Abstract
In recent years considerable progress has been made in treatment strategies for chronic lymphocytic leukemia (CLL). However, the disease remains incurable because of the development of chemoresistance. Strategies to overcome resistance mechanisms are therefore highly needed. At least two mechanisms contribute to the development of resistance to drugs; acquired mutations resulting in a dysfunctional p53 response and shifts in the balance between apoptosis-regulating proteins. Platinum-based compounds have been successfully applied in relapsed lymphoma and recently also in high-risk CLL. In this study we investigated the efficacy and mechanism of action of cisplatinum (CDDP) in chemorefractory CLL. Independent of p53-functional status, CDDP acted synergistically with fludarabine (F-ara-A). The response involved generation of reactive oxygen species (ROS), which led to specific upregulation of the proapoptotic BH3-only protein Noxa. Induction of Noxa resulted in cell death by apoptosis as inhibition of caspase activation completely abrogated cell death. Furthermore, drug-resistance upon CD40-ligand stimulation, a model for the protective stimuli provided in lymph nodes, could also be overcome by CDDP/F-ara-A. ROS accumulation resulted in Noxa upregulation mainly at the transcriptional level and this was, at least in part, mediated by the mitogen-activated protein kinase p38. Finally, Noxa RNA-interference markedly decreased sensitivity to CDDP/F-ara-A, supporting a key role for Noxa as mediator between ROS signaling and apoptosis induction. Our data indicate that interference in the cellular redox balance can be exploited to overcome chemoresistance in CLL.
Keywords: CLL, chemoresistance, p53, ROS, platinum-based compounds, Noxa
Introduction
The clinical course of chronic lymphocytic leukemia (CLL) is highly variable; however, most patients eventually develop symptomatic disease requiring treatment. Although important advances have been made in first and second line treatment strategies, cures are not attained. Various biological markers correlate with poor prognosis and response to treatment, such as IgVH mutational status, expression levels of CD38 and/or ZAP-70 and cytogenetic abnormalities (reviewed by Van Bockstaele et al., 2009). Especially deletion or mutation of the p53 locus (17p13) is associated with a very poor outcome (Zenz et al., 2008). Most drugs, including alkylating agents and nucleoside analogs, rely on intact p53 function for their activity, and treatment with these compounds results in selection and evolution of clones containing cytogenetic changes affecting the p53 response (Rosenwald et al., 2004). A second important contribution to chemoresistance in CLL is made by shifts in the balance between pro- and antiapoptotic proteins. Increased expression levels of Bcl-2 family members like Bfl-1/A1 (Morales et al., 2005) and in particular Mcl-1 (Pepper et al., 2008) have been associated with resistance to chemotherapy. Shifts in the apoptotic balance presumably arise in CLL cells residing in secondary lymphoid tissue. In these niches, cells receive stimuli from the microenvironment inhibiting apoptosis, and are consequently protected from the action of cytotoxic drugs (Munk Pedersen and Reed, 2004). Indeed, we found increased expression of Mcl-1, Bcl-xL and Bfl-1/A1 in lymphoid tissue-derived leukemia cells in comparison with peripheral blood-derived CLL cells (Smit et al., 2007). It is postulated that clones harboring acquired mutations originate from these niches (Munk Pedersen and Reed, 2004).
The prognosis for patients with fludarabine (F-ara-A) refractory disease is extremely poor, with a median survival of less than a year (Seymour et al., 1995). Therefore, it is highly necessary to develop therapeutic strategies that circumvent resistance mechanisms to cytotoxic agents to obtain sustained responses in these patients.
Several novel drugs are currently under investigation in clinical and preclinical studies for this category of patients (reviewed by Kater and Tonino, 2010), but although responses have been observed, when used as single agent these drugs generally do not induce long-term disease-free survival.
An approach to overcome drug resistance could be to alter the cellular apoptotic balance, independent of p53, to sensitize cells to alkylating agents and nucleoside analogs. Regimens containing platinum-based compounds have shown activity in CLL, importantly also in p53-dysfunctional cases. In a small phase I-II trial, the oxaliplatin, F-ara-A, cytarabine and rituximab (OFAR) regimen induced a response in 7 of 20 patients with a documented 17p deletion (Tsimberidou et al., 2008). We recently found marked activity of the rituximab, dexamethasone, cytarabine and cisplatinum (CDDP) (R-DHAP) regimen in 8 of 10 patients with F-ara-A refractory disease, including patients with proven p53 dysfunction (Tonino et al., 2010). Synergy between F-ara-A and platinum-based compounds was studied in in vitro studies and was found to result from inhibition of nucleotide excision repair by F-ara-A of DNA damage induced by platinum-based compounds in both a cell-free system and in CLL cells derived from patients with early-stage disease (in which p53 dysfunction is found in less than 5% of cases) (Li et al., 1997; Moufarij et al., 2006). CLL cells exhibit high DNA repair capacity and this was found to correlate with resistance to cytotoxic drugs (Geleziunas et al., 1991; Deriano et al., 2005). However, to what extent these repair pathways are involved in response to therapy in CLL, how these processes are regulated and how cell fate is decided on after DNA damage is not well known. As p53 is the central factor in the regulation of DNA repair pathways (Ford and Hanawalt, 1997), as well as in the initiation of the ensuing apoptotic response (reviewed by Rich et al., 2000), the above described synergy very likely depends on a functional p53 pathway. However, clinical effectiveness in p53-dysfunctional patients suggests that an alternative, p53 independent, mechanism is in play in the activity of the combination of CDDP and F-ara-A.
As elucidating p53-independent apoptosis pathways may yield useful information for the development of treatment strategies for chemorefractory patients, we studied how platinum-based compounds abrogate F-ara-A resistance in CLL. We found synergy between the two classes of drugs, irrespective of p53-functional status. Resistance resulting from CD40 ligation was also overcome. The apoptotic response towards CDDP/F-ara-A combination treatment was mediated by an increase in reactive oxygen species (ROS) and required upregulation of the proapoptotic BH3-only protein Noxa. These data stress the potential clinical relevance of targeting the redox balance to overcome drug resistance in CLL.
Results
Platinum-based compounds sensitize CLL cells to caspase-dependent apoptosis induction by F-ara-A, independent of p53 function
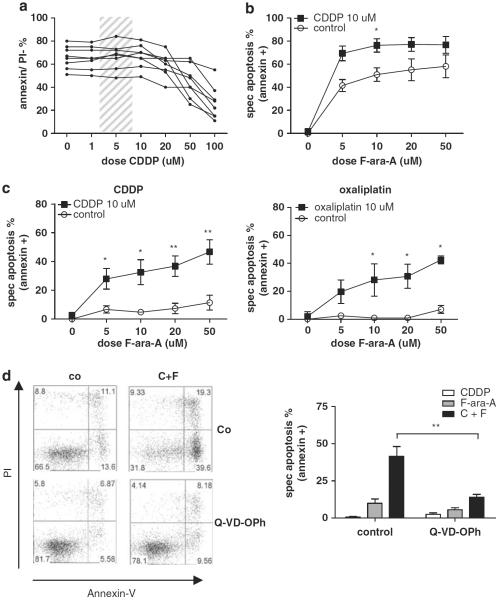
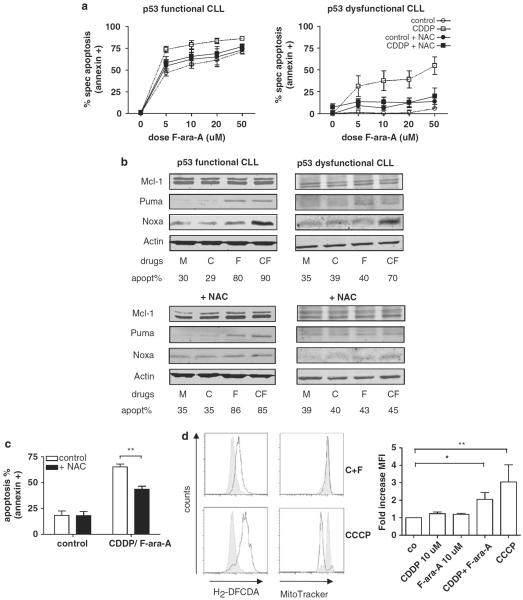
The response of CLL cells to increasing doses of CDDP (1–100 μm) was assessed after 24 and 48 h. After 48 h, cell death was seen only in doses above 50 μm. As the Cmax of CDDP in vivo is within the range indicated by the shaded area in Figure 1a (Monjanel-Mouterde et al., 2003), and does not exceed 10 μm, CDDP is not expected to be active as a single agent in CLL (Figure 1a). As expected, p53-functional CLL cells were sensitive to F-ara-A in increasing doses, whereas p53-dysfunctional CLL samples were resistant, even in doses much higher than those that can be achieved in vivo (Figures 1b and c). Addition of 10 μm CDDP, which by itself did not induce cell death, not only enhanced F-ara-A-induced cell death in p53-functional samples, but also synergistically induced cell death in p53-dysfunctional CLL. Significant synergy was already observed with 5 μm F-ara-A. Assessment of apoptosis by measuring both loss of mitochondrial membrane potential (MitoTracker staining) as well as phosphatidylserine-exposure (annexin-V/propidium iodide (PI) staining) yielded comparable results (presented are results from annexin-V/PI staining, Figures 1b and c; left graph).
