Abstract
Background
Shanghai fever, a community-acquired enteric illness associated with sepsis caused by Pseudomonas aeruginosa, was first described in 1918. The understanding of Shanghai fever is incomplete.
Objective
To delineate the clinical features and to examine the host and microbial factors associated with Shanghai fever.
Methods
We prospectively enrolled 27 consecutive previously healthy children with community-acquired P aeruginosa enteritis and sepsis between July 2003 and June 2012. An immunological investigation, including measurement of serum immunoglobulin levels and lymphocyte subpopulations, was performed. The clonal relationship of bacterial isolates was determined by multilocus sequence typing (MLST) and the virulence of isolates was measured using cellular and animal models.
Results
The median age of the patients was 7 months; 24 (89%) were aged <1 year. The most common clinical manifestations were fever (100%), diarrhoea (96%) and shock (81%). Leucopenia, thrombocytopenia, high C-reactive protein levels, coagulopathy and hypoalbuminaemia were the key laboratory findings. Necrotising enteritis with or without bowel perforation, ecthyma gangrenosum and seizures were main complications. The death rate was 15%. No common primary immune deficiency was identified. MLST genotypes indicated that isolates from Shanghai fever were non-clonal, but they shared similar phenotypes which were invariably cytotoxic, invasive and adhesive in cellular experiments and caused prolonged gut colonisation and more death than respiratory and laboratory control strains in mice.
Conclusions
Shanghai fever is a sporadic community-acquired disease of previously healthy infants that manifests as sepsis associated with P aeruginosa enteric disease. Both host and microbial factors play a role in pathogenesis.
Keywords: Bacterial Infection, Bacterial Pathogenesis, Infectious Diarrhoea, Sepsis, Enteric Infections
Significance of this study.
What is already known about this subject?
Pseudomonas aeruginosa is not a common enteric pathogen in healthy hosts.
Shanghai fever is a sepsis accompanied by enteric disease caused by P aeruginosa.
Hypogammaglobulinaemia is the most common underlying primary immune deficiency in previously healthy children with P aeruginosa sepsis.
What are the new findings?
The clinical features of Shanghai fever in a cohort of patients were clearly delineated.
No common primary immune deficiency was found in infants with Shanghai fever.
The high virulence of P aeruginosa strains derived from patients with Shanghai fever were confirmed by cellular and animal experiments.
Both host and microbial factors play a role in the pathogenesis.
How might it impact on clinical practice in the foreseeable future?
Understanding the mechanism, by which a specific group of virulent P aeruginosa causes enteric infection and sepsis in infants, may lead to increased awareness of the disease such that early initiation of effective treatment might be expected to reduce mortality and morbidity.
Introduction
Pseudomonas aeruginosa is one of the most important opportunistic pathogens in humans. Patients with chronic disease and compromised immune systems, such as cystic fibrosis, haematological malignancy, immunodeficiency or burns, are at greater risk of infection.1 2 The bloodstream and respiratory and urinary tracts are the common infection sites. Despite reports of antibiotic-associated diarrhoea caused by P aeruginosa, it is generally not considered a common cause of infectious diarrhoea in healthy hosts.3 4 Diarrhoea caused by P aeruginosa is seen almost exclusively in patients with prolonged antibiotic exposure.3 4 On the other hand, enteric disease associated with sepsis caused by P aeruginosa has been documented as early as 1918 with the description of ‘Shanghai fever’, a syndrome comprising fever, diarrhoea and sepsis.5 6 Cases of community-acquired P aeruginosa sepsis were subsequently reported in children without pre-existing conditions, primarily from Taiwan, Hong Kong and China.7–15 The disease usually leads to serious complications and is associated with high mortality.7–15 After more than 90 years, our understanding on Shanghai fever is still incomplete and its pathogenesis remains unknown. This study aimed to delineate the clinical features of Shanghai fever and to examine the host and microbial factors associated with the infection.
Patients and methods
Patients
The following criteria were used to diagnose Shanghai fever: (1) community-onset diarrhoea with fever, (2) sepsis and (3) growth of P aeruginosa from blood or another sterile body site. Twenty-seven consecutive children who met criteria for Shanghai fever in Chang Gung Children's Hospital in Taiwan from July 2003 to June 2012 were evaluated. All were healthy and without pre-existing conditions before the infection. Demographic, clinical and laboratory data of the patients were analysed. The institutional review board of the Chang Gung Memorial Hospital approved the study.
Standard immunological studies
Immunoglobulin levels, including IgG, IgM, IgA and IgE, were checked in all patients except two who died upon arrival at the hospital. Flow cytometry immunophenotyping of lymphocyte subpopulations was performed in 21 patients. All immunological studies were performed at the stage of recovery. The results were interpreted based on age-matched reference data.16 17
Bacterial strains
Of the 25 P aeruginosa isolates obtained from patients with Shanghai fever, 17 were available for analysis. Sixteen P aeruginosa isolates from the respiratory tracts of patients with nosocominal pneumonia were randomly collected and used for comparison. The cytotoxic P aeruginosa laboratory strains PA103 and PA14 and the non-cytotoxic but invasive strain PAO1 were used as phenotypic controls in cellular and animal experiments.18 Salmonella enterica server Typhimurium strain SL1344 was used as a positive control for the Madin–Darby canine kidney (MDCK) cell monolayer penetration assays.18 Non-invasive rabbit enterotoxigenic Escherichia coli strain RDEC-1 was used as a negative control.18
Antimicrobial susceptibility test and multilocus sequence typing (MLST)
The disk diffusion method was performed to determine the susceptibility of clinical P aeruginosa isolates to the following antimicrobial agents: gentamicin, amikacin, aztreonam, ceftazidime, cefepime, imipenem, meropenem, piperacillin and ciprofloxacin.19 MLST was performed as described by Curran et al.20 PCR products were sequenced by a DNA auto-sequencer and the sequences were compared with the P aeruginosa MLST database (http://www.pubmlst.org/paerugniosa) for allelic numbers. The isolates were designated a sequence type (ST) number according to their allelic profiles. STs with identical alleles in five or more loci were considered clonal clusters.
