Abstract
Objective
A systematic review was conducted to evaluate the impact of human factors (HF) on the risks associated with electronic cigarettes (e-cigarettes) and to identify research gaps. HF is the evaluation of human interactions with products and includes the analysis of user, environment and product complexity. Consideration of HF may mitigate known and potential hazards from the use and misuse of a consumer product, including e-cigarettes.
Methods
Five databases were searched through January 2014 and publications relevant to HF were incorporated. Voluntary adverse event (AE) reports submitted to the US Food and Drug Administration (FDA) and the package labelling of 12 e-cigarette products were analysed.
Results
No studies specifically addressing the impact of HF on e-cigarette use risks were identified. Most e-cigarette users are smokers, but data on the user population are inconsistent. No articles focused specifically on e-cigarette use environments, storage conditions, product operational requirements, product complexities, user errors or misuse.
Twelve published studies analysed e-cigarette labelling and concluded that labelling was inadequate or misleading. FDA labelling analysis revealed similar concerns described in the literature. AE reports related to design concerns are increasing and fatalities related to accidental exposure and misuse have occurred; however, no publications evaluating the relationship between AEs and HF were identified.
Conclusions
The HF impacting e-cigarette use and related hazards are inadequately characterised. Thorough analyses of user–product–environment interfaces, product complexities and AEs associated with typical and atypical use are needed to better incorporate HF engineering principles to inform and potentially reduce or mitigate the emerging hazards associated with e-cigarette products.
Introduction
‘Human factors’ (HF) analysis refers to the evaluation of how human users interact with products and takes the user characteristics, environment of use and product complexity into consideration.1 HF are analysed in order to inform the implementation of design principles that focus on user–environments–product interactions and thus minimise the hazards and risks associated with a product use. Human factors engineering (HFE) principles are intended to make products easier or harder (as appropriate) to use and to reduce or mitigate any potential hazards of product use, particularly hazards that are directly related to poor product design.2 Data from user profiles, product use studies and product malfunction or failure reports are analysed in order to improve the design of current and future products. HFE considers past and current product design, as well as the likely characteristics and behaviour of both intentional and unintentional product users, such as children who may have unattended access to the product.2 HFE has figured prominently in determining causative factors and remediation for consumer product failures, such as sudden unintended acceleration incidents in automobiles.3
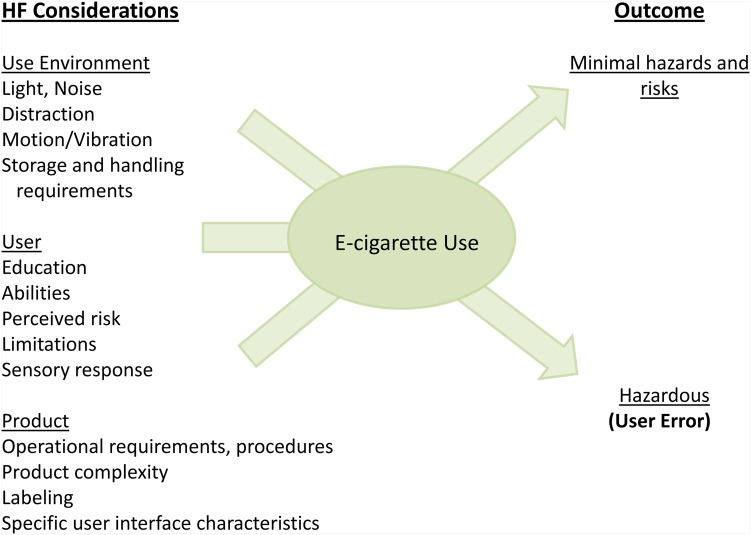
Electronic cigarettes (e-cigarettes) are battery-powered devices that aerosolise a chemical mixture (‘e-liquid’) that typically contains nicotine, propylene glycol, flavourings and other constituents. The intent of this paper is not to compare the risks of e-cigarettes versus traditional cigarettes but rather to review use-related risks of e-cigarettes and identify strategies to reduce hazards associated with these products. Published and voluntary reports to the US Food and Drug Administration (FDA) suggest that e-cigarette use may have risks unrecognised by the general public. Risk of exposure to e-liquids from leaks or spills, e-cigarette ignition and explosion, and exposure to contaminants in the e-liquid and aerosols are HF concerns. These risks can be exacerbated through poor product design, user behaviour or both. The interaction among HF considerations and their possible results is illustrated in figure 1.
Figure 1.
Human factors (HF) considerations and potential outcomes of e-cigarette use (adapted from an Food and Drug Administration guidance document related to HF engineering).2
Electronic cigarette use-related hazards should be identified, understood and addressed through the application of HFE to e-cigarette design processes in order to minimise consumer risks. To expand the understanding of these issues and identify gaps in knowledge, scientific literature, voluntary adverse events (AEs) reported to FDA, media reports and a sample of 12 e-cigarette product labelling were analysed.
Methods
Literature search and resource review
A systematic literature search of five reference databases (Web of Knowledge, PubMed, SciFinder, Embase and EBSCOhost) was conducted through January 2014 using search terms ‘electronic nicotine devices’ OR ‘electronic nicotine device’ OR ‘electronic nicotine delivery systems’ OR ‘electronic nicotine delivery system’ OR ‘electronic cigarettes’ OR ‘electronic cigarette’ OR ‘e-cigarettes’ OR ‘e-cigarette’ OR ‘e-cig’ OR ‘e-cigs’ to identify research and publications related to e-cigarettes. Titles and abstracts of the 455 articles identified were manually screened for HF and user hazards relevance. Included articles were (1) written in English; (2) accessible and non-confidential using FDA resources or publicly available; and (3) deal partly or exclusively with the relevant aspect of HF pertaining to e-cigarettes. The validity and strength of each study were determined based on a qualitative assessment of study objectives, design, population sampling strategies, depth of analysis, risk of bias and interpretation of results. Application of these criteria yielded a total of 31 articles for analysis; all articles were published between 2009 and 2014. We also reviewed the Center for Tobacco Products (CTP) daily news (news clips from a clipping service) for relevant information.
