Abstract
It has been reported that host defense against pulmonary K. pneumoniae infection requires IL-22, which has been proposed to be of T cell origin. Supporting a role for IL-22, we found that Il22−/− mice had decreased survival as compared with wild type mice after intratracheal infection with K. pneumoniae. Surprisingly, however, Rag2−/− mice did not differ from wild type mice in survival or levels of IL-22 in the lungs after infection with K. pneumoniae. By contrast, K. pneumoniae-infected Rag2−/−Il2rg−/− mice failed to produce IL-22. These data suggested a possible role for NK cells or other innate lymphoid cells (ILC) in host defense and production of IL-22. Unlike NK cell-like ILCs that produce IL-22 and display a surface phenotype of NK1.1−NKp46+CCR6+, lung NK cells showed the conventional phenotype, NK1.1+NKp46+CCR6−. Mice depleted of NK cells using anti-asialo GM1 showed decreased survival and higher lung bacterial counts as well as increased dissemination of K. pneumoniae to blood and liver as compared with control-treated mice. NK cell depletion also led to decreased production of IL-22 in the lung. Within one day after infection, although there was no increase in the number of lung NK cells, a subset of lung NK cells became competent to produce IL-22, and such cells were found in both wild type and Rag2−/− mice. Our data suggest that during pulmonary infection of mice with K. pneumoniae, conventional NK cells are required for optimal host defense, which includes the production of IL-22.
Introduction
The respiratory tract is an important mucosal site for entry of pathogens and thus requires a rapid and effective response to limit and/or clear infection. Th17-cell cytokines, which are critical for protection against various extracellular pathogens, have been shown to promote host defense against pulmonary infections (1-5). Roles for the Th17-cell cytokines IL-17 and IL-22 in protection against extracellular bacteria in the lung have been best characterized in the mouse model of pneumonia induced by Klebsiella pneumoniae (1, 2, 6). K. pneumoniae is an important agent in community-acquired and nosocomial pulmonary infection, and is of particular medical importance recently because of the threat from the world-wide spread of multi-drug resistant strains (7-10).
IL-17 and IL-22 were reported to be critical for host defense in the mouse model of K. pneumoniae pneumonia (1, 2, 6), and administration of an anti-IL-22 antibody to K. pneumoniae-infected mice resulted in 100% mortality within 24 h. This mortality was significantly more rapid than for control or Il17−/− mice, suggesting that IL-22 was particularly important in the initial response against K. pneumoniae in the lung (1). K. pneumoniae-induced IL-22 was proposed to be of T-cell origin (1).
IL-22 is a member of the IL-10 family of cytokines (11-13). The IL-22 receptor is expressed on several epithelial tissues, including human bronchial epithelial cells and mouse tracheal epithelial cells, but not on cells of hematopoietic origin (1, 14, 15). Studies have suggested a critical role for IL-22 in the maintenance of normal mucosal homeostasis and the protection of mucosal sites during infection (1, 15). The beneficial role of IL-22 in host defense against extracellular bacteria includes maintaining barrier integrity and up-regulation of bactericidal proteins (1, 16-19). The primary source of IL-22 has been thought to be Th17 cells and/or Th22 cells, the latter being a subset identified in human skin (16). Nonetheless, certain innate lymphoid cells (ILCs4), such as NK cells and other novel non-NK ILC subsets, can also produce IL-22 at mucosal sites in both mice and humans (17-23).
As prototypic ILCs, NK cells are a first line of defense against infection. Although NK cells have been viewed classically as being crucial in providing innate surveillance against viruses and intracellular bacteria through lysis of infected cells and production of IFN-γ (24-27), data also support a role for NK cells in host defense against extracellular bacteria (28). For example, it has been shown in mouse models that NK cells play protective roles in host defense against acute respiratory infection by S. aureus (29) and P. aeruginosa (30, 31) and infection of the gastrointestinal tract with the attaching and effacing bacterium, C. rodentium (32, 33). In addition, impairing NK cell responses is one mechanism whereby prior influenza infection increases susceptibility to subsequent S. aureus infection (29, 34). The mechanisms whereby NK cells protect against bacterial infections are not extensively characterized, but may include production of cytokines such as TNF-α and IFN-γ , production of chemokines to recruit additional leukocytes, interactions with macrophages to regulate bacterial clearance, and direct bacterial killing (32, 33, 35-37).
In our studies of the K. pneumoniae pneumonia model in mice, we found that, although IL-22 was indeed important for optimal host defense, T cells were not required for survival or for the production of IL-22. We found instead that NK cells were essential for protection against K. pneumoniae, including preventing extrapulmonary dissemination, that they contributed directly and/or indirectly to the production of IL-22 in the lungs of K. pneumoniae-infected mice, and could acquire the ability to make IL-22 in the absence of T cells. In addition, the lung NK cells exhibited a conventional phenotype rather than the phenotype of NK22/ILC22 cells described at other sites. Together, our data suggest novel and important roles for conventional NK cells in the earliest stages of anti-bacterial defense and maintenance of mucosal integrity in the respiratory tract.
Materials and Methods
Animals and reagents
Female 8-12 wk-old C57BL/6NTac, Rag2−/− (B6.129S6-Rag2tm1Fwa N12), Rag2−/−Il2rg−/−(B10;B6-Rag2tm1Fwa II2rgtm1Wjl) mice were obtained from Taconic Farms. Breeding pairs of BALB/c Il22−/− mice were generously provided by Dr. Lynette Fouser (Pfizer, Cambridge, MA), and the production of these mice was as previously described (16). BALB/cAnNTac, BALB/cAnNCr, and BALB/cJ mice were purchased from Taconic Farms, Division of Cancer Treatment and Diagnosis, NCI, and The Jackson Laboratory, respectively. All mice were housed under specific pathogen free conditions at the National Institutes of Health in an American Association for the Accreditation of Laboratory Animal Care-approved facility. Animal study protocols were approved by the Animal Care and Use Committee, NIAID, NIH. Three-to-five mice per group were used in each experiment, unless otherwise indicated.
Rabbit anti-mouse/rat asialo GM1 polyclonal antibody (ASGM1) was purchased from Cedarlane Laboratories, and reconstituted with 1 ml of sterile water. The antibody titer of the lot used in this study was approximately 1:1000 by immunoflocculation. Mice were injected intravenously with 30 μl of reconstituted antibody in 200 μl PBS one day before inoculation with K. pneumoniae and injected i.p. every three days over the course of the study. Rabbit serum was used as an antibody control.
K. pneumoniae inoculation model
Frozen stock aliquots of K. pneumoniae strain 43816, serotype 2 (American Type Culture Collection) were grown in tryptic soy broth (TSB) for 18 h at 37° C. One ml of the culture was added to 200 ml of fresh TSB, and grown for another 2 h until the bacteria reached log phase. Bacteria were pelleted by centrifugation at 6,000 rpm for 15 min at 4° C, washed twice with normal saline, and suspended in normal saline. Bacterial concentration was determined by measuring the optical density at 600 nm and comparing values with a predetermined standard curve, where 0.1 ODU was found to correspond to 2.8 × 108 bacteria per ml. For inoculation, mice were anesthetized via i.p. injection with ketamine/xylazine, the trachea was exposed, and a 30 μl inoculum of bacterial suspension or normal saline alone was administered via a 30-gauge needle. The inoculum of K. pneumoniae was 104 CFU for C57BL/6 mice, any mice on the C57BL/6 background, and 103 CFU for BALB/c and BALB/c Il22−/− mice. Inoculum sizes were determined based on survival data for wild-type BALB/c and C57BL/6 mice (data not shown). An aliquot of the inoculated K. pneumoniae suspension was serially diluted onto LB agar plates to confirm the dose of injected bacteria.
K. pneumoniae CFU in blood and tissues
At designated times post-infection, mice were anesthetized via i.p. injection with ketamine/xylazine. Heparinized blood was collected from the inferior vena cava. Lungs were perfused through the right ventricle with normal saline. Lungs and livers were removed and homogenized with normal saline. Bacterial burdens were determined in lung, liver as well as blood by plating 10-fold serial dilutions of tissue homogenates or blood on LB agar plates. After 24 h of incubation at 37° C, colonies were counted, and results calculated as log10 CFU per organ or per 1 ml blood.