Figure 1.
Synergism between F-ara-A and CDDP in CLL is independent of p53-functional status. (a) CLL cells of eight patients (patient nos. 2, 3, 5, 8, 11, 15, 18 and 22) were incubated for 48 h with CDDP at increasing doses (1–100 μm). Cell death was assessed by annexin-V/PI staining (as described in the Materials and methods section). The shaded area presents the concentration range attained on CDDP treatment in vivo (Monjanel-Mouterde et al., 2003). (b) CLL cells with functional p53 (n = 5; patient nos. 11, 12, 18, 21 and 22) were treated with increasing doses of F-ara-A (5–50 μm), with or without 10 μm CDDP, for 48 h. Cell death was assessed by annexin-V/PI staining. To correct for variation in base-line apoptosis levels (see a), specific apoptosis is depicted (as described in the Materials and methods section). Presented is mean + s.e.m. (*P<0.05; Mann–Whitney U-test). (c) CLL cells with dysfunctional p53 were treated with increasing doses of F-ara-A (5–50 μm), with or without 10 μm CDDP (n = 6; patients 1–6) or 10 μm oxaliplatin (n = 4; patient nos. 2, 3, 5 and 6) for 48 h. Cell death was assessed by annexin-V/PI staining. Presented is mean +s.e.m. (*P<0.05, **P<0.01; Mann–Whitney U-test). (d) Left panels: Cells of patient number 5 were treated with 10 μm CDDP and 10 μm F-ara-A (C+F) with or without preincubation with 20 μm Q-VD-OPh. Apoptosis was assessed after 48 h using annexin-V/PI-staining. Right graph: summarized data of 5 p53-dysfunctional CLL patients (patient nos. 1–3, 5 and 6). Bars represent mean+s.e.m. (**P<0.01; Mann–Whitney U-test).
Oxaliplatin (DACH oxalate-platinum), a third generation platinum-based compound with improved pharmacokinetics and toxicity profile in comparison with CDDP, is currently tested in chemorefractory CLL as component of the oxaliplatin, F-ara-A, cytarabine and rituximab (OFAR)-regimen. A similar synergistic effect was found by combining oxaliplatin with F-ara-A, indicating that the observed synergistic effect applies to platinum-based compounds in general (Figure 1c; right graph).
To analyze whether the observed cell death is caspase-dependent, synergy experiments were repeated in the presence of the general caspase inhibitor Q-VD-OPh. Addition of Q-VD-OPh significantly abrogated cell death in p53-functional (Supplementary Figure 1) as well in p53-dysfunctional cells (Figure 1d). Cell death could not be inhibited by the caspase-8 inhibitor Z-IETD-fmk (data not shown), indicating that the mitochondrial, rather than the extrinsic apoptosis pathway is involved.
Co-treatment of CLL cells with CDDP and F-ara-A results in upregulation of Noxa
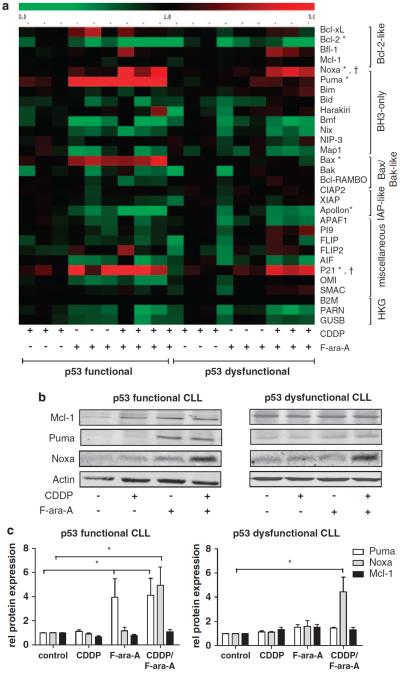
We have described earlier that F-ara-A-induced apoptosis relies on p53-dependent upregulation of the BH3-only molecule Puma (Mackus et al., 2005). To study whether the combination of CDDP and F-ara-A results in alterations in the balance between pro- and antiapoptotic proteins, the mRNA expression level of relevant apoptosis-regulating genes was assessed by reverse transcriptase-multiplex ligation-dependent probe amplification (RT-MLPA). Treatment with 10 μm CDDP did not result in significant modulation of gene expression in p53-functional and p53-dysfunctional CLL samples, in accordance with the absence of an apoptotic response. As expected, as the activity of F-ara-A depends on p53 (Rosenwald et al., 2004; Mackus et al., 2005; Mous et al., 2009), treatment with this drug induced significant upregulation of the p53-target genes Puma, p21 and Bax in p53-functional samples, whereas no upregulation of Puma and Bax was detected in p53-dysfunctional samples. Combination treatment with CDDP and F-ara-A induced significant changes in expression levels of only two genes: p21 and Noxa, irrespective of p53 function (Figure 2a). To test protein expression levels, cells were treated as indicated in the presence of Q-VD-OPh to prevent caspase-mediated breakdown of proteins. Noxa protein levels were clearly increased on combination treatment, whereas no induction occurred upon treatment with CDDP or F-ara-A as single drugs. Puma protein was upregulated in a p53-dependent manner (Figures 2b and c). Levels of the antiapoptotic Bcl-2 family-member Mcl-1, the principal binding partner of Noxa (Chen et al., 2005), remained stable, as did protein levels of antiapoptotic Bcl-2 and Bfl-1/A-1 and proapoptotic Bax and Bim (Figures 2b and c and data not shown).
Figure 2.
Co-treatment of CLL cells with F-ara-A and CDDP induces upregulation of Noxa. (a) Cells of three p53-functional (patient nos. 11, 18 and 22) and three p53-dysfunctional (patient nos. 2, 4 and 5) CLL patients were treated with 10 μm CDDP, 10 μm F-ara-A or with the combination in the presence of Q-VD-OPh. The mRNA expression level of 30 apoptosis-regulating genes was assessed by RT-MLPA (as described in the Materials and methods section). Gene expression following treatment was related to gene expression in untreated cells. The resulting matrix was imported in the program MultiExperiment Viewer (www.tigr.org/software/tm4), and values were assigned green or red colors; green for values between 0 and 1, indicating downregulation and red for values >1, indicating upregulation. The CLL samples are ordered as indicated below the matrix. In the right hand column, the genes are ordered by functional category (HKG, housekeeping genes; β2M, β2-microglobulin). Significant changes in expression are indicated with (*) for p53+ samples and (†) for p53− samples (P<0.05, Kruskal–Wallis test with post-hoc Dunn's test). (b) CLL cells were treated with 10 μM CDDP, 10 μM F-ara-A or the combination as indicated for 48 h in the presence of Q-VD-OPh to prevent caspase-dependent breakdown of proteins. Protein lysates were analyzed for Mcl-1, Puma and Noxa expression by western blot. Actin was used as loading control. Shown is a representative blot of both a p53-functional (patient no. 20) and a p53-dysfunctional CLL samples (patient no. 3). (c) Summarized data of the relative expression (compared with control) of Mcl-1, Puma and Noxa in four p53-functional (patient nos. 18–20 and 22) and four p53-dysfunctional (patient nos. 1–3 and 5) CLL samples on treatment as described in (b). Protein expression was quantified using Licor Odyssey software and corrected for the expression of actin. Bars represent mean + s.e.m. (*P< .05, Kruskal–Wallis test with post-hoc Dunn's test).