CTP AEs database analysis
AEs associated with e-cigarette use voluntarily reported to FDA/CTP between 2008 and 31 December 2013 were reviewed for their nature, seriousness, possible HF contribution and implications for product labelling. AE definitions applied to e-cigarettes were adapted from concepts in the regulations4 and guidance5 for investigational drugs and non-prescription products.
Labelling analysis of e-cigarette products by FDA/CTP
The labelling of 12 e-cigarette products from nine manufacturers (table 1)7–12 was analysed using a tool developed by the FDA. The tool is an unweighted list of 65 elements in broad categories, including claims; package design; product and manufacturer identification on the external package and main component parts; content, conspicuousness, readability and legibility of health cautions; and instructions for use. We scored each labelling element as ‘present’ or ‘absent’, included qualitative notations, and reported findings using descriptive statistics. The sample included products evaluated in published labelling analyses (n=7), products identified as popular by one or more independent analysts in late 2012 (n=8) and products for which AE reports had been received by the FDA (n=7).i The sample included rechargeable starter kits, cartridges and cartomizers, e-liquids for refilling cartridges and disposable e-cigarettes. FDA-approved labelling of a prescription13 and a non-prescription nicotine replacement product14 were used as references. All levels of package labelling were reviewed and the products were measured to evaluate whether there were any potential choking hazards and to examine whether the labelling properly addressed the risk.
Table 1.
Sample of e-cigarette products analysed by CTP for labelling (n=12)
| Brand name/manufacturer/(flavour(s)) | Type of product/identifiers/nicotine content | Purchase date | Data supporting inclusion (see code descriptions below) |
|---|---|---|---|
| NJOY® NPRO Sottera (five assorted regular flavour cartridges) |
Starter kit Model #03-NP-WHT-SK 18 mg nicotine |
11/21/2011 | A, B, C, D, E, F |
| Smoking Everywhere™ | Starter kit Nicotine content unknown |
2009 | A, D, E |
| Liberty Stix (Medium Mint) |
Cartridges 2-pack Nicotine content unknown |
10/31/2012 | E |
| NJOY® electronic cigarette refill cartridges Sottera (Traditional flavour) |
Cartridges SM0711 0.6% nicotine by volume |
11/21/2011 | A, B, C, D, E, F |
| SouthBeach Smoke Deluxe Cartridges (assorted full flavoured) | Cartridges 10-pack 16 mg nicotine |
10/31/2012 | B |
| Volcano Atomized Cartridges (CooCoo Coconut) | Cartridges 5 atomized cartridges 8 mg nicotine |
10/31/2012 | B |
| Greensmoke Variety Pack (5 FlavorMax™ Cartomizers) | Cartomizers 1.8% nicotine by volume |
10/31/2012 | B |
| Johnson Creek Original Smoke Juice (Red OakTM SolsticeTM) Johnson Creek |
e-Liquid 0.50 Fl. oz. ‘Low nicotine’:11 mg/mL |
10/31/2012 | D |
| NicQuid® (Midnight Express) |
e-Liquid 0 mg nicotine (0.0%) |
3/21/2013 | G |
| OneJoy Sottera |
Disposable e-cigarette ‘Equal to about 2 packs’ |
11/21/2011 | A, B, C, E, F |
| V2 Regular Full | Disposable e-cigarette ‘Equal to 40 cigarettes’ |
10/31/2012 | A, B, E |
| Blu® Lorillard |
Disposable e-cigarette ‘Equals over one Pack’ |
10/31/2012 | B, C |
A. ‘Top 5’ product among those named in adverse event reports to the FDA as of 12/31/2012.
B. ‘Top 20 selling brands’.7
C. ‘Top 5 in e-cigarette sales for unique brands sold in convenience and FDM Stores combined March 2011–March 2012’.8
D. Extends work of FDA/Division of Pharmaceutical Analysis (DPA), St Louis, Missouri , USA.9
F. ‘Most popular brand’ in the USA.12
G. This product was included in order to ensure that the sample included at least two of each of the types of products being marketed.
Results
Users and use environment
Per a recent comprehensive review of US e-cigarette users by Pepper and Brewer,15 adult e-cigarette use in the USA increased from 1% in 200916 to 6% in 201117 and current smokers are more likely to use e-cigarettes than former and never-smokers.16 18 Electronic cigarette use among US adolescent and young adults has also increased in recent years.15 19–23 Additional data characterising the user population are limited.ii
Although one study of e-cigarette use in schizophrenic patients was identified,24 no other studies evaluating specific user populations (eg, pregnant women, nursing mothers, older people, episodic users such as perioperative or hospitalised patients, users with chronic medical conditions/disabilities) were identified. Given that e-cigarettes are more complex than traditional cigarettes and some may require product assembly, component replacement or both, e-cigarette users may require additional skills to ensure appropriate use that are not required by traditional cigarettes users.25 Studies on the use environment were not identified, but e-cigarettes may be used in the work environment and public places.
Electronic cigarette product design
No articles specifically analysing e-cigarette operational requirements or complexity were identified. One US study identified leaking cartridges and noted that it was difficult to avoid contact with e-liquid when assembling or disassembling cartridges.10 An Italian study identified design concerns: specifically, the product could not be used as recommended, the product failed to produce mist when puffed, the atomizer had to be substituted and chargers were immediately replaced.26 Users indicated that acceptance of e-cigarettes could be improved by increasing manufacturing standards, by providing a battery capacity lasting at least 24 h, by reducing the weight of the product and by substituting the hard plastic mouthpiece.26 A study from the Czech Republic found that 4% of subjects (n=262) reported unspecified technical difficulties resulting in product dissatisfaction.27 Design issues identified in a 2010 survey conducted in England and France included leaking e-liquid, ‘broken down’ product, concern that the ‘vapour’ was not concentrated and difficult to inhale, and that the cartridges and batteries did not last long enough.28 No articles regarding the application of HFE to e-cigarette product design were identified.
