Abstract
Children with low aerobic fitness have altered brain function compared to higher-fit children. This study examined the effect of an 8-month exercise intervention on resting state synchrony. Twenty-two sedentary, overweight (body mass index ≥ 85th percentile) children 8–11 years old were randomly assigned to one of two after-school programs: aerobic exercise (n=13) or sedentary attention control (n=9). Before and after the 8-month programs, all subjects participated in resting state functional magnetic resonance imaging scans. Independent components analysis identified several networks, with four chosen for between-group analysis: salience, default mode, cognitive control, and motor networks. The default mode, cognitive control, and motor networks showed more spatial refinement over time in the exercise group compared to controls. The motor network showed increased synchrony in the exercise group with the right medial frontal gyrus compared to controls. Exercise behavior may enhance brain development in children.
Keywords: Resting state fMRI, aerobic exercise, obesity, development, default mode, cognitive control
Childhood obesity in the U.S. has tripled over the past 30 years (Ogden et al., 2012). Altered brain function and lower cognitive performance on a variety of tasks have been found in obese compared to leaner children (Bruce et al., 2011; Davis and Cooper, 2011; Yau et al., 2012). High adiposity and low fitness are often associated with one another (Ortega et al., 2008). Because cross-sectional studies of obesity often do not control for fitness (and vice versa), it is possible that studies reporting differences related to obesity might be confounded with low fitness. Exercise may improve children's brain function and development (Institute of Medicine, 2013) and in fact, fitness and exercise have been shown to benefit at least two specific aspects of children's higher-order cognition: memory and cognitive control (CC). Better relational memory performance as well as more accurate and less variable performance on CC tasks have been observed in higher-fit compared to lower-fit children (Chaddock et al., 2010; Chaddock et al., 2011; Wu CT et al., 2011). Children who are higher-fit also have shown altered brain function on CC tasks compared to lower-fit children (Chaddock et al., 2012; Voss et al., 2011). Furthermore, randomized controlled exercise trials have provided evidence that exercise leads to better memory and CC performance, as well as altered activation on CC tasks in children (Davis et al., 2007; Davis et al., 2011; Krafft et al., In press; Monti et al., 2012).
These relationships between fitness and higher-order cognition have been investigated by several studies to date using task-based functional magnetic resonance imaging (fMRI). A distinct but complementary technique that can be used to investigate brain function from a different perspective is task-free “resting state” fMRI (rsfMRI), during which data are acquired while participants are asked only to maintain a relaxed, wakeful state. Spontaneous blood oxygenation level-dependent (BOLD) signal changes during resting state reflect coherence in the functional organization of the brain (Fox and Raichle, 2007). Patterns of BOLD signal synchrony can reveal networks of regions that show co-activation. Despite the fact that rsfMRI does not involve a task, there is evidence that differences in resting state synchrony reflect a predisposition to utilize cognitive resources in a different manner during task-based activity (Rubia, In press; Kannurpatti et al., 2012).
A number of co-activated brain regions have been identified and labeled as distinct resting state networks (RSNs). Similar core regions exist in both adults and children (Beckmann et al., 2005; Biswal et al., 1995; Gordon et al., 2011), although RSNs in children are more diffuse and become more specialized and focal as they mature (Jolles et al., 2011; Stevens et al., 2009). Little is known about the relationship of resting state brain function with exercise, adiposity or fitness. Several of these networks are implicated in higher-order cognitive processes that have been altered by exercise, and indeed have shown differences in synchrony after exercise or between obese and lean participants (García-García et al., In press; Kullmann et al., 2012; Voss et al., 2010).
One network potentially associated with fitness and/or adiposity is the default mode network (DMN), which is likely important for memory (Buckner et al., 2008). The DMN is the most-studied RSN and is hypothesized to support self-referential processing (Kim, 2010), which includes functions such as autobiographical memory retrieval, considering the perspective of others, and envisioning the future (Buckner et al., 2008). This network generally consists of several distinct nodes: anterior cingulate cortex and medial prefrontal cortex, posterior cingulate cortex and precuneus, and bilateral inferior parietal regions. In one study, obese adults showed higher precuneus and lower right anterior cingulate functional synchrony as compared to lean participants (Kullmann et al., 2012), which the authors interpreted as possibly disrupted integration of cognitive and emotional stimuli. A study of older adults found that aerobic exercise training decreased synchrony between the DMN and a frontal executive network. This result indicates that these separate networks show greater differentiation after exercise training, which may be beneficial in older adults, who tend to show decreased specificity of functional brain networks as they age (Voss et al., 2010).
Given the growing evidence showing that CC performance and associated brain activation are different at various fitness levels (Wu CT et al., 2011; Colcombe et al., 2004; Voss et al., 2011) and that CC processes are particularly sensitive to exercise (Colcombe and Kramer, 2003), the CC network was of interest in this study. The CC network primarily includes frontal and parietal regions (Vincent et al., 2008). Another network related to CC processes is the salience network, putatively involved in assessing the relevance of internal and external stimuli in order to redirect attention to salient stimuli. It includes dorsal anterior cingulate and orbital fronto-insular cortices (Seeley et al., 2007), a network that has been altered in obesity. In one report, adults with obesity showed lower synchrony between the salience network and the putamen as compared to lean adults. García-García et al. (In press) suggested that this decrease could contribute to overeating through an imbalance between autonomic and reward processing of food stimuli.
All three of the RSNs mentioned above (DMN, CC, and salience) are putatively involved in higher-order cognitive processes that have been affected by obesity or fitness. A network involved in more basic processes that also may be affected by fitness or exercise is the motor network. Its main nodes are the bilateral primary motor cortices, along with supplementary motor cortex, thalamus, putamen, and cerebellum (Barber et al., 2012). Like the salience network, the motor network includes the insula, which is hypothesized to mediate interoceptive awareness (e.g., cravings) and thus could be implicated in obesity (Jastreboff et al., 2013; Mehta et al., 2012). Few studies thus far have investigated how exercise per se affects the motor network; however, there is evidence that skilled motor training affects synchrony with this network. For instance, training in sequential finger movements in one study caused altered synchrony with right postcentral gyrus and bilateral supramarginal gyri (increasing as behavioral performance improved, and decreasing later once there was no more further improvement in behavioral performance; Ma et al., 2011). In addition, training in a dynamic balance task caused increased synchrony between prefrontal, supplementary motor, and parietal areas (Taubert et al., 2011). It is possible that other complex motor training – for example, participation in a supervised aerobic exercise program, including activities such as basketball and jump rope – could alter motor circuitry synchrony.
A growing body of literature suggests that resting state synchrony is altered with excess weight or lower fitness, although few studies have investigated these effects in children. The current study used a randomized controlled trail with assignment of overweight children to 8 months of either exercise training or a sedentary control condition. It was hypothesized that exercise training would alter resting state synchrony as compared to sedentary control group in four networks: DMN, CC, salience, and motor. Specifically, based on evidence that exercise causes more mature, efficient patterns of brain activation (Chaddock-Heyman et al., 2013; Krafft et al., In press), we hypothesized that exercise would cause decreased synchrony between these resting state networks and brain regions outside of those networks, reflecting more specialized and focal patterns of resting state synchrony.
