Abstract
Objective
To review the available evidence regarding electronic cigarette (e-cigarette) product characterisation and design features in order to understand their potential impact on individual users and on public health.
Methods
Systematic literature searches in 10 reference databases were conducted through October 2013. A total of 14 articles and documents and 16 patents were included in this analysis.
Results
Numerous disposable and reusable e-cigarette product options exist, representing wide variation in product configuration and component functionality. Common e-cigarette components include an aerosol generator, a flow sensor, a battery and a nicotine-containing solution storage area. e-cigarettes currently include many interchangeable parts, enabling users to modify the character of the delivered aerosol and, therefore, the product's ‘effectiveness’ as a nicotine delivery product. Materials in e-cigarettes may include metals, rubber and ceramics. Some materials may be aerosolised and have adverse health effects. Several studies have described significant performance variability across and within e-cigarette brands. Patent applications include novel product features designed to influence aerosol properties and e-cigarette efficiency at delivering nicotine.
Conclusions
Although e-cigarettes share a basic design, engineering variations and user modifications result in differences in nicotine delivery and potential product risks. e-cigarette aerosols may include harmful and potentially harmful constituents. Battery explosions and the risks of exposure to the e-liquid (especially for children) are also concerns. Additional research will enhance the current understanding of basic e-cigarette design and operation, aerosol production and processing, and functionality. A standardised e-cigarette testing regime should be developed to allow product comparisons.
Keywords: Electronic nicotine delivery devices, Non-cigarette tobacco products, Nicotine
Background
Electronic cigarettes (e-cigarettes) comprise a subcategory of a broader range of products described as personal vaporisers (PV), advanced personal vaporisers (APV) or electronic nicotine delivery systems (ENDS). These products have a range of designs. Adequate characterisation of e-cigarette design features is necessary to evaluate the potential risks and benefits associated with their use.
Methods
Systematic literature searches were conducted through October 2013 to identify research related to e-cigarettes and electronic nicotine delivery systems. Ten reference databases (Web of Knowledge, PubMed, SciFinder, Legacy Tobacco Documents Library, Embase, EBSCOhost, Espacenet, Google Scholar, Google Patent and the US Patent Office) were searched using a set of relevant search terms used singly or in combination. Search terms included the following: ‘thermal runaway’ OR ‘battery fire’ OR ‘battery explosion’ OR ‘lithium battery explosion’ OR ‘electronic nicotine devices’ OR ‘electronic nicotine delivery systems’ OR ‘electronic cigarettes’ OR ‘e-cigarette’ OR ‘electronic’ AND ‘cigarette’.
To be considered for inclusion, the article or patent (granted and applications) had to (1) be written in English; (2) be publicly available; and (3) deal partly or exclusively with engineering design or operation, or lithium battery fires or explosions. The search yielded a total of 296 e-cigarette articles or documents that met the inclusion criteria. Article titles and abstracts (when titles provided insufficient detail) were then screened for relevance. In addition, thousands of battery and patent documents were identified; approximately 100 documents related to battery operation and 460 patents were screened for inclusion. Overall, the search yielded 54 articles and 28 patents for full-text review, which included a manual search of the reference lists of selected articles to identify additional relevant publications.
Following the full-text review, 14 articles and documents and 16 patent documents were deemed directly relevant for this analysis. The articles and patent documents were published between 2004 and 2013. The validity and strength of each study were determined based on a qualitative assessment of depth and breadth of analysis, uniqueness and relevance to engineering concerns.
Additional documents considered for review included conference presentations/posters, reports not published in peer-reviewed journals, national and international standards, and government reports. Three documents from either online sources or conference proceedings are cited. Two websites that provide e-cigarette design and operation information are also cited. Although not peer-reviewed, these websites and documents provide valuable insight on product design and operation.
Scientific review
Basic design and operation
e-cigarettes are generally designed to resemble traditional cigarettes in dimensions and, to some extent, graphic design. The common components for most e-cigarettes include an aerosol generator, a flow sensor, a battery and a solution (or e-liquid) storage area (see figure 1).
Figure 1.
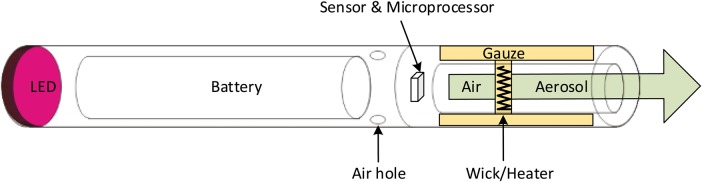
Typical e-cigarette configuration. This shows a wick/heater as aerosol generator, gauze saturated with e-liquid, a microprocessor (optional) to control operations and an LED (optional) to imitate a burning coal.
e-cigarettes currently are classified as either disposable or reusable. Disposable units do not have rechargeable batteries and are usually not refillable. They may have a light-emitting diode (LED). The e-liquid container or cartridge may be separate from the aerosol generator or atomizer; a combined atomizer and cartridge is called a cartomizer. Currently marketed e-cigarettes typically have an aerosol generator with a metal or ceramic heating element coiled around a wick bundle.