Literature review findings on e-cigarette products labelling and packaging
Twelve articles were identified that evaluated e-cigarette labeling9–12 29–36 (see online supplementary table S1): 11 studied e-liquid content using bench methods9 11 12 29–36; three evaluated broader aspects of product labelling10 11 31; and two commented on packaging design.31 36 Six studies were US based,9 10 29 30 33 35 and five others included one or more e-cigarette brands sold in the USA.11 12 31 34 36 Six of the eleven studies analysing the accuracy of labelled nicotine in e-liquids found higher than the labelled amount in one or more samples,9 11 12 32–34 and three studies found more than a twofold higher amount of nicotine.9 11 32 Bench analyses have also identified constituents of concern, including nicotine-related impurities,9 31 34 poisons or laboratory reagents, and drugs.9 31
Other published labelling and packaging analyses identified either global or specific concerns. Cheah et al11 analysed product design features, contents and eight labelling elements of 19 e-cigarettes brands (including three brands available in the USA) and concluded that product labelling and packaging had pervasive inadequate and misleading information. Trtchounian and Talbot10 analysed 25 elements of the starter kit instruction manuals for six products from five US manufacturers and concluded that important information about product contents, use and warnings was absent. A UK analysis noted that the e-cigarette packages analysed did not have choking warnings.31 One study found that none of the six e-cigarette packaging designs evaluated were in child-resistant (C-R) containers despite containing e-liquids with high nicotine concentration (14.8–87.2 mg/mL).33
Labelling analysis of e-cigarette products by FDA/CTP
Concerns regarding product and manufacturer labelling were identified in the majority of the samples analysed (eg, 8 of 12 products did not have lot or batch numbers). Most cartridges appeared to be interchangeable. Ingredient labelling was problematic for all products: five products did not provide ingredients, and of the seven product packages that listed ingredients, two did not provide consistent information across all levels of packaging. One e-liquid package listed peanut, a potentially fatal allergen, as an ingredient, but a warning was not provided.38 The 10 nicotine-containing products had ambiguities in content labelling such as qualitative descriptors or quantitative notations that were inexact. Eight products, including two that did not contain nicotine, warned of potential nicotine toxicity. Four warned of potential nicotine poisoning, but none warned of potential fatality.
Seven external package labels listed a health caution; however, warning and precautionary content occupied less than 30% of a back panel or 20–100% of one side panel, was printed in small font and may not be conspicuous to the readers.39–41 No products warned of the potential for overheating, explosions or burn injuries. Two products claimed to be non-flammable.
Five of the twelve products did not include instructions for use. Instructions for seven products did not provide step-by-step use instructions, were written above eighth grade level or both.39 42 None of the products had labelling stating that the product is intended as a smoking cessation aid; however, one product insert contained cessation lifestyle advice and five products' labelling suggested that the product is less risky than traditional cigarettes.
All products contained at least one component that qualified as a choking hazard.iii 43 Only one starter kit directly warned of this hazard on the external package, but in small font. Three cartridges and the two e-liquids appeared to have C-R packaging44 and meet the US consumer guidance.45 Eight products warned to ‘keep out of reach of children.’ Three products had neither C-R packaging nor the ‘keep out of reach of children’ warning.
AEs associated with e-cigarette use and possibly related to HFs
Literature review of AEs
Events related to e-cigarette product exposure reported to the American Association of Poison Control Centers (AAPCC) increased 42% from 2011 (n=256) to 2012 (n=438). More than 30% of cases involved children younger than 5 years (n=84 in 2011; n=172 in 2012) and intentional misuse increased 56% (n=12 in 2011 and n=27 in 2012). Approximately 15% of the exposures were allergic or idiosyncratic responses and 25% required medical treatment.46 47 Intentional misuse cases included a completed suicide by injecting e-liquid (serum nicotine level was 2000 ng/mL)48 and a suicide attempt with ingestion and dermal application of e-liquid.49 Limited information about state-level aggregate Poison Control Center data suggests that common routes of exposure in children are ingestion, inhalation and dermal contact.50–53 Outside the USA, e-liquid ingestion was reported in three suicide attempts in two adults from Denmark54 and an Israeli toddler's death.55
There were 14 e-cigarette ignition events injuring eight individuals, including at least two non-users; 13 were explosions of rechargeable devices. Two explosions occurred during use, resulting in burns, oral disfigurement56 and unilateral blindness.57 One disposable e-cigarette exploded while being removed from the package, resulting in sensory impairments and property damage.58 Eleven explosions during device charging have caused burns (n=2),59 60 smoke inhalation injuries (n=2)60–62 and property damage (n=8).52 63–69 Six reports suggest possible user-product interactions: three describe products left unattended while charging52 63 66 68; two state that the products lacked warnings or charging instructions,60 66 and one suggests that a charger intended for use with a different e-cigarette product may have been used.67
Analysis of product complaints and AEs voluntarily reported to the FDA
FDA received its first e-cigarette AE report in August 2008. As of 31 December 2013, FDA/CTP had received 91 voluntary reports with product or health effect complaints related to e-cigarettes, and 26 (29%) reports may have HF contributions. Six reports were exclusively about product quality, performance, labelling or advertising and did not involve AEs. Of the 20 reports containing AEs, 8 were serious including an infant death from choking on a flavour cartridge, 4 explosions causing burn injuries of three adults and one child,59 2 confirmed nicotine overdoses (one with cartridge overheating and one with intentional dual use of traditional cigarettes) and 1 possible nicotine overdose with psychotic symptoms reported after e-liquid ingestion.