2. Experimental Procedures
2.1. Participants
Participants (N = 37) were a subset of children in a larger randomized trial (N = 175), who were recruited from public schools around Augusta, GA and were eligible if they were 8-11 years old, overweight (BMI ≥ 85th percentile; Ogden et al., 2002), and inactive (no regular physical activity program ≥ 1 hr/week). Exclusions included any medical condition that would limit physical activity or affect study results (including neurological or psychiatric disorders). Children and parents completed written informed assent and consent in accordance with the Human Assurance Committee of the Medical College of Georgia. Each child's parent or guardian reported the child's age, sex, race, and health status. Parents also reported their own educational attainment, which was used as an index of socioeconomic status (1 = grade 7 or less; 2 = grades 8-9; 3 = grades 10-11; 4 = high school graduate; 5 = partial college; 6 = college graduate; 7 = post-graduate). The study took place at the Georgia Prevention Center at the Medical College of Georgia.
Resting state fMRI data were collected for 37 children at baseline before randomization to one of the two groups and 22 were scanned at post-test. Of the 15 participants lost after baseline, 4 refused to participate before randomization, 2 refused to continue partway through post-test MRI data collection (both from the control group), 8 dropped out during the course of the study (3 from the exercise group, 5 from the control group), and 1 was ruled out based on a neurological anomaly observed in the MRI scan (from the control group). The exercise group contained 16 children at baseline and 13 at post-test. The control group contained 17 children at baseline and 9 at post-test. The 10 children who participated in at least a portion of the study but refused to provide post-test rsfMRI data did not significantly differ from the participants in any of the baseline characteristics (variables listed in Table 1). Participants were included in analysis only if they had both baseline and post-test MRI data, resulting in a total of 22 participants (exercise group n=13, control group n=9). Characteristics of the sample are provided in Table 1.
2.2. Intervention
Participants were assigned randomly to one of two conditions, both of which were offered for 40 min/day: aerobic exercise or sedentary attention control. Randomization (balanced by race, sex, and school) was performed by the study statistician and concealed until after baseline testing was completed, at which point the study coordinator informed the families. Both groups were offered an after school program every school day for approximately 8 months (average number of days offered = 135, SD = 9). All participants were offered bus transportation after school to the Georgia Prevention Center where they spent half an hour on supervised homework time each day. All participants were provided with a snack each day. Lead instructors were rotated between the two groups every two weeks and assistants were rotated between the two groups every week. Both groups could earn points that were redeemed for small prizes weekly for performing desired behaviors. The reward schedule was periodically calibrated to keep the rewards offered to the groups similar.
The aerobic exercise group engaged in instructor-led aerobic activities (e.g., tag and jump rope). They wore heart rate monitors every day (S610i; Polar Electro, Oy, Finland) with which they could monitor their own performance and from which data were collected daily. Points in the exercise group were earned for an average daily heart rate above 150 beats per minute (bpm), with more points for higher average heart rates. 150 bpm was used as the goal for several reasons. This is an easily translatable public health approach that could be implemented with children anywhere, with no VO2 testing required. The variation in 70% of predicted maximum heart rate in children (220 – age) in this age range is only 2 bpm (146 – 148 bpm). We have successfully utilized this approach in previous studies to elicit a substantial dose of vigorous activity which improved aerobic fitness and reduced adiposity and diabetes risk, as well as improving executive function and achievement (Davis et al., 2011; Davis et al., 2012). We have found that this criterion permits every child, however fit, to maintain interest in participating. Finally, heart rate is a physiological index of effort that calibrates to a child's level of fitness such that as their fitness improves, more effort is required to achieve a given heart rate. Thus, it is fair across children that vary in fitness and keeps children moving at an appropriate, vigorous intensity. Participants in the exercise group had an average heart rate of 164 beats per minute (SD =10) during the intervention. The attention control group engaged in instructor-led sedentary activities (e.g., art and board games). Points in the control group were earned for participation and good behavior.
2.3. Data Collection and Analysis
2.3.1. Cognitive data
The Cognitive Assessment System (CAS), a standardized individual assessment of children's cognitive processes, was administered (Naglieri and Das, 1997). The Full Scale score of the CAS takes into account the four scales of the test (Planning, Attention, Simultaneous and Successive processing), each of which include 3 subtests. Analysis of this variable was conducted in SPSS Version 20 (IBM, Armonk, NY). A group by time ANOVA was conducted to investigate whether the groups significantly differed in how they changed over time.
2.3.2. MRI parameters
Images were acquired at the Medical College of Georgia on a GE Signa Excite HDx 3 Tesla MRI system (General Electric Medical Systems, Milwaukee, WI). For all MRI scans, head positions were stabilized with a vacuum pillow and/or foam padding. High-resolution T1-weighted structural brain images were acquired using a 3D FSPGR protocol (repetition time (TR) = 9.436 ms, echo time (TE) = 3.876 ms, flip angle = 20°, field of view (FOV) = 240 × 240 mm, acquisition matrix = 512 × 512, 120 contiguous axial slices, in-plane voxel dimensions = 0.469 × 0.469 mm, slice thickness = 1.3 mm, total scan time = 3 min, 33 sec). For resting state functional scans, one run was acquired during which participants were instructed to keep their eyes closed without falling asleep (TR = 5000 ms, TE = 25 ms, flip angle = 90°, FOV = 256 × 256 mm, acquisition matrix = 128 × 128, 60 interleaved axial slices, in-plane voxel dimensions = 2 × 2 mm, slice thickness = 2.4 mm [for all scans except 6 participants at baseline, who had a slice thickness of 2 mm], 112 volumes). A TR of 5000 ms was determined to be necessary to collect 60 slices. The first four volumes were discarded to allow for scanner stabilization. Each functional run was collected obliquely, with the slices aligned to the superior margin of the participants’ anterior commissure and the inferior margin of the posterior commissure.
2.3.3. MRI data analysis
Resting state fMRI analyses were conducted using FMRIB Software Libraries (FSL; (Jenkinson et al., 2012)) and Analysis of Functional Neuroimages (AFNI; (Cox, 1996)). Standard preprocessing (which has been previously used in other resting state studies; Camchong et al., In press) was applied in FMRIB Software Libraries (FSL version 5.0.1; Oxford, United Kingdom) for each subject. It consisted of motion correction using MCFLIRT (Jenkinson et al., 2002), non-brain removal using BET (Smith, 2002), spatial smoothing (full-width at half-maximum [FWHM] = 4 mm), grand-mean intensity normalization, and high-pass temporal filtering (.01 Hz). Due to the 5000 ms TR, slice time correction was not conducted, as the interpolation involved becomes less accurate with a TR over approximately 3000 ms and because there is evidence that this analysis step has a minimal impact on resting state data (Wu CW et al., 2011). Registration was carried out using FLIRT (Jenkinson et al., 2002; Jenkinson and Smith, 2001), with which each resting run was aligned to each individual's T1-weighted structural MR image, transformed into a standardized space based on a publicly available template created for 7-11 year olds in MNI space (Fonov et al., 2011; Fonov et al., 2009), and resampled to 2 × 2 × 2 mm voxels.
Data were inspected for excessive motion but no participants showed an average absolute motion greater than 1 mm of a shift in any of the three planes; therefore, no participants were excluded from analysis due to excessive motion. In order to limit any effect of smaller movements, probabilistic independent component analysis (PICA; Beckmann and Smith, 2004) was conducted as implemented in FSL's MELODIC for each participant to denoise individual data. Components that represented noise were selected by spatial and temporal characteristics as detailed by Kelly et al. (2010) including head motion (which appeared as “rim-like” artifacts around the brain) or scanner artifacts (such as slice dropouts, high-frequency noise, and field inhomogeneities) and removed. A group by time ANOVA conducted to look for difference in the sum of percent of total variance accounted for by components removed showed no group by time interactions in noise, F(1, 20) = .542, p = .470. In addition, six motion timecourses representing estimated motion in each plane (rotation and shift in x, y, and z planes) for each individual were removed.
