Abstract
Background
Serum levels of hepatitis B virus (HBV) DNA are an important predictor of the risk of hepatocellular carcinoma (HCC) in patients with chronic HBV infection. However, little is known about whether high levels of hepatitis B surface antigen (HBsAg) increase the risk for HCC.
Methods
We investigated 167 patients who were treated with nucleos(t)ide analogues (NA) for at least 2 years (median: 5.8 years, range: 2-13.1 years). Relationships between reduced levels of HBsAg and various factors were evaluated. In addition, we evaluated the usefulness of quantitative serum levels of HBV DNA and HBsAg as predictors of HCC development in patients receiving long-term NA therapy.
Results
HCC developed in 9 of the 167 NA-treated patients. In the 9 patients with HCC, HBV DNA was undetectable (<2.1 log copies/mL), but HBsAg levels were ≥2000 C.O.I. in 7 patients. No maternal transmission, long NA treatment period, HBV DNA levels <3.0 log copies/mL, and reduced hepatitis B e antigen levels during the first 24 weeks of treatment were a significant factor of HBsAg levels <2000 C.O.I..
Conclusions
Hepatocarcinogenesis was observed in patients with high HBsAg levels, despite the negative conversion of HBV DNA as a result of long-term NA therapy. Therefore, to suppress hepatocarcinogenesis, it is important to control not only HBV DNA levels but also HBsAg levels.
Key Words: HBV DNA, Hepatitis B surface antigen, Hepatitis B virus, Hepatocellular carcinoma, Nucleos(t)ide analogues
Introduction
Globally, approximately 400 million people are infected with the hepatitis B virus (HBV). Among them, half a million people develop cirrhosis or hepatocellular carcinoma (HCC) annually [1, 2]. Approximately 1 million people die annually from hepatitis B-induced HCC, underlining the fact that this is an important problem [3]. In Japan, HBV infection accounts for approximately 6% of HCC cases [4]. For this reason, patients with persistent hepatitis require antiviral interferon (IFN) or nucleos(t)ide analogue (NA) therapy. Anti-HBV therapy aims to suppress hepatitis through the continuous suppression of HBV. Additionally, the ultimate therapeutic goal is to improve vital prognosis through arrested cirrhosis or HCC development.
The risk of progression from chronic hepatitis B to cirrhosis is significantly affected by blood levels of HBV DNA. When the levels of HBV DNA are less than 4.0 log copies/mL, the risk of progression to cirrhosis is low. However, the risk of cirrhosis is reported to increase with an increase in the levels of HBV DNA above 4.0 log copies/mL [5]. Similarly, HBV DNA levels at the start of observation are thought to be associated with hepatocarcinogenesis. Because the risk of hepatocarcinoma increases as the HBV DNA level increases above 4.0 log copies/mL, therapies that control HBV DNA levels are important [6]. NAs are therapeutic agents that strongly control HBV DNA levels and also reduce alanine aminotransferase (ALT) levels. NA therapy is an epoch-making therapy that suppresses both hepatitis [7] and the onset of hepatocarcinoma [7, 8, 9, 10].
Recently, the levels of hepatitis B surface antigen (HBsAg), in addition to levels of HBV DNA, were linked with the risk of hepatocarcinogenesis in untreated hepatitis B patients [11]. Quantified HBsAg levels are increasingly recognized as a marker with which to evaluate the host immunological control of HBV replication and infection [12,13,14]. Low HBsAg levels in patients with HBV genotype B or C are considered to indicate a high likelihood of HBV clearance and lower hepatitis activity [13, 14, 15]. Studies of HBV genotype D have also defined patients with less than 1000 IU/mL of HBsAg as inactive virus carriers.
From the clinical perspective, we were interested in whether the incidence of HCC might vary in an HBsAg level-dependent manner in Japanese patients of genotype C in whom HBV DNA is controlled by long-term NA therapy. To address this interesting question, we enrolled 167 hepatitis B patients to whom we had administered NA therapy in our hospital for more 2 years. This study aimed to identify the predictors of HBsAg level reduction in the context of long-term NA administration. In addition, hepatocarcinogenesis during long-term NA administration about whether related to the amount of HBsAg, we investigated for the first time in Japan.
Patients and Methods
Patients
The subjects were 167 patients who had received NA therapy for more than 2 years and were selected from the hepatitis B patients with ALT levels ≥31 U/L and HBV DNA levels ≥4.0 log copies/mL who visited Kawasaki Hospital, Kawasaki Medical School, between 1999 and 2010. Seventy-two patients received 0.5 mg/day of entecavir (ETV), 57 patients received 100 mg/day of lamivudine (LMV), and 37 patients received adefovir dipivoxil (ADV) in addition to LMV to treat LMV-resistant virus. The median administration period of NA was 5.8 ± 2.8 years. The average age at the start of therapy, the male to female ratio, and the ratio of subjects with chronic hepatitis to those with cirrhosis were 49.2 ± 11.1 years, 112/55, and 126/41, respectively. The average HBV DNA level at the start of therapy was 6.8 ± 1.3 log copies/mL. Among the subjects, 3, 4, 144, and 16 carried A, B, C, and unknown HBV genotypes, respectively; thus, the majority of infections were of genotype C. Eighty-one patients (50.9%) were positive for the hepatitis B e antigen (HBeAg). The HBsAg levels were below 2000 C.O.I in 8 (4.7%) subjects and above 2000 C.O.I. in 159 (95.3%) subjects (table 1).
Table 1.