Twelve non-serious AE reports included non-specific physical complaints pertaining to advertisement, labelling or website issues (n=4), leakage of e-liquid resulting in oral or hand irritation (n=3); oral burns due to overheating or explosion (n=2); noxious smell and taste causing respiratory, gastrointestinal and constitutional symptoms (n=1); persistent after taste associated with difficulty using the charger (n=1); and possible product adulteration with marijuana (n=1). Complaints related to advertising, labelling, brochures or websites included unsubstantiated claims, inappropriate advertising from a healthcare provider's office, lack of product brochure; inappropriate labelling (“products labelled as nicotine-free and harm-free, despite FDA website information e-cigarettes may contain nicotine”), lack of health cautions in the retail setting and difficulty reaching ‘the company’.
Discussion
Although data on the contributions of HF to e-cigarette use are limited, many of the use-related concerns may be addressed by HFE in the product design, manufacture and dispensing processes. Currently, e-cigarette manufacturing is unregulated in the USA. There is significant variability in parameters such as the airflow rate required to produce aerosol, pressure drop, and lengths of time cartridges last, and aerosol production between and within e-cigarette brands.70
Electronic cigarette users
Although user characteristics have been explored in several studies, and consistently identify most e-cigarette users as smokers of traditional cigarette products, the studies identified are cross-sectional, and the data are limited and inconsistent across studies. Risks to individual users may be greater in those with underlying medical conditions, including asthma and hypersensitivity,2 and in youth, young adults and pregnant women. Given the complexity of some e-cigarette products, users with decreased manual dexterity, visual/hearing disabilities or limited cognitive skills may be at higher risk of use hazards.2 It is also unclear whether dual use of e-cigarettes and other nicotine-containing products will result in higher risks of nicotine toxicity and use-related hazards. Longitudinal national data, such as data from the ongoing Population Assessment of Tobacco and Health Study, a collaborative project between NIH and FDA/CTP, may provide a better understanding of the e-cigarette user population.
Electronic cigarette use environment
Electronic cigarettes may be used in many settings, including homes, public spaces (eg, parks and restaurants)71 and mobile environments (eg, automobiles). Although some states and localities have legislation limiting public use of e-cigarettes,71 the numbers and types of environments in which e-cigarettes are being used appear to be greater than those currently allowed for traditional cigarettes. Conditions such as ambient temperature and user distractions may impact appropriate product use2 and the storage environment may affect product quality, potency and function.2 In particular, the environment in which reusable devices are recharged may have important risk consequences. Research is needed to adequately characterise the environments in which e-cigarettes are used and stored.
Electronic cigarette design
The design and quality of e-cigarettes are diverse and inadequately characterised. Some of the known and potential associated e-cigarette risks, such as injuries and damage related to explosions, would likely be mitigated by improved product design and quality standards. For example, lithium batteries used in e-cigarettes carry a risk of overheating, explosion and electrolyte leakage due to their ability to store large amounts of energy in a compact space. Adoption of standardised manufacturing techniques with improved quality control processes may reduce battery hazards by reducing design flaws and manufacturing defects. These changes may also improve the tolerances associated with the environmental and storage conditions experienced during normal consumer use. In addition to design and manufacturing flaws, improper use and handling of the battery by the consumer can contribute to a condition known as ‘thermal runaway’ during which the internal battery temperature can increase, causing a fire or explosion.72 The use of industry-proven design safety features (such as overcharging protection circuits, thermal power cut-offs and internal overpressure relief mechanisms)73 and instructions for use that include the proper care and handling of the battery may help prevent thermal runaway.
Some e-cigarettes can be modified for use in ways other than intended, which may result in malicious adulteration, delivery of aerosols with higher concentrations of nicotine and toxins, and aerosolisation of products other than e-liquids, such as marijuana oils and waxes.25 Battery stacking and ‘dripping’ iv are examples of how the user can increase nicotine delivery. Designs that prevent product modification and use in ways other than intended may help to prevent or reduce adverse health effects associated with adulteration.2
Labelling and user interface
The literature, AE reports review and labelling analysis identified e-cigarette labelling inadequacies, including ambiguous, incomplete or misleading information, as well as information provided in fonts that are likely not conspicuous to the user. Given the complexity of e-cigarette products and potential risks, instructions to ensure appropriate use and adequate safety labelling may be needed for e-cigarettes.25 For example, all e-cigarettes in the CTP labelling analysis contain small parts that pose a choking risk to children and pets, and a fatal choking event has been reported. Products with C-R packaging design and with choking risk warnings may reduce or prevent choking hazards. In addition, ingestion of nicotine-containing e-liquids, especially those with high concentrations (eg, 87.2 mg nicotine/mL),32 can be toxic,v,74 and poisoning events have been reported.
Concerns have been raised that nicotine deposited on surfaces can be converted into carcinogens and then inhaled or ingested by both users and non-users.75 76 Although the magnitude of the risks of overdose and poisoning events remains undefined, adequate product labelling to warn of nicotine exposures and toxicity, and C-R packaging may help mitigate risks.
Incorporating HF approaches into e-cigarettes risk assessment
Additional information on e-cigarette users, use environment and the product–user interface is needed in order to apply HFE risk assessment strategies. Analytical HF approaches include function and task analysis, heuristic analysis and expert reviews. Empirical approaches (use studies) derive information from actual or simulated use of products. Usability testing prior to product marketing may reveal design problems and improve product labelling and directions for use.2 Postmarketing surveillance and evaluations are also critical for monitoring AEs and capturing unexpected user errors; this can inform consumer education and the development of additional safeguards with improved product design.
Limitations
To be included in this review, an article had to be relevant to HF, written in English and focus on the US market. Thus, it is possible that some articles may have been overlooked. AEs submitted to FDA are from voluntary reporters, may not include all cases and may lack accurate or complete information. For our labelling analysis, we were not the first to open some of the samples, which may have limited our ability to review all levels of packaging and may have affected the appearance of tamper-evident packaging features.
