Abstract
Abnormalities in the response of the orbitofrontal cortex (OFC) and dorsolateral prefrontal cortex (DLPFC) to negative emotional stimuli have been reported in acutely depressed patients. However, there is a paucity of studies conducted in unmedicated individuals with major depressive disorder in remission (rMDD) to assess whether these are trait abnormalities. To address this issue, 19 medication-free rMDD individuals and 20 healthy comparison (HC) participants were scanned using functional magnetic resonance imaging while performing an implicit emotion processing task in which they labeled the gender of faces depicting negative (fearful), positive (happy) and neutral facial expressions. The rMDD and HC groups were compared using a region-of-interest approach for two contrasts: fear vs. neutral and happy vs. neutral. Relative to HC, rMDD showed reduced activation in left OFC and DLPFC to fearful (vs. neutral) faces. Right DLPFC activation to fearful (vs. neutral) faces in the rMDD group showed a significant positive correlation with duration of euthymia. The findings support deficits in left OFC and DLPFC responses to negative emotional stimuli during euthymic periods of MDD, which may reflect trait markers of the illness or a ‘scar’ due to previous depression. Recovery may also be associated with compensatory increases in right DLPFC functioning.
Keywords: depression, functional magnetic resonance imaging, emotion processing
1. Introduction
There has been considerable progress in identifying the neural circuitry involved in major depressive disorder (MDD), however, a neural circuitry marker for the MDD trait has not yet been defined. Behavioral studies examining responses to emotional stimuli show relatively consistent findings in MDD of biases towards negative emotional stimuli that persist into remission when individuals with MDD are euthymic and medication-free (Bhagwagar et al., 2004; Leppanen et al., 2004). There have also been reports of a bias away from positive emotional stimuli in MDD patients during the acute and remitted illness stages (Gur et al., 1992; Surguladze et al., 2004; Harmer et al., 2009). Though the consistency of these reports implicate the neural circuitry that subserves emotional processing as a trait feature of MDD, there is a paucity of neuroimaging studies examining the neural correlates of these abnormalities in individuals who are in remission from MDD (rMDD) and medication-free. Conducting such studies in remitted individuals, could be a pivotal step forward in the identification of a trait marker for MDD (Bhagwagar and Cowen, 2008).
Neuroimaging studies performed during emotional processing in acutely depressed individuals have consistently identified abnormalities in the dorsolateral prefrontal cortex (DLPFC), ventrolateral prefrontal cortex (VLPFC) and the orbitofrontal cortex (OFC), key areas within the PFC involved in voluntary and automatic emotion processing (Kennedy et al., 2001; Lawrence et al., 2004; Drevets and Price, 2005; Keedwell et al., 2005; Johnstone et al., 2007; Fales et al., 2008; Fales et al., 2009; Hsu et al., 2010). Models of affective regulation suggest that regulation of emotion processing, and complex emotional behaviors, involves the engagement of DLPFC, VLPFC and OFC thought to exert top-down regulation (Ochsner et al., 2002; Phillips et al., 2008) of limbic and subcortical areas, including the subgenual anterior cingulate cortex (sgACC), amygdala and ventral striatum responsible for more rapid and automatic processing of emotional stimuli (Mayberg, 1997; Phillips et al., 2008).
In line with earlier positron emission tomography (PET) studies showing reduced left-sided DLPFC activation in individuals with MDD at “rest” (i.e. at baseline when not performing a specific task) (Baxter et al., 1989; Bench et al., 1993), more recent functional magnetic resonance imaging (fMRI) studies of acutely depressed patients who performed tasks requiring processing of implicit or explicit emotional face stimuli have reported reduced PFC activation and elevated sgACC and amygdala activation in response to negative facial expressions (Fu et al., 2004; Surguladze et al., 2005; Siegle et al., 2007). These findings have predominantly been localized to the left-hemisphere, consistent with theories of hemispheric localization of emotion (Davidson, 1992) and lesion-based studies in depressed patients (Shimoda and Robinson, 1999). Altered activity in both left and right OFC has also been found in individuals with MDD when acutely depressed and at rest (Ebert et al., 1991; Cohen et al., 1992; Drevets et al., 1992; Biver et al., 1994), as well as during the performance of implicit emotion processing tasks (Townsend et al., 2010) and reward-based tasks involving negative and positive feedback (Taylor Tavares et al., 2008). These suggest deficits in the appraisal of, and regulation of response to, emotionally valenced stimuli. Collectively, these findings suggest PFC dysregulation may represent a potential trait abnormality underlying emotion stimulus processing disturbances in individuals with MDD.
There are several neuroimaging studies in euthymic remitted MDD individuals to assess whether abnormal neural responses to emotional stimuli represent illness trait markers (Drevets et al., 1992; Liotti et al., 2002; Neumeister et al., 2006; Norbury et al., 2009; Victor et al., 2010). These studies have identified abnormalities in PFC systems. However, some studies included medicated subjects (Liotti et al., 2002), subjects with co-morbid illnesses (Neumeister et al., 2006; Norbury et al., 2009) or did not require individuals to have a family history of MDD (Norbury et al., 2009), making it difficult to draw conclusions about potential trait markers of vulnerability to MDD. Furthermore, most of these studies did not directly examine whether putative trait abnormalities in the processing of emotional stimuli were specific to negative stimuli or were also found in response to positive stimuli.
In the present study we examined neural activation during an implicit emotional face processing task using fMRI in fully recovered, medication-free individuals with MDD (rMDD), to determine whether dysfunction in brain regions subserving emotion processing persist into remission. Studying rMDD individuals who are medication-free offers an opportunity to investigate the disorder, without the confounding effects of medication, or symptom-induced (state-related) neural changes. In addition, we separately examined neural responses to negative (fearful) and positive (happy) facial expressions (relative to neutral) to assess whether disturbances are associated only with negative emotional processing, or whether they are also associated with positive emotional processing. We hypothesized that, relative to healthy control individuals, individuals with rMDD would show altered PFC neural system response to the processing of negative emotional stimuli. Whole brain exploratory analyses were performed to assess for potential regional differences not hypothesized, and for associations with trait anxiety scores and duration of euthymia in the rMDD group to assess for effects of trait anxiety and whether recovery is associated with shifts in the functioning of the circuitry.
