Abstract
The generation of a heterosubtypic memory T cell response is important for cross-protective immunity against unrelated strains of influenza virus. One way to facilitate the generation of the memory T cell population is to control the activity of immune modulatory agents. The enzyme, indoleamine 2,3-dioxygenase (IDO), is upregulated during influenza infection by the interferon response where IDO activity depletes tryptophan required in T cell response. In this study, IDO activity was pharmacologically inhibited with 1-methyl-tryptophan (1MT) during the primary response to influenza virus infection and the effect on the memory T cell response was evaluated. 1MT treatment improved the memory T cell response to influenza virus challenge by increasing interferon gamma expression by CD4 and CD8 T cells, and numbers of lung virus-specific CD8+ T cells, and increased the Th1 response as well as modifying the immunodominance hierarchy to increase the number of subdominant epitope specific CD8+ T cells, a feature which may be linked to decreased regulatory T cell function. These changes also accompanied evidence of accelerated lung tissue repair upon virus challenge. These findings suggest that modulation of IDO activity could be exploited in influenza vaccine development to enhance memory T cell responses and reduce disease burden.
Introduction
Influenza A virus is a worldwide health threat causing seasonal morbidity and mortality (50). Emerging strains of influenza continually threaten, as exemplified by the recent H1N1 influenza pandemic (43), producing illness in at-risk populations (1). Vaccination can diminish influenza transmission and disease severity in part by antibody-mediated protection. However, subtype-specific antibodies do not provide adequate protection against heterosubtypic influenza virus strains, whereas memory T cells can provide immunity via recognition of conserved viral epitopes (17,19). Therefore, inducing a robust memory T cell response is important for protection against a population of influenza subtypes.
Indoleamine 2,3-dioxygenase (IDO) is an immune modulatory enzyme expressed by antigen presenting cells (APCs) in response to proinflammatory mediators such as interferons (IFN) and TNF-α (4,14,32,46). APCs, including plasmacytoid dendritic cells (pDC), express IDO, which depletes tryptophan (Trp) and produces metabolites such as kynurenine (Kyn) (13), leading to activation of the GCN2 kinase pathway (14), which induces anergy in effector T cells (21), but upregulates regulatory T cells (Treg) (2,39). IDO also alters the cytokine environment during activation of T cells, promoting a Th2- over Th1-type cytokine response (56) and has been shown to compromise CD8+ T cell cytotoxicity (6,21).
Influenza virus infection has been shown to induce IDO (57) that may affect T cell priming and differentiation (23). Indeed, inhibition of IDO enhances the primary T cell response to influenza by increasing Th1 and virus-specific CD8+ T cells (15), but whether these changes are recapitulated in the memory T cell response to influenza virus challenge is not known.
Therefore, it is of importance to evaluate the relationship between IDO inhibition in the primary response and its potential impact on the memory response, particularly for virus-specific T cells for translation towards potentially implementing IDO inhibitors to improve the outcomes of vaccination. This study evaluates the hypothesis that inhibition of IDO enhances the memory T cell response against heterosubtypic infection. IDO inhibition by 1-methyl-tryptophan (1MT) resulted in a heightened memory T cell response characterized by higher IFNγ expression by CD4+ and CD8+ T cells and a broader repertoire of CD8+ T cells without compromising the response against immunodominant epitopes.
Materials and Methods
Influenza, mice, and IDO inhibition
Influenza A strains X31 (H3N2; A/Aichi/2/1968×A/Puerto Rico/8/1934) and PR8 (H1N1; A/Puerto Rico/8/1934) were propagated in 9-day-old embryonated chicken eggs, recovered from allantoic fluids, and stored at −80°C until use. Virus titers were determined by plaque assay using MDCK (16). Eight-to-ten week old female C57BL/6 mice (Charles River, Wilmington, MA) were anesthetized using 2,2,2-tribromoethanol (Avertin) (51) and intranasally (i.n.) infected with 103 plaque forming units (PFU) of X31 in 50 μL PBS. IDO was inhibited by oral administration of D,L-1-methyl-tryptophan (Sigma-Aldrich, St. Louis, MO) in drinking water (2 mg/mL with 2 mg/mL of aspartame) during the primary T cell response (15,25). Aspartame was added to increase palatability or used alone in the control group. Both solutions were filter-sterilized and provided to cohorts of mice ad libitum 3 days before virus infection and thereafter for 14 days and was replaced with a fresh solution every 5 days. Twenty-eight days after i.n. infection with X31, mice were i.n. intranasally challenged with 10 LD50 of PR8 (100 PFU) in 50 μL PBS. 1MT was not administered before or after challenge with PR8. All animal work was approved by Institutional Animal Care and Use Committee of the University of Georgia.
Cell preparation and flow cytometry
At various time points post PR8 challenge, mice were euthanized and cells in the airways were collected by bronchoalveolar lavage (BAL). Single cell suspensions were also prepared from the mediastinal lymph nodes (MLN) in PBS following passage through 100 μm cell-strainers (BD Biosciences, San Jose, CA). Cell numbers from the tissue samples were enumerated using a Z2-Coulter-Counter (Beckman-Coulter, Brea, CA). These cells were immune phenotyped as previously described (53). Cells were stained with antibodies against CD8, CD4, CD62L, CD44 (BD Biosciences) in combination with MHC-Class I Tetramers (Emory University, Atlanta, GA) loaded with influenza peptides: NP366–374: ASNENMETM (H-2Db), PA224–233: SSLENFRAYV (H-2Db), and PB1703–711: SSYRRPVGI (H-2Kb).
For intracellular IFNγ cytokine staining, the cells were fixed and permeabilized with fixation/permeabilization buffer (BD Biosciences), then stained with anti-IFNγ antibodies in permeabilization buffer for 30 min at 4°C as described previously (15). CD4 and CD8 T cells were analyzed by flow cytometry using a BD LSR-II (BD Biosciences) where at least 50,000 events were recorded following gating on T cells (BD FACSDiva, BD Biosciences) and analyzed with FlowJo (Tree Star Inc., Ashland, OR). CD4 and CD8 T cells were analyzed by first gating on CD4+ and CD8+ positive lymphocytes, followed by gating on phenotypes of interest (e.g., IFNγ expression, tetramer binding). The % positive population was then multiplied by the total BAL cell count to enumerate the cell population of interest.
High pressure liquid chromatography (HPLC)
HPLC was used to determine IDO activity by measuring the concentration of Trp and Kyn in clarified lung homogenates as previously described (33). Briefly, samples were suspended in 15 mM acetate buffer (Sigma-Aldrich) and deproteinated using trichloroacetic acid (Sigma-Aldrich). Samples were clarified by centrifugation and filtration. Finally, samples were analyzed on a 4.6×50 mm reverse phase C18 column (Restek, Bellefonte, PA). All reagents where applicable are HPLC grade. The area under the curve of Trp and Kyn were integrated and converted to concentrations from a standard curve.
Influenza Virus Titer by TCID50
Virus titer was measured by TCID50 (49). Briefly, extracted lungs were homogenized using a tissue-lyser (Eppendorf, Hamburg, Germany). The supernatant from the centrifuged lysate was diluted in MEM (HyClone) containing 100 μg/mL of streptomycin, 100 IU/mL of penicillin, 250 ng/mL of amphotericin B (Mediatech), and 1 μg/mL of TPCK-Trypsin (Worthington, Lakewood, NJ) in 96-well plate (Corning) over MDCK cells grown in DMEM (HyClone) with 5% FBS. The plates were incubated for 72 h and mixed with equal volume of 0.5% chicken erythrocytes in PBS, incubated for 1 h, and scored for agglutination.
