Abstract
Objectives
To assess the time course of changes in rapid muscle force/torque production capacity and neuromuscular activity of lower limb muscles in response to prolonged (∼2 h) match-play tennis under heat stress.
Methods
The rates of torque development (RTD) and electromyographic activity (EMG; ie, root mean square) rise were recorded from 0 to 30, –50, –100 and –200 ms during brief (3–5 s) explosive maximal isometric voluntary contractions (MVC) of the knee extensors (KE) and plantar flexors (PF), along with the peak RTD within the entirety of the torque-time curve. These values were recorded in 12 male tennis players before (prematch) and after (postmatch, 24 and 48 h) match-play in HOT (∼37°C) and COOL (∼22°C) conditions.
Results
The postmatch core temperature was greater in the HOT (∼39.4°C) vs COOL (∼38.7°C) condition (p<0.05). Reductions in KE RTD occurred within the 0–200 ms epoch after contraction onset postmatch and at 24 h, compared with prematch, independent of environmental conditions (p<0.05). A similar reduction in the KE peak RTD was also observed postmatch relative to prematch (p<0.05). No differences in KE RTD values were observed after normalisation to MVC torque. Furthermore, the rate of KE EMG activity rise remained unchanged. Conversely, the PF contractile RTD and rate of EMG activity rise were unaffected by the exercise or environmental conditions.
Conclusions
In the KE, a reduction in maximal torque production capacity following prolonged match-play tennis appears to account for the decrease in the rate of torque development, independent of environmental conditions, while remaining unchanged in the PF.
Keywords: Thermoregulation, Fatigue, Adaptations of skeletal muscle to exercise and altered neuromuscular activity, Exercise, Exercise physiology
Introduction
Success in modern tennis requires the ability to produce repetitive bursts of explosive strength, characterised by high levels of muscle force/torque production within the initial phase of contraction. A compromise in this ability is likely to impair match-play performance through reductions in the efficiency of on-court movements, as well as stroke proficiency.1 2 Delayed recovery (24–48 h) of explosive strength may also have important implications on performance during tournament play. Investigations into the neuromuscular consequences associated with prolonged (1–3 h) match-play tennis have reported maximal isometric voluntary contraction (MVC) strength losses to range between 5% and 20% during knee extension (KE) and plantar flexion (PF).3–7 However, measuring only maximal force production may underestimate the functional impairment of fatigued muscles in tennis, since it has been speculated that fast and forceful movements (eg, jumping, ∼0–5 m accelerations and direction changes) require contraction times in the order of 50–250 ms,8 9 which is considerably shorter than the time needed to reach maximal isometric force/torque levels (∼300 ms).10
Although ‘explosive’ muscle strength is difficult to quantify on the court during play, it can be measured as the contractile rate of force or torque development (RTD) within the early phase (ie, rising force or torque) of an MVC. The ability to develop force rapidly may be of greater importance than maximum muscle strength per se, especially considering that end-exercise reductions in RTD exceed (–15% to –25%) those in maximal strength (–10% to –12%) following a handball match11 and repeated cycling sprint exercise,12 although not in football play.13 Furthermore, explosive strength within the early phase of contraction (ie, <100 ms), rather than maximal strength, is a more likely determinant of injury risk.14 15 In the only available study investigating tennis players until now, bilateral leg press RTD was reduced immediately after the first match of a 3-day tournament, remaining depressed prior to the start of play in the following matches (ie, days 2 and 3).6 Some of the potential limitations with this study, however, include the use of a single measurement of force to characterise the rising force-time curve, and the absence of normalisation to MVC force, which would reveal the degree to which differences in RTD were the result of a discrepancy in maximal voluntary strength.
Nevertheless, increasing evidence suggests that the relative contribution of muscular9 and neural8 14 16 factors determining contractile RTD may differ at early (<100 ms) and late (>100 ms) phase epochs of the MVC. Measuring explosive torque production over different time periods following contraction onset,8 9 coupled with electromyogram (EMG) recordings (ie, as a proxy of the rate of agonist muscle activation), may help to delineate the contribution of neural factors in explosive muscular performance. This is especially relevant given that more pronounced alterations in early rather than late-phase RTD may be expected following an intervention.14 16 It is not surprising, therefore, that diminished RTD in the KE in fatigued football (7–8%)17 and handball (16–21%)11 players following simulated matches occurred concurrently with decreased EMG activity at selective epochs of rising muscle torque. To the best of our knowledge, the effect of match-play tennis-induced fatigue on rapid muscle force/torque capacity and accompanying neuromuscular activity has not been evaluated.
Moreover, the effects of environmental heat stress leading to hyperthermia on the contractile RTD and neuromuscular activity are poorly understood. Indeed, the contractile RTD recorded from 0 to 30, –50, –100 and –200 ms during explosive efforts failed to show any meaningful changes following localised warming (30–38°C) of the quadriceps muscle.18 Furthermore, in the absence of severe hyperthermia (ie, <38.5°C), heat exposure had no additional effect on the fatigue-induced reductions in contractile RTD following repeated-sprint cycling.12 Although it has been reported that reductions in KE strength are exacerbated after match-play tennis in the heat due to a greater decrement in voluntary drive,7 the question of whether similar or larger alterations in rapid muscle force characteristics (ie, RTD) also occur as a result of hyperthermia remains undetermined. As match-play tennis tournaments often require players to compete over consecutive days, sometimes in hot conditions up to 43°C,19–21 the extent to which hyperthermia exacerbates alterations in the contractile RTD is of practical relevance. Even though hyperthermia has limited influence on tennis-related physical performance,2 22 endurance capacity23 and repeated sprint ability24 have been shown to decrease under heat stress. A greater loss of endurance or sprint ability in the heat may therefore result in greater impaired on-court performance. For example, fatigue in the KE may impair explosive-type contractions that mediate lower-limb drive during the serve, and PF fatigue could diminish postural control, including the maintenance of dynamic ankle joint stability when positioning to the ball.1 Heat impairs the neuromuscular function of the PF and the KE differently,25–27 and given that isometric RTD measurements correlate with dynamic functional performance (ie, sprint and countermovement jump),28 29 the potential impact this has on joint stability requires further examination.14 15
The aim of this study was to assess the immediate and prolonged alterations in rapid muscle force/torque characteristics of the KE and PF in response to match-play tennis in temperate and hot environments. It was hypothesised that the development of hyperthermia during match-play tennis in the heat would exacerbate acute fatigue-induced impairments in RTD and delay the recovery process, compared with a match undertaken in temperate conditions.