CD40 ligation does not protect against the cytotoxic effect of CDDP and F-ara-A
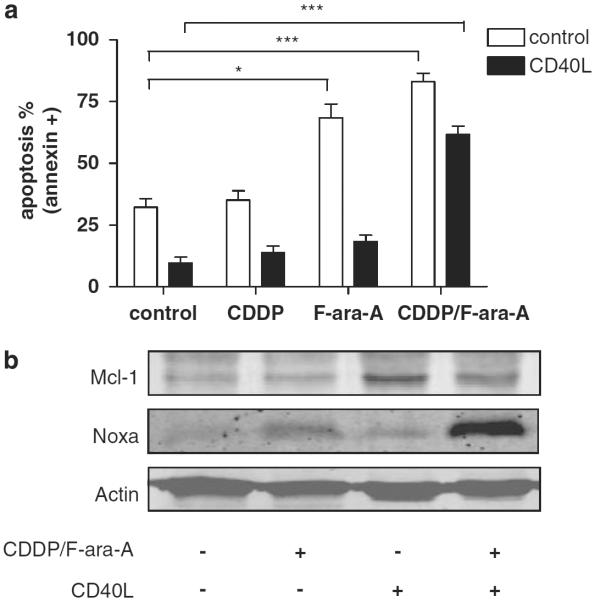
Next to the functional status of p53, protective stimuli derived from the microenvironment in secondary lymphoid tissue constitute a major determinant of drug resistance in CLL. The protective microenvironment can, in part, be mimicked in vitro by stimulating CLL cells with CD40-ligand, which results in increased expression levels of Bcl-xL, Bfl-1/A1 and Mcl-1 and decreased expression levels of Noxa (Kater et al., 2004; Hallaert et al., 2008; Vogler et al., 2009). To analyze whether combination treatment with CDDP and F-ara-A can also overcome chemoresistance due to these protective signals, CLL cells were cocultured with CD40-ligand expressing fibroblasts for 48 h and subsequently treated with CDDP and F-ara-A for 48 h. As shown in Figure 3a, cells cocultured with control fibroblasts were sensitive to F-ara-A, whereas CD40-ligand stimulation resulted in complete resistance to F-ara-A. Addition of CDDP to F-ara-A resulted in a significant increase in apoptosis levels despite CD40 ligation.
Figure 3.
CD40 ligation does not rescue CLL cells from apoptosis induced by CDDP/F-ara-A combination treatment. (a) Cells of six p53-functional CLL patients (patient nos. 9, 10, 13, 14, 17 and 21) were stimulated with CD40-ligand for 48 h, collected and treated with 10 μm CDDP, 10 μm F-ara-A or the combination for 48 h. As control, cells were cocultured with 3T3 fibroblasts. Apoptosis was assessed by annexin-V/PI staining. Bars represent mean+s.e.m. (*P<0.05, ***P<0.001; Mann-Whitney U-test). (b) Cells were treated as in (a), lysed and tested for expression levels of Mcl-1, Noxa and actin (patient no. 18; representative blot of four patients tested).
We have shown earlier that F-ara-A-mediated induction of Puma expression is not abrogated by CD40-ligand stimulation (Kater et al., 2004). Analysis of protein expression levels on combination treatment revealed strong upregulation of Noxa (Figure 3b). As Noxa selectively binds and inhibits Mcl-1 (Chen et al., 2005), these data suggest that induction of Mcl-1 expression is an important determinant of CD40-ligand-mediated chemoresistance. The decrease in Mcl-1 protein expression level in CD40-ligand-stimulated cells treated with CDDP and F-ara-A may be secondary to caspase activation (Clohessy et al., 2004).
Activity of CDDP is not correlated with p73 expression in CLL
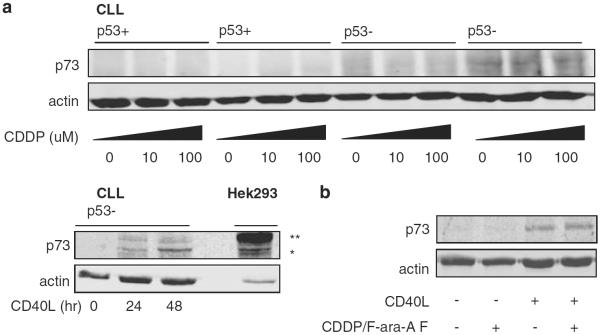
The observed synergy between CDDP and F-ara-A in p53-dysfunctional CLL samples implies activation of p53-independent apoptosis pathways. Previous studies in various solid cancers have indicated that the activity of CDDP involves p53-independent upregulation of p73, a member of the p53 family (Gong et al., 1999). As Noxa is reported to be a response gene of p73 (Flinterman et al., 2005), we studied whether p73 was upregulated by CDDP in CLL cells and whether the observed apoptosis was mediated by this protein. We first tested this hypothesis in the p53-dysfunctional prolymphocytic cell line Mec-1. Upon CDDP treatment, upregulation of p73 was seen in a time- and dose-dependent manner (data not shown). Next, we analyzed expression levels of p73 upon treatment with CDDP in p53-functional and -dysfunctional CLL cells. No upregulation of p73 protein levels were found when cells were treated with doses up to 100 μM (Figure 4a) and incubation up to 96 h (data not shown). CD40 ligation was used as a control, as this was demonstrated to induce upregulation of p73 protein levels in CLL (Dicker et al., 2006). Also treatment with F-ara-A or the combination of these drugs had no effect on p73 levels in CLL (Figure 4b).
Figure 4.
CDDP treatment does not induce p73 in CLL. (a) CLL cells of two patients with functional (patient nos. 19 and 20) and two with dysfunctional p53 (patient nos. 2 and 3) were treated with 10 or 100 μm CDDP; after 48 h, p73-protein expression levels were assessed by western blot. As control, CLL cells were cocultured with CD40-ligand expressing fibroblasts for 24 h and analyzed for p73 expression after an additional 24 or 48 h (patient no. 20). Lysates were obtained in the same experiment and loaded on separate gels. As antibody control a lysate of HEK293 cells transfected with a construct encoding HA-tagged p73 was used (*denotes the endogenous p73 band; **denotes the HA-tagged p73-band in transfected cells). (b) CLL cells were cocultured with CD40-ligand expressing or control 3T3 fibroblasts for 48 h and treated with 10 μm CDDP and 10 μm F-ara-A as indicated for 48 h. p73 and actin protein expression levels were analyzed by western blot (patient no. 18; representative blot of at least six patients tested).
N-acetylcysteine abrogates apoptosis as well as Noxa upregulation
The mechanism of action of platinum-based compounds is classically described to be mediated by DNA damage and the ensuing death response (Ali et al., 2005). However, CDDP binds to many cellular components; in fact only 10–15% is found in the nuclear compartment, whereas 75–85% is bound to proteins (Akaboshi et al., 1994). Once in the cell, platinum-based compounds are aquated and become highly reactive. In this form they rapidly bind cytoplasmatic proteins, among which antioxidant proteins like reduced glutathione (Hagrman et al., 2004) and constituents of mitochondria causing mitochondrial dysfunction (Garrido et al., 2008). We hypothesized that in CLL cells, disruption of the cellular redox balance and the generation of ROS, rather than direct DNA damage, might contribute to the apoptotic response upon treatment with CDDP. To study whether the activity of the combination of CDDP and F-ara-A involves generation of ROS, experiments were performed in the presence of the free radical scavenger N-acetylcysteine (NAC). As shown in Figure 5a, apoptosis upon CDDP/F-ara-A combination treatment in p53-dysfunctional cells was almost completely abrogated by NAC, whereas sensitivity to F-ara-A in p53-functional samples was unaffected by the addition of NAC. To confirm that cell death was abrogated by NAC through its ROS-scavenging properties, experiments were repeated with the alternative scavengers Tiron and butylated hydroxyl-anisole. These experiments yielded comparable results, but data analysis was hampered by toxicity of these compounds after 48 h (Supplementary Figure 2A). Upregulation of Noxa protein levels was abrogated by co-treatment with NAC, whereas expression levels of Puma remained unaffected (Figure 5b), which supports a functional role for Noxa in apoptosis induction upon CDDP/F-ara-A combination treatment. Furthermore, cell death upon combination treatment in CD40-ligand-stimulated cells could be diminished by the addition of NAC (and Tiron and butylated hydroxyl-anisole), indicating that also in this setting, death is mediated by ROS (Figure 5c and Supplementary Figure 2B).
Figure 5.
