Abstract
Plants growing in the Judea region are widely used in traditional medicine. This phytogeographic zone stands out in its climatic conditions and biodiversity. Consequently, both endemic and widely distributed Mediterranean plants growing in the area have unique chemotypes characterized by accumulation of relatively high levels of phytosteroids. Our comprehensive analysis revealed that many of the plants growing in the Judea region may hold a geroprotective potential. With this in mind, we undertook a wide screen of dozens of candidate herbal extracts for their cell protective, wound-healing, anti-inflammatory, and anti-cancer activities. The results obtained thus far have clearly shown that the extracts tested (1) protect normal human fibroblasts from genotoxic stress (prevent DNA double-strand beaks, increase cell survival and reduce the number of cells undergoing cellular senescence), (2) decrease secretion of pro-inflammatory cytokines, (3) promote wound healing, and (4) exert more pronounced cytotoxicity toward cancer cells.
Introduction
Although “magic bullets” still remain a dream of gerontology, we should not neglect any option in combating aging and age-related diseases (ARDs). Among valuable sources of geroprotectors could be medicinal herbs that for centuries have been used in folk medicine.1 Because herbal extracts are complex mixtures of active compounds that simultaneously act on hundreds of targets in the human genome,2 it is reasonable to suggest that their multi-target properties allow them to hold a considerable geroprotective potential3,4 and to be superior to conventional agents that affect only one target at a time.5 From the network-based perspective, partial inhibition of several targets could be more efficient than the complete inhibition of a single target,5–8 making the plant extracts a promising category for developing the next generation of pharmacological agents with a pleiotropic mode of action. In fact, ethno-medical knowledge could facilitate the discovery of new drugs by providing a preliminary list of the most promising candidate plants. Besides shortening the pipeline to drug discovery, using medicinal plants as a starting point has another advantage of potentially reducing the toxic side effects, because active compounds from plants that have already been used by humans are likely to be safer than those with no history of human use.
Plants growing in phytogeographic zones found at the intersection of different climates are of particular interest. The flora of these regions is subjected to highly stressful conditions that cause local herbs to accumulate relatively high levels of bioactive compounds. The Judea region in Israel is one of such stressful phytogeographic zones because it is situated between the Judean Hills, with an elevation of up to 1000 meters above sea level, a rainy Mediterranean climate, and the Dead Sea, which is the lowest place on earth (more than 400 meters below sea level), with constantly warm and dry conditions. As a result of this permanent stress, on a narrow strip of land of only 30 km in width, both endemic and widely distributed Mediterranean plants growing in the area have acquired unique chemotypes with various medicinal uses.9–11
Considering that the Judea region is a biodiversity spot and a valuable resource of plants with potential use in medicine, we have undertaken extensive data mining of scientific literature and ethno-pharmacological surveys to identify medicinal applications of the local vegetation.11(and references therein) The combined dataset of the plants growing in the Judea region (Judea Mountains, Judea Desert, and Dead Sea valley) contained 1291 plant species, of which 332 plants (25%) have been reported to have medicinal uses. Further analysis showed that a considerable portion of the medicinal plants exerted anti-inflammatory, anti-oxidant, anti-cancer, and anti-diabetic activities—all of which can be classified as geroprotective. Of note, among the potential targets of the Judean plants are many longevity- and ARD-associated genes.11 Thus, plant materials already known to be effective against at least one age-related disease (ARD) are more likely to be active against the others. With this in mind, we have screened the extracts from the selected Judean plants for potential geroprotective activities, using primary cultures of human foreskin fibroblasts (HFF) and human dermal fibroblasts (HDF) and human ovarian carcinoma cells as model systems. Here, we present the results of this screen, with a focus on the top hit extracts possessing cytoprotective, anti-inflammatory and wound-healing activities.