A between-subject analysis was carried out using a dual regression approach that allows for voxel-wise comparisons of resting functional synchrony (Filippini et al., 2009; Westlye et al., 2011; Zuo et al., 2010). First, preprocessed functional data for each subject (22 participants at both baseline and post-test, yielding 44 functional runs) were temporally concatenated across subjects to create a single 4D (three spatial dimensions × time) dataset. The concatenated dataset was decomposed using ICA to identify large-scale patterns of functional synchrony in the sample. The inclusion of all participants at both timepoints in the ICA analysis is in accordance with previously published rsfMRI studies using similar analyses (Licata et al., 2013; Martínez et al., In press). Thirty spatially-independent components were identified using automatic dimensionality estimation. Components of interest were selected using spatial correlation against a set of maps derived from a previous resting state study in children (Thomason et al., 2011). This method for selecting components of interest has been used in previous resting state studies (Camchong et al., 2011). The comparison maps were defined as follows: masks for four networks were created by generating regions of interest (ROIs; spheres of 10-mm radius) with centers of mass based the coordinates reported by the previous study (see Table 2). The four networks were default mode, salience, cognitive control, and motor. These networks were selected due to previous evidence suggesting that their associated cognitive processes are altered at different weight or fitness levels (Chaddock et al., 2012; García-García et al., In press; Kullmann et al., 2012; Taubert et al., 2011). The exercise and control groups did not differ in their mean effect (or representation) to any of these four components at either baseline or post-test (p > 0.29). These four masks were then spatially correlated with all 30 components automatically estimated by MELODIC, and the component that had the highest spatial correlation with each mask was selected for further analysis. A dual regression technique was used to identify, within each subject's fMRI dataset, subject-specific temporal dynamics and associated spatial maps. This technique involves two steps. First, the full set of group-ICA spatial maps is used in a linear model fit (spatial regression) against each individual's fMRI dataset. This step results in a matrix for each participant describing that individual's temporal dynamics for each ICA component. In the second step, each individual's matrix resulting from the first step is used in a linear model fit (temporal regression) against their own fMRI dataset to estimate the participant's spatial maps for each component. These subject-specific spatial maps resulting from dual regression analysis were then transformed into z-maps for group comparison (similarly to previous studies; Camchong et al., 2011).
Further group analysis was conducted using AFNI. In order to illustrate the networks that were selected, a one-sample t-test was performed across the individual z-maps corresponding to each component of interest for all participants at both baseline and post-test. These one-sample t-tests were thresholded at a voxelwise uncorrected p < .0001 for display purposes due to the large number of significant voxels. To investigate group by time changes in functional synchrony, a two-way group (exercise, control) by time (baseline, post-test) repeated measures ANOVA was performed using the individual z-maps. Results at p < .025 are reported. To protect against false positives, a threshold/cluster method derived from Monte Carlo simulations (accounting for the smoothness of the data and with a synchrony radius of 2 mm) was applied to the F-map (Ward, 1997). Based on these simulations, a family-wise alpha of 0.05 was preserved with three-dimensional clusters with a minimum volume of 169 voxels. The resulting clustered F-maps were used to identify regions with significant differences.
3. Results
3.1. Cognitive Performance
There was not a significant group by time interaction in CAS Full Scale scores. The groups also did not differ significantly at baseline on any of the characteristics listed in Table 1. The groups did not differ significantly in the percentage of days they attended the program out of the number of days offered (t(20) = .849, p = .406), with the groups attending an average of 3.4 days per week (M = 68% attendance, SD = 25%).
3.2. Salience Network
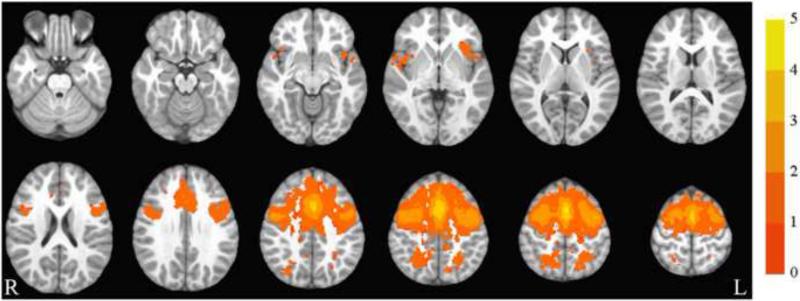
The salience network collapsed across group and time is illustrated in Figure 1. The group by time ANOVA showed no significant group by time interactions in functional synchrony with this network.
Figure 1.
Salience network. Axial slices (top left z = -26 through bottom right z = 62, spacing = 8 mm) displaying the salience network across all participants and both time points. Scale indicates z-value. The background anatomical image is the pediatric template that was used during alignment and is shown using radiological convention.
3.3. Default Mode Network
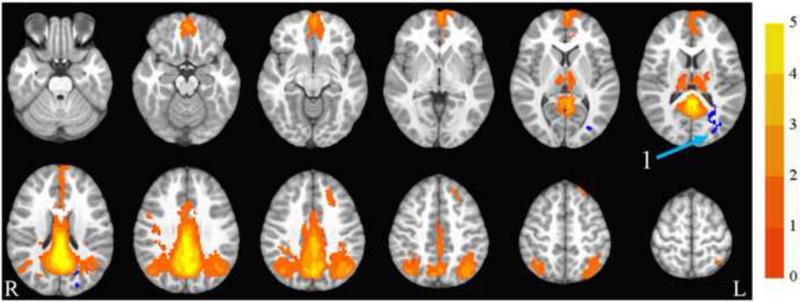
The default mode network collapsed across group and time is illustrated in Figure 2. The group by time ANOVA showed a significant interaction in the left middle occipital gyrus, extending into left cuneus, superior temporal gyrus, and posterior cingulate (shown in blue). In this region, the exercise group showed decreased synchrony over time compared to the control group. See Table 3 and Figure 2 for details.
Figure 2.
Default mode network. Axial slices (top left z = -26 through bottom right z = 62, spacing = 8 mm) displaying the default mode network across all participants and both time points. Scale indicates z-value. The arrow points to the left middle occipital gyrus, which showed a significant group by time interaction. The region is blue, indicating that the exercise group showed decreased synchrony with the DMN over time compared to the control group. The background anatomical image is the pediatric template that was used during alignment and is shown using radiological convention.
3.4. Cognitive Control Network
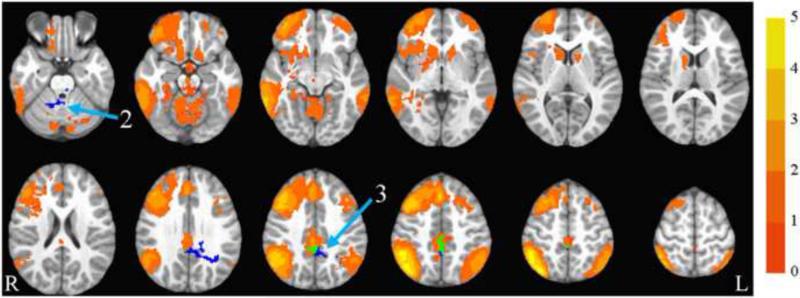
The cognitive control network collapsed across group and time is illustrated in Figure 3. The group by time ANOVA showed a significant interaction in the bilateral cingulate extending into bilateral precuneus, and in the right culmen. In both of these regions, the exercise group showed decreased synchrony over time compared to the control group. See Table 3 and Figure 3 for details.