Apoptosis and Noxa upregulation on CDDP/F-ara-A combination treatment are mediated by ROS. (a) p53-dysfunctional (n = 5; patient nos. 1–5) and p53-functional (n = 4; patient nos. 11, 12, 18 and 21) CLL samples were preincubated with 5 mM NAC for 30 min and subsequently treated with F-ara-A in increasing doses (5–50 μm), with or without 10 μm CDDP, for 48 h. Apoptosis was assessed by annexin-V/PI staining. Presented is mean + s.e.m. (b) CLL cells were treated for 48 h as indicated (M = control, C = 10 μm CDDP, F = 10 μm F-ara-A, CF = CDDP/F-ara-A both 10 μm) with or without preincubation with 5 mm NAC and analyzed for protein expression levels of Mcl-1, Puma, Noxa and actin. Experiments for protein collection were performed in the presence of Q-VD-OPh to prevent caspase-dependent breakdown of proteins. Percentage of apoptosis was assessed by annexin-V/PI staining (patient nos. 2 and 22; representative of six patients tested). (c) CLL cells, which have been cocultured with CD40L-expressing or control 3T3 fibroblast for 48 h, were treated for 48 h with 10 μm CDDP and 10 μm F-ara-A with or without preincubation with 5 mm NAC (n = 5; patient nos. 7, 15, 16, 21 and 22). Percentage of apoptosis was assessed by annexin-V/PI staining; bars represent mean + s.e.m. (**P<0.01; Mann–Whitney U-test). (d) Left panels: ROS content was determined by carboxy-H2-DCFDA staining and flow cytometry (as described in the Materials and methods section) after 6 h of treatment with 10 μm CDDP and 10 μm F-ara-A (C + F; open graph; upper panels) and compared with ROS content of untreated (gray graph) or CCCP (100 μm)-treated cells (open graph; lower panels) (patient no. 2). Loss of mitochondrial potential was assessed by MitoTracker staining. Right graph: summarized data of cellular ROS content after 6 h of treatment as indicated compared with ROS content of control sample (n = 4, patient nos. 1, 2, 3 and 5). Presented is mean + s.e.m. (*P<0.05, **P<0.01; Mann–Whitney U-test).
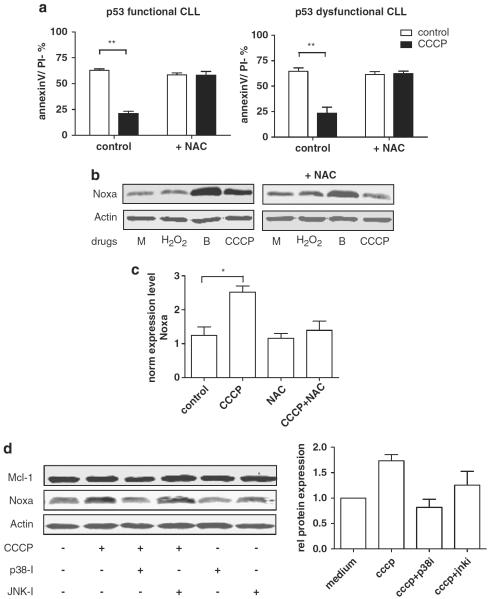
To further test our hypothesis, we performed direct assessment of both cellular glutathione as well as ROS levels. A significant but only partial decrease was found in glutathione levels within 6 h of treatment, not only in cells treated with high dose CDDP (100 μM), as expected, but also in cells treated with 10 μM CDDP in combination with 10 μM F-ara-A, whereas treatment with the drugs at this dose level alone, did not result in a significant modulation of glutathione levels (Supplementary Figure 2C). Direct assessment of cellular ROS content revealed an increase in ROS levels within 6 h of combination treatment (Figure 5d; upper left panels and right graph). This occurred well before the loss of mitochondrial membrane potential (upper left panels) and phosphatidylserine exposure (not shown), which supports a pivotal role for ROS in the induction of apoptosis. Carbonyl cyanide 3-chlorophenylhydrazone (CCCP), a potent uncoupler of oxidative phosphorylation and inducer of ROS, was used to confirm effects of ROS accumulation in CLL (Figure 5d; lower left panels).
Accumulation of ROS results in upregulation of Noxa, primarily on a transcriptional level
We further studied the mechanism by which accumulation of ROS results in upregulation of Noxa. First we determined what source of ROS is most likely accountable for induction of Noxa protein levels. Cells were treated with either CCCP or with hydrogen peroxide (H2O2, a common ROS intermediate generated by various extracellular and intracellular ROS producing sources). Treatment with 100 μM CCCP (and 300 μM H2O2; data not shown) strongly induced apoptosis within 24 h, independent of p53 function, and this could be completely abrogated by the addition of NAC (assessed by annexin-V/PI staining; Figure 6a). However, accumulation of Noxa protein was seen after 24 h of treatment with 100 μM CCCP (Figure 6b), but not with H2O2. The proteasome inhibitor bortezomib was used as positive control. These data suggest that mainly ROS derived from a mitochondrial source are required for the upregulation of Noxa.
Figure 6.
(a) p53-dysfunctional (n = 5; patient nos. 2–6) and p53-functional (n = 5; patient nos. 11, 19–22) CLL cells were treated with 100 μm CCCP for 24 h with or without preincubation with 5 mm NAC for 30 min. Apoptosis was assessed by annexin-V/PI staining. Presented is mean + s.e.m. (**P<0.01; Mann–Whitney U-test). (b) CLL cells were treated with 300 μm H2O2,, 30 nm bortezomib (b) or 100 μm CCCP (as indicated) for 24 h in the presence of Q-VD-OPh to prevent caspase-dependent breakdown of proteins. Protein lysates were analyzed for Noxa expression by western blot. Actin was used as loading control. Shown is a representative blot (patient no. 23) of three independent experiments. (c) Cells of three p53-functional (patient nos. 11, 18 and 22) CLL patients were treated with 100 μm CCCP with or without preincubation with 5 mm NAC for 30 min in the presence of Q-VD-OPh. The mRNA expression level of 30 apoptosis-regulating genes was assessed by RT-MLPA (as described in the Materials and methods section). Presented are the mRNA expression levels of Noxa normalized for β2-microglobulin. (*P<0.05, Student's t-test). For complete results of the assay see Supplementary Figure 3. (d) Left panels: CLL cells (patient no. 24) were treated with 100 μm CCCP with or without 10 μm of the MAPK-inhibitors p38-i (SB202190) or JNK-i (Sp600125; as indicated) for 24 h in the presence of Q-VD-OPh to prevent caspase-dependent breakdown of proteins. Protein lysates were analyzed for Noxa expression by western blot. Actin was used as loading control. Right graph: summarized data of the relative expression (compared with control) of Noxa in three CLL samples on treatment as described in (b). Protein expression was quantified using Licor Odyssey software and corrected for the expression of actin. Bars represent mean + s.e.m.
Next, we determined the level at which ROS-dependent Noxa induction is regulated. In accordance with our findings upon CDDP/F-ara-A treatment (Figure 2a), treatment with CCCP resulted in upregulation of Noxa mRNA levels, irrespective of p53-functional status (Figure 6c and Supplementary Figure 3), which was abrogated by the addition of NAC.
Apart from the p53 family, the mitogen-activated protein kinase (MAPK) signaling pathways have an important role in deciding cell fate on cellular stress, including oxidative stress (reviewed in McCubrey et al., 2006). Of these, mainly the JNK and p38 pathways orchestrate the apoptotic response to cellular stress and are hence grouped together and referred to as stress-activated protein kinases (SAPK). To investigate the role of stress-activated protein kinases, we treated cells with CCCP in the presence of specific pharmacological inhibitors of p38 en JNK. As shown in Figure 6b, p38 inhibition abrogated Noxa upregulation upon CCCP treatment, whereas JNK inhibition did not.
As recently has been shown in CLL cells, Noxa protein has a short half-life through rapid proteasomal dependent degradation (Baou et al., 2010). We measured proteasome activity in CLL cells treated with CCCP or bortezomib. Although some decrease in activity was observed upon CCCP treatment, effects were very limited, especially when compared with the decrease of proteasomal activity following treatment with bortezomib (decrease in activity 15–20% versus > 95%) (data not shown).
Knockdown of Noxa attenuates CDDP/F-ara-A-induced cell death
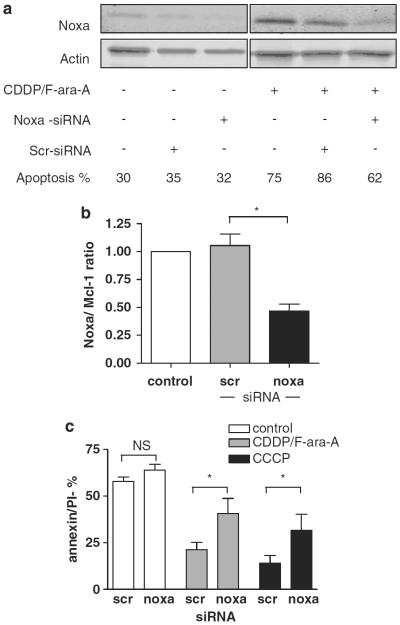
The functional relevance of Noxa upregulation in the apoptotic response upon treatment was further investigated by means of RNA interference. Cell viability was not affected by the nucleofection procedure (not shown). Nucleofection with Noxa small-interfering RNA (siRNA), but not a scrambled control siRNA, resulted in a 50–60% decrease in Noxa protein expression levels upon combination treatment (Figures 7a and b). Knockdown of Noxa significantly diminished sensitivity to CDDP/F-ara-A combination treatment and also to CCCP (Figure 7c). These data indicate a functional role for Noxa in the apoptotic response following drug-induced, p53-independent ROS accumulation in CLL cells.