Methods
Participants
Twelve male players with an International Tennis Federation (ITF) number of 1–3 participated in the study. The mean age, height, body mass, weekly training volume and years of practice were 22.0±4.4 years, 183.5±7.7 cm, 80.8±9.5 kg, 13.0±5.9 h/week and 16.4±3.6 years, respectively. They played an average of 17±10 tournaments and 65±23 matches/year. They were informed of the study aims, requirements and risks before being provided written informed consent.
Study design
Players completed two counterbalanced simulated matches on hardcourt surfaces separated by 72 or 144 h. They were paired according to level of play and competed against the same opponent in each match. One match was played indoors in temperate conditions (COOL: 21.8±0.1°C, 72.3±3.2% relative humidity, 19.4±0.3°C wet bulb globe temperature (WBGT)) and the other outside in hot conditions (HOT: 36.8±1.5°C, 36.1±11.3% relative humidity, 33.6±0.9°C WBGT). Wind velocity during the HOT matches was 0.7±0.2 m/s. The matches consisted of 20 min of effective play, that is, the time spent within play and excluding the time between points and games. Hence, matches were of typical length for a three set match (COOL: 102.1±19.0 min and HOT: 119.2±9.6 min). The characteristics of the matches are presented in a companion paper.30 One to 3 days prior to the start of the experiment, participants visited the testing and playing venues where they were thoroughly familiarised and accustomed to the neuromuscular tests. To ensure familiarisation, each participant had to produce a coefficient of variation in three successive MVC trials lower than 5%.
Experimental protocol
On arrival on match days (9:00), participants voided and inserted a telemetric temperature pill (VitalSense, Mini Mitter, Respironics, Herrsching, Germany) in the rectum (the length of a gloved index finger beyond the anal sphincter) for the measurement of core temperature. A standardised warm-up of running at 9 km/h for 5 min on the indoor court was then performed. This was followed by the pre-exercise (prematch) neuromuscular function assessment (see Neuromuscular function section). This assessment, along with the 24 and 48 h postmatch assessments, was preceded by a standardised warm-up consisting of 10 isometric contractions of 4 s duration interspaced with 10–20 s of recovery. Contraction intensity was progressively self-adjusted by the participant to attain maximal torque in the last three contractions. The neuromuscular function assessment was repeated ∼20 min after play (postmatch). Following the neuromuscular assessment, participants performed a 10 min tennis-specific warm-up consisting of various exchanges and serves in the condition of play. Core temperature and body mass were recorded prematch and postmatch. During play, participants consumed water and a commercially available sport drink (Gatorade, Chicago, Illinois, USA) ad libitum. On the days when the participants did not play, they performed the running warm-up and the neuromuscular assessment (24 and 48 h) at the same time of day as for a prematch, and then followed a standardised training programme led by a tennis coach (∼60 min of drills and exchanges) in the HOT environment.
Neuromuscular function
The neuromuscular assessment protocol was performed twice: once to evaluate the KE and once to evaluate the PF (2 min between series, counterbalanced order between participants). The participants performed three successful explosive voluntary contractions (separated by ≥30 s) on which a paired stimulus (doublet, 100 Hz) was superimposed. During all MVCs, the participants were carefully instructed to contract ‘as fast and as hard as possible’, sustaining the contraction for 3–5 s. Participants were asked to avoid any countermovement before torque onset; that is, they were reminded not to flex the knee/dorsiflex the foot immediately prior to knee extension/plantar flexion. They were strongly encouraged with verbal feedback and a visual display of the torque production. Contractions that had any discernible countermovement or pretension (ie, change of the baseline torque of >1.5 (KE) or >0.5 (PF) Nm during the 100 ms before contraction onset) were discarded and another attempt was made. To provide biofeedback on whether a countermovement had occurred, the resting torque level was displayed on a sensitive scale. The slope of the torque–time curve (10 ms time constant) was displayed throughout testing and the peak slope was used to provide visual performance feedback to participants after each contraction. The duration of the entire neuromuscular assessment (ie, for both muscle groups) was ∼10 min and was conducted in a temperate environment (∼22°C).
Recordings
Torque measurements
For KE torque measurements, participants were seated upright on a custom-built adjustable chair with the hips and knees flexed at 90°. Restraining straps placed across the chest and hips secured the participants in the chair to prevent extraneous movement, while the dynamometer (Captels, St Mathieu de Treviers, France) was attached 3–5 cm above the tip of the lateral malleoli. The PF torque was measured using a dynamometric pedal (Captels, St Mathieu de Treviers, France), with participants seated upright with the hips, knee and ankle flexed at 90°, 100° and 90°, respectively. The foot of the leg performing the MVC was secured to the dynamometric pedal with two restraining straps. During all contractions, the torque signals were amplified, sent through an A/D board and sampled at 2000 Hz by commercially available hardware and software (MP35 and BSL Pro V.3.6.7, Biopac Systems Inc, Santa Barbara, California, USA).
Electromyography
The EMG signals of the vastus lateralis, rectus femoris, soleus and gastrocnemius lateralis muscles were recorded on the right lower limbs via bipolar Ag/AgCl electrodes (Ambu Blue sensor T, Ambu A/S, Denmark; diameter = 9 mm; interdistance electrode=30 mm). Recording electrodes were fixed longitudinally over the muscle bellies. The reference electrode was attached to the right wrist. Low impedance between the two electrodes was obtained by abrading the skin with emery paper and cleaning with alcohol. The position of the electrodes was marked for consistent placement. EMG signals were amplified (gain=1000), filtered (bandwidth frequency 30–500 Hz) and recorded (sampling frequency=2000 Hz) by commercially available hardware (Biopac MP35, systems Inc) and software (Acqknowledge 3.6.7, Biopac Systems Inc).
