Abstract
Because apoB-containing lipoproteins are pro-atherogenic and their secretion by liver and intestine largely depends on microsomal triglyceride transfer protein (MTP) activity, MTP inhibition strategies are actively pursued. How decreasing the secretion of apoB-containing lipoproteins affects intracellular rerouting of cholesterol is unclear. Therefore, the aim of the present study was to determine the effects of reducing either systemic or liver-specific MTP activity on cholesterol metabolism and reverse cholesterol transport (RCT) using a pharmacological MTP inhibitor or a genetic model, respectively. Plasma total cholesterol and triglyceride levels were decreased in both MTP inhibitor-treated and liver-specific MTP knockout (L-Mttp−/−) mice (each P < 0.001). With both inhibition approaches, hepatic cholesterol as well as triglyceride content was consistently increased (each P < 0.001), while biliary cholesterol and bile acid secretion remained unchanged. A small but significant decrease in fecal bile acid excretion was observed in inhibitor-treated mice (P < 0.05), whereas fecal neutral sterol excretion was substantially increased by 75% (P < 0.001), conceivably due to decreased intestinal absorption. In contrast, in L-Mttp−/− mice both fecal neutral sterol and bile acid excretion remained unchanged. However, while total RCT increased in inhibitor-treated mice (P < 0.01), it surprisingly decreased in L-Mttp−/− mice (P < 0.05). These data demonstrate that: i) pharmacological MTP inhibition increases RCT, an effect that might provide additional clinical benefit of MTP inhibitors; and ii) decreasing hepatic MTP decreases RCT, pointing toward a potential contribution of hepatocyte-derived VLDLs to RCT.
Keywords: bile, high density lipoprotein, lipoprotein metabolism, liver metabolism, very low density lipoprotein, transintestinal cholesterol excretion, microsomal triglyceride transfer protein, reverse cholesterol transport
Cholesterol within apoB-containing lipoproteins is a major risk factor for the development of atherosclerotic CVD (1, 2). Plasma levels of apoB-containing lipoproteins are determined by hepatic production in the form of VLDLs and intestinal production of chylomicrons, in which cholesterol absorbed from the diet is packaged (3–5). For the assembly of both forms of apoB-containing lipoproteins, microsomal triglyceride transfer protein (MTP) expression is essential (4, 5). This is exemplified by reduced secretion of apoB-containing lipoproteins and the subsequent accumulation of hepatic triglycerides when MTP is absent in rodent models (6–9) or in human abetalipoproteinemia, a genetic disease characterized by MTP deficiency (10). On the other hand, overexpression of MTP increases secretion of apoB-containing lipoproteins (11, 12). Deletion of MTP, specifically in the intestine, was shown to reduce plasma cholesterol levels by about 45–50% (13, 14), illustrating the contribution of the intestine to systemic lipoprotein metabolism. The key role of MTP in determining circulating levels of apoB-containing lipoproteins and their importance for atherosclerotic CVD resulted in the development of systemically active pharmacological inhibitors that have recently entered phase 3 clinical trials (15, 16). Although a variable increase in hepatic triglyceride content was noticed, MTP inhibitors seem to have a reasonable safety profile and thus represent a viable therapeutic alternative to substantially decrease plasma levels of apoB-containing lipoproteins in patients not sufficiently responding to statin therapy (15). In addition, antisense oligonucleotides have been developed that specifically target hepatic MTP expression and thereby lower, at least in mouse models, circulating levels of pro-atherogenic apoB-containing lipoproteins (17).
HDLs, on the other hand, protect against atherosclerotic CVD (18). An established function of HDLs is to mediate reverse cholesterol transport (RCT), a pathway in which cholesterol from atherosclerotic lesions is transported back to the liver (19, 20). HDL cholesterol taken up into the liver can then follow different metabolic routes. In hepatocytes, it can either be i) used for conversion into bile acids (21), ii) secreted directly into the bile (21), iii) resecreted within newly formed HDL particles via the action of ABCA1 (22, 23), or iv) resecreted as part of VLDLs (24). The final step of RCT is the excretion of sterols, either bile acids or cholesterol, from the body via the feces, and several studies have indicated that inhibiting intestinal sterol uptake increases RCT (25–27). How pharmacological MTP inhibition or a decrease in hepatic MTP expression, clinically relevant interventions currently pursued, affect the rerouting of cholesterol between different compartments and pathways has not been investigated.