Figure 7.
Apoptosis induced by CDDP/F-ara-A combination treatment is mediated by Noxa. (a) Protein expression levels of Noxa and actin in untransfected cells and in cells nucleofected with Noxa or control (scr) siRNA, untreated (left panels) or treated with 10 μm CDDP and 10 μm F-ara-A for 48 h (right panels), were analyzed by western blot for control of knockdown of Noxa. Apoptosis was assessed by MitoTracker staining (patient no. 1). (b) Protein expression levels in four patients (patient nos. 1–3 and 5), treated as described in (a) were quantified using Licor Odyssey software and the Noxa/Mcl-1 ratio was calculated. Noxa/Mcl-1 ratio of untransfected cells was set to 1. Bars represent mean + s.e.m. (*P<0.05, Mann–Whitney U-test). (c) Cells of p53-dysfunctional CLL patients were transfected with Noxa RNAi or control scrambled (Scr) RNAi and treated with 10 μm CDDP and 10 μm F-ara-A for 48 h, 100 μm CCCP for 24 h or left untreated. Apoptosis was assessed using annexin-V/PI staining (n = 4; patient nos. 1–3 and 5). Bars represent mean + s.e.m. (*P<0.05, Mann–Whitney U-test).
Discussion
In this study we show that the combination of CDDP and F-ara-A induces apoptosis in CLL cells, independent of p53-functional status. The activity of this combination is mediated by the generation of ROS. The BH3-only protein Noxa was identified as an important factor in the ensuing apoptotic response. Noxa-mediated apoptosis induction in p53-dysfunctional cells by CCCP confirms the potency of ROS signaling in p53-independent apoptosis induction. Furthermore, our data suggest that the MAPK p38 links ROS accumulation to Noxa induction.
Initially we investigated whether apoptosis was mediated by p73, but although this protein mediates responses towards CDDP in various cancer types, this seems not to apply in CLL, as no upregulation of p73 upon CDDP (and/or F-ara-A) treatment was found. Activity of both drugs is classically described to be based on infliction of DNA damage (Pettitt 2003; Ali et al., 2005), and the subsequent activation of the DNA repair machinery in which p53, and possibly p73, have key roles. However the large majority of CLL cells, especially those derived from peripheral blood, are in G1 arrest. Active DNA replication does not prevail and in this respect CLL differs from other cancer types. It is therefore, conceivable, that alternative mechanisms of action of both drugs have a more prominent role in CLL.
Studies in various cancer types have shown that malignant cells are under increased oxidative stress due to enhanced ROS formation (Zhou et al., 2003). Therefore, the balance between ROS and antioxidant mechanisms is especially critical in cancer cells, and hence presents an attractive target for therapeutic intervention (reviewed by Fruehauf and Meyskens, 2007). Most likely, this also applies to CLL, as in comparison with normal lymphocytes, CLL cells are under enhanced oxidative stress, which is associated with oxidative damage to nuclear and mitochondrial DNA (Oltra et al., 2001; Zhou et al., 2003; Trachootham et al., 2008).
We found that responses to CDDP/F-ara-A combination treatment in CLL were mediated by the generation of ROS. Cellular ROS content increased within hours after the addition of drugs. Synergy between these drugs could furthermore be abrogated by the addition of ROS scavengers. Platinum-based compounds have been shown to stress the cellular redox system in various ways. CDDP induces mitochondrial dysfunction and an increase in ROS production by damaging mitochondrial DNA and disruption of the respiratory chain (Witte et al., 2005; Garrido et al., 2008). Furthermore, CDDP reacts with thiol-containing molecules like glutathione (Spitz et al., 1993; Hagrman et al., 2004), thereby depleting intracellular glutathione levels (Husain et al., 1998), and also inhibits thioreduxin reductase, an important component of the alternative cellular reducing machinery (Witte et al., 2005). We found a decrease in cellular glutathione levels upon combination treatment, whereas treatment with single drugs (in clinically relevant doses) did not significantly affect glutathione levels. However, as we found Noxa upregulation upon treatment with the uncoupler of oxidative phosphorylation CCCP, but not upon treatment with H2O2, accumulation of ROS from a mitochondrial source may be required to induce upregulation of Noxa. Clinically attainable doses of CDDP alone did not induce ROS accumulation in our experiments. It could be that the cellular antioxidant system is capable of neutralizing CDDP in the used dose within the time period of the experiments. Indeed, when we used supraphysiological concentrations of CDDP (50–100 mm), a decrease in glutathione levels, ROS production, Noxa upregulation and apoptosis were observed, which could be abrogated by the addition of NAC (data not shown). Although F-ara-A as a single agent does not seem to induce ROS production directly (Rosato et al., 2008), it has been found to enhance ROS production in leukemia cells when combined with other agents by a yet unrevealed mechanism (Maggio et al., 2004).
We found that apoptosis was the primary mechanism of cell death induced by combination treatment, as blocking caspase activation almost completely abrogated death. The BH3-only protein Noxa had an important role in this process. As the knockdown achieved in our nucleofection experiments was partial, it is conceivable that the role of Noxa in the apoptotic response may be underestimated. We have previously shown that in CLL mRNA expression levels of Noxa are high, independent of p53-functional status (Mackus et al., 2005). Although the Noxa/Mcl-1 balance was found to be decisive for cell fate in cells residing in the lymph node (and on CD40 stimulation as its in vitro counterpart; Smit et al., 2007), the role and regulation of Noxa in CLL-specific responses to drug treatment are not well known. Noxa is a p53-response gene in many cell types (Oda et al., 2000), but this is not the case in CLL (Mackus et al., 2005). In this study we show that, at least in CLL, Noxa is the sole BH3-only member that is induced on ROS signaling. These findings are of particular interest as they constitute an apoptosis pathway in CLL, which does not depend on p53. We found that ROS-mediated Noxa upregulation is mainly transcriptional. As inhibition of the MAPK/SAPK p38 abolished Noxa upregulation, this pathway may have an important role in the cellular reaction, including induction of Noxa, to oxidative stress. Although in recent years, it has been increasingly appreciated that MAPK activation may be the major component deciding cell fate upon CDDP treatment (reviewed in Brozovic and Osmak, 2007), we here describe a novel link between treatment-induced p38 activation and Noxa induction in CLL. Interestingly, in a very recent report, in keratinocytes Noxa upregulation following UVB irradiation was also found to be orchestrated by p38 MAPK in a p53-independent manner (Nys et al., 2010).
The potential relevance of ROS-dependent apoptosis in drug responses is supported by the recent observation that F-ara-A resistant cells are highly sensitive to beta-phenylethyl isothiocyanate, a compound that induces ROS accumulation by disabling the glutathione system (Trachootham et al., 2008) and data in various other reports that link the response to drugs, including bendamustine and histone deacetylase inhibitors (Zhou et al., 2003; Roue et al., 2008; Bhalla et al., 2009; Bouzar et al., 2009; Miller et al., 2009), to p53-independent ROS production. However, whether ROS production is required for the initiation of apoptosis, or is a consequence of this could not be conclusively determined for all of these compounds.
As major advances have recently been made in first- and second-line therapies for patients with CLL, it is expected that increasing numbers of patients, among whom a substantial number of p53-dysfunctional cases, will eventually need third-line therapy. Designing strategies that target the distinguishing biochemical features of malignancy may be a promising approach. The altered redox balance in cancer represents one of such features, as ROS adaptation may have a critical role in drug resistance. Our data indicate that pharmacological induction of ROS or abrogation of cellular adaptive mechanisms to ROS may be a sensible strategy in high-risk, chemoresistant CLL patients.
Materials and methods
Patient material and cell culture
Peripheral blood mononuclear cells of patients with CLL, collected during follow-up visits at the Department of Hematology, were isolated and subsequently frozen and stored as previously described (Hallaert et al., 2008). Patient characteristics are summarized in Table 1. Approval for these studies was acquired from the Amsterdam Academic Medical Center Medical Ethical Committee. Informed consent was obtained in accordance with the principles of Declaration of Helsinki. All samples included in these studies contained >90% CD5+CD19+ cells. p53 dysfunction (as assessed by RT-MLPA) was defined as the absence of upregulation of mRNA expression levels of Puma, Bax and p21 on 5 Gy radiation, as described (Mous et al., 2009).
