Abstract
Background:
Survivors of pediatric brain tumors are at-risk for late effects which may affect mobility within and access to the physical environment. This study examined the prevalence of and risk factors for restricted environmental access in survivors of childhood brain tumors and investigated the associations between reduced environmental access, health-related quality of life (HRQOL), and survivors’ social functioning.
Methods:
In-home evaluations were completed for 78 brain tumor survivors and 78 population-based controls matched on age, sex, and zip-code. Chi-square tests and multivariable logistic regression models were used to calculate odds ratios (OR) and 95% confidence intervals (CI) for poor environmental access and reduced HRQOL.
Results:
The median age of survivors was 22 years at the time of study. Compared to controls, survivors were more likely to report avoiding most dimensions of their physical environment, including a single flight of stairs (p<0.001), uneven surfaces (p<0.001), traveling alone (p=0.01), and traveling to unfamiliar places (p=0.001). Overall, survivors were 4.8 times more likely to report poor environmental access (95% CI, 2.0-11.5, p<0.001). In survivors, poor environmental access was associated with reduced physical function (OR=3.6, 95% CI, 1.0-12.8, p=0.04), general health (OR=6.0, 95% CI, 1.8-20.6, p=0.002), and social functioning (OR=4.3, 95% CI, 1.1-17.3, p=0.03).
Conclusions:
Adult survivors of pediatric brain tumors were more likely to avoid their physical environment than matched controls. Restricted environmental access was associated with reduced HRQOL and diminished social functioning. Interventions directed at improving physical mobility may have significant impact on survivor quality of life.
Keywords: CNS malignancies, survivorship, quality of life, environmental access
INTRODUCTION
Malignancies of the central nervous system account for nearly 20% of pediatric cancers in the United States [1]. While over 70% of children diagnosed with brain tumors achieve long-term survival, the consequences of tumor location within the CNS are considerable. Musculoskeletal, sensory, endocrine, neurologic, and cognitive complications have been documented [2].
Many late effects of pediatric brain tumors (BTs) become life-long chronic conditions and may adversely impact functional outcomes [3, 4]. Survivors are at-risk for restrictions in personal care and activities of daily living, including reduced school and work attendance [5]. Physical performance deficits are present in as many as 55% of BT survivors [4-6] with such impairments leading to limited participation in social roles [6] as well as contributing to diminished health-related quality of life (HRQOL) [7].
We recently described physical performance in a cohort of young adult survivors of childhood BTs that paralleled performance among adults in the sixth decade of life [6]. Impairments in muscle weakness, balance, and exercise tolerance were observed. Importantly, in older adults, these physical impairments are predictive of mobility disability, including restricted ability to navigate the physical environment [8, 9]. As young adult BT survivors exhibit physical performance deficits comparable to older adults, they may have a similar trajectory of disability, suggesting the need to better understand how mobility deficits impact the ability of survivors to interact with their environment.
Reduced engagement with the environment may have deleterious effects on social interactions, physical health status, and HRQOL in survivors of childhood BTs. To our knowledge, the extent to which BT survivors access or avoid their physical environment, and how this may be related to functional status, has not been investigated. The study aims were to (1) examine the prevalence of and risk factors for restricted environmental access in survivors, and (2) investigate the association between reduced environmental access, HRQOL, and the ability of survivors to participate in social roles.
MATERIALS AND METHODS
Participants
BT survivors ≥18 years of age, diagnosed <21 years of age, and treated between 1970 and 2000 were randomly recruited in blocks of 20 from St. Jude Children’s Research Hospital and the University of Minnesota Children’s Hospital. A population-based comparison group, without a history of childhood cancer, was enrolled and frequency matched to survivors by age group, sex, and zip-code using Melissa data services. Participants were compensated for participation. Home visits were performed to eliminate potential participation bias due to inability to travel. The study was approved by the institutional review boards at both hospitals. Informed consent was obtained from each study participant or legal guardian. See Ness et al [6] for additional details regarding participant recruitment.
Primary Outcomes
Environmental Access
The Environmental Analysis of Mobility Questionnaire (EAMQ) was used to assess environmental access over the past month [9, 10]. The EAMQ includes a series of questions that ask participants to indicate how frequently they encounter and avoid their physical environments. Questions encompass eight dimensions of the environment: distance (e.g., walking ten blocks), temporal (e.g., busy street), ambient (e.g., lighting), terrain (e.g., stairs), physical load (e.g., heavy objects), postural demand (e.g., reaching), attention (e.g., noise), and density (e.g., crowding). Each dimension has questions for avoidance and encounter frequency, which are not mutually exclusive. The overall avoidance and encounter scores were calculated separately by summing respective scores. The total score is a ratio of overall avoidance/overall encounter. Total avoidance/encounter ratios ≥0.5 were classified as poor environmental access. The EAMQ has high test-retest reliability for all dimensions as well as summary encounter and avoidance scores [9]. Participants also answered questions specific to social participation, including the the number of times each month they travel to specific places (e.g., friend’s home, restaurant, bank).
