Summary
Background
Currently there is a debate regarding whether tamoxifen used in breast cancer has an impact on lipid profiles. The aim of this study was to determine whether tamoxifen has an impact on the serum lipid profile in Taiwanese women.
Patients and Methods
Data of 109 patients were collected from the routine clinical follow-up for women with hormone receptor-positive breast cancer who were treated between July 2005 and March 2008. These patients were divided into 2 subgroups, based on their tumor grade and lymph node status. Subgroup 1 patients had tumor grade I/II and a negative lymph node status. Those patients with tumor grade III or a positive lymph node status were defined as subgroup 2.
Results
In the 109 patients, the mean serum total cholesterol (TC) levels after tamoxifen treatment, as well as the serum low-density lipoprotein cholesterol (LDL-C) levels, were lower than the baseline levels, with statistically significant differences. Treatment with tamoxifen lowered the serum TC and LDL-C levels in both subgroups.
Conclusions
The results indicate that tamoxifen has an impact on the serum lipid profile of breast cancer patients in Taiwan. Physicians should follow up the lipid profile in these patients.
KeyWords: Breast cancer, Tamoxifen, Metabolism, Lipid profile, Total cholesterol, Low-density lipoprotein cholesterol
Introduction
Many oncologists believe that breast cancer is a clinically heterogeneous disease with different responses to therapy [1, 2, 3, 4]. Of interest, some studies have suggested that adjuvant tamoxifen exhibits different effects on the lipid and liver profiles of cancer patients [5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25]. Researchers sought to determine the appropriate role of adjuvant tamoxifen in breast cancer patients regarding serum total cholesterol (TC), triglyceride (TG), high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), aspartate transaminase (AST), and alanine transaminase (ALT) levels. The large population-based study by Hernandez et al. [5] revealed that there were no associations between tamoxifen and the occurrence of atherosclerotic events. These findings are important in risk/benefit analyses as tamoxifen therapy in postmenopausal women is being replaced with aromatase inhibitors.
On the other hand, Esteva and Hortobagyi [7] reported that tamoxifen has an overall beneficial effect on lipid profiles. However, long-term data from tamoxifen clinical trials have failed to reveal a cardioprotective effect and patients treated with tamoxifen did not experience fewer cardiovascular events compared with those receiving a placebo [7]. Information on changes in lipid profiles induced by tamoxifen is still limited and confusing. To our knowledge, there is no similar study on the issue concerning the connection of tumor grade and lipid profile in Taiwan. Our aim was to investigate the significance of adjuvant tamoxifen with respect to lipid profiles and liver function in a group of homogeneously treated Taiwanese breast carcinoma patients.
One study in France suggested that tumor progression-related mechanisms would affect the membrane fatty acid profile in breast cancers [26]. Also, advanced breast cancer patients may receive more adjuvant hormone therapy and chemotherapy; therefore, the grouping factors for patients in this study included tumor grade and lymph node status.
Patients and Methods
This study was approved by the institutional review board and the ethics committee of Changhua Christian Hospital. We collected laboratory profiles of women with breast cancer who had received adjuvant endocrine therapy with tamoxifen between July 2005 and March 2008. 109 patients in the age range of 34–74 years were enrolled in this study.
Adjuvant chemotherapy was performed based on the recommendations of the St. Gallen guidelines or the National Comprehensive Cancer Network, with anthracycline-based chemotherapy regimens (in this study, mostly FEC: 500 mg/m2 5-fluorouracil (5-FU), 75–90 mg/m2 epirubicin, 500 mg/m2 cyclophosphamide). Taxanes (paclitaxel, docetaxel) were added to follow FEC therapy in a few high-risk patients. Tamoxifen was given only 4 weeks after completing adjuvant chemotherapy. Treatment after recurrence was based on multidisciplinary discussions and the two guidelines mentioned earlier.
The use of tamoxifen was according to the hormone status of breast cancer. There was a 1-month interval before tamoxifen prescription for those patients receiving adjuvant chemotherapies. Tamoxifen was prescribed at a dose of 10 mg twice a day. Before starting the first tamoxifen treatment, the biochemical markers, including serum TC, TG, HDL-C, LDL-C, AST, ALT, blood urea nitrogen and creatinine levels, were measured using a chemistry autoanalyzer (Hitachi 7600–110; Tokyo, Japan) as baseline information. In South Korea, the same apparatus had shown acceptable performance in the ‘within-day’ and ‘between-day’ precision, linearity, and accuracy [20]. In our hospital, the within-day and between-day coefficients of variations of all items were less than 3.0%.
