Abstract
Recent studies suggest that subjects with hypothyroidism under therapy with levothyroxine (L-T4) might develop oxidative stress. The aim of this study was to test a redox-balance modulator, fermented papaya-based nutraceutical (FPP), together with subclinical (SH) or mild hypothyroidism (MH) treatment in view of biochemical changes. A total of 60 females treated for SH-MH were divided into two matched groups and received either FPP 3 grams 1 sachet three times a day (t.i.d.) or placebo for 3 months. A significant baseline increase of all oxidative markers was observed in SH-MH (p<0.05 vs. control) and even more under T4 treatment (p<0.05). FPP caused a normalization of redox markers (p<0.01 vs. placebo). Thyroid supplementation accelerates mitochondrial oxygen consumption and oxidative stress, whereas a redox-modulator therapy is advisable, given the long-lasting treatment in such cases.
Introduction
It is known that thyroid hormones are associated with the redox-balance homeostatic regulation. Indeed, thyroid dysfunctions increase lipoperoxides, which constitute an autocatalytic mechanism leading to oxidative damage of cellular membranes.1 Such damage may bring about cell death and the production of toxic and reactive aldehyde metabolites, of which malondialdehyde (MDA) is the most important. Subclinical hypothyroidism (SH), defined as an elevated serum thyroid-stimulating hormone (TSH) level associated with serum thyroid hormone concentrations within the reference range, has been reported to affect 4–10% of Western populations.2,3 Moreover, although depression of metabolism due to hypothyroidism has been described to decrease oxidant production and thus theoretically protect tissues against oxidative damage, clinical evidence points out that, on the contrary, patients with hypothyroidism have an increased risk of developing atherosclerosis.4
SH is also considered either experimentally and clinically to be a definite risk factor for overall cardiovascular aging and disease.5,6 Accordingly, there are data that associate hypothyroid status to enhanced oxidative stress.7–9 However, this issue is far from being completely settled in that, for instance, when hypothyroid rats were treated with 3,3′,5-triiodothyronine (T3), a significant increase in the level of oxidative stress parameters in the mitochondrial fraction (MF) was recorded in adult rat brain.10 However, hydrogen peroxide (H2O2) content of the MF as well as post-mitochondrial fractions of cerebral cortex was elevated by induced hypothyroidism and reverted to normal level by subsequent treatment of T3. Indeed, when applying thyroid hormone supplementation (THS), it should be considered that T3 exerts calorigenic action by accelerating mitochondrial oxygen consumption through the triggering of the transcription of respiratory genes, which enhances reactive oxygen species (ROS) production.
Existing data suggest that THS calorigenesis is reached by either a short-term non-genomic signaling mechanism mediated by 3,5-diiodothyronine and T3, leading to the allosteric stimulation of cytochrome c oxidase and also a long-term pathway up-regulating nuclear and mitochondrial gene transcription via T3 signaling. This respiratory factor accounts for up to 25% of the net increase in total O2 consumption and, as a matter of fact, increased ROS is also a feature of hyper-thyroidism.11 Thus, it is not surprising that the finding of a very recent study suggesting that oxidative stress in subjects with primary hypothyroidism under therapy with levothyroxine (L-T4) might be the cause of the side effects following L-T4 therapy in relation to oxidative stress.12 This might be one of the reasons behind the discomfort and loss of working activity often experienced in these patients, not considering also further potential ROS-mediated subclinical phenomena to be manifested in the long run.
When considering a possible antioxidant intervention, we relied on a certified fermented papaya-based nutraceutical (FPP; Immun-Age®, ORI, Gifu, Japan, made under ISO 9001 and ISO 14001 from a patented biofermentation process of non-GMO Carica papaya), which has been shown to possess effective redox-modulating properties either in in vitro, experimental, and clinical settings, as summarized in a recent review.13 Thus, the aim of the present study was to test the redox-balance modulator FPP in association with treatment of SH or mild hypothyroidism (MH) in terms of clinical symptom score, oxidative stress, lipid profile, and gene expression involved in thyroid regulation.
Materials and Methods
Patients
The study included 60 generally healthy females, ages 18–55, not on the birth control pill or taking soy supplement. Exclusion criteria were main chronic diseases, relevant medications, major dyslipidemia disorders, heavy physical activity, and psychiatric disorders. All subjects presented with SH or MH and had been previously found to have normal levels of zinc, selenium, and copper.