Materials and Methods
Preparation of herbal extracts
Ethanol extracts were prepared from the aerial parts of plants. The timing of the plants collection was as close as possible to the period in which they are supposed to possess the highest level of therapeutic activity as determined by the available ethno-pharmacological knowledge. The collected plant tissues were dried for at least 2–3 days, ground to powder, dissolved in ethanol, and incubated at room temperature for 48 hr; they were then centrifuged at 2000 rpm for 10 min. The supernatant was evaporated by lyophilization, and the pellet was dissolved in a minimal amount of absolute ethanol (0.5 mL). The concentrations of the resultant stocks were in the range of 25–60 mg/mL.
Cell cultures
Primary cultures of HFF and HDF and the human ovarian SKOV3 carcinoma cell line were grown in Dulbecco modified Eagle medium (DMEM) or minimum essential medium-α (MEMα), respectively, supplemented with 10% fetal calf serum (FCS), 1% L-glutamine, and 1% penicillin/streptomycin. All products for cell cultures were from Biological Industries Israel Beit Haemek Ltd. Cell cultures were grown under standard conditions of 37°C and 5% CO2.
Cytotoxicity assay
Non-toxic concentrations of the herbal extracts were determined by the Neutral Red assay as described by us elsewhere.12 Briefly, the cells were plated in 96-well plates (5×103/well), and logarithmically-increasing concentrations of plant extracts were added 24 hr later. Cells were incubated for additional 24 hr, the medium was aspirated, and cells were washed with phosphate-buffered saline (PBS) and incubated with Neutral Red solution (0.21% in ethanol/water solution 1:100) for 2 hr. After extensive washing with PBS, cells were solubilized with Sorenson's buffer (30.5 mM disodium citrate, 19.4 mM HCl, 50% ethanol) for 15 min with agitation, and the intensity of color was read in an enzyme-linked immunosorbent assay (ELISA) reader at 570 nm. All samples were run in five to six replicates.
Treatments
To induce genotoxic stress, the cells were incubated with the indicated concentration of etoposide (Sigma Aldrich, USA) for 12 hr as described elsewhere.13 To test for the cytoprotective and other effects of plant extracts, they were added to the incubation medium at the indicated concentrations 30 min prior to etoposide and co-incubated for 24 hr.
Western blotting
The level of double-strand breaks (DSBs) was evaluated by western blotting, with the monoclonal anti-γ-H2AX foci antibody (1:2000 dilution; AbCam, cat. no. ab22551). The western blotting procedure was described by us elsewhere.14 Briefly, equal amounts of protein samples were mixed with Laemmli sample buffer and separated on 7.5 or 10% sodium dodecyl sulfate (SDS) polyacrylamide gels. Proteins were electroblotted onto nitrocellulose membranes in Tris-glycine buffer (25 mM Tris, 192 mM glycine, pH 8.3, 20% methanol) using a mini-Protean Trans-blot apparatus (BioRad). Filters were blocked with 5% non-fat milk in Tris-buffered saline+Tween 20 (TBS-T) buffer (100 mM Tris-HCl, pH 7.6, 150 mM NaCl, 0.1% Tween 20) for 1 hr, and probed with relevant primary antibodies for 1 hr at room temperature or overnight at 4°C. After incubation with horseradish peroxidase (HRPO)-conjugated secondary antibody, membranes were processed using an enhanced chemiluminescense kit (ECL; Amersham), as recommended by suppliers, and visualized on Kodak BioMax MR-1 films.
Enzyme-linked immunosrobent assay
After exposure to etoposide or etoposide+plant extract (see above), cells were allowed 24 hr to recover. The medium was replaced by the GIBCO opti-MEM reduced serum medium for a 3-day incubation. The medium collected from etoposide-treated and etoposide-untreated cultures was diluted 1:50, and samples were processed by ELISA for the measurement of interleukin- (IL-6) and IL-8, using materials supplied by and according to the protocols of R&D Systems (USA). Cytokine levels are expressed in pg/mL medium.