A wide variety of materials may be used in an e-cigarette. They include metals, ceramics, plastics, rubber, fibres and foams.1–5 Some materials may be aerosolised, possibly contributing to adverse health effects.
Although e-cigarettes range in complexity, the following describes the basic operation of a first-generation e-cigarette:
The user draws upon the e-cigarette, which activates an airflow sensor.
The airflow sensor detects pressure changes and prompts the flow of power to an LED and a heating element.
The e-liquid saturates a wick via capillary action and is then aerosolised by the heating element.6
The aerosolised droplets of e-liquid subsequently flow into the user's mouth and lungs.7
Detailed operation and components
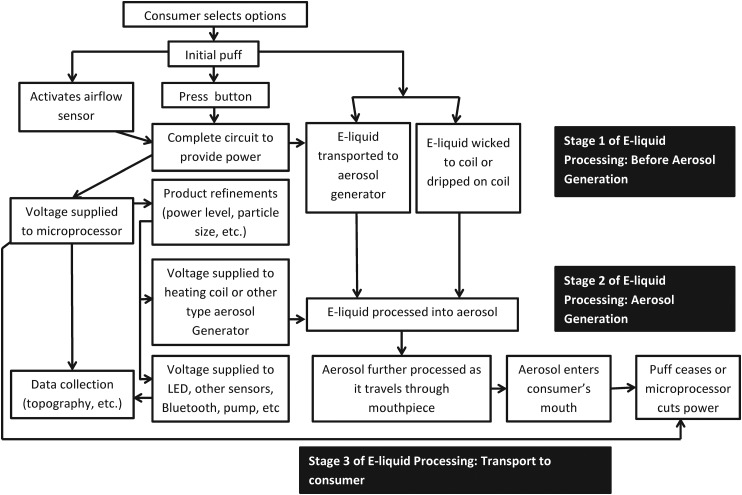
For some advanced e-cigarettes, prior to puffing, the consumer can select feature adjustments that determine heating element temperature, air flow rate or other functions. Figure 2 outlines a more detailed, but typical, e-cigarette operations cycle. The cycle is initiated by single or multiple sensor responses and/or the use of a button.8 The initiating sensor(s) may be an acoustic, pressure, touch, capacitive, optical, Hall Effect or electromagnetic field type.9 10 The sensor(s) and/or button initiates power flow to pumps, heating elements, LEDs and other elements.1 Anecdotal evidence suggests sensors or buttons may provide the ability to extend puff duration.11
Figure 2.
Basic e-cigarette operation. This flowchart outlines basic actions and functions to transform and deliver e-liquid-based aerosol.
The cartridge (or cartomizer) and sometimes the battery holder have air holes to help facilitate the flow of air required for puffing while also controlling for pressure drop. However, air holes may serve multiple purposes. For instance, the European and International Organization for Standardization (ISO) have ink pen lid standards that mandate air holes to prevent a child-choking hazard associated with pen lids.12
Aerosol production generally involves three stages: preprocessing, aerosol generation and postprocessing. The first stage involves the transport of the e-liquid to the aerosol generator. Capillary action through a wick is the primary means used by first-generation and possibly the majority of current e-cigarettes to control the delivery of e-liquid to the aerosolising element. Other possible transport mechanisms include programmed or mechanically controlled pumps, nozzles and diaphragms. The pump may be peristaltic, plunger, eccentric or screw, and powered by electrostatic, piezoelectric, magnetorestrictive, thermal contractive or thermal bubble processes.1 13 Additionally, fluid jet, micromesh, microetched screen or electropermeable membrane methods of transport are available.1 9
Another method of e-liquid delivery to the aerosol generator involves micro-pumps on microelectromechanical systems (MEMS). Miniaturised pumps and/or nozzles/jets deliver specifically programmed quantities and combinations of e-liquids to an aerosol generator.1 Alternately, a consumer may directly drip e-liquid onto a heating element before puffing.11 14
The second stage of aerosol processing involves aerosol generation, which involves (1) heating, in which the e-liquid comes in contact with the heating element as described above; and/or (2) mechanical processing, in which an ultrasonic vibration generator or other mechanical device produces an aerosol by mechanical dispersion.13 15
At least one e-cigarette (cigar) introduced to the overseas market uses ultrasonic vibration to produce an unheated aerosol of 0.5–1.5 μm particle size, and another described in patent documents combines heating and vibrating elements.16 2 In addition to heating and ultrasonic vibration, e-cigarettes may incorporate MEMS that use pumps and nozzles (jets) or ultrasonic piezoelectric elements for aerosol generation.
Possible heating element arrangements include straight line, multiple spiral, cluster, nozzle, laser or element combinations. Coil arrangements may incorporate wicks and coil covers (bridged) or no covers (debridged for ‘dripping’). The heating element's resistance, material and the voltage across it determine the current flow and element temperature. The heating element temperature and temperature duration influence the aerosol properties. Element degradation, fouling and other factors also influence the heating element temperature.