Cytotoxicity, penetration and adhesion assays
In vitro cytotoxicity of bacterial isolates to MDCK cells was evaluated by the method described by Hirakata et al.18 MDCK cells in Dulbecco's modified Eagle medium (Gibco, Grand Island, New York, USA) with 10% fetal bovine serum were seeded into 24-well tissue culture plates with a density of 1.5×105 per well. After 4 days of incubation at 37°C and 5% CO2, the cells reached a density of 1×106/well. Each bacterial isolate was added with a multiplicity of infection (MOI) of 100. Cytotoxicity was quantified by measurement of lactate dehydrogenase (LDH) in supernatants released from MDCK cells with the CytoTox 96 kit (Promega, Madison, Wisconsin, USA) 8 h after infection. The LDH value was given as a percentage, with 100% representing the amount of LDH released from cells completely lysed by the lysis solution in 45 min. The percentages were calculated according to the manufacturer's instructions. The method used for in vitro cytotoxicity against neutrophils was a modification of that used by El Solh et al.21 Neutrophils were collected from the whole blood of healthy volunteers and purified by density gradient centrifugation. The viability of neutrophils determined by trypan blue staining was >95%. Neutrophils were resuspended in RPMI-1640 (Roswell Park Memorial Institute, Gibco, Grand Island, New York, USA) medium. Approximately 5×106 neutrophils and bacterial isolates with an MOI of 100 were co-incubated in each well. Cytotoxicity was assessed 2 h after infection.
To assess the invasive phenotype of the strains, the MDCK cell monolayer penetration assay was used as previously described.18 Bacteria in basolateral medium were counted by plating appropriate dilutions at 1, 3, 6 and 8 h after inoculation. Strain RDEC-1 was used as a control to ensure the monolayer integrity.18 For adhesion assays, Caco-2 cells were seeded into six-well plates at a density of 1.5×105 cells/well. After 4 days of incubation, the cells grew to a density of 1.5×106/well. Bacteria were added to each well at an MOI of 100 and incubated for 1 h at 37°C. Non-adherent bacteria were eliminated by washing twice with phosphate-buffered saline. Cells were lysed with 0.05% Triton X-100 solution for 10 min. Bacteria were counted by serial dilution and plating. All the cellular studies were performed in three independent experiments.
Type III secretion system (TTSS)-encoding toxin genes
The PCR and sequencing were carried out to detect the presence of TTSS-encoding toxin genes using specific primers and methods described previously.22 The following genes were measured: exoU, exoS, exoT and exoY.
Animal studies
The 50% lethal doses (LD50) of two Shanghai fever isolates (S1 and S6) and the three control strains (PAO1, PA103 and PA14) were determined with the following procedure. Bacterial strains were cultured overnight with shaking at 37°C and then further subcultured for 3 h in fresh medium with shaking at 37°C. Subcultures were collected by centrifugation, then washed and diluted to appropriate ODs in phosphate-buffered saline. Bacterial suspension (50 μL) was injected into the tail veins of 6–8 week-old BALB/c mice. Each bacterial isolate was injected into a minimum of 10 mice. Mice were monitored every 8 h over the subsequent 96 h. Serial dilutions of each bacterial inoculum were plated onto selective media to determine the number of colony forming units (CFUs) injected into each group of mice. Mice showing signs of severe illness, as indicated by hypopnoea, tachypnoea, ruffled fur or absence of activity, were killed and scored as dead. The LD50 for each strain was calculated according to the method of Reed and Muench.23 Three independent experiments were performed. Approval from the animal care and use committee of Northwestern University was obtained before starting these experiments.
Fecal shedding of P aeruginosa in mice after oral challenge was also evaluated with the following procedure. P aeruginosa strains used in this experiment included the three laboratory strains (PAO1, PA103 and PA14), two respiratory isolates (R3 and R5) and five Shanghai fever isolates (S1, S2, S4, S6 and S8). P aeruginosa isolates were selected for this experiment based upon their adherence to Caco-2 cells: S1, S4 and R5 showed low adherence (below median), while S2, S6, S8 and R3 showed high adherence (above median). Five-week-old BALB/c mice weighing 20–25 g were used in the experiments. Individual litters (five mice per litter) were assigned to receive inoculation of one bacterial isolate. Mice were pretreated with clindamycin 0.6 mg intramuscularly daily for 5 days before bacterial challenge. A total of 0.1 mL of 0.45% sterile saline with or without a bacterial inoculum of 1×108 CFUs/mL was delivered to the mice through an orogastric tube daily for 3 days. To monitor bacterial shedding, stool samples were collected daily from individual mice by anal stimulation and plated on Pseudomonas isolation agar (Becton, Dickinson and Company, Sparks, Maryland, USA). The mice were observed daily. The study was terminated 30 days after bacterial inoculation. The fecal shedding study was performed independently three times. The institutional animal care and use committee of Chang Gung University approved the animal experiments.
Statistical analysis
The Student t test was used for continuous variables with normal distribution and the Mann–Whitney U test was used for continuous variables without normal distribution. Fisher's exact test or χ2 test was used for categorical data. Pearson correlation was used to measure the linear association between fecal shedding and adhesion. All reported p values were two-sided; p<0.05 was considered statistically significant.
Results
Clinical data
The patients had a median age of 7 months (range 3–25 months) and 24 (89%) were aged <1 year. The male-to-female ratio was 17:10. The median time from onset of first symptom to sepsis was 4 days (range 1–7 days). The median duration of fever was 8 days (range 1–26 days) and the median length of hospitalisation was 22 days (range 1–122 days). Seventeen (63%) patients developed the disease between July and October.