Health Related Quality of Life
HRQOL was measured using the Medical Outcomes Survey 36-Item Short Form Health Survey (SF-36) [11], Satisfaction with Life Scale (SWLS) [12], and Visual Analog Scale (VAS) [13]. The SF-36 is widely used and provides subscale scores for eight domains of HRQOL: general health, role physical, physical function, bodily pain, vitality, mental health, social function, and role emotional. Age and sex-specific norms were utilized and T-scores ≤40 were classified as poor HRQOL. The SWLS is a five-item questionnaire utilizing a 7-point Likert scale response format and provides a global measure of life satisfaction [12]. Scores ≤17 were classified as representing poor life satisfaction. The VAS is a single-item measure on which participants indicate their quality of life on a continuous line, anchored by ‘best possible’ and ‘worst possible’ quality of life [14].
Social Participation
Participants were asked open-ended questions about employment and current living situation. Employment was categorized as employed or student vs. unemployed, whereas independent living was categorized as living independently vs. living with family support or custodial care..
Predictors and Covariates
Demographic and Treatment Information
Demographic information was collected from study participants and/or caregivers/legal guardians. Four survivors required caregiver assistance to answer questions related to demographic information. Treatment information was obtained from medical records using trained data abstractors. Tumor type, surgical interventions, chemotherapy agents and doses, and. cranial and spinal radiation doses were recorded. Consistent with the approach described by Packer et al. [15], four different anatomic segments (frontal cortex, temporal lobe, posterior fossa, and parietal or occipital lobe) were identified and the maximum dose was estimated.
Physical Performance
Physical performance limitations were assessed with the Physical Performance Test (PPT) [16] and the Functional Status Index (FSI) [17]. The 7-item PPT includes a series of timed tasks: writing a sentence, eating, dressing, picking up a small object, placing an object on a shelf, standing and turning, and walking. Higher scores on the PPT indicate better physical performance [16]. The FSI is a self-report questionnaire that measures physical performance in three dimensions: assistance, difficulty, and pain. Lower scores on the FSI indicate less disability [17].
Cognitive Function
Cognitive performance was evaluated with the Kaufman Brief Intelligence Test–Version 2 (KBIT-2) [18], which provides an overall Intelligence Quotient (IQ) Composite. Age-adjusted scores were calculated based on population norms (M=100, SD=15) and scores falling ≤10th percentile were classified as impaired cognitive function.
Psychological Distress
Psychological distress was measured by the Brief-Symptom Inventory-18 (BSI-18) [19]. Sex-specific scores were calculated using standardized normative values (M=50, SD=10) and scores falling ≥90th percentile on the Global Severity Index (GSI) were classified as clinical levels of acute distress. This measure has previously been validated in adult survivors of childhood cancer [20].
Statistical Analysis
Descriptive statistics were calculated for survivor and comparison group demographic characteristics. Percentages for encounters and avoidances within each environmental dimension were compared between groups with the Chi-Square test or Fisher’s exact test. Multivariable linear regression models were used to compare the overall avoidance/encounter ratio and social participation between survivors and controls. Multivariate logistic regression was used to identify predictors of poor environmental access. Reduced HRQOL was examined with the Chi-Square test and odds ratios (OR) and 95% confidence intervals (CI) are reported. Associations between clinical and treatment variables and environmental access were evaluated using linear regression models; associations between poor environmental access and reduced HRQOL were evaluated with the Chi-Square test; and associations between poor environmental access and social outcomes were evaluated with logistic regression models.
RESULTS
Participants
Participants included 78 of the first 132 eligible BT survivors who were randomly selected for contact. BT participants did not differ from nonparticipants by sex, current age, age at diagnosis, years since diagnosis, or tumor type (p>0.50). Members of the population-based comparison group included 78 of 99 randomly selected individuals. See Figure 1 for a consort diagram of study participation. Sex and race distributions of BT survivors and comparison group members were identical. Comparison group members were slightly older (median 25 years; range 18-54 years) than BT survivors (median 22 years; range 18-58 years). Twenty-one survivors (27%) were treated with surgery only. Table 1 provides additional descriptive characteristics of survivors.
Fig. 1.