For laboratory analysis, overnight fasting blood samples were collected under identical conditions from the study patients. Repeated measurement of identical markers was obtained at several intervals (3–6 months) during the study period, depending on the various clinical conditions of the cases at the outpatient services. All the measurements relative to an individual patient were made at the same laboratory and with the same commercial kit. The patients with cholesterol reduction formula before beginning tamoxifen treatment, discontinuance of tamoxifen of their own accord, a history of diabetes mellitus, liver cirrhosis, history of other malignancy, or unstable serious coexisting medical conditions were excluded from this study. The normal range of the serum TG level is defined as less than 200 mg/dl. For patients with a serum TG level over 400 mg/dl, the TG level was reanalyzed the next month. If the TG level was still over 400 mg/dl, tamoxifen would be stopped or taken with a cholesterol reduction formula simultaneously, or replaced by an aromatase inhibitor for postmenopausal women. The biomarker data of these patients were not further analyzed after these modifications.
According to their tumor grade and lymph node status, 2 subgroups were identified. The subgroup 1 patients had tumor grade I/II and a negative lymph node status, whereas the subgroup 2 patients had tumor grade III or a positive lymph node status. The information regarding tumor characteristics, the differences in the adjuvant chemotherapy between the 2 subgroups and in the body mass index registered in the medical charts were also reviewed. The Bloom-Richardson grading system was used for tumor grading. This grading scheme is based on 3 morphologic features: degree of tumor tubule formation, tumor mitotic activity, and nuclear pleomorphism of the tumor cells. 7 possible scores are condensed into 3 Bloom-Richardson grades: I, II, or III.
Pearson chi-squared tests and independent t-tests were used to examine the differences between the 2 subgroups in terms of the demographic and clinical characteristics of the patients. Values of the continuous variables were presented as mean ± standard deviation (SD) in this study. Paired t-tests were applied in order to explore the differences of the lipid parameter values between the patients before and after receiving tamoxifen treatment. P values less than 0.05 were considered to be statistically significant. All statistical analyses were performed with SPSS statistical package (version 16.0).
Results
Among the 109 patients, the mean age was 49.4 ± 9.52 years (range 34–74 years); the mean follow-up interval was 167.0 ± 90.75 days (range 1–421 days); 67.5% were lymph node negative, 29.2% were lymph node positive, and for 3.3% of the patients data were missing. As shown in table 1, 98% of the patients were free from distant metastasis; tumor grades I, II, and III were determined in 9.2%, 54.2%, and 30.0% of the patients, respectively; in 6.7% of the patients, the tumor grade was unknown. The staging data revealed stage 0 and I at 47%, stage II at 38.3%, stage III at 10.0%, stage IV at 0.8%, and missing data at 3%. Histological categorization showed ductal carcinoma at 89%, lobular carcinoma at 1%, ductal carcinoma in situ at 5%, and other at 6%. A difference in adjuvant chemotherapy did exist between the 2 subgroups. The patient characteristics of the 2 subgroups are also provided in table 1.
Table 1.