Fever and diarrhoea were the most common clinical presentations (table 1). Twenty-three patients (85%) had severe necrotising enteritis, as defined and reported previously,11 and nine (33%) had bowel perforation requiring immediate surgical intervention. Intraoperative findings showed widespread patchy necrosis with fibrin coating of the small intestine or colon (figure 1A). Of patients with ecthyma gangrenosum (figure 1B), 10 had multiple sites affected. Seven patients (26%) had seizure; all of them had hyponatraemia. Two had meningitis with subsequent obstructive hydrocephalus. Both eventually developed severe chronic neurological sequelae, including brain atrophy and hydrocephalus.
Table 1.
Clinical features of patients with Shanghai fever
| Clinical manifestation | Number (%) |
|---|---|
| Initial presentations | |
| Fever and diarrhoea | 25 (93) |
| Dyspnoea/cyanosis | 2 (7) |
| Fever | 27 (100) |
| Diarrhoea | 26 (96) |
| Watery | 18 (67) |
| Greenish | 12 (44) |
| Mucoid | 11 (41) |
| Bloody | 7 (26) |
| Vomiting | 12 (44) |
| Dyspnoea | 10 (37) |
| Seizure | 7 (26) |
| Shock | 22 (81) |
| Necrotising enteritis | 23 (85) |
| Bowel perforation | 9 (33) |
| Ecthyma gangrenosum | 17 (63) |
Figure 1.
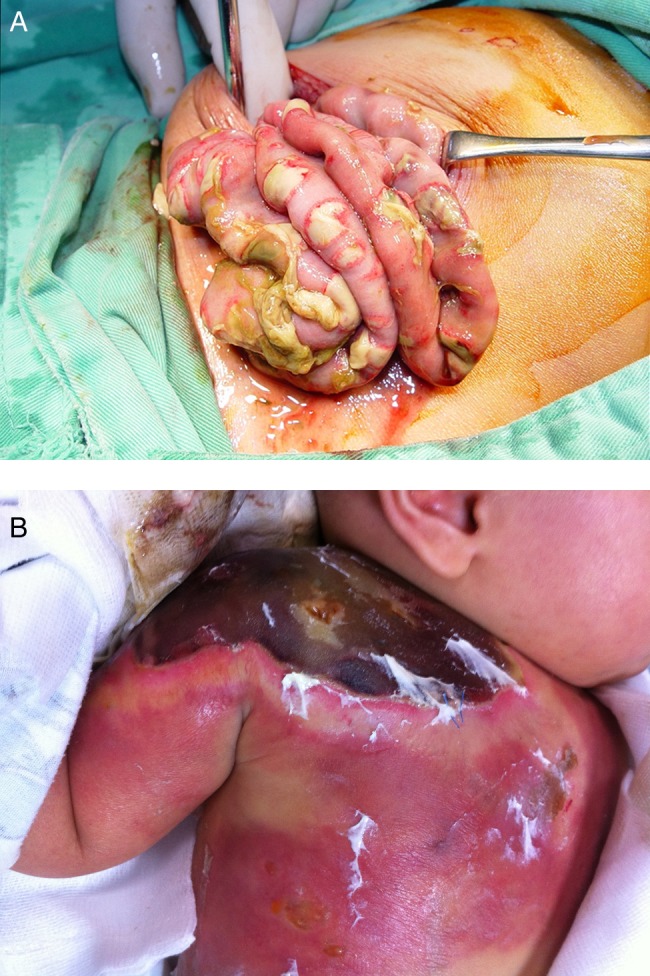
Pictures of necrotising enteritis and ecthyma gangrenosum. (A) Widespread patchy necrosis with fibrin coating on the small intestine. (B) A huge and extensive ecthyma gangrenosum.
Leucopenia, thrombocytopenia, high C-reactive protein (CRP) levels, coagulopathy and hypoalbuminaemia were the characteristic laboratory findings (table 2). Blood was the most common site of isolation for P aeruginosa. All the isolates were susceptible to the antimicrobial agents tested. One patient was co-infected with Salmonella and one with Campylobacter jejuni. Rotavirus antigen testing was performed in 16 patients; only one was positive.
Table 2.
Laboratory findings of patients with Shanghai fever
| Laboratory test | Median (range) | The number of patients with abnormal values,* no. /total no. (%) |
|---|---|---|
| Leucocyte count/mm3 | 2800 (900–22 400) | 16/27 (59) |
| Haemoglobin (g/dL) | 10.0 (7.3–13.1) | 12/27 (44) |
| Platelet count/mm3 | 77 000(1000–334 000) | 19/27 (70) |
| PT (s) | 16.9 (11.3–46.7) | 13/22 (59) |
| APTT (s) | 53.3 (33.7–120) | 14/22 (64) |
| D-dimer (ng/mL) | 1533 (0.25–10 000) | 12/17 (71) |
| C-reactive protein (mg/L) | 202 (37–484) | 19/27 (70) |
| AST (U/litre) | 41 (12–1164) | 9/21 (43) |
| ALT (U/L) | 36 (6–738) | 7/20 (35) |
| BUN (mg/dL) | 20 (4–54) | 11/24 (46) |
| Creatinine (mg/dL) | 0.6 (0.2–1.9) | 14/24 (58) |
| Albumin (g/dL) | 2.5 (1.1–3.5) | 17/20 (85) |
| Sodium (mEq/L) | 129 (118–141) | 13/26 (50) |
| Potassium (mEq/L) | 4.3 (2.7–6.3) | 3/26 (12) |
| Glucose (mg/L) | 141 (3–384) | 11/23 (48) |
| Sites of P aeruginosa isolation | ||
| Blood | 25/27 (93) | |
| Stool | 11/22 (50) | |
| Cerebrospinal fluid | 2/16 (13) | |
| Pus | 10/15 (67) | |
| Ascites | 8/11 (73) | |
*The abnormal values are as follows: leucocyte count <4000 mm3; haemoglobin <10 g/dL; platelet count <105/mm3; PT (prothrombin time) >1.5-fold; APTT (activated partial thromboplastin time) >1.5-fold; D-dimer >500 ng/mL; C-reactive protein >150 mg/L; AST (aspartate aminotransferase) >50 U/liter; ALT (alanine aminotransferase) >50 U/liter; BUN (blood urea nitrogen) >20 mg/dL; creatinine >0.5 mg/dL; albumin <3.0 g/dL; sodium <130 mEq/L; potassium <3.0 mEq/L; glucose >150 mg/dL.