Figure 3.
Cognitive control network. Axial slices (top left z = -26 through bottom right z = 62, spacing = 8 mm) displaying the cognitive control network across all participants and both time points. Scale indicates z-value. Arrow 2 points to the right culmen and arrow 3 points to the cingulate, both of which showed significant group by time interactions. Both regions are blue, indicating that the exercise group showed decreased synchrony with the cognitive control network over time compared to the control group. Where the clusters overlap with the cognitive control network, the overlap is in green. The background anatomical image is the pediatric template that was used during alignment and is shown using radiological convention.
3.5. Motor Network
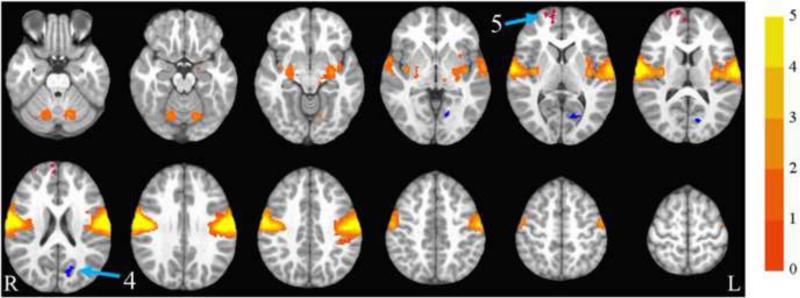
The motor network collapsed across group and time is illustrated in Figure 4. The group by time ANOVA showed a significant interaction in the right medial, superior, and middle frontal gyri (where the exercise group showed increased synchrony compared to the control group), and in the left cuneus (where the exercise group showed decreased synchrony compared to the control group). See Table 3 and Figure 4 for details.
Figure 4.
Motor network. Axial slices (top left z = -26 through bottom right z = 62, spacing = 8 mm) displaying the motor network across all participants and both time points. Scale indicates z-value. Arrow 4 points to the left cuneus and arrow 5 points to the right medial frontal gyrus, both of which showed significant group by time interactions. The left cuneus is blue, indicating that the exercise group showed decreased synchrony with the motor network over time compared to the control group. The right medial frontal gyrus is red, indicating that the interaction was in the opposite direction, with the exercise group showing increased synchrony with the motor network over time compared to the control group. The background anatomical image is the pediatric template that was used during alignment and is shown using radiological convention.
4. Discussion
Resting state synchrony, which reflects coherence in the functional organization of the brain independent of task performance, showed significant alterations due to exercise training that generally supported a pattern of greater specialization. These alterations were observed in overweight children randomized to an exercise intervention as compared to children assigned to a control condition over the course of 8 months. Results showed a pattern of decreased synchrony after exercise training with three resting state networks (with the sole exception of increased synchrony between the motor network and a frontal region). Previous studies suggest that a general pattern of refined synchrony (decreased between-network synchrony and increased within-network synchrony) reflects more mature, specialized, and efficient RSNs (Fair et al., 2007; Jolles et al., 2011; Kelly et al., 2009; Lopez-Larson et al., 2011; Stevens et al., 2009).
The developmental literature suggests an interesting pattern of resting state synchrony spatial refinement throughout development. There is evidence that RSNs are more specialized in young adults when compared to adolescents or children. Compared to adults, children show resting state networks that are defined more by anatomical proximity, whereas adults show patterns that are more associated with functional relationships (Fair et al., 2009). Networks in adults typically involved comparatively more long-range synchrony within the resting state networks but lower synchrony between separate networks, as well as lower synchrony between resting state networks and less functionally related regions (Jolles et al., 2011; Stevens et al., 2009). In this study, it is possible that exercise decreased influences between cognitive control, motor, and other networks, or simply that the networks became more specialized as a result of exercise. Lower mutual influences between networks may reflect greater flexibility in processing (Stevens et al., 2009) in that less synchrony between brain regions that do not have strong functional relationships with one another may allow them to act more independently to meet cognitive demands. Flexible use of strategies is one aspect of CC; CC has been improved by exercise in a previous randomized controlled trial (Davis et al., 2011). Although this interpretation needs to be further examined in longitudinal studies, it provides novel support for cross-sectional task-based work suggesting that children who are more fit exhibit greater flexible modulation of CC processes (Pontifex et al., 2010).
Decreased synchrony due to exercise was found in the DMN, which is hypothesized to support processes related to memory and self-referential processing (Kim, 2010), including autobiographical memory retrieval, considering the perspective of others, and envisioning the future (Buckner et al., 2008). The exercise group showed more refined synchrony associated with this network in the posterior cingulate, which is an important hub (Greicius et al., 2003). The decreased synchrony occurred outside the extent of activation of the posterior cingulate in the group map. A decrease in synchrony indicates that synchrony in this region became more focal and refined from baseline to post-test in the exercise group. The DMN also showed decreased synchrony in the exercise group with a visual region. Decreased synchrony was found with the middle occipital gyrus, which has been implicated in spatial localization (Renier et al., 2010), and the cuneus, which is important for comparatively basic visual processing and has been identified as a central hub of the visual RSN (Tomasi and Volkow, 2011). Finally, decreased DMN synchrony was seen in the superior temporal gyrus, which is part of an auditory RSN that has been identified in both children and adults (Cole et al., 2010; Thomason et al., 2011). Together, decreased synchrony in these regions seems to suggest that children in the exercise group show more a refined DMN over time as compared to the control group, including less synchrony with other distinct RSNs.
Refinement of RSNs was supported again by the CC network analysis, which showed decreased synchrony with the bilateral cingulate and precuneus after exercise. The cluster where a significant interaction was seen overlaps spatially with the CC network that was found in this study, possibly indicating that children in the exercise group demonstrated more specialized or focal synchrony of the CC network over time. Since the cingulate and precuneus are involved in both the CC and DMN networks, another possible interpretation is that synchrony between these two networks changed with exercise (Greicius et al., 2003). This difference is similar to an effect that was found in a previous study of older adults, where aerobic exercise training decreased synchrony between an executive network and the DMN (Voss et al., 2010), possibly leading to greater regulation between networks. Similarly, decreased synchrony was found between the CC network and the right cerebellum. This region is part of the CC network identified in at least one study in children (Gordon et al., 2011); however, the region that was found is similar to a region that has been identified as part of the salience network in adults (Habas et al., 2009). Overall, the exact network membership of the areas which show group by time differences in synchrony with the CC network is not yet clarified, as they may be involved in one or more networks, or perhaps shift roles during development. Therefore, the issue of whether exercise decreases inter-network synchrony or whether the CC network becomes more focal due to exercise has yet to be determined. Both processes may be at play and present topical questions for future studies. The pattern of change over the course of the intervention, however, is similar to that of the DMN analysis, with the exercise group showing decreased synchrony over time.