2. Materials and methods
2.1 Participants
A total of 44 participants [21 medication-free rMDD patients and 23 healthy comparison (HC) individuals] were recruited for the study. Five participants (2 rMDD and 3 HC) were subsequently excluded for poor behavioral performance and/or excessive movement in scanner (see sections 2.4 and 2.5 below), leaving a total of 39 participants who completed the study (Table 1). The presence or absence of Axis I psychiatric diagnoses and mood state were established by consensus of both a semi-structured clinical interview of an experienced clinician (AS, DM) and a Structured Clinical Interview for DSM-IV Axis 1 disorders (SCID) (First, 2002). Inclusion criteria for the rMDD participants included at least two past major depressive episodes (MDEs), age of onset of the first MDE was before the age of 25 years, duration of current period of euthymia a minimum of four months, Hamilton Depression Rating Scale, (Hamilton, 1960) score less than seven, and a Young Mania Rating Scale (Young et al., 1978) score less than twelve. Additional inclusion criteria included absence of another lifetime Axis I or II psychiatric diagnosis, a medication free period for at least 4 months and one or more first-degree relatives with a past or current diagnosis of MDD, which was obtained by administration of the structured Research Diagnostic Criteria Family History Questionnaire (FH-RDC) (Andreasen et al., 1977). The individuals in the rMDD group were ages 18-65 years, with mean age 33.6, SD± 13.5, 15 (79%) females. Individuals in the HC group were without personal current or past diagnosis of an Axis 1 disorder or a first-degree family member with a history of such illnesses and were ages 18-65 years, with mean age 35.8, SD± 12.10, 10 (50%) females. Participants with rMDD were recruited by referrals from a university-based medical center and advertisement, and HC participants by advertisement in the surrounding community. Exclusion criteria for both groups included significant current or lifetime medical condition (by history and physical examination), neurological condition, history of loss of consciousness for five minutes or more, current use of psychotropic medication, current or lifetime substance or alcohol dependence and/or abuse, or contraindication to magnetic resonance imaging scanning.
Table 1.
Participant demographics and clinical variables
| rMDD (n= 19) | HC (n= 20) | Statistics | P Value (two-tailed) | |
|---|---|---|---|---|
| Age at Scan (SD) | 33.6 (13.64) | 35.8 (12.10) | t(37) = 0.50 | 0.50 |
| Gender (%F) | 78 | 50 | χ2(1) = 3.5 | 0.06 |
| Handedness (R:L) | 18:1 | 19:1 | χ2(1) = .001 | 0.97 |
| Verbal IQ (AMNART) (SD)* | 123.55 (4.8) | 124.16 (2.25) | t(34) = .50 | 0.13 |
| Trait anxiety score STAI (SD) | 37.5 (8.65) | 27 (5.89) | t(37) = −4.48 | 0.001 |
| HAMD score (SD) | 1.79 (1.27) | 0.45 (0.99) | t(37) = −3.67 | 0.11 |
| Duration of Illness, months (SD) | 33.9 (25.7) | - | - | - |
| Lifetime number MDE's (SD) | 4.42 (6.45) | - | - | - |
| Duration of euthymia, months (SD) | 15.10 (12.26) | - | - | - |
Note: MDE's= Major Depressive Episodes. HAMD= Hamilton Depression Rating Scale. STAI= State-Trait Anxiety Inventory. AMNART= American version of the Nelson Adult reading test.
Information not available for 3 rMDD participants
Assessments also included the Edinburgh Handedness Inventory (Oldfield, 1971), the American version of the Nelson Adult Reading Test (AMNART) (Grober and Sliwinski, 1991), and the State Trait Anxiety Inventory (STAI) (Spielberger, 1983). All participants provided written informed consent in accordance with approval by the Yale Human Investigation Committee (HIC) and Hartford Hospital Institutional Review Board (IRB).
2.2 Event-related emotional face paradigm
Details pertaining to the event-related emotional face gender-labeling task have been described previously (Shah et al., 2008; Kalmar et al., 2009). Briefly, faces from the Ekman series (Ekman and Friesen, 1979) depicting negative emotional expressions (fear), positive emotional expressions (happiness) or neutrality were shown to participants via Eprime software on a computer attached to a projector (Psychology Software Tools; Pittsburgh, PA). Participants made a male-female discrimination by pressing one of two corresponding buttons on a button box. Each face was presented for 2 seconds and separated by 4, 8 or 12 second intervals, during which participants viewed a crosshair. Each run was comprised by 10 grey scale face stimuli (5 females, 5 males) with each actor exhibiting all 3 of the expressions yielding a total of 30 stimuli in each run. Order of face stimuli was counterbalanced for facial expression, sex, identity and the length of the inter-stimulus-interval. Participants completed 4 runs of the task (total duration 19 minutes, 20 seconds). Instructions were presented on the computer at the beginning of each run and subjects were asked to respond as quickly and accurately as possible. Detailed instructions and practice trials were completed on a computer outside the scanner prior to the scanning session.
2.3 MRI data acquisition
Participants were scanned using a 3-Tesla Siemens Allegra MR scanner (Siemens, Erlangen Germany) at the Olin Neuropsychiatric Research Center (Hartford Hospital, CT). A custom head cushion was used for head stabilization. T2* weighted images were acquired with a gradient echo planar imaging (EPI) sequence as follows: TR= 1.86s, TE= 27ms, FOV= 220mm× 220mm, matrix size= 64 × 64, voxel size= 3.44mm × 3.44mm × 4mm, slice thickness= 3mm with a 1mm slice gap, number of sequentially acquired slices= 36, flip angle= 70°.
2.4 Behavioral data analysis
Behavioral data were analyzed using a mixed-model repeated measures MANOVA with group as a between-subject factor, and facial expression type (fear, happy and neutral) as a within-subject factor. The multivariate statistic reported is Wilk's lambda. If sphericity assumptions were violated, Greenhouse-Geisser corrections were used. Participants were excluded (and their imaging data discarded) if their reaction time data was greater or less than two standard deviations of the group mean and/or if their mean accuracy was less than 90% resulting in the exclusion of 3 participants (all HC). Mean reaction times were computed for each participant across each of the emotions (i.e. fear, happy and neutral).
2.5 fMRI data processing and analysis
Data preprocessing was performed with SPM2 software (Wellcome Department of Imaging Neuroscience, Institute of Neurology, University College London, UK), running in Matlab 7.0 software. The first 5 images were discarded to account for the approach of the hemodynamic response to steady state, and to achieve a better magnetic stabilization. Images for each run were realigned to the 6th image of each run using the INRIAlign toolbox (Freire et al., 2002) to reduce interscan motion, creating an overall mean image from each run. Unusable imaging data due to susceptibility artifacts or translation motion greater than 3mm and rotational movement greater than 1 degree were discarded and not included in the analysis, resulting in the exclusion of 2 participants (1 rMDD, 1 HC). A mean image was constructed for each run from the realigned image volumes. This mean image volume was then used to determine parameters for spatial normalization into the Montreal Neurological Institute (MNI) standardized space employed in statistical parametric mapping (SPM2). The normalization parameters determined for the mean functional volume were then applied to the corresponding functional image volumes for each participant. Finally the normalized functional images were smoothed with a 12mm full-width half-maximum Gaussian filter. Event-related hemodynamic response amplitudes, along with their time derivative were estimated for each participant using a general linear model for each of the three event types (fearful, happy and neutral expressions) employed within the SPM2 (http://www.fil.ion.ucl.ac.uk/spm/) software (Buxton, 2002). A high pass filter of 128 seconds was used to remove low frequency artifact signals. Statistical maps for each subject were then created for the two emotion-type contrasts: fear minus neutral and happy minus neutral.