The final stage of aerosol processing occurs as the aerosol travels through the central air passage to the consumer. Unless the aerosol is heated, its temperature decreases as it flows and condensation occurs. Larger droplets that condense on the inside of the central air passage may be removed from the passage and subsequently discarded or reprocessed into an aerosol.17 To further simulate traditional smoking, the appearance of sidestream smoke is engineered into one e-cigarette design using pumps.13
Microprocessors, programmable logic units, integrated circuits and other electronic components may be incorporated into some e-cigarette products. The electronic components may be used to power components and in conjunction with a liquid crystal screen to display and/or record operating state parameters, such as battery life, use frequency per day, average use cycle and safety warnings. However, the microprocessor may also have additional functions such as the ability to control integrated MEMS (eg, pumps and/or motors) that deliver specifically programmed product quantities or concentrations. One patent describes Bluetooth communication protocol integration and multiple smoking software programs based on fluid type and user preference.13
The presence of microprocessors and memory chips in e-cigarettes raises concerns about the collection and use of personal privacy information. A microprocessor may facilitate consumer data collection for dissemination to a third party, either wirelessly by Bluetooth or through the universal serial bus (USB) interface when the battery is recharged.9 Data may include the smoker's personal information (eg, gender, age, address), smoking topography and possibly health-related data.9 18 Of further concern is the ability of the software and microprocessor to directly or covertly manipulate nicotine delivery, and software viruses.
Some e-cigarettes may be used while connected by USB cord to a power source. Additionally, some e-cigarettes may be powered by permanent rechargeable battery (a manufacturer-supplied sealed unit), a non-rechargeable battery or a user-replaceable battery (rechargeable or non-rechargeable). Portable chargeable carrying cases are available for remote e-cigarette charging for some brands. Nickel-cadmium (NiCad), nickel metal-hydride (NiMh), lithium ion (Li-ion), alkaline and lithium polymer (Li-poly), and lithium manganese (LiMn) batteries may be used to power e-cigarettes.19
Performance considerations
Several studies have described significant performance variability among e-cigarette brands and within the same brand/product and compared the performance of e-cigarettes with that of conventional cigarettes. The performance parameters investigated included pressure drop, airflow, aerosol products and puff count.
Trtchounian et al20 compared eight brands of traditional cigarettes to four brands of e-cigarettes. In a comparison of the first 10 puffs of each brand, three of the four e-cigarette brands required significantly higher average vacuums to produce an aerosol density comparable to traditional cigarettes. In the same study, when five brands (Trtchounian et al added additional brand) of e-cigarettes were tested, the vacuum required to produce an aerosol of standardised density increased for each brand as it was smoked. The puff count at which the increase would be required varied from a low of 24±12 for one brand to 121±26, and 114±71 for two brands at the high end. Puff count to entirely exhaust a cartridge varied for the e-cigarettes tested from low of 30±43 to a high of 313±115 for different brands.
Williams and Talbot followed up their collaboration with Trtchounian and tested four different brands of e-cigarettes that included duplicates of two brands.21 An analysis of the first 10 puffs showed that pressure drop, flow rate and aerosol density remained relatively constant for a given e-cigarette, but varied among brands. The investigators analysed the ventilation or air flow for each brand; they concluded that there was a good correlation between air hole area and pressure drop for half the e-cigarettes tested. The investigators concluded that “the airflow rate required to produce an aerosol varied significantly among e-cigarette brands and was usually higher than the airflow rate required to produce smoke from tobacco-containing cigarettes.”21 Additionally, they concluded that standard testing protocols typically used with traditional cigarettes are not appropriate for e-cigarettes, stating, “E-cigarette laboratory testing will require its own standard procedure, which is yet to be developed”.21
Test protocols and standardisation are a concern when comparing results from the two studies. In each study, puff counts are correlated with both puff volume and duration. The investigators produced aerosols of a particular density. This presupposes that density will drive the puff volume for all e-cigarettes. However, the satiating effect may be the driving factor; thus, standardisation of nicotine delivered/absorbed may be an alternate means of determining the appropriate test protocol. The effect of smoking topography on the studies’ results and conclusions is unclear and merits further investigation.
Design and aerosol production considerations
Anecdotal evidence suggests that larger capacity tanks, higher coil voltage and dripping configurations appear to be consumer innovations adopted by manufacturers. The e-cigarette forums are a potential source of information concerning emerging trends regarding e-cigarette design, use and maintenance. Currently marketed e-cigarettes may have thousands of interchangeable parts that modify the character of the delivered aerosol; connection adapters are available to further enable interchangeability. Currently advertised features of some e-cigarettes include
advanced power-on activation (multiple-button click-on feature)
auto shutoff (safety feature)
short circuit and over current protection (safety feature)
variable voltage ranges (eg, 3–6 V in 0.1 V increments).