In vitro wound-healing assay
Ibidi silicone wound healing inserts (Ibidi, USA, cat. no. 80241) were placed in 12-well plates. HDF or HFF were seeded in the inserts, according to the manufacturer's recommendation, and incubated overnight to achieve full confluence. The inserts were carefully removed from the plates, leaving a 500-μm wide gap between the monolayers. At that time, the medium was replaced by fresh DMEM for the control or supplemented with the indicated doses of the plant extracts. Kinetics of gap closure was measured using the Olympus IX2 series microscope (Digital Camera at 10–40×resolution). Image analysis of the free cell areas was performed using the ImageJ software (http://rsb.info.nih.gov/ij/).
Statistical evaluation
The statistical package for the social sciences (SPSS, Inc., Chicago, IL) software was used for the statistical evaluation of the results. Statistical evaluation was carried out using factorial analysis (analysis of variance [ANOVA]) to test for differences between the control and the experimental groups. Values of p<0.05 were considered statistically significant.
Results and Discussion
Dual effects on cell growth
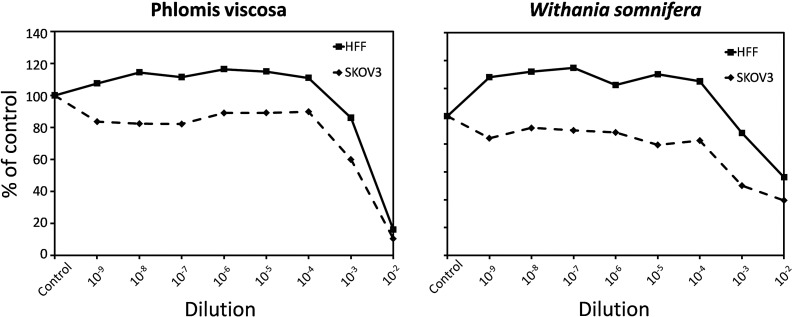
After a preliminary screen, six extracts from the Judean plants (Achillea santolina, Calotropis procera, Cistus creticus, Phlomis viscosa, Varthemia iphionoides, and Withania somnifera) were selected for further analysis. Most of the extracts display a dual dose-dependent effect on cell growth of HFF and HDF. While cytotoxic at high doses (10−3–10−2 dilutions), they promoted proliferation at lower doses (10−8–10−6 dilutions). An important point is that the activation of cell proliferation was not observed in cancer cells (data not shown). Moreover, in the case of P. viscosa and W. somnifera, the extracts stimulated HFF growth in a wide dose-range (10−9–10−4 dilutions; from 30 and 25 pg/mL to 3 and 2.5 μg/mL medium, respectively), whereas in SKOV3 ovarian carcinoma cells, the same doses exerted a slight cytotoxic effect (Fig. 1).
FIG. 1.
Effect of selected plant extracts (Phlomis viscosa, Withania somnifera) on cell growth in human foreskin fibroblasts (HFF; continuous line) and ovarian carcinoma SKOV3 cell (dashed line) cultures. The concentrations of the stocks were 30 and 25.2 mg/mL for the P. viscosa and W. somnifera extracts, respectively. The number of viable cells was measured by the Neutral Red assay and expressed as percent of control (untreated) cells. Each point represents the mean of five to six replicates. The standard errors of the means were less than 6% and as such were not indicated on the graph. Differences between treated and control cultures and between HFF and SKOV3 were highly significant (p<0.001; analysis of variance [ANOVA]).