In Vitro CTL restimulation assay
Memory T cells generated following X31 infection were expanded in vitro (26). Briefly, spleen and MLN-derived memory T cells were stimulated in vitro with syngeneic splenocytes infected with 1000 HAU/mL of X31 and mitotically inactivation with mitomycin C (Sigma-Aldrich) (44). Lymphocytes were restimulated for 6 days in RPMI-1640 with 10% FBS, antibiotics, 50 μM β-mercaptoethanol (Sigma), and 20 U/mL of mouse IL-2 (BD Biosciences). Expanded T cells were co-incubated at various effector-to-target ratios with H-2D/Kb-restricted MC57G target cells (fibroblasts from C57BL/6 mice) which were infected with 100 HAU PR8 overnight. Target cells were stained with PKH67 (Sigma-Aldrich). CTL and target cells were co-incubated 37°C for 4 h in 96-well V-bottom plates (Corning) and gently centrifuged (200 g for 1 min) to maximize cell contact. Cell cytotoxicity was analyzed by flow cytometry: MC57G (PKH67+) were gated and assessed for apoptosis as defined by binding of 7AAD+ and/or Annexin V+ (apoptosis) but not 7AAD alone (necrosis) (24).
Histopathology and immunohistochemistry
Lungs were removed, perfused with 10% buffered formalin through the heart and trachea, and fixed in 10% buffered formalin (Fisher Scientific) overnight. The sections were embedded in paraffin, cut in 5 μm-thick sections and stained with hematoxylin and eosin (20). IHC was performed on sections that were blocked with 1% bovine serum albumin in PBS and treated with Proteinase K (Dako, Carpentaria, CA) to minimize nonspecific staining and expose epitopes. Subsequently, sections were incubated with 1 μg/mL goat anti-influenza A H1N1 antibody (Meridian Life Science, Inc., Soca, ME) and then incubated with a biotinylated anti-goat rabbit antibody (Dako) for 10 min at room temperature. Finally, strepavidin/horseradish peroxidase (HRP) complex (Dako) was added according to manufacturer's instructions with color development by HRP substrate diaminobenzidine (DAB) addition. The sections were evaluated by light microscopy.
A histological score and remarkable inflammatory parameters for each lung was determined according to the following criteria: 0=no lung abnormality; 1=< 10% of airways inflamed; 2=10%–30% of airways inflamed; 3=30%–50% of airways inflamed and 4=> 50% of airways inflamed (20). The slides were evaluated without knowledge of the type of mouse or exposure to antigen. The area covered by an eyepiece grid was judged to be normal or abnormal.
Regulatory T cell (Treg) stimulation
Treg-mediated suppression of influenza-specific CD8+ T cell proliferation was evaluated (45). Two days after PR8 challenge, spleens and MLN from 1MT or control mice were removed and enriched for Tregs using a Treg Isolation Kit (Miltenyi Biotec, Auburn, CA). Tregs from age-matched naïve mice were used to address nonspecific suppressive activities. CD8+ T cells were negatively selected from spleens of X31-immune mice without 1MT treatment. The purity of enriched CD8+ T cells and Tregs was >90% by flow cytometry. Enriched Tregs were co-incubated with CD8+ T cells at specified ratios in the presence of X31-infected stimulator cells as described for in vitro restimulation of CTL assay. As a positive control, 2 μg/mL concanavalin A (Sigma) was added to a culture of CD8+ T cells only (no Treg). To evaluate Treg activity, 48 h after co-incubation with CD8+ T cells, 10 μM of EdU (5-ethynyl-2´-deoxyuridine, Invitrogen) was added for 2 hours, and the level of proliferation as determined by EdU incorporation. The Tregs from the co-culture were immunophenotyped by flow cytometry for intracellular granzyme B (eBioscience) and surface CTLA-4 (eBioscience) expression. Differences in the rate of CD8+ T cell proliferation in co-cultures with Tregs from the cohorts were used to measure Treg suppression.
Statistics
Statistical significance (p value<0.05) was tested between means of 1MT treated mice and controls using a Student's t-test. Exact p-values are listed when significant. All statistical analyses were performed using GraphPad Prism (GraphPad Software, San Diego, CA).
Results
1-Methyl-tryptophan (1MT) reduces IDO activity
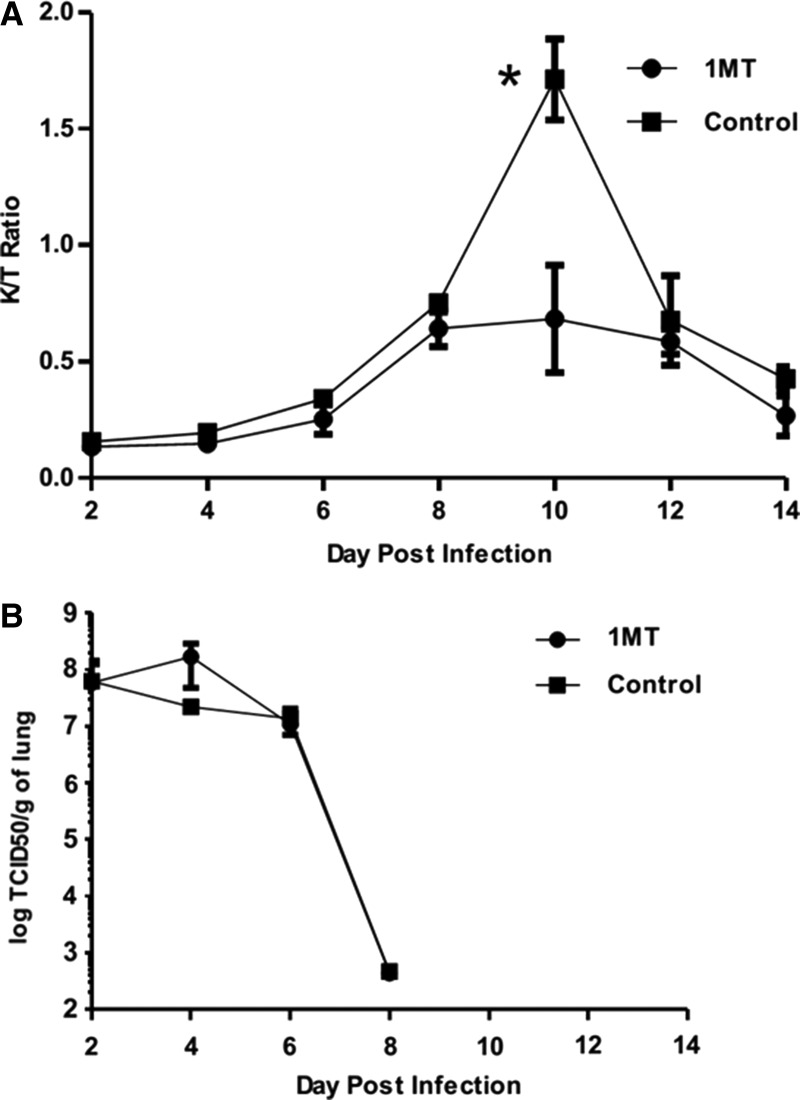
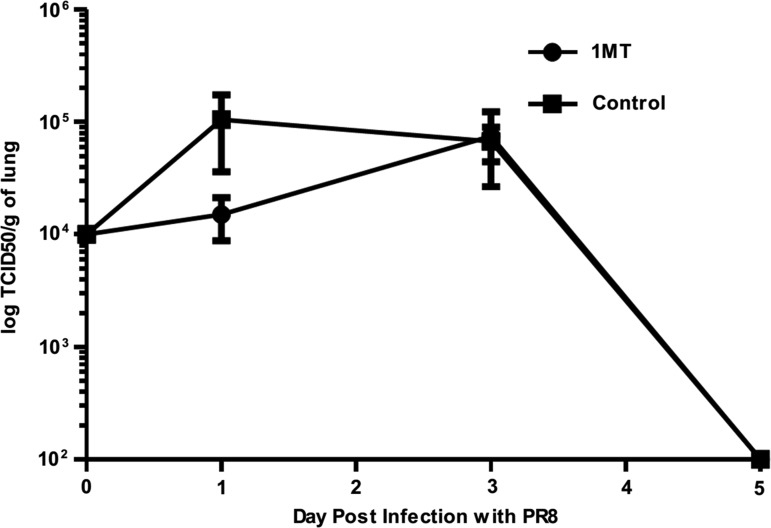
To determine IDO activity in the lungs following influenza (X31) infection, the ratio of IDO’s product (kynurenine, Kyn, to substrate (tryptophan, Trp) was measured in lung homogenates of infected mice (Fig. 1A). Where IDO is active, the product [Kyn] to substrate [Trp] ratio increases (29). X31 infection induced peak IDO activity between days 8 and 12 post-infection (pi), where significant (p=0.01) difference between 1MT-treated and control mice was evident at day 10 pi. This finding suggests that differences in the memory response are most likely due to IDO inhibition. Lung virus titers were not substantially affected by IDO inhibition (Fig. 1B) and consistent with virus clearance at day 8 pi (Fig. 1B) (36).