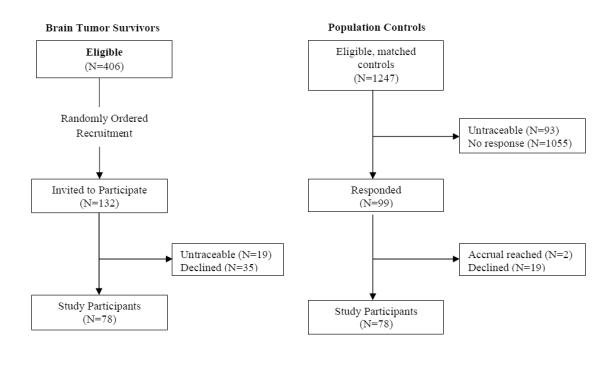
Consort diagram of study participation
Table 1.
Characteristics of brain tumor survivors
| Survivors (N=78) |
||
|---|---|---|
| N | % | |
| Sex | ||
| Female | 36 | 46 |
| Male | 42 | 54 |
| Age at Diagnosis, years | ||
| <5 | 22 | 28.2 |
| 5-9 | 26 | 33.3 |
| 10-14 | 21 | 26.9 |
| 15-20 | 9 | 11.5 |
| Time since Diagnosis, years | ||
| 5-9 | 12 | 15.4 |
| 10-14 | 30 | 38.5 |
| 15-19 | 22 | 28.2 |
| >20 | 14 | 17.9 |
| Tumor Type | ||
| Astrocytic | 40 | 51.3 |
| Medulloblastoma | 13 | 16.7 |
| Ependymoma | 9 | 11.5 |
| Other | 16 | 20.5 |
| Extent of Surgery | ||
| None/or Biopsy Only | 18 | 23.1 |
| Partial/Near Total Resection | 24 | 30.8 |
| Gross Total Resection | 36 | 46.1 |
| Chemotherapy | ||
| Yes | 24 | 30.8 |
| No | 54 | 69.2 |
| Radiation | ||
| None | 26 | 33.3 |
| Cranial | 29 | 37.2 |
| Craniospinal | 23 | 29.5 |
|
|
||
| Median | Range | |
|
|
||
| Segment Specific Dose, cGya | ||
| Spine | 3600 | 2430-6050 |
| Posterior Fossa | 5070 | 0-7020 |
| Temporal Lobe | 5040 | 0-7200 |
| Frontal Cortex | 3520 | 0-6000 |
| Occipital/Parietal Lobe | 3520 | 0-7020 |
cGy indicates centigray. Includes dose and boost to tumor bed.
Environmental Access
Across nearly all environmental dimensions, survivors were significantly more likely to report avoiding aspects of the physical environment compared to controls (Table 2). Specifically, survivors were significantly more likely to avoid navigating different environmental terrains, including a single flight of stairs (25.6% vs. 5.1%; p<0.001), curbs (20.5% vs. 2.6%; p<0.001), and uneven surfaces (28.2% vs. 5.1%; p<0.001), as well as traveling alone (48.7% vs. 28.2%; p=0.01) and to unfamiliar places (42.3% vs. 19.2%; p=0.001). Survivors were significantly more likely to report that they did not drive compared to controls (46.1% vs. 3.8%, p<0.01).
Table 2.
Environmental dimensions avoided and encountered by survivors and controls
| Encounter | Avoidance | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| Dimension | Survivor N (%) |
Control N (%) |
p-value | Survivor N (%) |
Control N (%) |
p-value |
| Distance | ||||||
| Walk long distances | 46 (59.0) | 48(61.5) | 0.90 | 35 (44.9) | 24 (30.8) | 0.05 |
| Temporal | ||||||
| Cross street with light | 50 (64.1) | 56 (71.8) | 0.42 | 22 (28.2) | 20 (25.6) | 0.65 |
| Cross busy street | 54 (69.2) | 49 (62.8) | 0.28 | 34 (43.6) | 22 (28.2) | 0.03 |
| Ambient | ||||||
| Dark | 67 (85.9) | 76 (97.4) | 0.03 | 32(41.0) | 17(21.8) | 0.01 |
| Rain | 64(82.1) | 71 (91.0) | 0.20 | 30(38.5) | 20 (25.6) | 0.07 |
| Snow | 60 (76.9) | 62 (79.5) | 0.93 | 38 (48.7) | 30(38.5) | 0.15 |
| Ice | 24 (30.8) | 34 (43.6) | 0.12 | 44 (56.4) | 41 (52.6) | 0.51 |
| Terrain | ||||||
| Single flight of stairs | 61 (78.2) | 63 (80.8) | 0.94 | 20 (25.6) | 4(5.1) | <0.001 |
| Two flights of stairs | 51 (65.4) | 51 (65.4) | 0.82 | 24 (30.8) | 11 (14.1) | <0.01 |
| Escalator | 41 (52.6) | 31 (39.7) | 0.08 | 16 (20.5) | 5 (6.4) | <0.01 |
| Curbs | 62 (79.5) | 65 (83.3) | 0.77 | 16 (20.5) | 2 (2.6) | <0.001 |
| Uneven Surfaces | 62 (79.5) | 69 (88.5) | 0.23 | 22 (28.2) | 4(5.1) | <0.001 |
| Physical Load | ||||||