Motor nerve stimulation
A high-voltage stimulator (Digitimer DS7AH, Digitimer, Hertfordshire, England) was used to deliver the doublet (square-wave stimuli of 0.2 ms duration) with a maximal voltage of 400 V. The femoral nerve was stimulated by placing a cathode (5 mm diameter) in the inguinal crease and an anode (5 cm×10 cm; Medicompex, SA, Ecublens, Switzerland) in the gluteal fold. The tibial nerve was stimulated using a cathode (9 mm diameter) placed in the popliteal cavity with compression supplied by a strap, and an anode (5 cm×10 cm) positioned beneath the patella. During the familiarisation session, an isometric recruitment curve using motor nerve stimulation was drawn on relaxed KE and PF muscles to individualise the optimal stimulus intensities. Briefly, the current was progressively increased in 10 mA increments until a plateau occurred in the maximal twitch amplitude. Supramaximal stimulations were ensured by increasing the final intensity by 50%, and kept constant for each participant throughout all experimental trials.
Data analysis
All analyses were performed using Spike 2 Software (Cambridge Electronic Design, Cambridge, UK). No differences were observed between the vastus lateralis and rectus femoris muscles or the soleus and gastrocnemius lateralis muscle amplitudes; thus, each EMG-dependent variable was averaged for these muscles to provide an overall representation of KE and PF muscle activity, respectively. During MVCs, the average root mean square (RMS) of each of the agonist muscles was calculated (ie, RMSMAX) over a 1 s period when the torque had reached a plateau (before the superimposed twitch). Similarly, the peak-to-peak amplitude of the superimposed maximum compound action potential (M-wave) response was measured for each agonist muscle, and averaged to give an average value for each muscle group. In order to normalise muscle activity for KE or PF, RMSMAX was divided by the respective M-wave of that muscle group to give a ratio RMSMAX/M-wave.
The contractile RTD (expressed as Nm/s) was derived from isometric measurements, as the average slope of the initial time phase of the torque-time curve at 0–30, 0–50, 0–100 and 0–200 ms, relative to the onset of contraction8 15 17 using a custom written program (Spike 2 Software, Cambridge Electronic Design, Cambridge, UK). The onset of muscle contraction was defined as the time point at which the torque curve exceeded the baseline by >4.5 Nm (KE) and >1.5 Nm (PF), corresponding to ∼2.5% of MVC torque values.31 The peak RTD was defined as the peak Δtorque/Δtime achieved during the initial 200 ms of the isometric contraction.32 In addition, the rate of muscle activation (expressed as mV/s) was measured as the RMS activity increase obtained at similar time intervals relative to onset integration (ie, activity). The onset of EMG integration was shifted 50 ms before the onset of contraction to account for the presence of electromechanical delay.8 12 The rate of torque development and EMG rise (KE or PF) were also normalised relative to MVC torque (%MVC) and maximal EMG activity (%RMSMAX/M-wave). The average of three trials was used for further analysis. Additional neuromuscular characteristics (ie, maximal strength, voluntary activation and twitch properties) are presented in a companion paper.7
Statistical analysis
All statistical calculations were performed using PASW software V.21.0 (SPSS, Chicago, Illinois, USA). Two-way repeated-measures analysis of variances (Time (prematch, postmatch, 24 vs 48 h)×Condition (COOL vs HOT)) were used to compare torque and muscle activation data for each muscle group (KE and PF) and time window (0–30, 0–50, 0–100 and 0–200 ms) independently for absolute and relative changes. Outcome variables were tested using Mauchly's procedure for sphericity. Whenever the data violated the assumption of sphericity, p values and adjusted degrees of freedom based on Greenhouse-Geisser correction were reported instead. Where significant effects were established, pairwise differences were identified using the Bonferroni post hoc analysis procedure adjusted for multiple comparisons. Values are expressed as means±SD. The significance level was set at p<0.05.
Results
Match-play responses
The increase in core temperature from the start to end of the match was greater in the HOT (37.6±0.3 to 39.4±0.5°C) compared to the COOL (37.5±0.3 to 38.7±0.2°C) condition (p<0.05). From prematch to postmatch, the level of body mass loss was similar between conditions (COOL: 81.2±9.6 to 80.9±9.8 kg and HOT: 80.7±9.6 to 80.2±10.3 kg).
Maximal strength
A larger reduction in the KE MVC torque occurred postmatch in the HOT (−22.0±10.9%) versus the COOL (−9.5±6.9%) condition compared with prematch values (table 1; p<0.05). The KE MVC torque was also lower in the HOT condition at 24 h relative to prematch (−10.6±7.3%) and COOL (−11.2±9.1%) condition values (p<0.05). There was a main effect of condition (p<0.01) on the RMSMAX/M-wave, but the interaction between condition × time did not reach significance (table 1; p=0.051).
Table 1.