Characteristics of patients before and after NA treatment
| NA treatment duration (years), n = 167 | 5.8±2.8 | |
|---|---|---|
| ETV, n = 72 | 4.4±1.9 | |
| LMV only, n = 57 | 6.5±3.1 | |
| LMV plus ADV, n = 38 | 7.3±2.8 | |
| Before NA treatment | After NA treatment | |
| Age (years) | 49.2 ± 11.1 | 56.7 ± 25.4 |
| Gender (male/female) | 112/55 | — |
| Chronic hepatitis/liver cirrhosis | 126/41 | — |
| HBV DNA (log copies/ml) | 6.8 ± 1.3 | 1.6 ± 1.5 |
| HBV genotype (A/B/C/ND) | 3/4/144/16 | — |
| HBeAg positive | 81 (50.9%) | 42 (25.6%) |
| HBsAg (< 2000/ ≥ 2000 C.O.I.) | 8/159 | 28/139 |
Values are mean ± standard deviation (SD). ND = no data; C.O.I. = cut off index.
Methods
Data were collected to measure the negative conversion rate of HBsAg, the negative conversion and seroconversion rates of HBeAg, the alanine aminotransferase (ALT) normalization (<30 IU/L) rate, and the negative conversion (<3.0 log copies/mL) rate of HBV DNA during the final evaluations of therapeutic effects in 167 patients with hepatitis B who had received NA for more than 2 years. Additionally, we verified the relationships of various factors (age, sex, chronic hepatitis/cirrhosis, HBV DNA level, HBV genotype, initial HBeAg level, initial HBsAg level, initial ALT value, IFN therapy history, the presence or absence of mother-to-child transmission, NA administration period, and HBV DNA and HBsAg levels at 24 weeks after the start of NA administration) with reduced HBsAg levels. Furthermore, we investigated the relationships between HCC incidence and the levels of HBV DNA and HBsAg at the final observation.
HBV Marker Assay
Serum HBsAg and anti-HBs antibody levels were measured by chemiluminescent enzyme immunoassay (CLEIA, Lumipulse System; Fujirebio, Tokyo, Japan). HBeAg and anti-HBe antibody levels were determined using commercially available enzyme-linked immunosorbent assay kits (EIA, Abbot Japan, Tokyo, Japan). HBV DNA was assayed using the COBAS Amplicor HBV Monitor Test (Roche Diagnostics, Tokyo, Japan), which has a dynamic range of 2.6 to 7.6 log copies/mL, or the COBAS TaqMan HBV Test, version 2.0 (Roche Diagnostics, Tokyo, Japan), which has a dynamic range of 2.1 to 9.0 log copies/mL.
Statistical Analysis
Statistical analyses were performed using the SAS statistical software package, version 9 (Cary, NC, USA). We used the Wilcoxon test, chi-square test, and Fisher's exact test for univariate analyses. Cumulative HCC incidence rates were analyzed according to the Kaplan-Meier method. We compared the cumulative incidence of HCC using the log-rank test. Significance was defined as p < 0.05 for all two-tailed tests.
Ethical Considerations
Informed consent was obtained from all participants. The study protocol complied with the ethical guidelines of the Declaration of Helsinki of 1975 (2004 revision) and was approved by the Ethics Committee of Kawasaki Hospital, Kawasaki Medical School.
Results
Virological and Biochemical Outcomes after Long-Term NA Therapy
After long-term (median: 5.8 years, range: 2-13.1 years) of NA therapy, the ALT normalization (<30 IU/L) rate was 83% (138/167). The proportion of subjects in whom the HBV DNA level decreased below 3.0 log copies/mL was 86% (143/167). The seroconversion rate was 31% (25/81) (fig. 1a).
Fig. 1.
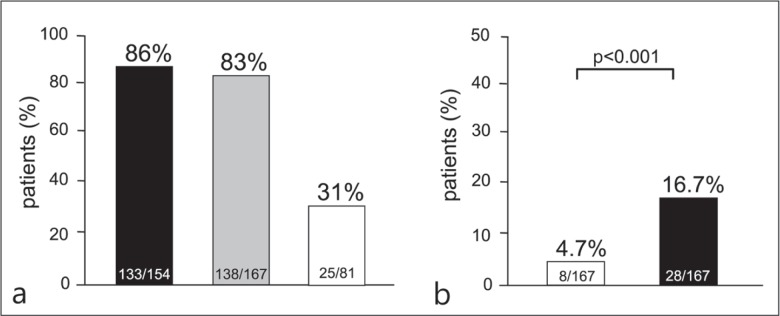
(a) Virological and biochemical outcomes after long-term NA therapy. ▪: undetectable HBV DNA (<3.0 log copies/mL); ▪: normalization of ALT (<30 IU/L); □: HBe seroconversion of hepatitis B e antigen-positive patients. (b) Percentages of patients with serum HBsAg levels <2000 C.O.I. before/after long-term NA therapy. □: HBsAg <2000 C.O.I. before NA treatment; ▪: HBsAg <2000 C.O.I. after NA treatment.
Percentage of Patients with Low HBsAg Levels before/after Long-Term NA Therapy
The proportion of patients with low HBsAg levels (<2000 C.O.I.) was 4.7% (8/167) prior to NA administration. However, after long-term NA administration, a significant increase to 16.7% (28/167) in the proportion of patients with low HBsAg levels was observed (p < 0.001) (fig. 1b).
Factors Related to Reduced HBsAg Levels
The 28 patients with low HBsAg values (<2000 C.O.I.) were compared with the 139 patients with high HBsAg values (≥2000 C.O.I.) at the observation after at least 2 years of long-term NA therapy (table 2). A long period of NA therapy (7.0 years vs. 5.5 years, p = 0.010) and non-mother-to-child transmission (66% vs. 74%, p = 0.042) were extracted as significant factors.
Table 2.
