Abstract
The aim of this study was to assess the immunomodulatory effect of KC-1317 (a symbiotic mixture containing Saccharomyces boulardii lysate in a cranberry, colostrum-derived lactoferrin, fragaria, and lactose mixture) supplementation in immune-compromised but otherwise healthy elderly subjects. A liquid formulation of KC-1317 was administered in a randomized controlled trial (RCT) fashion to healthy volunteers (65–79 years) previously selected for low natural killer (NK) cell activity, and this parameter was checked at the completion of the study. A significant improvement in NK cell activity of KC-1317 consumers was observed as compared to placebo at the end of 2 months. Although preliminary, these beneficial immune-modulatory effects of KC-1317 in aged individuals might indicate its employment within a wider age-management strategy.
Introduction
The gut microbiota represents a crucial check point of resistance to colonization by pathogens while modulating maturation and activity of both the innate and adaptive immune systems.1,2 In particular, the complex interaction between probiotic bacteria, resident flora, and mucosal surfaces is made possible because of the mucosal immune system and particularly the mucosa-associated lymphoid tissues. On the basis of the clinical evidence, it is suggested that the effects of probiotic bacteria over the mucosal immune response may be divided into local and systemic effects. Namely, this is recognition of bacteria that promote mucosal T cell function as the newly termed “immunobiotics” moves probiotic biology forward by focusing on a mechanism of outcome, i.e., immunomodulation at distant mucosal sites.3 Indeed, several lactobacillus strains have been reported to exert stimulatory properties on the immune system cellular population either in vitro4,5 or in animal models.6,7
Moreover, human intervention studies have shown that probiotics enhance innate immunity, including natural killer (NK) cell activity, phagocytic activity, and respiratory burst.8 In recent years, a number of Gram-positive lactic acid bacteria strains have been generated showing probiotic properties and immunomodulatory functions in healthy adults9,10 as well as elderly subjects.11,12 Recently, we have shown that KC-1317, a symbiotic mixture containing a microbial lysate (Saccharomyces boulardii lysate in a cranberry, colostrum-derived lactoferrin, fragaria, and lactose mixture, named, Italy) was able to counteract stress-induced gut hyperpermeability.13 This may help reduce translocation of the antigenic burden into the bloodstream. The aim of the present study was to further probe the possible more direct influence of KC-1317 on immunocompetence in healthy subjects with decreased NK cell activity.
Subjects and Methods
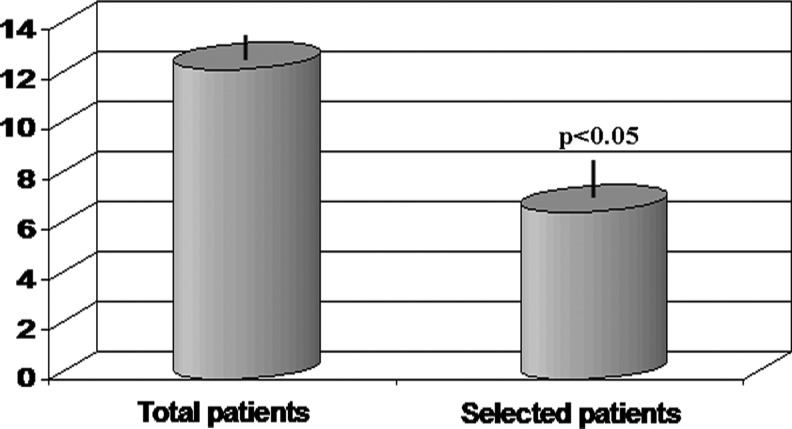
The inclusion criteria complied with the admission criteria for immunogerontological studies (SENIEUR protocol), a World Health Organization (WHO) performance status (PS) of grade 1, 2, or 3, and informed consent. Subjects who exhibited NK cell activity <10% (bottom 30% of population) were selected for the trial (48 out of the of the initially 186 screened). Blood samples were always withdrawn in the morning from all the subjects to avoid diurnal variation in NK cell activity. It was seen that average NK cell activity of the entire population was 12.4±0.54%, whereas the average activity of selected volunteers was 6.4±0.41%. The study population consisted of sedentary, non-smoker patients without infectious or viral disease, steroid or immunosuppressive therapy, major debilitating diseases, and prior or ongoing cancer. Patients were instructed to refrain from physical exercise; subjects under overt psychological and physical distress were excluded at the time of selection. Moreover, the web-based version of the National Institutes of Health Diet History Questionnaire (NIH DHQ) was used to assess diet history over the past month and throughout the study period; patients were given written and verbal instructions by a registered dietitian. The study was designed for a 9-week randomized, double-blinded, placebo-controlled trial.