| Carry heavy objects | 61 (78.2) | 74 (94.9) | 0.01 | 40(51.3) | 21 (26.9) | 0.001 |
| Open heavy doors | 53 (68.0) | 65 (83.3) | 0.05 | 15 (19.2) | 0(0) | <0.001 |
| Postural Demand | ||||||
| Reach above shoulders | 62 (79.5) | 71 (91.0) | 0.09 | 14(18.0) | 3 (3.9) | <0.01 |
| Reach below knees | 67 (85.9) | 69 (88.5) | 0.95 | 11 (14.1) | 3 (3.9) | 0.02 |
| Attention | ||||||
| Travel alone | 67 (85.9) | 72 (92.3) | 0.39 | 38 (48.7) | 22 (28.2) | 0.01 |
| Noisy or busy places | 61 (78.2) | 70 (89.7) | 0.10 | 20 (25.6) | 13 (16.7) | 0.14 |
| Unfamiliar places | 51 (65.4) | 62 (79.5) | 0.08 | 33 (42.3) | 15 (19.2) | 0.001 |
| Density | ||||||
| Crowded places | 44 (56.4) | 61 (78.2) | 0.01 | 32(41.0) | 24 (30.8) | 0.14 |
After adjusting for age and sex, survivors (M=0.44, SD=0.27) were more likely to have a higher total avoidance to encounter ratio compared with controls (M=0.24, SD=0.27), reflecting poorer environmental access across multiple dimensions (p<0.001). Specific differences were observed on the distance (p=0.004), ambient (p=0.03), terrain (p<0.001), physical load (p<0.001), postural demand (p=0.01), and attention dimensions (p<0.001) of the EAMQ. In a multivariable logistic regression model adjusting for age, sex, climate of the community, and employment status, survivors were 4.8 times more likely to report poor environmental access compared to controls (95% CI, 2.0-11.5; p<0.001) (Online Resource 1).
In analyses restricted to survivors, controlling for age and sex, we found that impaired cognition (p=0.002), lower physical performance (p=0.007), and reduced functional status (p=0.001) were significantly associated with restricted environmental access, though psychological distress was not (p=0.12). Vision loss (p=0.33), hearing loss (p=0.90), and obesity (p=0.27) were not significantly associated with access to the environment. A larger proportion of survivors who did not drive reported restricted environmental access (p=0.04), though no difference was observed in survivors treated with seizure medications (p=0.09). Results from a multivariable regression model examining the associations between treatment exposures and environmental access are provided in Table 3. Older age at the time of study completion was the only significant predictor of poor environmental access in survivors (p=0.03).
Table 3.
Impact of treatment on the EAMQ (entire scale) in survivors
| Univariate | Multivariable | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| Mean (SD) | 95% CI | p-value | LS mean (SE) | 95% CI | p-value | |
| Age at evaluation, years | - | - | 0.003 | - | - | 0.03 |
| Age at diagnosis, years | ||||||
| <5 | 0.56 (0.27) | 0.44-0.68 | 0.05 | 0.47 (0.08) | 0.31-0.63 | 0.76 |
| 5-20 | 0.40 (0.32) | 0.31-0.49 | 0.45 (0.05) | 0.34-0.55 | ||
| Sex | ||||||
| Male | 0.40(0.33) | 0.29-0.51 | 0.19 | 0.44 (0.06) | 0.31-0.57 | 0.60 |
| Female | 0.50 (0.29) | 0.40-0.59 | 0.48 (0.06) | 0.35-0.61 | ||
| Segmental radiation | ||||||
| Posterior fossa/spine | ||||||
| No | 0.42 (0.34) | 0.31-0.54 | 0.51 | 0.59 (0.09) | 0.41-0.76 | 0.08 |
| Yes | 0.47 (0.28) | 0.38-0.57 | 0.34 (0.09) | 0.16-0.52 | ||
| Temporal lobe | ||||||
| No | 0.37(0.34) | 0.25-0.50 | 0.10 | 0.36 (0.09) | 0.18-0.55 | 0.09 |
