Abstract
Men show an age-related decline in the circulating levels of testosterone (T) and dehydroepiandrosterone sulfate (DHEAS). Consequently, there is interest in developing androgen supplementation paradigms for old men that replicate the hormone profiles of young adults. In the present study, we used old (21–26 years old) male rhesus monkeys as a model to examine the efficacy of an androgen supplementation paradigm that comprised oral T administration (12 mg/kg body weight, dissolved in sesame oil/chocolate) in the evening, and two oral DHEA administrations, 3 hr apart (0.04 mg/kg body weight, dissolved in sesame oil/chocolate) in the morning. After 5 days of repeated hormone supplementation, serial blood samples were remotely collected from each animal hourly across the 24-hr day, and assayed for cortisol, DHEAS, T, 5α-dihydrotestosterone (DHT), estrone (E1), and 17β-estradiol (E2). Following androgen supplementation, T levels were significantly elevated and this was associated with a more sustained nocturnal elevation of T's primary bioactive metabolites, DHT and E1 and E2. Plasma DHEAS levels were also significantly elevated after androgen supplementation; DHEAS levels rose in the early morning and gradually declined during the course of the day, closely mimicking the profiles observed in young adults (7–12 years old); in contrast, cortisol levels were unaltered by the supplementation. Together the data demonstrate a non-invasive androgen supplementation paradigm that restores youthful circulating androgen levels in old male primates. Because this paradigm preserves the natural circulating circadian hormone patterns, we predict that it will produce fewer adverse side effects, such as perturbed sleep or cognitive impairment.
Introduction
The circulating levels of many steroid hormones show a marked decrease during aging, and this is thought to contribute to several age-associated pathologies, including cognitive decline, immune senescence, and skeleto-muscular changes.1,2 These hormonal changes are especially pronounced in post-menopausal women, who show a precipitous decrease in the synthesis and release of 17β-estradiol (E2) and progesterone from the ovaries, as well as a marked decrease in dehydroepiandrosterone sulfate (DHEAS) from the adrenal glands.3,4 In men, age-related steroid hormonal changes are less dramatic, but both testosterone (T) and DHEAS do show a significant age-related decrease in the circulation. Hormone therapy (HT) in post-menopausal women has been available for decades, and numerous clinical trials have been performed or are in progress to establish safe and effective HT paradigms. In contrast, HT in men has been less extensively studied and, despite a recent escalation in the promotion of HT in elderly men, the long-term safety and efficacy of these paradigms is unclear.5
Male rhesus monkeys show the same characteristic age-associated changes in circulating T and DHEAS levels as men, and also show many similar age-associated physiological changes.1,2,6,7 Consequently, they represent a pragmatic translational animal model in which to develop physiologically appropriate androgen supplementation paradigms. In pilot studies, we found that supplementing old male monkeys with oral T in the evening and oral DHEA in the morning elevates plasma T and DHEAS to levels typically observed in young animals. Our goal here was to extend the previous study8 by including more animals and examining the impact of this androgen supplementation paradigm on several other bioactive steroid metabolites, including 5α-dihydrotestosterone (DHT), estrone (E1), and E2.
Materials and Methods
Animals
The study used five young adult (7–12 years old) and nine old (21–26 years old) male rhesus monkeys (Macaca mulatta) and was approved by the Oregon Health & Science University (OHSU) Institutional Animal Care and Use Committee. The animals were cared for by the Oregon National Primate Research Center (ONPRC) Division of Comparative Medicine in accordance with the National Research Council's Guide for the Care and Use of Laboratory Animals. The animals were housed indoors under controlled conditions: 24°C temperature; 12-hr light, 12-hr dark photoperiods (lights on at 0700 hr); regular meals at 0800 and 1500 hr (LabDiet High Protein Monkey Chow, LabDiet, Inc., St. Louis, MO) supplemented with fresh fruit and vegetables; and fresh drinking water available ad libitum.