Therefore, the aim of the present study was to determine the effects of decreasing either systemic or hepatocyte-specific MTP activity on cholesterol metabolism and RCT. Our data demonstrate that pharmacological MTP inhibition is beneficial by increasing RCT, whereas liver-specific genetic MTP ablation decreases RCT. These data suggest that the metabolic effects of systemic MTP inhibition could be mainly mediated by impacting intestinal cholesterol handling. In a clinical perspective, our results indicate that MTP inhibition strategies that specifically target or at least include the intestinal compartment might be favorable.
MATERIALS AND METHODS
Experimental animals and treatments
All mice were housed in animal rooms with alternating 12 h periods of light (from 7:00 AM to 7:00 PM) and dark (from 7:00 PM to 7:00 AM) with ad libitum access to water and mouse chow diet (Arie Blok, Woerden, The Netherlands). Animal experiments were performed in accordance with national laws. All protocols were approved by the responsible ethics committee. C57BL/6J wild-type mice were obtained from Charles River (L'Arbresle, France). These mice were treated with the MTP inhibitor, BMS-212122 (kindly provided by Dr. David Gordon, Bristol-Myers Squibb), at a daily oral dose of 1 mg/kg/day for 2 weeks as described, resulting in a 63% decrease in hepatic MTP activity and an 86% decrease in intestinal MTP activity, while hepatic and intestinal MTP protein expression remained unchanged (supplementary Fig. I). As a consequence, hepatic VLDL as well as intestinal chylomicron production were virtually abolished (supplementary Fig. II). Liver-specific MTP knockout mice (L-Mttp−/−) were obtained by crossing floxed MTP knockout mice (6) with transgenic mice expressing Cre recombinase under the control of the hepatocyte-specific albumin promoter (Jackson Laboratory, Bar Harbor, ME; all on the C57BL/6J background) (28). Littermate controls not expressing Cre were used for all experiments. In L-Mttp−/− mice, no hepatic MTP protein expression was detectable and liver MTP activity was substantially lower, while intestinal MTP protein expression and activity remained unaltered (supplementary Fig. I).
Analysis of plasma lipids and lipoproteins
Mice were fasted for 4 h and blood was obtained by cardiac puncture under anesthesia. Aliquots of plasma were stored at −80°C until analysis. Commercially available reagents were used to measure plasma total cholesterol, triglycerides (Roche Diagnostics, Mannheim, Germany), and free cholesterol (Diasys, Holzheim, Germany). Pooled plasma samples were subjected to fast protein liquid chromatography (FPLC) gel filtration using a Superose 6 column (GE Healthcare, Little Chalfont, UK) as described previously (29). Individual fractions were assayed for cholesterol and triglyceride concentrations as detailed above.
Analysis of liver lipid composition
Liver tissue was homogenized and lipids were extracted according to the general procedure of Bligh and Dyer and redissolved in water containing 2% Triton X-100 (Sigma-Aldrich, Steinheim, Germany) (22). Total cholesterol, free cholesterol, and triglycerides were assayed as described above.
Bile collection and composition analysis
The gallbladder was cannulated under Hypnorm (fentanyl/fluanisone; 1 ml/kg body weight) and diazepam (10 mg/kg body weight) anesthesia, bile was collected for 30 min and production was determined gravimetrically. A humidified incubator was used to maintain body temperature. Biliary cholesterol, phospholipid, and bile acid concentrations were determined and the respective biliary secretion rates were calculated as described (30).
Analysis of fecal neutral sterol and bile acid secretion
Feces of animals individually housed in metabolic cages were collected over a 24 h period. Fecal samples were dried, weighed, and ground. Neutral sterol and bile acid composition was determined in a 50 mg fecal aliquot by gas liquid chromatography using a published protocol (22).
Cholesterol absorption studies
Fractional cholesterol absorption was determined as described before with an adapted plasma dual isotope ratio method using blood samples obtained at 0 and 72 h after intravenous (D7) and oral (D5) administration of stable isotopically labeled cholesterol (31).