Twenty-three patients (85%) received antipseudomonal antibiotics within 24 h of admission. Twenty-three patients (85%) survived. Three patients (11%) died within 24 h of admission and one (4%) died 3 days after hospitalisation. The four fatal cases (15%) had received antipseudomonal antibiotics on admission. All surviving patients had no severe infection during follow-up. No patient had short bowel syndrome after surgery.
Standard immunological studies
Hypogammaglobulinaemia was seen in five patients (table 3). One had persistently low serum IgG after 6 years of age (596 mg/dL). His IgG subclass was low for IgG1 before 6 years of age but was normal thereafter. His IgA and IgM levels returned to normal at 3 years of age. He did not receive intravenous immunoglobulin replacement therapy or antibiotic prophylaxis during follow-up and had no recurrent severe infection. Serum IgG levels returned to normal in two patients but the remaining two had no follow-up data because one died and the other was lost to follow-up. Three patients had an IgE level >1000 IU/mL but none had characteristic clinical features of hyper-IgE syndrome.
Table 3.
Immunological evaluation of patients with Shanghai fever
| Variables* | Median (range) | The number of patients with abnormal values (%)† |
|---|---|---|
| IgG (mg/dL) | 538.0 (147–1610) | 5 (20) |
| IgA (mg/dL) | 35.4 (6.6–158) | 4 (16) |
| IgM (mg/dL) | 68.9 (21.4–416) | 5 (20) |
| IgE (IU/mL) | 56.8 (6.4–1930) | 0 (0) |
| CD3 T lymphocytes | 64.0 (32.3–81.1) | 3 (14) |
| CD19 B lymphocytes | 21.4 (5.2–62.0) | 5 (24) |
| Natural killer cell | 7.6 (1.6–24.9) | 4 (19 |
| CD4 T lymphocytes | 43.1 (20.5–54.4) | 5 (24) |
| CD8 T lymphocytes | 19.4 (0.7–35.7) | 3 (14) |
| CD4:CD8 ratio | 2.3 (0.6–16.7) | 5 (24) |
| Naïve CD4 T lymphocytes | 79.8 (36.4–91.6) | 3 (14) |
| Memory CD4 T lymphocytes | 18.4 (13.3–46.3) | 0 (0) |
| Naïve CD8 T lymphocytes | 90.9 (68.4–97.2) | 0 (0) |
| Memory CD8 T lymphocytes | 7.7 (1.6–20.3) | 0 (0) |
| Activated T lymphocytes | 13.9 (1.0–33.4) | 3 (14) |
| Memory B lymphocytes‡ | 1.3 (0.3–4.9) |
*Lymphocyte subpopulations recognised included CD19 B lymphocytes, CD3 T lymphocytes, CD4 T lymphocytes, CD8 T lymphocytes, CD4:CD8 ratio, natural killer cell (CD3/CD16–56), naïve CD4 T lymphocytes (CD4/CD45RA), memory CD4 T lymphocytes (CD4/CD45RO), naïve CD8 T lymphocytes (CD8/CD45RA), memory CD8 T lymphocytes (CD8/CD45RO), activated T lymphocytes (HLADR/CD2) and memory B lymphocytes (CD27/CD19). Cells expressing the indicated markers for lymphocytes are shown as percentages.
‡No reference data at this age.
Lymphocyte subpopulation studies (table 3) showed that three patients had a reduced CD3 T lymphocyte count. Their CD4 T lymphocytes were also decreased. One of them also had low activated T lymphocyte counts. Nonetheless, their naïve and memory CD4 and CD8 T lymphocytes were within normal limits. Except for one patient with low CD4, CD8 and activated T lymphocytes counts, patients with low CD4 or CD8 T lymphocytes all had normal memory CD4, CD8 and activated T lymphocytes counts. The percentage of CD19 B lymphocytes was reduced in five patients, only one of whom had hypogammaglobulinaemia. Taken together, no definitive common primary immune deficiency was identified in these patients based upon both laboratory and clinical evaluations. Extremely young age (<1 year) was the only identified host factor predisposing to Shanghai fever.
MLST
MLST analysis of the P aeruginosa isolates from the patients with Shanghai fever showed 11 different STs, but none of the STs could be grouped into clonal clusters (see online supplementary figure 1). Five isolates were identified as ST244 and one as ST313. Nine new STs (ST1019–1027) were identified and reported to the P aeruginosa MLST database. The MLST patterns of Shanghai fever isolates were diverse, indicating that a single clone of P aeruginosa was not responsible for these cases.