Because most of the hormones under investigation show a circadian pattern of release into the circulation, serial blood samples needed to be collected from each animal to establish accurate plasma hormone profiles. As previously described,9 each animal was implanted with an intravenous catheter in the right subclavian vein, which was routed to a swivel assembly located at the top of the animal's cage. This setup allowed the animals to have unrestricted movement and enabled undisturbed blood collection from an adjacent room. Blood samples were collected hourly over a 24-hr period from 1900 hr to 1900 hr into EDTA-treated borosilicate tubes. Plasma was isolated and levels of cortisol, DHEAS, T, and E2 were assayed using a chemiluminescence-based automatic clinical platform (Roche Cobas e411, Roche Diagnostics, Indianapolis, IN) that has been validated for non-human primates. DHT was measured by a traditional ether extraction-column chromatography-radioimmunoassay; Sephadex LH-20 was used in column chromatography to separate DHT from T and other steroids, so that the DHT radioimmunoassay reached a specificity of at least 80% for DHT. E1 was measured using a commercial enzyme-linked immunosorbent assay (ELISA) kit (cat. no. IB59105, Immuno Biological laboratory, Minneapolis, MN). The overall intra-assay coefficient of variation was <6% for the Roche assays, <10% for the E1 ELISA, and <15% for the DHT assay.
Baseline 24-hr hormone profiles were established for each animal. In addition, 24-hr plasma hormone profiles were also established for each of the nine old animals after 5 days of androgen supplementation; this comprised oral T administration (12 mg/kg body weight, at 1900 hr) and two oral DHEA administrations (0.04 mg/kg body weight, at 700 and 1000 hr). Both T and DHEA were obtained from Sigma-Aldrich Corp (St. Louis, MO). Previously, it has been shown that when T is administered orally in oil, significant quantities bypass the liver, presumably because of reduced passage into the hepatic portal system and increased uptake by the lymphatic system, and elevate circulating T concentrations.10 Consequently, we dissolved the T in sesame oil at a concentration of 120 mg/mL and then mixed it with ∼12 grams of chocolate or placed it inside a 5-gram cookie, based on the animal's preference. Similarly, we suspended the DHEA in sesame oil (10 mg/mL) and mixed it with chocolate or placed it inside a cookie.
Results
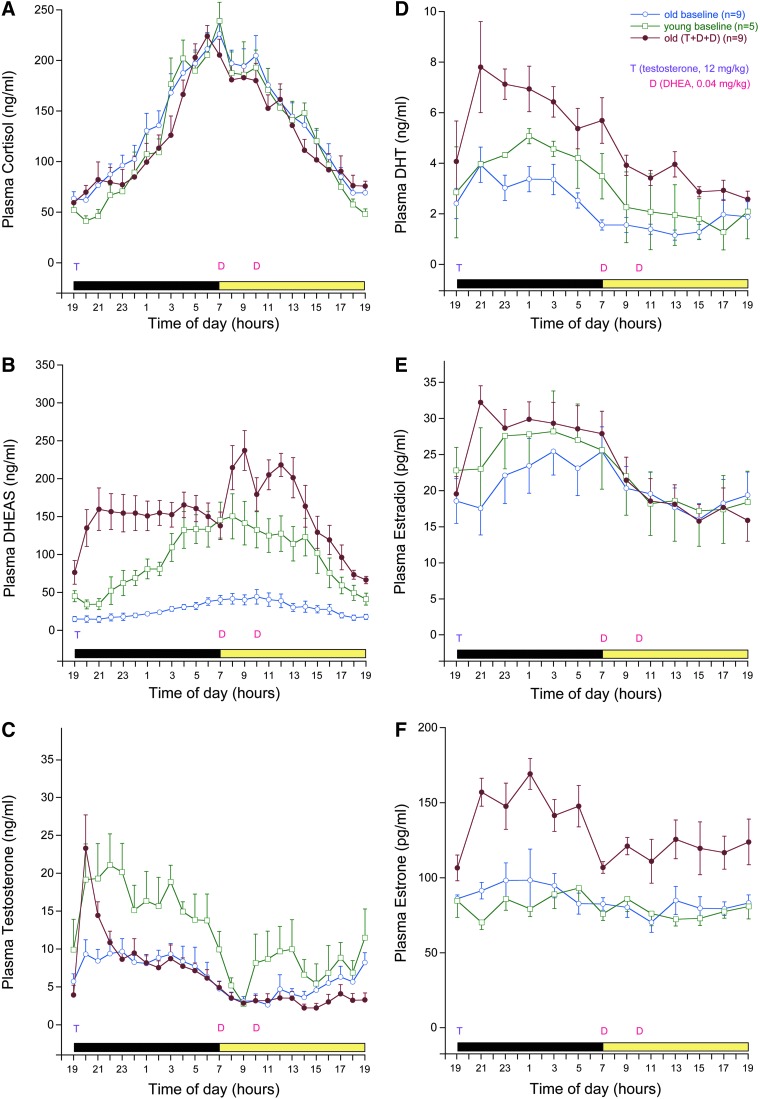
In both young and old male rhesus monkeys, plasma levels of cortisol, DHEAS, T, DHT, and E2 all showed distinct 24-hr profiles (Fig. 1A–E), whereas E1 levels were more consistent across the day (Fig. 1F). Overall baseline plasma cortisol profiles were similar between young and old animals (Fig. 1A), whereas both DHEAS and T levels showed a marked age-related attenuation (Fig. 1B, C), which was also reflected in an age-associated decline in the levels of their primary bioactive metabolites, DHT and E2 (Fig. 1D, E).