Patients were put on a 2-week washout period and then divided into two groups of 30 each, matched for age, routine biochemical status, dietary profile, and thyroid status assessment. Both groups received similar medical treatment for their hypothyroidism. One group was given FPP 3 grams 1 sachet twice a day for 3 months, while the other group was given a flavored sugar sachet as placebo. A matched group of normal thyroid function subjects was our healthy control (HC). Moreover, the web-based version of the National Institutes of Health Diet History Questionnaire was employed to assess diet history over the past month and throughout the study period. The subjects were given written and verbal instructions by a registered dietitian.
Biochemical tests
After a 2-week washout period, routine biochemistry was performed, and unbound free T3 (FT3), free thyroxine (FT4), and TSH levels were measured in the serum using the Microparticle Enzyme Immuno-Assay (Abbott Laboratories, Abbott Park, IL). The reference range for FT3 was 2.05–3.65 pg/mL; it was 0.71–1.85 ng/day for FT4, and 0.47–5.01 mIU/L for TSH. Plasma oxidized glutathione, superoxide dismutase, lipid hydro-peroxide, glutathione peroxidase, and MDA were assessed by spectrophotometric analysis.
RNA isolation and thyroid receptors gene expression analysis
Total mRNA was isolated from mononuclear cells with the RNeasy Mini Kit (Qiagen GmbH). The mRNA was then treated with DNase I (Gibco BRL) before reverse transcription with Moloney murine leukemia virus reverse transcriptase. Quantitative evaluation of TH receptors (TRα-1, TRβ-1) was carried out using real-time quantitative RT-PCR (qRT-PCR) with an ABI PRISM 7000 sequence detection apparatus (Applied Biosystems, Foster City, CA). Primers were designed with Primer Express software (Applied Biosystems). The 25-μL reaction mixture contained 12.5 μL of SYBR Green PCR Master Mix, 10 ng of cDNA template, and one pair of the primers 5′-GCT GCA GGC TGT GCT GCT A-3′ (forward) and 5′-CGA TCA TGC GGA GGT CAG T-3′ (reverse) for TRα-1, 5′-GTG TCT CAA GTG CCC AGA CCT T-3′ (forward), and 5′-CAC AGA GCT CGT CCT TGT CTA AGT AA-3′ (reverse) for TRβ-1. Relative quantification of TRα-1, TRβ mRNA expression was analyzed by the comparative threshold cycle (Ct) method. The relative quantitation value of the target, normalized to an endogenous control (housekeeping) gene and relative to a calibrator, is expressed as 2ΔCt.
Statistical analysis
All measurements were performed in duplicate and were repeated at least three times. SPSS 15.0 software was used for analysis of the data. Comparisons of data among groups were analyzed by the Kruskal–Wallis test. Further comparisons between either two groups were analyzed by the Nemenyi test. The values were considered to be statistically significant at p<0.05.
Results
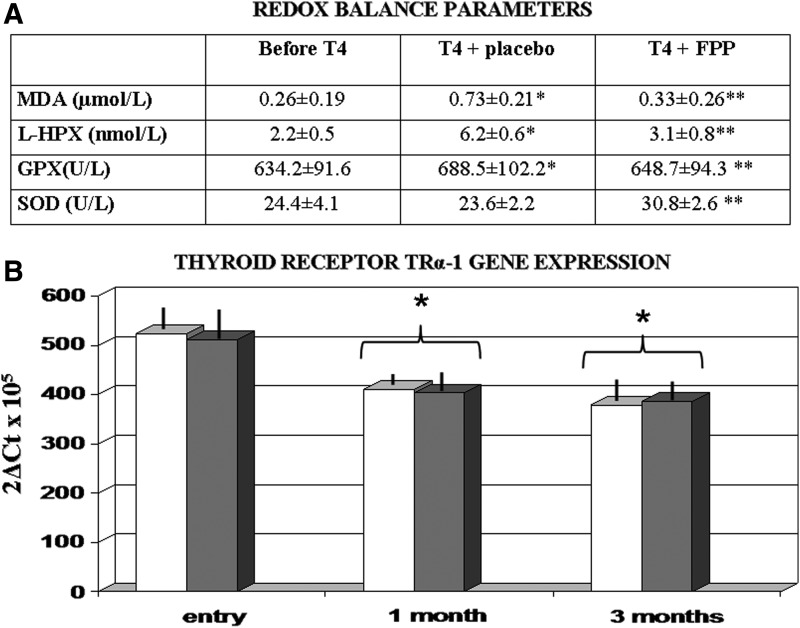
No reduced intake of main dietary trace minerals and vitamins appeared from the dietary questionnaire data, and lipid profile and thyroid hormone parameters remained unchanged and unaffected by FPP supplementation (data not shown). As compared to the HC group, a significant increase of all oxidative markers was observed in MH subjects (p<0.05) but not in SH subjects (Fig. 1A). Treatment with T4 brought about a further increase (p<0.05 vs. baseline) in MH subjects and a significant increase also in SH subjects. Although placebo was ineffective, FPP-supplemented individuals showed a significant normalization of redox markers in all tested subjects (p<0.01). As compared to baseline symptom score, treatment with FPP showed a non-significant trend improvement (NS). However, at study entry, only 6 (20%) subjects in the placebo group and 8 (27%) in the FPP-treated group reported symptoms affecting their quality of life. Although the limited number did not allow a deeper analysis, interestingly all 3 subjects in the FPP-treated group reported a long-standing gastrointestinal discomfort, often requiring lowering the dosage of the T3 therapy when cyclically being prescribed. This was in association with their established T4, and they reported a complete recovery. THS caused a significant down-regulation of TRα-1 mRNA (p<0.01 vs. baseline) but not of TRβ-1 genes, and this pattern was unaffected by FPP (Fig. 1B).