Table 1.
Patient characteristics
| Pt no. | Age | Rai-stadium | IgVH-mut statusa | Cytogeneticsb | p53 functionc |
|---|---|---|---|---|---|
| 1 | 64 | I | U | 17p− | Dysfunctional |
| 2 | 59 | I | U | 17p− | Dysfunctional |
| 3 | 54 | I | M | ND | Dysfunctional |
| 4 | 59 | II | poly | ND | Dysfunctional |
| 5 | 36 | III | U | 17p− | Dysfunctional |
| 6 | 82 | IV | M | 17p−, 13q− | Dysfunctionald |
| 7 | 83 | II | M | ND | Functional |
| 8 | 80 | 0 | U | Trisomy-12 | Functional |
| 9 | 64 | IV | M | 13q− | Functional |
| 10 | 67 | II | M | ND | Functional |
| 11 | 40 | II | M | 13q− | Functional |
| 12 | 61 | IV | M | Normal | Functional |
| 13 | 58 | III | M | Normal | Functional |
| 14 | 76 | I | M | 11q− | Functional |
| 15 | 67 | II | U | ND | Functional |
| 16 | 74 | 0 | M | 13q− | Functional |
| 17 | 62 | II | M | ND | Functional |
| 18 | 63 | I | M | Normal | Functional |
| 19 | 66 | 0 | U | ND | Functional |
| 20 | 49 | III | M | ND | Functional |
| 21 | 75 | 0 | M | 13q− | Functional |
| 22 | 73 | II | U | ND | Functional |
| 23 | 66 | II | M | Normal | Functional |
| 24 | 54 | III | U | Normal | Functional |
Abbreviations: IgVH, immunoglobulin heavy chain variable region; M, mutated; ND, not performed; pt, patient; poly, polyclonal; U, unmutated.
Mutational status of the immunoglobulin variable heavy chain.
As analyzed by FISH (fluorescence in situ hybridization).
p53 function as assessed by RT-MLPA as described in Mous et al. (2009) and in the Materials and methods section.
Patient sample was designated as p53-dysfunctional based on deletion of 17p and F-ara-A resistance in vitro.
Cells were cultured in Iscove's modified Dulbecco's medium (Invitrogen, Carlsbad, CA, USA), supplemented with 10% (v/v) heat-inactivated fetal calf serum (ICN Biomedicals, Meckenheim, Germany), 100 μg/ml gentamycin and 5 mml-glutamine (Invitrogen). Where indicated, CLL cells (1.5–2×106/ml) were stimulated with CD40-ligand for 48 h before drug treatment as previously described (Hallaert et al., 2008). In the synergy experiments the following drugs and reagents were used: CDDP (Mayne Pharma, Brussels, Belgium), oxaliplatin, F-ara-A, CCCP (Sigma-Aldrich, St Louis, MO, USA), H2O2 (Merck KGaA, Darmstadt, Germany), p38-i (SB202190), JNK-i (Sp600125; Enzo Life Sciences, Farmingdale, NY, USA), Q-VD-OPh (R & D systems, Minneapolis, MN, USA), Z-IETD-fmk, N-acetylcysteine, Tiron (4,5-dihydroxy-1,3-benzenedisulfonic acid disodium salt monohydrate), butylated hydroxyl-anisole (Sigma-Aldrich).
Assessment of apoptosis and ROS production
Apoptosis was assessed by annexin-V (IQ Products, Groningen, the Netherlands)/(PI) staining (Sigma-Aldrich) or MitoTracker Orange (Molecular Probes, Leiden, the Netherlands) staining as previously described (Smit et al., 2007), and analyzed by flow cytometry. Where indicated specific apoptosis is presented: (% apoptosistreated cells−% apoptosisuntreated cells)/% viableuntreated cells. For the measurement of cellular ROS content, cells which had been incubated with drugs for the indicated period of time, were harvested and washed with prewarmed phenol-red free Dulbecco's modified Eagle's medium (Invitrogen) and incubated with 10 μm carboxy-H2-DCFDA (C2938, Invitrogen) dissolved in warm Dulbecco's modified Eagle's medium at 37°C. After 30min, cells were washed and analyzed by flow cytometry.
mRNA isolation and RT-MLPA
CLL cells were incubated with Q-VD-OPh for 30 min at 37 °C before the addition of drugs as indicated. After the indicated period of time, cells were collected and total mRNA was isolated using the GenElute mammalian total mRNA miniprep kit (Sigma-Aldrich). A RT-MLPA assay (apoptosis kit R011-B1, MRC-Holland, Amsterdam, the Netherlands) was performed as described previously (Mackus et al., 2005). Expression levels were normalized for the expression of the housekeeping gene β2-microglobulin. For data presentation gene expression in treated cells was compared with expression in untreated cells. Genes that showed at least twofold increase or decrease in mean relative expression level on treatment were subjected to statistical testing for significance.
Protein isolation and western blot
CLL cells, incubated with drugs as indicated, were washed once in ice-cold phosphate buffered saline, and lysed by sonification in radioimmunoprecipitation buffer as previously described (Dicker et al., 2006). Protein content was measured using the BCA protein assay kit (Thermo Fisher Scientific Inc., Rockford, IL, USA). Around 30 to 100 μm of protein lysate was loaded onto each lane of a 7.5, 10 or 13% gradient SDS–PAGE gel (Bio-Rad, Hercules, CA, USA) and transferred onto a polyvinylidene fluoride microporous membrane (PVDF-FL, Millipore, Billerica, MA, USA). Membranes were probed with antibodies against β-actin (clone I-19; Santa Cruz Biotechnology, Santa Cruz, CA, USA), p73 (IMG-246, Imgenex, San Diego, CA, USA), Noxa (IMG-349, Imgenex), Puma (polyclonal, Cell signaling, Beverly, MA, USA), Bax and Mcl-1 (polyclonal, Pharmingen, Franklin Lakes, NJ, USA), and Bcl-2 (polyclonal, Alexis, Farmingdale, NY, USA). IRDye 680 donkey anti-rabbit IgG, IRDye 800 donkey anti-goat IgG or IRDye 800 donkey anti-mouse IgG (Westburg, Leusden, the Netherlands) were used as secondary antibody and blots were scanned on the Odyssey imager (LI-COR Biosciences, Lincoln, NE, USA). The indicated protein expression levels were quantified using LI-COR Odyssey software version 3.0.
Nucleofection
CLL cells were transfected using the Amaxa nucleofection technology (Lonza, Cologne, Germany) according to the manufacturer's recommendations and as described (Vogler et al., 2009). In short, CLL cells (5 × 106) were left to recuperate from thawing for 4–6 h and subsequently spinned down at 1300 r.p.m. for 5 min, resuspended in 100 μl of amaxa buffer and transfected with 3 μl of siRNA (stock concentration 100 μm) using programme X-01. Cells were immediately transferred into warm medium and after 1 h of incubation at 37 1C, drugs were added as indicated. The Noxa siRNA (s10709) and negative control siRNA-1 were used (Ambion, Austin, TX, USA).
Statistics
The Shapiro–Wilk normality test was performed to assess normal distribution of data sets. In case of Gaussian distribution of the data, a two-sided t-test was used to analyze differences between data sets. If there was no Gaussian distribution, a two-tailed Mann–Whitney U-test was used to analyze differences between groups of unpaired samples and a Wilcoxon-matched pairs test to analyze differences between paired samples. For the comparison of more than two sets of data, the Kruskal–Wallis test was used with a post-hoc Dunn's test in case of statistical difference. Statistic significance was set at a P-value <0.05.
Supplementary Material
Acknowledgements
We thank M Baou and GM Cohen of the MRC Toxicology Unit, University of Leicester, United Kingdom for sharing their experience with nucleofection-procedures. APK is personally supported by a `Veni' grant from the Netherlands Organization for Health Research and Development.
Footnotes
Conflict of interest The authors declare no conflict of interest.