FIG. 1.
1-Methyl-tryptophan (1MT) decreases IDO activity induced by influenza virus infection. X31-primed mice were treated with 1MT or vehicle control and assessed for IDO activity (A) and virus (B). (A) [Kyn]/[Trp] ratios±SEM in lung homogenate was measured by HPLC at the indicated time points post-X31 primary infection in mice treated with 1MT (circles) or vehicle control (squares). Data are representative of two independent experiments. Asterisk indicates statistical significance (p=0.01). (B) X31 lung virus titer (log TCID50) during primary infection. Data are representative of three independent experiments (n=3 per experimental group).
Inhibition of IDO activity increases memory Th1 response
To assess the effect of IDO inhibition on the memory T cell response to influenza virus, X31-primed mice was treated with 1MT or control and challenged with PR8 28 days post infection. After challenge with PR8, the total number of CD4+ and CD8+ effector (CD62Llo CD44hi) T cells in the BAL and MLN were determined at days 0, 1, 3, 5, 7, and 9 post-challenge (p.c.) (Table 1). The peak effector CD4+ T cell response in the BAL occurred between day 5 and 7 p.c. in the control and 1MT group, respectively, and at day 5 p.c. for both groups in the MLN. BAL also had higher numbers at day 5 p.c. in the 1MT group. There were no significant differences between the 1MT and control groups with respect to the number of effector T cells, so both CD4+ and CD8+ T cells further analyzed at the peak response to assess if IDO had an effect on specific antiviral parameters of the memory T cell response.
Table 1.
Effector T Cell Response to Heterologous Influenza Challenge
| Effector CD4+ T cell number±SEM (x1000) | ||||
|---|---|---|---|---|
| Day post challenge | 1MT BAL | Control BAL | 1MT MLN | Control MLN |
| 0 | 1±0 | 2±0 | 151±4 | 169±17 |
| 1 | 5±1 | 4±1 | 169±25 | 181±45 |
| 3 | 11±4 | 9±5 | 263±103 | 208±44 |
| 5 | 73±12 | 48±20 | 602±166 | 496±0 |
| 7 | 50±6 | 84±41 | 486±88 | 473±99 |
| 9 | 32±5 | 33±7 | 354±115 | 287±30 |
| Effector CD8+ T cell number±SEM (x1000) | ||||
|---|---|---|---|---|
| Day post challenge | 1MT BAL | Control BAL | 1MT MLN | Control MLN |
| 0 | 3±1 | 8±2 | 23±1 | 21±4 |
| 1 | 13±3 | 11±3 | 26±3 | 23±1 |
| 3 | 12±3 | 8±3 | 46±17 | 42±9 |
| 5 | 289±120 | 177±69 | 411±82 | 370±0 |
| 7 | 383±72 | 480±223 | 339±85 | 386±81 |
| 9 | 346±51 | 363±51 | 308±83 | 242±53 |
X31 primed mice treated with 1MT or vehicle control were challenged with PR8 28 days later. Number of effector (CD44hi CD62Llo) CD4+/CD8+ T cells presenet in airways (BAL) and MLN at day 0 (before challenge) through day 9 post challenge. Numbers are average cell numbers±SEM. Data are representative of three independent experiments.
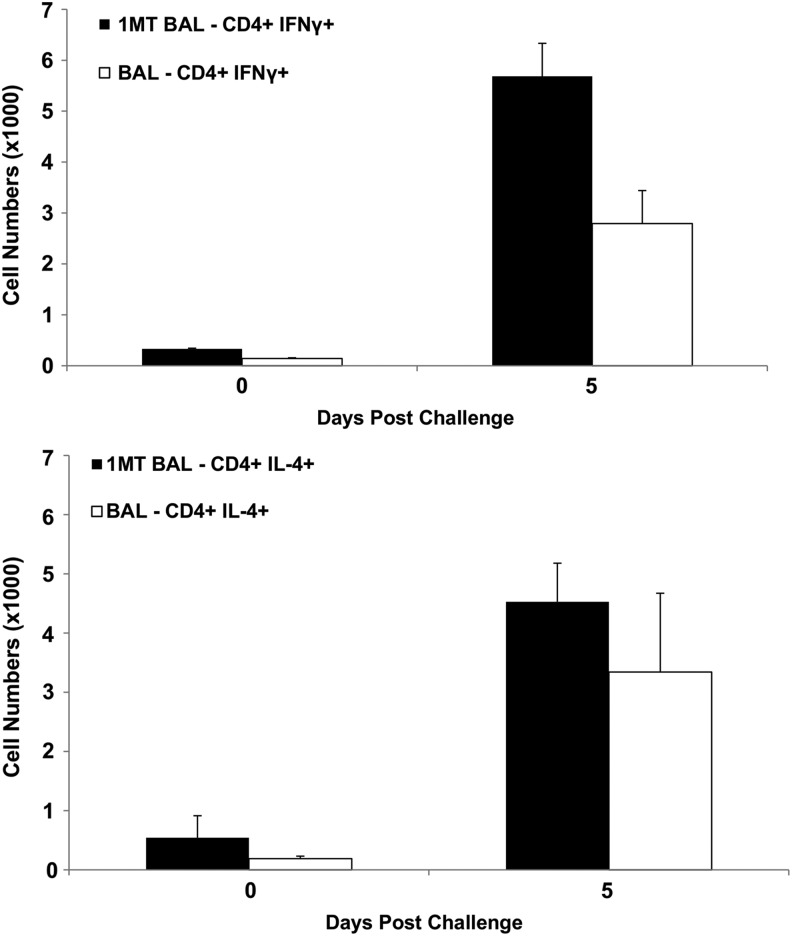
IDO inhibition has been associated with a greater Th1- (IFNγ+) over Th2- (IL-4+) type cytokine response (56). Given the importance of Th1-type cytokines in the response against influenza (18), memory CD4+ T cells were evaluated at day 5 and 7 p.c. to evaluate the effect of IDO on the Th1/Th2 axis. At day 5 p.c., the frequency of Th1 was significantly (p=0.04) higher in BAL of 1MT-treated mice compared to control (Fig. 2A). Th2 response in the BAL was equivalent between the groups (Fig. 2B), suggesting that IDO inhibition during priming promotes memory Th1.
FIG. 2.
IDO inhibition during the primary response increases the memory Th1 response to influenza virus challenge. X31-primed mice were treated with 1MT or vehicle control and rested 28 days prior to intranasal challenge with PR8 influenza virus. The numbers of CD4+ T cells expressing IFNγ (A) or IL-4 (B) in BAL following PR8 challenge are indicated. Numbers are averaged±SEM, and the data are representative of two independent experiments where n=3 per experimental group.