FIG. 1.
(A) Redox balance parameters. (*) p<0.01 vs. baseline at washout time; (**) p<0.05 vs. placebo group. (B) Thyroid receptors gene expression: Effect of thyroid hormone supplementation (THS) and supplementation. (White bars) Placebo-treated; (grey bars) fermented papaya-based neutraceutical (FPP)-treated. (*) p<0.05 vs. baseline at washout time. MDA, malondialdehyde; L-HPX, hydroperoxides; GPX, glutathione peroxidase; SOD, superoxidase dismutase.
Discussion
It is known that genomic and non-genomic molecular mechanisms are involved in the functionality of thyroid hormone. The genomic mechanisms mainly act in the interaction between T3 with nuclear thyroid hormone receptor (TR) proteins, such as TRβ1, and the development of intra-nuclear complexes of either co-activators or co-repressors. These modulate transcription by binding to the promoter regions of thyroid hormone-responsive genes.14 It has been shown experimentally that following thyroid hormone-enhanced ROS generation in the liver, damage to polyunsaturated fatty acids, proteins, and DNA occurs.15 There is also worsening hepatic injury caused by other injurious factors by amplification of ROS generation and macrophage hyperplasia- and hypertrophy-induced Kupffer cell activity.16 Indeed, in humans, hyperthyroidism is characterized by significant changes in redox balance, including increased levels of conjugated dienes, H2O2, and lipid hydroperoxides11 with reduced levels of thiols. However, literature data and our present study confirm that under normal THS either in SH and MH this pro-oxidant state increases the oxidative stress generation when the reduction in the anti-oxidant potential is not adequately compensated while an expected TR and redox gene up-regulation takes place.
It appears that not only can FPP counteract the thyroid hormone-induced oxidative stress, but it also does not impair the physiological primary hormone-related receptors. This may be speculatively advocated for by a possible enhancement of the inner mitochondrial efficiency due to better anti-oxidant mechanisms and avoiding the weakening anti-oxidant defense as shown by Zhang et al.17 It remains mandatory to implement a carefully tailor-made THS, because even limited increases of TSH may allow considerable health benefits.18 However, given the failure of vitamin C to counteract the associated redox imbalance during THS,19 these findings prove that FPP intervention might be an advisable integrative treatment to be associated with long-standing THS regimens. Redox-gene and other transcription factor regulation studies are underway, given the increased complexity of these metabolic pathways.20
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Catalá A. A synopsis of the process of lipid peroxidation since the discovery of the essential fatty acids. Biochem Biophys Res Commun 2010;399:318–323 [DOI] [PubMed] [Google Scholar]
- 2.Asvold BO, Vatten LJ, Bjøro T. Changes in the prevalence of hypothyroidism. The HUNT Study in Norway. Eur J Endocrinol 2013;169:613–620 [DOI] [PubMed] [Google Scholar]
- 3.Hollowell JG, Staehling NW, Flanders WD, Hannon WH, Gunter EW, Spencer CA. Serum TSH, T (4), and thyroid antibodies in the United States population (1988 to 1994): National Health and Nutrition Examination Survey (NHANES III) J Clin Endocrinol Metab 2002;87:489–499 [DOI] [PubMed] [Google Scholar]
- 4.Kutluturk F, Yuce S, Tasliyurt T, Yelken BM, Aytan P, Ozturk B, Yılmaz A. Changes in metabolic and cardiovascular risk factors before and after treatment in overt hypothyroidism. Med Glas (Zenica) 2013;10:348–353 [PubMed] [Google Scholar]