Neuromuscular parameters recorded during brief explosive maximal knee extensions prior to (pre) and following (post, 24 and 48 h) match-play tennis in COOL and HOT conditions
| Neuromuscular | Assessment time point | ||||
|---|---|---|---|---|---|
| Parameters | Condition | Pre | Post | 24 h | 48 h |
| MVC torque (Nm) | COOL | 224±36 | 202±27 * | 216±32 * | 223±38 |
| HOT † | 214±44 | 169±29 * | 195±39 * | 207±43 | |
| Peak RTD (Nm/s) | COOL | 1151±418 | 1082±429 * | 1184±410 | 1190±397 |
| HOT † | 1115±435 | 823±259 * | 923±231 | 1102±385 | |
| RMSMAX (mV) | COOL | 0.400±0.117 | 0.361±0.109 | 0.384±0.117 | 0.407±0.124 |
| HOT | 0.396±0.114 | 0.321±0.128 | 0.381±0.148 | 0.391±0.129 | |
| M-wave (mV) | COOL | 7.7±2.6 | 7.6±2.8 | 7.4±2.7 | 7.7±2.8 |
| HOT | 7.3±3.1 | 7.3±3.3 | 7.4±2.9 | 7.6±3.1 | |
| RMSMAX/M-wave (au) | COOL | 0.054±0.014 | 0.053±0.024 | 0.055±0.018 | 0.055±0.014 |
| HOT † | 0.057±0.016 | 0.045±0.012 | 0.054±0.019 | 0.055±0.016 | |
Values are expressed as means±SD.
*p<0.05, significantly different from pre. †p<0.05, significantly different from COOL. MVC torque, maximal voluntary contraction torque; RMSMAX, M-wave and RMSMAX/M-wave represent the average of root mean square, maximal M-waves and normalised electromyogram activities of vastus lateralis and rectus femoris muscles; Peak RTD, peak rate of torque development.
The PF MVC torque decreased (−11.2±13.6%) postmatch relative to prematch, with no difference between conditions, and was restored near baseline within 24 h (table 2).
Table 2.
Neuromuscular parameters recorded during brief explosive maximal plantar flexions prior to (pre) and following (post, 24 and 48 h) match-play tennis in COOL and HOT conditions
| Neuromuscular | Assessment time point | ||||
|---|---|---|---|---|---|
| Parameters | Condition | Pre | Post | 24 h | 48 h |
| MVC torque (Nm) | COOL | 83.5±11.9 | 75.8±10.3* | 80.4±11.2 | 83.0±14.3 |
| HOT** | 81.1±9.4 | 70.0±15.7* | 75.1±13.4 | 75.3±9.8 | |
| Peak RTD (Nm/s) | COOL | 372±67 | 372±73 | 381±82 | 353±83 |
| HOT | 330±65 | 324±64 | 359±107 | 380±106 | |
| RMSMAX (mV) | COOL | 0.227±0.070 | 0.239±0.086 | 0.238±0.100 | 0.245±0.095 |
| HOT | 0.223±0.084 | 0.214±0.075 | 0.248±0.085 | 0.232±0.088 | |
| M-wave (mV) | COOL | 7.1±2.6 | 6.8±2.3 | 7.7±1.7 | 7.7±2.1 |
| HOT | 7.2±2.4 | 7.3±1.8 | 7.7±1.8 | 7.3±2.3 | |
| RMSMAX/M-wave (au) | COOL | 0.034±0.009 | 0.036±0.011 | 0.032±0.013 | 0.033±0.012 |
| HOT | 0.032±0.012 | 0.030±0.008 | 0.033±0.011 | 0.033±0.012 | |
Values are expressed as means±SD.*p<0.05, significantly different from pre.
†p<0.05, significantly different from COOL.
MVC torque, maximal voluntary contraction torque; RMSMAX, M-wave and RMSMAX/M-wave represent the average of root mean square, maximal M-waves and normalised electromyogram activities of soleus and gastrocnemius lateralis muscles; Peak RTD, peak rate of torque development.
Rapid muscle characteristics
Knee extension
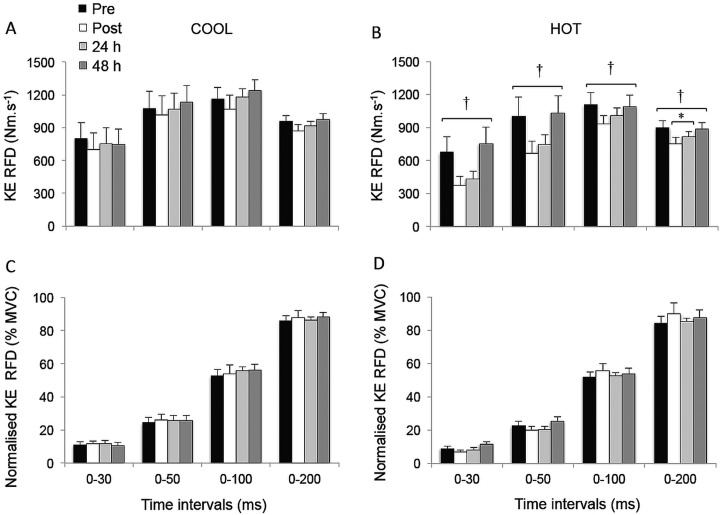
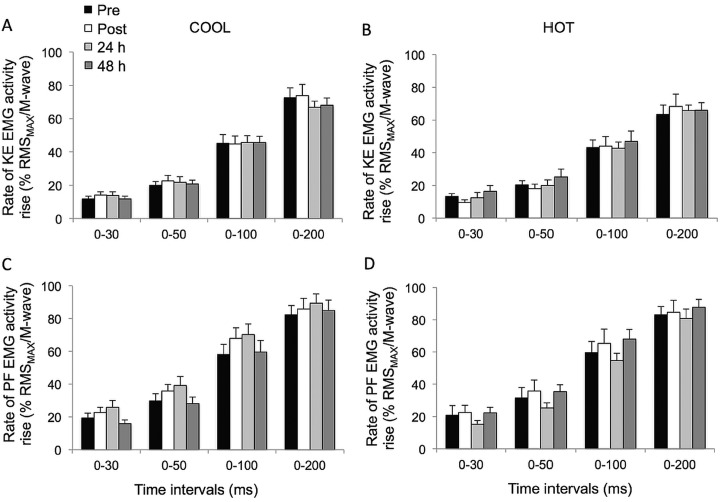
The KE peak RTD was significantly reduced postmatch in HOT and COOL conditions relative to prematch (both trials compounded: −12.8±12.4%; p<0.02; table 1). Overall, KE RTD values were lower throughout the HOT versus COOL condition in the epochs 0–30 (p=0.055), 0–50 (p=0.055), 0–100 (p=0.033) and 0–200 ms (p=0.005; figure 1A,B). KE RTD also displayed a significant main effect of time in the periods 0–100 (p=0.019) and 0–200 ms (p<0.001), independent of the condition, while failing to reach statistical significance in the periods 0–30 and 0–50 ms (p=0.076 and 0.106, respectively). Post hoc analysis revealed a reduction in KE RTD in the 200 ms epoch postmatch and at 24 h, relative to prematch (−12.0±6.9%; p<0.001). This reduction was fully restored after 48 h of recovery (p=0.013). A similar pattern (−8.9±12.8%) was observed for the 0–100 ms epoch, although statistical significance was not reached (p=0.072). When KE RTD was normalised to MVC torque, relative KE RTD values were unaffected by playing tennis in HOT or COOL conditions (figure 1C,D; p>0.139). The relative rate of KE EMG activity rise during any of the epochs did not change throughout the protocol (p>0.107) (figure 2).