Cytotoxicity, penetration and adhesion
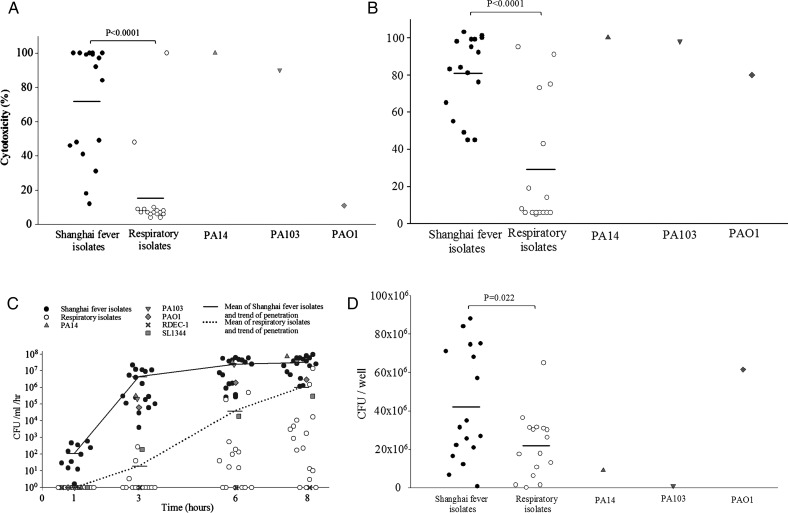
The P aeruginosa isolates were next evaluated for phenotypes suggestive of enhanced virulence. In the cytotoxicity assay to MDCK cells (figure 2A), 14/17 (82%) isolates from patients with Shanghai fever showed significantly higher cytotoxicity than PAO1 (more than five times higher release of LDH), with 10/17 (59%) having comparable LDH levels to PA103 and PA14. P aeruginosa isolates from patients with Shanghai fever were also significantly more cytotoxic than P aeruginosa isolates from nosocomial respiratory tract infection (p<0.0001). As shown in figure 2B, Shanghai fever isolates also showed higher cytotoxicity to neutrophils.
Figure 2.
In vitro virulence assays of Pseudomonas aeruginosa strains (17 isolates from patients with Shanghai fever; 16 from patients with nosocomial pneumonia; and PAO1, PA103 and PA14). (A) Cytotoxicity assays to Madin–Darby canine kidney (MDCK) cells. Isolates from patients with Shanghai fever were significantly more cytotoxic than isolates from patients with nosocomial pneumonia (p<0.0001). Horizontal lines represent means. (B) Cytotoxicity assays to neutrophils. Isolates from Shanghai fever were significantly more cytotoxic than respiratory isolates (p<0.0001). Horizontal lines represent means. (C) Penetration assays. Shanghai fever isolates penetrated the MDCK cell monolayer more rapidly than respiratory isolates (p<0.0001). The numbers of bacteria that penetrated the MDCK cell monolayers were significantly higher with Shanghai fever isolates than respiratory isolates (p=0.009) and laboratory strains (p=0.012) 3 h after inoculation. Shanghai fever isolates had significantly higher penetrative capability than the respiratory and laboratory strains. Horizontal lines represent the means at different time points. (D) Adhesion assays. Shanghai fever isolates were more adherent to Caco-2 cells than were respiratory isolates (p=0.022) and cytotoxic laboratory strains (PA103 and PA14) (p=0.001). Horizontal lines represent means. CFU, colony forming units.
In penetration assay (figure 2C), 11/17 isolates from patients with Shanghai fever (65%) were detected in the basolateral media 1 h after inoculation. All Shanghai fever isolates and the S enterica serovar Typhimurium SL1344 and P aeruginosa PAO1, PA103, PA14 strains were detected in the basolateral media 3 h after inoculation, whereas only three respiratory isolates were detected at this time. Shanghai fever strains penetrated the MDCK monolayer more rapidly than respiratory strains (p<0.0001). The CFUs of penetrating bacteria were significantly higher with Shanghai fever strains than with respiratory strains (p=0.009) and laboratory strains (p=0.012) 3 h after inoculation. Shanghai fever isolates penetrated MDCK cells to a greater extent than respiratory and laboratory strains. When P aeruginosa isolates and E coli strain RDEC-1 were co-incubated with MDCK cells, P aeruginosa isolates were detected alone in the basolateral media 1 and 3 h after inoculation. RDEC-1 bacteria were not found in the basolateral media until 6 h later, suggesting that P aeruginosa isolates transcytosed rather than disrupted MDCK cells in an early stage of the infection. The barrier function of MDCK cells was disrupted later owing to cytotoxicity.
In adhesion assays (figure 2D), Shanghai fever isolates were more adherent to Caco-2 cells than respiratory isolates (p=0.022) and cytotoxic laboratory strains (PA103 and PA14) (p=0.001). These in vitro assays showed that P aeruginosa isolates from patients with Shanghai fever are highly cytotoxic, invasive and adherent.
Distribution of TTSS-encoding effector genes
Of the 17 Shanghai fever isolates, 10 (59%) harboured the exoU gene, 7 (41%) exoS, 17 (100%) exoT and 15 (88%) exoY. The exoU and exoS genes were mutually exclusive. Shanghai fever isolates with exoU were significantly more cytotoxic than isolates with exoS (p=0.001). However, there were no differences in clinical manifestations, complications and mortality between patients infected by P aeruginosa isolates with exoU and those with exoS (see online supplementary table 1).
Animal studies
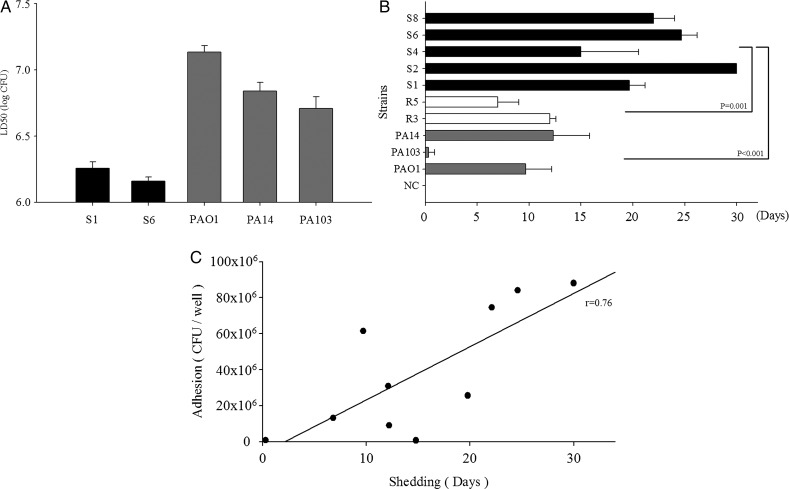
The LD50 to the mice of the Shanghai fever isolates determined by an intravenous infection model was extremely low, compared with that of the laboratory strains, suggesting high virulence of the isolates (figure 3A). Mice orally challenged with Shanghai fever strains of P aeruginosa shed bacteria significantly for longer times than those challenged with laboratory strains (PAO1, PA103, PA14) (p<0.001) and the respiratory stains (R3 and R5) (p=0.001) (figure 3B). P aeruginosa isolated from stools of mice showed identical TTSS-encoding effector gene patterns to those used in the challenge (data not shown). Fecal shedding in mice was significantly associated with bacterial adhesion to Caco-2 cells (r=0.76; p=0.011) (figure 3C).
