Abstract
Adipose TG lipase (ATGL) catalyzes the rate-limiting step in TG hydrolysis in most tissues. We have shown that hepatic ATGL preferentially channels hydrolyzed FAs to β-oxidation and induces PPAR-α signaling. Previous studies have suggested that liver FA binding protein (L-FABP) transports FAs from lipid droplets to the nucleus for ligand delivery and to the mitochondria for β-oxidation. To determine if L-FABP is involved in ATGL-mediated FA channeling, we used adenovirus-mediated suppression or overexpression of hepatic ATGL in either WT or L-FABP KO mice. Hepatic ATGL knockdown increased liver weight and TG content of overnight fasted mice regardless of genotype. L-FABP deletion did not impair the effects of ATGL overexpression on the oxidation of hydrolyzed FAs in primary hepatocyte cultures or on serum β-hydroxybutyrate concentrations in vivo. Moreover, L-FABP deletion did not influence the effects of ATGL knockdown or overexpression on PPAR-α target gene expression. Taken together, we conclude that L-FABP is not required to channel ATGL-hydrolyzed FAs to mitochondria for β-oxidation or the nucleus for PPAR-α regulation.
Keywords: peroxisome proliferator-activated receptor-α, β-oxidation, liver fatty acid binding protein, adipose triglyceride lipase
Adipose TG lipase (ATGL) was originally characterized as the major TG hydrolase in adipose tissue and heart, and more recent studies show it also has a predominant role in other tissues such as liver (1, 2). In addition to its effects on TG catabolism, ATGL influences the downstream partitioning of its FA products. We have shown that hepatic ATGL selectively channels hydrolyzes FAs to β-oxidation without affecting VLDL secretion (1). Consistent with these results, several other studies have also observed that hepatic ATGL overexpression or deletion affects FA oxidation but not VLDL secretion in vitro and in vivo (2, 3). Similar effects of ATGL on channeling hydrolyzed FAs to β-oxidation have also been observed in adipocytes, small intestine, and myocardial tissue (4–6). The mechanism through which ATGL channels FAs hydrolyzed at the surface of lipid droplets (LDs) to the mitochondria, however, is unknown.
In addition to its effects on FA trafficking, ATGL is also an important regulator of PPAR-α. We have shown that the expression of PPAR-α and its downstream target genes is decreased in mice or primary hepatocytes receiving ATGL shRNA (1). These results are consistent with microarray analysis of tissues in the whole-body ATGL KO mice (7) and work from others reporting that ATGL regulates PPAR-α in heart, small intestine, and brown adipose tissue (4–6). Although numerous metabolites can bind PPAR-α, FAs are thought to be the principal physiological ligands that activate PPAR-α (8–10). Given that ATGL produces FAs, it is assumed that ATGL regulates PPAR-α through the supply of FA ligands (11). However, data defining the mechanism through which ATGL regulates PPAR-α or how FAs are trafficked to PPAR-α are lacking.
Liver FA binding protein (L-FABP) is an abundant cytosolic FA carrier involved in long chain FA transport throughout the cytoplasm and channeling of FAs and acyl-CoAs to the mitochondria for oxidation (12). L-FABP has been shown to interact with mitochondria (13) and, more specifically, carnitine palmitoyltransferase 1 (CPT1), the rate-limiting step in mitochondrial FA oxidation (12, 14, 15). These observations have been taken to imply that L-FABP may mediate the transport of FAs to the mitochondria. In support of this, L-FABP gene ablation in cultured primary hepatocytes suppresses mitochondrial FA oxidation (16). Additionally, in vitro studies have determined that L-FABP binds PPAR-α with high affinity and further demonstrate that manipulating L-FABP expression alters PPAR-α-dependent transcription of genes coding for FA oxidative enzymes (17, 18). Yet, studies performed in vivo are inconsistent in regard to the role of L-FABP in mediating PPAR-α activity, with some studies showing that PPAR-α target gene expression is regulated independently from L-FABP (16, 19) and others showing dependence on L-FABP (20, 21).
Given the range of possible functional and physiological roles of L-FABP in FA oxidation, we questioned whether L-FABP is involved in the transport of a specific pool of FAs, ATGL-hydrolyzed FAs, to the mitochondria for oxidation and/or to the nucleus to regulate gene expression. To investigate the role of L-FABP in mediating the effects of ATGL, we used a strategy in which ATGL was either overexpressed (adenovirus expressing (Ad)-ATGL) or suppressed (ATGL shRNA) in both WT and L-FABP KO mice. Our findings reveal that despite its importance in hepatic FA transport, L-FABP is not required for the channeling of ATGL-hydrolzyed FAs to either the mitochondria for oxidation or to the nucleus for transcriptional regulation of PPAR-α.
MATERIALS AND METHODS
Animals, diets, and adenoviral administration
All animal protocols were approved by the University of Minnesota Institutional Animal Care and Use Committee. Male L-FABP KO mice on a C57BL/6J background were generated as previously described (22). All mice were housed under controlled temperature and lighting (20–22°C; 12:12 h light-dark cycle). Eight- to ten-week-old WT or L-FABP KO mice were injected via the tail vein with adenovirus expressing green fluorescent protein (Ad-GFP) or Ad-ATGL for overexpression studies or adenoviruses encoding ATGL shRNA or control shRNA targeting a nonspecific mRNA. Adenoviruses were generated as described previously (1, 23). All mice had free access to water and were fed a purified diet (TD.94045) from Harlan Teklad Premier Laboratory Diets (Madison, WI) for 1 week following adenoviral administration. The control diet contained protein (19% of total calories), carbohydrates (64%), and fat (17%), and the fat source was soybean oil (70 g/kg). Exactly 1 week after adenovirus injection, mice were euthanized for liver tissue and serum collection after a 4 h or overnight fast.
Primary hepatocyte isolation
Mouse primary hepatocytes were isolated by the collagenase perfusion method described previously (1). Hepatocytes were plated on collagen-coated multi-well tissue culture plates (Nunc) for 4 h with M199 plating media (Invitrogen) that contained 23 mM HEPES, 26 mM sodium bicarbonate, 10% FBS, 1% penicillin/streptomycin, 100 nM dexamethasone, 100 nM insulin, and 11 mM glucose. M199 maintenance media contained 23 mM HEPES, 26 mM sodium bicarbonate, 1% penicillin/streptomycin, 5.5 mM glucose, 100 μM carnitine, 10 nM dexamethasone, and 10 nM insulin. Cells were maintained in a humidified incubator at 37°C, 5% CO2.