It appears that the variable voltage units introduced the ability to increase heating element temperature. Increasing heating element temperature subsequently increases the temperature of the air containing the aerosol and increases the aerosol generation rate. The warmer air can hold more e-liquid mass per unit of air volume. Additionally, aerosol particle size may be altered by the temperature of the heating coil.5 Referencing Trtchounian et al20, Zhang et al22 noted, “Vaping technique would be especially important if vapers can generate a different range of particle sizes, or if particle sizes change over time.” Additional research is required to determine whether the increased coil temperature significantly increases the e-liquid mass available and/or alters aerosol particle size.
According to Etter et al23, particle size affects absorption and can directly affect aerosol toxicity. Two studies measured e-cigarette aerosol particle size. Ingebrethsen et al24 found that undiluted e-cigarette aerosols had particle diameters in the 250–450 nm range and particle density concentration of approximately 109 particles/cm3. However, according to the same report, these e-cigarette numbers differ somewhat from the 50–200 nm particle diameter modes reported in another study by Schripp et al.25 Zhang et al22 constructed an apparatus to determine in vitro particle size distribution and concluded through testing that “e-cigs and conventional reference cigarettes produce aerosols having generally similar particle sizes in the range of 100–600 nm.”
In 2012, Pellegrino et al compared particulate matter (PM) from aerosols of Italian brand e-cigarettes with the PM of conventional cigarettes. Data showed that concentration of fine and ultrafine PM was approximately 6–18 times higher for the conventional cigarettes than the e-cigarettes tested.26
A limited number of patents (granted and applications) were reviewed to determine what new features have been incorporated into products currently on the commercial market or potentially available to the market. One patent described ports designed to facilitate reprocessing of accumulated condensate.15 In their patent application, Tucker et al19 describe the following novel features: diffusers (figure 3), which enhance mixing; airflow diverters, which apparently allow the heating element to maintain a desired temperature, thus optimising nicotine delivery with puff intensity; multiple e-liquid tanks, each with its own wick heater; aroma strips to add fragrance to the outside of the e-cigarette (or into the aerosol); and a movable screen with openings to vary the airflow and thus the pressure drop. Liu describes multiple heating elements arranged in a series or in parallel, where aerosol streams converge or remain separate.27 In another patent application, Tucker et al28 describe an advanced dripping configuration. Alarcon and Healy describe extensive communications and data collection potential in one of their patent applications (table 4).9 Conley et al29 describe breath, saliva, sweat, and tissue or cell analysis and conveyance of the information to a healthcare professional as well as biometrics to couple a device to a particular user. Li et al30 describe a feature designed to shift aerosol particle size distribution towards a range of smaller particles through impacting the aerosol on a surface.
Figure 3.
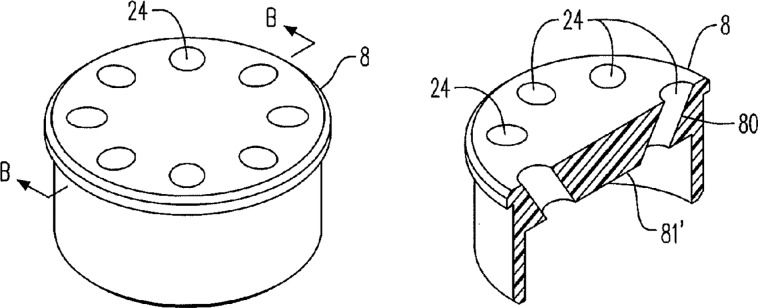
Diffuser. This diagram shows multiple angled openings that increase aerosol dispersion and buccal cavity contact. The numbers reference descriptive text in the patent.5 19
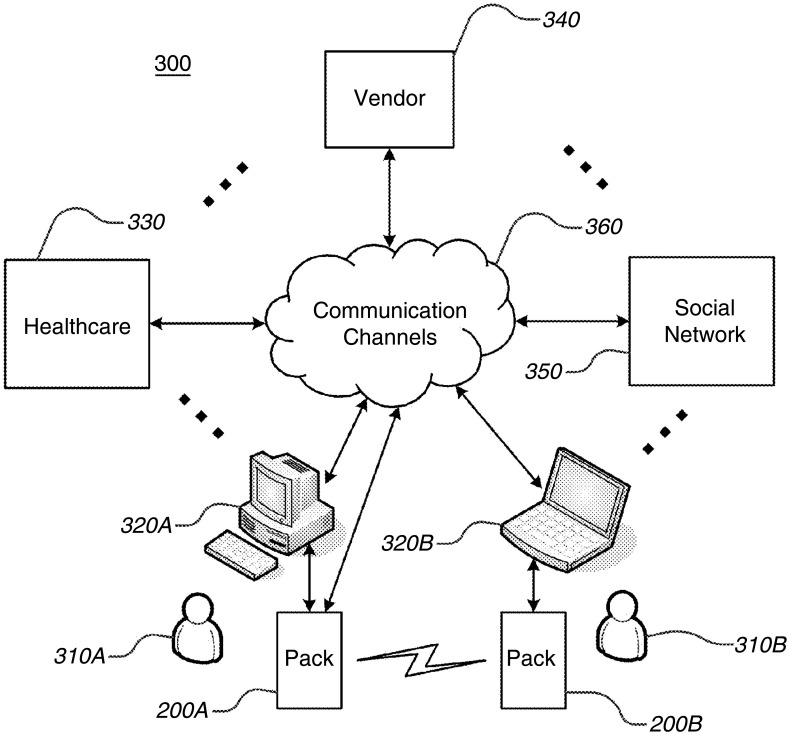
Figure 4.