Following this, at the group level, one-sample t-test contrast maps were used to assess regional positive and negative BOLD change within each subject group for the two emotion contrasts. To examine differences between the rMDD group and the HC group for the two emotion contrasts we used two approaches. Firstly, to test region-based hypotheses of group differences, we performed region of interest (ROI) analyses. ROIs were defined by WFU PickAtlas Utility (http://www.fmri.wfubmc.edu/cms/software#WFU_PickAtlas). Specifically, predefined anatomical masks (WFU Pickatlas Tool) were applied to define the following bilateral areas: DLPFC (BA 9/46), VLPFC (BA 45/47), OFC (BA 11/12), sgACC (BA 25), amygdala, and ventral striatum. To control for multiple statistical testing within the search volume of each of the bilateral ROIs, we maintained a false positive detection rate at P<0.05 at the voxel level for each of the contrasts and family-wise error (FWE) correction using a spatial extent threshold (Forman et al., 1995) with AlphaSim software implemented in AFNI. The number of contiguous voxels needed to maintain this false positive detection rate in each ROI was computed separately for each contrast and empirically determined by Monte Carlo simulations implemented in AlphaSim, which accounted for spatial correlations between BOLD signal changes in neighboring voxels (Ward, 2000). AlphaSim is a recommended approach for family-wise error correction as it provides adequate dual thresholding of both type-I error and cluster size while taking into account the smoothness of the data (Bennett et al., 2009).
Our second approach involved conducting exploratory analyses to assess patterns of activation occurring across the whole brain. A random effects analysis was carried out in SPM2 using a two-sample t-test for each contrast to generate two difference images (i.e. activation significantly greater in HC vs. rMDD and vice versa) across the brain. Due to the exploratory nature of this analysis, findings were considered significant at P<.001, uncorrected, Ke= 20 contiguous voxels. Inclusion of gender as a covariate did not alter the imaging results and were thus not included in all subsequent analyses.
2.6 Exploratory analyses examining relationships with clinical variables
Post-hoc analyses were performed to explore whether there was a relationship between activation during the processing of fearful (vs. neutral) and happy (vs. neutral) emotional stimuli across the brain including our ROIs, and trait anxiety scores, as well as duration of the current period of euthymia. We did this to assess if trait anxiety has modulating effects on neural activation that may persist into remission, and whether recovery is associated with changes in the neural circuitry underlying emotional processing. For each correlation trait anxiety and duration of euthymia scores were entered as regressors in a second-level analysis for the 2 contrasts (fear vs. neutral and happy vs. neutral) within SPM2. Where significant activations occurred that correlated with our covariates, a 2mm sphere was applied around the coordinates of the peak voxel within the significant cluster to extract out the average signal from the sphere. The values for each individual obtained from this analysis were then analyzed in SPSS to plot the data using Pearson's regression analyses. A threshold of P<.001, uncorrected, and a cluster threshold of Ke = 20 was used for each contrast.
3. Results
3.1 Behavioral data
There were no significant differences between the rMDD and HC groups in task performance. All 39 participants were highly accurate on the task (>90% correct). For response accuracy there was no significant main effect of group F(1, 37) = .790, P= 0.380, or emotion F(2, 36)= 1.14, P= .331, or significant group x emotion interaction F(2, 36) = 2.44, P= .10. Similarly, with regard to reaction time, there was no significant main effect of group F(1, 37) = 1.31, P= 0.26. There was a main effect of emotion F(2, 36) = 6.12, P= .005. Post-hoc simple contrast analyses with facial expression type as a within-subject factor indicated that all participants responded faster to happy faces compared to fearful faces F(2, 37) = 10.75, P= .002. There was also a significant group x emotion interaction F(2, 36) = 4.95, P= .013. Post-hoc independent t-tests with an adjusted threshold of P= .05/ (number of t-tests) used to compare groups across each emotion type, revealed no significant differences between the groups at the corrected or uncorrected level.
3.2 Functional magnetic resonance imaging results
In the following section, we present the ROI findings for each contrast (fear vs. neutral and happy vs. neutral). This is followed by the results of the whole-brain exploratory analyses for the same contrasts. The exclusion of two left-handed individuals (one MDD, one HC) had no significant effect on neural activation across the brain or in any of the ROIs for either contrast. Thus all subsequent analyses included all 39 participants.
3.2.1 ROI Analyses
For the fear vs. neutral face contrast, ROI analyses showed significantly lower activation in left OFC (BA 11) (t37= 3.85, P<0.001, PFWE<0.05, Ke= 866 voxels) and left DLPFC (BA 46) (t37= 2.55, P=0.008, PFWE<0.05, Ke= 136 voxels) in the rMDD group compared to the HC group (Figures 1 and 2 respectively). No significant between-group differences were found in any other ROI.
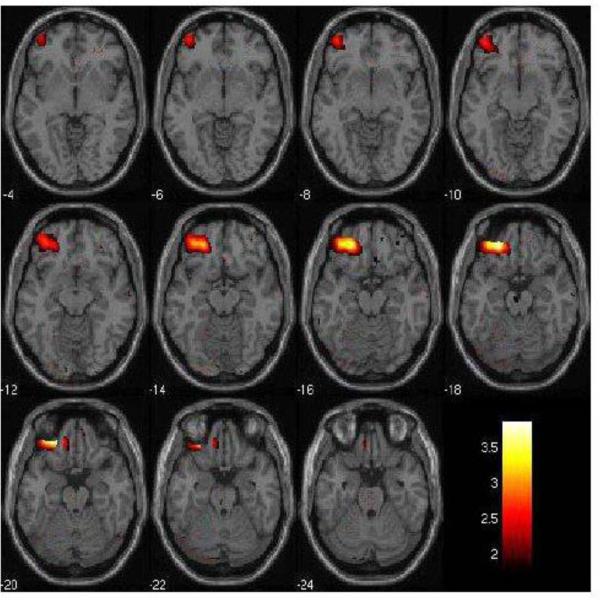
Figure 1. Left Orbitofrontal Cortex Decreases in rMDD individuals in the Fear vs. Neutral Condition.
The 2mm axial-oblique slices display the region of orbitofrontal cortex (BA11) activation decreases in the fearful vs. neutral face condition in the remitted major depressive disorder (rMDD, n=19) group compared to the healthy comparison (HC, n=20) group, at P<0.005, uncorrected. The differences survived correction for multiple comparisons (PFWE<0.05). Numbers to the left of the images are z-planes (Montreal Neurological Institute) in millimeters. The color bar shows the T values. The maxima was at coordinates x= −42mm, y= 22mm, z=−20mm; Ke= 866 voxels.