Supplementary Information accompanies the paper on the Oncogene website (http://www.nature.com/onc)
References
- Akaboshi M, Kawai K, Ujeno Y, Takada S, Miyahara T. Binding characteristics of (-)-(R)-2-aminomethylpyrrolidine(1,1-cyclobutanedicarboxylato)-2-platin um(II) to DNA, RNA and protein molecules in HeLa cells and its lethal effect: comparison with cis- and trans-diamminedichloroplatinums(II) Jpn J Cancer Res. 1994;85:106–111. doi: 10.1111/j.1349-7006.1994.tb02893.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali MS, Khan SR, Ojima H, Guzman IY, Whitmire KH, Siddik ZH, et al. Model platinum nucleobase and nucleoside complexes and antitumor activity: X-ray crystal structure of [PtIV(trans-1R,2R-diaminocyclohexane)trans-(acetate)2(9-ethylguanine)Cl]NO3.H2O. J Inorg Biochem. 2005;99:795–804. doi: 10.1016/j.jinorgbio.2004.12.015. [DOI] [PubMed] [Google Scholar]
- Baou M, Kohlhaas SL, Butterworth M, Vogler M, Dinsdale D, Walewska R, et al. Role of NOXA and its ubiquitination in proteasome inhibitor-induced apoptosis in chronic lymphocytic leukemia cells. Haematologica. 2010;95:1510–1518. doi: 10.3324/haematol.2010.022368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhalla S, Balasubramanian S, David K, Sirisawad M, Buggy J, Mauro L, et al. PCI-24781 induces caspase and reactive oxygen species-dependent apoptosis through NF-kappaB mechanisms and is synergistic with bortezomib in lymphoma cells. Clin Cancer Res. 2009;15:3354–3365. doi: 10.1158/1078-0432.CCR-08-2365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouzar AB, Boxus M, Defoiche J, Berchem G, Macallan D, Pettengell R, et al. Valproate synergizes with purine nucleoside analogues to induce apoptosis of B-chronic lymphocytic leukaemia cells. Br J Haematol. 2009;144:41–52. doi: 10.1111/j.1365-2141.2008.07426.x. [DOI] [PubMed] [Google Scholar]
- Brozovic A, Osmak M. Activation of mitogen-activated protein kinases by cisplatin and their role in cisplatin-resistance. Cancer Lett. 2007;251:1–16. doi: 10.1016/j.canlet.2006.10.007. [DOI] [PubMed] [Google Scholar]
- Chen L, Willis SN, Wei A, Smith BJ, Fletcher JI, Hinds MG, et al. Differential targeting of prosurvival Bcl-2 proteins by their BH3-only ligands allows complementary apoptotic function. Mol Cell. 2005;17:393–403. doi: 10.1016/j.molcel.2004.12.030. [DOI] [PubMed] [Google Scholar]
- Clohessy JG, Zhuang J, Brady HJ. Characterisation of Mcl-1 cleavage during apoptosis of haematopoietic cells. Br J Haematol. 2004;125:655–665. doi: 10.1111/j.1365-2141.2004.04949.x. [DOI] [PubMed] [Google Scholar]
- Deriano L, Guipaud O, Merle-Beral H, Binet JL, Ricoul M, Potocki-Veronese G, et al. Human chronic lymphocytic leukemia B cells can escape DNA damage-induced apoptosis through the nonhomologous end-joining DNA repair pathway. Blood. 2005;105:4776–4783. doi: 10.1182/blood-2004-07-2888. [DOI] [PubMed] [Google Scholar]
- Dicker F, Kater AP, Prada CE, Fukuda T, Castro JE, Sun G, et al. CD154 induces p73 to overcome the resistance to apoptosis of chronic lymphocytic leukemia cells lacking functional p53. Blood. 2006;108:3450–3457. doi: 10.1182/blood-2006-04-017749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flinterman M, Guelen L, Ezzati-Nik S, Killick R, Melino G, Tominaga K, et al. E1A activates transcription of p73 and Noxa to induce apoptosis. J Biol Chem. 2005;280:5945–5959. doi: 10.1074/jbc.M406661200. [DOI] [PubMed] [Google Scholar]
- Ford JM, Hanawalt PC. Expression of wild-type p53 is required for efficient global genomic nucleotide excision repair in UV-irradiated human fibroblasts. J Biol Chem. 1997;272:28073–28080. doi: 10.1074/jbc.272.44.28073. [DOI] [PubMed] [Google Scholar]
- Fruehauf JP, Meyskens FL., Jr Reactive oxygen species: a breath of life or death? Clin Cancer Res. 2007;13:789–794. doi: 10.1158/1078-0432.CCR-06-2082. [DOI] [PubMed] [Google Scholar]
- Garrido N, Perez-Martos A, Faro M, Lou-Bonafonte JM, Fernandez-Silva P, Lopez-Perez MJ, et al. Cisplatin-mediated impairment of mitochondrial DNA metabolism inversely correlates with glutathione levels. Biochem J. 2008;414:93–102. doi: 10.1042/BJ20071615. [DOI] [PubMed] [Google Scholar]
- Geleziunas R, McQuillan A, Malapetsa A, Hutchinson M, Kopriva D, Wainberg MA, et al. Increased DNA synthesis and repair-enzyme expression in lymphocytes from patients with chronic lymphocytic leukemia resistant to nitrogen mustards. J Natl Cancer Inst. 1991;83:557–564. doi: 10.1093/jnci/83.8.557. [DOI] [PubMed] [Google Scholar]
- Gong JG, Costanzo A, Yang HQ, Melino G, Kaelin WG, Jr, Levrero M, et al. The tyrosine kinase c-Abl regulates p73 in apoptotic response to cisplatin-induced DNA damage. Nature. 1999;399:806–809. doi: 10.1038/21690. [DOI] [PubMed] [Google Scholar]
- Hagrman D, Goodisman J, Souid AK. Kinetic study on the reactions of platinum drugs with glutathione. J Pharmacol Exp Ther. 2004;308:658–666. doi: 10.1124/jpet.103.059410. [DOI] [PubMed] [Google Scholar]
- Hallaert DY, Jaspers A, van Noesel CJ, Van Oers MH, Kater AP, Eldering E. c-Abl kinase inhibitors overcome CD40-mediated drug resistance in CLL: implications for therapeutic targeting of chemoresistant niches. Blood. 2008;112:5141–5149. doi: 10.1182/blood-2008-03-146704. [DOI] [PubMed] [Google Scholar]
- Husain K, Morris C, Whitworth C, Trammell GL, Rybak LP, Somani SM. Protection by ebselen against cisplatin-induced nephrotoxicity: antioxidant system. Mol Cell Biochem. 1998;178:127–133. doi: 10.1023/a:1006889427520. [DOI] [PubMed] [Google Scholar]
- Kater AP, Evers LM, Remmerswaal EB, Jaspers A, Oosterwijk MF, van Lier RA, et al. CD40 stimulation of B-cell chronic lymphocytic leukaemia cells enhances the anti-apoptotic profile, but also Bid expression and cells remain susceptible to autologous cytotoxic T-lymphocyte attack. Br J Haematol. 2004;127:404–415. doi: 10.1111/j.1365-2141.2004.05225.x. [DOI] [PubMed] [Google Scholar]
- Kater AP, Tonino SH. Standards for the treatment of relapsed chronic lymphocytic leukemia: a case-based study. Clin Lymphoma Myeloma Leuk. 2010;10(Suppl 1):S34–S41. doi: 10.3816/CLML.2010.s.005. [DOI] [PubMed] [Google Scholar]
- Li L, Liu X, Glassman AB, Keating MJ, Stros M, Plunkett W, et al. Fludarabine triphosphate inhibits nucleotide excision repair of cisplatin-induced DNA adducts in vitro. Cancer Res. 1997;57:1487–1494. [PubMed] [Google Scholar]
- Mackus WJ, Kater AP, Grummels A, Evers LM, Hooijbrink B, Kramer MH, et al. Chronic lymphocytic leukemia cells display p53-dependent drug-induced Puma upregulation. Leukemia. 2005;19:427–434. doi: 10.1038/sj.leu.2403623. [DOI] [PubMed] [Google Scholar]
- Maggio SC, Rosato RR, Kramer LB, Dai Y, Rahmani M, Paik DS, et al. The histone deacetylase inhibitor MS-275 interacts synergistically with fludarabine to induce apoptosis in human leukemia cells. Cancer Res. 2004;64:2590–2600. doi: 10.1158/0008-5472.can-03-2631. [DOI] [PubMed] [Google Scholar]
- McCubrey JA, Lahair MM, Franklin RA. Reactive oxygen species-induced activation of the MAP kinase signaling pathways. Antioxid Redox Signal. 2006;8:1775–1789. doi: 10.1089/ars.2006.8.1775. [DOI] [PubMed] [Google Scholar]