Communications features. This diagram shows bidirectional data transfer between the consumer, computer, pack, social networks and stakeholders. The numbers reference descriptive text in the patent.9
Safety considerations
Two safety issues are posed by contaminants in the e-liquid or ‘e-juice’ and the handling hazards associated with inadvertent skin contact. Due to a lack of manufacturing standards and controls, e-liquid purity often cannot be assured, and testing of some products has revealed the presence of hazardous substances.7 The nicotine in e-liquid can be hazardous if mishandled and can be toxic to infants and children at the levels present in e-liquid.31 Child-safe or child-resistant packaging, child safety locks (such as those present on cigarette lighters) and proper instruction on the safe handling of e-liquid can help mitigate some of these risks. One patent discussed the use of biometrics and sensors to identify consumers by age; this technology could possibly be used to prevent some child usage by using age screening.29
The use of e-cigarettes with illegal substances is a concern. One patent states that, “With slight modification of the solution storage container, the device and connecting structures of the present invention can be filled with conventional drug for pulmonary administration apparatus.”2 Another patent notes that the unit may be used with narcotics, steroids, the marijuana constituent tetrahydrocannabinol (THC) and other substances.29
Part interchangeability is of particular concern since the performance, risks and safety associated with a particular brand's configuration might change significantly when that configuration is modified using third-party products. One study showed some variability in cartomizer unit performance when batteries were exchanged with those of the same brand.21 In the same study, one e-cigarette stopped producing aerosol when parts were exchanged with another (same model) unit. Furthermore, brand-specific e-liquid cartridges performed ‘significantly better’ at producing aerosols than third-party cartridges provided by a vendor.
Other concerns involve materials used in the aerosol generation process, aging and fouling. e-cigarettes that use a heating mechanism to create a nicotine vapour emit metallic particles and even nanoparticles of heating coil components in the aerosol, such as tin, iron, nickel and chromium.3 Lead, nickel and chromium appear on the US Food and Drug Administration's (FDA) ‘harmful and potentially harmful constituents (HPHC) list’.32 The safety of the inhalation of these metallic particles and nanoparticles has not been studied and could be a cause for concern. The use of alternative aerosol generation mechanisms may mitigate some of these safety questions, although it is uncertain whether these alternatives may also generate particle and nanoparticle emissions. Moreover, long-term e-cigarette performance and the associated generation of HPHCs have not been studied. As e-cigarettes age and become fouled, the products they generate may change. Automatic or manual heating element cleaning should be design considerations.
Many e-cigarettes use lithium batteries due to their ability to store large amounts of energy in a compact amount of space. However, the inherent characteristics of lithium batteries can pose a risk of fire and explosion. Poor design, use of low-quality materials, manufacturing flaws and defects, and improper use and handling can all contribute to a condition known as ‘thermal runaway’, whereby the internal battery temperature can increase to the point of causing a battery fire or even an explosion.33 The use of overcharging protection circuits, thermal power cut-offs and internal overpressure relief mechanisms can help prevent and mitigate thermal runaway.34
Critical information/tool gaps
Additional scientific studies are needed to evaluate the safety and effectiveness of e-cigarettes. Topics for future research include the following:
Understanding of the designs and functions of products currently on the US market is incomplete.
Variable and increased voltage e-cigarettes appear to introduce the ability to deliver increased nicotine concentrations.35 Higher voltages and other features may introduce the ability to manipulate particle size and increase aerosol mass. Little research is available concerning these functions.
Knowledge of all the materials involved in aerosol production is lacking.
Hazards associated with the use of batteries require further study. Failure mechanisms and the frequency of burn, shock and explosion hazards are unknown.
The possible presence, function and capabilities of the software, sensors and microprocessors incorporated into e-cigarettes are unknown. It is not known what health or topographical data are being collected or how the data may influence or affect regulation and health policies. Software vulnerabilities are also unknown.
The absence of standardised testing protocols compromises comparisons across studies. Standardised test protocols that allow for meaningful testing, categorisation and comparison of e-cigarette test results would be a valuable research tool.
Knowledge of product lifecycles, degradation over time, third-party component performance and misuse is needed.
Conclusions
Although e-cigarettes share a basic design, engineering variations and user modifications result in differences in nicotine delivery and potential product risks. Performance appears to greatly vary among commercial products. e-cigarette aerosols may include HPHCs defined by the FDA. Battery explosions and risk of exposure to the e-liquid (especially for children) are also potential concerns. A number of safety features that could enhance consumer safety do not appear to be widely used.