Exercise caused decreased synchrony once more in the motor RSN. The decreased synchrony with this network was seen in the cuneus, which supports basic visual processing and is a central hub of the visual RSN (Tomasi and Volkow, 2011). While the motor network showed decreased synchrony in the exercise group with the cuneus, it was the only RSN to also show an opposing pattern of increased synchrony within the exercise group. This increase was found in frontal regions, including right medial frontal, middle frontal, and superior frontal gyri. These regions are associated with working memory and spatial attention (Hopfinger et al., 2000; Kirschen et al., 2005; Lepsien et al., 2005; McCarthy et al., 1996; Ptak and Schnider, 2011) and may be involved in regulating execution of complex motor sequences (Rao et al., 2008). For instance, increased synchrony between motor cortex and middle frontal gyrus has been related to improved motor recovery in stroke patients (Park et al., 2011). Additional evidence that this increase in synchrony is beneficial comes from a study of motor learning, where enhanced motor learning in young adults was related to increased synchrony between dorsolateral prefrontal cortex and supplementary motor area (Lin et al., 2012). The relationship between synchrony of these regions and motor performance is not yet clarified in children but our results indicate that this is an interesting area for further study. Another possible interpretation in the motor network is that because it includes the insula, interoceptive awareness (i.e., cravings) may be altered (Mehta et al., 2012). An increase in synchrony between frontal regions and this network may reflect greater top-down control of these processes in children assigned to the exercise group.
4.1. Conclusions
The results of this 8 month randomized controlled trial assessing the impact of an aerobic exercise condition in comparison to a rigorous control condition suggest that exercise per se causes decreased resting state synchrony associated with default mode, cognitive control, and motor networks in overweight children. Importantly, in this study, the results of the exercise intervention stand apart from nonspecific influences of an after school program (such as adult attention, interaction with peers, and snacks).
There are limitations of this study, including the relatively small sample size. These detailed imaging results were available for a subset of participants from a larger study powered to detect effects of exercise on cognition, fatness, and fitness. This study is one of the first to investigate effects of exercise on resting state synchrony in children and as such should be replicated with larger sample sizes. Another potential limitation is the relatively long TR (five seconds). This is unlikely to be a major limitation, however, given evidence that temporal resolution minimally influences resting state synchrony (Van Dijk et al., 2010).
Evidence from the developmental literature (Jolles et al., 2011; Stevens et al., 2009) suggests that refinement of resting state networks is often advantageous (though not always; see Gaffrey et al., 2012). The development of more specialized RSNs seen in the exercise group following the 8 month intervention is consistent with the pattern that is found in typical development. More specialization may allow for greater flexibility in neural recruitment (Stevens et al., 2009), a pattern which has been observed in task-based studies of fitness and effects of exercise (Pontifex et al., 2011). It is of note that the control group showed an increase in synchrony in brain regions where the exercise group showed a decrease. A potential interpretation is that the control group increased synchrony over time within specific brain regions as they developed a pattern of excessive synchrony associated with obesity. Cross-sectional studies in both adolescents and adults have indicated that obese participants show greater synchrony between various brain regions compared to their healthier peers (Nummenmaa et al., 2012; Olde Dubbelink et al., 2008). Future studies should compare the longitudinal development of brain function in obese and healthy weight children to investigate these possible differences in developmental trajectories. The only increase in synchrony was found in the motor network, which is involved in acquisition of motor skills (and may allow for better regulation of complex motor skills in the future; Lin et al., 2012). Another possibility is that altered synchrony with the motor network reflects altered regulation of interoceptive processing (with potential effects for food cravings; Jastreboff et al., 2013; Mehta et al., 2012).
Task-free resting state fMRI can be an important tool for investigating benefits of exercise for other reasons, as well. It can inform structural imaging findings, as functional synchrony has been associated with structural connectivity in previous studies, although there is evidence that function-structure relationships become more stable as children mature (Supekar et al., 2010). It also can serve as an efficient proxy for task-based functional imaging studies. Kannurpatti et al. (2012) have shown that resting state fMRI predicts both voxel level and individual subject level responses during task-based fMRI. Thus, resting state fMRI data can be useful in cases in which it is challenging to obtain task-based fMRI data. The ability to evaluate brain activation via functional networks in children by using a relatively simple resting state acquisition is less complicated than requiring stimulus presentation and task performance during data acquisition in the scanner.
Youth obesity is on the rise. As such, identification and evaluation of interventions that counteract any disadvantageous effects of overweight are critically important. The results from the current randomized controlled trial suggest that exercise may be a simple and effective intervention to cause more developed patterns of brain function which may enhance neurocognitive outcomes. Conversely, children's brain development may be adversely affected by a sedentary lifestyle.
EXERCISE ALTERS RSFMRI SYNCHRONY IN CHILDREN.
Children were randomized to 8 months of aerobic exercise or a control program
Three resting state networks (RSNs) showed greater refinement after exercise
The motor network showed increased synchrony within a frontal brain region
Exercise may cause more mature patterns of RSN synchrony in overweight children
Table 1.
Baseline Characteristics of Participants Included in Analysis
| Characteristic | Exercise group | Control group |
|---|---|---|
| n | 13 | 9 |
| Age (years) | 9.5 (0.6) | 9.6 (0.9) |
| Female | 77% | 56% |
| Black | 100% | 89% |
| Left-handed | 8% | 22% |
| Cognitive score (CAS Full Scale SS) | 94.2 (7.3) | 95.9 (8.9) |
| Parental education scale | 4.8 (1.0) | 4.3 (1.4) |
Mean and SD, where applicable. CAS = Cognitive Assessment System. SS = Standard Score.
Table 2.
MNI coordinates used to generate regions of interest
| Network | Region | x | y | z |
|---|---|---|---|---|
| Default mode | Posterior cingulate | 5 | −53 | 10 |
| Medial frontal gyrus | −2 | 57 | −6 | |
| Right angular gyrus | 49 | −64 | 22 | |
| Salience | Right insula | 39 | 19 | 2 |
| Left insula | −43 | 12 | 2 | |
| Anterior cingulate | −2 | 16 | 38 | |
| Cognitive control | Right middle frontal gyrus | 49 | 32 | 18 |
| Superior medial frontal gyrus | 1 | 29 | 46 | |
| Right posterior parietal cortex | 36 | −75 | 46 | |
| Left inferior parietal cortex | −51 | −53 | 54 | |
| Motor | Left precentral gyrus | −58 | −15 | 38 |
| Right precentral gyrus | 63 | −15 | 38 |
Table 3.