Our assumptions about the level of packaging intended for the retail consumer for the NicQuid® sample and the interpretation of ambiguous labelling content such as numbers and text descriptors were not confirmed with the manufacturers. We analysed 12 e-cigarette products that may or may not be representative of e-cigarette products at large. One-quarter of our sample was from a single manufacturer (NJOY), although the decision for inclusion was consistent with the product's market share12 and other published labelling analyses.9 10 Electronic cigarettes are increasingly popular and rapidly evolving. It is possible that some of the deficiencies we identified in labelling, especially for the earliest acquired samples, have already been addressed by the industry.
Conclusions
The impact of HFE on the hazards associated with e-cigarettes is not adequately characterised. HF risk management strategies may help to address e-cigarette related hazards. Given the complexity of e-cigarette products and the high concentrations of nicotine available for use, improvements in current labelling may help prevent or mitigate risks associated with e-cigarette use. AEs reported for e-cigarettes (explosions, burn injuries, poisonings, choking deaths and nicotine overdoses) may be reduced by improved product labelling and instructions, improved product and packaging design and improved manufacturing quality controls.
Future research priorities include collecting data to adequately characterise e-cigarette users, the user environment, identifying potential hazards associated with typical and atypical use, evaluating product complexities, determining appropriate manufacturing standards and design safety features, and determining the effectiveness of risk mitigation strategies.
What this paper adds.
This is the first comprehensive review applying principles of human factors engineering to e-cigarette-associated hazards.
Reports of adverse health effects and product malfunctions associated with e-cigarette use are increasing as the popularity of these products increases and human factors appear to contribute to a substantial number of the events, including some serious events.
Research is needed to further characterise the human factors that may cause or contribute to e-cigarette-associated adverse health effects and product malfunctions and to identify appropriate and effective risk mitigation strategies.
Supplementary Material
Acknowledgments
We would like to thank Drs Cathy Backinger, Priscilla Callahan-Lyon, Nancy Chang, Ii-Lun Chen, Carolyn Dresler, Corinne Husten and Sarah E. Johnson, as well as Mr Paul Aguilar and Ms. Deborah Neveleff, for their support of the project.
Contributors: All authors reviewed the final version of the paper and approved it for publication. LY conceptualised and developed the article topic, conducted the literature search on product use and user environment, assisted in analysing the FDA adverse events (AE) reports, and composed the majority of the article. SFR contributed to topic conceptualisation, conducted the literature search on AEs and product labelling/packaging, co-conducted the product labelling review, analysed the FDA AE reports, composed article sections pertaining to the labelling review and AEs, contributed to the discussion and summary article sections, and reviewed article drafts. JMC conducted the literature search on e-cigarette product design and composed the article section pertaining to product design. ELD reviewed study abstracts, co-conducted the product labelling review, assisted in reviewing the FDA AE reports, assisted in composing various article sections and reviewed article drafts.
Competing interests: None.
Provenance and peer review: Not commissioned; externally peer reviewed.
Some products were included in more than one category.
Refer to the Pepper and Brewer publication for additional information about e-cigarette users.
Section 1501 of the Federal Hazardous Substance Act (FHSA) (Pub L No. 86–613 [1960]) defines a test of object size using the small-parts test fixture (SPTF).43 The SPTF is a truncated cylinder with a diameter of 3.17 cm (1.25 in), simulating the mouth, and a depth between 2.54 and 5.71 cm (1.00 and 2.25 in), simulating the pharynx. An object is considered a small part if it fits completely within the SPTF. The SPTF was developed, in part, on the basis of data regarding the dimensions of airway foreign bodies recovered by bronchoscopy by Chevalier Jackson in the early 1900s.
‘Dripping’ is a term used to describe the application of e-liquid directly to the heating elements.25
The fatal dose of nicotine is estimated to be 30–60 mg for adults and 10 mg for children.74
References
- 1.American National Standard Institute/Association for the Advancement of Medical Instrumentation (AAMI) HE 75. Human factors engineering-design of medical devices. Arlington, VA: AAMI, 2009. http://www.aami.org/publications/standards/he75.html (accessed 19 Feb 2014) [Google Scholar]
- 2.United States Food and Drug Administration (FDA). Guidance for industry and FDA premarket and design control reviewers: medical device use-safety: incorporating human factors engineering into risk management. Washington, DC: U.S. Food and Drug Administration, 2000. http://www.fda.gov/downloads/MedicalDevices/DeviceRegulationandGuidance/GuidanceDocuments/ucm094461.pdf (accessed 19 Aug 2013) [Google Scholar]
- 3.Young D, Heckman G, Kim R. Human factors in sudden acceleration incidents. Proceedings of the Human Factors and Ergonomics Society Annual Meeting; 2011;Vol. 55:1938–42 [Google Scholar]