Current e-cigarette features have the potential to increase nicotine delivery though advanced aerosol production methods. Patents show that novel features are available that will likely further maximise aerosol properties and e-cigarette efficiency for delivering nicotine. e-cigarette forums and websites provide information on current e-cigarette use, misuse, innovations and concerns that may influence the commercial market.
Additional research will improve the current understanding of basic e-cigarette design and operation, aerosol production and processing, data collection capability, sensor and software/microprocessor functionality and vulnerability, performance variation, lifecycle degradation, unintended use and user safety. In addition, a standardised e-cigarette testing protocol should be developed to allow product comparisons. Although of significant importance, specific guidance for development of a standardised test regime is beyond the scope of this paper. e-cigarettes with unique designs may require specialised testing protocols.
What this paper adds.
To our knowledge, this is the first comprehensive review of the literature related to e-cigarette product design features and their potential health consequences.
e-cigarettes are highly engineered products representing a wide variation in product configuration, components and safety features; furthermore, flexibility in many e-cigarette designs allows user modifications. This results in cross-product and within-product differences in aerosol production, nicotine delivery and potential product use risks, making it difficult to evaluate the impact of e-cigarettes on individual users and the public health.
Additional research is required to ascertain the health consequences of e-cigarette use; a standardised e-cigarette testing regime should be developed in order to facilitate cross-product and within-product comparisons.
Acknowledgments
The authors thank Elizabeth L. Durmowicz, M.D., Deborah Neveleff, M.B.A., Paul Aguilar, M.P.H., Thomas Eads, Ph.D., M.P.A., and R. Philip Yeager, Ph.D., DABT, for their support and assistance.
Footnotes
Contributors: CJB performed the broad literature search on electronic cigarettes, and JMC performed the literature search on battery-related items. CJB and JMC cowrote the article; CJB, the lead author, focused on design, operation and performance, while JMC focused on batteries and exposure.
Competing interests: None.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Hon L, inventor; Best Partners Worldwide Limited, assignee. A flameless electronic atomizing cigarette. European Patent Specification EP1618803B1. 2006 Jan 25. http://worldwide.espacenet.com/publicationDetails/originalDocument?FT=D&date=20081203&DB=worldwide.espacenet.com&locale=en_EP&CC=EP&NR=1618803B1&KC=B1&ND=5 (accessed 13 Feb 2013)
- 2.Hon L, inventor; Best Partners Worldwide Limited, assignee. An aerosol electronic cigarette European Patent Specification. EP1736065B1. 2006 Dec 27. http://worldwide.espacenet.com/publicationDetails/originalDocument?FT=D&date=20090603&DB=worldwide.espacenet.com&locale=en_EP&CC=EP&NR=1736065B1&KC=B1&ND=4 (accessed 13 Feb 2013)
- 3.Williams M, Villarreal A, Bozhilov K, et al. Metal and silicate particles including nanoparticles are present in electronic cigarette cartomizer fluid and aerosol. PLoS ONE 2013;8:e57987 http://www.plosone.org/article/fetchObject.action?uri=info%3Adoi%2F10.1371%2Fjournal.pone.0057987&representation=PDF (accessed 16 July 2013) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Moflit B, Torrence K, Lam K, et al. New product focus team test plan & status report for Beijing Saybolt Ruyan Technologies product. University of California, San Francisco, Legacy Tobacco Documents Library, Philip Morris Collection 2004. Retrieved 2/21/2013 http://legacy.library.ucsf.edu/tid/sze91g00 (accessed 21 Feb 2013)
- 5.Tucker CS, Jordan GB. inventors; Altria Client Service Inc., assignee. Electronic smoking article and improved heating element. United States patent application. 20130213419 A1. 2013 Aug 22. http://appft.uspto.gov/netacgi/nph-Parser?Sect1=PTO2&Sect2=HITOFF&u=%2Fnetahtml%2FPTO%2Fsearch-adv.html&r=1&f=G&l=50&d=PG01&p=1&S1=20130213419.PGNR.&OS=DN/20130213419&RS=DN/20130213419 (accessed 26 Sep 2013)
- 6.Newton KD. Inventor. Electronic cigarette with liquid reservoir. United States patent 8528569 B1. 2013 Sep 10. http://patft.uspto.gov/netacgi/nph-Parser?Sect1=PTO1&Sect2=HITOFF&d=PALL&p=1&u=%2Fnetahtml%2FPTO%2Fsrchnum.htm&r=1&f=G&l=50&s1=8528569.PN.&OS=PN/8528569&RS=PN/8528569 (accessed 26 Sep 2013)
- 7.Wollscheid KA, Kremzner ME. Electronic cigarettes: Safety concerns and regulatory issues. Am J Health Syst Pharm 2009;66:1740–42 http://www.ajhp.org/content/66/19/1740.full (accessed 15 Feb 2013) [DOI] [PubMed] [Google Scholar]
- 8.Cheah NP, Chong NW, Tan J, et al. Electronic nicotine delivery systems: regulatory and safety challenges: Singapore perspective. Tob Control. 2014;23:119–25 http://tobaccocontrol.bmj.com/content/early/2012/11/30/tobaccocontrol-2012–050483.full.pdf+html?sid=05289f33–6563–407b-b9fc-850ba38895ff (accessed 05 Dec 2012) [DOI] [PubMed] [Google Scholar]
- 9.Alarcon R, Healy J, inventors; Electronic smoking device. United States patent application 20110265806 A1. 2011 Nov 03. http://appft.uspto.gov/netacgi/nph-Parser?Sect1=PTO1&Sect2=HITOFF&d=PG01&p=1&u=%2Fnetahtml%2FPTO%2Fsrchnum.html&r=1&f=G&l=50&s1=%2220110265806%22.PGNR.&OS=DN/20110265806&RS=DN/20110265806 (accessed 26 Sept 2013).