Cell adenoviral infection and radiolabeling
Ad-ATGL or Ad-GFP, which serves as a control virus, was generated as described previously (1). After 4 h of plating, primary hepatocytes were exposed to either Ad-ATGL or Ad-GFP at 100 multiplicities of infection for 24 h. Cells were pulsed with 500 μM oleate and 1 μCi [1-14C]oleate bound to FA-free BSA in a 3:1 molar ratio for 1.5 h (pulse period). Some cells were harvested for measurement of radiolabel incorporation into cellular lipid fractions. Parallel wells were washed with phosphate-buffered saline and new medium, void of FAs, was added for an additional 6 h (chase period) followed by collection of cells for lipid extraction. Lipid samples were separated into different fractions by TLC on 0.25 mm silica gel G plates in a hexane:ethyl ether:acetic acid (80:20:2, v/v/v) solvent system. TG fractions were identified and measured by AR-2000 radio-TLC imaging scanner. Total radiolabeled lipids were quantified by scintillation counter (LS6000IC, Beckman) after addition of Bio-Safe II cocktail.
Measurement of FA oxidation
During either the pulse or chase periods, as described above, radiolabeled media were transferred to Eppendorf tubes containing 20% BSA. Subsequently, perchloric acid was added to the tubes followed by vortexing. Following an overnight incubation at room temperature, the tubes were centrifuged and the supernatant fraction that contained [14C]-labeled acid-soluble metabolites (ASMs) was quantified via scintillation counting. In some experiments, HTS01037, a pan-FABP inhibitor, was dissolved in DMSO and applied to hepatocytes during the chase period at a final concentration of 100 μM.
RNA isolation, RT-PCR, and real-time quantitative PCR analysis
RNA was extracted with TRIzol from liver tissues followed by reverse transcription with SuperScript III First-Strand Synthesis SuperMix (Invitrogen). Gene expression was quantified using a SYBR GreenER Two-Step quantitative RT-PCR kit (Invitrogen) and an Applied Biosystems StepOne Plus real-time PCR system. Data were analyzed using the delta-delta CT method. For all analyses, gene expression was normalized to ribosomal protein L32. Melting curve analysis was performed on all samples to verify primer specificity.
Liver TG and FA analysis
TG was extracted from liver tissues or hepatocytes according to a method from Folch (24). Snap-frozen liver tissues were homogenized in sterile water and extracted twice with chloroform:methanol. The chloroform layer was isolated, dried under nitrogen gas, and redissolved in diluted Triton X-100. Colorimetric assays were used to quantify liver TG (Stanbio) and NEFAs (Sigma).
Serum measurements
Colorimetric kits were used for the determination of serum β-hydroxybutyrate (BHBA), NEFAs (both from Wako Chemicals), and serum TG (Stanbio).
Statistical analysis
Results are expressed as mean ± standard error of the mean. Statistical analysis was performed using an unpaired Student's t-test or, where appropriate, a two-way ANOVA test followed by Fischer's protected least significant difference. Values of P < 0.05 were considered statistically significant.
RESULTS
ATGL knockdown increases liver weights and TG content
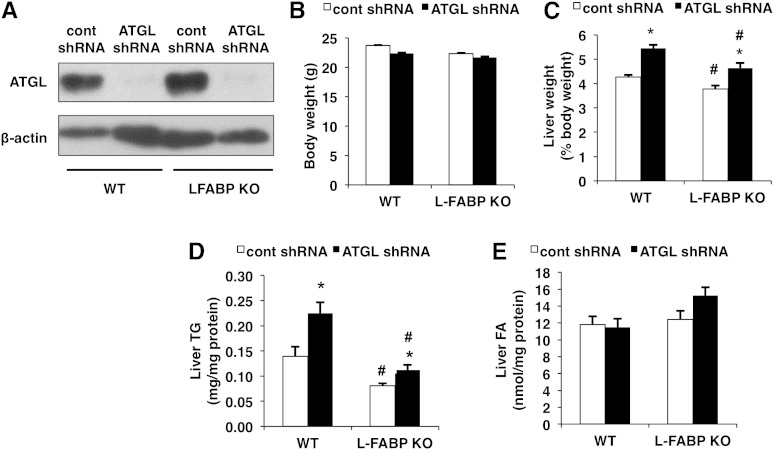
Given that the deletion of L-FABP has been shown to decrease hepatic TG accumulation (22) and ATGL is a major hepatic TG hydrolase (1), we investigated the effects of ATGL knockdown in L-FABP KO mice. ATGL shRNA administration suppressed protein expression of hepatic ATGL in both WT and L-FABP KO mice efficiently 7 days posttransduction (Fig. 1A). ATGL knockdown had no effect on body weight in WT or L-FABP KO mice when compared with control shRNA treatment (Fig. 1B). ATGL knockdown led to increased liver weight in WT mice and this effect was replicated in L-FABP KO mice (Fig. 1C). L-FABP ablation also resulted in reduced liver weights. ATGL knockdown led to enhanced hepatic TG accumulation in both WT and L-FABP KO mice, although the fold induction was less in the L-FABP KO mice (Fig. 1D). Consistent with previous findings, hepatic TG content was significantly lower in L-FABP KO mice compared with WT controls (21). Neither ATGL knockdown or L-FABP ablation affected the content of liver free FAs in either genotype (Fig. 1E).
Fig. 1.
ATGL knockdown increases liver weights and TG content. WT or L-FABP KO mice, at 8–10 weeks of age, were infected with control (cont) or ATGL shRNA adenoviruses and euthanized 7 days posttransduction (n = 8–10 per group). A: Protein expression of hepatic ATGL was determined via Western blot. Body (B) and liver (C) weights, the latter expressed as a % of body weight, were measured following an overnight fast. Liver TG (D) and NEFA (E) were quantified using a colorimetric enzymatic assay. Data are presented as mean ± SEM. *P < 0.05 versus control shRNA group; #P < 0.05 versus WT mice.