Cytoprotective effects
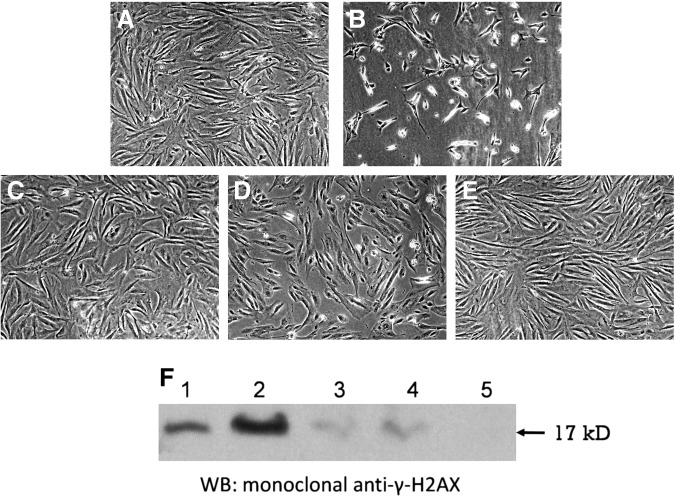
A common feature of various medicinal applications of the Judean plants suggests a cytoprotective activity.11 Therefore, these plants are good candidates for aiding the organism in resisting various forms of genotoxic stress. In fact, such a property of herbal preparations could contribute immensely to human well-being, because the capability to cope with genotoxic stress (such as thermal or oxidative insults, ionizing radiation, exposure to carcinogens, and inflammatory burden) is one of the important determinants of survival and longevity (for review, see refs. 15–17). With this in mind, we exposed cultured fibroblasts to etoposide, a well-known cytotoxic agent that induces formation of DNA DSBs,18,19 one of the most dangerous types of DNA damage. Specifically, on the basis of the DNA damage dose–response of cultured HFF to etoposide (data not shown) and literature data,13(and references therein) we selected the etoposide concentration of 100 μM. As seen in Fig. 2A–E, the extracts of V. iphionoides, C. procera, and A. santolina considerably reduced the cytotoxic effect of etoposide. We further examined whether the observed cytoprotective effect is attributed to attenuation of etoposide-induced DNA damage and found that supplementation of these plant extracts led to a remarkable reduction in the amount of DSBs (evaluated by the formation of γ-H2AX foci), even below the basal level (Fig. 2F).
FIG. 2.
Cytoprotective effects of selected plant extracts on etoposide-induced DNA damage and cytotoxicity. (A–E) Light microscopy of cultured human foreskin fibroblasts: (A) untreated cells; (B) treated with 100 μM etoposide; (C) treated with 100 μM etoposide and Varthemia iphionoides extract (162 ng/mL); (D) treated with 100 μM etoposide and Calotropis procera extract (40 ng/mL); (E) treated with 100 μM etoposide and Achillea santolina extract (45 ng/mL). (F) Western blot (WB) for double-strand breaks (DSBs) in human foreskin fibroblasts using the anti-γ-H2AX foci antibody. Lanes 1–5 correspond to A–E. The extracts were added to incubation medium 30 min prior to etoposide and co-incubated for 24 hr.
Secretion of pro-inflammatory cytokines
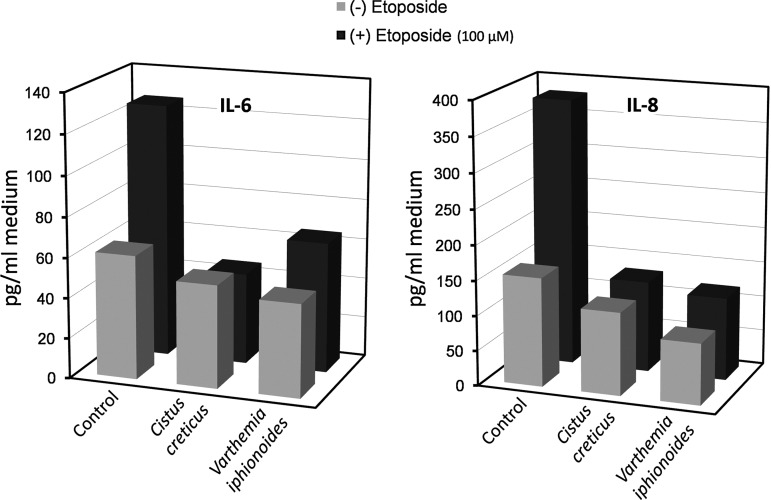
Another hallmark of aging and ARDs is activation of pathways leading to chronic inflammation (“inflammaging”),17,20 including the involvement of pro-inflammatory cytokines (tumor necrosis factor-α [TNF-α, IL-1β, IL-6), chemokines (IL-8), adhesion molecules, and other pro-inflammatory factors.21 The DNA damage response to etoposide was shown to be accompanied by overexpression of IL-6, IL-8, and others.13 Taking this into account, we have examined the impact of our extracts on the secretion of IL-6 and IL-8 by etoposide-treated versus control fibroblasts. Incubation with etoposide increased the secretion of these cytokines by approximately 2.5-fold as compared to the untreated control (Fig. 3). Addition of the V. iphionoides and C. creticus extracts led to a significant decrease (p<10−4 for all tested samples; ANOVA) in the cytokine levels, both in the controls and the etoposide-treated samples (Fig. 3). Of note, supplementation of these plant extracts ameliorated the etoposide-induced elevation in cytokine secretion to a level close to that of control cultures.