Figure 1.
Rate of torque development (RTD; A and B) and normalised RTD (% maximal voluntary contraction torque; C and D) during explosive isometric knee extensions obtained at 0–30, –50, –100 and –200 ms prior to (pre) and following (post, 24 and 48 h) match-play tennis in COOL and HOT conditions. *Significantly different from pre, p<0.05. †Significantly different from COOL, p<0.05.
Figure 2.
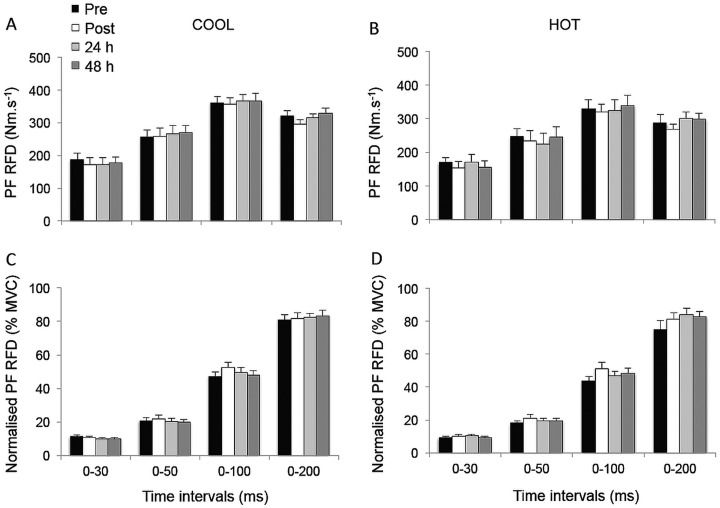
Rate of torque development (RTD; A and B) and normalised RTD (%MVC; C and D) during explosive isometric plantar flexions obtained at 0–30, –50, –100 and –200 ms prior to (pre) and following (post, 24 h and 48 h) match-play tennis in COOL and HOT conditions.
Plantar flexion
The PF RTD and normalised PF RTD (figure 3), as well as muscle activation (figure 2) values for any epoch throughout the experimental protocol, were not different between conditions (p>0.101); nor were there any interaction (p>0.109) or time effects (p>0.124).
Figure 3.
Rate of electromyogram activity rise (% RMSMAX/M-wave; C and D) during explosive isometric knee extensions (A and B) and plantar flexions (C and D) obtained at 0–30, –50, –100 and –200 ms prior to (pre) and following (post, 24 and 48 h) match-play tennis in COOL and HOT conditions.
Discussion
The aim of this study was to investigate the neuromuscular consequences of match-play tennis under severe heat stress on the contractile RTD and the underlying muscle recruitment during brief explosive MVCs of the lower limbs. In contrast to our hypothesis, the novel findings of this study are that despite the additional increase (∼0.7°C) in core temperature observed in the HOT compared with the COOL match,30 reductions in the KE peak RTD postmatch relative to prematch, as well as those noted in the 0–200 ms epoch postmatch and at 24 h compared with prematch, were similar between conditions, while accompanying muscle activation of the agonists was unchanged. These reductions occurred in parallel with decrements in peak twitch torque in the KE and PF postmatch relative to prematch, which were similar in HOT and COOL conditions and restored near baseline within 24 h.7 The reduction in RTD was primarily the result of a decrease in MVC torque postmatch, as normalised RTD remained unaffected by the conditions. Moreover, all explosive KE strength parameters were fully restored to baseline levels within 48 h. The present data further demonstrate that playing tennis in either HOT or COOL conditions has no detrimental effect on the contractile RTD and accompanying muscle activation capacity of the PF.
Peak RTD
An overall decline in KE peak RTD (–13%) occurred immediately after playing tennis for ∼2 h in HOT and COOL conditions (table 1). This finding appears to corroborate the data of Ojala and Häkkinen,6 reporting substantial reductions (∼38%) in bilateral leg press RTD (ie, presumably measured via the peak slope of the torque–time curve) in tennis players competing in a 3-day tournament (∼2 h matches). In the present study, KE peak RTD decreased to a lesser extent, which may explain why full recovery occurred after 24 h, while alterations persisted for at least 48 h after the 3-day tournament in the Ojala and Häkkinen study.6 However, the approach that consists of measuring the peak slope of the torque–time curve in isolation to provide information on the ability to produce explosive force is limited. Indeed, training studies have demonstrated that RTD and EMG in the early (<100 ms) and late (>100 ms) phases of rising muscle force/torque respond differently, which clearly emphasises the usefulness of the approach utilised in the current study (ie, separating into epochs).15 30 32
In one study attempting to isolate the effect of temperature on peak RTD, quadriceps muscle temperature was manipulated (30, 32, 34, 36 and 38°C) with hot-water and ice-water bags prior to performing maximal isometric knee extensions.33 The authors demonstrated faster peak RTD when the muscle temperature increased from 30°C to 36°C. Although this temperature effect is interesting and supports the positive dose/response relationship that exists between increases in muscle temperature and muscle power,34 it is unlikely that the difference in muscle temperature between conditions in the current study would have been significantly large enough to induce a variation in peak RTD. Rather, it appears that the fatigue induced via match-play tennis was the main factor inducing the deteriorations in RTD, especially considering that point duration, number of points and games played were similar between conditions.30 As with maximal torque and muscle activation levels,7 peak RTD measured in the PF was unaffected throughout the protocol.
