Abstract
Fibrodysplasia ossificans progressiva (FOP), a rare and disabling genetic condition characterized by congenital malformations of the great toes and progressive heterotopic endochondral ossification (HEO) which is the most catastrophic of HEO disorders in humans. Flare-ups of FOP are episodic; immobility is cumulative. Heterozygous activating mutations in activin receptor IA/activin-like kinase-2 (ACVR1/ALK2), a bone morphogenetic protein (BMP) type I receptor, exist in all sporadic and familial cases of FOP. The discovery of the FOP gene established a critical milestone in our understanding FOP, and revealed a highly conserved therapeutic target in the BMP signaling pathway. This discovery has advanced efforts to develop novel therapies for this disabling disorder of tissue metamorphosis. While effective treatment for FOP will likely be based on interventions that modulate overactive ACVR1/ALK2 signaling, or that specifically block postnatal HEO, current management is focused on early diagnosis, assiduous avoidance of injury or iatrogenic harm, symptomatic amelioration of painful flare-ups, and optimization of residual function.
Keywords: fibrodysplasia ossificans progressiva, heterotopic ossification, activin receptor IA/activin-like kinase-2 (ACVR1/ALK2), BMP signaling
CLINICAL AND BASIC SCIENCE FEATURES OF FOP
Epidemiology and Clinical Features
Fibrodysplasia ossificans progressiva (FOP) is a rare and disabling disorder with a worldwide prevalence of approximately one in two million individuals. There is no ethnic, racial, gender, or geographic predisposition [1, 2].
Two clinical features define classic FOP: malformations of the great toes and progressive heterotopic endochondral ossification (HEO) in characteristic anatomic patterns. Individuals with FOP appear normal at birth except for malformations of the great toes that are present in all classically affected individuals. During the first decade of life, most children with FOP develop episodic, painful inflammatory soft tissue swellings (or flare-ups). While some flare-ups regress spontaneously, most transform soft connective tissues including aponeuroses, fascia, ligaments, tendons, and skeletal muscles into mature heterotopic bone. Ribbons, sheets, and plates of heterotopic bone replace skeletal muscles and connective tissues through a process of endochondral ossification that leads to an armament-like encasement of bone and permanent immobility. Minor trauma such as intramuscular immunizations, mandibular blocks for dental work, muscle fatigue, blunt muscle trauma from bumps, bruises, falls, or influenza-like viral illnesses can trigger painful new flare-ups of FOP leading to progressive heterotopic ossification (reviewed in [3]. Surgical removal of heterotopic bone provokes explosive and painful new bone growth.
HEO in FOP progresses in characteristic anatomic and temporal patterns that mimic the patterns of normal embryonic skeletal formation. Heterotopic ossification (HO) typically occurs first in the dorsal, axial, cranial, and proximal regions of the body and later seen in the ventral, appendicular, caudal, and distal regions [4]. Several skeletal muscles including the diaphragm, tongue, and extra-ocular muscles are spared from FOP. Cardiac muscle and smooth muscle are not involved.
HEO in FOP is episodic, but disability is cumulative [5]. Most patients with FOP are confined to a wheelchair by the third decade of life, and require lifelong assistance in performing activities of daily living. Severe weight loss may result following ankylosis of the jaw. Pneumonia or right-sided heart failure may complicate rigid fixation of the chest wall. The median age of survival is approximately 40 years, and death often results from complications of thoracic insufficiency syndrome [6].
Anomalies of the Normotopic Skeleton in FOP
While malformation of the great toes is characteristic of FOP, other developmental anomalies are frequently observed, particularly in the thumbs and cervical spine. Stiffness of the neck is an early finding in most patients and can precede the appearance of HEO at that site. Crawling is often limited due to lack of neck extension. Characteristic anomalies of the cervical spine include large posterior elements, tall narrow vertebral bodies, and fusion of the facet joints between C2 and C7. Although the cervical spine often becomes ankylosed early in life, any minimal residual movement may eventually result in painful arthritic symptoms [7].
Other skeletal anomalies associated with FOP include short malformed thumbs, clinodactyly, short broad femoral necks, and proximal medial tibial osteochondromas [8,9].
Radiographic Features of FOP
Radiographic and bone scan findings suggest normal modeling and remodeling of the heterotopic skeleton [10]. Bone scans are abnormal before conventional radiographs can detect HO. Computed tomography and magnetic resonance imaging of early lesions have been described. While these evaluation methods are generally superfluous from a diagnostic standpoint, they can provide a useful three-dimensional perspective of the disease process [11]. The definitive diagnosis of FOP can be made by simple clinical evaluation that associates rapidly appearing soft tissue lesions with malformations of the great toes [12, 13].
Histopathology of FOP Lesions
The histopathology of FOP lesions has been well described [14-17]. Early FOP lesions contain an intense mononuclear and perivascular infiltration of macrophages, mast cells, and lymphocytes. The precise roles of these cells in the evolution of FOP flare-ups are unknown, although focal inflammation from any cause is a known trigger of disease activity. Subsequent migration of mononuclear inflammatory cells into affected muscle precedes widespread death of skeletal muscle.
Following a rapid and destructive inflammatory stage, there is an intense fibroproliferative phase associated with robust angiogenesis and neovascularity. Early fibroproliferative lesions are histologically indistinguishable from aggressive juvenile fibromatosis. As lesions mature, fibroproliferative tissue undergoes an avascular condensation into cartilage followed by a revascularization stage with osteogenesis in a characteristic process of HEO. Resultant new ossicles of heterotopic bone appear histologically normal with mature lamellar bone and often contain marrow elements.
Mast cells have been identified at every histological stage of FOP lesion formation, and are found in much greater abundance compared with normal skeletal muscle and nonlesional FOP muscle. In fact, during the intense fibroproliferative stage of the lesion, mast cells are found at a density much higher than in any other inflammatory myopathy [18].
All stages of histological development are present in an active FOP lesion, suggesting that different regions within the lesion mature at different rates. Although heterotopic bone formation in FOP is similar in some respects to bone formation in embryonic skeletal development and postnatal fracture healing, an important difference is the lack of inflammation in primary skeletal formation.
More recent studies indicate that inflammatory cells of hematopoietic origin may be involved in the induction of heterotopic ossification [19] and contribute to the late osteogenic stage [20], while multipotent stem-like cells of vascular origin contribute to the fibroproliferative, chondrogenic and osteogenic stages of heterotopic ossification [21-23].
Laboratory Findings in FOP
Routine biochemical evaluations are usually normal, although the serum alkaline phosphatase activity and the erythrocyte sedimentation rate (ESR) may be increased, especially during disease flare-ups [24]. C-reactive protein elevation is a more specific test than ESR for monitoring the acute inflammatory phase of heterotopic ossification after spinal cord injury, but has not been studied in FOP [25]. Urinary basic fibroblast growth factor levels may be elevated during disease flare-ups coinciding with the pre-osseous angiogenic phase of early fibroproliferative lesions [25]. Circulating osteogenic cells may also herald early heterotopic bone formation, but remain a research tool [20].
The Immune System & FOP
Evidence from all levels of investigation suggests involvement of the innate immune system in FOP. The presence of macrophages, lymphocytes and mast cells in early FOP lesions, macrophage and lymphocyte-associated death of skeletal muscle, flare-ups following viral infections, the intermittent timing of flare-ups, and the beneficial response of early flare-ups to corticosteroids all support involvement of the innate immune system in the pathogenesis of FOP lesions [19, 27, 28]. Recent experimental studies in murine animal models strongly support the role of the innate immune system in inducing heterotopic ossification [22, 29, 30].
GENETICS OF FOP
Most cases of FOP arise as a result of a spontaneous new mutation. A paternal age effect has been reported [31]. When observed, genetic transmission is autosomal dominant and can be inherited from either mothers or fathers; maternal mosaicism may exist [32, 33]. Fewer than ten small multigenerational families are known worldwide [32]. Phenotypic heterogeneity is observed [34, 35].
Both genetic and environmental factors affect the phenotype of FOP. A study of three pairs of monozygotic twins with FOP found that within each pair, congenital toe malformations were identical. However, postnatal heterotopic ossification varied greatly depending on life history and environmental exposure to viral illnesses and to soft tissue trauma. Genetic determinants strongly influence disease phenotype during prenatal development while environmental factors strongly influence postnatal progression of heterotopic ossification [36].
The FOP Gene and the BMP Signaling Pathway
The classic and consistent FOP phenotype of great toe malformations and progressive HEO suggested that the primary molecular pathology might involve the bone morphogenetic protein (BMP) signaling pathway [37]. A number of seminal discoveries provided evidence of profound dysregulation of the BMP signaling pathway in cells from FOP patients [14, 38-49].