FIG. 3.
Effect of plants extracts (Varthemia iphionoides and Cistus Creticus, 162 and 60 ng/mL medium, respectively) on the secretion of pro-inflammatory cytokines interleukin-6 (IL-6) (left diagram) and IL-8 (right diagram) by the primary cultures of human dermal fibroblasts. Black columns, etoposide-treated cultures; grey columns, etoposide-untreated cultures. Results are presented as mean of triplicates from one of two representative experiments. The standard errors of the means were less than 5%. The effect of plant extracts was highly significant (p<10−4).
Wound healing
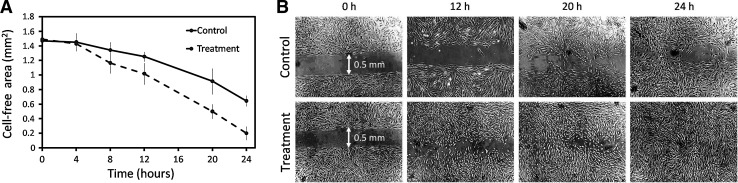
In various organs, including the skin, the rate of wound healing (WH) decreases with advanced age.22(and references therein) Therefore, another geroprotective property that was measured in this study was the ability of the extracts to modulate the rate of WH using the in vitro model. The most potent effect on the rate of gap closure was observed for the extract of P. viscosa (Fig. 4). Addition of this extract led to a significant acceleration of WH (p=0.003) and almost complete closure of the cell-free area after 24 hr of incubation (0.2 vs. 0.65 mm2 in extract-treated and control cultures, respectively; p<0.05) (Fig. 4A). The differences in the course of gap closure are clearly seen in the light microscopy photos taken at various time points (Fig. 4B).
FIG. 4.
(A) The time course of gap closure in cultured human foreskin fibroblasts treated with the Phlomis viscosa extract (0.3 ng/mL medium). Results are presented as mean±standard error (SE) of three independent experiments. Differences between treated and control cultures were significant (p=0.003; anlaysis of variance [ANOVA]). (B) Light microscopy photos of gap closure at various time intervals.
Concluding Remarks
This work represents the first screen aimed at uncovering the geroprotective potential of the plants growing in the Judea region. The results obtained thus far have clearly shown that several extracts tested (1) protect normal human fibroblasts from genotoxic stress by preventing DSBs, (2) decrease secretion of pro-inflammatory cytokines (IL-6 and IL-8), (3) promote in vitro WH, and (4) exert more pronounced cytotoxicity toward cancer cells. The natural development of this work would be high-performance liquid chromatography (HPLC) fractionation of the top hit extracts to identify groups of compounds that stand behind the geroprotective properties. We have already obtained fractions from the extract of V. iphionoides that are enriched in various water-soluble compounds, polysaccharides, polyphenols, flavonoids, and saponins. From this perspective, it would be of special interest to examine the fractions containing the polysaccharides, polyphenols, and flavonoids, as compounds belonging to these chemical classes have already been shown to possess geroprotective activities. Examples include myricetin found in many edible plants,23 tyrosol,24 and secoiridoid polyphenols25 from olive oil, and the whole-apple extracts that are enriched in flavonoids.26 The above compounds or extracts increased the resistance to stress and extended life span in the nematode Caenorhabditis elegans24–26 or exhibited anti-cancer activity and prevented induction of cellular senescence.26 With regard to the reported data and on the basis of results obtained in this study, the Judean plants warrant further experimental investigation for their geroprotective activity.