IDO inhibition affects CD8+ T cell epitope specificity
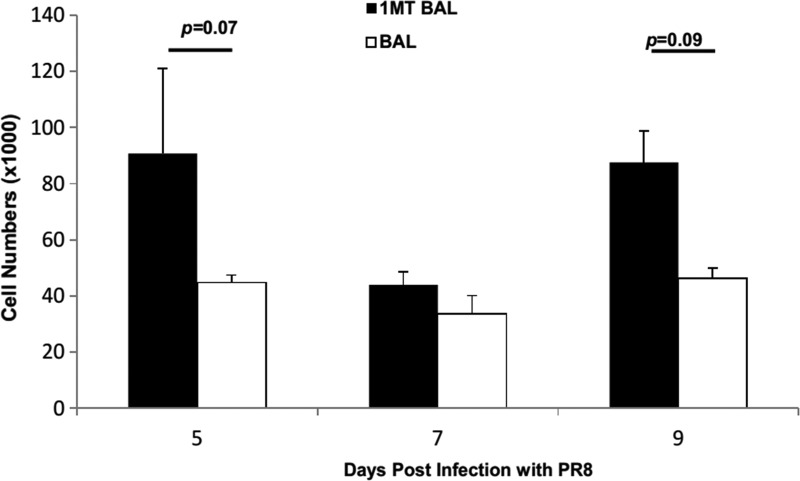
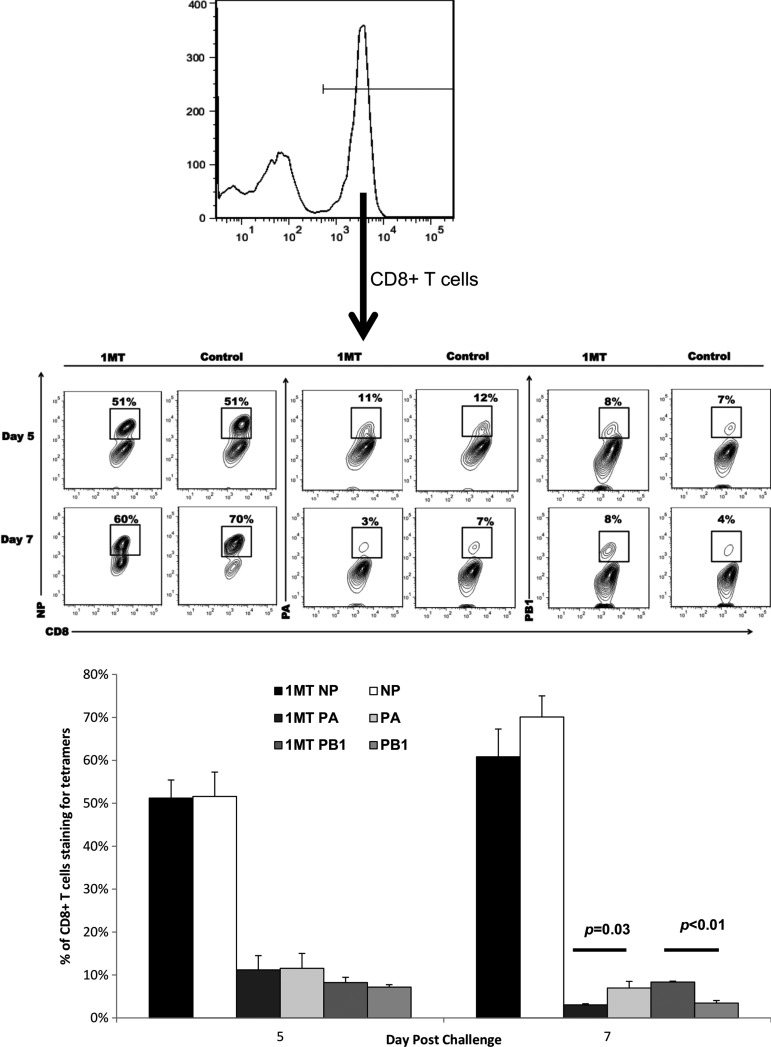
The effect of IDO inhibition was examined for CD8+ cells. 1MT treatment did not result in statistically significant higher numbers of memory CD8+ IFN7+ T cells in BAL (p=0.07, 0.09 at days 5 and 9 p.c., respectively) (Fig. 3). However, when virus specificity and cytokine expression were taken into account, significant differences were evident. First, the immunodominance pattern (NP366–374>PA224–233>PB1703–711) (5) shifted (Fig. 4). The response was equivalent at day 5, but at day 7, there was a decrease in the fraction of CD8+ T cells reactive to NP and PA (p=0.03), but a two-fold increase in reactivity against the subdominant epitope PB1 (p<0.01). Although these differences appear minor, it should be noted that the response toward subdominant epitopes is typically small to begin with (5), so what may appear as a minor change compared to dominant epitopes recognized likely has substantial biological significance, and importantly, these changes are attributable to changes in the repertoire which occurred in the primary response due to IDO inhibition (15).
FIG. 3.
X31 primed mice were treated with 1MT or vehicle control and rested 28 days prior to intranasal challenge with PR8 influenza virus. At 5, 7, and 9 days post challenge, the number of CD8+ T cells expressing IFNγ in the BAL were determined. Numbers are average cell numbers±SEM.
FIG. 4.
IDO activity during the primary CD8+ T cell response to influenza modifies immunodominance in influenza challenged mice. BAL cells from 1MT and control treated groups challenged with PR8 were stained for CD8+ expression and specificity against influenza NP, PA, or PB1 epitopes. Contour plots indicated populations from cells were initially gated for CD8+ expression (top histogram). Percentages in the contour plots (middle panel) and bar graphs (bottom panel) indicate the average frequency (n=3) of CD8+ T cells that are specific for the indicated epitopes±SEM at day 5 or 7 post-challenge.