In order to identify the chromosomal locus for the FOP gene, a genome-wide linkage analysis was conducted using a subset of five families with the most stringent and unambiguous features of FOP. This approach linked FOP to the chromosome 2q23-24 region. The gene encoding activin receptor type IA/activin-like kinase 2 (ACVR1/ALK2), a BMP type I receptor, was identified in the linkage interval. DNA sequencing of the ACVR1/ALK2 gene determined that a recurrent heterozygous missense mutation in the glycine-serine (GS) activation domain (c.617G>A; R206H) occurs in all sporadic or familial classically affected individuals [8, 40, 43, 51-52]. Recently, additional mutations have been identified in the GS-domain and kinase domain of ACVR1 in individuals with atypical forms of FOP [8, 53-57]. Noggin mutations have been reported but are erroneous [58].
Structural and Functional Consequences of the FOP Mutation
Protein homology modeling of the mutated ACVR1/ALK2 receptor predicts destabilization of the glycine-serine (GS) activation domain, consistent with an overactive BMP signaling pathway as the underlying cause of the ectopic chondrogenesis, osteogenesis, and joint fusions seen in FOP [43, 59]. The identified mutation is consistent with previous findings of increased BMP signaling pathway in FOP cells and provides a rational basis for understanding both the postnatal heterotopic ossification and the congenital skeletal malformations that are ignominious signatures of this devastating disease. Models of protein structure are being developed to understand both inter-and intramolecular interactions of the mutant receptor [59].
The GS domain of all BMP type I receptors is a critical site for binding and activation of pathway-specific Smad signaling proteins and is a binding site of FKBP12 (also called FKBP1A), an inhibitory protein that prevents uninhibited low-level constitutive activation of the BMP type I receptor in the absence of ligand [60, 61]. FKBP12 also recruits a Smad – Smurf ubiquitin ligase complex that regulates the concentration of the receptor at the membrane [62]. Leaky activation of BMP signaling and accumulation of BMP type I receptors at the cell membrane in FOP cells supports aberrant association with FKBP12 in FOP [reviewed in [40]. FKBP12 binding to the GS domain is altered, leading to promiscuous ACVR1/ALK2 activity [38, 40, 63-67]. Exactly how the R206H mutation in ACVR1/ALK2 specifically perturbs BMP signaling in FOP is presently unknown but could involve dysregulation of BMP receptor oligomerization, internalization, degradation and/or intensity and duration of downstream signaling. This is presently the subject of intense investigation [64, 68].
Animal Models of FOP
Animal models of FOP are important in deciphering the pathophysiology of FOP and in testing possible therapies. Laboratory-generated animal models with some features of FOP have provided the opportunity to better understand the biology of BMP-associated heterotopic ossification and to study the effectiveness and safety of currently available and emerging therapies [22, 30, 38, 45, 69-72]. Development of a knock-in mouse model carrying the classic FOP disease-causing mutation in ACVR1/ALK2 is critical in order to establish specificity of treatment for FOP as well as investigate many previously unexplored aspects of the condition. Such a genetically engineered knock-in mouse is in progress [73, 74].
DIAGNOSIS OF FOP
FOP is commonly misdiagnosed as aggressive juvenile fibromatosis, lymphedema, or soft tissue sarcoma. Clinicians often fail to associate the rapidly developing soft tissue swellings that appear on the head, neck, and upper back with the malformed great toes. The misdiagnosis of FOP approaches 90 per cent of affected individuals worldwide [75]. Children often undergo unnecessary and harmful diagnostic biopsies that exacerbate the progression of the condition [76]. This can be particularly dangerous at any anatomic site, but especially so in the neck, back, and jaw where asymmetric HEO can lead to rapidly progressive spinal deformity, exacerbation of thoracic insufficiency syndrome, or rapid ankylosis of the temporomandibular joints. The high rate of misdiagnosis of FOP may be due, at least in part, to the inadequate descriptions of FOP in most textbooks of medicine, pediatrics, oncology and podiatry. The correct diagnosis of FOP can be made clinically even before radiographic evidence of heterotopic ossification is seen, if soft tissues lesions are associated with symmetrical malformations of the great toes.
Genetic Testing & FOP
Definitive genetic testing of FOP is now available and can confirm a diagnosis of FOP prior to the appearance of heterotopic ossification. Clinical suspicion of FOP early in life on the basis of malformed great toes can lead to early clinical diagnosis, confirmatory diagnostic genetic testing (if appropriate), and the avoidance of harmful diagnostic and treatment procedures. Clinicians should be aware of the early diagnostic signs of FOP, which are congenital malformation of the great toes and episodic soft tissue swelling even before the appearance of heterotopic ossification. This awareness should prompt genetic consultation and testing (if appropriate) and the institution of assiduous precautions to prevent injury and iatrogenic harm [77]. At the present time, genetic testing is available on a clinical and research basis at several laboratories. Please contact the corresponding author for more information.
MEDICAL MANAGEMENT OF FOP AND THERAPEUTIC HORIZONS
Current Medical Management of FOP
The rarity, variable severity and episodic clinical course of FOP pose substantial uncertainties when evaluating experimental therapies [78,79]. Accordingly, medical intervention is currently supportive.
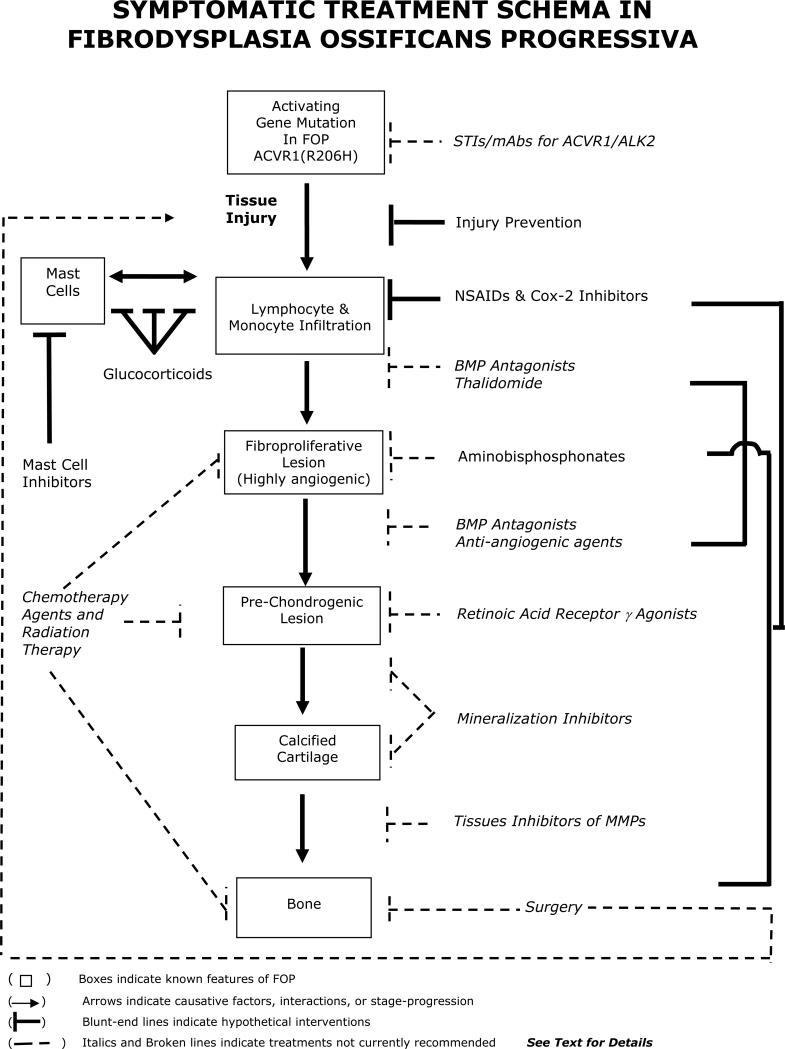
Guidelines for symptomatic management of disease flare-ups have been published, and highlight the anecdotal utility of glucocorticoids in managing new flare-ups affecting the function of major joints in the appendicular skeleton [79]. Anecdotally, nonsteroidal anti-inflammatory medications, cyclo-oxygenase-2 inhibitors, leukotriene inhibitors and mast cell stabilizers are useful anecdotally in managing chronic discomfort and ongoing flare-ups, but, to date, there is no proven efficacy with any therapy in altering the natural history of the disease [79]. A recent report documented the failure of bone marrow transplantation to cure the condition, but suggested that chronic immunosuppression may have some utility, although its general use is not recommended due in part to the potential of severe morbidity in the face of severe restrictive chest wall disease [19]. Figure 1 summarizes the current and potential treatment options in FOP.
Figure 1.