Cell isolation from lung, spleen and lymph node
Naïve non-infected or infected mice were anesthetized via i.p. injection with ketamine/xylazine. To obtain lung cell suspensions, lungs were perfused with PBS through the right ventricle of the heart, then lungs were cut into small pieces and digested with 1 mg/ml Collagenase D (Roche) and 50 U/ml DNase I (Sigma-Aldrich) in PBS for 30 min at 37 °C with vortexing every 10 min. Samples were mashed through 70 μm cell strainers, washed with complete RPMI media (supplemented with 10% FBS, 1 mM pyruvate, 1 mM non essential amino acids, and 1mM L-glutamine). Spleens and mediastinal lymph nodes were mechanically disrupted using a syringe plunger in complete RPMI and cells were filtered similarly. Remaining erythrocytes in lung and spleen samples were lysed with ACK lysis buffer. Single cell suspensions were used for subsequent analysis.
Staining of cells and analysis by flow cytometry
Fluorophore-conjugated antibodies against mouse CD45, CD3, NK1.1, NKp46, DX5, KLRG1, and CD27 were purchased from BD Biosciences, and anti-mouse IL-22 was purchased from Biolegend. Blue-fluorescent reactive dye for distinguishing live and dead cells was purchased from Invitrogen, and dead cells were excluded from all analyses. NK cells were identified by their scatter profile, and as having the surface phenotype CD45+CD3−NK1.1+ among cells from C57BL/6 wild type and Rag2−/− mice and CD45+CD3−DX5+ among cells from BALB/c Il22−/− mice. Absolute numbers of cells per sample were calculated by adding a known number of fluorescent counting beads (Spherotech) to each of the samples before analysis on the flow cytometer. Intracellular staining for IL-22 and IFN-γ in isolated lung cells was done after activating cells for 4 hours with Leukocyte Activation Cocktail with Golgiplug (BD Biosciences). Cells were fixed and permeabilized using the Cytofix/Cytoperm Plus kit according to the manufacturer's instructions (BD Bioscience). All samples were analyzed on an LSR II System flow cytometer (BD Biosciences), and the data were analyzed using FlowJo software (version 8.2, Tree Star).
Cytokine assays
Mouse lungs were homogenized with T-PER tissue protein extraction reagent (Pierce) supplemented with complete mini protease inhibitor cocktail tablets (Roche) at a proportion of 1 tablet/10 ml of T-PER reagent. Lung homogenates were centrifuged at 10,000 × g for 5 min and supernatants were collected for cytokine analysis. IL-22 levels in lung homogenates were determined using a mouse IL-22 ELISA Construction Kit (Antigenix) according to the manufacturer's instructions.
RT-PCR
Total RNA was purified using Trizol Plus RNA purification kit (Invitrogen) according to the manufacture's instruction. We used 50 ng of RNA with the Platinum Quantitative RT-PCR ThermoScript One Step System (Invitrogen) to perform RT-PCR. Primers and probes for Gapdh, Il17a, Ifng, Tnfa and Il6 were purchased from Applied Biosystems (catalog numbers Mm99999915_g1, Mm00439619_m1, Mm00801778_m1, Mm00443258_m1, mm00446190_m1 respectively), and primers and probes for Il22 were as previously described (16, 20). The reactions were run on an Applied Biosystems 7900HT system using the standard protocol provided by Invitrogen. All PCR reactions used an annealing temperature of 60 °C.
Statistics
All quantitative data are shown as mean ± SD unless otherwise indicated. All samples were compared using 2-tailed, unpaired Student's t test. Survival analysis was performed using the Gehan-Breslow-Wilcoxon test. A p value less than 0.05 was considered significant.
Results
Reduced survival of IL-22-deficient mice during pulmonary infection with K. pneumoniae
It has been reported that treatment with anti-IL-22 antibody leads rapidly to 100% mortality after pulmonary infection of C57BL/6 mice with K. pneumoniae, strain 43816, serotype 2 (1). We used the same strain of K. pneumoniae in two experiments to infect a total of 17 BALB/c Il22−/− mice and 20 BALB/c wild-type controls. As shown in Fig.1, Il22−/− mice started to die at Day 2 and wild-type mice started to die at Day 3 after infection. At Days 2, 3, 4, 5, 7, 8, 12, and 17 after infection, 1, 6, 2, 1, 0, 0, 2, and 0 Il22−/− mice, and 0, 1, 1, 4, 1, 2, 0, and 2 wild-type mice died, respectively. These data demonstrated that Il22−/− mice had significantly reduced survival as compared with wild type mice (p < 0.05), although these results are not as dramatic as those reported using anti-IL-22 antibody (1). To help rule out an artifactual difference between the Il22−/− and wild-type mice due to the commercial source of the wild-type controls, which could differ in their intestinal flora in ways that might affect responses to K. pneumoniae, we not only compared survival between the Il22−/− and BALB/cAnNTac mice, as shown in Fig. 1, but also, in a separate experiment, among Il22−/−, BALB/cAnNTac, BALB/cAnNCr, and BALB/cJ mice. Survival was similar among wild-type BALB/c mice irrespective of the commercial colony, and survival was reduced for the Il22−/− mice versus each of the wild-type groups (p < 0.05, data not shown).
Figure 1.
IL-22-deficient mice are more susceptible to infection with K. pneumoniae. Survival of BALB/c wild-type (WT, closed squares) and BALB/c Il22−/− (open circles) mice after inoculation in the trachea with 103 CFU of K. pneumoniae. Data are combined from two independent experiments, where total n = 20 in wild-type group, n = 17 in Il22−/− group. *p < 0.05 for the Il22−/− curve as compared to the curve for wild-type mice (Gehan-Breslow-Wilcoxcon Test).
A possible role for NK cells in host defense and IL-22 production
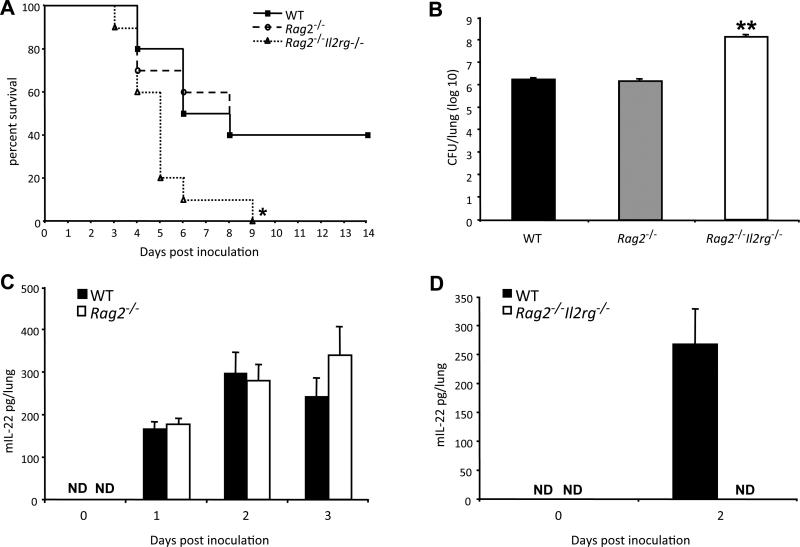
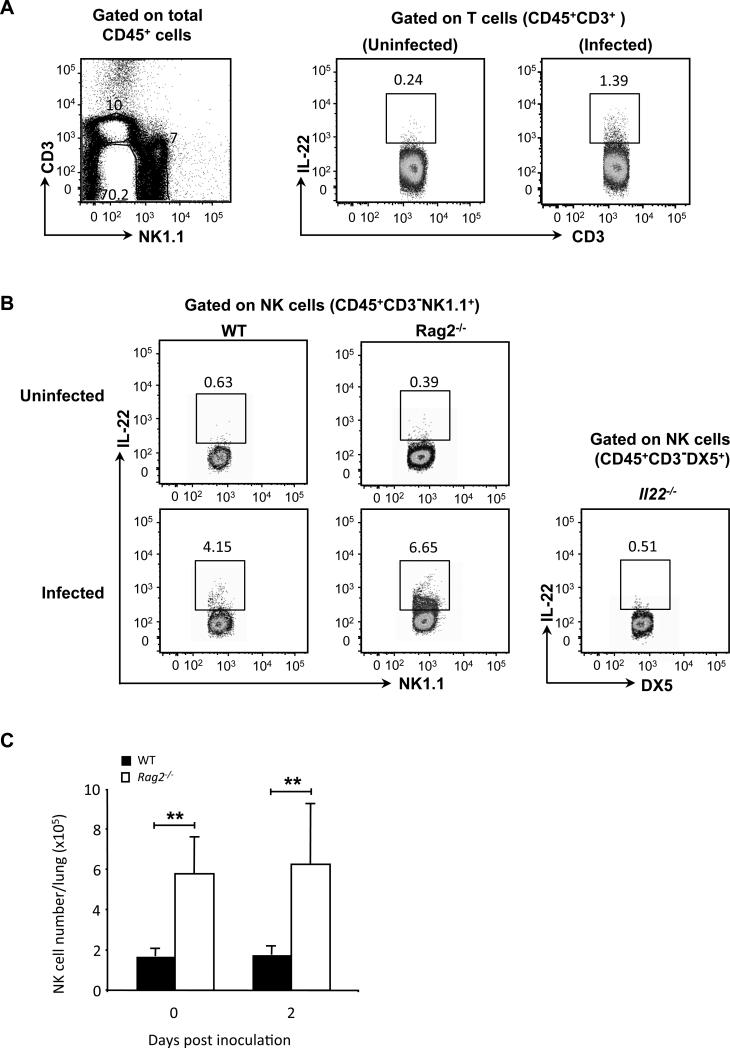
It has been proposed that IL-22 production in the first few days after pulmonary infection with K. pneumoniae is of T-cell origin (1). Given the importance of IL-22 in this model, we were surprised to find that infected Rag2−/− mice did not differ from wild-type mice in survival (Fig. 2A), lung bacterial count (Fig. 2B), and levels of IL-22 in the lungs (Fig. 2C). By contrast, K. pneumoniae-infected Rag2−/−Il2rg−/− mice showed decreased survival (Fig. 2A, p < 0.05 for Rag2−/−Il2rg−/− versus Rag2−/− and versus WT), had higher bacterial counts as compared with wild type and Rag2−/− mice (Fig. 2B, p < 0.01 for Rag2−/−Il2rg−/− versus Rag2−/− and versus WT), and failed to produce IL-22 (Fig. 2D). These data suggested a possible role for NK cells, or other populations of innate lymphocytes, in host defense and the production of IL-22. With regard to non-NK cell innate lymphocytes, a subset of lung resident ILCs (Lin−CD90+CD25+) was recently identified in both human and mouse that were shown to have a modest ability to produce IL-22 after stimulation with IL-23 (21). However, in our experiments we failed to detect a clear population of these cells in the lung either before or after infection with K. pneumoniae (Supplemental Fig. 1).