Summary of the patient characteristics
| Subgroup 1 (n = 49) | Subgroup 2 (n = 60) | P | Total (n = 109) | |
|---|---|---|---|---|
| Mean age at diagnosis (SD), years | 51.29 (10.10) | 47.93 (8.83) | 0.067 | 49.44 (9.52) |
| Interval (SD), days | 182.22 (97.03) | 154.58 (84.07) | 0.114 | 167.01 (90.75) |
| Tumor size, n (%) | ||||
| T0 | 8 (16) | 9 (15) | 0.471 | 17 (16) |
| ≤ 2 cm, T1 | 26 (53) | 26 (43) | 52 (48) | |
| > 2 cm and ≤ 5 cm, T2 | 14 (29) | 22 (37) | 36 (33) | |
| > 5 cm, T3 | 0 (0) | 2 (3) | 2 (2) | |
| T4 | 0 (0) | 1 (2) | 1 (1) | |
| Unknown | 1 (2) | 0 | 1 (1) | |
| Distant metastasis, n (%) | ||||
| No | 49 (100) | 58 (96) | 1.000 | 107 (98) |
| Yes | 0 (0) | 1 (2) | 1 (1) | |
| Unknown | 0 | 1 (2) | 1 (1) | |
| Stage, n (%) | ||||
| < II | 33 (67) | 18 (30) | < 0.001* | 51 (47) |
| ≥ II | 14 (29) | 41 (68) | 55 (50) | |
| Unknown | 2 (4) | 1 (2) | 3 (3) | |
| Pathological type, n (%) | ||||
| Ductal | 42 (86) | 55 (92) | 0.604 | 97 (89) |
| Lobular | 1 (2) | 0 (0) | 1 (1) | |
| Ductal carcinoma in situ | 3 (6) | 2 (3) | 5 (5) | |
| Other | 3 (6) | 3 (5) | 6 (6) | |
| Adjuvant chemotherapy, n (%) | ||||
| Yes | 27 (55.10) | 47 (78.33) | 0.010* | 74 (68) |
| No | 22 (44.90) | 13 (21.67) | 35 (32) | |
| Body mass index, n (%) | ||||
| < 24 | 24 (49) | 35 (58) | 0.657 | 59 (54) |
| 24–27 | 12 (25) | 14 (23) | 27 (25) | |
| ≥ 27 | 10 (20) | 10 (17) | 19 (17) | |
| Unknown | 3 (6) | 1 (2) | 4 (4) |
SD = Standard deviation; interval = days after starting tamoxifen.
Statistically significant.
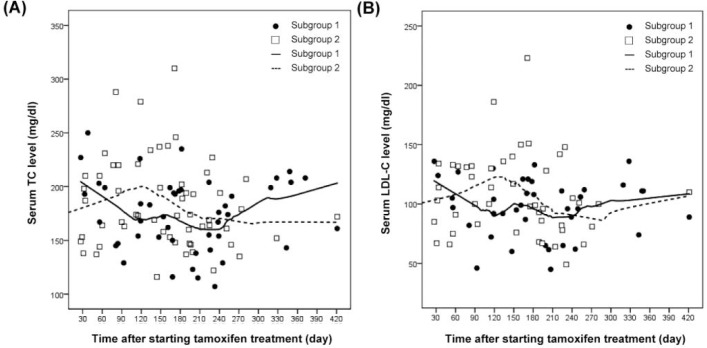
The mean serum TC levels after tamoxifen treatment, as well as the LDL-C levels, were significantly lower than their baseline levels (table 2). The change in the mean serum TG levels in these patients was not statistically significant; the same was true for the mean serum levels of HDL-C, AST and ALT. Table 3 shows the effects of tamoxifen on the biochemical risk factors between the 2 subgroups. The percentages of cases with feasible TC data in these 109 patients displayed 3 months at 91.7%, 6 months at 95.4%, and 12 months at 56.8%. The percentages of cases with feasible TG data in these 109 patients revealed 3 months at 92.7%, 6 months at 95.4%, and 12 months at 57.8%. The percentages of cases with feasible HDL-C data in these 109 patients revealed 3 months at 83.5%, 6 months at 79.8%, and 12 months at 51.4%. The percentages of cases with feasible LDL-C data in these 109 patients revealed 3 months at 83.5%, 6 months at 78.9%, and 12 months at 52.3%. Figure 1 shows the grouping trends for serum TC and LDL-C.
Table 2.
Influence of adjuvant tamoxifen on the serum lipid profiles of breast cancer patients
| Variables | Baseline | After tamoxifen | P |
|---|---|---|---|
| TC, mg/dl (n = 95) | 196.71 ± 36.58 | 179.58 ± 35.50 | < 0.001* |
| TG, mg/dl (n = 96) | 145.15 ± 118.18 | 132.89 ± 84.89 | 0.282 |
| HDL-C, mg/dl (n = 76) | 58.43 ± 11.89 | 61.11 ± 14.21 | 0.015* |
| LDL-C, mg/dl (n = 75) | 124.35 ± 33.60 | 102.73 ± 26.67 | < 0.001* |
| AST, U/l (n = 66) | 29.00 ± 16.08 | 27.44 ± 16.90 | 0.467 |
| ALT, U/l (n = 69) | 30.78 ± 26.19 | 26.87 ± 24.40 | 0.161 |
Data shown as mean ± SD.