- Miller CP, Rudra S, Keating MJ, Wierda WG, Palladino M, Chandra J. Caspase-8 dependent histone acetylation by a novel proteasome inhibitor, NPI-0052: a mechanism for synergy in leukemia cells. Blood. 2009;113:4289–4299. doi: 10.1182/blood-2008-08-174797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monjanel-Mouterde S, Ciccolini J, Bagarry D, Zonta-David M, Duffaud F, Favre R, et al. Population pharmacokinetics of cisplatin after 120-h infusion: application to routine adaptive control with feedback. J Clin Pharm Ther. 2003;28:109–116. doi: 10.1046/j.1365-2710.2003.00468.x. [DOI] [PubMed] [Google Scholar]
- Morales AA, Olsson A, Celsing F, Osterborg A, Jondal M, Osorio LM. High expression of bfl-1 contributes to the apoptosis resistant phenotype in B-cell chronic lymphocytic leukemia. Int J Cancer. 2005;113:730–737. doi: 10.1002/ijc.20614. [DOI] [PubMed] [Google Scholar]
- Moufarij MA, Sampath D, Keating MJ, Plunkett W. Fludarabine increases oxaliplatin cytotoxicity in normal and chronic lymphocytic leukemia lymphocytes by suppressing inter-strand DNA crosslink removal. Blood. 2006;108:4187–4193. doi: 10.1182/blood-2006-05-023259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mous R, Jaspers A, Luijks DM, Mellink CH, Van Oers MH, Kater AP, et al. Detection of p53 dysfunction in chronic lymphocytic leukaemia cells through multiplex quantification of p53 target gene induction. Leukemia. 2009;23:1352–1355. doi: 10.1038/leu.2009.71. [DOI] [PubMed] [Google Scholar]
- Munk Pedersen I, Reed J. Microenvironmental interactions and survival of CLL B-cells. Leuk Lymphoma. 2004;45:2365–2372. doi: 10.1080/10428190412331272703. [DOI] [PubMed] [Google Scholar]
- Nys K, Van Laethem A, Michiels C, Rubio N, Piette JG, Garmyn M, et al. A p38(MAPK)/HIF-1pathway initiated by UVB irradiation is Required to induce Noxa and apoptosis of human keratinocytes. J Invest Dermatol. 2010;130:2269–2276. doi: 10.1038/jid.2010.93. [DOI] [PubMed] [Google Scholar]
- Oda E, Ohki R, Murasawa H, Nemoto J, Shibue T, Yamashita T, et al. Noxa, a BH3-only member of the Bcl-2 family and candidate mediator of p53-induced apoptosis. Science. 2000;288:1053–1058. doi: 10.1126/science.288.5468.1053. [DOI] [PubMed] [Google Scholar]
- Oltra AM, Carbonell F, Tormos C, Iradi A, Saez GT. Antioxidant enzyme activities and the production of MDA and 8-oxo-dG in chronic lymphocytic leukemia. Free Radic Biol Med. 2001;30:1286–1292. doi: 10.1016/s0891-5849(01)00521-4. [DOI] [PubMed] [Google Scholar]
- Pepper C, Lin TT, Pratt G, Hewamana S, Brennan P, Hiller L, et al. Mcl-1 expression has in vitro and in vivo significance in chronic lymphocytic leukemia and is associated with other poor prognostic markers. Blood. 2008;112:3807–3817. doi: 10.1182/blood-2008-05-157131. [DOI] [PubMed] [Google Scholar]
- Pettitt AR. Mechanism of action of purine analogues in chronic lymphocytic leukaemia. Br J Haematol. 2003;121:692–702. doi: 10.1046/j.1365-2141.2003.04336.x. [DOI] [PubMed] [Google Scholar]
- Rich T, Allen RL, Wyllie AH. Defying death after DNA damage. Nature. 2000;407:777–783. doi: 10.1038/35037717. [DOI] [PubMed] [Google Scholar]
- Rosato RR, Almenara JA, Maggio SC, Coe S, Atadja P, Dent P, et al. Role of histone deacetylase inhibitor-induced reactive oxygen species and DNA damage in LAQ-824/fludarabine antileukemic interactions. Mol Cancer Ther. 2008;7:3285–3297. doi: 10.1158/1535-7163.MCT-08-0385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenwald A, Chuang EY, Davis RE, Wiestner A, Alizadeh AA, Arthur DC, et al. Fludarabine treatment of patients with chronic lymphocytic leukemia induces a p53-dependent gene expression response. Blood. 2004;104:1428–1434. doi: 10.1182/blood-2003-09-3236. [DOI] [PubMed] [Google Scholar]
- Roue G, Lopez-Guerra M, Milpied P, Perez-Galan P, Villamor N, Montserrat E, et al. Bendamustine is effective in p53-deficient B-cell neoplasms and requires oxidative stress and caspase-independent signaling. Clin Cancer Res. 2008;14:6907–6915. doi: 10.1158/1078-0432.CCR-08-0388. [DOI] [PubMed] [Google Scholar]
- Seymour JF, Robertson LE, O'Brien S, Lerner S, Keating MJ. Survival of young patients with chronic lymphocytic leukemia failing fludarabine therapy: a basis for the use of myeloablative therapies. Leuk Lymphoma. 1995;18:493–496. doi: 10.3109/10428199509059650. [DOI] [PubMed] [Google Scholar]
- Smit LA, Hallaert DY, Spijker R, de Goeij B, Jaspers A, Kater AP, et al. Differential Noxa/Mcl-1 balance in peripheral versus lymph node chronic lymphocytic leukemia cells correlates with survival capacity. Blood. 2007;109:1660–1668. doi: 10.1182/blood-2006-05-021683. [DOI] [PubMed] [Google Scholar]
- Spitz DR, Phillips JW, Adams DT, Sherman CM, Deen DF, Li GC. Cellular resistance to oxidative stress is accompanied by resistance to cisplatin: the significance of increased catalase activity and total glutathione in hydrogen peroxide-resistant fibroblasts. J Cell Physiol. 1993;156:72–79. doi: 10.1002/jcp.1041560111. [DOI] [PubMed] [Google Scholar]
- Tonino SH, van Gelder M, Eldering E, Van Oers MH, Kater AP. R-DHAP is effective in fludarabine-refractory chronic lymphocytic leukemia. Leukemia. 2010;24:652–654. doi: 10.1038/leu.2009.240. [DOI] [PubMed] [Google Scholar]
- Trachootham D, Zhang H, Zhang W, Feng L, Du M, Zhou Y, et al. Effective elimination of fludarabine-resistant CLL cells by PEITC through a redox-mediated mechanism. Blood. 2008;112:1912–1922. doi: 10.1182/blood-2008-04-149815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsimberidou AM, Wierda WG, Plunkett W, Kurzrock R, O'Brien S, Wen S, et al. Phase I–II study of oxaliplatin, fludarabine, cytarabine, and rituximab combination therapy in patients with Richter's syndrome or fludarabine-refractory chronic lymphocytic leukemia. J Clin Oncol. 2008;26:196–203. doi: 10.1200/JCO.2007.11.8513. [DOI] [PubMed] [Google Scholar]
- Van Bockstaele F, Verhasselt B, Philippe J. Prognostic markers in chronic lymphocytic leukemia: a comprehensive review. Blood Rev. 2009;23:25–47. doi: 10.1016/j.blre.2008.05.003. [DOI] [PubMed] [Google Scholar]
- Vogler M, Butterworth M, Majid A, Walewska RJ, Sun XM, Dyer MJ, et al. Concurrent up-regulation of BCL-XL and BCL2A1 induces approximately 1000-fold resistance to ABT-737 in chronic lymphocytic leukemia. Blood. 2009;113:4403–4413. doi: 10.1182/blood-2008-08-173310. [DOI] [PubMed] [Google Scholar]
- Witte AB, Anestal K, Jerremalm E, Ehrsson H, Arner ES. Inhibition of thioredoxin reductase but not of glutathione reductase by the major classes of alkylating and platinum-containing anticancer compounds. Free Radic Biol Med. 2005;39:696–703. doi: 10.1016/j.freeradbiomed.2005.04.025. [DOI] [PubMed] [Google Scholar]
- Zenz T, Krober A, Scherer K, Habe S, Buhler A, Benner A, et al. Monoallelic TP53 inactivation is associated with poor prognosis in chronic lymphocytic leukemia: results from a detailed genetic characterization with long-term follow-up. Blood. 2008;112:3322–3329. doi: 10.1182/blood-2008-04-154070. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Hileman EO, Plunkett W, Keating MJ, Huang P. Free radical stress in chronic lymphocytic leukemia cells and its role in cellular sensitivity to ROS-generating anticancer agents. Blood. 2003;101:4098–4104. doi: 10.1182/blood-2002-08-2512. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.