ATGL overexpression has minimal effects on liver weight and TG content in 4 h-fasted mice
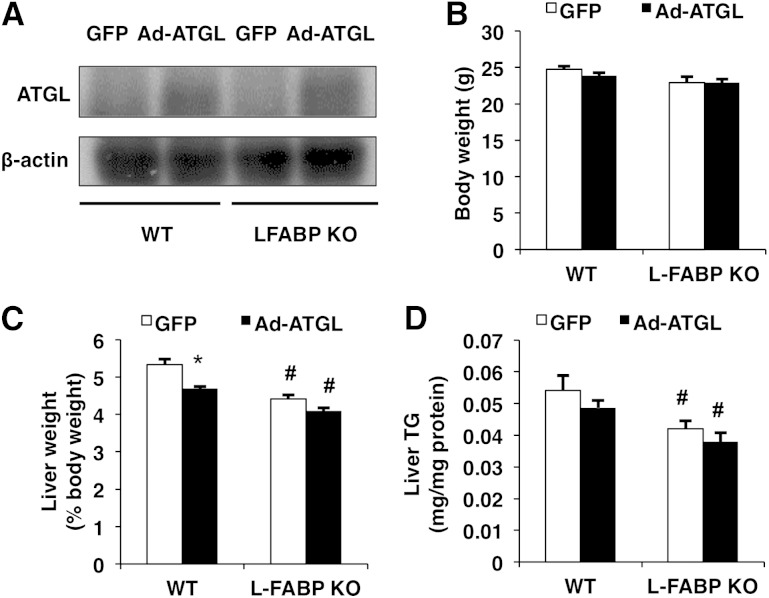
To further examine the importance of L-FABP in mediating the effects of ATGL on hepatic lipid metabolism, we also overexpressed ATGL in both WT and L-FABP KO mice. Administration of Ad-ATGL adenovirus increased the protein expression of hepatic ATGL in both WT and L-FABP KO mice 7 days postinfection (Fig. 2A). Consistent with the findings above, overexpressing ATGL did not alter body weight of WT or L-FABP KO mice (Fig. 2B). Increasing the expression of hepatic ATGL decreased liver weights only in WT mice (Fig. 2C). In addition, L-FABP ablation also resulted in lower liver weights. Hepatic TG content was reduced in L-FABP KO mice, whereas ATGL overexpression had no significant effect (Fig. 2D). The lack of effect of ATGL on liver TG may stem from the low basal TG levels in 4 h-fasted mice.
Fig. 2.
ATGL overexpression has minimal effects on liver weight and TG content in 4 h-fasted mice. WT or L-FABP KO mice, at 8–10 weeks of age, were injected with Ad-GFP or Ad-ATGL adenoviruses and euthanized 7 days postinfection (n = 8–10 per group). A: Hepatic ATGL protein expression was quantified via Western blot. Body (B) and liver (C) weights were measured following a 4 h fast. Liver TG (D) was quantified using a colorimetric enzymatic assay. Data are presented as mean ± SEM. *P < 0.05 versus GFP; #P < 0.05 versus WT mice.
ATGL overexpression increases serum BHBA regardless of genotype
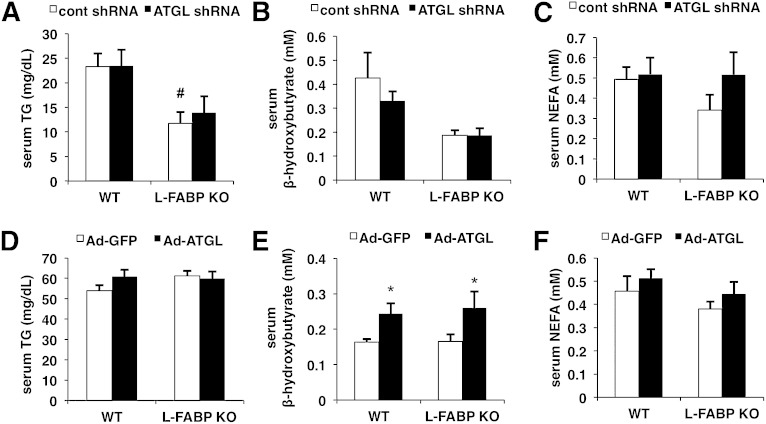
We next determined the effect of ATGL knockdown on serum TG, BHBA, and NEFA. ATGL knockdown had no effect on serum TG, BHBA, or NEFA in either genotype (Fig. 3A–C). However, serum TG levels in overnight-fasted L-FABP KO mice were lower than those of WT mice treated with control shRNA adenovirus after an overnight fast (Fig. 3A). Two previous studies showed no change in serum TG levels with L-FABP deletion, but the discrepancies in fasting duration and mouse gender may account for the differences (22, 25). The main effect of L-FABP KO on lowering serum BHBA approached significance (P = 0.06) in mice fasted for 16 h (Fig. 3B), but not 4 h (Fig. 3E). Overexpressing ATGL did not alter serum TG or NEFA in WT or L-FABP KO mice, but increased BHBA in both mouse lines (Fig. 3D–F).
Fig. 3.
ATGL overexpression increases serum BHBA regardless of genotype. WT or L-FABP KO mice, at 8–10 weeks of age, were transduced with control (cont) or ATGL shRNA adenovirus (n = 8–10 per group) (A–C) or Ad-GFP or Ad-ATGL adenovirus (n = 8–10 per group) (D–F). Mice were fed with purified control diet and euthanized 7 days postinfection. Serum TG (A, D), BHBA (B, E), and NEFA (C, F) were measured with a colorimetric enzymatic kit after an overnight (A–C) or 4 h fast (D–F). Data are presented as mean ± SEM. *P < 0.05 versus GFP; #P < 0.05 versus WT mice.