TC = Total cholesterol, TG = triglyceride, HDL-C = high-density lipoprotein cholesterol, LDL-C = low-density lipoprotein cholesterol, AST = aspartate transaminase, ALT = alanine transaminase.
Statistically significant.
Table 3.
Effects of tamoxifen on the biochemical risk factors between the 2 subgroups
| Subgroup 1 |
Subgroup 2 |
|||||
|---|---|---|---|---|---|---|
| Biochemical risk factors | Baseline | After tamoxifen | P | Baseline | After tamoxifen | P |
| TC, mg/dl (subgroup 1, n = 43; subgroup 2, n = 52) | 195.70 (27.80) | 177.60 (32.82) | < 0.001* | 197.54 (42.76) | 181.21 (37.81) | < 0.001* |
| TG, mg/dl (subgroup 1, n = 43; subgroup 2, n = 53) | 137.79 (104.86) | 127.02 (77.15) | 0.304 | 149.49 (128.81) | 137.64 (91.13) | 0.514 |
| HDL-C, mg/dl (subgroup 1, n = 34; subgroup 2, n = 42) | 59.97 (11.63) | 62.15 (12.21) | 0.144 | 57.19 (12.10) | 60.26 (15.74) | 0.055 |
| LDL-C, mg/dl (subgroup 1, n = 34; subgroup 2, n = 41) | 120.00 (24.22) | 100.42 (22.77) | < 0.001* | 127.95 (39.70) | 104.65 (29.67) | < 0.001* |
| AST, U/l (subgroup 1, n = 25; subgroup 2, n = 41) | 32.44 (20.69) | 26.76 (13.96) | 0.117 | 26.90 (12.32) | 27.85 (18.62) | 0.721 |
| ALT, U/l (subgroup 1, n = 27; subgroup 2, n = 42) | 37.37 (36.14) | 27.52 (21.88) | 0.021* | 26.55 (16.20) | 26.45 (26.15) | 0.979 |
Data shown as mean ± SD. Paired t-tests were applied.
TC = Serum total cholesterol, TG = triglyceride, HDL-C = high-density lipoprotein cholesterol, LDL-C = low-density lipoprotein cholesterol, AST = aspartate transaminase, ALT = alanine transaminase.
Statistically significant.
Fig. 1.
Serum lipid profile changes in different subgroups of breast cancer patients. (A) TC, (B) LDL-C.
Discussion
We present herein the results of a study that examined tamoxifen in relation to lipid profiles. Of interest, we found statistically significant decreases in serum TC and LDL-C after tamoxifen treatment in Taiwanese breast cancer patients.
The results demonstrated that the serum TC levels were reduced by tamoxifen, in agreement with the results of comparable series [7, 10, 11, 12]. However, the results of this study are in contrast with those of a Japanese study: Hozumi et al. [9] reported that the serum TC levels remained unchanged after tamoxifen treatment. Their patient population was composed of postmenopausal and lymph node-negative women. Their blood samples were collected before and at 2 months after the initiation of tamoxifen treatment. Our data indicated serum TC level differences between subgroup 1 (both grade I/II and a negative lymph node status) and subgroup 2 (grade III or a positive lymph node status) at 4 months after the initiation of tamoxifen treatment. Figure 1 shows that there was a turning point in the serum TC level, and the LDL-C level, at around 4 months after starting tamoxifen treatment. Thereafter, the serum levels of TC and LDL-C tended to stabilize. The timing of blood sampling might explain the differences between the findings of Hozumi et al. [9] and this study, or the disparity might be caused by population differences in terms of grade and lymph node status. Tan et al. [21] reported that genetic polymorphisms influence the epirubicin metabolism, as well as the in vitro activity of tamoxifen. Genetic polymorphisms and epirubicin may influence tamoxifen and the lipid profile in vivo. In this study, patients in subgroup 2 who had more advanced breast cancer more likely received epirubicin chemotherapy, which may be related to the turning point found in this study, shown in figure 1.