Figure 3.
Results of animal experiments. (A) The 50% lethal doses (LD50) of Pseudomonas aeruginosa strains to mice by intravenous challenge. Two Shanghai fever isolates (S1 and S6) and the three laboratory strains (PAO1, PA103 and PA14) were tested. Virulence of Shanghai fever isolates was greater than that of laboratory strains. (B) Duration of fecal shedding of P aeruginosa after oral challenge. Stool collected from each mouse before oral challenge was confirmed negative for P aeruginosa. Negative controls (NC) were mice challenged with normal saline. Mice challenged with Shanghai fever isolates of P aeruginosa (S1, S2, S4, S6 and S8) showed significantly longer shedding time than those challenged with laboratory strains (PAO1, PA103 and PA14) (p<0.001) or respiratory isolates (R3 and R5) (p=0.001). The results are expressed as mean±SD. The study was terminated at day 30, but S2 shed for more than 30 days. (C) The correlation between adhesion in cellular model and fecal shedding in mice was 0.76 (p=0.011).
Discussion
Although P aeruginosa can be a gastrointestinal pathogen, most of these infections are mild. Two exceptions are typhlitis or rectal abscesses in neutropenic patients due to chemotherapy and necrotising enterocolitis in premature infants. Shanghai fever is yet another exception that was previously ignored. Nearly 100 cases of community-acquired P aeruginosa sepsis have been reported7–15; most of the reported cases are from East Asia.9–11 13–15 In East Asia, community-acquired P aeruginosa sepsis in a previously healthy child is referred to as Shanghai fever. This illness is characterised by sporadic and community-onset necrotising enteritis with sepsis. Interestingly, the clinical manifestations of these infections appear to be different among patients from Eastern and Western countries. As in the present series, fever and diarrhoea are the most common presentations in East Asia, whereas fever and skin lesions are more common in cases reported from North America and Europe.7–15
Shanghai fever has three main complications. First, widespread patchy necrotising bowel lesions with or without bowel perforation may occur. Although most patients have extensive necrotising enteritis, short bowel syndrome is rare, with only one reported case.9 Second, ecthyma gangrenosum occurs in more than 50% of the patients with Shanghai fever, as seen in this study.10 11 In contrast, this cutaneous manifestation occurs in only 1.3–2.8% of patients with Pseudomonas bacteraemia,24 many of whom are profoundly neutropenic. However, none of the patients with Shanghai fever in this study were neutropenic. Ecthyma gangrenosum was the most common extraintestinal complication in our study and also often affected multiple sites. Third, seizures are frequently associated with Shanghai fever.15 Cerebrospinal fluid studies showed neither pleocytosis nor increased protein concentration in patients without meningitis. Hyponatraemia may be the main cause of these seizures. In this study, Shanghai fever complicated with meningitis resulted in severe neurological sequelae. Meningitis is the most devastating complication of Shanghai fever.
In Shanghai fever, concurrent infection with other enteropathogenic micro-organisms has been postulated to increase susceptibility to P aeruginosa infection through local effects on the gastrointestinal mucosa.7 9 Rotavirus is the most common cause of gastroenteritis in children aged <5 years. Yet only one of the patients with Shanghai fever had concomitant rotavirus infection. In addition, the majority of the Shanghai fever cases occurred in the hot and humid season, which is not a peak season for rotavirus infection.
The reported death rate of Shanghai fever ranges from 23% to 89%.9 13–15 In the report with the highest death rate, infection with P aeruginosa had not been considered at the time of sepsis onset.13 Previous studies suggested that antipseudomonal antibiotics given within 24 h of presentation are significantly associated with lower fatality.9 15 Increased awareness and early recognition of this disease with prompt administration of antipseudomonal antibiotics and surgical intervention may be the reason for the lower mortality in this series than in previously published series.9 13–15
Community-acquired P aeruginosa sepsis can be the initial manifestation of underlying immune deficiency. The most common underlying problem in such patients is reportedly hypogammaglobulinaemia.7 Although immunoglobulin studies were performed on less than half of the patients in previous reports from Taiwan and Hong Kong, in those patients only one had hypogammaglobulinaemia.14 However, transient or persistent hypogammaglobulinaemia was not mentioned in that patient. One of our patients had a prolonged hypogammaglobulinaemia. His lymphocyte subset study showed persistently decreased CD4 T lymphocytes, naïve CD4 T lymphocytes and inverse CD4/CD8 ratio after 6 years of age. This particular patient was HIV negative. The patient had no subsequent recurrent infection and therefore was probably a case of transient hypogammaglobulinaemia of infancy.
Analysis of lymphocyte subsets is diagnostic in many cases of immune deficiency. Declining CD4 T lymphocytes in HIV-infected patients increased the risk of P aeruginosa disease.25 CD4 T lymphocytes may defend against P aeruginosa infection. Data in this series indicated that none of the patients had common humoral or cellular immune deficiency. Nevertheless, specific immune deficiency could not be ruled out.