Figure 2.
Rag2−/−Il2rg−/−, but not Rag2−/− mice are compromised in their defense against K. pneumoniae infection. Mice were inoculated in the trachea with 104 CFU of K. pneumoniae or normal saline alone. (A) Survival of C57BL/6 wild-type (WT, closed squares), Rag2−/− (open circles) and Rag2−/−Il2rg−/− (open triangles) mice. Data are from ten mice per group in one representative experiment of two performed. *p < 0.05 for the Rag2−/−Il2rg−/− curve as compared to the other curves (Gehan-Breslow-Wilcoxcon Test). (B) K. pneumoniae burden in the lung two days after infection in C57BL/6 wild-type (WT, filled bar), Rag2−/− (striped bar) and Rag2−/−Il2rg−/− (open bar) mice. Data are from five mice per group in one representative experiment of two performed. For this and subsequent figures, error bars show SD's. **p < 0.01 for Rag2−/−Il2rg−/− versus other groups. (C) ELISA for IL-22 in the lungs of wild-type (WT, closed bars) and Rag2−/− (open bars) mice. “Day 0” data are from mice inoculated with normal saline alone and killed on Day 1. ND indicates not detectable. Data are from four mice per group in one representative experiment of three performed. (D) ELISA for IL-22 in the lungs of wild-type (WT, closed bars) and Rag2−/−Il2rg−/− mice. “Day 0” data are from mice inoculated with normal saline alone and killed on Day 2. ND indicates not detectable. Data are from 3-5 mice per group in one representative experiment of two performed.
NK cells contribute to host defense against K. pneumoniae
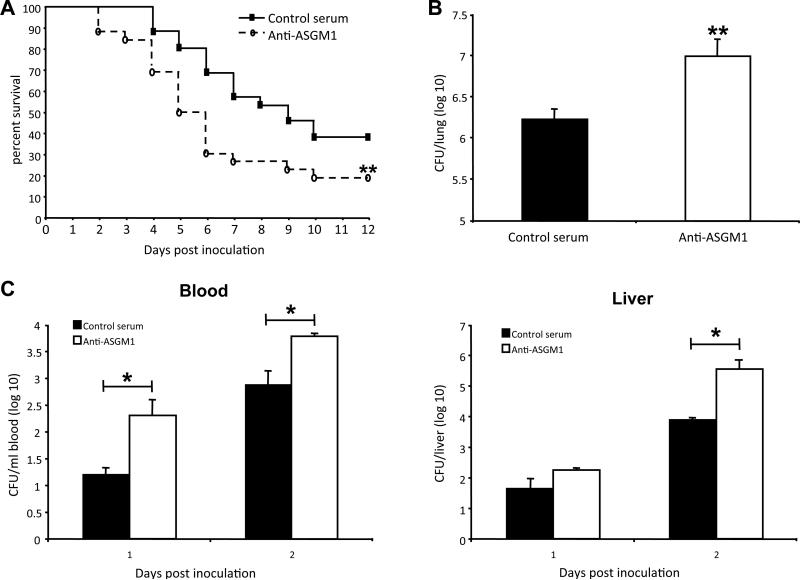
To examine the involvement of NK cells in this model, a non-activating antibody, polyclonal rabbit anti-mouse/rat asialo ganglio-N-tetraosylceramide (ASGM1) (38) was used successfully to deplete NK cells in vivo (data not shown). NK cell depletion resulted in decreased survival (Fig. 3A, p < 0.01) and higher lung bacterial counts (Fig. 3B, p < 0.01). One feature of K. pneumoniae infection is the dissemination of bacteria to the bloodstream. The effect of NK cells on K. pneumoniae dissemination was examined by comparing peripheral blood bacterial counts as well as liver bacterial counts after infection between NK-cell depleted and non-depleted mice. Mice treated with anti-ASGM1 displayed significantly increased peripheral blood bacterial counts at Day 1 and Day 2 post inoculation as compared to control antibody treated mice (Fig. 3C, p < 0.05 for both days). NK cell depletion also resulted in a significant increase in liver bacterial counts at Day 2 after infection (Fig. 3C, right panel, p < 0.05). Consistent with these findings, blood bacterial counts were significantly higher in the Rag2−/−Il2rg−/− versus wild-type and Rag2−/− mice at Day 2 after infection (data not shown).
Figure 3.
NK-cell depletion compromises host defense against K.pneumoniae infection. C57BL/6 wild-type mice were injected with anti-ASGM1 or control rabbit serum in PBS, and after 24 h mice were inoculated in the trachea with 104 CFU of K. pneumoniae. (A) Survival of anti-ASGM1-treated mice (open circles) and control rabbit serum-treated mice (closed squares). Data are combined from two independent experiments, where total n = 26 in each group. **p < 0.01 for the anti-ASGM1-treated curve as compared to the curve for control serum-treated mice (Gehan-Breslow-Wilcoxcon Test). (B) Lung CFU in anti-ASGM1-treated mice (open bar) and in control serum-treated mice (filled bar) at Day 2 after infection. Data are from 5 mice per group in one representative experiment of four performed. **p < 0.01 for anti-ASGM1-treated versus control serum-treated mice. (C) Blood (left panel) and liver (right panel) CFU in anti-ASGM1-treated mice (open bars) and control serum-treated mice (filled bars) at Day1 and Day 2 after infection. Data are from 4 mice per group in one representative experiment of two performed. *p < 0.05 for anti-ASGM1-treated versus control serum-treated mice.