Characteristics of patients with HBsAg <2000 C.O.I, and HBsAg ≥2000 C.O.I, after long-term NA therapy
| HBsAg <2000 C.O.I. (n = 28) | HBsAg ≥2000 C.O.I. (n = 139) | p value | |
|---|---|---|---|
| Age (years) | 49.1 ± 12.9 | 49.8 ± 12.4 | 0.630 |
| Gender (male/female) | 20/8 | 92/47 | 0.587 |
| Chronic hepatitis/liver cirrhosis | 17/11 | 109/30 | 0.057 |
| HBV DNA (log copies/ml) | 6.8 ± 1.3 | 6.6 ± 1.4 | 0.480 |
| HBV genotype C (%) | 96 | 95 | 0.826 |
| HBeAg positive (%) | 35 | 54 | 0.156 |
| ALT (IU/L) | 155 ± 190 | 134 ± 156 | 0.343 |
| Previous IFN treatment (%) | 37 | 24 | 0.175 |
| Maternal transmission (%) | 66 | 74 | 0.042 |
| Period of NA treatment (years) | 7.0 ± 3.1 | 5.5 ± 2.8 | 0.010 |
| At 24 weeks; HBV DNA < 3.0 log copies/ml (%) | 88 | 67 | 0.031 |
| HBeAg negative (%) | 81 | 52 | 0.005 |
Values are mean ± standard deviation (SD).
Additionally, an evaluation of viral dynamics during therapy revealed that HBsAg levels decreased significantly both in patients with low HBV DNA levels (<3.0 log copies/mL; 87.5% vs. 66.9%, p = 0.031) and in HBeAg-negative patients (81% vs. 52%, p = 0.005) at 24 weeks after the start of NA therapy. Cirrhosis or a history of IFN therapy was more frequently noted, with marginal significance, in patients with HBsAg levels below 2000 C.O.I. On the other hand, no significant differences were observed with regard to age; sex; genotype; or levels of HBV DNA, HBeAg, or ALT before therapy.
Factors Related to Liver Carcinogenesis
Comparison of background factors was conducted for the 9 patients who developed HCC during long-term NA therapy and for the 158 patients who did not develop liver cancer during the treatment period. Among the factors that were present before NA therapy, being male (p = 0.03) and the presence of hepatic cirrhosis (p = 0.04) were significant factors for the development of HCC. However, the ALT level normalization, low HBsAg level, and HBV-DNA negativation at the last observation at the end of the long-term NA therapy were not found to be significant factors for the development of HCC (table 3).
Table 3.
Characteristics of patients with HCC and without HCC after long-term NA therapy
| Without HCC (n = 158) | With HCC (n = 9) | p value | |
|---|---|---|---|
| Before NA treatment | |||
| Age (years) | 48.9 | 54.8 | 0.115 |
| Gender (male/female) | 104/54 | 9/0 | 0.033 |
| Chronic hepatitis/liver cirrhosis | 122/36 | 4/5 | 0.040 |
| HBV DNA (log copies/ml) | 6.7 | 7.1 | 0.840 |
| HBV genotype C (%) | 96 | 88 | 0.358 |
| HBeAg positive (%) | 22 | 52 | 0.180 |
| ALT (IU/L) | 142 | 72 | 0.675 |
| Previous IFN treatment (%) | 26 | 33 | 0.627 |
| Maternal transmission (%) | 50 | 71 | 0.306 |
| Period of NA treatment (years) | 5.7 | 6.8 | 0.213 |
| At HCC | |||
| HBV DNA < 2.1 log copies/ml (%) | 69 | 78 | 0.552 |
| HBsAg < 2000 C.O.I. (%) | 16 | 11 | 0.652 |
| ALT < 40 (IU/L) | 91 | 100 | 0.500 |
Incidence of HCC in Relation to HBV DNA and HBsAg Levels
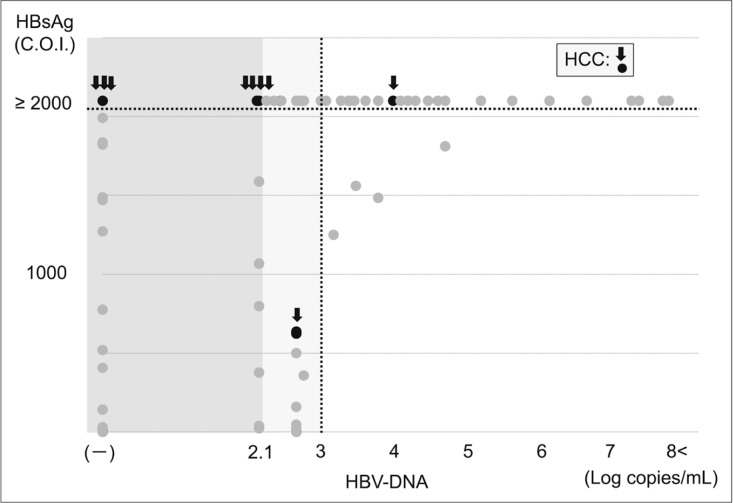
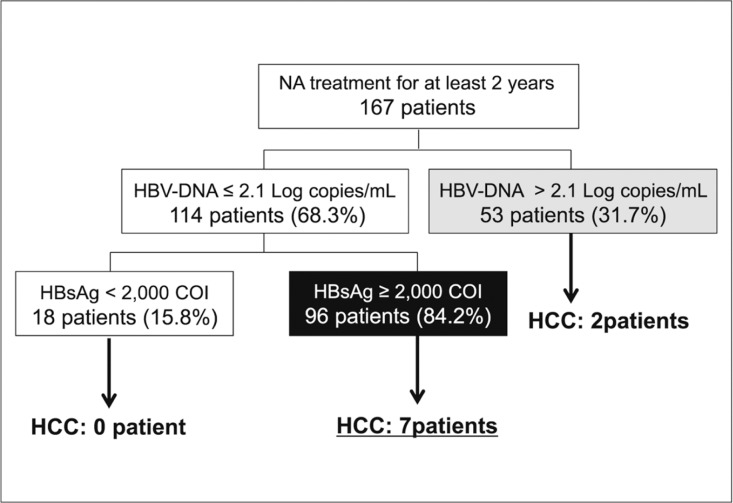
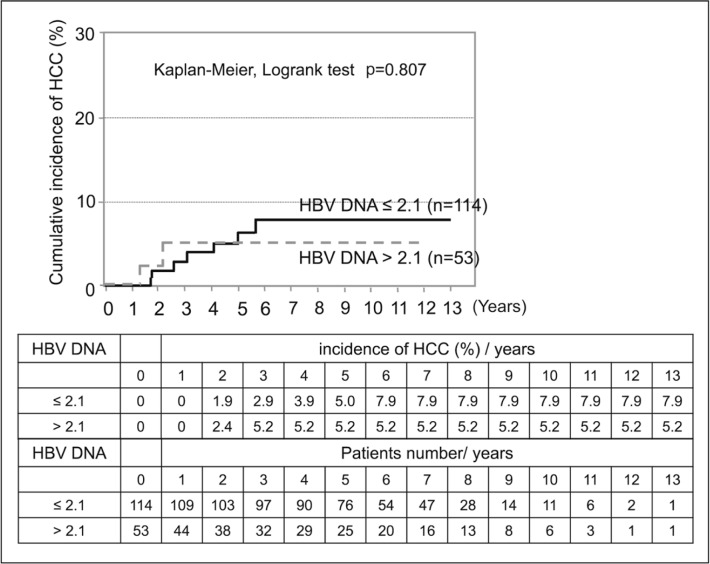
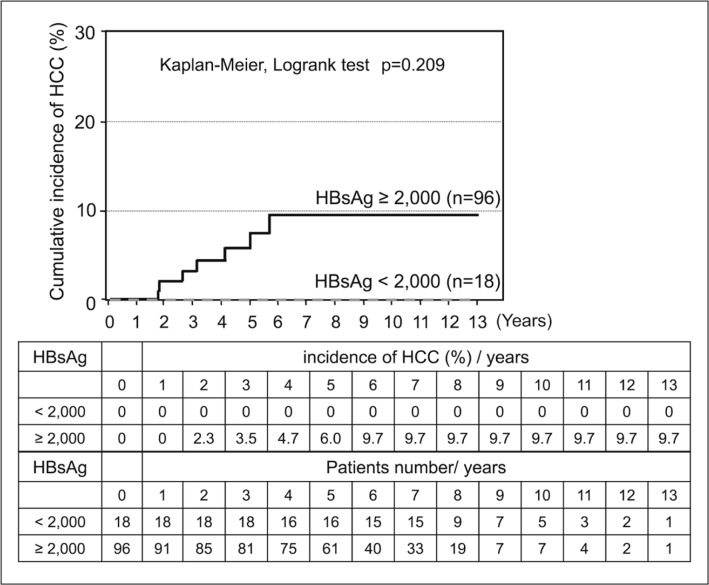