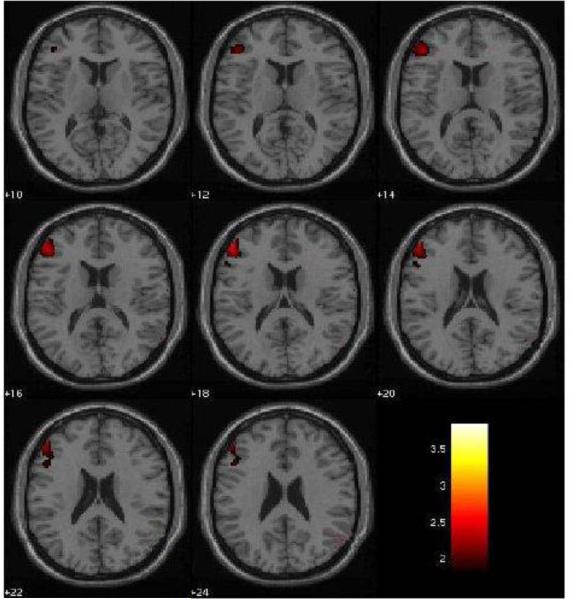
Figure 2. Left Dorsolateral Prefrontal Cortex Decreases in rMDD individuals in the Fear vs. Neutral Condition.
The 2mm axial-oblique slices display the region of dorsolateral prefrontal cortex (BA9) activation decreases in the fearful vs. neutral face condition in the remitted major depressive disorder (rMDD, n=19) group compared to the healthy comparison (HC, n=20) group, at P<0.005, uncorrected. The differences survived correction for multiple comparisons (PFWE<0.05). Numbers to the left of the images are z-planes (Montreal Neurological Institute) in millimeters. The color bar shows the T values. The maxima was at coordinates x= −50, y= 40, z= 20; Ke= 136 voxels.
To further clarify these findings we performed exploratory analyses comparing each emotion condition (fear or neutral) with the baseline BOLD response (i.e. no face). For fear vs. baseline, consistent with our findings for fear vs. neutral, we found reduced activation in left DLPFC (BA 46) in rMDD relative to HC (P=0.003, uncorrected, Ke= 118 voxels).
There were no significant between-group differences for the happy vs. neutral face contrast in any of our ROIs. Comparing each emotion condition (happy or neutral) with baseline BOLD response revealed no significant differences.
3.2.2 Whole-brain Analyses
For the fear vs. neutral face contrast, in addition to the findings in the hypothesized regions, the voxel-wise whole-brain exploratory analyses revealed lower activation in rMDD group compared to HC in a cluster in right parietal lobe (t37= 3.79, P<0.001 uncorrected, Ke= 22) and left occipital lobe (t37= 2.52, P<0.001 uncorrected, Ke=31). However, these results did not survive FWE correction at P<0.05.
For the happy vs. neutral face contrast there were no additional regions of group differences.
3.3 Correlation analyses between neuroimaging data and clinical variables
No significant relationships were found between the ROIs and trait anxiety scores in the rMDD group for either the fearful or happy (vs. neutral) contrasts.
Correlation analyses revealed a significant, positive relationship between right DLPFC activation and duration of euthymia in rMDD individuals during the presentation of fearful (vs. neutral) faces (r(19)= .69, P<0.001) (Figure 3). No significant relationships were found between DLPFC activation and duration of euthymia for happy vs. neutral face contrast in the DLPFC or in any of our ROIs in the rMDD group.
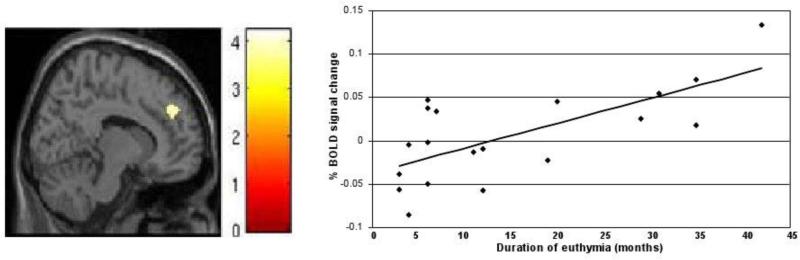
Figure 3. Association between Dorsolateral Prefrontal Cortex Activation and Duration of Euthymia.
Left: The sagittal image (Montreal Neurological Institute plane x= 12mm) displays the right dorsolateral prefrontal cortex region (BA9) in which response during the fearful vs. neutral face condition was significantly associated with duration of the current euthymic period in the participants with remitted major depressive disorder (n=19), at P<0.001. The color bar shows the T values. The maxima was at coordinates x=12, y= 46, z= 34; Ke= 75 voxels. Right: The graph demonstrates the positive correlation between the months of the current euthymic period in the participants with remitted major depressive disorder (n=19) and the percent of blood oxygen level dependent signal change extracted from the peak of the right dorsolateral prefrontal region shown in the image to the left, r= 0.69, r2= 0.47.
Together, the findings support diminished response of the left OFC and left DLPFC during the implicit emotional stimulus processing in rMDD individuals, especially when the stimuli are of a negative valence, and that recovery of MDD may include compensatory changes in the right DLPFC.
4. Discussion
In this study we used fMRI during an implicit negative and positive emotion stimulus processing task to investigate differences in blood oxygen level dependent response in fully recovered individuals with MDD. Relative to HC participants, individuals with rMDD showed significantly reduced activation in the left OFC (BA 11) and left DLPFC (BA 46) during the processing of faces depicting negative emotions. The rMDD individuals were euthymic, unmedicated, had a family history of MDD and were without psychiatric comorbidity, suggesting that the findings may represent a trait marker for MDD. Duration of euthymia was significantly associated with increases in right DLPFC activation. This suggests that recovery from MDD may be associated with compensatory changes in the right DLPFC.
Significant PFC reductions in the MDD group were found only during the processing of negative emotional stimuli. This finding is significant as this is one of the few studies in rMDD that has separately examined abnormalities in the processing of positive and negative emotions. The findings suggest that abnormalities in the processing of stimuli of a negative emotional valence may be a trait feature of MDD. These findings are consistent with previous neuroimaging studies in rMDD. For example, in a PET study using a mood challenge paradigm to induce sad mood, Liotti et al., (2002) reported significantly lower activation in medial OFC (BA 10/11) in rMDD individuals compared to HC. Norbury et al., (2009) reported altered DLPFC activation in response to fearful faces during an explicit face-matching paradigm in rMDD individuals. Our findings are also consistent with a number of previous studies in acutely depressed and remitted MDD individuals which have similarly reported abnormalities in the OFC and DLPFC in response to negatively valenced stimuli including masked emotional faces (Fu et al., 2004; Neumeister et al., 2006; Fales et al., 2009), and negative emotional distracters (Fales et al., 2008; Wang et al., 2008). Together these findings support cognitive theories of MDD in which abnormal responses to negative emotional stimuli are central to the disorder (Beck et al., 1979). Whilst there are some studies that have reported abnormalities in the processing of happy facial stimuli in individuals with MDD when acutely depressed (i.e. during a major depressive episode) (Surguladze et al., 2005) and in unmedicated, remitted MDD individuals (Kerestes et al., 2011), the most predominant findings have been in response to negative emotional stimuli, including consistent findings in remitted individuals, suggesting these may represent a trait marker specific to MDD. The prominence of the findings in response to negative emotional stimuli in MDD contrasts with that observed in individuals with Bipolar Disorder (BD) where more pronounced functional abnormalities in response to positive emotional stimuli have been reported (Lawrence et al., 2004; Blumberg et al., 2005). These impairments in emotional processing of positive stimuli have been reported in BD individuals in all phases of the illness including mania (Altshuler et al., 2005; Chen et al., 2006), depression (Malhi et al., 2004), and euthymia (Hassel et al., 2008). This suggests that abnormal responses to negative but not positive emotional stimuli may help to distinguish the MDD from the BD trait. Future studies are needed which directly compare neural responses to positive and negative emotional stimuli in remitted individuals with MDD and BD to help characterize the different nature of the deficits in emotional processing.