Neutropenia is the major risk factor for P aeruginosa infection in patients receiving chemotherapy. Although 59% of the patients in our study had neutropenia at the onset of sepsis, their neutrophil counts recovered as their clinical condition improved. P aeruginosa isolates with higher cytotoxicity to neutrophils were associated with persistent lung infection.21 ExoU-mediated impairment of phagocytes plays a role in the pathogenesis of pneumonia caused by P aeruginosa.26 Our in vitro cytotoxicity assays showed that Shanghai fever isolates possessed higher cytotoxicity to neutrophils than respiratory isolates. Neutropenia in patients with Shanghai fever was probably induced by P aeruginosa infection rather than a predisposing factor for the infection. The high cytotoxicity of Shanghai fever isolates to neutrophils may facilitate the development of Shanghai fever.
Furthermore, this study is in accord with a previous observation that infants accounted for >80% of reported cases of community-acquired P aeruginosa sepsis.9–11 13–15 Young age appears to be the only host risk factor identified for Shanghai fever.
The virulence of P aeruginosa is multifactorial and combinatorial.27 TTSS is one of the most important determinants of virulence in P aeruginosa. Four TTSS effectors have been identified as virulence factors: ExoU, ExoS, ExoT and ExoY.28 29 The two distinct bacterial phenotypes, cytotoxic and invasive, correlate with the presence of the exoU and exoS genes, respectively.30 This study of isolates from patients with Shanghai fever is consistent with earlier findings that strains bearing exoU are highly cytotoxic. In a global P aeruginosa population structure study, 73% of isolates harboured exoS and only 23% exoU.31 Among isolates from patients with cystic fibrosis, 98% carried exoS.31 Our study found that isolates harbouring exoU were in the majority (59%). ExoU-mediated cytotoxicity may be an important P aeruginosa virulence trait in Shanghai fever. A neutropenic murine model of P aeruginosa gastrointestinal colonisation and dissemination also found that strains expressing ExoU were more virulent than strains lacking it.32
Gastrointestinal colonisation with subsequent invasion into the bloodstream is the presumed mechanism of P aeruginosa bacteraemia in neutropenic patients. In agreement with previous studies showing that P aeruginosa isolates from blood were more adherent than sputum isolates to Caco-2 cells,33 we found that Shanghai fever isolates were more adherent than respiratory isolates. The highly adherent phenotypes of these isolates may explain their ability to colonise and persist in the gastrointestinal tract of mice after oral inoculation. Likewise, the Shanghai fever isolates were highly cytotoxic and were able to rapidly penetrate through epithelial cell monolayers in cell culture experiments, traits that may facilitate invasion into the bloodstream during human infections. Despite the shared phenotypes of the P aeruginosa isolates from patients with Shanghai fever, these isolates appeared genotypically diverse. This observation is consistent with a previous consensus that there are no specific clones of P aeruginosa with a specific disease selection.31 Additional studies are necessary to determine whether the enhanced virulence phenotypes of P aeruginosa causing Shanghai fever are from different combinations of diverse virulence genes or whether they share specific pathogenicity islands.
Shanghai fever is a distinct and fulminant P aeruginosa enteric disease in infants. Both host and microbial factors contribute to its pathogenesis.
Supplementary Material
Acknowledgments
The authors thank Professor David P Speert for providing the PAO1 and PA103, Professor Robert E W Hancock and Professor David P Speert for their helpful ideas and advice and Dr Jeng-Chang Chen for providing the photograph.
Footnotes
Contributors: C-H Chuang: contributed to the experimental plan, performed research, analysed the data and wrote the manuscript; Y-HW, H-JC and H-LC: contributed to the experimental plan, performed research and analysed the data; Y-CH and T-YL: analysed the data, commented on the manuscript; EAO, JPA and ARH: performed research, analysed the data, commented on the manuscript; C-H Chiu: conceived the overall research plan, analysed the data and wrote the manuscript.
Funding: This work was in part supported by St Paul's Hospital grant SMRPG380031 and Chang Gung Memorial Hospital grants CMRPG340235 and CMRPG3B0071 to C-H Chiu. The study was also supported, in part, by National Institutes of Health grants R01AI075191 (ARH), R01AI053674 (ARH), F32AI089068 (EAO) and T32AI0007476 (JPA).
Competing interests: None.