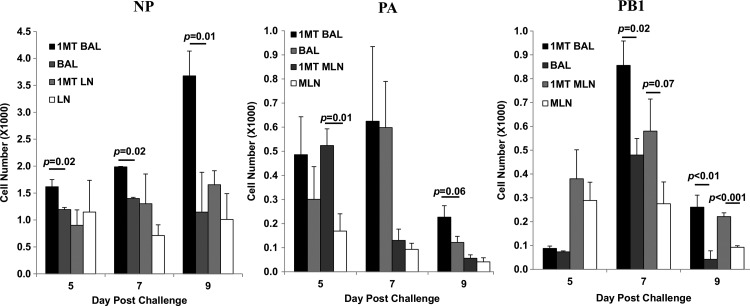
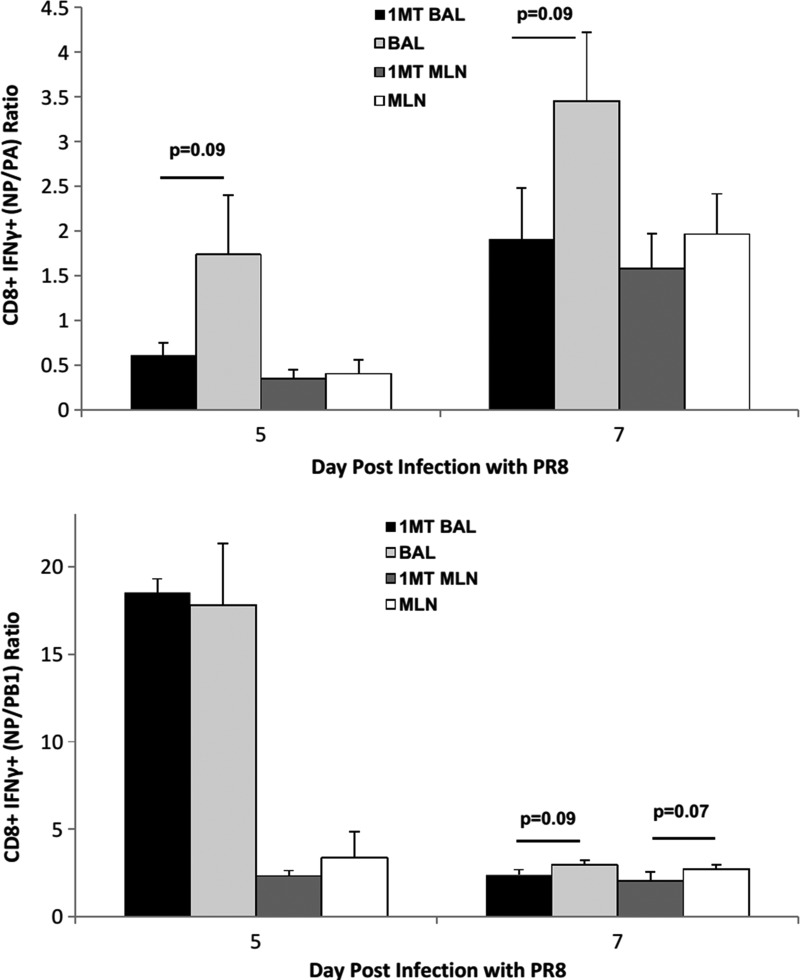
To determine the relationship between immunodominance and the kinetics of the response, the number of CD8+ IFNγ+ T cells against NP, PA, and PB1 was determined at day 5 p.c. onwards (Fig. 5). IFNγ was measured because expression by CD8+ T cells supports Th1 function and effector cell recruitment (33,55), virus clearance, and protection (7). IFNγ+ NP-specific cells in the BAL of 1MT-treated mice were significantly higher at day 5, 7, and 9 p.c. (p<0.05 for all days) compared to controls. The late resurgence of NP-specific cells at day 9 pi in 1MT treated mice may reflect a second memory T cell population that migrated to the airways, although no substantial differences were detected in the MLN. PA-specific cells in the MLN at day 5 p.c. (p=0.01), and BAL at day 9 p.c. (p=0.06) were higher in 1MT treated mice compared to controls. PB1-specific cells were also higher at day 7 p.c. in BAL (p=0.02), and remained higher through day 9 p.c. in 1MT-treated mice. Cells in MLN were also higher at day 7 p.c. (p=0.07), through day 9 (p=0.001). The ratio of NP- to PA-specific CD8+ T cells were lower in 1MT BAL at day 5 and 7 p.c. (p=0.09; Fig. 6), as were NP- to PB1-specific CD8+ T cells in both MLN (p=0.07) and BAL (p=0.09) at day 7 p.c.
FIG. 5.
See Figure 3 for introductory information. The number of IFNγ+CD8+ T cells in the BAL and MLN specific for NP, PA, and PB1 epitopes±SEM were determined.
FIG. 6.
See Figure 3 for introductory information. The average ratio±SEM of NP-specific CD8+ IFNγ+ T cells to PA and PB1 specific CD8+ IFNγ+ T cells at day 5 and 7 p.c. was determined. Data are representative of three independent experiments (n=3 per experimental group).
In vitro CTL assays revealed no difference in the cytotoxicity of these cells (data not shown); thus the effector function as revealed by CTL cytotoxicity of the memory CD8+ T cells was functionally equivalent.
IDO inhibition decreases CTLA-4 expression on virus-specific Tregs
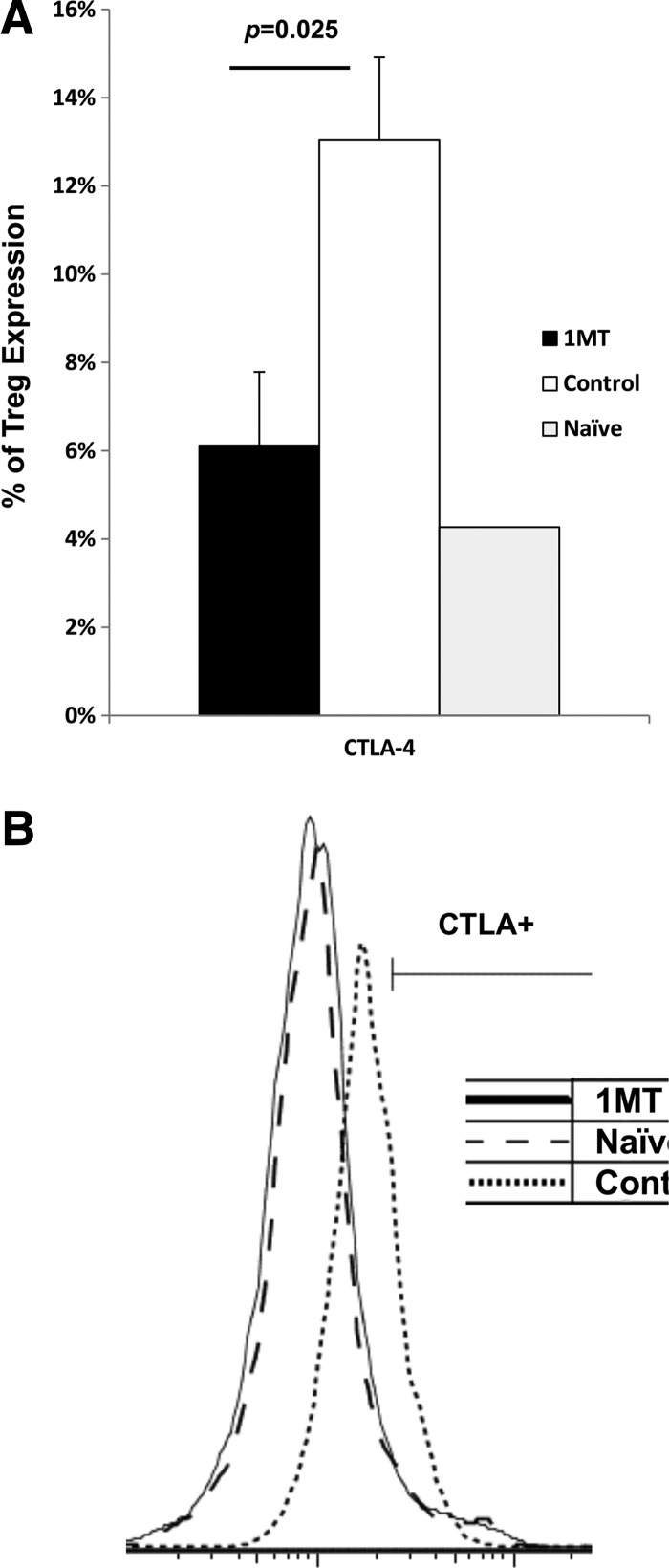
IDO may affect Tregs' suppression of effector T cells by modifying CTLA-4 mediated contact-dependent suppression as its expression and activity is affected by IDO (11,42). The number of CD4+ Foxp3+ (Tregs) responding to PR8 challenge was not changed by 1MT treatment (data not shown). Therefore, Tregs were further analyzed for granzyme B and CTLA-4 expression, effector molecules which mediate contact dependent IDO-related suppression (40). Fewer Tregs were evident expressing CTLA-4 following virus stimulation (p=0.025) (Fig. 7), and Tregs expressed less CTLA-4 on the cell surface on a per-cell basis based on median fluorescence intensity (MFI) (p=0.05) (data not shown). Therefore, IDO inhibition results in fewer Tregs expressing CTLA-4. No difference in granzyme B expression was evident (data not shown).
FIG. 7.