L-FABP ablation does not attenuate ATGL-mediated β-oxidation
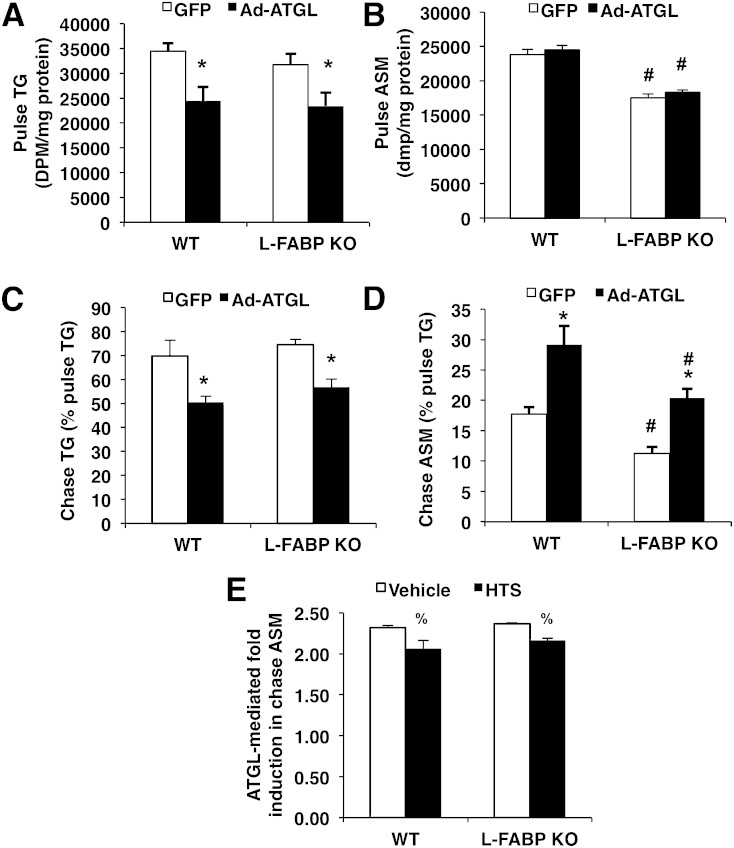
Our previous studies have shown that hepatic ATGL regulates β-oxidation in primary hepatocyte cultures (1). Thus, we performed pulse-chase experiments with [1-14C]oleate in primary mouse hepatocytes isolated from WT or L-FABP KO mice to gain insights into the role of L-FABP in influencing ATGL-induced changes in β-oxidation. During the pulse period, overexpressing ATGL decreased incorporation of [14C]oleate into the TG fraction of primary hepatocytes derived from both WT and L-FABP KO mice to a similar extent (Fig. 4A) without any change to the specific activity of TG (Ad-GFP: 205,909 ± 15,337 dpm/mg TG; Ad-ATGL: 202,420 ± 20,362 dpm/mg TG). ASM production during the pulse period was unaltered in response to ATGL overexpression, but was reduced in L-FABP KO mice compared with WT mice (Fig. 4B). To determine the importance of L-FABP on the oxidation of FAs derived primarily from intracellular TG hydrolysis (i.e., ATGL-hydrolyzed FAs), we quantified ASMs as a measure of FA oxidation during the chase period. ATGL overexpression resulted in ASM in both WT and L-FABP KO mice, suggesting that L-FABP is not required for ATGL-mediated increase in β-oxidation (Fig. 4D). These effects were not due to an effect of L-FABP on lipolysis, as ATGL increased the amount of radiolabeled TG lost during the chase period equally in WT and L-FABP KO hepatocytes (Fig. 4C). To ensure that this observation was not due to a compensatory effect by other FABP isoforms, we also utilized a pan-specific FABP inhibitor, HTS01037, during the chase period in hepatocytes isolated from WT and L-FABP KO mice. Although HTS01037 selectively inhibits FABP4, it inhibits all FABP isoforms with Ki values of less than 10 μM (26), well below the 100 μM concentration used in the current study. HTS01037 resulted in a small, but significant, attenuation of the ATGL-mediated increase in ASM during the chase period regardless of the mouse line, suggesting that other FABP isoforms do not play a major role in mediating the oxidation of ATGL-derived FAs (Fig. 4E). Taken together, these data indicate that L-FABP is not a major carrier of ATGL-hydrolyzed FAs and may be more important for channeling exogenous FAs for the purpose of mitochondrial β-oxidation.
Fig. 4.
L-FABP deletion does not attenuate ATGL-mediated β-oxidation. Primary mouse hepatocytes were isolated from 8–10-week-old WT or L-FABP KO mice and transduced with Ad-GFP or Ad-ATGL for 24 h, at which time pulse (1.5 h) and chase (6 h) experiments were performed with 500 μM [1-14C]oleate. [1-14C]oleate incorporation into cell TG during the pulse period (A) and radiolabeled TG remaining after the chase period (C). ASMs from media were quantified at the end of pulse (B) and chase (D) periods. A pan-specific FABP inhibitor, HTS01037 (100 μM), was added during the chase period, and radiolabel incorporation into ASMs was quantified (E). ATGL-mediated fold induction was calculated as the Ad-ATGL values divided by the Ad-GFP values. Data are presented as mean ± SEM. *P < 0.05 versus GFP; #P < 0.05 versus WT mice; %P < 0.05 versus vehicle.
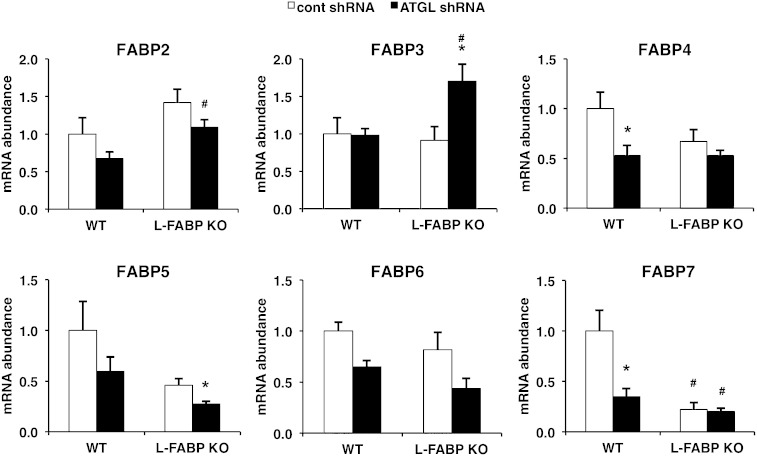
L-FABP ablation or ATGL knockdown alters the expression of other FABP isoforms
We quantified mRNA abundance of FABP2–7 to further test whether either L-FABP ablation or ATGL knockdown influenced expression of other FABP isoforms. Only FABP7 was significantly reduced in response to L-FABP ablation alone (Fig. 5). ATGL knockdown decreased the expression of several FABP isoforms in the WT or L-FABP backgrounds. In contrast, FABP3 was significantly increased following ATGL knockdown in the L-FABP KO mice, but not in the WT mice. Thus, based on mRNA abundance, if any compensation occurred in response to L-FABP ablation and ATGL knockdown it would likely be due to FABP3.
Fig. 5.
Changes in FABP isoform expression in response to ATGL knockdown or L-FABP ablation. WT or L-FABP KO mice, at 8–10 weeks of age, were infected with control or ATGL shRNA adenovirus (n = 8–10 per group) followed by harvesting of liver tissues 7 days later after an overnight fast. Data are presented as mean ± SEM. *P < 0.05 versus control shRNA group; #P < 0.05 versus WT mice.