Contrary to our supposition, our data indicate that the TG levels remained unchanged before and after tamoxifen treatment, which is in contrast with the findings of previous studies [7, 8, 9, 10, 11, 12, 19]. Estava and Hortobagyi [7] reported that tamoxifen increased the TG levels 12 weeks after initiation of the drug. However, they also mentioned that long-term data from clinical trials have failed to demonstrate a cardioprotective effect, and patients treated with tamoxifen did not experience fewer cardiovascular events compared with those receiving a placebo [7]. The reason might be an apolipoprotein E (APOE) polymorphism. Chang et al. [18] reported that the effects of tamoxifen on serum TG levels are modified by the APOE polymorphism. Breast cancer patients with the APOE4 allele have lower serum TG levels when undergoing tamoxifen therapy [18]. The genomic differences between races might explain the different TG levels found in this study.
The results of the LDL-C level reduction by tamoxifen are consistent with previous series [7, 10, 11, 12, 17, 18, 19]. Tumor grade and lymph node status were not independent factors for the serum TC and LDL-C levels. The serum TC and LDL-C levels decreased in patients of both subgroup 1 and subgroup 2.
A study by Chajès et al. [26] suggested that malignant transformation- and tumor progression-related mechanisms would affect the membrane fatty acid profile in breast cancers. This study revealed that subgroups 1 and 2 displayed similar distributions of clinicopathological characteristics, such as age, tumor size, metastasis, and body mass index. However, the trends of the serum TC and LDL-C levels between the subgroups were not similar. This difference may not be related to the lymph node status and tumor grade. A difference in adjuvant chemotherapy did exist between the 2 subgroups (P = 0.01) and could influence the effects on the lipid profiles. On the other hand, a lower stage was observed in the patients of subgroup 1 in this study. In spite of these findings, the steroid and xenobiotic receptor (SXR) was not investigated in the 2 subgroups. Verma et al. [3] reported that tamoxifen has the ability to activate a heterodimer of the SXR that is highly expressed in the liver. They also revealed that, in MCF-7 breast cancer cells treated with SXR activators, the SXR target gene CYP3A4 was induced. The changes in the serum TC and LDL-C levels in subgroup 2 of this study might be related to the SXR inhibitors, which may modulate the CYP3A4 and tamoxifen activities [21].
There are potential limitations to this study. In this study, no data on dietary effects, body weight, lifestyle, or cardiac events are available. Balance is achieved on some covariates as shown in table 1. However, in such a small number of patients, imbalances in unobserved covariates could greatly bias the results. According to the research by Lee et al. [23], a multiple regression model indicates a harmful effect of dietary fat (odds ratio (OR): 2.6, 95% confidence interval (CI): 1.4–5.0) for the highest versus the lowest quartile in Taiwanese women. The finding is compatible with concerns about dietary effects.
Furthermore, the tamoxifen lipid profile data were presented only for the first year. The short follow-up period is indeed a limitation of this study. However, other breast cancer researchers also used short follow-up periods such as 12 weeks [7], 3 months [10], 2 months [9] and 36 months [11].
In this study, the small number of patients divided into 2 heterogeneous groups with longer follow-up is also a limitation, especially concerning the single parameters (ALT, AST data for only half of the patients). However, the most confounding factors, including age, tumor size, metastasis, pathological type, and body mass index, have been assessed and demonstrated no statistically significant difference between subgroups 1 and 2 (table 1).
The estrogen receptor and the glucocorticoid receptor belong to a group of type I steroid hormone receptors. Glucocorticoid stimulates fat breakdown in adipose tissue. The fatty acids released by lipolysis are used for the production of energy in tissues like muscle. The released glycerol provides the substrate for gluconeogenesis. Based on these observations, it seems reasonable to consider steroid hormone receptors while discussing tamoxifen, the estrogen receptor, and the lipid profile [3, 5, 24, 25]. Furthermore, a new class of steroid hormone receptors has recently been elucidated: Along with the well-documented intracellular receptors, cell membrane receptors have been shown to exist. Their cellular responses are much quicker than those of the intracellular receptors [24, 25].
This study indicates that tamoxifen tends to have an impact on the serum lipid profiles in Taiwanese breast cancer patients. Notably for tumor grade I/II and node-negative patients, tamoxifen does have a positive effect on the serum TC and LDL-C levels in patients with grade III tumors or positive nodes. Physicians should follow up the lipid profiles in these patients.
Disclosure Statement
The authors declare that they have no competing interests.
Acknowledgement
This study was supported by a grant from the Changhua Christian Hospital (98-CCH-IRP-10). Editorial support was provided by Ms. Yu-Fen Wang, MS.