IDO-induced Tregs have different expression patterns of suppressive surface molecules. Mice treated with 1MT or control during infection with X31 was challenged with PR8 28 days after infection. Two days after challenge with PR8, Treg cells from the spleen and MLN were examined. Tregs were co-incubated with X31 infected stimulator cells at a 1:1 ratio. Two days after co-incubation, the culture was analyzed for % CTLA-4 expression on Tregs (A) (n=3) with a representative histogram (B). Age-matched naïve mice were used as a negative control.
IDO inhibition accelerates lung tissue repair in PR8 challenged mice
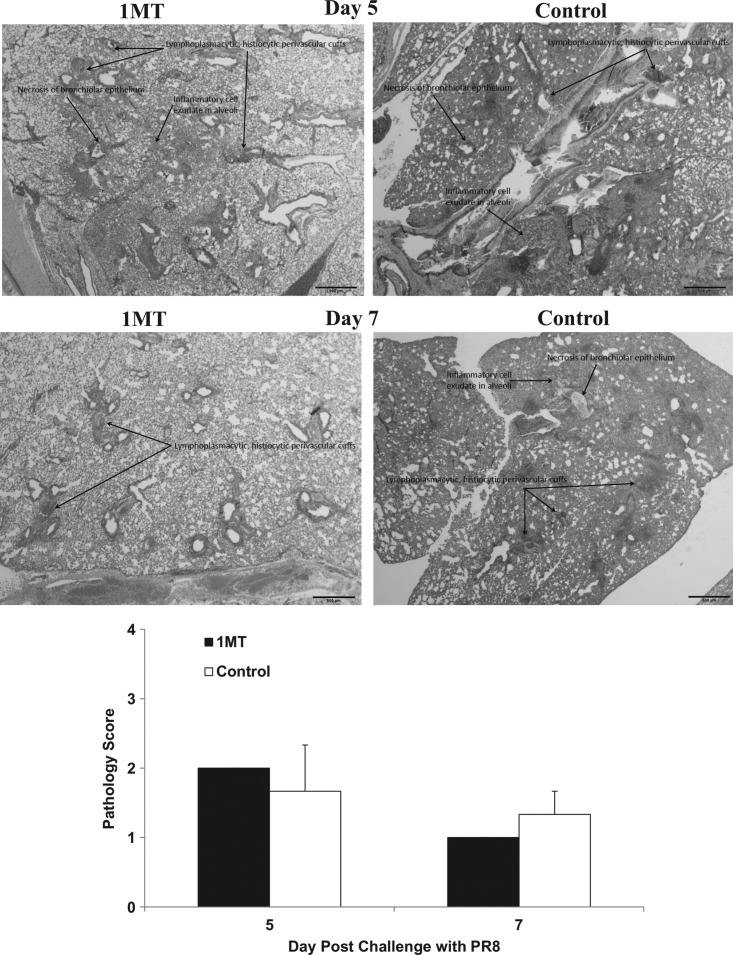
IDO inhibition increases pulmonary memory T cell infiltration and cytokine responses as a consequence of IDO inhibition, which can affect lung pathogenesis. Specifically, heightened IFNγ expression by Th1 and virus-specific CD8+ T cells may mitigate pathology (55). Evaluation of overall gross pathology at days 5 and 7 p.c. showed no substantial differences related to IDO inhibition (Fig. 8). At day 5 p.c., control lungs had increased fibrin deposition in the alveoli with neutrophil involvement in the bronchioles which may delay tissue repair (30) compared to 1MT treated mice's lungs. At day 7 p.c., lungs from control mice exhibited increased necrosis, alveolar exudate, and increased neutrophil recruitment compared to 1MT-treated mice lungs. These observations are consistent with increased fibrin deposition, as fibrin from the alveolar capillaries is expressed to control exudates. Since IFNγ regulates fibrinolysis (38), the increased IFNγ cytokine environment afforded by IDO inhibition may promote lung repair due to 1MT treatment.
FIG. 8.
Inhibition of IDO during the primary immune response to influenza virus infections decreases histopathology associated with a lethal influenza challenge independent of virus clearance. X31 primed mice, which were treated with 1MT or vehicle control were rested 28 days prior to challenge with PR8. Representative lung sections following PR8 challenge are shown for IMT and control mice. Average gross pathology scores±SEM are shown. A histological score for each lung was determined according to the following criteria: 0=no lung abnormality; 1=<10% of airways inflamed; 2=10%–30% of airways inflamed; 3=30%–50% of airways inflamed, and 4=>50% of airways inflamed. Scale bars=500 μm.
To determine if virus load was also affected by IDO, levels of infectious virus were determined in the lungs but no differences in infectious virion (Fig. 9) or antigen (IHC, data not shown) were evident. These findings indicate that, although 1MT treatment is associated with an improved memory T cell response, the outcome does not have a statistically significant effect on virus clearance, similar to what was seen in the primary response (15).
FIG. 9.
See Figure 8 for introductory information. Kinetics of infectious virus levels by TCID50 following challenge with PR8. Data are representative of three independent experiments (n=3 per experimental group).
Discussion
Inhibition of IDO activity during the primary response to influenza infection modifies aspects of the memory T cell response to secondary influenza challenge. These changes are characterized by higher numbers of memory Th1 (IFNγ+) cells and virus-specific CD8+ IFNγ+ T cells that may be linked to an increased memory T cell precursor frequency and Th1-response in the primary response due to IDO inhibition (15). It is important to emphasize that no IDO inhibition was employed prior to or after PR8 challenges, so the differences observed in the secondary response are linked to the effects of IDO treatment in the primary response.
Specifically, IFNγ from increased Th1 and virus-specific CD8+ T cells in the primary response due to IDO inhibition (15) may have promoted the development of memory Th1 and virus-specific T cells (31) and increased burst size which gave rise to increased memory T cell precursor frequency (12,27). These differences should be considered in the context of repeat seasonal influenza challenge in the human population, where individuals experience influenza antigen in the form of vaccination and natural challenges multiple times through their lifetime to shape the immune repertoire. Because differences in memory T cells were seen just from IDO inhibition in the primary response, one might anticipate that these effects could be sustained or even amplified if IDO inhibitors were applied. For example, NP- and PA-specific CD8+ T cells still dominated the response, but the increase in PB1-specific CD8+ T cells is notable. These changes are consistent with broadened epitope specificity and may be a favorable feature to promote immunity against heterosubtypic influenza challenges and escape mutants (54).
Although the frequency and epitope specificity of the BAL-derived T cells were different in 1MT treated mice, the ability of these T cells to kill their targets was not measurably affected by IDO. It is well known that memory T cell response in this model is robust, so minor changes in the immunodominance profile or having higher number of IFNγ+ CTLs may not reveal a detectable difference in the capacity to secondary virus challenges. This is evident by the fact that PR8 is already cleared by day 5 (Fig. 9), but differences in CD4+ and CD8+ T cells is seen at day 5 p.c. and onwards. Challenge with influenza strains that persist longer may reveal differences in clearance patterns and CTL cytotoxicity. Although virus clearance was not affected, this is the first study to report that the memory T cell response was modified by IDO inhibition during the primary response.
In addition to altering the memory T cell response, 1MT-treated mice had lower pulmonary neutrophil infiltrates which may exacerbate pathology (41). Higher Th1 and IFNγ+ CD8+ T cell response by IDO inhibition may have contributed to the accelerated tissue repair, as IFNγ antagonizes Th2 development and activity. This is generally favorable in influenza infections, as Th2 responses promote eosinophilia (34), and is associated with a pathological response without virus clearance (18). IFNγ also modulates pathology by controlling inflammation, antagonizes neutrophil-mediated damage (55), which with increased cytokine expression is largely responsible for influenza induced immunopathology (38). Finally, IFNγ also mediates fibrinolysis (38,52) to restore normal pulmonary functions.