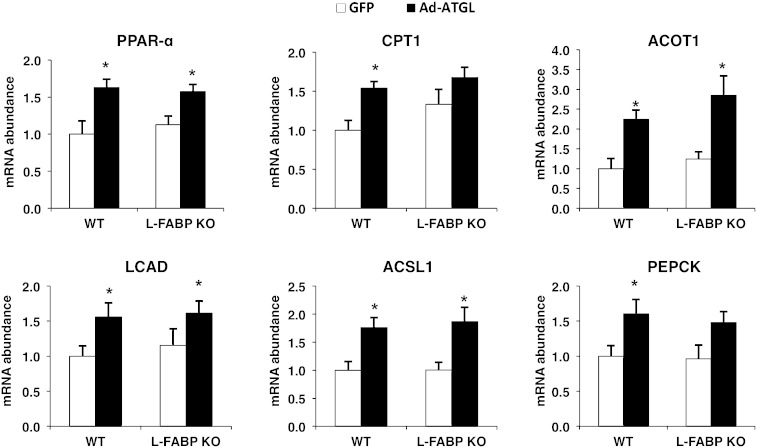
L-FABP is not required for the induction of PPAR-α target genes following ATGL overexpression
We have previously shown that hepatic ATGL promotes FA oxidation via changes in the expression of PPAR-α and its target genes (1). Other work has demonstrated that L-FABP binds directly to PPAR-α and may play a role in PPAR-α activation (17). To further examine whether L-FABP serves as a carrier of ATGL-hydrolyzed FAs to the nucleus to influence oxidative genes, we also quantified PPAR-α target gene expression in WT or L-FABP KO mice treated with Ad-ATGL adenovirus. If L-FABP mediates the effects of ATGL on FA trafficking, we would expect that the induction of FA oxidation and PPAR-α target gene expression following ATGL overexpression would be attenuated in L-FABP KO mice and that no reduction in the same outcomes would be observed following ATGL knockdown in L-FABP mice. In WT mice, hepatic ATGL overexpression led to approximately a 0.4- to 2-fold increase in PPAR-α and its target genes (Fig. 6). Despite the absence of L-FABP, adenoviral-mediated overexpression of ATGL still induced gene expression of PPAR-α, acyl-CoA thioesterase (ACOT1), long chain acyl-CoA dehydrogenase (LCAD), and acyl-CoA synthetase 1 (ACSL1) (0.3- to 2.5-fold) in L-FABP KO mice [CPT1 and phosphoenolpyruvate carboxykinase (PEPCK) did not reach significance at P < 0.05]. These results suggest that L-FABP is not required to mediate the effects of ATGL overexpression on PPAR-α activity.
Fig. 6.
L-FABP is not required for the induction of PPAR-α target genes following ATGL overexpression. WT or L-FABP KO mice, at 8–10 weeks of age, were transduced with Ad-GFP or Ad-ATGL adenovirus (n = 8–10 per group). Liver tissues were collected 7 days posttransduction after a 4 h fast. Data are presented as mean ± SEM. *P < 0.05 versus GFP; #P < 0.05 versus WT mice.
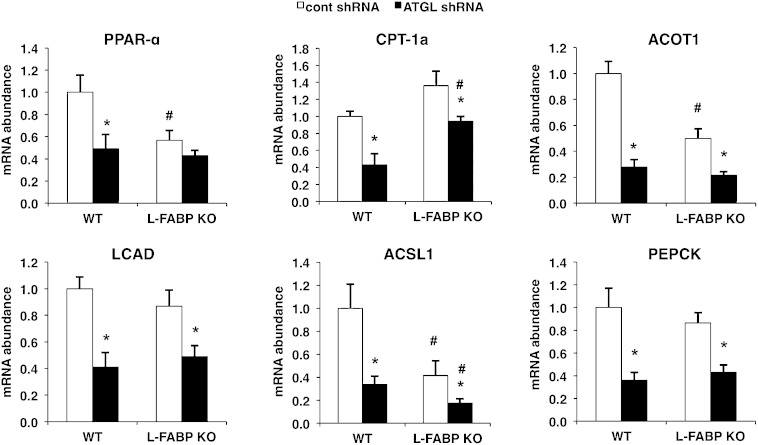
L-FABP deletion does not mediate the effects of ATGL knockdown on PPAR-α target gene expression
We next investigated the effect of ATGL knockdown on PPAR-α-regulated genes in L-FABP KO mice. A subset of genes, including PPAR-α, ACOT1, and ACSL1, was downregulated in L-FABP KO compared with WT mice injected with control shRNA adenovirus (Fig. 7), consistent with an important role of L-FABP in modulating oxidative gene expression during fasting. ATGL knockdown resulted in a downregulation of all PPAR-α target genes examined, with the exception of PPAR-α itself, and these effects were largely the same in WT and L-FABP KO mice. Thus, these data support the overexpression studies and show that L-FABP does not mediate the effects of ATGL on the expression of PPAR-α target genes, and imply that an alternate mechanism links changes in ATGL activity to transcriptional regulation.
Fig. 7.
L-FABP deletion does not mediate the effects of ATGL knockdown on PPAR-α target gene expression. WT or L-FABP KO mice, at 8–10 weeks of age, were infected with control (cont) or ATGL shRNA adenovirus (n = 8–10 per group) followed by harvesting of liver tissues 7 days later after an overnight fast. Data are presented as mean ± SEM. *P < 0.05 versus control shRNA group; #P < 0.05 versus WT mice.
DISCUSSION
It is well-established that L-FABP plays a central role in hepatic FA trafficking and energy metabolism. Its high expression level and ability to interact with mitochondria and nuclear transcription factors suggest that it may act as a FA sensor that alters metabolism directly via changes in intracellular FA handling as well as transcriptional regulation of metabolic pathways. To date, most studies have focused on the role of L-FABP in modulating exogenous FA metabolism, especially during fasting, high fat feeding, or in various disease models (20, 21, 25, 27, 28). The effects of L-FABP on the metabolism and signaling properties of endogenous FAs, such as those derived from lipolysis, are unknown. The present study sought to determine whether L-FABP is important in modulating the effects of ATGL on the signaling and channeling of hydrolyzed FAs.