Subjects were randomly distributed in two groups that were matched as for baseline NK cell activity (6.5%–7.3%) and for dietary and lifestyle questionnaire answers. Group A received 100 mL of fruit juice as a placebo; group B received 90 mL of placebo that had 10 mL of KC-1317 added. The sealed drink bottles were number coded for each participant and provided on a weekly basis to the study subjects. There was no noticeable difference in taste between the placebo and the S. boulardii lysate-added samples. The subjects were asked to consume the supplement twice daily, in the morning and evening for 2 months while avoiding any other food supplements or vitamins. Peripheral blood samples were withdrawn at two different time points during the trial—at selection and after completion of trial.
Isolation of lymphocytes
Lymphocytes were isolated from freshly withdrawn heparinized venous blood by the standard verographine method. Three milliliters of blood were layered on 3 mL of verographine and centrifuged at 2500 rpm for 30 min. The lymphocyte ring formed on the density interface was aspirated and washed three times with potassium buffer solution (pH 7.3) by centrifugation at 1500 rpm for 10 min, and then resuspended in RPMI-1640 (Sigma). The resulting lymphocyte suspension containing 5×106 cells/mL was applied for the following study.
NK cell culture and activity assay
NK cell activity was measured from the blood at the indicated time points without further priming. K562, the NK-sensitive erythromyeloid cell line, was continuously maintained in vitro in Corning 25-cm2 tissue culture flasks in RPMI-1640 (Gibco Laboratories, Grand Island, NY) supplemented with 10% fetal bovine serum (FBS) with low-dose lipopolysaccharide (LPS) supplementation (Gibco Laboratories, Grand Island, NY), 100 μg/mL streptomycin (Whittaker Bioproducts, Walkersville, MD), 100 units/mL penicillin, 0.1 mM non-essential amino acids, and 2 mM L-glutamine (Gibco Laboratories, Grand Island, NY). The cells were sub-cultured every 3–4 days and were incubated at 37°C and 5% CO2 atmosphere. Viable target cells (1×106/mL) were enumerated by microscopy of Trypan Blue–stained cell preparations and 5×106 cells were collected and washed twice with phosphate-buffered saline (PBS) before incubation with the dye Calcein AM (16 μM) at 37°C for 30 min. After incubation, the target cells were washed twice with PBS and resuspended in 1 mL of complete medium composed of RPMI-1640 medium, 0.75 mM glutamine, and 10% newborn calf serum (Sigma, Dorset, UK) and incubated for 4 hr at 37°C and 5% CO2 with effector cells (peripheral blood mononuclear cells [PBMCs]) in a ratio of 1:50 in 1 mL of medium, i.e., 104 K-562 cells: 5×105 lymphocytes. K-562 cells alone were used as a control. Post-incubation, cells were centrifuged, washed with PBS (twice), and suspended in 100 μL of PBS. Twenty microliters of propidium iodide at 1 mg/mL was added to the samples prior to analysis on the flow cytometer. Fluorescence measurements of the suspension were performed at λEX-495 nm and λEM -530 nm. The results were expressed as the percentage lysis of the target cells, as described below:
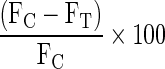 |
where FC is intensity for control K 562 and FT is intensity for K-562 in presence of NK cells. All experimental means were calculated from triplicate values.
Statistical analysis
All the values are presented as mean and standard deviation. All data were analyzed using SPSS (v. 15.0). Significant differences among treatments were evaluated by two-way analysis of variance or the Student t-test when applicable. A probability value of <0.05 was considered significant. When transforming the data did not result in normal distribution of the parameter, non-parametrical testing (Mann–Whitney) was performed. Values are reported as median with range.