Analysis of hepatic gene expression by real-time PCR
Total RNA from mouse livers and small intestines was isolated using TRIzol reagent (Invitrogen, Carlsbad, CA) and quantified using a NanoDrop 2000c UV-vis spectrophotometer (Thermo Scientific, Wilmington, DE) as described before (29). cDNA synthesis from 1 μg of total RNA was performed using reagents from Invitrogen. Real-time quantitative PCR was performed on a 7900HT fast real-time PCR system (Applied Biosystems, Foster City, CA). PCR primers and fluorogenic probes were designed with Primer Express software (Applied Biosystems) and synthesized by Eurogentec (Seraing, Belgium). mRNA expression levels were calculated relative to the average of the housekeeping gene, cyclophilin, and further normalized to the expression levels of the respective controls.
In vivo macrophage-to-feces RCT studies
Experiments were performed as described before using thioglycollate-elicited primary peritoneal macrophages from C57BL/6J donor mice loaded in vitro with 50 μg/ml acetylated LDL and 3 μCi/ml [3H]cholesterol (Perkin Elmer, Boston, MA) for 24 h to become foam cells (29). These were injected intraperitoneally into individually housed recipient mice. Counts in plasma collected 6, 24, and 48 h after macrophage injection were assessed directly by liquid scintillation counting (Packard 1600CA Tri-carb; Packard, Meriden, CT). Tracer uptake into the liver was determined at 48 h after injection following solubilization of the tissue (Solvable; Packard) and radioactivity was determined by liquid scintillation counting (32). Feces were collected over a 48 h period, dried, weighed, and ground. Aliquots were separated into neutral sterol and bile acid fractions (22), and radioactivity was determined by liquid scintillation counting. Counts were expressed relative to the administered dose.
Statistics
Statistical analyses were performed using GraphPad Prism (GraphPad Software, San Diego, CA). Data are presented as means ± SEM. Statistical differences between two groups were assessed using the Mann-Whitney U test. P values below 0.05 were considered statistically significant.
RESULTS