Previous findings that show ATGL selectively channels hydrolyzed FAs exclusively to β-oxidation provide novel insights into the regulation of intracellular lipid trafficking (1, 2, 4–6). To better understand the mechanisms responsible for this selective partitioning of FAs, we tested the importance of L-FABP in mediating these effects. In contrast to its clear effects on channeling of exogenous FAs to oxidative pathways, L-FABP did not mediate the trafficking of hydrolyzed FAs derived from ATGL to β-oxidation. Consistent with the lack of effect on intracellular FA trafficking, L-FABP did not modulate serum TG, BHBA, or NEFA in mice with manipulated hepatic ATGL expression. The lack of effect of L-FABP ablation on oxidation of FAs derived from ATGL was surprising, and suggests that either another lipid carrier is responsible for channeling hydrolyzed FAs or that this pool of FAs does not require a protein carrier for their shuttling to the mitochondria. To address the former possibility, we measured oxidation of ATGL-derived FAs in the presence of a pan L-FABP inhibitor and found that the inhibitor only resulted in a small (∼15–20%) attenuation of ATGL-induced FA oxidation. The results from this study suggest that there may be some compensation by other FABP isoforms, but it is unlikely to be a major contributor to the oxidation of hydrolyzed FAs. We also found that FABP3 was modestly increased in L-FABP KO mice treated with ATGL shRNA, although it is questionable whether this increase in a relatively low-abundance hepatic FABP isoform (29) could compensate for the lack of L-FABP. Previous findings show other L-FABP isoforms or putative FA transporters, such as sterol carrier proteins (SCP-2 and SCP-x), are not upregulated in L-FABP KO mice (20, 22). We found that only FABP7 was significantly decreased in the L-FABP KO mice. Although the current study does not preclude the importance of other FA carriers, the data do not support a major role of other FABP isoforms in compensating for L-FABP ablation.
An alternate potential mechanism explaining the selective channeling of hydrolyzed FAs to oxidative pathways may involve a direct interaction between LDs and mitochondria where FAs are “handed off” to downstream enzymes independent of FA carriers. In fact, mitochondria are often observed to be closely located to LDs in adipocytes, heart, and liver (30–35). Given that oxidative and metabolically active tissues often have higher rates of lipolysis and FA oxidation, conditions that increase energy demand may enhance these interactions. In exercise-trained dogs, goats, and humans, every LD in muscle tissue is observed to be in contact with at least one mitochondrion (36, 37). In addition, mitochondria and LDs also form chains of alternating organelles in type I fibers of human skeletal muscle (38). When lipolysis is activated with resveratrol in hepatocytes, more mitochondria are observed to directly attach to LDs than those treated with vehicle (39). Notably, perilipin 5, a LD protein commonly found in oxidative tissues, plays an important role in linking LDs to mitochondria in cardiomyocytes and muscle tissues (40, 41). However, despite increased LD-mitochondria interactions in response to perilipin 5 overexpression, oxidation of hydrolyzed FAs is not altered (40). Thus, the importance of LD-mitochondria interactions, or the mechanism through which hydrolyzed FAs are oxidized, remains largely unknown.
Several studies have shown that L-FABP mediates the effects of exogenous FAs, especially polyunsaturated FAs, on PPAR-α gene expression and activity (17, 42, 43). L-FABP physically interacts with PPAR-α and it is thought that this interaction facilitates transfer of FA ligands (18). Whether L-FABP is critical in regulating activation of PPAR-α by ATGL-hydrolyzed FAs is unknown. Herein, we reveal that L-FABP ablation does not mediate the effects of ATGL on PPAR-α target gene expression in comparison with WT mice, suggesting that L-FABP is not a major transporter of ATGL-hydrolyzed FAs to the nucleus. Moreover, ATGL overexpression or knockdown and L-FABP ablation did not influence FA levels in the liver. These data imply that additional factors beyond FABP and intracellular TG hydrolysis control intracellular FA levels, and that perhaps ATGL and L-FABP regulate distinct pools of FAs that are differentially channeled.
The finding that L-FABP ablation does not mediate the effects of ATGL on PPAR-α target gene expression is suggestive of a more complex mechanism linking lipolysis to transcriptional regulation. These data are in agreement with a previous study from our laboratory showing that administration of fenofibrate, a PPAR-α agonist, is unable to rescue PPAR-α target gene expression or normalize hepatic TG levels (1). In contrast, administration of Wy-14643 to ATGL KO mice rescues their cardiomyopathy and normalizes cardiac oxidative gene expression (6). It is unclear if these discrepancies between the two studies are due to differences between PPAR-α agonists or the target tissues evaluated. A recent study showed that ATGL was required for lipolytic stimulation of adipocytes to activate PPAR-α reporter constructs that were generated by fusing the ligand binding domain of PPAR-α to the C-terminal LD binding domain of perilipin 1 so that PPAR-α was bound to LDs (44). However, the physiological significance of this model compared with the predominant nuclear localization of PPAR-α remains to be determined.
In summary, this study demonstrates that L-FABP, despite being a major FA carrier and an abundant cytosolic protein, is not necessary for the shuttling of FAs hydrolyzed by ATGL to the mitochondria. Furthermore, L-FABP plays no essential role in delivering ATGL-hydrolyzed FAs to the nucleus to modulate oxidative genes. These findings allude to a more complex mechanism linking ATGL to downstream changes in hepatic energy metabolism.
Acknowledgments
The authors express their gratitude to Katie Ress and Ellen Fischer for their excellent technical support.
Footnotes
Abbreviations:
- ACOT1
- acyl-CoA thioesterase 1
- ACSL1
- acyl-CoA synthetase 1
- Ad-ATGL
- adenovirus expressing adipose TG lipase
- Ad-GFP
- adenovirus expressing green fluorescent protein
- ASM
- acid-soluble metabolite
- ATGL
- adipose TG lipase
- BHBA
- β-hydroxybutyrate
- CPT1
- carnitine palmitoyltransferase 1
- LCAD
- long chain acyl-CoA dehydrogenase
- LD
- lipid droplet
- L-FABP
- liver FA binding protein
- PEPCK
- phosphoenolpyruvate carboxykinase
This work was supported by National Institutes of Health Grants DK090364 (D.G.M.); DK56260, DK52574, and HL38180 (N.O.D.); and DK050456 (Minnesota Obesity Center).