We observed evidence for lateralization of the emotional processing deficits in rMDD. The reduced left-sided OFC and DLPFC activation in rMDD individuals is consistent with theories of the lateralization of emotions and findings of depression in association with lesions in left frontal cortex (Robinson and Starkstein, 1989; Fedoroff et al., 1992; Jorge et al., 1993). A variation of this theory suggests that in healthy individuals left lateral PFC subserves approach behaviors (Wager et al., 2003), suggesting that left PFC lesions and/or abnormal activation in this area may contribute to the manifestation of behavioral constriction in MDD. Though at the trend-level, left-sided reductions in parietal and occipital lobe activation observed in rMDD patients during the processing of negative faces suggest that left hemisphere abnormalities may include these posterior association cortices required for the rapid perception and evaluation of faces (Haxby et al., 2000).
Converging lines of evidence from structural neuroimaging and postmortem studies of individuals with MDD provide further support for PFC trait markers in MDD. These studies provide evidence for structural differences in MDD that may underlie the functional differences observed. For example, magnetic resonance imaging (MRI) studies have reported reductions in OFC grey matter volume in acutely depressed (Lai et al., 2000; Wagner et al., 2008), and remitted (Bremner et al., 2002) individuals with MDD relative to HC subjects. Postmortem studies also suggest cellular alterations that may contribute to the differences. For example, significant reductions in neuronal size and glial densities in the OFC and DLPFC have been documented in MDD (Rajkowska et al., 1999; Cotter et al., 2002).
In the rMDD group, duration of remission was associated with activation in the right DLPFC (BA 9), such that the longer duration of euthymia in rMDD individuals, the greater the activation in the right DLPFC to negative facial expressions. We speculate that this additional recruitment of the contralateral PFC may represent a compensatory response in at least a subset of rMDD patients that contributes to recovery. These findings are important, as there has been little previous study of the changes in regional function in recovery from mood disorders. Future longitudinal studies examining DLPFC activation could provide valuable insights into the role of the DLPFC in recovery from depression and how it might be targeted in the design of future interventions.
A limitation to interpreting the findings as trait markers of vulnerability to MDD is that all of the rMDD participants were required to have experienced at least two previous major depressive episodes. While this criterion reduced sample heterogeneity in order to study recurrent MDD, it also raises the possibility that the group differences found reflect vulnerability to depressive relapse, or a residual abnormality due to previous episodes of the illness, rather than biological markers that predispose initial onset of MDD. In addition, the lack of an acutely depressed group to which direct comparisons could be made with the remitted group makes it difficult to identify state vs. trait markers of the illness. Future longitudinal studies examining unaffected offspring at-risk are required in order to advance our understanding of vulnerability markers of the illness that might be targeted for prevention. Indeed, abnormal activations in the amygdala and nucleus accumbens to negative facial expressions have been reported in children at-risk for MDD (Monk et al., 2008). Similarly, studies examining siblings of MDD patients, as well as first-episode patients will provide valuable insight to understanding trait markers of MDD. We did not detect differences in the sgACC, amygdala or ventral striatum, areas implicated previously in MDD studies (Drevets et al., 1997; Sheline et al., 2001; Neumeister et al., 2006; Victor et al., 2010). Possible reasons for this could include that previous findings were related to state, comorbidity or medication; however, differences in these regions may not have been detected because of insufficient power owing to the small sample size in the present study, or methodological differences across studies related to task design and emotional stimuli employed. Finally, there was a gender imbalance in the rMDD group. We covaried for gender in our analyses, however we did not have sufficient power to assess gender effects so it is not clear whether the findings generalize to both genders. Replication of the present findings in larger cohorts of females and males with rMDD is thus required.
In conclusion, the findings suggest that clinical recovery in unmedicated MDD individuals is associated with enduring, trait-like abnormalities in the left OFC and DLPFC in the context of processing negative emotional stimuli. Further, the right DLPFC may mediate a compensatory response during recovery. Collectively these findings could have significant clinical implications for the identification of trait markers of MDD, as well as for identifying mechanisms contributing to recovery in MDD individuals.
Supplementary Material
Acknowledgments
We would like to thank the patients who participated in the study, Russell Starankewicz for his MRI technical assistance, the Connecticut Mental Health Center and the ONRC staff for their contributions to the study.
Funding/Support
The authors were supported by grants from the National Institute of Mental Health Grant Nos. R01 MH69747 (HPB), R01 MH070902 (HPB), RC1 MH088366 (HPB), K23 MH077914 (ZB), CTSA UL1 RR024139 (ZB), R01 MH076971 (MLP), K01 MH083001 (CDL), R37 MH43775 (GP), R01 MH074797 (GP), R01 MH077945 (HPB, GP), K01MH086621 (FW), the Department of Veterans Affairs Research Enhancement Award Program (REAP) (HPB), the National Alliance for Research in Schizophrenia and Depression (Great Neck, New York) (ZB, HPB, FW, CDL), the Attias Family Foundation (HPB), Women's Health Research at Yale (New Haven, Connecticut) (HPB), and the Klingenstein Third Generation Foundation (New York, New York) (FW).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of Interest
Dr Bhagwagar is an employee of Bristol-Myers Squibb though the study was conceived, designed and data collected before he started working for BMS. None of the statements in the paper represent the views of BMS. None of the other authors have financial disclosures to report.