Systemic MTP inhibition decreases plasma triglycerides by lowering hepatic and intestinal production of apoB-containing lipoproteins
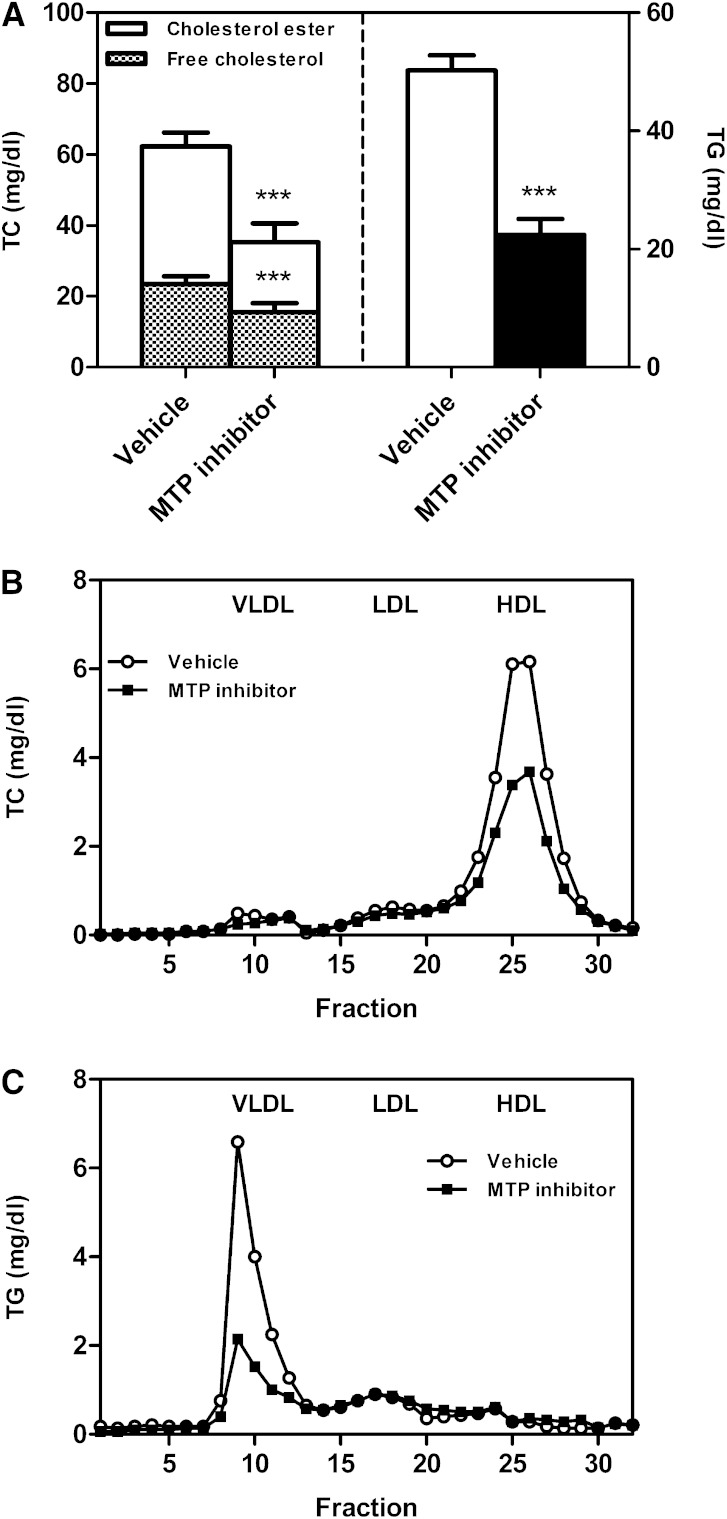
To determine the effect of pharmacological inhibition of MTP activity, C57BL/6J wild-type mice on chow diet were treated with the MTP inhibitor BMS-212122 for 2 weeks and compared with vehicle-treated controls. Plasma total cholesterol as well as triglyceride levels were decreased in MTP inhibitor-treated mice (each P < 0.001, Fig. 1A). Both free cholesterol and cholesterol esters contributed to the decrease of total cholesterol (each P < 0.001, Fig. 1A). FPLC profiles indicated a decrease in HDL cholesterol (Fig. 1B) and in VLDL triglycerides (Fig. 1C), attributable to decreased hepatic VLDL and intestinal chylomicron production as a result of MTP inhibition (supplementary Fig. I).
Fig. 1.
Impact of systemic MTP inhibition on plasma lipids. After 2 weeks of MTP inhibitor treatment mice were fasted for 4 h and a blood sample was taken. A: Plasma total cholesterol and triglyceride levels. Data are presented as means ± SEM. Statistically significant differences from control mice are indicated as ***P < 0.001. FPLC profiles of pooled plasma samples for the distribution of total cholesterol (B) and triglycerides (C) over the different lipoprotein subclasses as indicated. At least n = 6 for each group.
Systemic MTP inhibition does not impact biliary sterol secretion but increases fecal neutral sterol excretion by decreasing cholesterol absorption
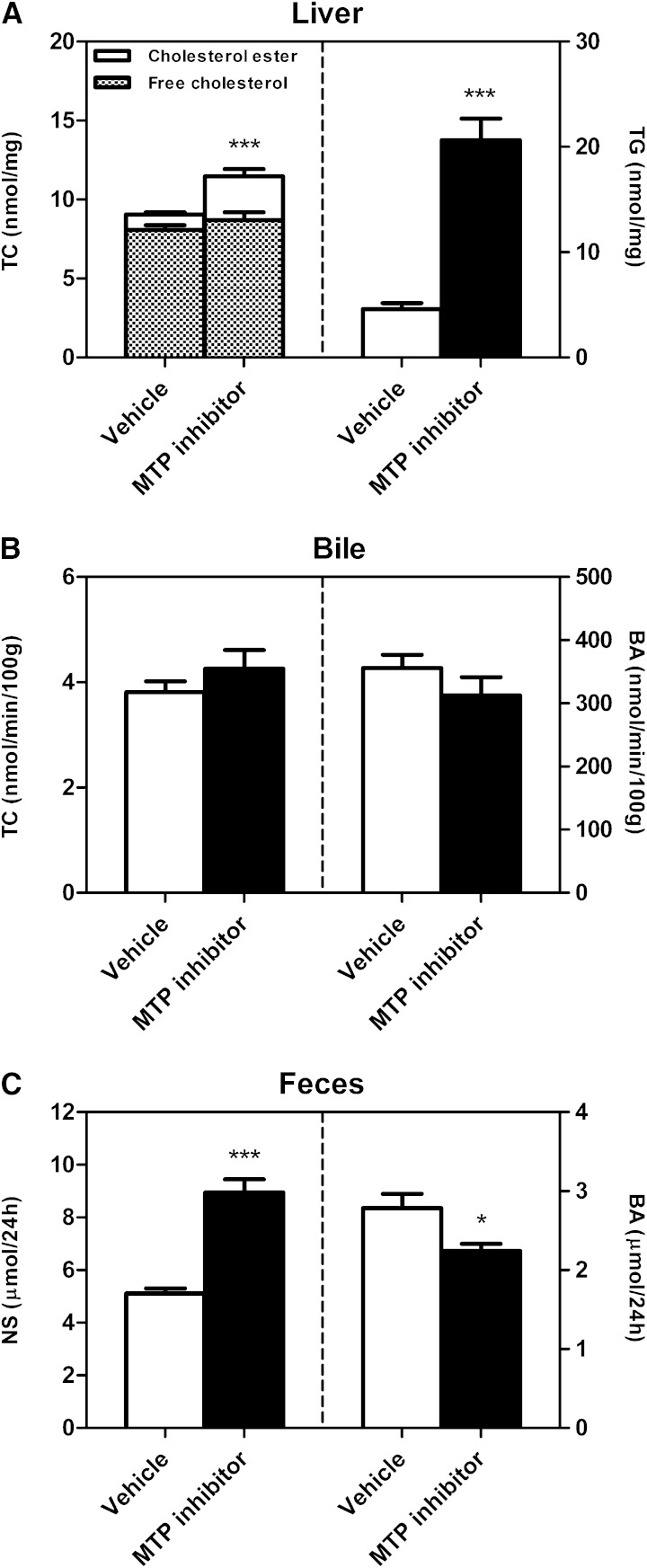
Body weight and liver weight were unchanged upon MTP inhibitor treatment (data not shown). Liver total and esterified cholesterol, as well as triglyceride content, were increased in mice receiving MTP inhibitor compared with controls (each P < 0.001, Fig. 2A). There was a tendency toward an increase in free cholesterol (Fig. 2A). Hepatic expression of the SREBP2 target genes, HMG-CoAR (P < 0.001, Table 1) and LDLR (P < 0.05, Table 1), were significantly lower in MTP inhibitor-treated mice, likely as a consequence of increased hepatic cholesterol accumulation. To explore whether systemic MTP inhibition would also translate