Decreased production of lung IL-22 after infection in NK-cell depleted mice
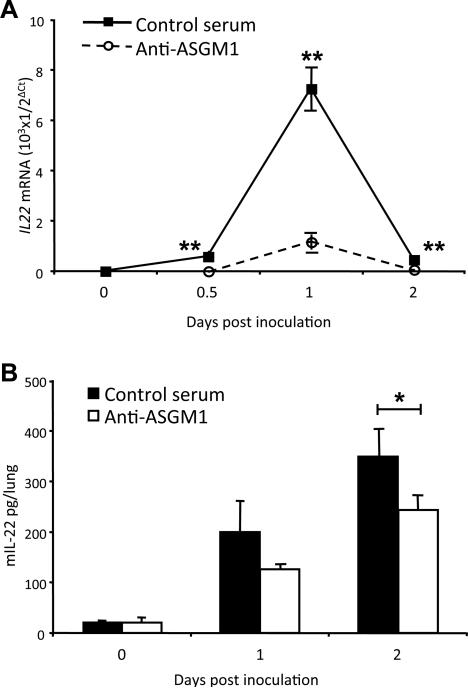
To test our hypothesis that NK cells contribute to IL-22 production in the lung after K. pneumoniae infection, we examined the effect of NK cell-depletion on lung IL-22 as measured by real-time RT-PCR (Fig. 4A) and ELISA (Fig. 4B). Our results showed that NK cell depletion decreased the production of IL-22 in the lung at two days after infection (Fig.4A, p < 0.01; Fig. 4B, p < 0.05), although results for mRNA and protein were discordant, with a dramatic reduction in the former and a more modest reduction in the latter. The effect of NK-cell depletion on the induction of mRNAs for other cytokines, including IL-17A, IFN-γ, TNF-α and IL-6, which have been shown to be critical in protection against infection with K. pneumoniae (2, 6, 39-44), was also examined by real-time RT-PCR. NK cell depletion had no effect on the induction of mRNA for IL-17A, but significantly decreased the induction of mRNAs for IFN-γ, TNF-α and IL-6 in the lung at one day after infection (Supplemental Fig. 2).
Figure 4.
NK-cell depletion results in decreased production of IL-22 in lungs of infected mice. C57BL/6 wild-type mice were treated with anti-ASGM1 or control rabbit serum, and after 24 h mice were inoculated in the trachea with 104 CFU of K. pneumoniae or normal saline alone. Lungs were homogenized at various times for quantification of Il22 mRNA and protein. (A) Il22 mRNA was measured by real time RT-PCR in lungs from mice treated with anti-ASGM1 (open circles) or control serum (closed squares). “Day 0” data are from mice inoculated with normal saline alone and killed at 0.5 days. Data are from 3 mice per group in 1 representative experiment of two performed. **p < 0.01 for control serum-treated versus anti-ASGM1-treated mice. (B) ELISA for IL-22 in the lungs of anti-ASGM1-treated (open bars) and control serum-treated (closed bars) mice. “Day 0” data are from mice inoculated with normal saline alone and killed on Day 1. Data are from 4 mice per group in one representative experiment of three performed. *p < 0.05 for control serum-treated versus anti-ASGM1-treated mice.
Lung NK cells show conventional but not NK22/ILC22 surface phenotype
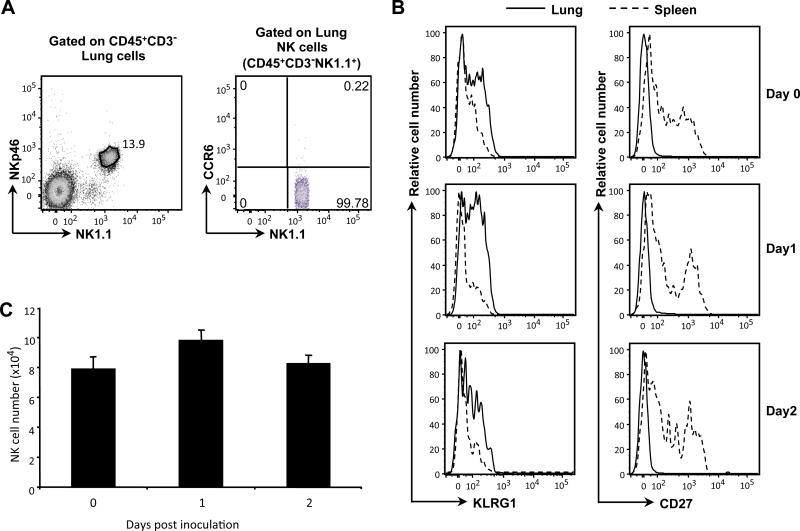
In contrast to conventional NK cells, a population of NK-like cells identified in the mouse intestinal tract, so-called “NK22” or “ILC22” cells, are NKp46+CCR6+, and are limited to NK1.1-negative or NK1.1-low subsets. To determine if these NK-like cells are present in mouse lung, we examined the expression of NK1.1, NKp46 and CCR6 on NK cells from lungs of wild type mice. We found no CD3−NK1.1−NKp46+CCR6+cells. On the contrary, all CD3−NKp46+ cells in the lung were NK1.1+ (Fig. 5A), and lacked CCR6 expression. Because NK cell maturation is associated with KLRG1 up-regulation and CD27 down-regulation (45, 46), we examined the expression of KLRG1 and CD27 on lung versus splenic NK cells. Overall, lung NK cells showed higher expression of KLRG1 and decreased expression of CD27 as compared with splenic NK cells, suggesting that the cells in the lung were more mature (Fig. 5B). Neither expression of these markers (Fig. 5B), nor the numbers of NK cells in the lung (Fig. 5C) was altered over the first two days after infection with K. pneumoniae.
Figure 5.
Lung NK cells show conventional, mature surface phenotype. Lung cells were isolated from uninfected and K. pneumoniae-infected C57BL/6 wild-type mice. (A) Surface expression of NK1.1, NKp46 and CCR6 in CD45+CD3− lung cells of uninfected mice. Data are from one mouse, representative of three mice from one experiment, representative of two experiments performed. (B) Expression of KLRG1 and CD27 on NK cells (CD45+CD3−NK1.1+) from lung versus spleen in uninfected mice or mice at Day 1 and Day 2 after infection by inoculation into the trachea of 104 CFU of K. pneumoniae or normal saline alone. Cells from the lungs are shown using a solid line histogram and cells from the spleens are shown using a dashed line histogram. “Day 0” data are from mice inoculated with normal saline alone and killed on Day 1. Data are of cells pooled from 3 mice per group in one representative experiment of two performed. (C) Numbers of lung NK cells (CD45+CD3−NK1.1+) in uninfected mice or mice at Day 1 and Day 2 after infection. “Day 0” data are from mice inoculated with normal saline alone and killed on Day 1. Data are from 5 mice per group in one representative experiment of two performed.
A subset of lung NK cells produces IL-22 early after infection with K. pneumoniae
Our NK cell-depletion experiments suggested a direct and/or indirect contribution of NK cells to IL-22 production in the K. pneumoniae-infected lungs. In order to examine whether or not lung NK cells have the ability to produce IL-22 early after infection, intracellular staining of IL-22 on lung NK cells and T cells was performed following ex vivo stimulation of cells isolated from lungs after two days of infection as well as from lungs of uninfected mice. In wild-type animals, IL-22 was detected in lung T cells by intracellular staining (Fig. 6A), although only in cells taken from infected mice. These data also show that the infected lungs do not contain a significant population of CD3+NK1.1+ cells (Fig. 6A, left panel). Just as for lung T cells, lung NK cells of uninfected wild-type mice were unable to produce IL-22, whereas a subset of NK cells from K. pneumoniae-infected mice were competent to produce IL-22 at Day 2 after infection (Fig. 6B). Similarly, only the NK cells taken from infected lungs were able to produce IFN-γ and the IL-22-producing NK cells co-express IFN-γ (Supplemental Fig. 3). In fact, a subset of lung NK cells – but not NK cells in the spleen or in the mediastinal lymph nodes - acquired the ability to produce IL-22 as early as 24 h after infection (Supplemental Fig. 4).
Figure 6.