Results
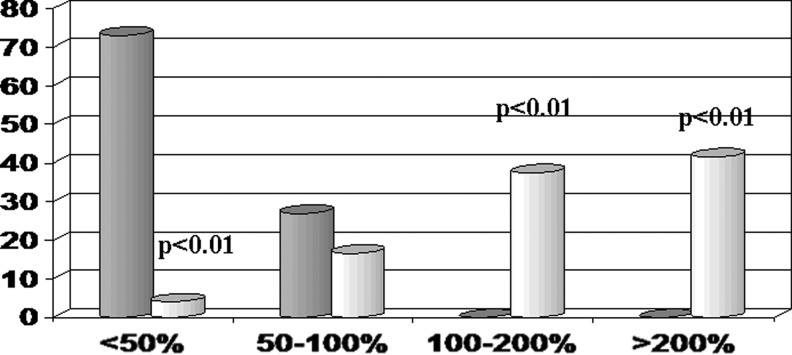
The subjects confirmed compliance with the dietary regimes. No significant differences were observed in routine biochemistry, body mass index, and white blood cell (WBC) count nor in daily dietary intake and energy expenditure between the two groups The baseline activity of NK cells of the volunteers in the two groups was comparable (6.2%–7.4%). On the basis of the questionnaire, it was also apparent that neither type of diet (vegetarian, western, mediterranean-like) nor the gender had any effect on NK cell activity. As compared to placebo-treated group, the KC-1317–treated group showed an overall significant increase of NK activity to 12.8±1.6% (data not shown, p<0.01). Only one subject (4.1%) responded poorly (<50% increase in NK cell activity; Fig. 1). In particular, 4 subjects (16.6%), 9 subjects (37.5%), and 10 subjects (41.6%) showed a mild (<100% increase in NK cell activity), moderate (100%–200% increase in NK cell activity), and intense (>200% increase in NK cell activity) response, respectively. The former and the latter two values were significantly different from placebo-treated group (p<0.01) (Fig. 2).
FIG. 1.
Proportion of volunteers showing natural killer (NK) cell activity change: Effect of KC-1317 supplementation.
FIG. 2.
The natural killer (NK) cell cytolytic activity of total and selected healthy elderly subjects.
Discussion
Aging is associated with impaired specific immune response.14 The loss of the ability of T cells of the elderly people to generate an immune response equal to that of T cells of the young is unquestionably an age-related phenomenon, and this deficiency can expose to the increased susceptibility of elderly subjects to infections, cancers, and autoimmune diseases15 as well as to the limitation incurred when immune therapy is envisaged in this population.16 Several studies have suggested that the monitoring of cytolytic activity of the immune cells both in vivo and in vitro can be related to the overall immunity status of an individual. Although, other members of cell-mediated pathways play significant roles in the regulation of immunity, this variable has been used and successfully modulated with probiotic interventions,6–12,17,18 although controversial data exist even using the same strain.19 Indeed, to date, a lack of consistency among some experimental models and different methods of preparation and administration of the bacteria represent factors yet to be fully explored.
Our present data suggest that KC-1317 is efficiently enhancing the immune system in healthy elderly and at a comparatively low dose. One cannot rule out the possibility of a synergistic action of the symbiotic milieu of KC-1337.20 Although these data need larger and longer-term studies together with correlations with several other health status markers, they offer a promising avenue to be pursued within a wider global health strategy by providing a safe dietary supplement enhancing innate cellular immune function.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Kelly D., Conway S., Aminov R. Commensal gut bacteria: Mechanisms of immune modulation. Trends Immunol 2005;26:326–333 [DOI] [PubMed] [Google Scholar]
- 2.Mazmanian SK, Liu CH, Tzianabos AO, Kasper DL. An immunomodulatory molecule of symbiotic bacteria directs maturation of the host immune system. Cell Mol Biol 2005;122:107–118 [DOI] [PubMed] [Google Scholar]
- 3.Matsuzaki T, Chin J. Modulating immune responses with probiotic bacteria. Immunol Cell Biol 2000;78:67–73 [DOI] [PubMed] [Google Scholar]