HCC was observed in 9 of 167 patients (5.3%) who received long-term NA therapy; of these, 2 patients were positive for HBV DNA (>2.1 log copies/mL) at the onset of HCC. However, the remaining 7 patients developed HCC despite a negative conversion of HBV DNA (<2.1 log copies/mL) and all of these 7 patients had high HBsAg levels (>2000 C.O.I.). Patients who had low levels of both HBV DNA and HBsAg did not develop HCC (figs 2, 3, 4 and 5).
Fig. 2.
HCC risk and levels of HBV DNA and HBsAg after long-term NA therapy (scatter plot). Among patients with low viral loads under long-term NA therapy, HCC were frequently observed in patients with hight HBsAg levels. ○: hepatocarcinogenesis; ○: no hepatocarcinogenesis.
Fig. 3.
HCC risk a1nd levels of HBV DNA and HBsAg after long-term NA therapy (flow-chart). HCC developed in 9 of 167 patients who were treated with NA. In 7 of the 9 HCC patients, HBV DNA levels were below 2.1 log copies/mL, although HBsAg levels were >2000 C.O.I.
Fig. 4.
HCC risk of HBV DNA levels after long-term NA therapy.—: HBV DNA ≤2.1 log copies/mL; --: HBV DNA >2.1 log copies/mL.
Fig. 5.
HCC risk and HBsAg levels in patients with HBV DNA levels ≤2.1 log copies/mL after long-term NA therapy. —: HBsAg ≥2000 C.O.I. and HBV DNA ≤2.1 log copies/mL; --: HBsAg < 2000 C.O.I. and HBV DNA ≤ 2.1 log copies/mL.
Discussion
HBV DNA levels have been considered an important factor related to hepatitis B-induced carcinogenesis. The rate of carcinogenesis is known to increase proportionally with increased HBV DNA levels [6]. NAs are epoch-making hepatitis B therapeutic agents because they reduce HBV DNA and ALT levels, resulting in the suppression of hepatitis [7]. Moreover, NAs have been shown to significantly reduce hepatocarcinogenesis [8,9]. In a matched control study of patients receiving long-term ETV administration versus untreated controls, the 5-year carcinogenic rates were 3.7% and 13.7%, respectively, indicating a significant suppression of carcinogenesis in the ETV group [10].
On the other hand, HBsAg levels, in addition to HBV DNA levels, were recently reported to be related to the carcinogenic risk. Tseng et al. [11] observed the natural courses of 2688 cases of chronic hepatitis B, excluding those with cirrhosis, for an average of 14.7 years and reported that male sex, old age, a high serum ALT level, HBeAg positivity, genotype C, an HBV DNA level ≥ 2000 IU/mL, and an HBsAg level ≥ 1000 IU/mL are significant predictors of hepatocarcinogenesis. In an analysis stratified by HBV DNA levels, the HBV DNA level was not extracted as a factor related to carcinogenesis in patients with HBV DNA levels below 2000 IU/mL. On the other hand, the researchers reported the importance of the HBsAg level as a hepatocarcinogenesis-related factor because old age, a high ALT value, and an HBsAg level >1000 IU/mL were significantly related to hepatocarcinogenesis. Additionally, of a total of 5055 hepatitis B cases with natural progression or intervention, only 2 patients of 231 patients with negative conversion of HBsAg developed hepatic carcinoma, indicating that HBsAg could be predictive of hepatocarcinogenesis [16].