These changes are likely attributable to T cell priming during the early response to influenza virus infection via resident antigen presenting cells (APCs) in the airways (9) and stimulation of some TLR pathways that affect IDO activity (10,37). APCs expressing IDO are involved in the regulation of T cell activation, differentiation, and expansion (2). Tregs are also upregulated in response to IDO, and in turn, further upregulate IDO activity in DC by a positive feedback mechanism (2,37,39) by CTLA-4/B7 ligation (8). This is the first report to show that IDO regulates CTLA-4 expression in virus-specific Tregs, and this aspect is particularly relevant because Treg CTLA-4 expression disrupt virus-specific effector T cell function (28). Specifically, Tregs mediate selective suppression of specific subpopulations of influenza-specific CD8+ T cells which could account for the disparity in virus-specific CD8+ T cell response (22). Although granzyme B was not apparently affected by IDO, PD-1 (47) and TGF-β (28) may also reduce antiviral T cell responses and will be explored in the future as additional possible suppressive mediators in response to influenza.
Another consideration is the other arm of the Treg differentiation, Th17-type cells. The effect of IDO on the Treg/Th17 axis has been examined in noninfectious disease models (2,48), but presently the only report examining this for influenza virus comes from our laboratory (15) which addressed how IDO inhibition can affect Th17 cells. Indeed, it would be important to understand this aspect better for evaluating how inhibiting IDO activity could contribute to the design of influenza vaccines.
The cornerstone of developing a good vaccine is the production of high frequencies of effector memory precursors which is sustained as the vaccinated individuals' age. Future work will concentrate on seeing to what extent that the changes observed due to IDO inhibition in the resting memory population will be sustained, including the recall of virus-specific T cells and cytokine expression patterns. It is possible that IDO modifies one or more of these pathways during influenza infections and vaccinations; the use of IDO inhibitors during influenza vaccination offers intriguing prospects.
Acknowledgments
This study was supported by the Georgia Research Alliance and National Institute of Health's U01 grant # AI083005-01.
Author Disclosure Statement
The authors declare no competing financial interests.
References
- 1.2009 Hospitalized patients with novel influenza A (H1N1) virus infection–California, April–May, 2009. MMWR Morb Mortal Wkly Rep 2009;58:536–541 [PubMed] [Google Scholar]
- 2.Baban B, Chandler PR, Sharma MD, et al. IDO activates regulatory cells and blocks their conversion into Th17-like T cells. J Immunol 2009;183:2475–2483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baumgarth N, and Kelso A. In vivo blockade of gamma interferon affects the influenza virus-induced humoral and the local cellular immune response in lung tissue. J Virol 1996;70:4411–4418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Belladonna ML, Volpi C, Bianchi R, et al. Cutting edge: Autocrine TGF-beta sustains default tolerogenesis by IDO-competent dendritic cells. J Immunol 2008;181:51945198. [DOI] [PubMed] [Google Scholar]
- 5.Belz GT, Xie W, and Doherty PC. Diversity of epitope and cytokine profiles for primary and secondary influenza a virus-specific CD8+ T cell responses. J Immunol 2001;166:4627–4633 [DOI] [PubMed] [Google Scholar]
- 6.Boasso A, Hardy AW, Anderson SA, et al. HIV-induced type I interferon and tryptophan catabolism drive T cell dysfunction despite phenotypic activation. PLoS One 2008;3:e2961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bot A, Bot S, and Bona CA. Protective role of gamma interferon during the recall response to influenza virus. J Virol 1998;72:6637–6645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cecchinato V, Tryniszewska E, Ma ZM, et al. Immune activation driven by CTLA-4 blockade augments viral replication at mucosal sites in simian immunodeficiency virus infection. J Immunol 2008;180:5439–5447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chaperot L, Blum A, Manches O, et al. Virus or TLR agonists induce TRAIL-mediated cytotoxic activity of plasmacytoid dendritic cells. J Immunol 2006;176:248–255 [DOI] [PubMed] [Google Scholar]
- 10.Chen W, Liang X, Peterson AJ, et al. The indoleamine 2,3-dioxygenase pathway is essential for human plasmacytoid dendritic cell-induced adaptive T regulatory cell generation. J Immunol 2008;181:5396–5404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Coquerelle C, Oldenhove G, Acolty V, et al. Anti-CTLA-4 treatment induces IL-10-producing ICOS+ regulatory T cells displaying IDO-dependent anti-inflammatory properties in a mouse model of colitis. Gut 2009;58:1363–1373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Doherty PC, Hou S, and Tripp RA. CD8+ T-cell memory to viruses. Curr Opin Immunol 1994;6:545–552 [DOI] [PubMed] [Google Scholar]
- 13.Fallarino F, Grohmann U, Vacca C, et al. T cell apoptosis by tryptophan catabolism. Cell Death Differ 2002;9:1069–1077 [DOI] [PubMed] [Google Scholar]
- 14.Fitzgerald P, Cassidy Eugene M, Clarke G, et al. Tryptophan catabolism in females with irritable bowel syndrome: Relationship to interferon-gamma, severity of symptoms and psychiatric co-morbidity. Neurogastroenterol Motil 2008;20:1291–1297 [DOI] [PubMed] [Google Scholar]
- 15.Fox JM, Sage LK, Huang L, et al. Inhibition of indoleamine 2,3-dioxygenase enhances the T-cell response to influenza virus infection. J Gen Virol 2013;94:1451–1461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gabbard J, Velappan N, Di Niro R, et al. A humanized anti-M2 scFv shows protective in vitro activity against influenza. Protein Eng Des Sel 2009;22:189–198 [DOI] [PubMed] [Google Scholar]
- 17.Ge X, Tan V, Bollyky PL, et al. Assessment of seasonal influenza A virus-specific CD4 T-cell responses to 2009 pandemic H1N1 swine-origin influenza A virus. J Virol 2010;84:3312–3319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Graham MB, Braciale VL, and Braciale TJ. Influenza virus-specific CD4+ T helper type 2 T lymphocytes do not promote recovery from experimental virus infection. J Exp Med 1994;180:1273–1282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gras S, Kedzierski L, Valkenburg SA, et al. Cross-reactive CD8+ T-cell immunity between the pandemic H1N1-2009 and H1N1-1918 influenza A viruses. Proc Natl Acad Sci USA 2010;107:12599–12604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gudmundsson G, and Hunninghake GW. Interferon-gamma is necessary for the expression of hypersensitivity pneumonitis. J Clin Invest 1997;99:2386–2390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guillonneau C, Mintern JD, Hubert FX, et al. Combined NKT cell activation and influenza virus vaccination boosts memory CTL generation and protective immunity. Proc Natl Acad Sci USA 2009;106:3330–3335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Haeryfar SM, DiPaolo RJ, Tscharke DC, et al. Regulatory T cells suppress CD8+ T cell responses induced by direct priming and cross-priming and moderate immunodominance disparities. J Immunol 2005;174:3344–3351 [DOI] [PubMed] [Google Scholar]
- 23.Harty JT, and Badovinac VP. Shaping and reshaping CD8+ T-cell memory. Nat Rev Immunol 2008;8:107–119 [DOI] [PubMed] [Google Scholar]