References
- Altshuler LL, Bookheimer SY, Townsend J, Proenza MA, Eisenberger N, Sabb F, Mintz J, Cohen MS. Blunted activation in orbitofrontal cortex during mania: a functional magnetic resonance imaging study. Biol Psychiatry. 2005;58:763–769. doi: 10.1016/j.biopsych.2005.09.012. [DOI] [PubMed] [Google Scholar]
- Andreasen NC, Endicott J, Spitzer RL, Winokur G. The family history method using diagnostic criteria. Reliability and validity. Arch Gen Psychiatry. 1977;34:1229–1235. doi: 10.1001/archpsyc.1977.01770220111013. [DOI] [PubMed] [Google Scholar]
- Baxter LR, Jr., Schwartz JM, Phelps ME, Mazziotta JC, Guze BH, Selin CE, Gerner RH, Sumida RM. Reduction of prefrontal cortex glucose metabolism common to three types of depression. Archives of General Psychiatry. 1989;46:243–250. doi: 10.1001/archpsyc.1989.01810030049007. [DOI] [PubMed] [Google Scholar]
- Beck AT, Rush AJ, Shaw BF, Emery G. Cognitive therapy of depression. Guildford Press; New York: 1979. [Google Scholar]
- Bench CJ, Friston KJ, Brown RG, Frackowiak RS, Dolan RJ. Regional cerebral blood flow in depression measured by positron emission tomography: the relationship with clinical dimensions. Psychological Medicine. 1993;23:579–590. doi: 10.1017/s0033291700025368. [DOI] [PubMed] [Google Scholar]
- Bennett CM, Wolford GL, Miller MB. The principled control of false positives in neuroimaging. Soc Cogn Affect Neurosci. 2009;4:417–422. doi: 10.1093/scan/nsp053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhagwagar Z, Cowen PJ. ‘It's not over when it's over’: persistent neurobiological abnormalities in recovered depressed patients. Psychological Medicine. 2008;38:307–313. doi: 10.1017/s0033291707001250. [DOI] [PubMed] [Google Scholar]
- Bhagwagar Z, Cowen PJ, Goodwin GM, Harmer CJ. Normalization of enhanced fear recognition by acute SSRI treatment in subjects with a previous history of depression. American Journal of Psychiatry. 2004;161:166–168. doi: 10.1176/appi.ajp.161.1.166. [DOI] [PubMed] [Google Scholar]
- Biver F, Goldman S, Delvenne V, Luxen A, De Maertelaer V, Hubain P, Mendlewicz J, Lotstra F. Frontal and parietal metabolic disturbances in unipolar depression. Biol Psychiatry. 1994;36:381–388. doi: 10.1016/0006-3223(94)91213-0. [DOI] [PubMed] [Google Scholar]
- Blumberg HP, Donegan NH, Sanislow CA, Collins S, Lacadie C, Skudlarski P, Gueorguieva R, Fulbright RK, McGlashan TH, Gore JC, Krystal JH. Preliminary evidence for medication effects on functional abnormalities in the amygdala and anterior cingulate in bipolar disorder. Psychopharmacology (Berl) 2005;183:308–313. doi: 10.1007/s00213-005-0156-7. [DOI] [PubMed] [Google Scholar]
- Bremner JD, Vythilingam M, Vermetten E, Nazeer A, Adil J, Khan S, Staib LH, Charney DS. Reduced volume of orbitofrontal cortex in major depression. Biol Psychiatry. 2002;51:273–279. doi: 10.1016/s0006-3223(01)01336-1. [DOI] [PubMed] [Google Scholar]
- Buxton RB. Introduction to Functional Magnetic Resonance Imaging:Principles and Techniques. Cambridge University Press; Cambridge: 2002. [Google Scholar]
- Chen CH, Lennox B, Jacob R, Calder A, Lupson V, Bisbrown-Chippendale R, Suckling J, Bullmore E. Explicit and implicit facial affect recognition in manic and depressed States of bipolar disorder: a functional magnetic resonance imaging study. Biol Psychiatry. 2006;59:31–39. doi: 10.1016/j.biopsych.2005.06.008. [DOI] [PubMed] [Google Scholar]
- Cohen RM, Gross M, Nordahl TE, Semple WE, Oren DA, Rosenthal N. Preliminary data on the metabolic brain pattern of patients with winter seasonal affective disorder. Archives of General Psychiatry. 1992;49:545–552. doi: 10.1001/archpsyc.1992.01820070039006. [DOI] [PubMed] [Google Scholar]
- Cotter D, Mackay D, Chana G, Beasley C, Landau S, Everall IP. Reduced neuronal size and glial cell density in area 9 of the dorsolateral prefrontal cortex in subjects with major depressive disorder. Cereb Cortex. 2002;12:386–394. doi: 10.1093/cercor/12.4.386. [DOI] [PubMed] [Google Scholar]
- Davidson RJ. Anterior cerebral asymmetry and the nature of emotion. Brain and Cognition. 1992;20:125–151. doi: 10.1016/0278-2626(92)90065-t. [DOI] [PubMed] [Google Scholar]
- Drevets WC, Price JL. Neuroimaging and neuropathological studies of mood disorders. In: Licinio JWM, editor. Biology of Depression: from Novel Insights to Therapeutic Strategies. Wiley–VCH Verlag GmbH; Weinheim: 2005. pp. 427–466. [Google Scholar]
- Drevets WC, Price JL, Simpson JR, Jr., Todd RD, Reich T, Vannier M, Raichle ME. Subgenual prefrontal cortex abnormalities in mood disorders. Nature. 1997;386:824–827. doi: 10.1038/386824a0. [DOI] [PubMed] [Google Scholar]
- Drevets WC, Videen TO, Price JL, Preskorn SH, Carmichael ST, Raichle ME. A functional anatomical study of unipolar depression. The Journal of Neuroscience. 1992;12:3628–3641. doi: 10.1523/JNEUROSCI.12-09-03628.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebert D, Feistel H, Barocka A. Effects of sleep deprivation on the limbic system and the frontal lobes in affective disorders: a study with Tc-99m-HMPAO SPECT. Psychiatry Res. 1991;40:247–251. doi: 10.1016/0925-4927(91)90016-j. [DOI] [PubMed] [Google Scholar]
- Ekman P, Friesen WV. Pictures of Facial Affect. Consulting Psychologists; Palo Alto: 1979. [Google Scholar]
- Fales CL, Barch DM, Rundle MM, Mintun MA, Mathews J, Snyder AZ, Sheline YI. Antidepressant treatment normalizes hypoactivity in dorsolateral prefrontal cortex during emotional interference processing in major depression. Journal of Affective Disorders. 2009;112:206–211. doi: 10.1016/j.jad.2008.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fales CL, Barch DM, Rundle MM, Mintun MA, Snyder AZ, Cohen JD, Mathews J, Sheline YI. Altered emotional interference processing in affective and cognitive-control brain circuitry in major depression. Biological Psychiatry. 2008;63:377–384. doi: 10.1016/j.biopsych.2007.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedoroff JP, Starkstein SE, Forrester AW, Geisler FH, Jorge RE, Arndt SV, Robinson RG. Depression in patients with acute traumatic brain injury. American Journal of Psychiatry. 1992;149:918–923. doi: 10.1176/ajp.149.7.918. [DOI] [PubMed] [Google Scholar]
- First MB. The DSM series and experience with DSM-IV. Psychopathology. 2002;35:67–71. doi: 10.1159/000065121. [DOI] [PubMed] [Google Scholar]