Acknowledgments
This work was funded by the Israel Ministry of Science and Technology (to A.B.), the European Comission FP7 Health Research Grant number HEALTH-F4-2008-202047 (to V.E.F.), and the Fund in Memory of Dr. Amir Abramovich. The authors thank Dr. Gilad Lehmann for his assistance with statistical analysis and Dmitri Taranukha for help in preparing the manuscript for submission.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Farnsworth NR, Akerele O, Bingel AS, Soejarto DD, Guo Z. Medicinal plants in therapy. Bull World Health Organ 1985;63:965–981 [PMC free article] [PubMed] [Google Scholar]
- 2.Schmidt B, Ribnicky DM, Poulev A, Logendra S, Cefalu WT, Raskin I. A natural history of botanical therapeutics. Metabolism 2008;57(7 Suppl 1):S3–S9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ho YS, So KF, Chang RC. Anti-aging herbal medicine—how and why can they be used in aging-associated neurodegenerative diseases? Ageing Res Rev 2010;9:354–362 [DOI] [PubMed] [Google Scholar]
- 4.Kitagishi Y, Kobayashi M, Matsuda S. Protection against cancer with medicinal herbs via activation of tumor suppressor. J Oncol 2012;2012:236530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Csermely P, Agoston V, Pongor S. The efficiency of multi-target drugs: The network approach might help drug design. Trends Pharmacol Sci 200526:178–182 [DOI] [PubMed] [Google Scholar]
- 6.Csermely P, Korcsmáros T, Kiss HJ, London G, Nussinov R. Structure and dynamics of molecular networks: A novel paradigm of drug discovery: A comprehensive review. Pharmacol Ther 2013;138:333–408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Budovsky A, Abramovich A, Cohen R, Chalifa-Caspi V, Fraifeld V. Longevity network: Construction and implications. Mech Ageing Dev 2007;128:117–124 [DOI] [PubMed] [Google Scholar]
- 8.Tacutu R, Budovsky A, Wolfson M, Fraifeld VE. MicroRNA-regulated protein-protein interaction networks: How could they help in searching for pro-longevity targets? Rejuvenation Res 2010;13:373–377 [DOI] [PubMed] [Google Scholar]
- 9.Lev E. Reconstructed material medica of the Medieval and Ottoman al-Sham. J Ethnopharmacol 2002;80:167–179 [DOI] [PubMed] [Google Scholar]
- 10.Tamir H, Satovic Z, Gorelick J, Danin A, Fischer R, Chaimovitsh D, Dudai N. Intraspecific variation of Chiliadenus iphionoides essential oil in Israel. Chem Biodivers 2011;8:1065–1082 [DOI] [PubMed] [Google Scholar]
- 11.Budovsky A, Fraifeld VE. Medicinal plants growing in the Judea region network approach for searching potential therapeutic targets. Network Biol 2012;2:84–94 [Google Scholar]
- 12.Fraifeld V, Seidman R, Sagi O, Muradian K, Wolfson M. Aurintricarboxylic acid decreases proliferative potential of SKOV3 and MCF7 human carcinoma cells. Anticancer Res 2001;21:1975–1978 [PubMed] [Google Scholar]
- 13.Chien Y, Scuoppo C, Wang X, Fang X, Balgley B, Bolden JE, Premsrirut P, Luo W, Chicas A, Lee CS, Kogan SC, Lowe SW. Control of the senescence-associated secretory phenotype by NF-κB promotes senescence and enhances chemosensitivity. Genes Dev 2011;25:2125–2136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ofir R, Seidman R, Rabinski T, Krup M, Yavelsky V, Weinstein Y, Wolfson M. Taxol-induced apoptosis in human SKOV3 ovarian and MCF7 breast carcinoma cells is caspase-3 and caspase-9 independent. Cell Death Differ 2002;9:636–642 [DOI] [PubMed] [Google Scholar]