A subset of lung NK cells produces IL-22 after K. pneumoniae infection. (A) Wild-type C57BL/6 mice were inoculated in the trachea with 104 CFU of K. pneumoniae or normal saline alone. Two days after infection, lung cells were isolated and activated with PMA/ionomycin for 4 h, and intracellular staining was done to detect IL-22 in lung T cells. Left panel: Dot plot for identifying lung T and NK cells. Right panel: Intracellular staining for IL-22 in lung T cells (CD45+CD3+) from uninfected and infected WT mice. (B) Wild-type C57BL/6 mice (WT) and Rag2−/− mice were inoculated in the trachea with 104 CFU of K. pneumoniae or normal saline alone. BALB/c Il22−/− mice (Il22−/−), used as a negative control for IL-22 staining, were inoculated in the trachea with 103 CFU of K. pneumoniae. Two days after infection, lung cells were isolated and activated with PMA/ionomycin for 4 h, and intracellular staining was done to detect IL-22 in lung NK cells. Staining is shown for CD45+CD3−NK1.1+ cells from WT mice and Rag2−/−mice, and for CD45+CD3− DX5+ cells from Il22−/− mice. Gating to identify IL-22+ cells was set based on staining of the cells from the Il22−/− mice. Data are of cells pooled from 4-5 WT or Rag2−/− mice and 3 Il22−/− mice in one representative experiment of two performed. (C) C57BL/6 wild-type mice (WT, filled bar) and Rag2−/− mice (open bar) were inoculated in the trachea with 104 CFU of K. pneumoniae or normal saline alone. Two days after infection, lung cells were isolated to quantify numbers of NK cells (CD45+CD3−NK1.1+). “Day 0” data are from mice inoculated with normal saline alone. Data are from 4-5 mice per group in one representative experiment of two performed. **p < 0.01 for the Rag2−/− versus wild-type mice.
Although the number of lung NK cells was about 50-70% of the number of lung T cells in infected animals, the frequency of IL-22 expressing NK cells was approximately 3-5 fold of that in T cells. Similarly, we presumed that NK cells could be responsible for the production of IL-22 after infection of the Rag2−/− mice. As shown in Fig. 6B, infection induced NK cells to become competent to produce IL-22 in the Rag2−/− mice at a frequency comparable to the frequency in wild-type mice. In addition, as has been described previously (47, 48), the Rag2−/− mice contained significantly more NK cells in the lung as compared to wild-type mice (Fig. 6C, p < 0.01 and p = 0.01 at Day 0 and Day 2, respectively), although here too infection did not alter lung NK cell numbers. Taken together, these data suggest that NK cells could be induced to produce IL-22 early after infection, that such induction did not require T cells, and that lung NK cells could be a source of IL-22 early after infection in both Rag2−/−, and, more importantly, in wild-type mice.
Discussion
This study has focused on the role of lung NK cells in the production of IL-22 and host defense in a model of bacterial pneumonia using the gram-negative pathogen, K. pneumoniae. Previous work has shown that pre-treatment with anti-IL-22 antibody resulted in 100% mortality after 24 h of infection (1). Consistent with these findings, we demonstrated that Il22−/− mice had decreased survival as compared with wild type mice, although our results showed a less dramatic effect of eliminating IL-22 than the report using antibody-dependent neutralization. We are not able explain the difference in the magnitudes of the effects. One possibly relevant difference between the antibody neutralization experiments and our own was that the former were done in C57BL/6 mice, whereas the Il22−/− mice we used were in the BALB/c background (and were compared with BALB/c wild-type mice). Another possible factor, of course, is that the life-long absence of IL-22 produced effects that partially mitigated the lack of IL-22 during K. pneumoniae infection. In any case, the data establish a critical role for IL-22 in host defense during K. pneumoniae pneumonia.
Published data using models of infection with K. pneumoniae or other pathogens suggest several mechanisms whereby IL-22 mediates protection of the host through activities both at epithelial surfaces and systemically. These include stimulating epithelial cell proliferation, enhancing the integrity of the epithelial barrier, activating an acute phase response, and inducing the production of antimicrobial peptides and immune mediators such as chemokines and cytokines(1, 15, 18, 49-52). In combination, these activities limit microbial replication and invasion (52-54).
Although the IL-22 produced during K. pneumoniae pneumonia has been proposed to be of T cell origin (1), we found no differences in survival or lung bacterial burden or in lung IL-22 in Rag2−/− vs. wild-type mice. On the other hand, host defense was significantly compromised in Rag2−/−Il2rg−/− mice, which showed reduced survival and increased bacteria in the lung, and produced no detectable IL-22. Together these results suggested that an ILC contributed to control of pulmonary infection with K. pneumoniae through mechanisms that were, at least in part, IL-22-dependent.
We tested this possibility by eliminating NK cells using anti-ASGM1. Treatment with anti-ASGM1 resulted in increased mortality and bacterial burdens in lung, as well as blood and liver, associated with diminished induction of IL-22 and other protective cytokines. Given that IL-22 is of particular importance in maintaining barrier integrity and limiting bacterial invasion (1, 15, 52), a deficiency of IL-22 might have contributed to the extrapulmonary dissemination seen in the NK-cell depleted mice. NK cells have also been reported to limit bacterial dissemination through mechanisms that are IL-22-independent (32).
Anti-ASGM1 is used routinely for NK cell depletion, and has the advantage of being depleting without being activating (38). Nonetheless, there are reports of ASGM1 being expressed on various non-NK cells, and/or of anti-ASGM1 depleting non-NK cells in certain experimental models (55-58). For this and other reasons, it was informative to demonstrate that NK cells isolated from infected lungs were able to produce IL-22. Acquiring this ability occurred as soon as one day after infection and was not T-cell dependent, since IL-22 could be made by the NK cells from both wild-type and Rag2−/− mice. Taken together, these data suggested that NK cells could serve as an early and direct source of IL-22 in the lungs of infected animals. NK cells might also contribute to the production of IL-22 through indirect effects mediated by IL-6 and TNF-α NK cell products (59) that can induce IL-22 in T cells (60) Consistent with this hypothesis, we found that depletion of NK cells also led to decreases in expression of Il6 and Tnfa in infected lungs. The diminished expression of Tnfa expression might have had broader consequences, since TNF-α has been shown to synergize with IL-22 in promoting an inflammatory response (61, 62), and the loss of TNF-α might have compounded the effects of decreased IL-22 in the infected, NK cell-depleted mice.
It is of interest that, even after pharmacological activation ex vivo, NK cells were only able to produce IL-22 if isolated from infected, but not non-infected lungs. Similarly, only the NK cells taken from infected lungs were able to produce detectable IFN-γ, consistent with reports in other models of infection (63, 64). Because we found no increase in NK cell number in the lungs during infection, these data are consistent with changes in NK cell functionality occurring in situ rather than the recruitment of new, IL-22-producing NK cells. In addition, the surface phenotype of the lung NK cells, which suggested that they were more mature than the NK cells in the spleen, did not change during the infection.
Within the T cell population, in addition to Th17 cells (and the Th22 cells in human skin), it has been reported that lung invariant natural killer T cells (iNKT) are able to produce IL-22 (65). Nonetheless, we do not believe that iNKT cells were important in our experiments, since we found very few NKT cells in lungs of naïve or K. pneumoniae-infected mice. Other candidates as sources of IL-22 include non-NK cell populations of ILCs. A subset of lung resident ILCs (Lin−CD90+CD25+) capable of producing IL-22 was recently identified in both human and mouse (21). A second report found very few Lin−CD90+ ILCs in lungs of naïve mice (47), and in our experiments we failed to detect a clear population of these cells in the lung either before or after infection with K. pneumoniae.
Other innate lymphocytes of possible relevance are the NK22/ILC22 cells described in the intestinal tract. We found no cells, either in uninfected or infected lungs, with the surface phenotype NKp46+CCR6+NK1.1-negative or low, which is characteristic of NK22/ILC22 cells. On the contrary, we found that all the lung NK cells showed a conventional phenotype. Moreover, the IL-22 producing NK cells from infected lungs were also able to make IFN-γ, in contrast to ILC22 cells (36). Our data are consistent with recent reports in an influenza virus model (15, 66), in which IL-22 was produced by conventional lung NK cells, which did not express RORγt and were absent from Il15ra−/− mice (15). However, these two reports differed somewhat in their findings on the importance of IL-22 in host defense, since in one study neutralizing IL-22 had little effect on clinical outcomes (66), while in the second study Il22−/− mice showed persistent weight loss after influenza infection (15). A third report found that NK cells were not a source of IL-22 after influenza infection, and the authors attributed their apparently discrepant findings to differences in strains of influenza virus (67). Although we showed that NK cells are an important component of host defense in the K. pneumoniae pneumonia model, the Rag2−/−Il2rg−/− mice died earlier and in greater numbers than the NK-cell depleted animals. Given the apparent lack of an effect of eliminating T cells, as demonstrated using the Rag2−/− mice, this finding suggests potential contributions from non-NK, non-T, γc-dependent cells. It is also possible, however, that T cells provided functions in the wild-type, NK-cell depleted mice that were not apparent when comparing Rag2−/− versus wild-type animals. This latter alternative is plausible given the expansion of the NK cell population that we found in the lungs of the Rag2−/− mice, which may have masked any deficit in IL-22 and other components of host defense resulting from the absence of T cells.