REFERENCES
- 1.Ong K. T., Mashek M. T., Bu S. Y., Greenberg A. S., Mashek D. G. 2011. Adipose triglyceride lipase is a major hepatic lipase that regulates triacylglycerol turnover and fatty acid signaling and partitioning. Hepatology. 53: 116–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wu J. W., Wang S. P., Alvarez F., Casavant S., Gauthier N., Abed L., Soni K. G., Yang G., Mitchell G. A. 2011. Deficiency of liver adipose triglyceride lipase in mice causes progressive hepatic steatosis. Hepatology. 54: 122–132. [DOI] [PubMed] [Google Scholar]
- 3.Reid B. N., Ables G. P., Otlivanchik O. A., Schoiswohl G., Zechner R., Blaner W. S., Goldberg I. J., Schwabe R. F., Chua S. C., Jr, Huang L-S. 2008. Hepatic overexpression of hormone-sensitive lipase and adipose triglyceride lipase promotes fatty acid oxidation, stimulates direct release of free fatty acids, and ameliorates steatosis. J. Biol. Chem. 283: 13087–13099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ahmadian M., Abbott M. J., Tang T., Hudak C. S. S., Kim Y., Bruss M., Hellerstein M. K., Lee H-Y., Samuel V. T., Shulman G. I., et al. 2011. Desnutrin/ATGL is regulated by AMPK and is required for a brown adipose phenotype. Cell Metab. 13: 739–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Obrowsky S., Chandak P. G., Patankar J. V., Povoden S., Schlager S., Kershaw E. E., Bogner-Strauss J. G., Hoefler G., Levak-Frank S., Kratky D. 2013. Adipose triglyceride lipase is a TG hydrolase of the small intestine and regulates intestinal PPARα signaling. J. Lipid Res. 54: 425–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Haemmerle G., Moustafa T., Woelkart G., Büttner S., Schmidt A., van de Weijer T., Hesselink M., Jaeger D., Kienesberger P. C., Zierler K., et al. 2011. ATGL-mediated fat catabolism regulates cardiac mitochondrial function via PPAR-α and PGC-1. Nat. Med. 17: 1076–1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Haemmerle G., Lass A., Zimmermann R., Gorkiewicz G., Meyer C., Rozman J., Heldmaier G., Maier R., Theussl C., Eder S., et al. 2006. Defective lipolysis and altered energy metabolism in mice lacking adipose triglyceride lipase. Science. 312: 734–737. [DOI] [PubMed] [Google Scholar]
- 8.Chakravarthy M. V., Lodhi I. J., Yin L., Malapaka R. R. V., Xu H. E., Turk J., Semenkovich C. F. 2009. Identification of a physiologically relevant endogenous ligand for PPARalpha in liver. Cell. 138: 476–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Forman B. M., Chen J., Evans R. M. 1997. Hypolipidemic drugs, polyunsaturated fatty acids, and eicosanoids are ligands for peroxisome proliferator-activated receptors alpha and delta. Proc. Natl. Acad. Sci. USA. 94: 4312–4317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kliewer S. A., Sundseth S. S., Jones S. A., Brown P. J., Wisely G. B., Koble C. S., Devchand P., Wahli W., Willson T. M., Lenhard J. M., et al. 1997. Fatty acids and eicosanoids regulate gene expression through direct interactions with peroxisome proliferator-activated receptors alpha and gamma. Proc. Natl. Acad. Sci. USA. 94: 4318–4323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zechner R., Zimmermann R., Eichmann T. O., Kohlwein S. D., Haemmerle G., Lass A., Madeo F. 2012. FAT SIGNALS–lipases and lipolysis in lipid metabolism and signaling. Cell Metab. 15: 279–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Atshaves B. P., Martin G. G., Hostetler H. A., McIntosh A. L., Kier A. B., Schroeder F. 2010. Liver fatty acid-binding protein and obesity. J. Nutr. Biochem. 21: 1015–1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bordewick U., Heese M., Börchers T., Robenek H., Spener F. 1989. Compartmentation of hepatic fatty-acid-binding protein in liver cells and its effect on microsomal phosphatidic acid biosynthesis. Biol. Chem. Hoppe Seyler. 370: 229–238. [DOI] [PubMed] [Google Scholar]
- 14.Bhuiyan A. K., Pande S. V. 1994. Carnitine palmitoyltransferase activities: effects of serum albumin, acyl-CoA binding protein and fatty acid binding protein. Mol. Cell. Biochem. 139: 109–116. [DOI] [PubMed] [Google Scholar]
- 15.Woldegiorgis G., Bremer J., Shrago E. 1985. Substrate inhibition of carnitine palmitoyltransferase by palmitoyl-CoA and activation by phospholipids and proteins. Biochim. Biophys. Acta. 837: 135–140. [DOI] [PubMed] [Google Scholar]
- 16.Erol E., Kumar L. S., Cline G. W., Shulman G. I., Kelly D. P., Binas B. 2004. Liver fatty acid binding protein is required for high rates of hepatic fatty acid oxidation but not for the action of PPARalpha in fasting mice. FASEB J. 18: 347–349. [DOI] [PubMed] [Google Scholar]
- 17.Wolfrum C., Borrmann C. M., Borchers T., Spener F. 2001. Fatty acids and hypolipidemic drugs regulate peroxisome proliferator-activated receptors alpha - and gamma-mediated gene expression via liver fatty acid binding protein: a signaling path to the nucleus. Proc. Natl. Acad. Sci. USA. 98: 2323–2328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hostetler H. A., McIntosh A. L., Atshaves B. P., Storey S. M., Payne H. R., Kier A. B., Schroeder F. 2009. L-FABP directly interacts with PPARalpha in cultured primary hepatocytes. J. Lipid Res. 50: 1663–1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tan N-S., Shaw N. S., Vinckenbosch N., Liu P., Yasmin R., Desvergne B., Wahli W., Noy N. 2002. Selective cooperation between fatty acid binding proteins and peroxisome proliferator-activated receptors in regulating transcription. Mol. Cell. Biol. 22: 5114–5127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McIntosh A. L., Atshaves B. P., Landrock D., Landrock K. K., Martin G. G., Storey S. M., Kier A. B., Schroeder F. 2013. Liver fatty acid binding protein gene-ablation exacerbates weight gain in high-fat fed female mice. Lipids. 48: 435–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Newberry E. P., Kennedy S. M., Xie Y., Luo J., Crooke R. M., Graham M. J., Fu J., Piomelli D., Davidson N. O. 2012. Decreased body weight and hepatic steatosis with altered fatty acid ethanolamide metabolism in aged L-Fabp -/- mice. J. Lipid Res. 53: 744–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Newberry E. P., Xie Y., Kennedy S., Han X., Buhman K. K., Luo J., Gross R. W., Davidson N. O. 2003. Decreased hepatic triglyceride accumulation and altered fatty acid uptake in mice with deletion of the liver fatty acid-binding protein gene. J. Biol. Chem. 278: 51664–51672. [DOI] [PubMed] [Google Scholar]