into changes in biliary sterol secretion, a 30 min continuous bile cannulation experiment was performed. Bile flow (data not shown), as well as biliary cholesterol, bile acid (Fig. 2B), and phospholipid secretion (data not shown) remained unchanged compared with controls. Next, the impact of MTP inhibition on fecal sterol excretion was investigated. A small decrease in fecal bile acid excretion was observed (P < 0.05, Fig. 2C), mainly in ω-muricholic, α-muricholic, and deoxycholic acid (Table 2). Fecal neutral sterol excretion, on the other hand, was significantly increased by 75% (P < 0.001; Fig. 2C, Table 2). This latter finding is likely attributable to a decrease in intestinal cholesterol absorption, because intestinal mRNA expression of NPC1L1 was 50% lower in mice receiving MTP inhibitor (Table 1), and patients with abetalipoproteinemia lacking intestinal MTP (4, 10) as well as intestine-specific MTP knockout mice exhibit decreased intestinal lipid absorption (14, 33). Consistent with this hypothesis, subsequent determination of intestinal cholesterol absorption confirmed a 46% decrease in the mice treated with the pharmacological MTP inhibitor (48.1 ± 4.0% vs. 25.9 ± 1.7%, P < 0.001).
Fig. 2.
Impact of systemic MTP inhibition on liver lipids and biliary as well as fecal sterol excretion. A: Hepatic contents of total cholesterol, free cholesterol, cholesterol esters, and triglycerides. After 2 weeks of MTP inhibitor treatment mice were fasted for 4 h, the liver was excised, and cholesterol as well as triglyceride contents were determined as detailed in Materials and Methods. B: Biliary secretion rates of total cholesterol and bile acids. Continuous bile cannulation experiments for 30 min were performed after 2 weeks of inhibitor treatment as described in Materials and Methods. C: Mass fecal neutral sterol and bile acid excretion levels. After 2 weeks of inhibitor treatment feces were collected for a 24 h period, processed, and analyzed as detailed in Materials and Methods. Data are presented as means ± SEM, at least n = 6 for each group. Statistically significant differences from control mice are indicated as *P < 0.05, ***P < 0.001.