- 4.Office of the Federal Register. Code of Federal Regulations (annual edition). Title 21 Food and Drugs. Subpart B: Investigational New Drug Application. Parts 312.32 IND safety reporting. 75 FR 59961, 29 Sept 2010 Washington, DC: U.S. Government Printing Office, 2013 [Google Scholar]
- 5.International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use. ICH Harmonised Tripartite Guideline: Clinical safety data management—definitions and standards for expedited reporting. E2A. Step 4 version:2-3 Geneva, Switzerland: ICH, 1994 [Google Scholar]
- 6.Office of the Federal Register. Code of Federal Regulations (annual edition). Title 21 Food and Drugs. Part H: Serious adverse event reports. Section 379aa(a) Serious adverse event reporting for nonprescription drugs: Definitions. 75 FR 59961, 29 Sept 2010 Washington, DC: U.S. Government Printing Office, 2010 [Google Scholar]
- 7.Modi N, Schmid B, Miller R. UBS Investment Research: Clearing the Smoke on E-cigarettes. US Tobacco 14 May 2012 [Google Scholar]
- 8.RTI International. Quarter 1 report: Trends in tobacco use, tobacco industry marketing practices, and nature of online conversations about tobacco—Final report prepared for USDHHS/FDA/CTP/OHCE. Project Number 0212926.011.001, Table 3–7, pp. 3–15 Research Triangle Park, NC: RTI, 2012 [Google Scholar]
- 9.Trehy M, Ye W, Hadwiger ME, et al. Analysis of electronic cigarette cartridges, refill solutions, and smoke for nicotine and nicotine related impurities. J Liquid Chromatogr Relat Technol 2011;34:1442–58 [Google Scholar]
- 10.Trtchounian A, Talbot P. Electronic nicotine delivery systems: Is there a need for regulation? Tob Control 2011;20:47–52 [DOI] [PubMed] [Google Scholar]
- 11.Cheah NP, Chong NWL, Tan J, et al. Electronic nicotine delivery systems: Regulatory and safety challenges: Singapore perspective. Tob Control 2014;23:119–25 [DOI] [PubMed] [Google Scholar]
- 12.Goniewicz ML, Kuma T, Gawron M, et al. Nicotine levels in electronic cigarettes. Nicotine Tob Res 2013;15:158–66 [DOI] [PubMed] [Google Scholar]
- 13.Pfizer. Nicotrol Inhaler external package. NDA 020714 New York, NY: Pfizer, Pharmacia & UpJohn Company, LLC, 2006 [Google Scholar]
- 14.Glaxo Smith Kline (GSK). Nicorette® Lozenge (Nicotine Polacrilex) 4 mg external package. NDA 018612 Moon Township, PA: GlaxoSmithKline Consumer Healthcare, L.P., 2012 [Google Scholar]
- 15.Pepper JK, Brewer NT. Electronic nicotine delivery system (electronic cigarette) awareness, use, reactions and beliefs: a systematic review. Tob Control. Published Online First: 20 Nov 2013. doi:10.1136/tobaccocontrol-2013-051122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Regan AK, Promoff G, Dube SR, et al. Electronic nicotine delivery systems: adult use and awareness of the ‘e-cigarette’ in the USA. Tob Control 2013;22:19–23 [DOI] [PubMed] [Google Scholar]
- 17.King BA, Alam S, Promoff G, et al. Awareness and ever use of electronic cigarettes among U.S. adults, 2010–2011. Nicotine Tob Res 2013;15:1623–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pearson JL, Richardson A, Niaura RS, et al. E-cigarette awareness, use, and harm perceptions in US adults. Am J Public Health 2012;102:1758–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McMillen R, Maduka J, Winickoff J. Use of emerging tobacco products in the United States. J Environ Public Health 2012;989:474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Centers for Disease Control and Prevention. Electronic cigarette use among middle and high school students—United States, 2011–2012. MMWR 2013;62:729–30 [PMC free article] [PubMed] [Google Scholar]
- 21.Centers for Disease Control and Prevention. Tobacco product use among middle and high school students—United States, 2011–2012. MMWR 2013;62:893–7 [PMC free article] [PubMed] [Google Scholar]
- 22.Pepper JK, Reiter PL, McRee AL, et al. Adolescent males’ awareness of and willingness to try electronic cigarettes. J Adolesc Health 2013;52:144–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Choi K, Forster J. Characteristics associated with awareness, perceptions, and use of electronic nicotine delivery systems among young US Midwestern adults. Am J Public Health 2013;103:556–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Caponnetto P, Auditore R, Russo C, et al. Impact of an electronic cigarette on smoking reduction and cessation in schizophrenic smokers: a prospective 12-month pilot study. Int J Environ Res Public Health 2013;10:446–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McQueen A, Tower S, Summer W. Interviews with “vapers”: implications for future research with electronic cigarettes. Nicotine Tob Res 2011;13:860–7 [DOI] [PubMed] [Google Scholar]
- 26.Polosa R, Caponnetto P, Morjaria JB, et al. Effect of an electronic nicotine delivery device (e-cigarette) on smoking reduction and cessation: a prospective 6-month pilot study. BMC Public Health 2011;11:786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kralikova E, Kubatova S, Truneckova K, et al. The electronic cigarette: What proportion of smokers have tried it and how many use it regularly? Addiction 2012;107:1528–9 [DOI] [PubMed] [Google Scholar]
- 28.Etter JF, Bullen C. Electronic cigarette: users profile, utilization, satisfaction and perceived efficacy. Addiction 2011;106:2017–28 [DOI] [PubMed] [Google Scholar]