| Yes | 0.50 (0.29) | 0.41-0.58 | 0.56 (0.06) | 0.44-0.68 | ||
| Frontal cortex | ||||||
| No | 0.38(0.33) | 0.28-0.48 | 0.04 | 0.37(0.08) | 0.21-0.52 | 0.17 |
| Yes | 0.53 (0.28) | 0.43-0.63 | 0.56 (0.09) | 0.37-0.74 | ||
| Occipital/parietal lobe | ||||||
| No | 0.42 (0.33) | 0.32-0.52 | 0.40 | 0.48 (0.09) | 0.29-0.66 | 0.81 |
| Yes | 0.48 (0.29) | 0.38-0.59 | 0.44 (0.08) | 0.29-0.60 | ||
| Shunt placement | ||||||
| No | 0.41 (0.30) | 0.33-0.50 | 0.18 | 0.42 (0.06) | 0.30-0.55 | 0.32 |
| Yes | 0.52(0.35) | 0.37-0.66 | 0.50 (0.07) | 0.37-0.63 | ||
| Tumor type | ||||||
| Astrocytic | 0.44 (0.33) | 0.33-0.55 | 0.15 | 0.45 (0.06) | 0.32-0.58 | 0.22 |
| Medulloblastoma | 0.56(0.23) | 0.42-0.71 | 0.50(0.10) | 0.31-0.70 | ||
| Ependymoma | 0.53 (0.30) | 0.31-0.76 | 0.59(0.12) | 0.35-0.83 | ||
| Other | 0.31(0.32) | 0.14-0.49 | 0.30(0.10) | 0.11-0.49 | ||
| Chemotherapy | ||||||
| No | 0.41 (0.32) | 0.33-0.50 | 0.17 | 0.48 (0.07) | 0.35-0.61 | 0.77 |
| Yes | 0.53 (0.29) | 0.40-0.65 | 0.45 (0.08) | 0.28-0.61 | ||
Health-Related Quality of Life
In unadjusted analyses, survivors were significantly more likely to report reduced HRQOL than comparison group members across several domains including physical function (OR=9.97, 95% CI, 2.21-45.0, p<0.001), role physical (OR=21.8, 95% CI, 2.82-168.6, p<0.001) general health (OR=23.5, 95% CI, 3.05-181.0, p<0.001), and bodily pain (OR=7.72, 95% CI, 1.68-35.5, p=0.003). No significant differences were reported on the VAS or SWLS. Among survivors, those with poor environmental access were more likely to report diminished vitality (OR=4.44, 95% CI=1.09-18.0, p=0.03) and restricted social function (OR=4.27, 95% CI=1.05-17.3, p=0.03) (Table 4).
Table 4.
Restricted environmental access and reduced health-related quality of life in survivors
| Physical Function | Role Physical | General Health | Bodily Pain | VASa | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| N(%) | OR(95%CI) | N(%) | OR(95%CI) | N(%) | OR(95%CI) | N(%) | OR(95%CI) | N(%) | OR(95%CI) | |
|
| ||||||||||
| Restricted | 10(28.6) | 3.6(1.0-12.8) | 13(37.1) | 7.3(1.9-28.5) | 14(40.0) | 6.0(1.8-20.1) | 9(25.7) | 3.1(0.9-11.2) | 6(17.7) | 4.1(0.8-21.7) |
| Unrestricted | 4(10.0) | 1.0 | 3(7.5) | 1.0 | 4(10.0) | 1.0 | 4(10.0) | 1.0 | 2(5.0) | 1.0 |
| Mental Health | Role Emotional | Social Function | Vitality | SWLSb | ||||||
|
|
||||||||||
| N(%) | OR(95%CI) | N(%) | OR(95%CI) | N(%) | OR(95%CI) | N(%) | OR(95%CI) | N(%) | OR(95%CI) | |
|
|
||||||||||
| Restricted | 4(11.8) | 0.9(0.2-3.8) | 7(20.0) | 3.1(0.7-13.0) | 9(25.7) | 4.3(1.1-17.3) | 9(26.5) | 4.4(1.1-18.0) | 8(23.5) | 3.8(0.9-15.7) |
| Unrestricted | 5(12.5) | 1.0 | 3(7.5) | 1.0 | 3(7.5) | 1.0 | 3(7.5) | 1.0 | 3(7.5) | 1.0 |
VAS=visual analog scale.
SWLS=satisfaction with life scale.
Social Participation
In multivariable models adjusting for age and sex, survivors differed from controls with respect to the overall frequency in which they engaged in functional living and social activities in their community (p=0.003). Specifically, survivors reported going to a bank (p=0.048), friend’s home (p=0.003), and restaurant or café (p=0.035) less frequently each month compared to controls.