Significant Clusters in the Whole-Brain Group by Time Analysis of Synchrony with Each Resting State Network
| Anatomical location | Direction of synchrony change over time (in exercise group with respect to control group) | MNI coordinates of center of mass (x,y,z) | Voxels |
|---|---|---|---|
| Salience network | |||
| No significant group by time interactions | |||
| Default mode network | |||
| 1. Left middle occipital gyrus, extending into left cuneus, superior temporal gyrus, and posterior cingulate | Exercise decreased | 28, 73, 14 | 291 |
|
Cognitive control network | |||
| 2. Right culmen, extending into right cerebellar tonsil | Exercise decreased | −17, 47, −32 | 424 |
| 3. Bilateral cingulate, extending into bilateral precuneus | Exercise decreased | 8, 44, 37 | 625 |
|
Motor network | |||
| 4. Left cuneus | Exercise decreased | 10, 72, 12 | 181 |
| 5. Right medial frontal gyrus, extending into right superior and middle frontal gyri | Exercise increased | −4, −66, 18 | 247 |
Acknowledgements
The project described was supported by the National Institutes of Health (R01 HL087923 and HL087923-03S2) and the National Science Foundation Graduate Research Fellowship Program.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- American College of Sports Medicine . ACSM's Guidelines for Exercise Testing and Prescription. 6th ed. Lippincott Williams & Wilkins; Baltimore, MD: 2000. [Google Scholar]
- Barber AD, Srinivasan P, Joel SE, Caffo BS, Pekar JJ, Mostofsky SH. Motor “dexterity”?: evidence that left hemisphere lateralization of motor circuit connectivity is associated with better motor performance in children. Cereb Cortex. 2012;22:51–59. doi: 10.1093/cercor/bhr062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckmann CF, DeLuca M, Devlin JT, Smith SM. Investigations into resting-state connectivity using independent component analysis. Philos Trans R Soc Lond B Biol Sci. 2005;360:1001–1013. doi: 10.1098/rstb.2005.1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckmann CF, Smith SM. Probabilistic independent component analysis for functional magnetic resonance imaging. IEEE Trans Med Imaging. 2004;23:137–152. doi: 10.1109/TMI.2003.822821. [DOI] [PubMed] [Google Scholar]
- Biswal B, Yetkin FZ, Haughton VM, Hyde JS. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn Reson Med. 1995;34:537–541. doi: 10.1002/mrm.1910340409. [DOI] [PubMed] [Google Scholar]
- Bruce AS, Martin LE, Savage CR. Neural correlates of pediatric obesity. Prev Med. 2011;52:S29–S35. doi: 10.1016/j.ypmed.2011.01.018. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Andrews-Hanna JR, Schacter DL. The brain's default network: anatomy, function, and relevance to disease. Ann N Y Acad Sci. 2008;1124:1–38. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- Camchong J, MacDonald AW, Bell C, Mueller BA, Lim KO. Altered functional and anatomical connectivity in schizophrenia. Schizophr Bull. 2011;37:640–650. doi: 10.1093/schbul/sbp131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camchong J, Stenger A, Fein G. Resting-state synchrony during early alcohol abstinence can predict subsequent relapse. Cereb Cortex. doi: 10.1093/cercor/bhs190. (In press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaddock-Heyman L, Erickson KI, Voss MW, Knecht AM, Pontifex MB, Castelli DM, Hillman CH, Kramer AF. The effects of physical activity on functional MRI activation associated with cognitive control in children: a randomized controlled intervention. Front Hum Neurosci. 2013;7:72. doi: 10.3389/fnhum.2013.00072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaddock L, Erickson KI, Prakash RS, Kim JS, Voss MW, VanPatter M, Pontifex MB, Raine LB, Konkel A, Hillman CH, Cohen NJ, Kramer AF. A neuroimaging investigation of the association between aerobic fitness, hippocampal volume, and memory performance in preadolescent children. Brain Res. 2010;1358:172–183. doi: 10.1016/j.brainres.2010.08.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaddock L, Erickson KI, Prakash RS, Voss MW, VanPatter M, Pontifex MB, Hillman CH, Kramer AF. A functional MRI investigation of the association between childhood aerobic fitness and neurocognitive control. Biol Psychol. 2012;89:260–268. doi: 10.1016/j.biopsycho.2011.10.017. [DOI] [PubMed] [Google Scholar]
- Chaddock L, Hillman CH, Buck SM, Cohen NJ. Aerobic fitness and executive control of relational memory in preadolescent children. Med Sci Sports Exerc. 2011;43:344–349. doi: 10.1249/MSS.0b013e3181e9af48. [DOI] [PubMed] [Google Scholar]
- Colcombe SJ, Kramer AF. Fitness effects on the cognitive function of older adults: a meta-analytic study. Psychol Sci. 2003;14:125–130. doi: 10.1111/1467-9280.t01-1-01430. [DOI] [PubMed] [Google Scholar]
- Colcombe SJ, Kramer AF, Erickson KI, Scalf P, McAuley E, Cohen NJ, Webb A, Jerome GJ, Marquez DX, Elavsky S. Cardiovascular fitness, cortical plasticity, and aging. Proc Natl Acad Sci U S A. 2004;101:3316–3321. doi: 10.1073/pnas.0400266101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole DM, Smith SM, Beckmann CF. Advances and pitfalls in the analysis and interpretation of resting-state FMRI data. Front Syst Neurosci. 2010;4:8. doi: 10.3389/fnsys.2010.00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Davis CL, Cooper S. Fitness, fatness, cognition, behavior, and academic achievement among overweight children: Do cross-sectional associations correspond to exercise trial outcomes? Prev Med. 2011;52:S65–S69. doi: 10.1016/j.ypmed.2011.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis CL, Pollock NK, Waller JL, Allison JD, Dennis BA, Bassali R, Meléndez A, Boyle CA, Gower BA. Exercise dose and diabetes risk in overweight and obese children: a randomized controlled trial. JAMA. 2012;308:1103–1112. doi: 10.1001/2012.jama.10762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis CL, Tomporowski PD, Boyle CA, Waller JL, Miller PH, Naglieri JA, Gregoski M. Effects of aerobic exercise on overweight children's cognitive functioning: a randomized controlled trial. Res Q Exerc Sport. 2007;78:510–519. doi: 10.1080/02701367.2007.10599450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis CL, Tomporowski PD, McDowell JE, Austin BP, Miller PH, Yanasak NE, Allison JD, Naglieri JA. Exercise improves executive function and achievement and alters brain activation in overweight children: a randomized, controlled trial. Health Psychol. 2011;30:91–98. doi: 10.1037/a0021766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fair DA, Dosenbach NUF, Church JA, Cohen AL, Brahmbhatt S, Miezin FM, Barch DM, Raichle ME, Petersen SE, Schlaggar BL. Development of distinct control networks through segregation and integration. PNAS. 2007;104:13507–13512. doi: 10.1073/pnas.0705843104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fair DA, Cohen AL, Power JD, Dosenbach NU, Church JA, Miezin FM, Schlaggar BL, Petersen SE. Functional brain networks develop from a “local to distributed” organization. PLoS Comput Biol. 2009;5:e1000381. doi: 10.1371/journal.pcbi.1000381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filippini N, MacIntosh BJ, Hough MG, Goodwin GM, Frisoni GB, Smith SM, Matthews PM, Beckmann CF, Mackay CE. Distinct patterns of brain activity in young carriers of the APOE-ε4 allele. Proc Natl Acad Sci U S A. 2009;106:7209–7214. doi: 10.1073/pnas.0811879106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonov VS, Evans AC, Botteron K, Almli CR, McKinstry RC, Collins DL. Unbiased average age-appropriate atlases for pediatric studies. NeuroImage. 2011;54:313–327. doi: 10.1016/j.neuroimage.2010.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonov VS, Evans AC, McKinstry RC, Almli CR, Collins DL. Unbiased nonlinear average age-appropriate brain templates from birth to adulthood. NeuroImage. 2009;47:S102. [Google Scholar]