- 29.Alliance Technologies, LLC. Characterization of Regal Cartridges for electronic cigarettes. Prepared for in Life LLC (Irvine, CA), LIMS #20090304. St. Louis, MO: Alliance Technologies, LLC, 2009. http://truthaboutecigs.com/science/8.pdf (accessed 29 Aug 2013) [Google Scholar]
- 30.Alliance Technologies, LLC. Chemical composition of “Instead” electronic cigarette smoke juice and vapor. LIMS # 20090399. St. Louis, MO: Alliance Technologies, LLC, 2009. http://truthaboutecigs.com/science/13.pdf (accessed 29 Aug 2013) [Google Scholar]
- 31.Commission on Human Medicines Working Group on Nicotine Containing Products. Assessment of the constituents of four e-cigarette products. May 2011. (CHMWG2011/th). http://www.mhra.gov.uk/home/groups/comms-ic/documents/websiteresources/con286843.pdf (accessed 2 Sep 2013)
- 32.Villa AF, Saviuc P, Gazin V, et al. Electronic cigarettes: Risk assessment [abstract #131]. Abstracts of the 2012 International Congress of the European Association of Poison Centers and Clinical Toxicologists; 25 May-1 June 2012, London (UK). Clin Toxicol 2012;50:309 [Google Scholar]
- 33.Kirschner RI, Gerona R, Jacobitz KL. Nicotine content of liquid for electronic cigarettes [Abstract #240]. 2013 Annual Meeting of the North American Congress of Clinical Toxicology (NACCT). Clin Toxicol 2013;51:684 [Google Scholar]
- 34.Etter JF, Zather E, Svensson S. Analysis of refill liquids for electronic cigarettes. Addiction 2013;108:1671–79 [DOI] [PubMed] [Google Scholar]
- 35.Cameron JM, Howell DN, White JR, et al. Variable and potentially fatal amounts of nicotine in e-cigarette nicotine solutions. Tob Control 2014;23:77–8 [DOI] [PubMed] [Google Scholar]
- 36.Goniewicz ML, Hajek P, McRobbie H. Nicotine content of electronic cigarettes, its release in vapour and its consistency across batches: regulatory implications. Addiction 2014;109:500–7 [DOI] [PubMed] [Google Scholar]
- 37.U.S. Food and Drug Administration. Guidance for Industry: Nasal spray and inhalation solution, suspension, and spray drug products—Chemistry, Manufacturing, and Controls Documentation, July 2002. http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM070575.pdf (accessed 20 Feb 2014)
- 38.Sicherer SH, Miñoz-Furlong A, Godbold JH, et al. U.S. prevalence of self-reported peanut, tree nut, and sesame allergy: 11-year follow-up. J Allergy Clin Immunol 2010;125:1322–6 [DOI] [PubMed] [Google Scholar]
- 39.U.S. Food and Drug Administration. Guidance on Medical Device Patient Labeling; Final Guidance for Industry and FDA Reviewers, 2001. http://www.fda.gov/downloads/MedicalDevices/DeviceRegulationandGuidance/GuidanceDocuments/UCM070801.pdf (accessed 29 Oct 2013)
- 40.U.S. Centers for Disease Control and Prevention. Cigarette package health warnings and interest in quitting smoking-14 countries, 2008–2010. MMWR 2011;60: 645–50 [PubMed] [Google Scholar]
- 41.World Health Organization. Guidelines for implementation of Article 11 of the WHO Framework Convention on Tobacco Control (Packaging and labelling of tobacco products). Geneva, Switzerland: World Health Organization, 2008. http://www.who.int/fctc/guidelines/article_11.pdf (accessed 6 Feb 2014) [Google Scholar]
- 42.Microsoft Office. Online instructions for setting up automated reading ease and reading grade level analysis. 2010. http://office.microsoft.com/en-us/word-help/test-your-document-s-readability-HP010354286.aspx (accessed 3 Jun 2013)
- 43.U.S. Consumer Product Safety Commission. Small Parts Regulations: Toys and products intended for use by children under 3 years old. Code of Federal Regulations, 2001. Title 16 Part 1501 and 1500,50–53. http://www.cpsc.gov/PageFiles/111656/regsumsmallparts.pdf (accessed 19 Aug 2013) [Google Scholar]
- 44.American Society for Testing and Materials International. Standard classification of child-resistant packages. ASTM D3475-12 West Conshohocken, PA: ASTM, 2012 [Google Scholar]
- 45.Consumer Product Safety Commission. Poison Prevention Packaging: A Guide for Healthcare Professionals. CPSC 384 Washington, DC: CPSC, 2005. http://www.cpsc.gov//PageFiles/116503/384.pdf (accessed 29 Oct 2013) [Google Scholar]
- 46.Bronstein AC, Spyker DA, Cantilena LR, Jr, et al. 2011 Annual report of the American Association of Poison Control Centers’ National Poison Data System (NPDS): 29th annual report. Clin Toxicol (Phila) 2012;50: 911–1164 [DOI] [PubMed] [Google Scholar]
- 47.Mowry JB, Spyker DA, Cantilena LR, Jr, et al. 2012 Annual report of the American Association of Poison Control Centers’ National Poison Data System (NPDS): 30th Annual Report. Clin Toxicol (Phila) 2013;51:949–1229 [DOI] [PubMed] [Google Scholar]
- 48. doi: 10.1007/s13181-014-0380-9. Thornton SL, Oller L, Sawyer T. Fatal intravenous injection of electronic nicotine delivery system refilling solution. J Med Toxicol. Published Online First: 6 Feb 2014.doi:10.1007/s13181-014-0380-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Valento M. Nicotine poisoning following ingestion of e-liquid [abstract #239]. 2013 Annual Meeting of the North American Congress of Clin Toxicol (NACCT). Clin Toxicol 2013;51:683–4 [Google Scholar]
- 50.Cantrell FL. Adverse effects of e-cigarette exposures. J Community Health. Published Online First: 15 December 2013. 10.1007/s10900-013-9807-5 [DOI] [PubMed] [Google Scholar]
- 51.Ordonez J, Forrester MB, Kleinschmidt K. Electronic cigarette exposures reported to poison centers [abstract #242]. 2013 Annual Meeting of the North American Congress of Clin Toxicol (NACCT). Clin Toxicol 2013;51:685 [Google Scholar]