Early and late-phase RTD
A downward-shift was observed in the contractile RTD after match-play tennis that was unrelated to environmental conditions, reflecting an overall fatigue-induced reduction (range 6–16%) in rapid muscle force/torque characteristics for the KE (figure 1A,B). Slower rates of muscle force production in the KE may negatively affect functional performance during on-court movements (eg, one-step accelerations and rapid direction changes) and stroke proficiency during intense training or competition.35 These effects may persist during tournament play when efforts are repeated on consecutive days. As with MVC torque results, more pronounced RTD-type fatigue occurred in the KE versus PF, where no significant changes occurred throughout the protocol. We have recently reported that PF late-phase RTD (ie, measured in the 200 ms epoch) was not compromised in 17 semiprofessional soccer players undertaking a 90 min match in either a temperate (21°C) or a hot (43°C) environment.13 This most likely relates to muscle fibre composition. Indeed, the KE have a higher percentage of type II fibres, which contribute to explosive-type contractions (eg, lower-limb drive during the power serve), whereas type I fibres, which contribute more to postural control tasks, including the maintenance of dynamic ankle joint stability (eg, positioning to the ball), are found in a higher proportion in the PF.36
Tennis players run an average of 3 m per shot for a total of 8–15 m in the pursuit of one point, completing 1300–3600 m/h of play.37 These repeated accelerations–decelerations, executed in a restricted space, certainly have the potential to contribute to the impairment in RTD. Accordingly, diminished KE RTD in fatigued football (7–8%)17 and handball (16–21%)11 players following simulated matches have been reported previously. Our data extend these observations to indicate that reductions in late-phase KE RTD persist 24 h into recovery, regardless of environmental conditions. Nonetheless, a direct comparison among studies must be made with caution, as manual and automated methods can be used to detect contraction onset,38 39 and the most suitable time intervals (eg, 0–30, –50, –100 and –200 ms vs fixed 50 ms intervals from force/torque onset) to assess RTD still need to be agreed on.
The effects of fatigue on the contractile RTD became increasingly clear on the later phases of the rising muscle torque, since statistical differences were reached only for the late-phase time period (ie, 200 ms epoch). The failure for rapid muscle torque capacity parameters to reach statistical significance during the early phase of MVC (0–30, –50 and –100 ms) may be due to the marked within-participant variability reported for explosive KE torque over the initial 50 ms40; that is, the lower reliability of RTD measures at early time intervals reduces the likelihood of detecting changes. The variability of RTD40 and EMG41 measures in the very initial phase of the contraction (30–50 ms) is an important consideration when examining temporal adaptations in explosive torque characteristics, requiring large sample sizes to overcome the inherent variability. Another explanation may lie with the dependence on maximal strength as a good determinant of explosive strength. When executing explosive contractions, Andersen and Aagaard9 reported that the contractile RTD becomes increasingly dependent on MVC torque as the time from torque onset increases. Accordingly, in the current study, reductions in explosive torque production (ie, RTD) from prematch to postmatch were associated with reductions in strength (ie, MVC torque; figure 1C,D). Therefore, when controlling for fatigue-induced losses in maximal strength, our results indicate a maintained relative ability to generate explosive efforts. This observation confirms the significant influence of maximal strength in maintaining normalised RTD values after playing tennis either in HOT or COOL conditions.
Potential underpinning mechanisms
In the current study, decreases in late-phase RTD were not associated with a detectable change in the rate of muscle activation in the agonists, as measured via EMG signals (figure 2). As such, the decrement in RTD could not be ascribed to corresponding alterations in efferent neural drive (ie, changes in motor unit recruitment and/or firing frequency). However, in a companion paper, we reported that voluntary muscle activation during brief and sustained MVCs, measured via twitch interpolation, was reduced in the KE, but not PF.7 Therefore, a reduction in agonist muscle activation cannot be overlooked, especially since significant decreases in the rate of muscle activation of the quadriceps, as measured by surface EMG using a fixed electromechanical delay to determine onset rise, were reported after match-play soccer17 and handball.11 Nonetheless, future studies should directly measure EMG onset so as to avoid potential confounding effects (eg, temperature and fatigue).
Other neuromuscular mechanisms potentially involved in the alterations of contractile KE RTD may relate to an impairment in peripheral muscle properties, as evidenced by reductions in involuntary twitch characteristics (ie, peak twitch torque as well as maximum rates of torque development and relaxation), following match-play tennis in HOT and COOL conditions (same players as in the current study).7 Moreover, a reduction in hopping ability following prolonged match-play tennis,22 which is indicative of a reduction in leg stiffness, may be associated with deterioration of the muscle–tendon complex and a concomitant decrease in contractile RTD.42 Another possibility could also be that fatigue-induced reductions in the contractile RTD resulted from shifts in the optimal muscle length for torque production, as demonstrated after eccentric damage.43 Whatever the exact underpinning mechanism responsible for the reduction in RTD, it is important to note that competing under severe heat stress does not appear to exacerbate the impairments in the contractile RTD.
Conclusion
This is the first study to directly compare the acute and delayed effects of prolonged match-play tennis in HOT versus COOL conditions on changes in rapid muscle torque production capacity and neuromuscular activity in the lower limb of high-level players. In the KE, a reduction in maximal torque following prolonged (∼2 h) match-play tennis in HOT and COOL conditions accounted for the decrease in peak and late-phase (200 ms) rapid muscle torque production capacity, the latter persisting for 24 h, as no changes in the rates of quadriceps muscle activation (RMS activity) were found. Alternatively, the contractile rate of torque development and muscle activation remained unchanged in the PF. In contrast to our hypothesis, competing under severe heat stress did not exacerbate tennis-induced alterations (match and recovery kinetics) in the contractile rate of torque development, despite a ∼0.7°C core temperature difference between conditions.