There are only a small number of studies on roles for NK cells in bacterial infections of the lung, particularly with regard to extracellular organisms, and both protective and deleterious effects have been described, depending on the model (68). NK cell production of IFN-γ and TNF-α has been implicated in those cases in which NK cells have beneficial activities (68). Our study shows that during pulmonary infection of mice with K. pneumoniae, NK cells are required for optimal host defense, which includes the production of IL-22 in the lung. As far as we are aware, this is the first description of a role for conventional NK cells in producing IL-22 in host defense against extracellular bacteria.
These observations may have clinical relevance. In cases of infection with multi-drug resistant K. pneumoniae, not only are there few treatment options among the available antimicrobials, but in colonized and susceptible patients, the adaptive immune system has often been severely compromised, either secondary to underlying disease or iatrogenic factors (10). Based on our data, it is possible that augmenting NK cell function might provide therapeutic benefit in this infection, as has been reported in mouse models of viral infection (69) and bacterial sepsis (70). Identifying the factors responsible for enabling NK cells to become producers of IL-22, IFN-γ and other protective cytokines in response to infection with K. pneumoniae may provide avenues worth pursuing in this regard.
Supplementary Material
Footnotes
This work was supported by the Intramural Research Program of the National Institute of Allergy and Infectious Diseases.
Abbreviation used in this paper: ILC, innate lymphoid cell
References
- 1.Aujla SJ, Chan YR, Zheng M, Fei M, Askew DJ, Pociask DA, Reinhart TA, McAllister F, Edeal J, Gaus K, Husain S, Kreindler JL, Dubin PJ, Pilewski JM, Myerburg MM, Mason CA, Iwakura Y, Kolls JK. IL-22 mediates mucosal host defense against Gram-negative bacterial pneumonia. Nat. Med. 2008;14:275–281. doi: 10.1038/nm1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ye P, Garvey PB, Zhang P, Nelson S, Bagby G, Summer WR, Schwarzenberger P, Shellito JE, Kolls JK. Interleukin-17 and lung host defense against Klebsiella pneumoniae infection. Am. J. Respir. Cell Mol. Biol. 2001;25:335–340. doi: 10.1165/ajrcmb.25.3.4424. [DOI] [PubMed] [Google Scholar]
- 3.Zenewicz LA, Flavell RA. Recent advances in IL-22 biology. Int. Immunol. 2011;23:159–163. doi: 10.1093/intimm/dxr001. [DOI] [PubMed] [Google Scholar]
- 4.Kudva A, Scheller EV, Robinson KM, Crowe CR, Choi SM, Slight SR, Khader SA, Dubin PJ, Enelow RI, Kolls JK, Alcorn JF. Influenza A inhibits Th17-mediated host defense against bacterial pneumonia in mice. J. Immunol. 2011;186:1666–1674. doi: 10.4049/jimmunol.1002194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fernandes BF, Rezende AB, Alves CC, Teixeira FM, Farias RE, Ferreira AP, Teixeira HC. Splenic autotransplantation restores IL-17 production and antibody response to Streptococcus pneumoniae in splenectomized mice. Transpl. Immunol. 2010;22:195–197. doi: 10.1016/j.trim.2009.12.002. [DOI] [PubMed] [Google Scholar]
- 6.Happel KI, Zheng M, Young E, Quinton LJ, Lockhart E, Ramsay AJ, Shellito JE, Schurr JR, Bagby GJ, Nelson S, Kolls JK. Cutting edge: roles of Toll-like receptor 4 and IL-23 in IL-17 expression in response to Klebsiella pneumoniae infection. J. Immunol. 2003;170:4432–4436. doi: 10.4049/jimmunol.170.9.4432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hirsch EB, Tam VH. Detection and treatment options for Klebsiella pneumoniae carbapenemases (KPCs): an emerging cause of multidrug-resistant infection. J. Antimicrob. Chemother. 2010;65:1119–1125. doi: 10.1093/jac/dkq108. [DOI] [PubMed] [Google Scholar]
- 8.Bradford PA, Bratu S, Urban C, Visalli M, Mariano N, Landman D, Rahal JJ, Brooks S, Cebular S, Quale J. Emergence of carbapenem-resistant Klebsiella species possessing the class A carbapenem-hydrolyzing KPC-2 and inhibitor-resistant TEM-30 beta-lactamases in New York City. Clin. Infect. Dis. 2004;39:55–60. doi: 10.1086/421495. [DOI] [PubMed] [Google Scholar]
- 9.Gupta N, Limbago BM, Patel JB, Kallen AJ. Carbapenem-resistant Enterobacteriaceae: epidemiology and prevention. Clin. Infect. Dis. 2011;53:60–67. doi: 10.1093/cid/cir202. [DOI] [PubMed] [Google Scholar]
- 10.Snitkin ES, Zelazny AM, Thomas PJ, Stock F, Henderson DK, Palmore TN, Segre JA. Tracking a hospital outbreak of carbapenem-resistant Klebsiella pneumoniae with whole-genome sequencing. Sci. Transl. Med. 2012;4:148ra116. doi: 10.1126/scitranslmed.3004129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wolk K, Kunz S, Asadullah K, Sabat R. Cutting edge: immune cells as sources and targets of the IL-10 family members? J. Immunol. 2002;168:5397–5402. doi: 10.4049/jimmunol.168.11.5397. [DOI] [PubMed] [Google Scholar]
- 12.Wolk K, Sabat R. Interleukin-22: a novel T- and NK-cell derived cytokine that regulates the biology of tissue cells. Cytokine Growth Factor Rev. 2006;17:367–380. doi: 10.1016/j.cytogfr.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 13.Pestka S, Krause CD, Sarkar D, Walter MR, Shi Y, Fisher PB. Interleukin-10 and related cytokines and receptors. Annu. Rev. Immunol. 2004;22:929–979. doi: 10.1146/annurev.immunol.22.012703.104622. [DOI] [PubMed] [Google Scholar]
- 14.Whittington HA, Armstrong L, Uppington KM, Millar AB. Interleukin-22: a potential immunomodulatory molecule in the lung. Am. J. Respir. Cell Mol. Biol. 2004;31:220–226. doi: 10.1165/rcmb.2003-0285OC. [DOI] [PubMed] [Google Scholar]
- 15.Kumar P, Thakar MS, Ouyang W, Malarkannan S. IL-22 from conventional NK cells is epithelial regenerative and inflammation protective during influenza infection. Mucosal. Immunol. 2013;6:69–82. doi: 10.1038/mi.2012.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zheng Y, Danilenko DM, Valdez P, Kasman I, Eastham-Anderson J, Wu J, Ouyang W. Interleukin-22, a T(H)17 cytokine, mediates IL-23-induced dermal inflammation and acanthosis. Nature. 2007;445:648–651. doi: 10.1038/nature05505. [DOI] [PubMed] [Google Scholar]
- 17.Satoh-Takayama N, Vosshenrich CA, Lesjean-Pottier S, Sawa S, Lochner M, Rattis F, Mention JJ, Thiam K, Cerf-Bensussan N, Mandelboim O, Eberl G, Di Santo JP. Microbial flora drives interleukin 22 production in intestinal NKp46+ cells that provide innate mucosal immune defense. Immunity. 2008;29:958–970. doi: 10.1016/j.immuni.2008.11.001. [DOI] [PubMed] [Google Scholar]
- 18.Zheng Y, Valdez PA, Danilenko DM, Hu Y, Sa SM, Gong Q, Abbas AR, Modrusan Z, Ghilardi N, de Sauvage FJ, Ouyang W. Interleukin-22 mediates early host defense against attaching and effacing bacterial pathogens. Nat. Med. 2008;14:282–289. doi: 10.1038/nm1720. [DOI] [PubMed] [Google Scholar]