FIG. 1.
Effect of androgen supplementation on the circulating steroid hormone levels in male rhesus monkeys. Serial blood samples were remotely collected from young (7–12 years old, n=5) and old (21–26 years old, n=9) males. Samples were again collected from the same old animals after 5 days of androgen supplementation, which consisted of oral testosterone (T) administration (12 mg/kg body weight, at 1900 hr) and two oral dehydroepiandrosterone (DHEA) administrations (0.04 mg/kg body weight, at 700 and 1000 hr). In each panel, the light cycle is represented along the abscissa, with a black bar indicating light off and a pale bar indicating light on. Each data point represents the mean, and the vertical lines represent standard errors of the mean. The data demonstrate no obvious effect of aging or androgen supplementation on plasma cortisol levels (A). In contrast, the supplementation helped to restore the age-related decline in plasma levels of dehydroepiandrosterone sulfate (DHEAS) (B) and T (C), and elevated plasma levels of the principal bioactive metabolites, 5α-dihydrotestosterone (DHT) (D), 17β-estradiol (E), and estrone (F). Importantly, the androgen supplementation paradigm restored the characteristic youthful 24-hr patterns of these steroid hormones. Color images available online at www.liebertpub.com/rej
After daily androgen supplementation, the old animals showed an increase in plasma levels of T and DHEAS, but not cortisol (Fig. 1A–C). Although the resulting nocturnal elevation of plasma T levels was sustained for only a few hours, this was to be expected because the steroid is rapidly converted to either DHT and/or E2, which in turn exert bioactivity via androgen and estrogen receptors, respectively (Fig. 1D, E). Overall, the steroid hormone profiles of the old androgen-supplemented animals more closely resembled those of the young adults; however, some of the hormones, including DHEAS (Fig. 1B), DHT (Fig. 1D), and E1 (Fig. 1F), a less potent estrogen than E2, showed even higher levels. Importantly, the androgen supplementation paradigm resulted in robust maintenance of 24-hr plasma profiles.
Discussion
In both men and male rhesus monkeys, circulating levels of T and DHEAS show a 24-hr pattern as well as a significant age-related decrease.2,7 Consequently, our goal was to develop an androgen supplementation paradigm for old males, involving natural hormones and a non-invasive route of administration. Moreover, one of our primary objectives was to develop a supplementation paradigm that would maintain, or enhance, the circadian profiles that these hormones show in young adults. The rationale for the latter objective stems from our recent finding that individual rhesus monkeys with perturbed activity–rest cycles also show significant cognitive impairment and attenuated immune response.1 Our overall hypothesis is that a more natural, 24-hr androgen supplementation paradigm would be a safer and more effective therapy for old males. We initially performed considerable pilot studies to determine effective doses of T and DHEAS and to establish optimal route and time of hormone administration. The protocol described here represents the one we ultimately adopted for an ongoing monkey study that examines the therapeutic benefits of androgen supplementation on age-related changes in cognition, sleep–wake cycles, immune function, and metabolism.
As expected, when we administered T to the old animals, their plasma T levels rapidly increased to young adult levels. Due to the relatively high clearance rate of testosterone, however, this elevation was sustained for only a few hours. On the other hand, plasma levels of DHT and E2, the primary bioactive metabolites of T, rapidly increased in the circulation, and the plasma profiles of these two steroids more closely resembled those of young adult males, both in terms of magnitude as well as circadian pattern. Because disrupted sleep–wake cycles and circadian rhythms are known to adversely affect physiological functions, such as cognitive performance and immune function,1 our early evening oral administration of testosterone represents an androgen supplementation paradigm that is more physiological, and potentially less detrimental, than methods commonly in use.