- 5.Sarati LI, Martinez CR, Artés N, Arreche N, López-Costa JJ, Balaszczuk AM, Fellet AL. Hypothyroidism: Age-related influence on cardiovascular nitric oxide system in rats. Metabolism 2012;61:1301–1311 [DOI] [PubMed] [Google Scholar]
- 6.Rhee CM, Curhan GC, Alexander EK, Bhan I, Brunelli SM. Subclinical hypothyroidism and survival: The effects of heart failure and race. J Clin Endocrinol Metab 2013;98:2326–2336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nanda N, Bobby Z, Hamide A. Oxidative stress and protein glycation in primary hypothyroidism. Male/female difference. Clin Exp Med 2008;8:101–108 [DOI] [PubMed] [Google Scholar]
- 8.Kebapcilar L, Akinci B, Bayraktar F. Plasma thiobarbituric acid-reactive substance levels in subclinical hypothyroidism. Med Princ Pract 2007;16:432–436 [DOI] [PubMed] [Google Scholar]
- 9.Haribabu A, Reddy VS, Pallavi C, Bitla AR, Sachan A, Pullaiah P, Suresh V, Rao PV, Suchitra MM. Evaluation of protein oxidation and its association with lipid peroxidation and thyrotropin levels in overt and subclinical hypothyroidism. Endocrine 2012;44:152–157 [DOI] [PubMed] [Google Scholar]
- 10.Das K, Chainy GBN. Thyroid hormone influences antioxidant defense system in adult rat brain. Neurochem Res 2004;29:1755–1766 [DOI] [PubMed] [Google Scholar]
- 11.Bednarek J, Wysocki H, Sowinski J. Oxidation products and antioxidant markers in plasma of patients with Graves, disease and toxic multinodular goiter: effect of methimazole treatment. Free Radic Res 2004;38:659–664 [DOI] [PubMed] [Google Scholar]
- 12.Cornelli U, Belcaro G, Ledda A, Feragalli B. Oxidative stress following administration of levothyroxine in subjects suffering from primary hypothyroidism. Panminerva Med 2011;53:95–98 [PubMed] [Google Scholar]
- 13.Marotta F, Catanzaro R, Yadav H, Jain S, Tomella C, Polimeni A, Mantello P. Functional foods in genomic medicine: A review of fermented papaya preparation research progress. Acta Biomed 2012;83:21–29 [PubMed] [Google Scholar]
- 14.Cheng SY, Leonard JL, Davis PJ. Molecular aspects of thyroid hormone actions. Endocr Rev 2010; 31:139–170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Andican G, Gelisgen R, Civelek S, Seven A, Seymen O, Altug T, Yigit G, Burcak G. Oxidative damage to nuclear DNA in hyperthyroid rat liver: Inability of vitamin C to prevent the damage. J Toxicol Environ Health 2004;67:413–420 [DOI] [PubMed] [Google Scholar]
- 16.Videla LA. Energy metabolism, thyroid calorigenesis, and oxidative stress: Functional and cytotoxic consequences. Redox Rep 2000;5:265–275 [DOI] [PubMed] [Google Scholar]
- 17.Zhang N, Tong YJ, Shan ZY, Teng WP. Effect of chronic mild and moderate iodine excess on thyroid anti-oxidative ability of iodine deficiency and non-iodine deficiency Wistar rats. Zhonghua Yi Xue Za Zhi 2006;86:1274–1278 [PubMed] [Google Scholar]
- 18.Taylor PN, Razvi S, Pearce SH, Dayan C. A review of the clinical consequences of variation in thyroid function within the reference range. J Clin Endocrinol Metab 2013;98:3562–3571 [DOI] [PubMed] [Google Scholar]
- 19.Fernández V, Tapia G, Varela P, Romanque P, Cartier-Ugarte D, Videla LA. Thyroid hormone-induced oxidative stress in rodents and humans: A comparative view and relation to redox regulation of gene expression. Comp Biochem Physiol C Toxicol Pharmacol 2006;142:231–239 [DOI] [PubMed] [Google Scholar]
- 20.Flamant F, Gauthier K, Samarut J. Thyroid hormones signalling is getting more complex: STORMs are coming. Mol Endocrinol 2007;21:321–333 [DOI] [PubMed] [Google Scholar]