Forty-three percent of survivors who reported poor environmental access were living independently. In contrast, 80% of survivors reporting satisfactory environmental access were living independently, although this difference was not statistically significant. The association between employment status and environmental access approached statistical significance, indicating that survivors with poor environmental access were less likely to be currently employed (OR=0.36, 95% CI, 0.12-1.04, p=0.06).
DISCUSSION
To our knowledge, this is the first study to report on environmental access and its impact on HRQOL and social participation in a cohort of adult survivors of childhood BTs. The results indicate that BT survivors are more likely to avoid specific aspects of their physical environment compared to individuals of the same age, sex, and living in the same communities. Additionally, survivors were less likely to engage in expected social activities and roles within their communities. Poor environmental access was associated with reduced HRQOL among survivors. These results suggest that access to the physical environment has important consequences for daily functioning and social integration of BT survivors.
Beyond reporting reduced environmental access, we found that survivors were more likely to actively avoid many aspects of their physical environment than comparison adults. Specifically, survivors were more likely to avoid dark conditions, uneven terrain, physical demands, unfamiliar places, and traveling alone. Importantly, the use of a zip-code matched comparison group reduced the influence of potentially confounding aspects of the immediate physical environment (i.e., climate, street lighting, public transportation) on environmental access. Consistent with other studies, our results show that actively avoiding aspects of the physical environment has the potential to restrict daily functioning [9]. Accordingly, we found that survivors were less likely to engage in developmentally appropriate functional activities such as working, banking, and living independently. Further, survivors were less likely to engage in social activities including going to a friend’s home or going out to a restaurant. These findings are similar to reports that adult survivors of childhood BTs are more socially isolated [21] and experience less social independence [22] than their healthy counterparts. However, our results further suggest that social restrictions may be related to reduced environmental access.
In this cohort, survivors with lower physical performance scores, reduced functional status, and impaired cognition were more likely to report restricted environmental access than survivors who did not demonstrate such deficits. In older adults, similar patterns of physical functioning have been identified as precursors to mobility disability [8] which may be mediated by components of the physical environment [9]. As such, interventions with the potential to reduce environmental barriers may be a critical step toward preventing the onset of mobility disability in survivors. Such intervention efforts should capitalize on promoting access to and utilization of existing services and assistive technologies for individuals with disabilities. Targeted efforts to enhance independent mobility, balance, and coordination through individualized rehabilitation programming will also be important.
The association between environmental access and HRQOL has not previously been reported in survivors of childhood BTs, but is consistent with research among brain and spinal cord injury patients. Access to the environment has been associated with positive life satisfaction in adults with spinal cord injury [23], while environmental barriers are associated with reduced life satisfaction in patients with brain [24] and spinal cord injuries [25]. Similarly, we found that poor environmental access was associated with reduced physical function, general health, vitality, and social functioning. It is important to consider the potential bidirectional associations between these factors. Specifically, limitations on measures of physical performance and functional status were associated with poor environmental access, which may result in difficulty navigating the physical environment. However, actively avoiding one’s physical environment may precipitate or exacerbate risks for health problems associated with inactivity. Likewise, restricting oneself from accessing the environment and participating in social opportunities may potentiate feelings of isolation.
While we did not find an association between treatment variables and environmental access in survivors, older age significantly predicted poor environmental access. Previous literature suggests that disabled older adults are more likely to report avoidance of physical challenges in their environment than are nondisabled older adults [10]. Although our sample was previously reported to demonstrate greater physical performance limitations than matched comparisons [6], it is important to note that the median age for survivors in our study was only 22 years. This suggests that even young adult BT survivors may be vulnerable to barriers restricting mobility within the physical environment. These findings have important implications as physical activity and social engagement are known contributors to cardiovascular [26, 27], cognitive [28], and emotional health, [29, 30] and are likely of high import for survivors with established risk for developing chronic health conditions [3].
Several limitations need to be considered when interpreting these results. The participation rate among survivors was only 59%, thus survivors who participated may have worse or better environmental access than those who did not participate. However, every effort was made to allow eligible participants to enroll by performing evaluations in their homes and offering flexibility with timing of visits. Survivors in our study were recruited from pediatric oncology centers and may not represent the larger population of BT survivors, including those primarily treated and followed at neuro-surgical centers. Given the small number of survivors reporting reduced HRQOL and restricted social outcomes we had limited power to detect statistically significant associations between these variables and poor environmental access. Moreover, environmental access was based on self-report. Observing survivors interact with and negotiate their physical environment may provide insight into specific environmental barriers and potential intervention targets for these patients.
In summary, adult survivors of childhood BTs report, on average, worse environmental access, HRQOL, and social participation than age, sex, and zip-code matched comparisons. In survivors, limited access to the environment is associated with reduced HRQOL. Given the potential consequences of restricted environmental access on physical, social, and cognitive health, intervention efforts directed at increasing survivor engagement in their physical environments may be warranted.