- 10.Terry N, Minskoff N, inventors; Personal vaporizing inhaler with mouthpiece cover. United States patent application 20110277780A1. 2011 Nov 17. http://worldwide.espacenet.com/publicationDetails/originalDocument?FT=D&date=20111117&DB=EPODOC&locale=en_EP&CC=US&NR=2011277780A1&KC=A1&ND=4 (accessed 18 Nov 2013).
- 11.E-Dripping.com. How do you Drip with an Electronic Cigarette? http://edripping.com/about-ecigarettes/e-cigarette-dripping-how-to/ (accessed 25 Jul 2013)
- 12.European Child Satiety Alliance (ECOSA). A guide to child safety regulations and standards in Europe. AD, Netherlands 2003. http://www.childsafetyeurope.org/publications/info/child-safety-regulations-standards.pdf (accessed 31 Jan 2013)
- 13.Katase M, inventor. Electronic cigarette. United States patent application 20050016550 A1. 2005 Jul 25. http://www.google.com/patents?hl=en&lr=&vid=USPATAPP10886508&id=SY-SAAAAEBAJ&oi=fnd&dq=ELECTRONIC+CIGARETTE&printsec=abstract (accessed 8 Feb 2013)
- 14.E-Cigarette Forum. E-cigarette Technical; Tips and Tricks, Do You Drip? http://www.e-cigarette-forum.com/forum/tips-tricks/9069-do-you-drip-2.html (accessed 29 Aug 2013)
- 15.Alelov E, inventor. Inhalation device including substance usage controls. United States patent 8550069 B2. 2013 Oct 8. http://worldwide.espacenet.com/publicationDetails/originalDocument?FT=D&date=20131008&DB=worldwide.espacenet.com&locale=en_EP&CC=US&NR=8550069B2&KC=B2&ND=4 (accessed 18 Nov 2013)
- 16.Pauly J, Li Q, Barry MB. Tobacco-free electronic cigarettes and cigars deliver nicotine and generate concern. Tob Control 2007;16:357 http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2598554/ (accessed 15 Feb 2013) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hon L, inventor; Ruyan Investment (Holdings) Limited, assignee. Electronic atomization cigarette. United States patent 8,490,628 B2. 2013 July 23. http://patft.uspto.gov/netacgi/nph-Parser?Sect1=PTO1&Sect2=HITOFF&d=PALL&p=1&u=%2Fnetahtml%2FPTO%2Fsrchnum.htm&r=1&f=G&l=50&s1=8490628.PN.&OS=PN/8490628&RS=PN/8490628 (accessed 26 Sep 2013)
- 18.Marangos B, inventor; Electronic cigarette. Canadian patent application 02731485. 2011 Feb 09. http://worldwide.espacenet.com/publicationDetails/originalDocument?CC=CA&NR=2731485A1&KC=A1&FT=D&ND=4&date=20120809&DB=worldwide.espacenet.com&locale=en_EP (accessed 18 Nov 2013)
- 19.Tucker CS, Jordan GB, Smith BS, et al. inventors; Altria Client Service Inc., assignee. Electronic cigarette. United States patent application 20130192623 A1. 2013 Aug 1. http://appft.uspto.gov/netacgi/nph-Parser?Sect1=PTO1&Sect2=HITOFF&d=PG01&p=1&u=%2Fnetahtml%2FPTO%2Fsrchnum.html&r=1&f=G&l=50&s1=%2220130192623%22.PGNR.&OS=DN/20130192623&RS=DN/20130192623 (accessed 26 Sep 2013).