- 19.Cella M, Fuchs A, Vermi W, Facchetti F, Otero K, Lennerz JK, Doherty JM, Mills JC, Colonna M. A human natural killer cell subset provides an innate source of IL-22 for mucosal immunity. Nature. 2009;457:722–725. doi: 10.1038/nature07537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hedrick MN, Lonsdorf AS, Shirakawa AK, Richard Lee CC, Liao F, Singh SP, Zhang HH, Grinberg A, Love PE, Hwang ST, Farber JM. CCR6 is required for IL-23-induced psoriasis-like inflammation in mice. J. Clin. Invest. 2009;119:2317–2329. doi: 10.1172/JCI37378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Monticelli LA, Sonnenberg GF, Abt MC, Alenghat T, Ziegler CG, Doering TA, Angelosanto JM, Laidlaw BJ, Yang CY, Sathaliyawala T, Kubota M, Turner D, Diamond JM, Goldrath AW, Farber DL, Collman RG, Wherry EJ, Artis D. Innate lymphoid cells promote lung- tissue homeostasis after infection with influenza virus. Nat. Immunol. 2011;12:1045–1054. doi: 10.1031/ni.2131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sonnenberg GF, Monticelli LA, Elloso MM, Fouser LA, Artis D. CD4(+) lymphoid tissue-inducer cells promote innate immunity in the gut. Immunity. 2011;34:122–134. doi: 10.1016/j.immuni.2010.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Spits H, Di Santo JP. The expanding family of innate lymphoid cells: regulators and effectors of immunity and tissue remodeling. Nat. Immunol. 2011;12:21–27. doi: 10.1038/ni.1962. [DOI] [PubMed] [Google Scholar]
- 24.Orange JS, Wang B, Terhorst C, Biron CA. Requirement for natural killer cell-produced interferon gamma in defense against murine cytomegalovirus infection and enhancement of this defense pathway by interleukin 12 administration. J. Exp. Med. 1995;182:1045–1056. doi: 10.1084/jem.182.4.1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tay CH, Szomolanyi-Tsuda E, Welsh RM. Control of infections by NK cells. Curr. Top. Microbiol. Immunol. 1998;230:193–220. doi: 10.1007/978-3-642-46859-9_12. [DOI] [PubMed] [Google Scholar]
- 26.Vankayalapati R, Garg A, Porgador A, Griffith DE, Klucar P, Safi H, Girard WM, Cosman D, Spies T, Barnes PF. Role of NK cell-activating receptors and their ligands in the lysis of mononuclear phagocytes infected with an intracellular bacterium. J. Immunol. 2005;175:4611–4617. doi: 10.4049/jimmunol.175.7.4611. [DOI] [PubMed] [Google Scholar]
- 27.Weiss ID, Wald O, Wald H, Beider K, Abraham M, Galun E, Nagler A, Peled A. IFN-gamma treatment at early stages of influenza virus infection protects mice from death in a NK cell-dependent manner. J. Interferon. Cytokine. Res. 2010;30:439–449. doi: 10.1089/jir.2009.0084. [DOI] [PubMed] [Google Scholar]
- 28.Lodoen MB, Lanier LL. Natural killer cells as an initial defense against pathogens. Curr. Opin. Immunol. 2006;18:391–398. doi: 10.1016/j.coi.2006.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Small CL, McCormick S, Gill N, Kugathasan K, Santosuosso M, Donaldson N, Heinrichs DE, Ashkar A, Xing Z. NK cells play a critical protective role in host defense against acute extracellular Staphylococcus aureus bacterial infection in the lung. J. Immunol. 2008;180:5558–5568. doi: 10.4049/jimmunol.180.8.5558. [DOI] [PubMed] [Google Scholar]
- 30.Borchers MT, Harris NL, Wesselkamper SC, Zhang S, Chen Y, Young L, Lau GW. The NKG2D-activating receptor mediates pulmonary clearance of Pseudomonas aeruginosa. Infect. Immun. 2006;74:2578–2586. doi: 10.1128/IAI.74.5.2578-2586.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wesselkamper SC, Eppert BL, Motz GT, Lau GW, Hassett DJ, Borchers MT. NKG2D is critical for NK cell activation in host defense against Pseudomonas aeruginosa respiratory infection. J. Immunol. 2008;181:5481–5489. doi: 10.4049/jimmunol.181.8.5481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hall LJ, Murphy CT, Hurley G, Quinlan A, Shanahan F, Nally K, Melgar S. Natural killer cells protect against mucosal and systemic infection with the enteric pathogen Citrobacter rodentium. Infect. Immun. 2013;81:460–469. doi: 10.1128/IAI.00953-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Reid-Yu SA, Small CL, Coombes BK. CD3(−) NK1.1(+) cells aid in the early induction of a Th1 response to an attaching and effacing enteric pathogen. Eur. J. Immunol. 2013;43:2638–2649. doi: 10.1002/eji.201343435. [DOI] [PubMed] [Google Scholar]
- 34.Small CL, Shaler CR, McCormick S, Jeyanathan M, Damjanovic D, Brown EG, Arck P, Jordana M, Kaushic C, Ashkar AA, Xing Z. Influenza infection leads to increased susceptibility to subsequent bacterial superinfection by impairing NK cell responses in the lung. J. Immunol. 184:2048–2056. doi: 10.4049/jimmunol.0902772. [DOI] [PubMed] [Google Scholar]
- 35.Kupz A, Scott TA, Belz GT, Andrews DM, Greyer M, Lew AM, Brooks AG, Smyth MJ, Curtiss R, 3rd, Bedoui S, Strugnell RA. Contribution of Thy1+ NK cells to protective IFN-gamma production during Salmonella typhimurium infections. Proc. Natl. Acad. Sci. USA. 2013;110:2252–2257. doi: 10.1073/pnas.1222047110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vivier E, Raulet DH, Moretta A, Caligiuri MA, Zitvogel L, Lanier LL, Yokoyama WM, Ugolini S. Innate or adaptive immunity? The example of natural killer cells. Science. 2011;331:44–49. doi: 10.1126/science.1198687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Horowitz A, Stegmann KA, Riley EM. Activation of natural killer cells during microbial infections. Front. Immunol. 2011;2:88. doi: 10.3389/fimmu.2011.00088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Scott MJ, Hoth JJ, Gardner SA, Peyton JC, Cheadle WG. Natural killer cell activation primes macrophages to clear bacterial infection. Am. Surg. 2003;69:679–686. [PubMed] [Google Scholar]
- 39.Tsai WC, Strieter RM, Wilkowski JM, Bucknell KA, Burdick MD, Lira SA, Standiford TJ. Lung-specific transgenic expression of KC enhances resistance to Klebsiella pneumoniae in mice. J. Immunol. 1998;161:2435–2440. [PubMed] [Google Scholar]
- 40.Yoshida K, Matsumoto T, Tateda K, Uchida K, Tsujimoto S, Iwakurai Y, Yamaguchi K. Protection against pulmonary infection with Klebsiella pneumoniae in mice by interferon-gamma through activation of phagocytic cells and stimulation of production of other cytokines. J. Med. Microbiol. 2001;50:959–964. doi: 10.1099/0022-1317-50-11-959. [DOI] [PubMed] [Google Scholar]
- 41.Ruan S, Young E, Luce MJ, Reiser J, Kolls JK, Shellito JE. Conditional expression of interferon-gamma to enhance host responses to pulmonary bacterial infection. Pulm. Pharmacol. Ther. 2006;19:251–257. doi: 10.1016/j.pupt.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 42.Moore TA, Perry ML, Getsoian AG, Newstead MW, Standiford TJ. Divergent role of gamma interferon in a murine model of pulmonary versus systemic Klebsiella pneumoniae infection. Infect. Immun. 2002;70:6310–6318. doi: 10.1128/IAI.70.11.6310-6318.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Laichalk LL, Kunkel SL, Strieter RM, Danforth JM, Bailie MB, Standiford TJ. Tumor necrosis factor mediates lung antibacterial host defense in murine Klebsiella pneumonia. Infect. Immun. 1996;64:5211–5218. doi: 10.1128/iai.64.12.5211-5218.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sutherland RE, Olsen JS, McKinstry A, Villalta SA, Wolters PJ. Mast cell IL-6 improves survival from Klebsiella pneumonia and sepsis by enhancing neutrophil killing. J. Immunol. 2008;181:5598–5605. doi: 10.4049/jimmunol.181.8.5598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Huntington ND, Tabarias H, Fairfax K, Brady J, Hayakawa Y, Degli- Esposti MA, Smyth MJ, Tarlinton DM, Nutt SL. NK cell maturation and peripheral homeostasis is associated with KLRG1 up-regulation. J. Immunol. 2007;178:4764–4770. doi: 10.4049/jimmunol.178.8.4764. [DOI] [PubMed] [Google Scholar]