Some tissues, including the brain, express the necessary enzymes to convert DHEA into T, as well as into DHT, E2, and E1. Consequently, it is plausible that the DHEA supplementation increased DHT, E2, and E1 levels within these tissues through intracrine conversion and may have also contributed to the enhanced levels observed in the circulation. DHEA supplementation has been shown to significantly enhance cognitive function in rodent studies, but so far the evidence for a pro-cognitive effect of DHEA in humans is not obvious.5 Perhaps a more physiological DHEA administration paradigm, such as the one proposed here, would prove more effective.
Given that DHEA supplements are available in the United States without prescription, and that T supplementation is widely promoted for elderly men, the present study has important translational potential. In summary, we propose that this steroid regimen, of combined T and DHEA supplementation, at physiologically appropriate times of the day, which closely mimics the circadian hormone profiles of young animals, represents an improved paradigm for assessing the physiological effects of hormone supplementation during aging. Furthermore, T has the potential to be converted to E2, as well as to androgenic metabolites such as DHT, both of which have been implicated in improved cognitive performance. Consequently, we hypothesize that this androgen supplementation protocol will result in memory improvement in old male monkeys and possibly also in elderly men.
Acknowledgments
We wish to thank the ONPRC Endocrine Technology and Support Core for help with the hormone measurements. This work was supported by National Institutes of Health (NIH) grants AG-023477, AG-029612, AG-036670, and OD-011092.
Author Disclosure Statement
None of the authors has competing financial interests.
References
- 1.Messaoudi I, Urbanski HF, Kohama SG. Integrative models of aging in the nonhuman primate. In: Williams RM, ed. Monkeys: Biology, Behavior and Disorders. Nova Science Publishers, Hauppauge, NY, 2011, Chapter 1, pp. 1–54 [Google Scholar]
- 2.Urbanski HF, Sorwell K. Age-related changes in neuroendocrine rhythmic function in the rhesus macaque. Age (Dodr) 2012;34:1111–1121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Downs JL, Urbanski HF. Neuroendocrine changes in the aging reproductive axis of female rhesus macaques (Macacaca mulatta). Biol Reprod 2006;75:539–546 [DOI] [PubMed] [Google Scholar]
- 4.Sorwell KG, Kohama SG, Urbanski HF. Perimenopausal regulation of steroidogenesis in the nonhuman primate. Neurobiol Aging 2012;33:1487..e1–e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Corona G, Rastrelli G, Giagulli VA, Sila A, Sforza A, Forti G, Mannucci E, Maggi M. Dehydroepiandrosterone supplementation in elderly men: A meta-analysis study of placebo-controlled trials. J Clin Endocrinol Metab 2013;98:3615–3626 [DOI] [PubMed] [Google Scholar]
- 6.Downs JL, Mattison JA, Ingram DK, Urbanski HF. Effect of age and caloric restriction on circadian adrenal steroid rhythms in rhesus macaques. Neurobiol Aging 2008;29:1412–1422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sitzmann BD, Brown DI, Garyfallou VT, Mattison JA, Ingram DK, Roth GS, Ottinger MA, Urbanski HF. Impact of moderate calorie restriction on testicular morphology and endocrine function in adult rhesus macaques (Macaca mulatta). Age (Dordr) 2014;26:183–197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sorwell KG, Garten J, Renner L, Weiss A, Garyfallou VT, Kohama SG, Neuringer M, Urbanski HF. Hormone supplementation during aging: How much and when? Rejuvenation Res 2012;15:128–131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Urbanski HF. Circadian variation in the physiology and behavior of humans and nonhuman primates. In: Raber J, ed. Animal Models of Behavioral Analysis. Neuromethods 50 Springer, New York, 2011, pp. 217–235 [Google Scholar]
- 10.Amory JK, Bremner WJ. Oral testosterone in oil plus dutasteride in men: A pharmacokinetic study. J Clin Endocrinol Metab 2005;90:2610–2617 [DOI] [PubMed] [Google Scholar]