- 4.Ibnou-Zekri N, Blum S, Schiffrin E, von der Weid T. Divergent patterns of colonization and Immune response elicited from two intestinal lactobacillus strains that display similar properties in vitro. Infect Immun 2003;71:428–436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Haller D, Blum S, Bode C, Hammes WP, Schriffin EJ. Activation of human peripheral blood mononuclear cells by nonpathogenic bacteria in vitro: Evidence of NK cells as primary targets. Infect Immun 2000;68:752–759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Perdigon G, de Macias N, Alvarez S, Oliver G, Gobbato N. Systemic augmentation of the immune response in mice by feeding fermented milks with Lactobacillus casei and Lactobacillus acidophilus. Immunology 1998;63:17–23 [PMC free article] [PubMed] [Google Scholar]
- 7.Rodes L, Khan A, Paul A, Coussa-Charley M, Marinescu D, Tomaro-Duchesneau C, Shao W, Kahouli I, Prakash S. Effect of probiotics Lactobacillus and Bifidobacterium on gut-derived lipopolysaccharides and inflammatory cytokines: An in vitro study using a human colonic microbiota model. J Microbiol Biotechnol 2013;23:518–526 [DOI] [PubMed] [Google Scholar]
- 8.Gill HS, Rutherfurd KJ, Cross ML. Dietary probiotic supplementation enhances natural killer cell activity in the elderly: An investigation of age-related immunological changes. J Clin Immunol 2001;21:264–271 [DOI] [PubMed] [Google Scholar]
- 9.Klein A, Friedrich U, Vogelsang H, Jahreis G. Lactobacillus acidophilus 74-2 and Bifidobacterium animalis subsp lactis DGCC 420 modulate unspecific cellular immune response in healthy adults. Eur J Clin Nutr 2008;62:584–593 [DOI] [PubMed] [Google Scholar]
- 10.Olivares M, Diaz-Ropero MP, Sierra S, et al. Oral intake of Lactobacillus fermentum CECT5716 enhances the effects of influenza vaccination. Nutrition 2007;23:254–260 [DOI] [PubMed] [Google Scholar]
- 11.Gill HS, Rutherfurd KJ. Probiotic supplementation to enhance natural immunity in the elderly: effects of a newly characterized immunostimulatory strain Lactobacillus rhamnosus HN001 (DR20 (TM)) on leucocyte phagocytosis. Nutr Res 2001;21:183–189 [Google Scholar]
- 12.Del Piano M, Ballare M, Montino F, Orsello M, Garello E, Ferrari P, Masini C, Strozzi GP, Sforza F. Clinical experience with probiotics in the elderly on total interal nutrition. J Clin Gastroenterol 2004;38:s111–s114 [DOI] [PubMed] [Google Scholar]
- 13.Takadanohara H, Catanzaro R, Chui de H, He F, Yadav H, Ganguli A, Sakata Y, Solimene U, Minelli E, Kobayashi R, Nagamachi Y, Marotta F. Beneficial effect of a symbiotic preparation with S. boulardii lysate in mild stress-induced gut hyper-permeability. Acta Biomed 2012;83:208–216 [PubMed] [Google Scholar]
- 14.Salvioli S, Monti D, Lanzarini C, Conte M, Pirazzini C, Bacalini MG, Garagnani P, Giuliani C, Fontanesi E, Ostan R, Bucci L, Sevini F, Yani SL, Barbieri A, Lomartire L, Borelli V, Vianello D, Bellavista E, Martucci M, Cevenini E, Pini E, Scurti M, Biondi F, Santoro A, Capri M, Franceschi C. Immune system, cell senescence, aging and longevity—inflamm-aging reappraised. Curr Pharm Des 2013;19:1675–1679 [PubMed] [Google Scholar]
- 15.Maruyama M. Age-associated decline in the immune system. Nihon Rinsho 2013;71:993–998 [PubMed] [Google Scholar]
- 16.Gravekamp C. The impact of aging on cancer vaccination. Curr Opin Immunol 2011;23:555–560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dong H, Rowland I, Tuohy KM, Thomas LV, Yaqoob P. Selective effects of Lactobacillus casei Shirota on T cell activation, natural killer cell activity and cytokine production. Clin Exp Immunol 2010;161:378–388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Takeda K, Okumura K. Effects of a fermented milk drink containing Lactobacillus casei strain Shirota on the human NK-cell activity. J Nutr 2007;137:791S–793S [DOI] [PubMed] [Google Scholar]
- 19.Seifert S, Bub A, Franz CM, Watzl B. Probiotic Lactobacillus casei Shirota supplementation does not modulate immunity in healthy men with reduced natural killer cell activity. J Nutr 2011;141:978–984 [DOI] [PubMed] [Google Scholar]
- 20.Liu KY, Comstock SS, Shunk JM, Monaco MH, Donovan SM. Natural killer cell populations and cytotoxic activity in pigs fed mother's milk, formula, or formula supplemented with bovine lactoferrin. Pediatr Res 2013;74:402–407 [DOI] [PubMed] [Google Scholar]