Symptomatic Treatment of FOP
(Adapted from: The International Clinical Consortium on FOP. The Medical Management of Fibrodysplasia Ossificans Progressiva: Current Treatment Considerations. Clin Proc Intl Clin Consort FOP 2011; 4:1-100)
Surgical release of joint contractures is generally unsuccessful and risks new, trauma-induced HO. Osteotomy of heterotopic bone or surgical removal of heterotopic bone to mobilize joints is generally counterproductive because additional HO develops at the operative site. Rarely, a joint may be repositioned surgically to improve the patient's overall functional status. Spinal bracing is ineffective and surgical intervention is associated with numerous complications [80].
Challenges of Therapeutic Assessment in FOP
Flare-ups of FOP are sporadic and unpredictable, and there is great individual variability in the rate of disease progression. Several large studies on the natural history of FOP have confirmed that it is impossible to predict the occurrence, duration or severity of an FOP flare-up, although characteristic anatomic patterning has been described. The rarity of FOP and the unpredictable nature of the condition make it extremely difficult to assess any therapeutic intervention, a fact recognized as early as 1918 by Julius Rosenstirn [81]:
“The disease was attacked with all sorts of remedies and alternatives for faulty metabolism; every one of them with more or less marked success observed solely by its original author but pronounced a complete failure by every other follower. In many cases, the symptoms of the disease disappear often spontaneously, so the therapeutic effect (of any treatment) should not be unreservedly endorsed.”
These words ring true today as they did when they were written nearly a century ago. Presently, there is no proven effective prevention or treatment for FOP. With the discovery of the FOP gene and emerging understanding of the pathology and molecular genetics of FOP, new pharmacologic strategies will emerge to definitively treat FOP [3, 29, 82, 83]. Presently, physicians are faced with an increasing number of potential medical interventions. Unfortunately, clinical experience using these medications for FOP is mostly anecdotal.
Individuals who have FOP also develop the same common problems as anyone in the general population. Generally, the safest way to diagnose and treat these problems in a patient with FOP is to ask the question: “How would I evaluate this patient if he or she did not have FOP?” Following that, the “FOP filter” can be applied to ask: “Given the nature of the possible intercurrent medical problem, and the relative risks that particular problem presents in relation to FOP, are there any diagnostic or treatment procedures that should or should not be undertaken or are there alternative diagnostic procedures might be more appropriate?” Using this approach, diagnostic dilemmas can often be resolved and appropriate care delivered.
SPECIAL MEDICAL CONSIDERATIONS
In addition to common medical problems that individuals with FOP might have, there are a number of special medical considerations for FOP patients that are worthy of very special attention. They are presented below.
Prophylactic Issues in FOP
Dental therapy must involve assiduous attention to prophylaxis of caries and must avoid intramuscular injection of local anesthetics, especially mandibular blocks and stretching of the jaw 84-86]. All intramuscular injections must be avoided [27, 79]. In addition, prevention of falls is crucial [87]. Prophylaxis against influenza and pneumonia, as well as measures to prevent respiratory infection and cardiopulmonary complications of restrictive chest well disease, are vitally important to help minimize flare-ups [87-89].
Additional Complications of FOP
Although ankylosis of the temporomandibular joint is common in later stages of FOP, submandibular heterotopic ossification is relatively uncommon [90, 91]. Submandibular swelling can be a life-threatening complication, especially when associated with massive anterior neck swelling and difficulty in swallowing [90, 91]. Efforts to reduce swelling, including treatment with glucocorticoids and respiratory support, are recommended [90, 91].
Hearing impairment occurs in about half of FOP patients. The onset is usually in childhood or adolescence with slow progression during the lifetime. Hearing loss is most often conductive, possibly due to middle ear ossification; in some patients, the hearing impairment is neurologic [92]. Hearing aids are often helpful. Learning disabilities and special needs should be addressed by appropriate specialists.
Patients with FOP develop thoracic insufficiency syndrome (TIS). This life-threatening complication of cardiopulmonary function can cause pneumonia and right-sided heart failure. It is associated with costovertebral malformations and orthotopic ankylosis of the costovertebral joints, ossification of intercostal muscles, paravertebral muscles and aponeuroses, and progressive spinal deformity including kyphoscoliosis or thoracic lordosis. Prophylactic measures to maximize pulmonary function and minimize respiratory compromise help to reduce morbidity and mortality from TIS in patients with FOP [8, 88, 89].
Careful attention should be directed toward the prevention and therapy of intercurrent chest infections. Such measures include prophylactic pneumococcal pneumonia and influenza vaccinations (subcutaneous injection), chest physiotherapy, and antibiotic treatment at early stages of chest infection. Upper abdominal surgery interferes with diaphragmatic respiration and should be avoided if possible. Sleep studies to assess sleep apnea and positive pressure assisted breathing devices such as bipap masks without the use of supplemental oxygen may be helpful [6].
Patients with FOP who have advanced TIS and who use unmonitored oxygen have a high risk of death caused by sudden correction of oxygen tension in the presence of chronic carbon dioxide retention that suppresses respiratory drive. Patients who have FOP and severe TIS should not use supplemental oxygen in an unmonitored setting [6, 88].
Patients with FOP have approximately a two-fold higher prevalence of kidney stones than the general population. Immobilization coupled with increased bone turnover is a significant risk factor. As in other susceptible populations, a low fiber diet, deficient water intake, excess animal protein intake, and history of urinary tract infections increase the risk of developing kidney stones in FOP [93]. Extracorporeal shock wave lithotripsy, uteroscopic stone removal, percutaneous nephrolithotomy, and laser lithotripsy have all been used as treatment modalities.
Anesthesia in Patients with FOP
General anesthesia is particularly dangerous in patients with FOP. Guidelines for general anesthesia have been reported [79]. Overstretching of the jaw for intubation may cause additional trauma to the TMJ, and lead to disease flare-ups. In older patients whose TMJs are ankylosed, oral access for intubation may not be possible. General anesthesia in FOP patients should be accomplished through an awake fiber-optic nasal intubation under light sedation so that the patient can control secretions. This should be performed by well-trained anesthesia teams who are familiar and experienced with this type of procedure [79].
Rehabilitation Issues in FOP
As heterotopic bone accumulates in FOP, range of motion is progressively lost leading to nearly complete immobility. Present and future rehabilitation approaches should be focused on enhancing activities of daily living. Occupational therapy and vocational education consultations may be useful. Despite the widespread HEO and progressive disability, most patients lead productive and fulfilling lives [94].
Future Treatment Strategies for Inhibiting Skeletal Metamorphosis in FOP
Clinical management of FOP remains symptomatic [3]. The discovery of the FOP gene along with emerging insights into the pathophysiology of ACVR1/ALK2-mediated heterotopic ossification reveals at least four long-term approaches to the treatment and/or prevention of FOP [83]. These approaches include blocking activity of the mutant FOP receptor, diverting the responding mesenchymal stem cells (MSCs) to a soft tissue fate, inhibiting inflammatory and neuro-inflammatory triggers of FOP flare-ups, and altering the inductive and/or conducive microenvironments that promote the formation of FOP lesions.
Blocking Activity of the Mutant FOP Receptor
Signal transduction inhibitors (STIs) have the potential for development into powerful therapeutic agents. Recently, the small molecule STI, dorsomorphin, was identified in a screen for compounds that perturb dorsoventral axis formation in zebrafish [95]. Dorsomorphin and its derivatives inhibit all type I BMP receptors (ALK2, ALK3, and ALK6), and block BMP-mediated SMAD phosphorylation, target gene transcription, and chondro-osseous differentiation [29]. Importantly, Dorsomorphin blocks all BMP-specific SMAD signaling in cells in which the FOP gene was overexpressed [68]. Residues close to the ATP-binding site of ALK2 are being exploited to achieve selectivity, even among closely related receptor serine-threonine kinases such as BMPRIA/ALK3, BMPRIB/ALK6 and ACVR1/ALK2. However, a safe and effective STI for treating FOP will inhibit ALK2 preferentially over ALK3 and ALK6 [95]. STIs designed to specifically block ALK2 must have specificity, efficacy, and tolerance to resistance, acceptable safety profiles, and lack rebound effect before they can be tested in clinical trials for FOP. Additional studies in in vivo models of classic FOP are needed to determine potential efficacy of this class of molecules in preventing inflammation-induced flare-ups of the condition.
Diverting MSCs and Chondroprogenitor Cells from an Osseous to a Soft Tissue Fate
In a recent landmark study, Shimono et al. demonstrated a novel approach that blocks FOP-like HEO after the induction events that lead to HEO have begun [96]. The authors build on well-established findings that retinoic acid is a potent skeletal teratogen that inhibits chondrogenesis, a crucial function they exploit to inhibit heterotopic chondrogenesis using retinoic acid receptor gamma (RARγ) agonists [78].