- 20.Trtchounian A, Williams M, Talbot P. Conventional and electronic cigarettes (e-cigarettes) have different smoking characteristics. Nicotine Tob Res 2010;12:905–12 http://ntr.oxfordjournals.org/content/12/9/905.abstract?sid=7ee8d8e7-dc0f-4b26-ab8f-719f7a2fb312 (accessed 2 Nov 2012) [DOI] [PubMed] [Google Scholar]
- 21.Williams M, Talbot P. Variability among electronic cigarettes in the pressure drop, airflow rate, and aerosol production. Nicotine Tob Res 2011;13:1276–83 http://ntr.oxfordjournals.org/content/13/12/1276.abstract?sid=d4f38075–063c-4812-be93–604f99e02a3e (accessed 02 Nov 2013) [DOI] [PubMed] [Google Scholar]
- 22.Zhang Y, Sumner W, Chen D. In vitro particle size distributions in electronic and conventional cigarette aerosols suggest comparable deposition patterns. Nicotine Tob Res 2012;15:501–8 [DOI] [PubMed] [Google Scholar]
- 23.Etter JF, Bullen C, Flouris AD, et al. Electronic nicotine delivery systems: a research agenda. Tob Control 2011;20:243–48 Retrieved 11/02/2012. http://tobaccocontrol.bmj.com/content/20/3/243.abstract?sid=68114618–566f-4315-a6e5-b1a6c2d6a9e7 (accessed 02 Nov 2012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ingebrethsen BJ, Cole SK, Alderman SL. Electronic cigarette aerosol particle size distribution measurements. Inhal Toxicol 2012;24:976–84 [DOI] [PubMed] [Google Scholar]
- 25.Schripp T, Markewitz D, Uhde E, et al. Does e-cigarette consumption cause passive vaping? Indoor Air 2013;23:25–31 [DOI] [PubMed] [Google Scholar]
- 26.Pellegrino RM, Tinghino B, Mangiaracina G, et al. Electronic cigarettes: an evaluation of exposure to chemicals and fine particulate matter (PM). Ann Ig 2012;24:279–88 [PubMed] [Google Scholar]
- 27.Liu Q, inventor; Electronic cigarette, electronic cigarette flare and atomizer thereof. European patent application EP2641490A1. 2013 Sep 25. http://worldwide.espacenet.com/publicationDetails/originalDocument?FT=D&date=20130925&DB=worldwide.espacenet.com&locale=en_EP&CC=EP&NR=2641490A1&KC=A1&ND=5 (accessed 18 Nov 2013)
- 28.Tucker CS, Kobal G, Jordan GB, et al. inventors; Altria Client Service Inc., assignee. Electronic smoking article. United States patent application. 20130213418 A1. 2013 Aug 22. http://appft.uspto.gov/netacgi/nph-Parser?Sect1=PTO1&Sect2=HITOFF&d=PG01&p=1&u=%2Fnetahtml%2FPTO%2Fsrchnum.html&r=1&f=G&l=50&s1=%2220130213418%22.PGNR.&OS=DN/20130213418&RS=DN/20130213418 (accessed 26 Sep 2013)
- 29.Conley GD, Hillenbrandt DC, Mandela M, et al. inventors; Fuma International, assignee. Electronic vaporizer. United States patent application 20130220315 A1. 2013 Aug 29. http://appft.uspto.gov/netacgi/nph-Parser?Sect1=PTO1&Sect2=HITOFF&d=PG01&p=1&u=%2Fnetahtml%2FPTO%2Fsrchnum.html&r=1&f=G&l=50&s1=%2220130220315%22.PGNR.&OS=DN/20130220315&RS=DN/20130220315.
- 30.Li S, Karles G, Munmaya KM, et al. inventors; Altria Client Service Inc., assignee. Electronic smoking article. United States patent application 20130192621 A1. 2013 Aug 1. http://appft.uspto.gov/netacgi/nph-Parser?Sect1=PTO2&Sect2=HITOFF&u=%2Fnetahtml%2FPTO%2Fsearch-adv.html&r=1&p=1&f=G&l=50&d=PG01&S1=20130192621&OS=20130192621&RS=20130192621 (accessed 21 Nov 2013)
- 31.Connolly GN, Richter P, Aleguas A, et al. Unintentional child poisonings through ingestion of conventional and novel tobacco products. Pediatrics 2010;125:896–9 [DOI] [PubMed] [Google Scholar]
- 32.U.S. Food and Drug Administration. Harmful and Potentially Harmful Constituents in Tobacco Products and Tobacco Smoke; Established List. Docket No. FDA-2012-N-0143. Federal Register 2012;64. http://www.fda.gov/downloads/TobaccoProducts/GuidanceComplianceRegulatoryInformation/UCM297981.pdf
- 33.Wang Q, Ping P, Zhao X, et al. Thermal runaway caused fire and explosion of lithium ion battery. Journal of power sources 2012;208:210–24 [Google Scholar]
- 34.Ballard GE, inventor; Standard Oil Company, assignee. Explosion resistant battery cells. United States patent 4397919. 1983 Aug 9
- 35.Shihadeh A, Salman R, Zainab Balhas Z, et al. Factors influencing the toxicant content of electronic cigarette vapor: device characteristics and puff topography. Poster session presented at annual meeting of the Society for Research on Nicotine and Tobacco Boston, MA: 2013. from Joseph Lisko, CDC. Atlanta, GA; (ivv0@cdc.gov). 15 April 2013 [Google Scholar]