What are the new findings?
In the knee extensors (KE), peak and late-phase (0–200 ms epoch) contractile rates of torque development (RTD) decreased postmatch in HOT and COOL conditions, recovering fully within 48 h.
Contractile RTD values were not different after normalisation to maximal voluntary contraction torque, indicating that postmatch strength losses accounted for the decrease in RTD.
No changes in the rates of quadriceps muscle activation (RMS activity) were found.
In the plantar flexors, explosive muscle strength and activation capacity were preserved following prolonged match-play tennis in HOT and COOL conditions.
How might the findings impact on clinical practice?
When interpreting fatigue-induced changes in rapid muscle torque production characteristics and neuromuscular activity in response to match-play tennis in HOT and COOL conditions, alterations in the contractile rates of torque development should be analysed using peak slope values and those obtained in the late phase (>100 ms) of the rising muscle contraction.
Enhancing and/or preserving maximal voluntary strength capacity of the knee extensors through resistance training may offer an advantage in generating explosive strength by improving fatigue resistance during play.
Acknowledgments
The authors would like to thank all the players for their efforts. They are very grateful to Tim Colijn and Samuel Rota for their involvement in the protocol. They also thank the staff from Research and Education Centre (Aspetar) for their precious help with data collection and statistical analyses.
Footnotes
Contributors: OG, SR and JDP were involved with study design, data collection and analyses. OG drafted the manuscript that was reviewed and approved in its final version by JDP and SR.
Funding This project was funded by the Aspire Zone Research Foundation.
Competing interests: None.
Ethics approval: The study was approved by the Shafallah Medical Genetics Center Ethical Research Committee and conformed to the current Declaration of Helsinki guidelines.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Girard O, Millet GP. Neuromuscular fatigue in racquet sports. Neurol Clin 2008;26:181–94 [DOI] [PubMed] [Google Scholar]
- 2.Hornery D, Farrow D, Mujika I, et al. Fatigue in tennis: mechanisms of fatigue and effect on performance. Sports Med 2007;37:199–212 [DOI] [PubMed] [Google Scholar]
- 3.Girard O, Lattier G, Maffiuletti NA, et al. Neuromuscular fatigue during a prolonged intermittent exercise: application to tennis. J Electromyogr Kinesiol 2008;18:1038–46 [DOI] [PubMed] [Google Scholar]
- 4.Girard O, Racinais S, Micallef JP, et al. Spinal modulations accompany peripheral fatigue during prolonged tennis playing. Scand J Med Sci Sports 2011;21:455–64 [DOI] [PubMed] [Google Scholar]
- 5.Fabre JB, Martin V, Gondin J, et al. Effect of playing surface properties on neuromuscular fatigue in tennis. Med Sci Sports Exerc 2012;44:2182–9 [DOI] [PubMed] [Google Scholar]
- 6.Ojala T, Häkkinen K. Effects of the Tennis tournament on players’ physical performance, hormonal responses, muscle damage and recovery. J Sports Sci Med 2013;12:240–8 [PMC free article] [PubMed] [Google Scholar]
- 7.Périard J, Racinais S, Knez WL, et al. Neuromuscular adjustments of the knee extensors and plantar flexors following match-play tennis in the heat. Br J Sports Med 2014;48:i45–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aagaard P, Simonsen EB, Andersen JL, et al. Increased rate of force development and neural drive of human skeletal muscle following resistance training. J Appl Physiol 2002;93:1318–26 [DOI] [PubMed] [Google Scholar]
- 9.Andersen LL, Aagaard P. Influence of maximal muscle strength and intrinsic muscle contractile properties on contractile rate of force development. Eur J Appl Physiol 2006;96:46–52 [DOI] [PubMed] [Google Scholar]
- 10.Thorstensson A, Karlsson J, Viitasalo HT, et al. Effect of strength training on EMG of human skeletal muscle. Acta Physiol Scand 1976;98:232–6 [DOI] [PubMed] [Google Scholar]
- 11.Thorlund JB, Michalsik LB, Madsen K, et al. Acute fatigue-induced changes in muscle mechanical properties and neuromuscular activity in elite handball players following a handball match. Scand J Med Sci Sports 2008;18:462–72 [DOI] [PubMed] [Google Scholar]
- 12.Girard O, Racinais S, Bishop D. Hot conditions improve power output during repeated cycling sprints without modifying neuromuscular fatigue characteristics. Eur J Appl Physiol 2013;113:359–69 [DOI] [PubMed] [Google Scholar]
- 13.Nybo L, Girard O, Mohr M, et al. Markers of muscle damage and performance recovery after exercise in the heat. Med Sci Sports Exerc 2013;45:860–8 [DOI] [PubMed] [Google Scholar]
- 14.de Ruiter CJ, Kooistra RD, Paalman MI, et al. Initial phase of maximal voluntary and electrically stimulated knee extension torque development at different knee angles. J Appl Physiol 2004;97:1693–701 [DOI] [PubMed] [Google Scholar]
- 15.Suetta C, Aagaard P, Rosted A, et al. Training-induced changes in muscle CSA, muscle strength, EMG and rate of force development in elderly subjects after long-term unilateral disuse. J Appl Physiol 2004;97:1954–61 [DOI] [PubMed] [Google Scholar]
- 16.Tillin NA, Jimenez-Reyes P, Pain MT, et al. Neuromuscular performance of explosive power athletes versus untrained individuals. Med Sci Sports Exerc 2010;42:781–90 [DOI] [PubMed] [Google Scholar]