HBsAg was first discovered in 1965 as the “Australia antigen” from the sera of Aboriginal people by Blumberg et al. [17]. In 1968, Okochi et al. reported relationships between the Australia antigen and hepatitis [18]. HBsAg is produced via multiple pathways in the lifecycle of HBV and uses cccDNA as a template. Therefore, HBsAg levels are reported to correlate with cccDNA levels [19, 20, 21, 22]. Indeed, hepatitis B patients with HBsAg levels below 1-2 × 103 IU/mL and HBV DNA levels below 2 × 103 IU/mL have a low risk of hepatitis recrudescence. Because the HBsAg level reflects HBV replication, it appears necessary to focus on HBsAg as well as on HBV DNA in hepatocarcinogenesis [23, 24, 25]. Additionally, it was reported that HBV DNA levels above 3 log copies/mL at the termination of NA therapy are likely to lead to recrudescence and that, despite the negative conversion of HBV DNA, high HBsAg and HBeAg levels are likely to lead to recrudescence in patients who received NA [26]. Therefore, the HBsAg level is an important indicator of both hepatocarcinogenesis and hepatitis recrudescence.
Moreover, the therapeutic goals for chronic hepatitis B put forth in the guidelines of the American Association for the Study of Liver Diseases, the European Association for the Study of the Liver, and the Asian Pacific Association for the Study of the Liver are improvements in the quality of life and survival rates through the prevention of hepatic cirrhosis, decompensated cirrhosis, end-stage liver diseases, HCC, and progression to death. Furthermore, the guidelines mention that the ideal endpoint is the “disappearance of HBsAg” [27, 28, 29].
The disappearance rate of HBsAg during the natural course of infection has been reported in several studies as 0.5-3.0% per year [27, 28, 29]. In our previous study, the disappearance rate of HBsAg in cirrhosis patients was 0.9% per year [30]. The significant factors that led to the disappearance of HBsAg within 3 years were reported to be HBsAg levels <2000 IU/mL or a decrease in HBsAg levels at an annual rate of 0.5 log IU/mL [31]. However, because the annual disappearance rate of HBsAg was the lowest in patients infected with HBV genotype C in our present study [32], the HBV subgenotype C2/Ce observed in many Japanese is an independent risk factor for HCC [33]. Therefore, we consider it necessary to reduce HBsAg levels as much as possible through antiviral therapy to suppress hepatocarcinogenesis.
The negative conversion rates of HBsAg after a year of NA therapy were reported to be 2, 0-1, and 0% in ETV-, LAM-, and ADV-treated patients, respectively [7, 34, 35, 36, 37, 38, 39]. In our study, HBsAg levels decreased to below 2000 C.O.I. in 16.7% of all patients who received long term NA therapy over 2 years; these proportions were 4.2 and 26.3% in ETV- and LMV (with or without ADV)-treated patients, respectively. However, among all patients, this level of reduction was seen in only four patients (2.3%) with a negative conversion of HBsAg. The significant factors that led to reductions of HBsAg levels below 2000 C.O.I during long-term NA therapy were a long period of NA therapy, non-mother-to-child transmission, a low HBV DNA level at 24 weeks after the start of NA therapy, and HBeAg negativity at 24 weeks after the start of NA therapy. These patients were expected to have reduced HBsAg levels, although they accounted for no more than 16.7% of the total number of patients.
In the present study, hepatocarcinogenesis was observed in 9 of 167 patients (5.3%) who received long-term NA therapy, and 2 of these patients showed a positive conversion of HBV DNA. However, all 7 remaining patients had HBsAg levels above 2000 C.O.I., despite the negative conversion of HBV DNA. This indicates that patients with high HBsAg levels should be observed for hepatocarcinogenesis even in cases with successful negative conversion of HBV DNA during NA therapy. On the basis of the above results, we consider that both HBV DNA and HBsAg levels are important in hepatocarcinogenesis.
NA are reported low effective in the reduction of HBsAg levels because of difficulties in elimination of cccDNA, although NA strongly reduces HBV DNA levels by inhibiting HBV replication through reverse transcription [19]. On the other hand, after reviewing reductions in HBsAg levels in response to NA therapy and IFN therapy in 11 studies, Liaw reported that IFN therapy reduced HBsAg levels more efficiently than NA therapy did [40]. Furthermore, other reports state that the negative conversion rate of HBsAg after 48 weeks of pegylated-IFN administration is 3-7%, which is higher than the 0-2% rate reported for NA therapy [34, 41]. Moreover, a 5-year follow-up after the termination of pegylated-IFN therapy showed that the negative conversion rate of HBsAg was further elevated in a time-dependent manner, achieving a rate of 12% at 5 years [42, 43]. Similarly, a study in Japan, where genotype C accounts for the majority of hepatitis B infections, also demonstrated that the negative conversion rate of HBsAg was elevated in a time-dependent manner, although not as markedly in genotype C as in genotypes A and B; this elevation resulted in a negative conversion rate of 11% at 10 years [44].
Our present study revealed that 8 of 9 patients who developed HCC had high HBsAg levels during long-term NA therapy. Therefore, to achieve the suppression of hepatocarcinogenesis, it may be important to reduce HBsAg levels not only by NA therapy, but also by combining NA with IFN. This is the goal of hepatitis B therapy, as the suppressive effects of IFN therapy on carcinogenesis have been previously reported [45, 46].
In conclusion, despite the negative conversion of HBV DNA during long-term NA therapy, hepatocarcinogenesis was observed in patients with high HBsAg levels. Therefore, the control of both HBV DNA and HBsAg levels is important for the suppression of hepatocarcinogenesis.
Conflict of Interest
All authors declare that they have no conflicts of interest.