Supplementary Material
Acknowledgements
Funding
This work was supported by grant RSGPB-06-210-01-CPPB from the American Cancer Society (K.K.N.). Additional funding at St. Jude Children’s Research Hospital was provided by the American Lebanese-Syrian Associated Charities (ALSAC) and, at the University of Minnesota, by the Masonic Cancer Center.
Footnotes
Portions of this work were presented at the 14th International Symposium on Pediatric Neuro-Oncology, Vienna, Austria, June 2010.
Conflict of Interest
The authors declare that they have no conflict of interest.
REFERENCES
- 1.National Cancer Institute (1975-2008) SEER Cancer Statistics Review. http://seer.cancer.gov/csr/1975_2008/. Accessed August 25, 2011.
- 2.Armstrong GT, Liu Q, Yasui Y, et al. Long-term outcomes among adult survivors of childhood central nervous system malignancies in the Childhood Cancer Survivor Study. J Natl Cancer Inst. 2009;101:946–958. doi: 10.1093/jnci/djp148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Oeffinger KC, Mertens AC, Sklar CA, et al. Chronic health conditions in adult survivors of childhood cancer. N Engl J Med. 2006;355:1572–1582. doi: 10.1056/NEJMsa060185. [DOI] [PubMed] [Google Scholar]
- 4.Hudson MM, Mertens AC, Yasui Y, et al. Health status of adult long-term survivors of childhood cancer: a report from the Childhood Cancer Survivor Study. JAMA. 2003;290:1583–1592. doi: 10.1001/jama.290.12.1583. [DOI] [PubMed] [Google Scholar]
- 5.Ness KK, Mertens AC, Hudson MM, et al. Limitations on physical performance and daily activities among long-term survivors of childhood cancer. Ann Intern Med. 2005;143:639–647. doi: 10.7326/0003-4819-143-9-200511010-00007. [DOI] [PubMed] [Google Scholar]
- 6.Ness KK, Morris EB, Nolan VG, et al. Physical performance limitations among adult survivors of childhood brain tumors. Cancer. 2010;116:3034–3044. doi: 10.1002/cncr.25051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ness KK, Gurney JG, Zeltzer LK, et al. The impact of limitations in physical, executive, and emotional function on health-related quality of life among adult survivors of childhood cancer: a report from the Childhood Cancer Survivor Study. Arch Phys Med Rehabil. 2008;89:128–136. doi: 10.1016/j.apmr.2007.08.123. [DOI] [PubMed] [Google Scholar]
- 8.Fried LP, Bandeen-Roche K, Chaves PH, Johnson BA. Preclinical mobility disability predicts incident mobility disability in older women. J Gerontol A Biol Sci Med Sci. 2005;55:M43–52. doi: 10.1093/gerona/55.1.m43. [DOI] [PubMed] [Google Scholar]
- 9.Shumway-Cook A, Patla A, Stewart AL, Ferrucci L, Ciol MA, Guralnik JM. Assessing environmentally determined mobility disability: self-report versus observed community mobility. J Am Geriatr Soc. 2005;53:700–704. doi: 10.1111/j.1532-5415.2005.53222.x. [DOI] [PubMed] [Google Scholar]
- 10.Shumway-Cook A, Patla A, Stewart A, Ferrucci L, Ciol MA, Guralnik JM. Environmental components of mobility disability in community-living older persons. J Am Geriatr Soc. 2003;51:393–398. doi: 10.1046/j.1532-5415.2003.51114.x. [DOI] [PubMed] [Google Scholar]
- 11.Ware JE, Jr., Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992;30:473–483. [PubMed] [Google Scholar]
- 12.Diener E, Emmons RA, Larsen RJ, Griffin S. The Satisfaction With Life Scale. J Pers Assess. 1985;49:71–75. doi: 10.1207/s15327752jpa4901_13. [DOI] [PubMed] [Google Scholar]
- 13.Selby PJ, Chapman JA, Etazadi-Amoli J, Dalley D, Boyd NF. The development of a method for assessing the quality of life of cancer patients. Br J Cancer. 1984;50:13–22. doi: 10.1038/bjc.1984.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.de Boer AG, van Lanschot JJ, Stalmeier PF, et al. Is a single-item visual analogue scale as valid, reliable and responsive as multi-item scales in measuring quality of life? Qual Life Res. 2004;13:311–320. doi: 10.1023/B:QURE.0000018499.64574.1f. [DOI] [PubMed] [Google Scholar]