- 17.Thorlund JB, Aagaard P, Madsen K. Rapid muscle force capacity changes after soccer match play. Int J Sports Med 2009;30:273–8 [DOI] [PubMed] [Google Scholar]
- 18.Dewhurst S, Macaluso A, Gizzi L, et al. Effects of altered muscle temperature on neuromuscular properties in young and older women. Eur J Appl Physiol 2010;108:451–5 [DOI] [PubMed] [Google Scholar]
- 19.Mountjoy M, Alonso J-M, Bergeron MF, et al. Hyperthermic-related challenges in aquatics, athletics, football, tennis and triathlon. Br J Sports Med 2012;46:800–4 [DOI] [PubMed] [Google Scholar]
- 20.Hornery DJ, Farrow D, Mujika I, et al. An integrated physiological and performance profile of professional tennis. Br J Sports Med 2007;41:531–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Morante SM, Brotherhood JR. Autonomic and behavioural thermoregulation in tennis. Br J Sports Med 2008;42:679–85 [DOI] [PubMed] [Google Scholar]
- 22.Girard O, Christian R, Racinais S, et al. Heat stress does not exacerbate tennis-induced alterations in physical performance. Br J Sports Med 2014;48:i39–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Périard JD, Cramer MN, Chapman PG, et al. Cardiovascular strain impairs prolonged self-paced exercise in the heat. Exp Physiol 2011;96:134–44 [DOI] [PubMed] [Google Scholar]
- 24.Drust B, Rasmussen P, Mohr M, et al. Elevations in core and muscle temperature impairs repeated sprint performance. Acta Physiol Scand 2005;183:181–90 [DOI] [PubMed] [Google Scholar]
- 25.Racinais S, Girard O. Neuromuscular failure is unlikely to explain the early exercise cessation in hot ambient conditions. Psychophysiology 2012;49:853–65 [DOI] [PubMed] [Google Scholar]
- 26.Périard JD, Caillaud C, Thompson MW. Central and peripheral fatigue during passive and exercise-induced hyperthermia. Med Sci Sports Exerc 2011;43: 16–57–65 [DOI] [PubMed] [Google Scholar]
- 27.Racinais S, Gaoua N, Grantham J. Hyperthermia impairs short-term memory and peripheral motor drive transmission. J Physiol 2008;586:4751–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.de Ruiter CJ, Vermeulen G, Toussaint HM, et al. Isometric knee-extensor torque development and jump height in volleyball players. Med Sci Sports Exerc 2007;39:1336–46 [DOI] [PubMed] [Google Scholar]
- 29.Tillin NA, Pain MT, Folland J. Explosive force production during isometric squats correlates with athletic performance in rugby union players. J Sports Sci 2013;31:66–76 [DOI] [PubMed] [Google Scholar]
- 30.Périard J, Racinais S, Knez WL, et al. Thermal physiological and perceptual strain mediate alterations in match-play tennis under heat stress. Br J Sports Med 2014;48:i32–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Andersen LL, Andersen JL, Zebis MK, et al. Early and late rate of force development: differential adaptive responses to resistance training? Scand J Med Sci Sports 2010;20:162–9 [DOI] [PubMed] [Google Scholar]
- 32.Oliveira F, Rizatto G, Denadai B. Are early and late force development differently influenced by fast-velocity resistance training? Clin Physiol Funct Imaging 2013;33:282–7 [DOI] [PubMed] [Google Scholar]
- 33.Zhou S, Carey MF, Snow RJ, et al. Effects of muscle fatigue and temperature on electromechanical delay. Electromyogr Clin Neurophysiol 1998;38:67–73 [PubMed] [Google Scholar]
- 34.Racinais S, Oksa J. Temperature and neuromuscular function. Scand J Med Sci Sports 2010;20:1–18 [DOI] [PubMed] [Google Scholar]
- 35.Mendez-Villanueva A, Fernandez-Fernandez J. Exercise induced homeostatic perturbations provoked by singles tennis match play with reference to development of fatigue. Br J Sports Med 2007;41:717–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Johnson MA, Polgar J, Weightman D, et al. Data on the distribution of fibre types in thirty-six human muscles: anautopsy study. J Neurol Sci 1973;18:111–29 [DOI] [PubMed] [Google Scholar]
- 37.Fernandez-Fernandez J, Sanz-Rivas D, Mendez-Villanueva A. A review of the activity profile and physiological demands of tennis match play. Strength Cond J 2009;31:15–26 [DOI] [PubMed] [Google Scholar]
- 38.Thompson BJ, Ryan ED, Herda TJ, et al. Consistency of rapid muscle force characteristics: influence of muscle contraction onset detection methodology. J Electromyogr Kinesiol 2012;22:893–900 [DOI] [PubMed] [Google Scholar]
- 39.Tillin NA, Pain MT, Folland JP. Identification of contraction onset during explosive contractions. J Electromyogr Kinesiol 2013;23:991–4 [DOI] [PubMed] [Google Scholar]
- 40.Buckthorpe MW, Hannah R, Pain M, et al. Reliability of neuromuscular measurements during explosive isometric contractions, with special reference to electromyography normalization techniques. Muscle Nerve 2012;46:566–76 [DOI] [PubMed] [Google Scholar]
- 41.Blazevich AJ, Horne S, Cannavan D, et al. Effect of contraction mode of slow-speed resistance training on the maximum rate of force development in the human quadriceps. Muscle Nerve 2008;38:1133–46 [DOI] [PubMed] [Google Scholar]
- 42.Bojsen-Møller J, Magnusson SP, Rasmussen LR, et al. Muscle performance during maximal isometric and dynamic contractions is influenced by the stiffness of the tendinous structures. J Appl Physiol 2005;99:986–94 [DOI] [PubMed] [Google Scholar]
- 43.Prasarrwuth O, Allen TJ, Butler JE, et al. Length-dependent changes in voluntary activation, maximum voluntary torque and twitch responses after eccentric damage in humans. J Physiol 2006;571:253–2 [DOI] [PMC free article] [PubMed] [Google Scholar]