- 46.Sun JC, Beilke JN, Lanier LL. Adaptive immune features of natural killer cells. Nature. 2009;457:557–561. doi: 10.1038/nature07665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Le-Barillec K, Magalhaes JG, Corcuff E, Thuizat A, Sansonetti PJ, Phalipon A, Di Santo JP. Roles for T and NK cells in the innate immune response to Shigella flexneri. J. Immunol. 2005;175:1735–1740. doi: 10.4049/jimmunol.175.3.1735. [DOI] [PubMed] [Google Scholar]
- 48.Grundy MA, Sentman CL. Immunodeficient mice have elevated numbers of NK cells in non-lymphoid tissues. Exp. Cell Res. 2006;312:3920–3926. doi: 10.1016/j.yexcr.2006.08.019. [DOI] [PubMed] [Google Scholar]
- 49.Wolk K, Kunz S, Witte E, Friedrich M, Asadullah K, Sabat R. IL-22 increases the innate immunity of tissues. Immunity. 2004;21:241–254. doi: 10.1016/j.immuni.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 50.Liang SC, Tan XY, Luxenberg DP, Karim R, Dunussi-Joannopoulos K, Collins M, Fouser LA. Interleukin (IL)-22 and IL-17 are coexpressed by Th17 cells and cooperatively enhance expression of antimicrobial peptides. J. Exp. Med. 2006;203:2271–2279. doi: 10.1084/jem.20061308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Liang SC, Nickerson-Nutter C, Pittman DD, Carrier Y, Goodwin DG, Shields KM, Lambert AJ, Schelling SH, Medley QG, Ma HL, Collins M, Dunussi-Joannopoulos K, Fouser LA. IL-22 induces an acute-phase response. J. Immunol. 2010;185:5531–5538. doi: 10.4049/jimmunol.0904091. [DOI] [PubMed] [Google Scholar]
- 52.Rutz S, Eidenschenk C, Ouyang W. IL-22, not simply a Th17 cytokine. Immunol. Rev. 2013;252:116–132. doi: 10.1111/imr.12027. [DOI] [PubMed] [Google Scholar]
- 53.Sonnenberg GF, Fouser LA, Artis D. Border patrol: regulation of immunity, inflammation and tissue homeostasis at barrier surfaces by IL-22. Nat. Immunol. 2011;12:383–390. doi: 10.1038/ni.2025. [DOI] [PubMed] [Google Scholar]
- 54.Sonnenberg GF, Monticelli LA, Alenghat T, Fung TC, Hutnick NA, Kunisawa J, Shibata N, Grunberg S, Sinha R, Zahm AM, Tardif MR, Sathaliyawala T, Kubota M, Farber DL, Collman RG, Shaked A, Fouser LA, Weiner DB, Tessier PA, Friedman JR, Kiyono H, Bushman FD, Chang KM, Artis D. Innate lymphoid cells promote anatomical containment of lymphoid-resident commensal bacteria. Science. 2012;336:1321–1325. doi: 10.1126/science.1222551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wiltrout RH, Santoni A, Peterson ES, Knott DC, Overton WR, Herberman RB, Holden HT. Reactivity of anti-asialo GM1 serum with tumoricidal and non-tumoricidal mouse macrophages. J. Leukoc. Biol. 1985;37:597–614. doi: 10.1002/jlb.37.5.597. [DOI] [PubMed] [Google Scholar]
- 56.Lee U, Santa K, Habu S, Nishimura T. Murine asialo GM1+CD8+ T cells as novel interleukin-12-responsive killer T cell precursors. Jpn. J. Cancer. Res. 1996;87:429–432. doi: 10.1111/j.1349-7006.1996.tb00241.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nishikado H, Mukai K, Kawano Y, Minegishi Y, Karasuyama H. NK cell-depleting anti-asialo GM1 antibody exhibits a lethal off-target effect on basophils in vivo. J. Immunol. 2011;186:5766–5771. doi: 10.4049/jimmunol.1100370. [DOI] [PubMed] [Google Scholar]
- 58.Schmitt DM, O'Dee DM, Brown MJ, Horzempa J, Russo BC, Morel PA, Nau GJ. Role of NK cells in host defense against pulmonary type A Francisella tularensis infection. Microbes. Infect. 2013;15:201–211. doi: 10.1016/j.micinf.2012.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hall LJ, Clare S, Dougan G. NK cells influence both innate and adaptive immune responses after mucosal immunization with antigen and mucosal adjuvant. J. Immunol. 2010;184:4327–4337. doi: 10.4049/jimmunol.0903357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ouyang W, Rutz S, Crellin NK, Valdez PA, Hymowitz SG. Regulation and functions of the IL-10 family of cytokines in inflammation and disease. Annu. Rev. Immunol. 2011;29:71–109. doi: 10.1146/annurev-immunol-031210-101312. [DOI] [PubMed] [Google Scholar]
- 61.Eyerich S, Eyerich K, Pennino D, Carbone T, Nasorri F, Pallotta S, Cianfarani F, Odorisio T, Traidl-Hoffmann C, Behrendt H, Durham SR, Schmidt-Weber CB, Cavani A. Th22 cells represent a distinct human T cell subset involved in epidermal immunity and remodeling. J. Clin. Invest. 2009;119:3573–3585. doi: 10.1172/JCI40202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Guilloteau K, Paris I, Pedretti N, Boniface K, Juchaux F, Huguier V, Guillet G, Bernard FX, Lecron JC, Morel F. Skin Inflammation Induced by the Synergistic Action of IL-17A, IL-22, Oncostatin M, IL-1{alpha}, and TNF-{alpha} Recapitulates Some Features of Psoriasis. J. Immunol. 2010;184:5263–5270. doi: 10.4049/jimmunol.0902464. [DOI] [PubMed] [Google Scholar]
- 63.Lopez MC, Duckett NS, Baron SD, Metzger DW. Early activation of NK cells after lung infection with the intracellular bacterium, Francisella tularensis LVS. Cell. Immunol. 2004;232:75–85. doi: 10.1016/j.cellimm.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 64.Byrne P, McGuirk P, Todryk S, Mills KH. Depletion of NK cells results in disseminating lethal infection with Bordetella pertussis associated with a reduction of antigen-specific Th1 and enhancement of Th2, but not Tr1 cells. Eur. J. Immunol. 2004;34:2579–2588. doi: 10.1002/eji.200425092. [DOI] [PubMed] [Google Scholar]
- 65.Paget C, Ivanov S, Fontaine J, Renneson J, Blanc F, Pichavant M, Dumoutier L, Ryffel B, Renauld JC, Gosset P, Si-Tahar M, Faveeuw C, Trottein F. Interleukin-22 is produced by invariant natural killer T lymphocytes during influenza A virus infection: potential role in protection against lung epithelial damages. J. Biol. Chem. 2012;287:8816–8829. doi: 10.1074/jbc.M111.304758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Guo H, Topham DJ. Interleukin-22 (IL-22) production by pulmonary Natural Killer cells and the potential role of IL-22 during primary influenza virus infection. J. Virol. 2010;84:7750–7759. doi: 10.1128/JVI.00187-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ivanov S, Renneson J, Fontaine J, Barthelemy A, Paget C, Fernandez EM, Blanc F, De Trez C, Van Maele L, Dumoutier L, Huerre MR, Eberl G, Si-Tahar M, Gosset P, Renauld JC, Sirard JC, Faveeuw C, Trottein F. Interleukin-22 reduces lung inflammation during influenza A virus infection and protects against secondary bacterial infection. J.Virol. 2013;87:6911–6924. doi: 10.1128/JVI.02943-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Culley FJ. Natural killer cells in infection and inflammation of the lung. Immunology. 2009;128:151–163. doi: 10.1111/j.1365-2567.2009.03167.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gibbert K, Joedicke JJ, Meryk A, Trilling M, Francois S, Duppach J, Kraft A, Lang KS, Dittmer U. Interferon-alpha subtype 11 activates NK cells and enables control of retroviral infection. PLoS Patho. 2012;8:e1002868. doi: 10.1371/journal.ppat.1002868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Inoue S, Unsinger J, Davis CG, Muenzer JT, Ferguson TA, Chang K, Osborne DF, Clark AT, Coopersmith CM, McDunn JE, Hotchkiss RS. IL-15 prevents apoptosis, reverses innate and adaptive immune dysfunction, and improves survival in sepsis. J. Immunol. 2010;184:1401–1409. doi: 10.4049/jimmunol.0902307. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.