- 15.Moskalev AA, Smit-McBride Z, Shaposhnikov MV, Plyusnina EN, Zhavoronkov A, Budovsky A, Tacutu R, Fraifeld VE. Gadd45 proteins: Relevance to aging, longevity and age-related pathologies. Ageing Res Rev 2012;11:51–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moskalev AA, Shaposhnikov MV, Plyusnina EN, Zhavoronkov A, Budovsky A, Yanai H, Fraifeld VE. The role of DNA damage and repair in aging through the prism of Koch-like criteria. Ageing Res Rev 2013;12:661–684 [DOI] [PubMed] [Google Scholar]
- 17.López-Otín C, Blasco MA, Partridge L, Serrano M, Kroemer G. The hallmarks of aging. Cell 2013;153:1194–1217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Narita M, Nũnez S, Heard E, Narita M, Lin AW, Hearn SA, Spector DL, Hannon GJ, Lowe SW. Rb-mediated heterochromatin formation and silencing of E2F target genes during cellular senescence. Cell 2003;113:703–716 [DOI] [PubMed] [Google Scholar]
- 19.Nakamura AJ, Chiang YJ, Hathcock KS, Horikawa I, Sedelnikova OA, Hodes RJ, Bonner WM. Both telomeric and non-telomeric DNA damage are determinants of mammalian cellular senescence. Epigenetics Chromatin 2008;1:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Franceschi C, Capri M, Monti D, Giunta S, Olivieri F, Sevini F, Panourgia MP, Invidia L, Celani L, Scurti M, Cevenini E, Castellani GC, Salvioli S. Inflammaging and anti-inflammaging: A systemic perspective on aging and longevity emerged from studies in humans. Mech Ageing Dev 2007;128:92–105 [DOI] [PubMed] [Google Scholar]
- 21.Chung HY, Cesari M, Anton S, Marzetti E, Giovannini S, Seo AY, Carter C, Yu BP, Leeuwenburgh C. Molecular inflammation: Underpinnings of aging and age-related diseases. Ageing Res Rev 2009;8:18–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yanai H, Budovsky A, Tacutu R, Fraifeld VE. Is rate of skin wound healing associated with aging or longevity phenotype? Biogerontology 2011;12:591–597 [DOI] [PubMed] [Google Scholar]
- 23.Büchter C, Ackermann D, Havermann S, Honnen S, Chovolou Y, Fritz G, Kampkötter A, Wätjen W. Myricetin-mediated lifespan extension in Caenorhabditis elegans is modulated by DAF-16. Int J Mol Sci 2013;14:11895–11914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cañuelo A, Gilbert-López B, Pacheco-Liñán P, Martínez-Lara E, Siles E, Miranda-Vizuete A. Tyrosol, a main phenol present in extra virgin olive oil, increases lifespan and stress resistance in Caenorhabditis elegans. Mech Ageing Dev 2012;21:563–574 [DOI] [PubMed] [Google Scholar]
- 25.Menendez JA, Joven J, Aragonès G, Barrajón-Catalán E, Beltrán-Debón R, Borrás-Linares I, Camps J, Corominas-Faja B, Cufí S, Fernández-Arroyo S, Garcia-Heredia A, Hernández-Aguilera A, Herranz-López M, Jiménez-Sánchez C, López-Bonet E, Lozano-Sánchez J, Luciano-Mateo F, Martin-Castillo B, Martin-Paredero V, Pérez-Sánchez A, Oliveras-Ferraros C, Riera-Borrull M, Rodríguez-Gallego E, Quirantes-Piné R, Rull A, Tomás-Menor L, Vazquez-Martin A, Alonso-Villaverde C, Micol V, Segura-Carretero A. Xenohormetic and anti-aging activity of secoiridoid polyphenols present in extra virgin olive oil: A new family of gerosuppressant agents. Cell Cycle 2013;12:555–578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vayndorf EM, Lee SS, Liu RH. Whole apple extracts increase lifespan, healthspan and resistance to stress in Caenorhabditis elegans. J Funct Foods 2013;5:1236–1243 [DOI] [PMC free article] [PubMed] [Google Scholar]