RARγ agonists effectively inhibit HEO during a wide treatment window that includes the pre-chondrogenic fibroproliferative phase, up to, but not including, the ossification phase. Remarkably, when RARγ agonists are discontinued, no significant rebound effect occurs, indicating that the RARγ effect may be irreversible, perhaps through the mechanisms that redirect cell fate decisions in pre-chondrogenic mesenchymal stem cells to a non-osseous lineage. In vitro studies and mouse models show that both the pre-chondrogenic and chondrogenic stages of HEO are exquisitely sensitive to the inhibitory effects of RARγ agonists, which block BMP signaling and the skeletogenic potential of progenitor cells by promoting the proteosome-regulated degradation of BMP pathway-specific phosphorylated Smads [78, 96].
Thus, the study by Shimono et al identifies RARγ agonists as a class of compounds that profoundly inhibit BMP-induced chondrogenesis required for the cartilaginous scaffold of HEO (Figure 2). The beauty of this approach is that it does not just target the BMP signaling pathway, but modifies a specific pathological process of tissue metamorphosis that requires the BMP signaling pathway to cause disabling disease [78].
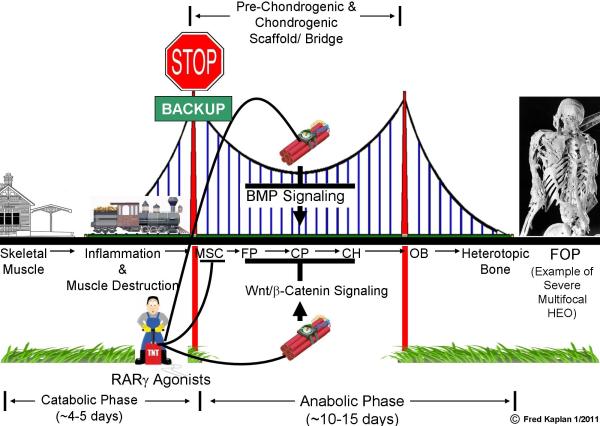
Figure 2. RAR Gamma Agonists Derail Heterotopic Endochondral Ossification.
Tissue metamorphosis in HEO involves two major phases - a catabolic (destructive) phase of acute inflammation and tissue destruction followed by an anabolic (formative) phase of tissue neogenesis involving the formation of a transient cartilaginous scaffold, and its replacement with mature heterotopic bone. A key feature of HEO is the formation of a bridging cartilaginous scaffold that is under control of the BMP and Wnt/β-catenin signaling pathways. RARγ agonists inhibit BMP signaling and promote Wnt/β-catenin signaling in cells that build the cartilaginous scaffold, disrupting the bridge and de-railing HEO. RARγ agonists can re-program MSCs to a non-HEO soft tissue fate, thus effectively backing-up the train into the station if it has not yet reached the bridge. [MSC (mesenchymal stem cells); FP (fibroproliferative cells); CP (cartilage progenitor cells); CH (chondrocytes); OB (osteoblasts). Arrows in pathways signify stimulation; blunt-ended lines signify inhibition. Arrows along the railroad track between cell types indicate stages of progression of HEO. The length of the train on the track indicates the well-established finding that contiguous stages of HEO occur simultaneously in different anatomic areas of the lesion. The time in days for each stage is an estimate, and varies with the age of the patient or the animal model studied. (Adapted from: Kaplan FS, Shore EM. Derailing heterotopic ossification and RARing to go. Nat Med 2011; 17:420-421).
Inhibiting the Inflammatory and Neuro-inflammatory Triggers of FOP Flare-ups
In FOP and related common disorders of acquired heterotopic endochondral ossification (HEO), sensory nerves regulate the innate immune system and amplify the formation of heterotopic bone. Substance P, an 11 amino acid neurotransmitter and potent neuroinflammatory protein, plays a key role in this metamorphosis, and provides a critical link between the sensory branch of the nervous system and the innate immune system in the induction and amplification of HO. The sensory innervation of the innate immune system is BMP pathway dependent, and unveils a myriad of unanticipated new targets for the treatment of FOP. Non-hereditary forms of HEO are commonly associated with injuries to peripheral nerves, the spinal cord, or the brain; yet, a distinct causative relationship between HEO and the nervous system has eluded discovery [97]. Using four different mouse models of HEO as well as early lesional biopsy specimens from FOP patients and those with various forms of acquired HEO, recent work establishes a common mechanism underlying nearly all forms [98].
TRPV1 (Transient Receptor Potential Vanilloid 1) is a cationic channel receptor located on the sensory nerve endings in muscle and other soft connective tissue. Soft tissue injury produces prostaglandins and bradykinins that trigger the bone morphogenetic protein (BMP)-sensitized cationic channel receptor to release calcium and magnesium ions that generate an electrochemical signal. This signal enters the spinal cord and continues up to the brain, causing the sensation of pain. However, HEO is induced when the signal reaches the nucleus of the sensory nerve cells in the dorsal root ganglia and triggers the sensory neurons to manufacture and release Substance P. Substance P then travels in a retrograde manner down the nerve and is released at the nerve ending in the injured muscle where it binds to Substance P receptors (NK1R) on tissue mast cells. Once bound to the mast cells, Substance P triggers the release of inflammation-inducing and edema-causing modulators that amplify the innate inflammatory response, further stimulating HEO (Figure 3).
Figure 3. Neuroinflam0matory Pathways in Heterotopic Ossification.
This schematic depicts the major neuroinflammatory pathways described in the accompanying text. PG/BK= prostaglandins/bradykinins, inflammatory molecules that are released at sites of local soft tissue injury. TRPV1= Transient Receptor Potential Vanilloid 1, a calcium/magnesium (Ca++/Mg++) cation channel receptor expressed at the terminals of primary sensory neurons. PPTA=preprotachykinin, the gene that makes Substance P (SP) and several other inflammatory neuropeptides. DRG= dorsal root ganglia, clusters of primary sensory nerve cells (located near the spinal cord) at each spinal level, the site where Substance P is made, and the site from where pain and temperature signals enter the central nervous system. ACVR1 (R206H)=the FOP mutation.
The increased BMP signaling that results from FOP (and likely from other sporadic forms of HO) sensitizes the TRPV cation channel receptor and also likely increases the synthesis of NK1R. NK1R=neurokinin 1 receptor (the cell surface receptor for Substance P), expressed on many inflammatory cells, most notably mast cells and macrophages (M).
A subgroup of endothelial cells is converted to mesenchymal stem cells (MSCs) in an endothelial to mesenchymal transition (EMT) under the influence of inflammation and the FOP gene. MSCs transform into cartilage and bone cells by a process of heterotopic endochondral ossification (HEO), a process negatively regulated by RARγ. Green=nerve fibers. Blue=mast cells and macrophages. Dashed lines=transport of chemicals or neurotransmitters. Cupped ends=receptors. Arrows=stimulation. Blunt ended lines=inhibition. ? =Possible pathways.
Experiments using genetically engineered and pharmacologically-manipulated HEO forming mice show that blocking any major control point in the sensory nerve pathway - the TRPV1 ion channel, the dorsal root ganglion cells, the preprotachykinin (PPTA) gene that encodes Substance P, the neurokinin 1 receptor (NK1R; the receptor for Substance P), the tissue mast cells that express NK1R, or the c-kit gene (required for mast cell development) - curtails or abolishes HEO [98].
The neurological innervation of the innate immune system is a complex feedback system that allows the body to ramp-up an inflammatory response when needed, but control it so that it does not run amuck. Such a mechanism likely arose to regulate the balance between fighting infection or healing wounds on one hand and controlling a runaway innate immune system that could kill the host on the other hand [99]. However, complexity has its price. When such a system goes awry, disease processes such as HEO and FOP are potentiated.
Inhibiting the Mutant FOP Allele
In a study by Kaplan et al., the authors generated inhibitory RNA (RNAi) duplexes capable of specifically suppressing the mutant copy of the ACVR1/ALK2 gene in connective tissue progenitor cells from FOP patients. This RNAi approach decreased the elevated BMP signaling in FOP cells to levels observed in control cells [100]. The cells used in the experiments were adult stem cells obtained directly from deciduous teeth of FOP patients and thus contained both damaged and normal ACVR1/ALK2 receptors found in all classically affected FOP patients.
While this RNAi approach provides proof-of-principle for using allele–specific inhibition of ACVR1/ALK2 in the treatment of FOP, its in vivo utility must be confirmed in mouse models of classic FOP prior to its consideration for human use. Additionally, other hurdles for RNAi therapy remain to be overcome, most notably safe delivery of the siRNA to cells in the body.
Table 1.