References
- 1.Alexe G, Dalgin GS, Scanfeld D, Tamayo P, Mesirov JP, Ganesan S, Delisi C, Bhanot G. Breast cancer stratification from analysis of micro-array data of micro-dissected specimens. Genome Inform. 2007;18:130–140. [PubMed] [Google Scholar]
- 2.Horwitz KB, Dye WW, Harrell JC, Kabos P, Sartorius CA. Rare steroid receptor-negative basal-like tumorigenic cells in luminal subtype human breast cancer xenografts. Proc Natl Acad Sci USA. 2008;105:5774–5779. doi: 10.1073/pnas.0706216105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Verma S, Tabb MM, Blumberg B. Activation of the steroid and xenobiotic receptor, SXR, induces apoptosis in breast cancer cells. BMC Cancer. 2009;9:3. doi: 10.1186/1471-2407-9-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yin WJ, Lu JS, Di GH, Lin YP, Zhou LH, Liu GY, Wu J, Shen KW, Han QX, Shen ZZ, Shao ZM. Clinicopathological features of the triple-negative tumors in Chinese breast cancer patients. Breast Cancer Res Treat. 2009;115:325–333. doi: 10.1007/s10549-008-0096-0. [DOI] [PubMed] [Google Scholar]
- 5.Hernandez RK, Sorensen HT, Jacobsen J, Pedersen L, Lash TL. Tamoxifen treatment in Danish breast cancer patients and 5-year risk of arterial atherosclerotic events: a null association. Cancer Epidemiol Biomarkers Prev. 2008;17:2509–2511. doi: 10.1158/1055-9965.EPI-08-0570. [DOI] [PubMed] [Google Scholar]
- 6.Elefsiniotis IS, Pantazis KD, Ilias A, Pallis L, Mariolis A, Glynou I, Kada H, Moulakakis A. Tamoxifen induced hepatotoxicity in breast cancer patients with pre-existing liver steatosis: The role of glucose intolerance. Eur J Gastroenterol Hepatol. 2004;16:593–598. doi: 10.1097/00042737-200406000-00013. [DOI] [PubMed] [Google Scholar]
- 7.Esteva FJ, Hortobagyi GN. Comparative assessment of lipid effects of endocrine therapy for breast cancer: implications for cardiovascular disease prevention in postmenopausal women. Breast. 2006;15:301–312. doi: 10.1016/j.breast.2005.08.033. [DOI] [PubMed] [Google Scholar]
- 8.Farrell GC. Drugs and steatohepatitis. Semin Liver Dis. 2002;22:185–194. doi: 10.1055/s-2002-30106. [DOI] [PubMed] [Google Scholar]
- 9.Hozumi Y, Kawano M, Saito T, Miyata M. Effect of tamoxifen on serum lipid metabolism. J Clin Endocrinol Metab. 1998;83:1633–1635. doi: 10.1210/jcem.83.5.4753. [DOI] [PubMed] [Google Scholar]
- 10.Lewis S. Do endocrine treatments for breast cancer have a negative impact on lipid profiles and cardiovascular risk in postmenopausal women? Am Heart J. 2007;153:182–188. doi: 10.1016/j.ahj.2006.10.034. [DOI] [PubMed] [Google Scholar]
- 11.Liu CL, Yang TL. Sequential changes in serum triglyceride levels during adjuvant tamoxifen therapy in breast cancer patients and the effect of dose reduction. Breast Cancer Res Treat. 2003;79:11–16. doi: 10.1023/a:1023348021773. [DOI] [PubMed] [Google Scholar]
- 12.Markopoulos C, Polychronis A, Zobolas V, Xepapadakis G, Papadiamantis J, Koukouras D, Lappas H, Gogas H. The effect of exemestane on the lipidemic profile of postmenopausal early breast cancer patients: preliminary results of the TEAM Greek sub-study. Breast Cancer Res Treat. 2005;93:61–66. doi: 10.1007/s10549-005-3783-0. [DOI] [PubMed] [Google Scholar]
- 13.McClain CJ, Mokshagundam SP, Barve SS, Song Z, Hill DB, Chen T, Deaciuc I. Mechanisms of non-alcoholic steatohepatitis. Alcohol. 2004;34:67–79. doi: 10.1016/j.alcohol.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 14.Nowak AK, Stockler MR, Chow PK, Findlay M. Use of tamoxifen in advanced-stage hepatocellular carcinoma. A systematic review. Cancer. 2005;103:1408–1414. doi: 10.1002/cncr.20963. [DOI] [PubMed] [Google Scholar]