- Forman SD, Cohen JD, Fitzgerald M, Eddy WF, Mintun MA, Noll DC. Improved assessment of significant activation in functional magnetic resonance imaging (fMRI): use of a cluster-size threshold. Magnetic Resonance in Medicine. 1995;33:636–647. doi: 10.1002/mrm.1910330508. [DOI] [PubMed] [Google Scholar]
- Freire L, Roche A, Mangin JF. What is the best similarity measure for motion correction in fMRI time series? IEEE Transactions on Medical Imaging. 2002;21:470–484. doi: 10.1109/TMI.2002.1009383. [DOI] [PubMed] [Google Scholar]
- Fu CH, Williams SC, Cleare AJ, Brammer MJ, Walsh ND, Kim J, Andrew CM, Pich EM, Williams PM, Reed LJ, Mitterschiffthaler MT, Suckling J, Bullmore ET. Attenuation of the neural response to sad faces in major depression by antidepressant treatment: a prospective, event-related functional magnetic resonance imaging study. Archives of General Psychiatry. 2004;61:877–889. doi: 10.1001/archpsyc.61.9.877. [DOI] [PubMed] [Google Scholar]
- Grober E, Sliwinski M. Development and validation of a model for estimating premorbid verbal intelligence in the elderly. Journal of Clinical and Experimental Neuropsychology. 1991;13:933–949. doi: 10.1080/01688639108405109. [DOI] [PubMed] [Google Scholar]
- Gur RC, Erwin RJ, Gur RE, Zwil AS, Heimberg C, Kraemer HC. Facial emotion discrimination: II. Behavioral findings in depression. Psychiatry Research. 1992;42:241–251. doi: 10.1016/0165-1781(92)90116-k. [DOI] [PubMed] [Google Scholar]
- Hamilton M. A rating scale for depression. Journal of Neurology, Neurosurgery and Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harmer CJ, O'Sullivan U, Favaron E, Massey-Chase R, Ayres R, Reinecke A, Goodwin GM, Cowen PJ. Effect of acute antidepressant administration on negative affective bias in depressed patients. Am J Psychiatry. 2009;166:1178–1184. doi: 10.1176/appi.ajp.2009.09020149. [DOI] [PubMed] [Google Scholar]
- Hassel S, Almeida JR, Kerr N, Nau S, Ladouceur CD, Fissell K, Kupfer DJ, Phillips ML. Elevated striatal and decreased dorsolateral prefrontal cortical activity in response to emotional stimuli in euthymic bipolar disorder: no associations with psychotropic medication load. Bipolar Disord. 2008;10:916–927. doi: 10.1111/j.1399-5618.2008.00641.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haxby JV, Hoffman EA, Gobbini MI. The distributed human neural system for face perception. Trends Cogn Sci. 2000;4:223–233. doi: 10.1016/s1364-6613(00)01482-0. [DOI] [PubMed] [Google Scholar]
- Hsu DT, Langenecker SA, Kennedy SE, Zubieta JK, Heitzeg MM. fMRI BOLD responses to negative stimuli in the prefrontal cortex are dependent on levels of recent negative life stress in major depressive disorder. Psychiatry Res. 2010;183:202–208. doi: 10.1016/j.pscychresns.2009.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnstone T, van Reekum CM, Urry HL, Kalin NH, Davidson RJ. Failure to regulate: counterproductive recruitment of top-down prefrontal-subcortical circuitry in major depression. The Journal of Neuroscience. 2007;27:8877–8884. doi: 10.1523/JNEUROSCI.2063-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorge RE, Robinson RG, Arndt SV, Starkstein SE, Forrester AW, Geisler F. Depression following traumatic brain injury: a 1 year longitudinal study. Journal of Affective Disorders. 1993;27:233–243. doi: 10.1016/0165-0327(93)90047-n. [DOI] [PubMed] [Google Scholar]
- Kalmar JH, Wang F, Chepenik LG, Womer FY, Jones MM, Pittman B, Shah MP, Martin A, Constable RT, Blumberg HP. Relation between amygdala structure and function in adolescents with bipolar disorder. J Am Acad Child Adolesc Psychiatry. 2009;48:636–642. doi: 10.1097/CHI.0b013e31819f6fbc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keedwell PA, Andrew C, Williams SC, Brammer MJ, Phillips ML. A double dissociation of ventromedial prefrontal cortical responses to sad and happy stimuli in depressed and healthy individuals. Biological Psychiatry. 2005;58:495–503. doi: 10.1016/j.biopsych.2005.04.035. [DOI] [PubMed] [Google Scholar]
- Kennedy SH, Evans KR, Kruger S, Mayberg HS, Meyer JH, McCann S, Arifuzzman AI, Houle S, Vaccarino FJ. Changes in regional brain glucose metabolism measured with positron emission tomography after paroxetine treatment of major depression. American Journal of Psychiatry. 2001;158:899–905. doi: 10.1176/appi.ajp.158.6.899. [DOI] [PubMed] [Google Scholar]
- Kerestes R, Ladouceur CD, Meda S, Nathan PJ, Blumberg HP, Maloney K, Ruf B, Saricicek A, Pearlson GD, Bhagwagar Z, Phillips ML. Abnormal prefrontal activity subserving attentional control of emotion in remitted depressed patients during a working memory task with emotional distracters. Psychol Med. 2011:1–12. doi: 10.1017/S0033291711001097. [DOI] [PubMed] [Google Scholar]
- Lai T, Payne ME, Byrum CE, Steffens DC, Krishnan KR. Reduction of orbital frontal cortex volume in geriatric depression. Biol Psychiatry. 2000;48:971–975. doi: 10.1016/s0006-3223(00)01042-8. [DOI] [PubMed] [Google Scholar]
- Lawrence NS, Williams AM, Surguladze S, Giampietro V, Brammer MJ, Andrew C, Frangou S, Ecker C, Phillips ML. Subcortical and ventral prefrontal cortical neural responses to facial expressions distinguish patients with bipolar disorder and major depression. Biol Psychiatry. 2004;55:578–587. doi: 10.1016/j.biopsych.2003.11.017. [DOI] [PubMed] [Google Scholar]
- Leppanen JM, Milders M, Bell JS, Terriere E, Hietanen JK. Depression biases the recognition of emotionally neutral faces. Psychiatry Research. 2004;128:123–133. doi: 10.1016/j.psychres.2004.05.020. [DOI] [PubMed] [Google Scholar]
- Liotti M, Mayberg HS, McGinnis S, Brannan SL, Jerabek P. Unmasking disease-specific cerebral blood flow abnormalities: mood challenge in patients with remitted unipolar depression. Am J Psychiatry. 2002;159:1830–1840. doi: 10.1176/appi.ajp.159.11.1830. [DOI] [PubMed] [Google Scholar]
- Malhi GS, Lagopoulos J, Ward PB, Kumari V, Mitchell PB, Parker GB, Ivanovski B, Sachdev P. Cognitive generation of affect in bipolar depression: an fMRI study. Eur J Neurosci. 2004;19:741–754. doi: 10.1111/j.0953-816x.2003.03159.x. [DOI] [PubMed] [Google Scholar]
- Mayberg HS. Limbic-cortical dysregulation: a proposed model of depression. J Neuropsychiatry Clin Neurosci. 1997;9:471–481. doi: 10.1176/jnp.9.3.471. [DOI] [PubMed] [Google Scholar]