Summary of key practice points
| Activities: Avoid soft tissue injuries, contact sports, overstretching of soft tissues, and muscle fatigue. Avoid biopsies, surgical removal of heterotopic bone, intramuscular injections, and all non-emergent surgical procedures. |
| Anesthesia: If general anesthesia is required, perform awake intubation by nasotracheal fiber-optic technique. Highly-skilled FOP-aware anesthesiologists should be present for all elective intubations. |
| Falls: Locked upper limbs may accentuate head and neck trauma from falls. Epidural hematomas are common (surgical emergency). Use protective headgear in children who have upper limb involvement. All head and neck injuries must be evaluated immediately on an emergent basis. |
| Flare-up: (Back/chest): Use non-steroidal anti-inflammatory medications or cox-2 inhibitors with GI precautions. Use analgesics, muscle relaxants, and local applications of ice packs, as needed. |
| Flare-up: (Limbs/throat): Prednisone – 2 mgs/kg once daily (up to 100 mgs daily) in AM (per oral) for four days; begin within first 24 hours of flare-up. Keep medication on-hand for emergencies. Use analgesias and/or muscle relaxants, as needed, with GI precautions. Local application of ice packs may also be helpful. |
| Flare-ups (Protection): Most flare-ups result from over-use and soft tissue injuries. Prednisone - 2 mgs/kg, (per oral) once daily for three days to prevent flare-up after severe soft-tissue injury. Do not use after minor bumps or bruises. |
| Hearing: Conductive hearing impairment is common. Perform periodic audiology evaluations. Hearing aids may improve conductive hearing loss. |
| Immunizations: Avoid all intramuscular immunizations. Subcutaneous immunizations are acceptable when FOP is quiescent. Avoid immunizations during flare-ups. |
| Influenza: Administer influenza vaccines subcutaneously, but never during flare-ups. Avoid live attenuated flu vaccine as it may cause flu-like symptoms and exacerbate FOP. Household contacts of FOP patients should be immunized annually. Cough suppression may alleviate undo stress on chest musculature. |
| IV's: Superficial IV access and venipuncture is acceptable. Traumatic IV's and arterial punctures may cause heterotopic ossification. |
| Limb swelling: Lymphedema and transient neuropathy may occur with flare-ups of limbs. Elevate legs while sleeping and recumbent. Use support stockings. Take one baby aspirin daily with food. Rule-out deep vein phlebitis with Doppler ultrasound. |
| Occupational therapy (OT): Perform periodic OT evaluations as activities of daily living change. |
| Physiotherapy: Avoid passive range of motion. Warm water hydrotherapy may be helpful. |
| Prednisone: For flare-ups (as noted above), pre-operatively and for three days post-operatively (for emergent procedures and for minor and elective procedures including dental surgery), and for prophylaxis following major soft tissue injury (severe trauma). |
| Pulmonary function: Perform baseline pulmonary function tests (PFTs) and echocardiogram. Repeat periodically. Supplemental oxygen should not be used in an unmonitored setting. |
| School: Use school aides to protect and assist children. Request medical letter and preschool evaluation. |
| Surgery: Avoid surgery, except in emergencies. |
| Teeth: Avoid mandibular blocks, over-stretching of the jaw, and muscle fatigue. |
Revised from Kaplan et. al. [1]
ACKNOWLEDGMENTS
This work was supported in part by the International Fibrodysplasia Ossificans Progressiva Association, the Center for Research in FOP and Related Disorders, the Ian Cali Endowment for FOP Research, the Whitney Weldon Endowment for FOP Research, the Isaac & Rose Nassau Professorship of Orthopaedic Molecular Medicine, and by grants from the Rita Allen Foundation, and the US National Institutes of Health (NIH R01-AR41916).
The International FOP Association
The International FOP Association (IFOPA) was founded in June 1988 to educate patients, doctors and the public about FOP; to support medical research into FOP; and to support patients with FOP and their families by providing a network of communication to help end the isolation that accompanies this rare and severely disabling condition. Additional information can be found on the IFOPA website (www.ifopa.org). In recent years, many regional FOP organizations have arisen worldwide to support patient-related activities.
REFERENCES
- 1.Morales-Piga A, Kaplan FS. Osteochondral diseases and fibrodysplasia ossificans progressiva. Adv Exp Med Biol. 2010;686:335–348. doi: 10.1007/978-90-481-9485-8_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shore EM, Feldman GJ, Xu M, Kaplan FS. The genetics of fibrodysplasia ossificans progressiva. Clin Rev Bone Miner Metab. 2005;3:201–204. [Google Scholar]
- 3.Kaplan FS, Le Merrer M, Glaser DL, Pignolo RJ, Goldsby RE, Kitterman JA, Groppe J, Shore EM. Fibrodysplasia ossificans progressiva. Best Pract Res Clin Rheumatol. 2008;22:191–205. doi: 10.1016/j.berh.2007.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cohen RB, Hahn GV, Tabas JA, Peeper J, Levitz CL, Sando A, Sando N, Zasloff M, Kaplan FS. The natural history of heterotopic ossification in patients who have fibrodysplasia ossificans progressiva. A study of forty-four patients. J Bone Joint Surg Am. 1993;75:215–219. doi: 10.2106/00004623-199302000-00008. [DOI] [PubMed] [Google Scholar]
- 5.Rocke DM, Zasloff M, Peeper J, Cohen RB, Kaplan FS. Age- and joint-specific risk of initial heterotopic ossification in patients who have fibrodysplasia ossificans progressiva. Clin Orthop Relat Res. 1994:243–248. [PubMed] [Google Scholar]
- 6.Kaplan FS, Glaser DL. Thoracic insufficiency syndrome in patients with fibrodysplasia ossificans progressiva. Clin Rev Bone Miner Metab. 2005;3:213–216. [Google Scholar]
- 7.Schaffer AA, Kaplan FS, Tracy MR, O'Brien ML, Dormans JP, Shore EM, Harland RM, Kusumi K. Developmental anomalies of the cervical spine in patients with fibrodysplasia ossificans progressiva are distinctly different from those in patients with Klippel-Feil syndrome: clues from the BMP signaling pathway. Spine. 2005;30:1379–1385. doi: 10.1097/01.brs.0000166619.22832.2c. [DOI] [PubMed] [Google Scholar]
- 8.Kaplan FS, Xu M, Seemann P, Connor JM, Glaser DL, Carroll L, Delai P, Fastnacht-Urban E, Forman SJ, Gillessen-Kaesbach G, Hoover-Fong J, Koster B, Pauli RM, Reardon W, Zaidi SA, Zasloff M, Morhart R, Mundlos S, Groppe J, Shore EM. Classic and atypical fibrodysplasia ossificans progressiva (FOP) phenotypes are caused by mutations in the bone morphogenetic protein (BMP) type I receptor ACVR1. Hum Mutat. 2009;30:379–390. doi: 10.1002/humu.20868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Deirmengian GK, Hebela NM, O'Connell M, Glaser DL, Shore EM, Kaplan FS. Proximal tibial osteochondromas in patients with fibrodysplasia ossificans progressiva. J Bone Joint Surg Am. 2008;90:366–374. doi: 10.2106/JBJS.G.00774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kaplan FS, Strear CM, Zasloff MA. Radiographic and scintigraphic features of modeling and remodeling in the heterotopic skeleton of patients who have fibrodysplasia ossificans progressiva. Clin Orthop Relat Res. 1994:238–247. [PubMed] [Google Scholar]
- 11.Reinig JW, Hill SC, Fang M, Marini J, Zasloff MA. Fibrodysplasia ossificans progressiva: CT appearance. Radiology. 1986;159:153–157. doi: 10.1148/radiology.159.1.3952301. [DOI] [PubMed] [Google Scholar]
- 12.Mahboubi S, Glaser DL, Shore EM, Kaplan FS. Fibrodysplasia ossificans progressiva. Pediatr Radiol. 2001;31:307–314. doi: 10.1007/s002470100447. [DOI] [PubMed] [Google Scholar]