References
- 1.Sorrell MF, Belongia EA, Costa J, Gareen IF, Grem JL, Inadomi JM, Kern ER, McHugh JA, Petersen GM, Rein MF, Strader DB, Trotter HT. National Institutes of Health consensus development conference statement: management of hepatitis B. Hepatology. 2009;49(Suppl):S4–S12. doi: 10.1002/hep.22946. [DOI] [PubMed] [Google Scholar]
- 2.Kim DY, Han KH. Epidemiology and surveillance of hepatocellular carcinoma. Liver Cancer. 2012;1:2–14. doi: 10.1159/000339016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fontana RJ. Management of patients with decompensated HBV cirrhosis. Semin Liver Dis. 2003;23:89–100. doi: 10.1055/s-2003-37591. [DOI] [PubMed] [Google Scholar]
- 4.Nissen NN, Martin P. Hepatocellular carcinoma: the high-risk patient. J Clin Gastroenterol. 2002;35(Suppl 2):S79–S85. doi: 10.1097/00004836-200211002-00003. [DOI] [PubMed] [Google Scholar]
- 5.Iloeje UH, Yang HI, Su J, Jen CL, You SL, Chen CJ, Risk Evaluation of Viral Load Elevation and Associated Liver Disease/Cancer-In HBV (the REVEAL-HBV) Study Group Predicting cirrhosis risk based on the level of circulating hepatitis B viral load. Gastroenterology. 2006;130:678–686. doi: 10.1053/j.gastro.2005.11.016. [DOI] [PubMed] [Google Scholar]
- 6.Chen CJ, Yang HI, Su J, Jen CL, You SL, Lu SN, Huang GT, Iloeje UH, REVEAL-HBV Study Group Risk of hepatocellular carcinoma across a biological gradient of serum hepatitis B virus DNA level. JAMA. 2006;295:65–73. doi: 10.1001/jama.295.1.65. [DOI] [PubMed] [Google Scholar]
- 7.Lai CL, Chien RN, Leung NW, Chang TT, Guan R, Tai DI, Ng KY, Wu PC, Dent JC, Barber J, Stephenson SL, Gray DF, Asia Hepatitis Lamivudine Study Group A one-year trial of lamivudine for chronic hepatitis B. N Engl J Med. 1998;339:61–68. doi: 10.1056/NEJM199807093390201. [DOI] [PubMed] [Google Scholar]
- 8.Liaw YF, Sung JJ, Chow WC, Farrell G, Lee CZ, Yuen H, Tanwandee T, Tao QM, Shue K, Keene ON, Dixon JS, Gray DF, Sabbat J, Cirrhosis Asian Lamivudine Multicentre Study Group Lamivudine for patients with chronic hepatitis B and advanced liver disease. N Engl J Med. 2004;351:1521–1531. doi: 10.1056/NEJMoa033364. [DOI] [PubMed] [Google Scholar]
- 9.Matsumoto A, Tanaka E, Rokuhara A, Kiyosawa K, Kumada H, Omata M, Okita K, Hayashi N, Okanoue T, Iino S, Tanikawa K, Inuyama Hepatitis Study Group Efficacy of lamivudine for preventing hepatocellular carcinoma in chronic hepatitis B: A multicenter retrospective study of 2795 patients. Hepatol Res. 2005;32:173–184. doi: 10.1016/j.hepres.2005.02.006. [DOI] [PubMed] [Google Scholar]
- 10.Hosaka T, Suzuki F, Kobayashi M, et al. Long-term entecavir treatment reduces hepatocellular carcinoma incidence in patients with hepatitis B virus infection. Hepatology. 2013;58:98–107. doi: 10.1002/hep.26180. [DOI] [PubMed] [Google Scholar]
- 11.Tseng TC, Liu CJ, Yang HC, Su TH, Wang CC, Chen CL, Kuo SF, Liu CH, Chen PJ, Chen DS, Kao JH. High levels of hepatitis B surface antigen increase risk of hepatocellular carcinoma in patients with low HBV load. Gastroenterology. 2012;142:1140–1149. doi: 10.1053/j.gastro.2012.02.007. e3, quiz e13-e14. [DOI] [PubMed] [Google Scholar]
- 12.Chan HL, Wong VW, Wong GL, Tse CH, Chan HY, Sung JJ. A longitudinal study on the natural history of serum hepatitis B surface antigen changes in chronic hepatitis B. Hepatology. 2010;52:1232–1241. doi: 10.1002/hep.23803. [DOI] [PubMed] [Google Scholar]
- 13.Tseng TC, Liu CJ, Su TH, et al. Serum hepatitis B surface antigen levels predict surface antigen loss in hepatitis B e antigen seroconverters. Gastroenterology. 2011;141:517–525. doi: 10.1053/j.gastro.2011.04.046. e2. [DOI] [PubMed] [Google Scholar]
- 14.Tseng TC, Liu CJ, Yang HC, Su TH, Wang CC, Chen CL, Kuo SF, Liu CH, Chen PJ, Chen DS, Kao JH. Determinants of spontaneous surface antigen loss in hepatitis B e antigen-negative patients with a low viral load. Hepatology. 2012;55:68–76. doi: 10.1002/hep.24615. [DOI] [PubMed] [Google Scholar]
- 15.Chan HL, Wong GL, Tse CH, Chan HY, Wong VW. Viral determinants of hepatitis B surface antigen seroclearance in hepatitis B e antigen-negative chronic hepatitis B patients. J Infect Dis. 2011;204:408–414. doi: 10.1093/infdis/jir283. [DOI] [PubMed] [Google Scholar]
- 16.Arase Y, Ikeda K, Suzuki F, et al. Long-term outcome after hepatitis B surface antigen seroclearance in patients with chronic hepatitis B. Am J Med. 2006;119:e9-71.e16. doi: 10.1016/j.amjmed.2005.02.033. [DOI] [PubMed] [Google Scholar]
- 17.Blumberg BS, Alter HJ, Visnich S. A “new” antigen in leukemia sera. JAMA. 1965;191:541–546. doi: 10.1001/jama.1965.03080070025007. [DOI] [PubMed] [Google Scholar]