- Fox MD, Raichle ME. Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat Rev Neurosci. 2007;8:700–711. doi: 10.1038/nrn2201. [DOI] [PubMed] [Google Scholar]
- Gaffrey MS, Luby JL, Botteron K, Repovš G, Barch DM. Default mode network connectivity in children with a history of preschool onset depression. J Child Psychol Psychiatry. 2012;53:964–972. doi: 10.1111/j.1469-7610.2012.02552.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García-García I, Jurado MÁ, Garolera M, Segura B, Sala-Llonch R, Marqués-Iturria I, Pueyo R, Sender-Palacios MJ, Vernet-Vernet M, Narberhaus A, Junqué C. Alterations of the salience network in obesity: A resting-state fMRI study. Hum Brain Mapp. doi: 10.1002/hbm.22104. (In press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon EM, Lee PS, Maisog JM, Foss-Feig J, Billington ME, VanMeter J, Vaidya CJ. Strength of default mode resting-state connectivity relates to white matter integrity in children. Dev Sci. 2011;14:738–751. doi: 10.1111/j.1467-7687.2010.01020.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greicius MD, Krasnow B, Reiss AL, Menon V. Functional connectivity in the resting brain: A network analysis of the default mode hypothesis. Proc Natl Acad Sci U S A. 2003;100:253–258. doi: 10.1073/pnas.0135058100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habas C, Kamdar N, Nguyen D, Prater K, Beckmann CF, Menon V, Greicius MD. Distinct cerebellar contributions to intrinsic connectivity networks. J Neurosci. 2009;29:8586–8594. doi: 10.1523/JNEUROSCI.1868-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopfinger JB, Buonocore MH, Mangun GR. The neural mechanisms of top-down attentional control. Nat Neurosci. 2000;3:284–291. doi: 10.1038/72999. [DOI] [PubMed] [Google Scholar]
- Institute of Medicine . Educating the Student Body Taking Physical Activity and Physical Education to School. The National Academies Press; Washington, DC: 2013. [PubMed] [Google Scholar]
- Jastreboff AM, Sinha R, Lacadie C, Small DM, Sherwin RS, Potenza MN. Neural correlates of stress- and food- cue-induced food craving in obesity: association with insulin levels. Diabetes Care. 2013;36:394–402. doi: 10.2337/dc12-1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkinson M, Bannister P, Brady M, Smith S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. NeuroImage. 2002;17:825–841. doi: 10.1016/s1053-8119(02)91132-8. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Beckmann CF, Behrens TE, Woolrich MW, Smith SM. FSL. NeuroImage. 2012;62:782–790. doi: 10.1016/j.neuroimage.2011.09.015. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Smith S. A global optimisation method for robust affine registration of brain images. Medical Image Anal. 2001;5:143–156. doi: 10.1016/s1361-8415(01)00036-6. [DOI] [PubMed] [Google Scholar]
- Jolles DD, van Buchem MA, Crone EA, Rombouts SA. A comprehensive study of whole-brain functional connectivity in children and young adults. Cereb Cortex. 2011;21:385–391. doi: 10.1093/cercor/bhq104. [DOI] [PubMed] [Google Scholar]
- Kannurpatti SS, Rypma B, Biswal BB. Prediction of task-related BOLD fMRI with amplitude signatures of resting-state fMRI. Front Syst Neurosci. 2012;6:7. doi: 10.3389/fnsys.2012.00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly AMC, DiMartino A, Uddin LQ, Shehzad Z, Gee DG, Reiss PT, Margulies DS, Castellanos FX, Milham MP. Development of anterior cingulate functional connectivity from late childhood to early adulthood. Cereb Cortex. 2009;19:640–657. doi: 10.1093/cercor/bhn117. [DOI] [PubMed] [Google Scholar]
- Kelly RE, Alexopoulos GS, Wang Z, Gunning FM, Murphy CF, Morimoto SS, Kanellopoulos D, Jia Z, Lim KO, Hoptman MJ. Visual inspection of independent components: Defining a procedure for artifact removal from fMRI data. J Neurosci Methods. 2010;189:233–245. doi: 10.1016/j.jneumeth.2010.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H. Dissociating the roles of the default-mode, dorsal, and ventral networks in episodic memory retrieval. NeuroImage. 2010;50:1648–1657. doi: 10.1016/j.neuroimage.2010.01.051. [DOI] [PubMed] [Google Scholar]
- Kirschen MP, Chen SH, Schraedley-Desmond P, Desmond JE. Load- and practice-dependent increases in cerebro-cerebellar activation in verbal working memory: an fMRI study. NeuroImage. 2005;24:462–472. doi: 10.1016/j.neuroimage.2004.08.036. [DOI] [PubMed] [Google Scholar]
- Kullmann S, Heni M, Veit R, Ketterer C, Schick F, Häring HU, Fritsche A, Preissl H. The obese brain: Association of body mass index and insulin sensitivity with resting state network functional connectivity. Hum Brain Mapp. 2012;33:1052–1061. doi: 10.1002/hbm.21268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krafft CE, Schwarz NF, Chi L, Weinberger AL, Schaeffer DJ, Pierce JE, Rodrigue AL, Yanasak NE, Miller PH, Tomporowski PD, Davis CL, McDowell JE. An eight month randomized controlled exercise trial alters brain activation during cognitive tasks in overweight children. Obesity. doi: 10.1002/oby.20518. (In press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lepsien J, Griffin IC, Devlin JT, Nobre AC. Directing spatial attention in mental representations: Interactions between attentional orienting and working-memory load. NeuroImage. 2005;26:733–743. doi: 10.1016/j.neuroimage.2005.02.026. [DOI] [PubMed] [Google Scholar]
- Licata SC, Nickerson LD, Lowen SB, Trksak GH, Maclean RR, Lukas SE. The hypnotic zolpidem increases the synchrony of BOLD signal fluctuations in widespread brain networks during a resting paradigm. NeuroImage. 2013;70:211–22. doi: 10.1016/j.neuroimage.2012.12.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin CH, Chiang MC, Wu AD, Iacoboni M, Udompholkul P, Yazdanshenas O, Knowlton BJ. Enhanced motor learning in older adults is accompanied by increased bilateral frontal and fronto-parietal connectivity. Brain Connect. 2012;2:56–68. doi: 10.1089/brain.2011.0059. [DOI] [PubMed] [Google Scholar]
- Lopez-Larson MP, Anderson JS, Ferguson MA, Yurgelun-Todd D. Local brain connectivity and associations with gender and ge. Dev Cogn Neurosci. 2011;1:187–197. doi: 10.1016/j.dcn.2010.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma L, Narayana S, Robin DA, Fox PT, Xiong J. Changes occur in resting state network of motor system during 40 weeks of motor skill learning. NeuroImage. 2011;58:226–233. doi: 10.1016/j.neuroimage.2011.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martínez K, Solana AB, Burgaleta M, Hernández-Tamames JA, Alvarez-Linera J, Román FJ, Alfayate E, Privado J, Escorial S, Quiroga MA, Karama S, Bellec P, Colom R. Changes in resting-state functionally connected parietofrontal networks after videogame practice. Hum Brain Mapp. doi: 10.1002/hbm.22129. (In press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy G, Puce A, Constable T, Krystal JH, Gore JC, Goldman-Rakic P. Activation of human prefrontal cortex during spatial and nonspatial working memory tasks measured by functional MRI. Cereb Cortex. 1996;6:600–611. doi: 10.1093/cercor/6.4.600. [DOI] [PubMed] [Google Scholar]
- Mehta S, Melhorn SJ, Smeraglio A, Tyagi V, Grabowski T, Schwartz MW, Schur EA. Regional brain response to visual food cues is a marker of satiety that predicts food choice. Am J Clin Nutr. 2012;96:989–999. doi: 10.3945/ajcn.112.042341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monti JM, Hillman CH, Cohen NJ. Aerobic fitness enhances relational memory in preadolescent children: The FITKids randomized control trial. Hippocampus. 2012;22:1876–1882. doi: 10.1002/hipo.22023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naglieri JA. Essentials of CAS Assessment. John Wiley & Sons, Inc.; Hoboken, NJ: 1999. [Google Scholar]