- 13.Kaplan FS, Glaser DL, Shore EM, Deirmengian GK, Gupta R, Delai P, Morhart P, Smith R, Le Merrer M, Rogers JG, Connor JM, Kitterman JA. The phenotype of fibrodysplasia ossificans progressiva. Clin Rev Bone Miner Metab. 2005;3:183–188. [Google Scholar]
- 14.Hegyi L, Gannon FH, Glaser DL, Shore EM, Kaplan FS, Shanahan CM. Stromal cells of fibrodysplasia ossificans progressiva lesions express smooth muscle lineage markers and the osteogenic transcription factor Runx2/Cbfa-1: clues to a vascular origin of heterotopic ossification? J Pathol. 2003;201:141–148. doi: 10.1002/path.1413. [DOI] [PubMed] [Google Scholar]
- 15.Gannon FH, Valentine BA, Shore EM, Zasloff MA, Kaplan FS. Acute lymphocytic infiltration in an extremely early lesion of fibrodysplasia ossificans progressiva. Clin Orthop Relat Res. 1998:19–25. [PubMed] [Google Scholar]
- 16.Kaplan FS, Tabas JA, Gannon FH, Finkel G, Hahn GV, Zasloff MA. The histopathology of fibrodysplasia ossificans progressiva. An endochondral process. J Bone Joint Surg Am. 1993;75:220–230. doi: 10.2106/00004623-199302000-00009. [DOI] [PubMed] [Google Scholar]
- 17.Pignolo RJ, Suda RK, Kaplan FS. The fibrodysplasia ossificans progressiva lesion. Clin Rev Bone Miner Metab. 2005;3:195–200. [Google Scholar]
- 18.Gannon FH, Glaser D, Caron R, Thompson LD, Shore EM, Kaplan FS. Mast cell involvement in fibrodysplasia ossificans progressiva. Hum Pathol. 2001;32:842–848. doi: 10.1053/hupa.2001.26464. [DOI] [PubMed] [Google Scholar]
- 19.Kaplan FS, Glaser DL, Shore EM, Pignolo RJ, Xu M, Zhang Y, Senitzer D, Forman SJ, Emerson SG. Hematopoietic stem-cell contribution to ectopic skeletogenesis. J Bone Joint Surg Am. 2007;89:347–357. doi: 10.2106/JBJS.F.00472. [DOI] [PubMed] [Google Scholar]
- 20.Suda RK, Billings PC, Egan KP, Kim JH, McCarrick-Walmsley R, Glaser DL, Porter DL, Shore EM, Pignolo RJ. Circulating osteogenic precursor cells in heterotopic bone formation. Stem Cells. 2009;27:2209–2219. doi: 10.1002/stem.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Medici D, Shore EM, Lounev VY, Kaplan FS, Kalluri R, Olsen BR. Conversion of vascular endothelial cells into multipotent stem-like cells. Nat Med. 2010;16:1400–1406. doi: 10.1038/nm.2252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lounev VY, Ramachandran R, Wosczyna MN, Yamamoto M, Maidment AD, Shore EM, Glaser DL, Goldhamer DJ, Kaplan FS. Identification of progenitor cells that contribute to heterotopic skeletogenesis. J Bone Joint Surg Am. 2009;91:652–663. doi: 10.2106/JBJS.H.01177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Horwitz EM. Building bone from blood vessels. Nat Med. 2010;16:1373–1374. doi: 10.1038/nm1210-1373. [DOI] [PubMed] [Google Scholar]
- 24.Lutwak L. Myositis ossificans progressiva. Mineral, metabolic and radioactive calcium studies of the effects of hormones. Am J Med. 1964;37:269–293. doi: 10.1016/0002-9343(64)90011-7. [DOI] [PubMed] [Google Scholar]
- 25.Estrores IM, Harrington A, Banovac K. C-reactive protein and erythrocyte sedimentation rate in patients with heterotopic ossification after spinal cord injury. J Spinal Cord Med. 2004;27:434–437. doi: 10.1080/10790268.2004.11752233. [DOI] [PubMed] [Google Scholar]
- 26.Kaplan F, Sawyer J, Connors S, Keough K, Shore E, Gannon F, Glaser D, Rocke D, Zasloff M, Folkman J. Urinary basic fibroblast growth factor. A biochemical marker for preosseous fibroproliferative lesions in patients with fibrodysplasia ossificans progressiva. Clin Orthop Relat Res. 1998;346:59–65. [PubMed] [Google Scholar]
- 27.Lanchoney TF, Cohen RB, Rocke DM, Zasloff MA, Kaplan FS. Permanent heterotopic ossification at the injection site after diphtheria-tetanus-pertussis immunizations in children who have fibrodysplasia ossificans progressiva. J Pediatr. 1995;126:762–764. doi: 10.1016/s0022-3476(95)70408-6. [DOI] [PubMed] [Google Scholar]
- 28.Kaplan FS, Shore EM, Gupta R, Billings PC, Glaser DL, Pignolo RJ, Graf D, Kamoun M. Immunological features of fibrodysplasia ossificans progressiva and the dysregulated BMP4 Pathway. Clin Rev Bone Miner Metab. 2005;3:189–193. [Google Scholar]
- 29.Yu PB, Deng DY, Lai CS, Hong CC, Cuny GD, Bouxsein ML, Hong DW, McManus PM, Katagiri T, Sachidanandan C, Kamiya N, Fukuda T, Mishina Y, Peterson RT, Bloch KD. BMP type I receptor inhibition reduces heterotopic ossification. Nat Med. 2008;14:1363–1369. doi: 10.1038/nm.1888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kan L, Liu Y, McGuire TL, Berger DM, Awatramani RB, Dymecki SM, Kessler JA. Dysregulation of local stem/progenitor cells as a common cellular mechanism for heterotopic ossification. Stem Cells. 2009;27:150–156. doi: 10.1634/stemcells.2008-0576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rogers JG, Chase GA. Paternal age effect in fibrodysplasia ossificans progressiva. J Med Genet. 1979;16:147–148. doi: 10.1136/jmg.16.2.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kaplan FS, McCluskey W, Hahn G, Tabas JA, Muenke M, Zasloff MA. Genetic transmission of fibrodysplasia ossificans progressiva. Report of a family. J Bone Joint Surg Am. 1993;75:1214–1220. doi: 10.2106/00004623-199308000-00011. [DOI] [PubMed] [Google Scholar]
- 33.Janoff HB, Muenke M, Johnson LO, Rosenberg A, Shore EM, Okereke E, Zasloff M, Kaplan FS. Fibrodysplasia ossificans progressiva in two half-sisters: evidence for maternal mosaicism. Am J Med Genet. 1996;61:320–324. doi: 10.1002/(SICI)1096-8628(19960202)61:4<320::AID-AJMG4>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 34.Virdi AS, Shore EM, Oreffo RO, Li M, Connor JM, Smith R, Kaplan FS, Triffitt JT. Phenotypic and molecular heterogeneity in fibrodysplasia ossificans progressiva. Calcif Tissue Int. 1999;65:250–255. doi: 10.1007/s002239900693. [DOI] [PubMed] [Google Scholar]
- 35.Janoff HB, Tabas JA, Shore EM, Muenke M, Dalinka MK, Schlesinger S, Zasloff MA, Kaplan FS. Mild expression of fibrodysplasia ossificans progressiva: a report of 3 cases. J Rheumatol. 1995;22:976–978. [PubMed] [Google Scholar]
- 36.Hebela N, Shore EM, Kaplan FS. Three pairs of monozygotic twins with fibrodysplasia ossificans progressiva: the role of environment in the progression of heterotopic ossification. Clin Rev Bone Miner Metab. 2005;3:205–208. [Google Scholar]
- 37.Kaplan FS, Tabas JA, Zasloff MA. Fibrodysplasia ossificans progressiva: a clue from the fly? Calcif Tissue Int. 1990;47:117–125. doi: 10.1007/BF02555995. [DOI] [PubMed] [Google Scholar]
- 38.Shen Q, Little SC, Xu M, Haupt J, Ast C, Katagiri T, Mundlos S, Seemann P, Kaplan FS, Mullins MC, Shore EM. The fibrodysplasia ossificans progressiva R206H ACVR1 mutation activates BMP-independent chondrogenesis and zebrafish embryo ventralization. J Clin Invest. 2009;119:3462–3472. doi: 10.1172/JCI37412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Billings PC, Fiori JL, Bentwood JL, O'Connell MP, Jiao X, Nussbaum B, Caron RJ, Shore EM, Kaplan FS. Dysregulated BMP signaling and enhanced osteogenic differentiation of connective tissue progenitor cells from patients with fibrodysplasia ossificans progressiva (FOP). J Bone Miner Res. 2008;23:305–313. doi: 10.1359/JBMR.071030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kaplan FS, Groppe J, Pignolo RJ, Shore EM. Morphogen receptor genes and metamorphogenes: skeleton keys to metamorphosis. Ann N Y Acad Sci. 2007;1116:113–133. doi: 10.1196/annals.1402.039. [DOI] [PubMed] [Google Scholar]
- 41.O'Connell MP, Billings PC, Fiori JL, Deirmengian G, Roach HI, Shore EM, Kaplan FS. HSPG modulation of BMP signaling in fibrodysplasia ossificans progressiva cells. J Cell Biochem. 2007;102:1493–1503. doi: 10.1002/jcb.21370. [DOI] [PubMed] [Google Scholar]