- 18.Okochi K, Murakami S. Observations on Australia antigen in Japanese. Vox Sang. 1968;15:374–385. doi: 10.1111/j.1423-0410.1993.tb04532.x. [DOI] [PubMed] [Google Scholar]
- 19.Werle-Lapostolle B, Bowden S, Locarnini S, Wursthorn K, Petersen J, Lau G, Trepo C, Marcellin P, Goodman Z, Delaney WE, 4th, Xiong S, Brosgart CL, Chen SS, Gibbs CS, Zoulim F. Persistence of cccDNA during the natural history of chronic hepatitis B and decline during adefovir dipivoxil therapy. Gastroenterology. 2004;126:1750–1758. doi: 10.1053/j.gastro.2004.03.018. [DOI] [PubMed] [Google Scholar]
- 20.Volz T, Lutgehetmann M, Wachtler P, Jacob A, Quaas A, Murray JM, Dandri M, Petersen J. Impaired intrahepatic hepatitis B virus productivity contributes to low viremia in most HBeAg-negative patients. Gastroenterology. 2007;133:843–852. doi: 10.1053/j.gastro.2007.06.057. [DOI] [PubMed] [Google Scholar]
- 21.Chan HL, Wong VW, Tse AM, Tse CH, Chim AM, Chan HY, Wong GL, Sung JJ. Serum hepatitis B surface antigen quantitation can reflect hepatitis B virus in the liver and predict treatment response. Clin Gastroenterol Hepatol. 2007;5:1462–1468. doi: 10.1016/j.cgh.2007.09.005. [DOI] [PubMed] [Google Scholar]
- 22.Brunetto MR. A new role for an old marker, HBsAg. J Hepatol. 2010;52:475–477. doi: 10.1016/j.jhep.2009.12.020. [DOI] [PubMed] [Google Scholar]
- 23.Chen CH, Lee CM, Wang JH, Tung HD, Hung CH, Lu SN. Correlation of quantitative assay of hepatitis B surface antigen and HBV DNA levels in asymptomatic hepatitis B virus carriers. Eur J Gastroenterol Hepatol. 2004;16:1213–1218. doi: 10.1097/00042737-200411000-00021. [DOI] [PubMed] [Google Scholar]
- 24.Brunetto MR, Oliveri F, Colombatto P, Moriconi F, Ciccorossi P, Coco B, Romagnoli V, Cherubini B, Moscato G, Maina AM, Cavallone D, Bonino F. Hepatitis B surface antigen serum levels help to distinguish active from inactive hepatitis B virus genotype D carriers. Gastroenterology. 2010;139:483–490. doi: 10.1053/j.gastro.2010.04.052. [DOI] [PubMed] [Google Scholar]
- 25.Jaroszewicz J, Calle Serrano B, Wursthorn K, Deterding K, Schlue J, Raupach R, Flisiak R, Bock CT, Manns MP, Wedemeyer H, Cornberg M. Hepatitis B surface antigen (HBsAg) levels in the natural history of hepatitis B virus (HBV)-infection: a European perspective. J Hepatol. 2010;52:514–522. doi: 10.1016/j.jhep.2010.01.014. [DOI] [PubMed] [Google Scholar]
- 26.Matsumoto A, Tanaka E, Suzuki Y, Kobayashi M, Tanaka Y, Shinkai N, Hige S, Yatsuhashi H, Nagaoka S, Chayama K, Tsuge M, Yokosuka O, Imazeki F, Nishiguchi S, Saito M, Fujiwara K, Torii N, Hiramatsu N, Karino Y, Kumada H. Combination of hepatitis B viral antigens and DNA for prediction of relapse after discontinuation of nucleos(t)ide analogs in patients with chronic hepatitis B. Hepatol Res. 2012;42:139–149. doi: 10.1111/j.1872-034X.2011.00910.x. [DOI] [PubMed] [Google Scholar]
- 27.Lok AS, McMahon BJ. Chronic hepatitis B: update 2009. Hepatology. 2009;50:661–662. doi: 10.1002/hep.23190. [DOI] [PubMed] [Google Scholar]
- 28.European Association For The Study Of The Liver EASL clinical practice guidelines: Management of chronic hepatitis B virus infection. J Hepatol. 2012;57:167–185. doi: 10.1016/j.jhep.2012.02.010. [DOI] [PubMed] [Google Scholar]
- 29.Liaw YF, Kao JH, Piratvisuth T, et al. Asian-Pacific consensus statement on the management of chronic hepatitis B: a 2012 update. Hepatol Int. 2012;6:531–561. doi: 10.1007/s12072-012-9365-4. [DOI] [PubMed] [Google Scholar]
- 30.Mahmood S, Niiyama G, Kamei A, Izumi A, Nakata K, Ikeda H, Suehiro M, Kawanaka M, Togawa K, Yamada G. Influence of viral load and genotype in the progression of Hepatitis B-associated liver cirrhosis to hepatocellular carcinoma. Liver Int. 2005;25:220–225. doi: 10.1111/j.1478-3231.2005.01077.x. [DOI] [PubMed] [Google Scholar]
- 31.Seto WK, Wong DK, Fung J, Hung IF, Fong DY, Yuen JC, Tong T, Lai CL, Yuen MF. A large case-control study on the predictability of hepatitis B surface antigen levels three years before hepatitis B surface antigen seroclearance. Hepatology. 2012;56:812–819. doi: 10.1002/hep.25718. [DOI] [PubMed] [Google Scholar]
- 32.Simonetti J, Bulkow L, McMahon BJ, Homan C, Snowball M, Negus S, Williams J, Livingston SE. Clearance of hepatitis B surface antigen and risk of hepatocellular carcinoma in a cohort chronically infected with hepatitis B virus. Hepatology. 2010;51:1531–1537. doi: 10.1002/hep.23464. [DOI] [PubMed] [Google Scholar]