- 24.Hoppner M, Luhm J, Schlenke P, et al. A flow-cytometry based cytotoxicity assay using stained effector cells in combination with native target cells. J Immunol Methods 2002;267:157–163 [DOI] [PubMed] [Google Scholar]
- 25.Hou DY, Muller AJ, Sharma MD, et al. Inhibition of indoleamine 2,3-dioxygenase in dendritic cells by stereoisomers of 1-methyl-tryptophan correlates with antitumor responses. Cancer Res 2007;67:792–801 [DOI] [PubMed] [Google Scholar]
- 26.Hou S, and Doherty PC. Partitioning of responder CD8+ T cells in lymph node and lung of mice with Sendai virus pneumonia by LECAM-1 and CD45RB phenotype. J Immunol 1993;150:5494–5500 [PubMed] [Google Scholar]
- 27.Hou S, Hyland L, Ryan KW, et al. Virus-specific CD8+ T-cell memory determined by clonal burst size. Nature 1994;369:652–654 [DOI] [PubMed] [Google Scholar]
- 28.Hryniewicz A, Boasso A, Edghill-Smith Y, et al. CTLA-4 blockade decreases TGF-beta, IDO, and viral RNA expression in tissues of SIVmac251-infected macaques. Blood 2006;108:3834–3842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Huengsberg M, Winer JB, Gompels M, et al. Serum kynurenine-to-tryptophan ratio increases with progressive disease in HIV-infected patients. Clin Chem 1998;44:858–862 [PubMed] [Google Scholar]
- 30.Idell S. Coagulation, fibrinolysis, and fibrin deposition in acute lung injury. Crit Care Med 2003;31:S213–220 [DOI] [PubMed] [Google Scholar]
- 31.Janssen EM, Lemmens EE, Wolfe T, et al. CD4+ T cells are required for secondary expansion and memory in CD8+ T lymphocytes. Nature 2003;421:852–856 [DOI] [PubMed] [Google Scholar]
- 32.Jurgens B, Hainz U, Fuchs D, et al. Interferon-gamma-triggered indoleamine 2,3-dioxygenase competence in human monocyte-derived dendritic cells induces regulatory activity in allogeneic T cells. Blood 2009;114:3235–3243 [DOI] [PubMed] [Google Scholar]
- 33.Laich A, Neurauter G, Widner B, and Fuchs D. More rapid method for simultaneous measurement of tryptophan and kynurenine by HPLC. Clin Chem 2002;48:579–581 [PubMed] [Google Scholar]
- 34.Li L, Xia Y, Nguyen A, et al. Th2-induced eotaxin expression and eosinophilia coexist with Th1 responses at the effector stage of lung inflammation. J Immunol 1998;161:3128–3135 [PubMed] [Google Scholar]
- 35.Mahanonda R, Sa-Ard-Iam N, Montreekachon P, et al. IL-8 and IDO expression by human gingival fibroblasts via TLRs. J Immunol 2007;178:1151–1157 [DOI] [PubMed] [Google Scholar]
- 36.Marshall DR, Turner SJ, Belz GT, et al. Measuring the diaspora for virus-specific CD8+ T cells. Proc Natl Acad Sci USA 2001;98:6313–6318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mellor AL, Baban B, Chandler PR, et al. Cutting edge: CpG oligonucleotides induce splenic CD19+ dendritic cells to acquire potent indoleamine 2,3-dioxygenase-dependent T cell regulatory functions via IFN Type 1 signaling. J Immunol 2005;175:5601–5605 [DOI] [PubMed] [Google Scholar]
- 38.Mullarky IK, Szaba FM, Winchel CG, et al. In situ assays demonstrate that interferon-gamma suppresses infection-stimulated hepatic fibrin deposition by promoting fibrinolysis. J Thromb Haemost 2006;4:1580–1587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Munn DH, Sharma MD, Baban B, et al. GCN2 kinase in T cells mediates proliferative arrest and anergy induction in response to indoleamine 2,3-dioxygenase. Immunity 2005;22:633–642 [DOI] [PubMed] [Google Scholar]
- 40.Munn DH, Sharma MD, and Mellor AL. Ligation of B7-1/B7-2 by human CD4+ T cells triggers indoleamine 2,3-dioxygenase activity in dendritic cells. J Immunol 2004;172:4100–4110 [DOI] [PubMed] [Google Scholar]
- 41.Narasaraju T, Yang E, Samy RP, et al. Excessive neutrophils and neutrophil extracellular traps contribute to acute lung injury of influenza pneumonitis. Am J Pathol 2011;179:199–210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Onodera T, Jang MH, Guo Z, et al. Constitutive expression of IDO by dendritic cells of mesenteric lymph nodes: functional involvement of the CTLA-4/B7 and CCL22/CCR4 interactions. J Immunol 2009;183:5608–5614 [DOI] [PubMed] [Google Scholar]
- 43.Perez-Padilla R, de la Rosa-Zamboni D, Ponce de Leon S, et al. Pneumonia and respiratory failure from swine-origin influenza A (H1N1) in Mexico. N Engl J Med 2009;361:680–689 [DOI] [PubMed] [Google Scholar]
- 44.Ponchio L, Duma L, Oliviero B, et al. Mitomycin C as an alternative to irradiation to inhibit the feeder layer growth in long-term culture assays. Cytotherapy 2000;2:281–286 [DOI] [PubMed] [Google Scholar]
- 45.Robertson SJ, Messer RJ, Carmody AB, and Hasenkrug KJ. In vitro suppression of CD8+ T cell function by Friend virus-induced regulatory T cells. J Immunol 2006;176:3342–3349 [DOI] [PubMed] [Google Scholar]
- 46.Robinson CM, Hale PT, and Carlin JM. The role of IFN-gamma and TNF-alpha-responsive regulatory elements in the synergistic induction of indoleamine dioxygenase. J Interferon Cytokine Res 2005;25:20–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sharma MD, Baban B, Chandler P, et al. Plasmacytoid dendritic cells from mouse tumor-drainig lymph nodes directly activate mature Tregs via indoleamine 2,3-dioxygenase. J Clin Invest 2007;117:2570–2582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sharma MD, Hou DY, Liu Y, et al. Indoleamine 2,3-dioxygenase controls conversion of Foxp3+ Tregs to TH17-like cells in tumor-draining lymph nodes. Blood 2009;113:6102–6111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Smith JH, Nagy T, Driskell E, et al. Comparative pathology in ferrets infected with H1N1 influenza A viruses isolated from different hosts. J Virol 2011;85:7572–7581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Thompson WW, Shay DK, Weintraub E, et al. Influenza-associated hospitalizations in the United States. JAMA 2004;292:1333–1340 [DOI] [PubMed] [Google Scholar]
- 51.Tripp RA, Topham DJ, Watson SR, and Doherty PC. Bone marrow can function as a lymphoid organ during a primary immune response under conditions of disrupted lymphocyte trafficking. J Immunol 1997;158:3716–3720 [PubMed] [Google Scholar]
- 52.Tumpey TM, Garcia-Sastre A, Taubenberger JK, et al. Pathogenicity of influenza viruses with genes from the 1918 pandemic virus: Functional roles of alveolar macrophages and neutrophils in limiting virus replication and mortality in mice. J Virol 2005;79:14933–14944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Turner SJ, Olivas E, Gutierrez A, et al. Disregulated influenza A virus-specific CD8+ T cell homeostasis in the absence of IFN-gamma signaling. J Immunol 2007;178:7616–7622 [DOI] [PubMed] [Google Scholar]
- 54.Welsh RM, Che JW, Brehm MA, and Selin LK. Heterologous immunity between viruses. Immunol Rev 2010;235:244–266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wiley JA, Cerwenka , Harkema JR, et al. Production of interferon-gamma by influenza hemagglutinin-specific CD8 effector T cells influences the development of pulmonary immunopathology. Am J Pathol 2001;158:119–130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Xu H, Oriss TB, Fei M, et al. Indoleamine 2,3-dioxygenase in lung dendritic cells promotes Th2 responses and allergic inflammation. Proc Natl Acad Sci USA 2008;105:6690–6695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yoshida R, Urade Y, Tokuda M, and Hayaishi O. Induction of indoleamine 2,3-dioxygenase in mouse lung during virus infection. Proc Natl Acad Sci USA 1979;76:4084–4086 [DOI] [PMC free article] [PubMed] [Google Scholar]