- 42.Fiori JL, Billings PC, de la Pena LS, Kaplan FS, Shore EM. Dysregulation of the BMP-p38 MAPK signaling pathway in cells from patients with fibrodysplasia ossificans progressiva (FOP). J Bone Miner Res. 2006;21:902–909. doi: 10.1359/jbmr.060215. [DOI] [PubMed] [Google Scholar]
- 43.Shore EM, Xu M, Feldman GJ, Fenstermacher DA, Cho TJ, Choi IH, Connor JM, Delai P, Glaser DL, LeMerrer M, Morhart R, Rogers JG, Smith R, Triffitt JT, Urtizberea JA, Zasloff M, Brown MA, Kaplan FS. A recurrent mutation in the BMP type I receptor ACVR1 causes inherited and sporadic fibrodysplasia ossificans progressiva. Nat Genet. 2006;38:525–527. doi: 10.1038/ng1783. [DOI] [PubMed] [Google Scholar]
- 44.de la Pena LS, Billings PC, Fiori JL, Ahn J, Kaplan FS, Shore EM. Fibrodysplasia ossificans progressiva (FOP), a disorder of ectopic osteogenesis, misregulates cell surface expression and trafficking of BMPRIA. J Bone Miner Res. 2005;20:1168–1176. doi: 10.1359/JBMR.050305. [DOI] [PubMed] [Google Scholar]
- 45.Glaser DL, Economides AN, Wang L, Liu X, Kimble RD, Fandl JP, Wilson JM, Stahl N, Kaplan FS, Shore EM. In vivo somatic cell gene transfer of an engineered Noggin mutein prevents BMP4-induced heterotopic ossification. J Bone Joint Surg Am. 2003;85-A:2332–2342. doi: 10.2106/00004623-200312000-00010. [DOI] [PubMed] [Google Scholar]
- 46.Ahn J, Serrano de la Pena L, Shore EM, Kaplan FS. Paresis of a bone morphogenetic protein-antagonist response in a genetic disorder of heterotopic skeletogenesis. J Bone Joint Surg Am. 2003;85-A:667–674. doi: 10.2106/00004623-200304000-00013. [DOI] [PubMed] [Google Scholar]
- 47.Gannon FH, Kaplan FS, Olmsted E, Finkel GC, Zasloff MA, Shore E. Bone morphogenetic protein 2/4 in early fibromatous lesions of fibrodysplasia ossificans progressiva. Hum Pathol. 1997;28:339–343. doi: 10.1016/s0046-8177(97)90133-7. [DOI] [PubMed] [Google Scholar]
- 48.Shafritz AB, Shore EM, Gannon FH, Zasloff MA, Taub R, Muenke M, Kaplan FS. Overexpression of an osteogenic morphogen in fibrodysplasia ossificans progressiva. N Engl J Med. 1996;335:555–561. doi: 10.1056/NEJM199608223350804. [DOI] [PubMed] [Google Scholar]
- 49.Roush W. Protein builds second skeleton. Science. 1996;273:1170. doi: 10.1126/science.273.5279.1170. [DOI] [PubMed] [Google Scholar]
- 50.Shore EM, Kaplan FS. Inherited human diseases of heterotopic bone formation. Nat Rev Rheumatol. 2010;6:518–527. doi: 10.1038/nrrheum.2010.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Couzin J. Biomedical research. Bone disease gene finally found. Science. 2006;312:514–515. doi: 10.1126/science.312.5773.514b. [DOI] [PubMed] [Google Scholar]
- 52.Kaplan FS. The key to the closet is the key to the kingdom: a common lesson of rare diseases. Orphan Disease Update. 2006;24:1–9. [Google Scholar]
- 53.Shore EM, Kaplan FS. Insights from a rare genetic disorder of extra-skeletal bone formation, fibrodysplasia ossificans progressiva (FOP). Bone. 2008;43:427–433. doi: 10.1016/j.bone.2008.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bocciardi R, Bordo D, Di Duca M, Di Rocco M, Ravazzolo R. Mutational analysis of the ACVR1 gene in Italian patients affected with fibrodysplasia ossificans progressiva: confirmations and advancements. Eur J Hum Genet. 2009;17:311–318. doi: 10.1038/ejhg.2008.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Furuya H, Ikezoe K, Wang L, Ohyagi Y, Motomura K, Fujii N, Kira J, Fukumaki Y. A unique case of fibrodysplasia ossificans progressiva with an ACVR1 mutation, G356D, other than the common mutation (R206H). Am J Med Genet A. 2008;146A:459–463. doi: 10.1002/ajmg.a.32151. [DOI] [PubMed] [Google Scholar]
- 56.Gregson CL, Hollingworth P, Williams M, Petrie KA, Bullock AN, Brown MA, Tobias JH, Triffitt JT. A novel ACVR1 mutation in the glycine/serine-rich domain found in the most benign case of a fibrodysplasia ossificans progressiva variant reported to date. Bone. 2011;48:654–658. doi: 10.1016/j.bone.2010.10.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Petrie KA, Lee WH, Bullock AN, Pointon JJ, Smith R, Russell RG, Brown MA, Wordsworth BP, Triffitt JT. Novel mutations in ACVR1 result in atypical features in two fibrodysplasia ossificans progressiva patients. PLoS One. 2009;4:e5005. doi: 10.1371/journal.pone.0005005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Xu MQ, Feldman G, Le Merrer M, Shugart YY, Glaser DL, Urtizberea JA, Fardeau M, Connor JM, Triffitt J, Smith R, Shore EM, Kaplan FS. Linkage exclusion and mutational analysis of the noggin gene in patients with fibrodysplasia ossificans progressiva (FOP). Clin Genet. 2000;58:291–298. doi: 10.1034/j.1399-0004.2000.580407.x. [DOI] [PubMed] [Google Scholar]
- 59.Groppe JC, Shore EM, Kaplan FS. Functional modeling of the ACVR1 (R206H) mutation in FOP. Clin Orthop Relat Res. 2007;462:87–92. doi: 10.1097/BLO.0b013e318126c049. [DOI] [PubMed] [Google Scholar]
- 60.Wang T, Li BY, Danielson PD, Shah PC, Rockwell S, Lechleider RJ, Martin J, Manganaro T, Donahoe PK. The immunophilin FKBP12 functions as a common inhibitor of the TGF beta family type I receptors. Cell. 1996;86:435–444. doi: 10.1016/s0092-8674(00)80116-6. [DOI] [PubMed] [Google Scholar]
- 61.Chen YG, Liu F, Massague J. Mechanism of TGFbeta receptor inhibition by FKBP12. EMBO J. 1997;16:3866–3876. doi: 10.1093/emboj/16.13.3866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yamaguchi T, Kurisaki A, Yamakawa N, Minakuchi K, Sugino H. FKBP12 functions as an adaptor of the Smad7-Smurf1 complex on activin type I receptor. J Mol Endocrinol. 2006;36:569–579. doi: 10.1677/jme.1.01966. [DOI] [PubMed] [Google Scholar]
- 63.Groppe JC, Wu J, Shore EM, Kaplan FS. In vitro analyses of the dysregulated R206H ALK2 kinase-FKBP12 interaction associated with heterotopic ossification in FOP. Cells Tissues Organs. 2011;194:291–295. doi: 10.1159/000324230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kaplan FS, Pignolo RJ, Shore EM. The FOP metamorphogene encodes a novel type I receptor that dysregulates BMP signaling. Cytokine Growth Factor Rev. 2009;20:399–407. doi: 10.1016/j.cytogfr.2009.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kaplan FS, Shen Q, Lounev V, Seemann P, Groppe J, Katagiri T, Pignolo RJ, Shore EM. Skeletal metamorphosis in fibrodysplasia ossificans progressiva (FOP). J Bone Miner Metab. 2008;26:521–530. doi: 10.1007/s00774-008-0879-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Song GA, Kim HJ, Woo KM, Baek JH, Kim GS, Choi JY, Ryoo HM. Molecular consequences of the ACVR1 (R206H) mutation of fibrodysplasia ossificans progressiva. J Biol Chem. 2010;285:22542–22553. doi: 10.1074/jbc.M109.094557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.van Dinther M, Visser N, de Gorter DJ, Doorn J, Goumans MJ, de Boer J, ten Dijke P. ALK2 R206H mutation linked to fibrodysplasia ossificans progressiva confers constitutive activity to the BMP type I receptor and sensitizes mesenchymal cells to BMP-induced osteoblast differentiation and bone formation. J Bone Miner Res. 2010;25:1208–1215. doi: 10.1359/jbmr.091110. [DOI] [PubMed] [Google Scholar]
- 68.Fukuda T, Kohda M, Kanomata K, Nojima J, Nakamura A, Kamizono J, Noguchi Y, Iwakiri K, Kondo T, Kurose J, Endo K, Awakura T, Fukushi J, Nakashima Y, Chiyonobu T, Kawara A, Nishida Y, Wada I, Akita M, Komori T, Nakayama K, Nanba A, Maruki Y, Yoda T, Tomoda H, Yu PB, Shore EM, Kaplan FS, Miyazono K, Matsuoka M, Ikebuchi K, Ohtake A, Oda H, Jimi E, Owan I, Okazaki Y, Katagiri T. Constitutively activated ALK2 and increased SMAD1/5 cooperatively induce bone morphogenetic protein signaling in fibrodysplasia ossificans progressiva. J Biol Chem. 2009;284:7149–7156. doi: 10.1074/jbc.M801681200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Olmsted EA, Gannon FH, Wang ZQ, Grigoriadis AE, Wagner EF, Zasloff MA, Shore EM, Kaplan FS. Embryonic overexpression of the c-Fos proto-oncogene. A murine stem cell chimera applicable to the study of fibrodysplasia ossificans progressiva in humans. Clin Orthop Relat Res. 1998;346:1–94. [PubMed] [Google Scholar]