- Naglieri JA, Das JP. Cognitive Assessment System: Interpretive Handbook. Riverside Publishing; Itasca, IL: 1997. [Google Scholar]
- Nummenmaa L, Hirovnen J, Hannukainen JC, Immonen H, Lindroos MM, Salminen P, Nuutila P. Dorsal striatum and its limbic connectivity mediate abnormal anticipatory reward processing in obesity. PLoS One. 2012;7:e31089. doi: 10.1371/journal.pone.0031089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of obesity and trends in body mass index among us children and adolescents, 1999-2010. JAMA. 2012;307:483–490. doi: 10.1001/jama.2012.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogden CL, Kuczmarski RJ, Flegal KM, Mei Z, Guo S, Wei R, Grummer-Strawn LM, Curtin LR, Roche AF, Johnson CL. Centers for Disease Control and Prevention 2000 growth charts for the United States: Improvements to the 1977 National Center for Health Statistics version. Pediatrics. 2002;109:45–60. doi: 10.1542/peds.109.1.45. [DOI] [PubMed] [Google Scholar]
- Olde Dubbelink KTE, Felius A, Verbunt JPA, van Dijk BW, Berendse HW, Stam CJ, Delemarre-van de Waal HA. Increased resting-state functional connectivity in obese adolescents; a magnetoencephalographic pilot study. PLoS ONE. 2008;3:e2827. doi: 10.1371/journal.pone.0002827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortega FB, Ruiz JR, Castillo MJ, Sjöström M. Physical fitness in childhood and adolescence: a powerful marker of health. Int J Obes. 2008;32:1–11. doi: 10.1038/sj.ijo.0803774. [DOI] [PubMed] [Google Scholar]
- Park CH, Chang WH, Ohn SH, Kim ST, Bang OY, Pascual-Leone A, Kim YH. Longitudinal changes of resting-state functional connectivity during motor recovery after stroke. Stroke. 2011;42:1357–1362. doi: 10.1161/STROKEAHA.110.596155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pontifex MB, Raine LB, Johnson CR, Chaddock L, Voss MW, Cohen NJ, Kramer AF, Hillman CH. Cardiorespiratory fitness and the flexible modulation of cognitive control in preadolescent children. Cogn Neurosci. 2011;23:1332–1345. doi: 10.1162/jocn.2010.21528. [DOI] [PubMed] [Google Scholar]
- Ptak R, Schnider A. The attention network of the human brain: Relating structural damage associated with spatial neglect to functional imaging correlates of spatial attention. Neuropsychologica. 2011;49:3063–3070. doi: 10.1016/j.neuropsychologia.2011.07.008. [DOI] [PubMed] [Google Scholar]
- Rao H, Di X, Chan RC, Ding Y, Ye B, Gao D. A regulation role of the prefrontal cortex in the fist-edge-palm task: Evidence from functional connectivity analysis. NeuroImage. 2008;41:1345–1351. doi: 10.1016/j.neuroimage.2008.04.026. [DOI] [PubMed] [Google Scholar]
- Renier LA, Anurova I, De Volder AG, Carlson S, VanMeter J, Rauschecker JP. Preserved functional specialization for spatial processing in the middle occipital gyrus of the early blind. Neuron. 2010;68:138–148. doi: 10.1016/j.neuron.2010.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubia K. Functional brain imaging across development. Eur Child Adolesc Psychiatry. doi: 10.1007/s00787-012-0291-8. (In press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H, Reiss AL, Greicius MD. Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci. 2007;27:2349–2356. doi: 10.1523/JNEUROSCI.5587-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith S. Fast robust automated brain extraction. Hum Brain Mapp. 2002:143–155. doi: 10.1002/hbm.10062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens MC, Pearlson GD, Calhoun VD. Changes in the interaction of resting-state neural networks from adolescence to adulthood. Hum Brain Mapp. 2009;30:2356–2366. doi: 10.1002/hbm.20673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Supekar K, Uddin LQ, Prater K, Amin H, Greicius MD, Menon V. Development of functional and structural connectivity within the default mode network in young children. NeuroImage. 2010;52:290–301. doi: 10.1016/j.neuroimage.2010.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taubert M, Lohmann G, Margulies DS, Villringer A, Ragert P. Long-term effects of motor training on resting-state networks and underlying brain structure. NeuroImage. 2011;57:1492–1498. doi: 10.1016/j.neuroimage.2011.05.078. [DOI] [PubMed] [Google Scholar]
- Thomason ME, Dennis EL, Joshi AA, Joshi SH, Dinov ID, Chang C, Henry ML, Johnson RF, Thompson PM, Toga AW, Glover GH, Van Horn JD, Gotlib IH. Resting-state fMRI can reliably map neural networks in children. NeuroImage. 2011;55:165–175. doi: 10.1016/j.neuroimage.2010.11.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasi D, Volkow ND. Association between functional connectivity hubs and brain networks. Cereb Cortex. 2011;21:2003–2013. doi: 10.1093/cercor/bhq268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Dijk KR, Hedden T, Venkataraman A, Evans KC, Lazar SW, Buckner RL. Intrinsic functional connectivity as a tool for human connectomics: theory, properties, and optimization. J Neurophysiol. 2010;103:297–321. doi: 10.1152/jn.00783.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent JL, Kahn I, Snyder AZ, Raichle ME, Buckner RL. Evidence for a frontoparietal control system revealed by intrinsic functional connectivity. J Neurophysiol. 2008;100:3328–3342. doi: 10.1152/jn.90355.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voss MW, Chaddock L, Kim JS, VanPatter M, Pontifex MB, Raine LB, Cohen NJ, Hillman CH, Kramer AF. Aerobic fitness is associated with greater efficiency of the network underlying cognitive control in preadolescent children. Neuroscience. 2011;199:166–176. doi: 10.1016/j.neuroscience.2011.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voss MW, Prakash RS, Erickson KI, Basak C, Chaddock L, Kim JS, Alves H, Heo S, Szabo AN, White SM, Wójcicki TR, Mailey EL, Gothe N, Olson EA, McAuley E, Kramer AF. Plasticity of brain networks in a randomized intervention trial of exercise training in older adults. Front Aging Neurosci. 2010;2:32. doi: 10.3389/fnagi.2010.00032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward BD. Simultaneous inference for fMRI data. Biophysics Research Institute, Medical College of Wisconsin; Milwaukee, WI: 1997. [Google Scholar]
- Welk GJ, Morrow JR. FITNESSGRAM Reference Guide. The Cooper Institute; Dallas, TX: 2008. [Google Scholar]
- Westlye ET, Lundervold A, Rootwelt H, Lundervold AJ, Westlye LT. Increased hippocampal default mode synchronization during rest in middle-aged and elderly APOE ε4 carriers: relationships with memory performance. J Neurosci. 2011;31:7775–7783. doi: 10.1523/JNEUROSCI.1230-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu CT, Pontifex MB, Raine LB, Chaddock L, Voss MW, Kramer AF, Hillman CH. Aerobic fitness and response variability in preadolescent children performing a cognitive control task. Neuropsychology. 2011;25:333–341. doi: 10.1037/a0022167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu CW, Chen CL, Liu PY, Chao YP, Biswal BB, Lin CP. Empirical evaluations of slice-timing, smoothing, and normalization effects in seed-based, resting-state functional magnetic resonance imaging analyses. Brain Connect. 2011;1:401–410. doi: 10.1089/brain.2011.0018. [DOI] [PubMed] [Google Scholar]
- Yau PL, Castro MG, Tagani A, Tsui WH, Convit A. Obesity and metabolic syndrome and functional and structural brain impairments in adolescence. Pediatrics. 2012;130:e856–64. doi: 10.1542/peds.2012-0324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuo XN, Kelly C, Adelstein JS, Klein DF, Castellanos FX, Milham MP. Reliable intrinsic connectivity networks: Test–retest evaluation using ICA and dual regression approach. NeuroImage. 2010;49:2163–2177. doi: 10.1016/j.neuroimage.2009.10.080. [DOI] [PMC free article] [PubMed] [Google Scholar]