- 52.Gilger L, Tomasch M, Alter M, et al. Vaping, the latest phenomenon to hit the cigarette industry, is spurring health concerns. Knox News. 2 January 2014. http://www.knoxnews.com/news/2014/jan/02/vaping-latest-phenomenon-hit-cigarette-industry-sp/?print=1 (accessed 3 Jan 2014)
- 53.Orti C. E-cigarette poisoning on the rise. ABC 8 Eyewitness News. 9 January 2014. http://www.klkntv.com/story/24411448/e-cigarette-poisoning-on-the-rise (accessed 10 Jan 2014)
- 54.Christensen LB, van't Veen T, Bang J. Three cases of attempted suicide by ingestion of nicotine liquid used in e-cigarettes [abstract #85]. In XXXIII International Congress of the European Association of Poisons Centers and Clinical Toxicologists (EAPCCT). Clin Toxicol 2013;51:290 [Google Scholar]
- 55.Kloosterman K. Electronic cigarette kills toddler in Israel. Green Prophet. 29 May 2013. http://www.greenprophet.com/2013/05/electronic-cigarette-kills-toddler-in-israel/ (accessed 31 May 2013)
- 56.Los Angeles Times. Are smokeless cigarettes safer? E-cig explodes in smoker's mouth. February 16, 2012. http://latimesblogs.latimes.com/nationnow/2012/02/electronic-cigarette-explodes-mans-mouth.html (accessed 16 Feb 2012)
- 57.Kurwicki H. Man says e-cig caused temporary blindness. WWAY News. 12 December 2013. http://www.wwaytv3.com/2013/12/12/only-3-man-says-e-cig-caused-temporary-blindness (accessed 13 Dec 2013)
- 58.Fox23 News. Electronic cigarette explodes in Muskogee woman's hand. 18 April 2012. http://www.fox23.com/mostpopular/story/Electronic-cigarette-explodes-in-Muskogee-womans/ek2×6P6rvkyLu5cMrvGwVQ.cspx (accessed 19 Aug 2013)
- 59.McFall M. Exploding e-cigarette set toddler's clothes afire, Utah fire marshal says. The Salt Lake Tribune. 24 September 2013. http://www.sltrib.com/sltrib/news/56916022-78/fire-schofield-cigarette-marshal.html.csp (accessed 1 Oct 2013)
- 60.Downing M. Sherman man's e-cigarette explodes while charging. News 12 KXII.com. 16 July 2013. http://www.kxii.com/news/headlines/E-cigarette-explodes-in-Texoma-mans-home-215771641.html (accessed 17 Jul 2013)
- 61.Edwards A. E-cigarette battery cause for bomb scare at OKC apartment. KFOR-TV. 16 April 2013. http://kfor.com/2013/04/16/woman-in-good-condition-after-explosion-at-apartment-complex/ (accessed 29 Oct 2013)
- 62.Koco.com. Police: ‘Explosion’ at apartment complex came from e-cigarette-1 person taken to hospital. 16 April 2013. http://www.koco.com/news/oklahomanews/okc/Police-Explosion-at-apartment-complex-came-from-e-cigarette/-/11777584/19768650/-/po4rsj/-/index.html (accessed 16 Apr 2013)
- 63.Arkell H.E-cigarette wrecked car when it exploded ‘like a firework’ while being charged overnight leaving seats destroyed and windows blackened. MailOnline. 24 September 2013. http://www.dailymail.co.uk/news/article-2430393/E-cigarette-wrecked-car-EXPLODED-like-firework-charged-overnight-leaving-seats-destroyed-windows-blackened.html (accessed 1 Oct 2013)
- 64.Christie J. No smoke, but lots of fire! Another e-cigarette explosion smolders home as officials warn you don't need to ‘light up’ to be in danger. Mail Online. 3 October 2013. http://www.dailymail.co.uk/news/article-2442715/E-cigarette-explosion-causes-Wisconsin-home.html (accessed 11 Oct 2013)
- 65.DeMasters T. E-cigarette explodes while charging. Fox 13 News. 9 December 2013. http://fox13now.com/2013/12/09/e-cigarette-explodes-while-charging/ (accessed 13 Dec 2013)
- 66.Gilger L. Phoenix Fire Department Officials warn electronic cigarette users about fire danger. ABC15.com. 31 December 2013
- 67.Huffington Post. Elizabeth Wilkowski, Atlanta woman, claims e-cigarette explosions almost destroyed her home. 4 September 2013. http://www.huffingtonpost.com/2013/09/04/elizabeth-wilkowski-e-cigarette-explosion_n_3865233.html (accessed 5 Sep 2013)
- 68.Reiser L. Lighting up: E-cigarette charger sparks car fire in AZ. CBS5 (KPHO Broadcasting Corporation). 9 May 2013. http://www.ksla.com/story/22071692/lighting-up-e-cigarette-charger-sparks-car-fire-in-az (accessed 20 May 2013)
- 69.Trent W. Exploding electronic cigarette blamed for fire. Azfamily.com. 11 November 2013. http://www.azfamily.com/news/consumer/Exploding-electronic-cigarette-blamed-for-fire-231499581.html (accessed 12 Nov 2013)
- 70.Williams M, Talbot P. Variability among electronic cigarettes in the pressure drop, airflow rate, and aerosol production. Nicotine Tob Res 2011;13:1276–83 [DOI] [PubMed] [Google Scholar]
- 71.Blumenfeld K. Electronic Cigarettes White Paper. Summit, NJ: Global Advisors Smoke free Policy (GASP), 2013. http://www.njgasp.org/E-Cigs_White_Paper.pdf (accessed 14 Nov 2013) [Google Scholar]
- 72.Wang Q, Ping P, Zhao X, et al. Thermal runaway caused fire and explosion of lithium ion battery. J Power Sources 2012;208:210–24 [Google Scholar]
- 73.Ballard GE. inventor; Standard Oil Company, assignee. Explosion resistant battery cells. U.S. Patent 4,397,919. 1983. Washington, DC: U.S. Patent and Trademark Office.
- 74.Henningfield JE, Zatari GS. Electronic nicotine delivery systems: emerging science foundation for policy. Tob Control 2010;19:89–90 [DOI] [PubMed] [Google Scholar]
- 75.Sleiman M, Gundel LA, Pankow JF, et al. Formation of carcinogens indoors by surface-mediated reactions of nicotine with nitrous acid, leading to potential third hand smoke hazards. PNAS 2010;107:6576–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Connolly GN, Richter P, Aleguas A, et al. Unintentional child poisonings through ingestion of conventional and novel tobacco products. Pediatrics 2010;125: 896–9 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.