Ethics approval: Institutional review board of Chang Gung Memorial Hospital.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Bodey GP, Bolivar R, Fainstein V, et al. Infections caused by Pseudomonas aeruginosa. Rev Infect Dis 1983;5:279–313 [DOI] [PubMed] [Google Scholar]
- 2.Grisaru-Soen G, Lerner-Geva L, Keller N, et al. Pseudomonas aeruginosa bacteremia in children: analysis of trends in prevalence, antibiotic resistance and prognostic factors. Pediatr Infect Dis J 2000;19:959–63 [DOI] [PubMed] [Google Scholar]
- 3.Adlard PA, Kirov SM, Sanderson K, et al. Pseudomonas aeruginosa as a cause of infectious diarrhoea. Epidemiol Infect 1998;121:237–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kim SW, Peck KR, Jung SI, et al. Pseudomonas aeruginosa as a potential cause of antibiotic-associated diarrhea. J Korean Med Sci 2001;16:742–4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dold H. On pyocyaneus sepsis and intestinal infections in Shanghai due to Bacillus pyocyaneus. Chin Med J 1918;32:435 [Google Scholar]
- 6.Lepow ML. Pseudomonas aeruginosa colonization and infection of the gastrointestinal tract. In: Baltch AL, Smith RP, eds. Pseudomonas aeruginosa infections and treatment. New York: Marcel Dekker Press, 1994:421–40 [Google Scholar]
- 7.Chusid MJ, Hillmann SM. Community-acquired Pseudomonas aeruginosa sepsis in previously healthy infants. Pediatr Infect Dis J 1987;6:681–4 [DOI] [PubMed] [Google Scholar]
- 8.Martin-Ancel A, Borque C, del Castillo F. Pseudomonas sepsis in children without previous medical problems. Pediatr Infect Dis J 1993;12:258–60 [DOI] [PubMed] [Google Scholar]
- 9.Huang YC, Lin TY, Wang CH. Community-acquired Pseudomonas aeruginosa sepsis in previously healthy infants and children: analysis of forty-three episodes. Pediatr Infect Dis J 2002;21:1049–52 [DOI] [PubMed] [Google Scholar]
- 10.Yeung CK, Lee KH. Community acquired fulminant Pseudomonas infection of the gastrointestinal tract in previously healthy infants. J Paediar Child Health 1998;34:584–7 [DOI] [PubMed] [Google Scholar]
- 11.Tsai MJ, Teng CJ, Teng RJ, et al. Necrotizing bowel lesions complicated by Pseudomonas septicaemia in previously healthy infants. Eur J Pediatr 1996;155:216–18 [DOI] [PubMed] [Google Scholar]
- 12.Viola L, Langer A, Pulitano S, et al. Serious Pseudomonas aeruginosa infection in healthy children: case report and review of the literature. Pediatr Int 2006;48:330–3 [DOI] [PubMed] [Google Scholar]
- 13.He SJ, Jin YM, Huang AR, et al. Clinical analysis of community-acquired Pseudomonas aeruginosa septic shock. Zhonghua Er Ke Za Zhi 2008;46:333–9 [PubMed] [Google Scholar]
- 14.Kuo KC, Kuo HC, Huang LT, et al. The clinical implications of ABO blood groups in Pseudomonas aeruginosa sepsis in children. J Microbiol Immunol Infect 2013;46:109–14 [DOI] [PubMed] [Google Scholar]
- 15.Zhang Q, Smith JC, Zhu Q, et al. A five-year review of Pseudomonas aeruginosa bacteremia in children hospitalized at a single center in southern China. Int J Infect Dis 2012;16:e628–32 [DOI] [PubMed] [Google Scholar]
- 16.Shearer WT, Rosenblatt HM, Gelman RS, et al. Lymphocyte subsets in healthy children from birth through 18 years of age: the Pediatric AIDS Clinical Trials Group P1009 study. J Allergy Clin Immunol 2003;112:973–80 [DOI] [PubMed] [Google Scholar]
- 17.Imai K, Slupphaug G, Lee WI, et al. Human uracil-DNA glycosylase deficiency associated with profoundly impaired immunoglobulin class-switch recombination. Nat Immunol 2003;4:1023–8 [DOI] [PubMed] [Google Scholar]
- 18.Hirakata Y, Finlay BB, Simpson DA, et al. Penetration of clinical isolates of Pseudomonas aeruginosa through MDCK epithelial cell monolayers. J Infect Dis 2000;181:765–9 [DOI] [PubMed] [Google Scholar]
- 19.Clinical and Laboratory Standards Institute. Performance standards for antimicrobial susceptibility testing. Twenty-Second informational supplement. Document M100-S22 Wayne, PA: CLSI, 2012 [Google Scholar]
- 20.Curran B, Jonas D, Grundmann H, et al. Development of a multilocus sequence typing scheme for the opportunistic pathogen Pseudomonas aeruginosa. J Clin Microbiol 2004;42:5644–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.El Solh AA, Akinnusi ME, Wiener-Kronish JP, et al. Persistent infection with Pseudomonas aeruginosa in ventilator-associated pneumonia. Am J Respir Crit Care Med 2008;178:513–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stepinska M, Trafny EA. Diverse type III secretion phenotypes among Pseudomonas aeruginosa strains upon infection of murine macrophage-like and endothelial cell lines. Microb Pathog 2008;44:448–58 [DOI] [PubMed] [Google Scholar]
- 23.Reed L, Muench J. A simple method for estimating fifty percent endpoints. Am J Hyg 1938;27:493–7 [Google Scholar]
- 24.Zomorrodi A, Wald ER. Ecthyma gangrenosum: considerations in a previously healthy child. Pediatr Infect Dis J 2002;21:1161–4 [DOI] [PubMed] [Google Scholar]
- 25.Sorvillo F, Beall G, Turner PA, et al. Incidence and determinants of Pseudomonas aeruginosa infection among persons with HIV: association with hospital exposure. Am J Infect Control 2001;29:79–84 [DOI] [PubMed] [Google Scholar]
- 26.Diaz MH, Hauser AR. Pseudomonas aeruginosa cytotoxin ExoU is injected into phagocytic cells during acute pneumonia. Infect Immun 2010;78:1447–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee DG, Urbach JM, Wu G, et al. Genomic analysis reveals that Pseudomonas aeruginosa virulence is combinatorial. Genome Biol 2006;7:R90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shaver CM, Hauser AR. Relative contributions of Pseudomonas aeruginosa ExoU, ExoS and ExoT to virulence in the lung. Infect Immun 2004;72:6969–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vance RE, Rietsch A, Mekalanos JJ. Role of the type III secreted exoenzymes S, T and Y in systemic spread of Pseudomonas aeruginosa PAO1 in vivo. Infect Immun 2005;73:1706–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fleiszig SM, Zaidi TS, Preston MJ, et al. Relationship between cytotoxicity and corneal epithelial cell invasion by clinical isolates of Pseudomonas aeruginosa. Infect Immun 1996;64:2288–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pirnay JP, Bilocq F, Pot B, et al. Pseudomonas aeruginosa population structure revisited. PLoS One 2009;4:e7740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Koh AY, Priebe GP, Pier GB. Virulence of Pseudomonas aeruginosa in a murine model of gastrointestinal colonization and dissemination in neutropenia. Infect Immun 2005;73:2262–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hirakata Y, Izumikawa K, Yamaguchi T, et al. Adherence to and penetration of human intestinal Caco-2 epithelial cell monolayers by Pseudomonas aeruginosa. Infect Immun 1998;66:1748–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.