- 70.Fukuda T, Scott G, Komatsu Y, Araya R, Kawano M, Ray MK, Yamada M, Mishina Y. Generation of a mouse with conditionally activated signaling through the BMP receptor, ALK2. Genesis. 2006;4:159–167. doi: 10.1002/dvg.20201. [DOI] [PubMed] [Google Scholar]
- 71.Kan L, Hu M, Gomes WA, Kessler JA. Transgenic mice overexpressing BMP4 develop a fibrodysplasia ossificans progressiva (FOP)-like phenotype. Am J Pathol. 2004;65:1107–1115. doi: 10.1016/S0002-9440(10)63372-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kaplan FS, Shore EM, Pignolo RJ, Glaser DL. Animal models of fibrodysplasia ossificans progressiva. Clin Rev Bone Miner Metab. 2005;3:229–234. [Google Scholar]
- 73.Chakkalakal SA, Zhang D, Culbert A, Wright AC, Maidment ADA, Kaplan FS, Shore EM. The ACVR1 R206H mutation recapitulates the clinical phenotype of FOP in a knock-in mouse model. J Bone Miner Res. 2010;25:s4. [Google Scholar]
- 74.Chakkalakal SA, Zhang D, Raabe T, Richa J, Hankenson K, Kaplan FS, Shore EM. ACVR1 knock-in mouse model for fibrodysplasia ossificans progressiva. J Bone Miner Res. 2008;23:s57. doi: 10.1002/jbmr.1637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kitterman JA, Kantanie S, Rocke DM, Kaplan FS. Iatrogenic harm caused by diagnostic errors in fibrodysplasia ossificans progressiva. Pediatrics. 2005;116:e654–661. doi: 10.1542/peds.2005-0469. [DOI] [PubMed] [Google Scholar]
- 76.Zaghloul KA, Heuer GG, Guttenberg MD, Shore EM, Kaplan FS, Storm PB. Lumbar puncture and surgical intervention in a child with undiagnosed fibrodysplasia ossificans progressiva. J Neurosurg Pediatr. 2008;1:91–94. doi: 10.3171/PED-08/01/091. [DOI] [PubMed] [Google Scholar]
- 77.Kaplan FS, Xu M, Glaser DL, Collins F, Connor M, Kitterman J, Sillence D, Zackai E, Ravitsky V, Zasloff M, Ganguly A, Shore EM. Early diagnosis of fibrodysplasia ossificans progressiva. Pediatrics. 2008;121:e1295–1300. doi: 10.1542/peds.2007-1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kaplan FS, Shore EM. Derailing heterotopic ossification and RARing to go. Nat Med. 2011;17:420–421. doi: 10.1038/nm0411-420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.The International Clinical Consortium on FOP The Medical Management of Fibrodysplasia Ossificans Progressiva: Current Treatment Considerations. Clin Proc Intl Clin Consort FOP. 2011;4:1–100. [Google Scholar]
- 80.Shah PB, Zasloff MA, Drummond D, Kaplan FS. Spinal deformity in patients who have fibrodysplasia ossificans progressiva. J Bone Joint Surg Am. 1994;76:1442–1450. doi: 10.2106/00004623-199410000-00002. [DOI] [PubMed] [Google Scholar]
- 81.Rosenstirn J. A contribution to the study of myositis ossificans progressiva. Ann Surg. 1918;8:485–520. 591–637. doi: 10.1097/00000658-191811000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kaplan FS, Glaser DL, Pignolo RJ, Shore EM. A new era for fibrodysplasia ossificans progressiva: a druggable target for the second skeleton. Expert Opin Biol Ther. 2007;7:705–712. doi: 10.1517/14712598.7.5.705. [DOI] [PubMed] [Google Scholar]
- 83.Kaplan FS, Groppe J, Shore EM. When one skeleton is enough: Approaches and strategies of the treatment of fibrodysplasia ossificans progressiva. Drug Discov Today Ther Strateg. 2009;5:255–262. doi: 10.1016/j.ddstr.2008.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Nussbaum BL, O'Hara I, Kaplan FS. Fibrodysplasia ossificans progressiva: report of a case with guidelines for pediatric dental and anesthetic management. ASDC J Dent Child. 1996;63:448–450. [PubMed] [Google Scholar]
- 85.Luchetti W, Cohen RB, Hahn GV, Rocke DM, Helpin M, Zasloff M, Kaplan FS. Severe restriction in jaw movement after routine injection of local anesthetic in patients who have fibrodysplasia ossificans progressiva. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1996;81:21–25. doi: 10.1016/s1079-2104(96)80141-7. [DOI] [PubMed] [Google Scholar]
- 86.Nussbaum BL, Grunwald Z, Kaplan FS. Oral and dental healthcare and anesthesia for persons with fibrodysplasia ossificans progressiva. Clin Rev Bone Miner Metab. 2005;3:239–242. [Google Scholar]
- 87.Glaser DL, Rocke DM, Kaplan FS. Catastrophic falls in patients who have fibrodysplasia ossificans progressiva. Clin Orthop Relat Res. 1998;346:110–116. [PubMed] [Google Scholar]
- 88.Kaplan FS, Zasloff MA, Kitterman JA, Shore EM, Hong CC, Rocke DM. Early mortality and cardiorespiratory failure in patients with fibrodysplasia ossificans progressiva. J Bone Joint Surg Am. 2010;92:686–691. doi: 10.2106/JBJS.I.00705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kussmaul WG, Esmail AN, Sagar Y, Ross J, Gregory S, Kaplan FS. Pulmonary and cardiac function in advanced fibrodysplasia ossificans progressiva. Clin Orthop Relat Res. 1998;346:104–109. [PubMed] [Google Scholar]
- 90.Janoff HB, Zasloff MA, Kaplan FS. Submandibular swelling in patients with fibrodysplasia ossificans progressiva. Otolaryngol Head Neck Surg. 1996;114:599–604. doi: 10.1016/S0194-59989670253-X. [DOI] [PubMed] [Google Scholar]
- 91.Leavitt BD, Teeples TJ, Viozzi CF. Submandibular space swelling in a patient with fibrodysplasia ossificans progressiva: a diagnostic dilemma. J Oral Maxillofac Surg. 2009;67:668–673. doi: 10.1016/j.joms.2008.07.011. [DOI] [PubMed] [Google Scholar]
- 92.Levy CE, Lash AT, Janoff HB, Kaplan FS. Conductive hearing loss in individuals with fibrodysplasia ossificans progressiva. Am J Audiol. 1999;8:29–33. doi: 10.1044/1059-0889(1999/011). [DOI] [PubMed] [Google Scholar]
- 93.Glaser DL, Kaplan FS. Treatment considerations for the management of fibrodysplasia ossificans progressiva. Clin Rev Bone Miner Metab. 2005;3:243–250. [Google Scholar]
- 94.Levy CE, Berner TF, Bendixen R. Rehabilitation for individuals with fibrodysplasia ossificans progressiva. Clin Rev Bone Miner Metab. 2005;3:251–256. [Google Scholar]
- 95.Hong CC, Yu PB. Applications of small molecule BMP inhibitors in physiology and disease. Cytokine Growth Factor Rev. 2009;20:409–418. doi: 10.1016/j.cytogfr.2009.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Shimono K, Tung WE, Macolino C, Chi AH, Didizian JH, Mundy C, Chandraratna RA, Mishina Y, Enomoto-Iwamoto M, Pacifici M, Iwamoto M. Potent inhibition of heterotopic ossification by nuclear retinoic acid receptor-gamma agonists. Nat Med. 2011;17:454–460. doi: 10.1038/nm.2334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Pignolo RJ, Foley KL. Nonhereditary heterotopic ossification: Implications for injury, arthropathy, and aging. Clin Rev Bone Miner Metabol. 2005;3:261–266. [Google Scholar]
- 98.Kan L, Lounev VY, Pignolo RJ, Duan L, Liu Y, Stock SR, McGuire TL, Lu B, Gerard NP, Shore EM, Kaplan FS, Kessler JA. Substance P signaling mediates BMP-dependent heterotopic ossification. J Cell Biochem. 2011;112:2759–2772. doi: 10.1002/jcb.23259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Tracey KJ. Cell biology. Ancient neurons regulate immunity. Science. 2011;332:673–674. doi: 10.1126/science.1206353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kaplan J, Kaplan FS, Shore EM. Restoration of normal BMP signaling levels and osteogenic differentiation in FOP mesenchymal progenitor cells by mutant allele-specific targeting. Gene Ther. 2011 Oct 20; doi: 10.1038/gt.2011.152. doi: 10.1038/gt.2011.152. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]