- 15.Verset G, Verslype C, Reynaert H, Borbath I, Langlet P, Vandebroek A, Peeters M, Houbiers G, Francque S, Arvanitakis M, Van Laethem JL. Efficacy of the combination of long-acting release octreotide and tamoxifen in patients with advanced hepatocellular carcinoma: a randomised multicentre phase III study. Br J Cancer. 2007;97:582–588. doi: 10.1038/sj.bjc.6603901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yuvaraj S, Premkumar VG, Shanthi P, Vijayasarathy K, Gangadaran SG, Sachdanandam P. Effect of coenzyme Q(10), riboflavin and niacin on tamoxifen treated postmenopausal breast cancer women with special reference to blood chemistry profiles. Breast Cancer Res Treat. 2009;114:377–384. doi: 10.1007/s10549-008-0012-7. [DOI] [PubMed] [Google Scholar]
- 17.Montagnani A, Gonnelli S, Cadirni A, Caffarelli C, Del Santo K, Pieropan C, Campagna MS, Montomoli M, Petrioli R, Nuti R. The effects on lipid serum levels of a 2-year adjuvant treatment with exemestane after tamoxifen in postmenopausal women with early breast cancer. Eur J Intern Med. 2008;19:592–597. doi: 10.1016/j.ejim.2007.05.016. [DOI] [PubMed] [Google Scholar]
- 18.Chang NW, Chen FN, Wu CT, Lin CF, Chen DR. Apolipoprotein E4 allele influences the response of plasma triglyceride levels to tamoxifen in breast cancer patients. Clin Chim Acta. 2009;401:144–147. doi: 10.1016/j.cca.2008.12.005. [DOI] [PubMed] [Google Scholar]
- 19.Liberopoulos E, Karabina SA, Tselepis A, Bairaktari E, Nicolaides C, Pavlidis N, Elisaf M. Are the effects of tamoxifen on the serum lipid profile modified by apolipoprotein E phenotypes? Oncology. 2002;62:115–120. doi: 10.1159/000048256. [DOI] [PubMed] [Google Scholar]
- 20.Cho SE, Nam JW, Hong KS. Performance evaluation of the Hitachi 7600–110 chemistry autoanalyzer. Korean J Clin Pathol. 2001;21:331–337. [Google Scholar]
- 21.Tan SH, Lee SC, Goh BC, Wong J. Pharmacogenetics in breast cancer therapy. Clin Cancer Res. 2008;14:8027–8041. doi: 10.1158/1078-0432.CCR-08-0993. [DOI] [PubMed] [Google Scholar]
- 22.Rodriguez-Antona C, Ingelman-Sundberg M. Cytochrome P450 pharmacogenetics and cancer. Oncogene. 2006;25:1679–1691. doi: 10.1038/sj.onc.1209377. [DOI] [PubMed] [Google Scholar]
- 23.Lee MM, Chang IY, Horng CF, Chang JS, Cheng SH, Huang A. Breast cancer and dietary factors in Taiwanese women. Cancer Causes Control. 2005;16:929–937. doi: 10.1007/s10552-005-4932-9. [DOI] [PubMed] [Google Scholar]
- 24.Norman AW, Mizwicki MT, Norman DP. Steroid-hormone rapid actions, membrane receptors and a conformational ensemble model. Nat Rev Drug Discov. 2004;3:27–41. doi: 10.1038/nrd1283. [DOI] [PubMed] [Google Scholar]
- 25.Pitroda SP, Khodarev NN, Beckett MA, Kufe DW, Weichselbaum RR. Muc1-induced alterations in a lipid metabolic gene network predict response of human breast cancers to tamoxifen treatment. Proc Natl Acad Sci USA. 2009;106:5837–5841. doi: 10.1073/pnas.0812029106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chajès V, Lanson M, Fetissof F, Lhuillery C, Bougnoux P. Membrane fatty acids of breast carcinoma: contribution of host fatty acids and tumor properties. Int J Cancer. 1995;63:169–175. doi: 10.1002/ijc.2910630204. [DOI] [PubMed] [Google Scholar]