- 15.Packer RJ, Gurney JG, Punyko JA, et al. Long-term neurologic and neurosensory sequelae in adult survivors of a childhood brain tumor: childhood cancer survivor study. J Clin Oncol. 2003;21:3255–3261. doi: 10.1200/JCO.2003.01.202. [DOI] [PubMed] [Google Scholar]
- 16.Reuben DB, Siu AL. An objective measure of physical function of elderly outpatients. The Physical Performance Test. J Am Geriatr Soc. 1990;38:1105–12. doi: 10.1111/j.1532-5415.1990.tb01373.x. [DOI] [PubMed] [Google Scholar]
- 17.Jette AM. The Functional Status Index: reliability and validity of a self-report functional disability measure. J Rheumatol Suppl. 1987;14(suppl.):15–21. [PubMed] [Google Scholar]
- 18.Kaufman A, Kaufman Kaufman N. Brief Intelligence Test. 2 NCS Pearson, Inc; Circle Pines, MN: 2004. [Google Scholar]
- 19.Derogatis L. Brief Symptom Inventory (BSI): Administration, scoring, and procedures manual. NCS Pearson; Minneapolis, MN: 2000. [Google Scholar]
- 20.Recklitis CJ, Parsons SK, Shih MC, Mertens A, Robison LL, Zeltzer L. Factor structure of the brief symptom inventory 18 in adult survivors of childhood cancer: results from the childhood cancer survivor study. Psychol Assess. 2006;18:22–32. doi: 10.1037/1040-3590.18.1.22. [DOI] [PubMed] [Google Scholar]
- 21.Boydell KM, Stasiulis E, Greenberg M, Greenberg C, Spiegler B. I'll show them: the social construction of (in)competence in survivors of childhood brain tumors. J Pediatr Oncol Nurs. 2008;25:164–174. doi: 10.1177/1043454208315547. [DOI] [PubMed] [Google Scholar]
- 22.Koch SV, Kejs AM, Engholm G, Moller H, Johansen C, Schmiegelow K. Leaving home after cancer in childhood: a measure of social independence in early adulthood. Pediatr Blood Cancer. 2006;47:61–70. doi: 10.1002/pbc.20827. [DOI] [PubMed] [Google Scholar]
- 23.Richards JS, Bombardier CH, Tate D, Dijkers M, Gordon W, Shewchuk R, et al. Access to the environment and life satisfaction after spinal cord injury. Arch Phys Med Rehabil. 1999;80:1501–1506. doi: 10.1016/s0003-9993(99)90264-2. [DOI] [PubMed] [Google Scholar]
- 24.Whiteneck GG, Gerhart KA, Cusick CP. Identifying environmental factors that influence the outcomes of people with traumatic brain injury. J Head Trauma Rehabil. 2004;19:191–204. doi: 10.1097/00001199-200405000-00001. [DOI] [PubMed] [Google Scholar]
- 25.Whiteneck G, Meade MA, Dijkers M, Tate DG, Bushnik T, Forchheimer MB. Environmental factors and their role in participation and life satisfaction after spinal cord injury. Arch Phys Med Rehabil. 2004;85:1793–1803. doi: 10.1016/j.apmr.2004.04.024. [DOI] [PubMed] [Google Scholar]
- 26.Ikeda A, Iso H, Kawachi I, Yamagishi K, Inoue M, Tsugane S. Social support and stroke and coronary heart disease: the JPHC study cohorts II. Stroke. 2008;39:768–775. doi: 10.1161/STROKEAHA.107.496695. [DOI] [PubMed] [Google Scholar]
- 27.Thompson PD. Exercise and physical activity in the prevention and treatment of atherosclerotic cardiovascular disease. Arterioscler Thromb Vasc Biol. 2003;23:e42–49. doi: 10.1161/01.ATV.0000087143.33998.F2. [DOI] [PubMed] [Google Scholar]
- 28.Seeman TE, Miller-Martinez DM, Stein Merkin S, Lachman ME, Tun PA, Karlamangla AS. Histories of social engagement and adult cognition: midlife in the U.S. study. J Gerontol B Psychol Sci Soc Sci. 2011;66(suppl.):i141–152. doi: 10.1093/geronb/gbq091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Strawbridge WJ, Deleger S, Roberts RE, Kaplan GA. Physical activity reduces the risk of subsequent depression for older adults. Am J Epidemiol. 2002;156:328–334. doi: 10.1093/aje/kwf047. [DOI] [PubMed] [Google Scholar]
- 30.Glass TA, De Leon CF, Bassuk SS, Berkman LF. Social engagement and depressive symptoms in late life: longitudinal findings. J Aging Health. 2006;18:604–628. doi: 10.1177/0898264306291017. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


