the first stage of the study, no patients dropped out and all recovered well from the surgical procedures. None of the 17 implants of the Ficoll-group were lost, and only one implant out of 33 failed in the BMAC group before prosthetic loading (Fig. 1). After loading, 49 implants osseointegrated and remained functional. Implant survival of the Ficoll group was 100% compared with the BMAC group, which had 93.4% survival (95% confidence interval, 0.849–1). The difference between the groups was not significant (p=0.381).
FIG. 1.
Kaplan-Meier plots of implant survival with 95% confidence intervals of implant survival for bone marrow aspirate concentrate (BMAC). Confidence intervals for Ficoll are not defined for this model because there were no events. Implants at risk are indicated at the bottom.
Discussion
Results of the present study on implant survival show that the difference between the clinically feasible BMAC method was not significantly (p=0.381) different from the approved, but clinically impractical Ficoll method. Similar results were observed in the split mouth study of Rickert et al.,8 in which sinus augmentation with the BMAC method was compared with the conventional method, which involves biomaterial being mixed with autologous bone. In that study, 91% of the dental implants osseointegrated in the BMAC site, whereas 100% osseointegrated in the control. As in the present study, no implants were lost after functional loading.8
Conclusion
This study indicates that the BMAC method is a clinically effective and a suitable chair-side alternative to the established Ficoll method for hard tissue regeneration.
Abbreviations Used
- BBM
bovine bone mineral
- BMAC
bone marrow aspirate concentrate
- MSC
mesenchymal stem cell
Acknowledgments
This study received technical support from Harvest Technologies and Geistlich Biomaterials. Financial support for in vitro work was given by the Camlog Foundation.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Duttenhoefer F, Souren C, Menne D, et al. . Long-term survival of dental implants placed in the grafted maxillary sinus: systematic review and meta-analysis of treatment modalities. PLoS ONE. 2013;8:e75357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Smiler D, Soltan M. Bone marrow aspiration: technique, grafts, and reports. Implant Dent. 2006;15:229–235 [DOI] [PubMed] [Google Scholar]
- 3.Sauerbier S, Stricker A, Kuschnierz J, et al. . In vivo comparison of hard tissue regeneration with human mesenchymal stem cells processed with either the Ficoll method or the BMAC method. Tissue Engineering Part C: Methods 2010;16:215–223 [DOI] [PubMed] [Google Scholar]
- 4.Hermann PC, Huber SL, Herrler T, et al. . Concentration of bone marrow total nucleated cells by a point-of-care device provides a high yield and preserves their functional activity. Cell Transplant. 2008;16:1059–1069 [PubMed] [Google Scholar]
- 5.Wongchuensoontorn C, Liebehenschel N, Schwarz U, et al. . Application of a new chair-side method for the harvest of mesenchymal stem cells in a patient with nonunion of a fracture of the atrophic mandible–a case report. J Craniomaxillofac Surg. 2009;37:155–161 [DOI] [PubMed] [Google Scholar]
- 6.Buser D, Ingimarsson S, Dula K, et al. . Long-term stability of osseointegrated implants in augmented bone: a 5-year prospective study in partially edentulous patients. Int J Periodontics Restorative Dent. 2002;22:109–117 [PubMed] [Google Scholar]
- 7.Therneau TM, Grambsch PM. Expected survival. In: Modeling Survival Data: Extending the Cox Model. Dietz K, Gail M. (eds.) Springer-Verlag: Heidelberg, Germany; pp. 261–281; 2000 [Google Scholar]
- 8.Rickert D, Vissink A, Slot WJ, et al. . Maxillary sinus floor elevation surgery with BioOss® mixed with a bone marrow concentrate or autogenous bone: test of principle on implant survival and clinical performance. Int J Oral Maxillofac Surg. 2013;29:1–5 [DOI] [PubMed] [Google Scholar]