- 23.Miyoshi H., Perfield J. W., 2nd, Souza S. C., Shen W-J., Zhang H-H., Stancheva Z. S., Kraemer F. B., Obin M. S., Greenberg A. S. 2007. Control of adipose triglyceride lipase action by serine 517 of perilipin A globally regulates protein kinase A-stimulated lipolysis in adipocytes. J. Biol. Chem. 282: 996–1002. [DOI] [PubMed] [Google Scholar]
- 24.Folch J., Lees M., Stanley G. H. S. 1957. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 226: 497–509. [PubMed] [Google Scholar]
- 25.Martin G. G., Danneberg H., Kumar L. S., Atshaves B. P., Erol E., Bader M., Schroeder F., Binas B. 2003. Decreased liver fatty acid binding capacity and altered liver lipid distribution in mice lacking the liver fatty acid-binding protein gene. J. Biol. Chem. 278: 21429–21438. [DOI] [PubMed] [Google Scholar]
- 26.Hertzel A. V., Hellberg K., Reynolds J. M., Kruse A. C., Juhlmann B. E., Smith A. J., Sanders M. A., Ohlendorf D. H., Suttles J., Bernlohr D. A. 2009. Identification and characterization of a small molecule inhibitor of fatty acid binding proteins. J. Med. Chem. 52: 6024–6031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Newberry E. P., Kennedy S. M., Xie Y., Sternard B. T., Luo J., Davidson N. O. 2008. Diet-induced obesity and hepatic steatosis in L-Fabp / mice is abrogated with SF, but not PUFA, feeding and attenuated after cholesterol supplementation. Am. J. Physiol. Gastrointest. Liver Physiol. 294: G307–G314. [DOI] [PubMed] [Google Scholar]
- 28.Newberry E. P., Xie Y., Kennedy S. M., Luo J., Davidson N. O. 2006. Protection against Western diet-induced obesity and hepatic steatosis in liver fatty acid-binding protein knockout mice. Hepatology. 44: 1191–1205. [DOI] [PubMed] [Google Scholar]
- 29.Zschiesche W., Kleine A. H., Spitzer E., Veerkamp J. H., Glatz J. F. 1995. Histochemical localization of heart-type fatty-acid binding protein in human and murine tissues. Histochem. Cell Biol. 103: 147–156. [DOI] [PubMed] [Google Scholar]
- 30.Cohen A. W., Razani B., Schubert W., Williams T. M., Wang X. B., Iyengar P., Brasaemle D. L., Scherer P. E., Lisanti M. P. 2004. Role of caveolin-1 in the modulation of lipolysis and lipid droplet formation. Diabetes. 53: 1261–1270. [DOI] [PubMed] [Google Scholar]
- 31.Novikoff A. B., Novikoff P. M., Rosen O. M., Rubin C. S. 1980. Organelle relationships in cultured 3T3-L1 preadipocytes. J. Cell Biol. 87: 180–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Blanchette-Mackie E. J., Dwyer N. K., Barber T., Coxey R. A., Takeda T., Rondinone C. M., Theodorakis J. L., Greenberg A. S., Londos C. 1995. Perilipin is located on the surface layer of intracellular lipid droplets in adipocytes. J. Lipid Res. 36: 1211–1226. [PubMed] [Google Scholar]
- 33.Blanchette-Mackie E. J., Scow R. O. 1983. Movement of lipolytic products to mitochondria in brown adipose tissue of young rats: an electron microscope study. J. Lipid Res. 24: 229–244. [PubMed] [Google Scholar]
- 34.Jacob S. 1987. Lipid droplet accumulation in the heart during fasting. Acta Histochem. 82: 149–152. [DOI] [PubMed] [Google Scholar]
- 35.Vilaró S., Llobera M. 1988. Uptake and metabolism of Intralipid by rat liver: an electron-microscopic study. J. Nutr. 118: 932–940. [DOI] [PubMed] [Google Scholar]
- 36.Vock R., Hoppeler H., Claassen H., Wu D. X., Billeter R., Weber J. M., Taylor C. R., Weibel E. R. 1996. Design of the oxygen and substrate pathways. VI. Structural basis of intracellular substrate supply to mitochondria in muscle cells. J. Exp. Biol. 199: 1689–1697. [DOI] [PubMed] [Google Scholar]
- 37.Tarnopolsky M. A., Rennie C. D., Robertshaw H. A., Fedak-Tarnopolsky S. N., Devries M. C., Hamadeh M. J. 2007. Influence of endurance exercise training and sex on intramyocellular lipid and mitochondrial ultrastructure, substrate use, and mitochondrial enzyme activity. Am. J. Physiol. Regul. Integr. Comp. Physiol. 292: R1271–R1278. [DOI] [PubMed] [Google Scholar]
- 38.Shaw C. S., Jones D. A., Wagenmakers A. J. M. 2008. Network distribution of mitochondria and lipid droplets in human muscle fibres. Histochem. Cell Biol. 129: 65–72. [DOI] [PubMed] [Google Scholar]
- 39.Shiozaki M., Hayakawa N., Shibata M., Koike M., Uchiyama Y., Gotow T. 2011. Closer association of mitochondria with lipid droplets in hepatocytes and activation of Kupffer cells in resveratrol-treated senescence-accelerated mice. Histochem. Cell Biol. 136: 475–489. [DOI] [PubMed] [Google Scholar]
- 40.Wang H., Sreenevasan U., Hu H., Saladino A., Polster B. M., Lund L. M., Gong D., Stanley W. C., Sztalryd C. 2011. Perilipin 5, a lipid droplet-associated protein, provides physical and metabolic linkage to mitochondria. J. Lipid Res. 52: 2159–2168. [Erratum. 2013. J. Lipid Res. 54: 3539.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bosma M., Minnaard R., Sparks L. M., Schaart G., Losen M., de Baets M. H., Duimel H., Kersten S., Bickel P. E., Schrauwen P., et al. 2012. The lipid droplet coat protein perilipin 5 also localizes to muscle mitochondria. Histochem. Cell Biol. 137: 205–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wolfrum C., Börchers T., Sacchettini J. C., Spener F. 2000. Binding of fatty acids and peroxisome proliferators to orthologous fatty acid binding proteins from human, murine, and bovine liver. Biochemistry. 39: 1469–1474. [DOI] [PubMed] [Google Scholar]
- 43.Petrescu A. D., Huang H., Martin G. G., McIntosh A. L., Storey S. M., Landrock D., Kier A. B., Schroeder F. 2013. Impact of L-FABP and glucose on polyunsaturated fatty acid induction of PPARα-regulated β-oxidative enzymes. Am. J. Physiol. Gastrointest. Liver Physiol. 304: G241–G256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mottillo E. P., Bloch A. E., Leff T., Granneman J. G. 2012. Lipolytic products activate peroxisome proliferator-activated receptor (PPAR) α and δ in brown adipocytes to match fatty acid oxidation with supply. J. Biol. Chem. 287: 25038–25048. [DOI] [PMC free article] [PubMed] [Google Scholar]