- Monk CS, Klein RG, Telzer EH, Schroth EA, Mannuzza S, Moulton JL, 3rd, Guardino M, Masten CL, McClure-Tone EB, Fromm S, Blair RJ, Pine DS, Ernst M. Amygdala and nucleus accumbens activation to emotional facial expressions in children and adolescents at risk for major depression. Am J Psychiatry. 2008;165:90–98. doi: 10.1176/appi.ajp.2007.06111917. [DOI] [PubMed] [Google Scholar]
- Neumeister A, Drevets WC, Belfer I, Luckenbaugh DA, Henry S, Bonne O, Herscovitch P, Goldman D, Charney DS. Effects of a alpha 2C-adrenoreceptor gene polymorphism on neural responses to facial expressions in depression. Neuropsychopharmacology. 2006;31:1750–1756. doi: 10.1038/sj.npp.1301010. [DOI] [PubMed] [Google Scholar]
- Norbury R, Selvaraj S, Taylor MJ, Harmer C, Cowen PJ. Increased neural response to fear in patients recovered from depression: a 3T functional magnetic resonance imaging study. Psychological Medicine. 2009;40:425–432. doi: 10.1017/S0033291709990596. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Bunge SA, Gross JJ, Gabrieli JD. Rethinking feelings: an FMRI study of the cognitive regulation of emotion. J Cogn Neurosci. 2002;14:1215–1229. doi: 10.1162/089892902760807212. [DOI] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Phillips ML, Ladouceur CD, Drevets WC. A neural model of voluntary and automatic emotion regulation: implications for understanding the pathophysiology and neurodevelopment of bipolar disorder. Molecular Psychiatry. 2008;13:833–857. doi: 10.1038/mp.2008.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips ML, Travis MJ, Fagiolini A, Kupfer DJ. Medication effects in neuroimaging studies of bipolar disorder. American Journal of Psychiatry. 2008;165:313–320. doi: 10.1176/appi.ajp.2007.07071066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajkowska G, Miguel-Hidalgo JJ, Wei J, Dilley G, Pittman SD, Meltzer HY, Overholser JC, Roth BL, Stockmeier CA. Morphometric evidence for neuronal and glial prefrontal cell pathology in major depression. Biol Psychiatry. 1999;45:1085–1098. doi: 10.1016/s0006-3223(99)00041-4. [DOI] [PubMed] [Google Scholar]
- Robinson RG, Starkstein SE. Mood disorders following stroke: new findings and future directions. J Geriatr Psychiatry. 1989;22:1–15. [PubMed] [Google Scholar]
- Shah MP, Wang F, Kalmar JH, Chepenik LG, Tie K, Pittman B, Jones MM, Constable RT, Gelernter J, Blumberg HP. Role of variation in the serotonin transporter protein gene (SLC6A4) in trait disturbances in the ventral anterior cingulate in bipolar disorder. Neuropsychopharmacology. 2008;34:1301–1310. doi: 10.1038/npp.2008.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheline YI, Barch DM, Donnelly JM, Ollinger JM, Snyder AZ, Mintun MA. Increased amygdala response to masked emotional faces in depressed subjects resolves with antidepressant treatment: an fMRI study. Biological Psychiatry. 2001;50:651–658. doi: 10.1016/s0006-3223(01)01263-x. [DOI] [PubMed] [Google Scholar]
- Shimoda K, Robinson RG. The relationship between poststroke depression and lesion location in long-term follow-up. Biol Psychiatry. 1999;45:187–192. doi: 10.1016/s0006-3223(98)00178-4. [DOI] [PubMed] [Google Scholar]
- Siegle GJ, Thompson W, Carter CS, Steinhauer SR, Thase ME. Increased amygdala and decreased dorsolateral prefrontal BOLD responses in unipolar depression: related and independent features. Biological Psychiatry. 2007;61:198–209. doi: 10.1016/j.biopsych.2006.05.048. [DOI] [PubMed] [Google Scholar]
- Spielberger CD. State-Trait Inventory (Form Y) Manual. Mind Garden; Redwood City: 1983. [Google Scholar]
- Surguladze S, Brammer MJ, Keedwell P, Giampietro V, Young AW, Travis MJ, Williams SC, Phillips ML. A differential pattern of neural response toward sad versus happy facial expressions in major depressive disorder. Biological Psychiatry. 2005;57:201–209. doi: 10.1016/j.biopsych.2004.10.028. [DOI] [PubMed] [Google Scholar]
- Surguladze SA, Young AW, Senior C, Brebion G, Travis MJ, Phillips ML. Recognition accuracy and response bias to happy and sad facial expressions in patients with major depression. Neuropsychology. 2004;18:212–218. doi: 10.1037/0894-4105.18.2.212. [DOI] [PubMed] [Google Scholar]
- Taylor Tavares JV, Clark L, Furey ML, Williams GB, Sahakian BJ, Drevets WC. Neural basis of abnormal response to negative feedback in unmedicated mood disorders. Neuroimage. 2008;42:1118–1126. doi: 10.1016/j.neuroimage.2008.05.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townsend JD, Eberhart NK, Bookheimer SY, Eisenberger NI, Foland-Ross LC, Cook IA, Sugar CA, Altshuler LL. fMRI activation in the amygdala and the orbitofrontal cortex in unmedicated subjects with major depressive disorder. Psychiatry Res. 2010;183:209–217. doi: 10.1016/j.pscychresns.2010.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Victor TA, Furey ML, Fromm SJ, Ohman A, Drevets WC. Relationship between amygdala responses to masked faces and mood state and treatment in major depressive disorder. Archives of General Psychiatry. 2010;67:1128–1138. doi: 10.1001/archgenpsychiatry.2010.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wager TD, Phan KL, Liberzon I, Taylor SF. Valence, gender, and lateralization of functional brain anatomy in emotion: a meta-analysis of findings from neuroimaging. Neuroimage. 2003;19:513–531. doi: 10.1016/s1053-8119(03)00078-8. [DOI] [PubMed] [Google Scholar]
- Wagner G, Koch K, Schachtzabel C, Reichenbach JR, Sauer H, Schlosser Md RG. Enhanced rostral anterior cingulate cortex activation during cognitive control is related to orbitofrontal volume reduction in unipolar depression. Journal of Psychiatry and Neuroscience. 2008;33:199–208. [PMC free article] [PubMed] [Google Scholar]
- Wang L, LaBar KS, Smoski M, Rosenthal MZ, Dolcos F, Lynch TR, Krishnan RR, McCarthy G. Prefrontal mechanisms for executive control over emotional distraction are altered in major depression. Psychiatry Research: Neuroimaging. 2008;163:143–155. doi: 10.1016/j.pscychresns.2007.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward BD. AFNI AlphaSim documentation. Biophysics Research Institute, Medical College of Wisconsin; Milwaukee, WI: 2000. Simultaneous inference for fMRI data. [Google Scholar]
- Young RC, Biggs JT, Ziegler VE, Meyer DA. A rating scale for mania: reliability, validity and sensitivity. British Journal of Psychiatry. 1978;133:429–435. doi: 10.1192/bjp.133.5.429. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.