- 33.Tanaka Y, Mukaide M, Orito E, Yuen MF, Ito K, Kurbanov F, Sugauchi F, Asahina Y, Izumi N, Kato M, Lai CL, Ueda R, Mizokami M. Specific mutations in enhancer II/core promoter of hepatitis B virus subgenotypes C1/C2 increase the risk of hepatocellular carcinoma. J Hepatol. 2006;45:646–653. doi: 10.1016/j.jhep.2006.06.018. [DOI] [PubMed] [Google Scholar]
- 34.Lau GK, Piratvisuth T, Luo KX, Marcellin P, Thongsawat S, Cooksley G, Gane E, Fried MW, Chow WC, Paik SW, Chang WY, Berg T, Flisiak R, McCloud P, Pluck N, Peginterferon Alfa-2a HBeAg-Positive Chronic Hepatitis B Study Group Peginterferon Alfa-2a, lamivudine, and the combination for HBeAg-positive chronic hepatitis B. N Engl J Med. 2005;352:2682–2695. doi: 10.1056/NEJMoa043470. [DOI] [PubMed] [Google Scholar]
- 35.Dienstag JL, Schiff ER, Wright TL, Perrillo RP, Hann HW, Goodman Z, Crowther L, Condreay LD, Woessner M, Rubin M, Brown NA. Lamivudine as initial treatment for chronic hepatitis B in the United States. N Engl J Med. 1999;341:1256–1263. doi: 10.1056/NEJM199910213411702. [DOI] [PubMed] [Google Scholar]
- 36.Chang TT, Gish RG, de Man R, Gadano A, Sollano J, Chao YC, Lok AS, Han KH, Goodman Z, Zhu J, Cross A, DeHertogh D, Wilber R, Colonno R, Apelian D, BEHoLD AI463022 Study Group A comparison of entecavir and lamivudine for HBeAg-positive chronic hepatitis B. N Engl J Med. 2006;354:1001–1010. doi: 10.1056/NEJMoa051285. [DOI] [PubMed] [Google Scholar]
- 37.Lai CL, Gane E, Liaw YF, Hsu CW, Thongsawat S, Wang Y, Chen Y, Heathcote EJ, Rasenack J, Bzowej N, Naoumov NV, Di Bisceglie AM, Zeuzem S, Moon YM, Goodman Z, Chao G, Constance BF, Brown NA, Globe Study Group Telbivudine versus lamivudine in patients with chronic hepatitis B. N Engl J Med. 2007;357:2576–2588. doi: 10.1056/NEJMoa066422. [DOI] [PubMed] [Google Scholar]
- 38.Marcellin P, Chang TT, Lim SG, Tong MJ, Sievert W, Shiffman ML, Jeffers L, Goodman Z, Wulfsohn MS, Xiong S, Fry J, Brosgart CL, Adefovir Dipivoxil 437 Study Group Adefovir dipivoxil for the treatment of hepatitis B e antigen-positive chronic hepatitis B. N Engl J Med. 2003;348:808–816. doi: 10.1056/NEJMoa020681. [DOI] [PubMed] [Google Scholar]
- 39.Marcellin P, Heathcote EJ, Buti M, Gane E, de Man RA, Krastev Z, Germanidis G, Lee SS, Flisiak R, Kaita K, Manns M, Kotzev I, Tchernev K, Buggisch P, Weilert F, Kurdas OO, Shiffman ML, Trinh H, Washington MK, Sorbel J, Anderson J, Snow-Lampart A, Mondou E, Quinn J, Rousseau F. Tenofovir disoproxil fumarate versus adefovir dipivoxil for chronic hepatitis B. N Engl J Med. 2008;359:2442–2455. doi: 10.1056/NEJMoa0802878. [DOI] [PubMed] [Google Scholar]
- 40.Liaw YF. Clinical utility of hepatitis B surface antigen quantitation in patients with chronic hepatitis B: a review. Hepatology. 2011;54:E1–E9. doi: 10.1002/hep.24473. [DOI] [PubMed] [Google Scholar]
- 41.Janssen HL, van Zonneveld M, Senturk H, Zeuzem S, Akarca US, Cakaloglu Y, Simon C, So TM, Gerken G, de Man RA, Niesters HG, Zondervan P, Hansen B, Schalm SW, HBV 99-01 Study Group Rotterdam Foundation for Liver Research Pegylated interferon alfa-2b alone or in combination with lamivudine for HBeAg-positive chronic hepatitis B: a randomised trial. Lancet. 2005;365:123–129. doi: 10.1016/S0140-6736(05)17701-0. [DOI] [PubMed] [Google Scholar]
- 42.Lampertico P, Viganò M, Colombo M. Treatment of HBeAg-negative chronic hepatitis B with pegylated interferon. Liver Int. 2011;31(Suppl 1):90–94. doi: 10.1111/j.1478-3231.2010.02386.x. [DOI] [PubMed] [Google Scholar]
- 43.Keating GM. Peginterferon-α-2a (40 kD): A review of its use in chronic hepatitis B. Drugs. 2009;69:2633–2660. doi: 10.2165/11203660-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 44.Suzuki F, Arase Y, Suzuki Y, Akuta N, Sezaki H, Seko Y, Kawamura Y, Hosaka T, Kobayashi M, Saito S, Ikeda K, Kobayashi M, Kumada H. Long-term efficacy of interferon therapy in patients with chronic hepatitis B virus infection in Japan. J Gastroenterol. 2012;47:814–822. doi: 10.1007/s00535-012-0548-5. [DOI] [PubMed] [Google Scholar]
- 45.Lin SM, Yu ML, Lee CM, Chien RN, Sheen IS, Chu CM, Liaw YF. Interferon therapy in HBeAg positive chronic hepatitis reduces progression to cirrhosis and hepatocellular carcinoma. J Hepatol. 2007;46:45–52. doi: 10.1016/j.jhep.2006.08.021. [DOI] [PubMed] [Google Scholar]
- 46.Yang YF, Zhao W, Zhong YD, Xia HM, Shen L, Zhang N. Interferon therapy in chronic hepatitis B reduces progression to cirrhosis and hepatocellular carcinoma: a meta-analysis. J Viral Hepat. 2009;16:265–271. doi: 10.1111/j.1365-2893.2009.01070.x. [DOI] [PubMed] [Google Scholar]