TABLE 1.
Hepatic and proximal intestinal mRNA expression in vehicle- versus MTP inhibitor-treated wild-type mice and wild-type versus L-Mttp−/− mice
| Vehicle | MTP Inhibitor | Wild-type | L-Mttp−/− | |
| Hepatic mRNA expression | ||||
| Mtp | 1.00 ± 0.04 | 0.96 ± 0.01 | 1.00 ± 0.06 | 0.13 ± 0.09c |
| Hmgcoar | 1.00 ± 0.06 | 0.62 ± 0.05c | 1.00 ± 0.12 | 0.35 ± 0.06c |
| Abca1 | 1.00 ± 0.08 | 0.99 ± 0.02 | 1.00 ± 0.06 | 0.88 ± 0.07 |
| Sr-b1 | 1.00 ± 0.04 | 1.08 ± 0.01 | 1.00 ± 0.07 | 0.79 ± 0.05a |
| Ldlr | 1.00 ± 0.05 | 0.78 ± 0.01a | 1.00 ± 0.07 | 0.65 ± 0.06b |
| Small intestine mRNA expression | ||||
| Mtp | 1.00 ± 0.06 | 1.21 ± 0.05 | 1.00 ± 0.05 | 0.74 ± 0.05a |
| Abca1 | 1.00 ± 0.21 | 0.63 ± 0.03 | 1.00 ± 0.18 | 0.61 ± 0.16 |
| Abcg5 | 1.00 ± 0.09 | 0.65 ± 0.03b | 1.00 ± 0.06 | 0.86 ± 0.12 |
| Abcg8 | 1.00 ± 0.09 | 0.67 ± 0.03b | 1.00 ± 0.08 | 0.98 ± 0.13 |
| Npc1l1 | 1.00 ± 0.13 | 0.51 ± 0.03b | 1.00 ± 0.13 | 1.24 ± 0.11 |
| Ldlr | 1.00 ± 0.10 | 1.01 ± 0.18 | 1.00 ± 0.11 | 0.95 ± 0.08 |
Mice were fasted for 4 h and livers were excised, weighed, and stored at −80°C until analysis. Individual genes are expressed as a percentage of the housekeeping gene, cyclophilin, and further normalized to the expression levels of the respective controls. Data are presented as means ± SEM, at least n = 6 for each group.
P < 0.05 versus control mice.
P < 0.01 versus control mice.
P < 0.001 versus control mice.
TABLE 2.
Fecal excretion of neutral sterol and bile acid species in vehicle- and MTP inhibitor-treated wild-type mice
| Vehicle | MTP Inhibitor | |
| Fecal output (g/24 h) | 0.85 ± 0.07 | 0.87 ± 0.07 |
| Neutral sterol species in feces | ||
| Cholesterol (nmol/24 h) | 3,799.20 ± 213.87 | 6,318.96 ± 469.60b |
| Dihydrocholesterol (nmol/24 h) | 385.76 ± 12.66 | 417.26 ± 17.62 |
| Coprostanol (nmol/24 h) | 930.97 ± 95.82 | 2,208.34 ± 490.14a |
| Bile acid species in feces | ||
| Allocholic acid (nmol/24 h) | 68.64 ± 7.02 | 59.97 ± 4.49 |
| α-Muricholic acid (nmol/24 h) | 153.77 ± 12.85 | 97.95 ±3.26b |
| β-Muricholic acid (nmol/24 h) | 206.10 ± 17.19 | 190.64 ± 7.24 |
| ω-Muricholic acid (nmol/24 h) | 894.72 ± 67.29 | 713.17 ± 42.57a |
| Cholic acid (nmol/24 h) | 199.34 ± 18.54 | 169.75 ± 16.01 |
| Deoxycholic acid (nmol/24 h) | 1,154.51 ± 90.40 | 934.82 ± 40.57a |
| Hyodeoxycholic acid (nmol/24 h) | 107.31 ± 7.81 | 74.76 ± 7.71a |
Feces were collected over a 24 h period. Data are presented as means ± SEM, at least n = 6 for each group.
P < 0.05 versus control mice.
P < 0.001 versus control mice.
Systemic MTP inhibition increases macrophage-to-feces RCT
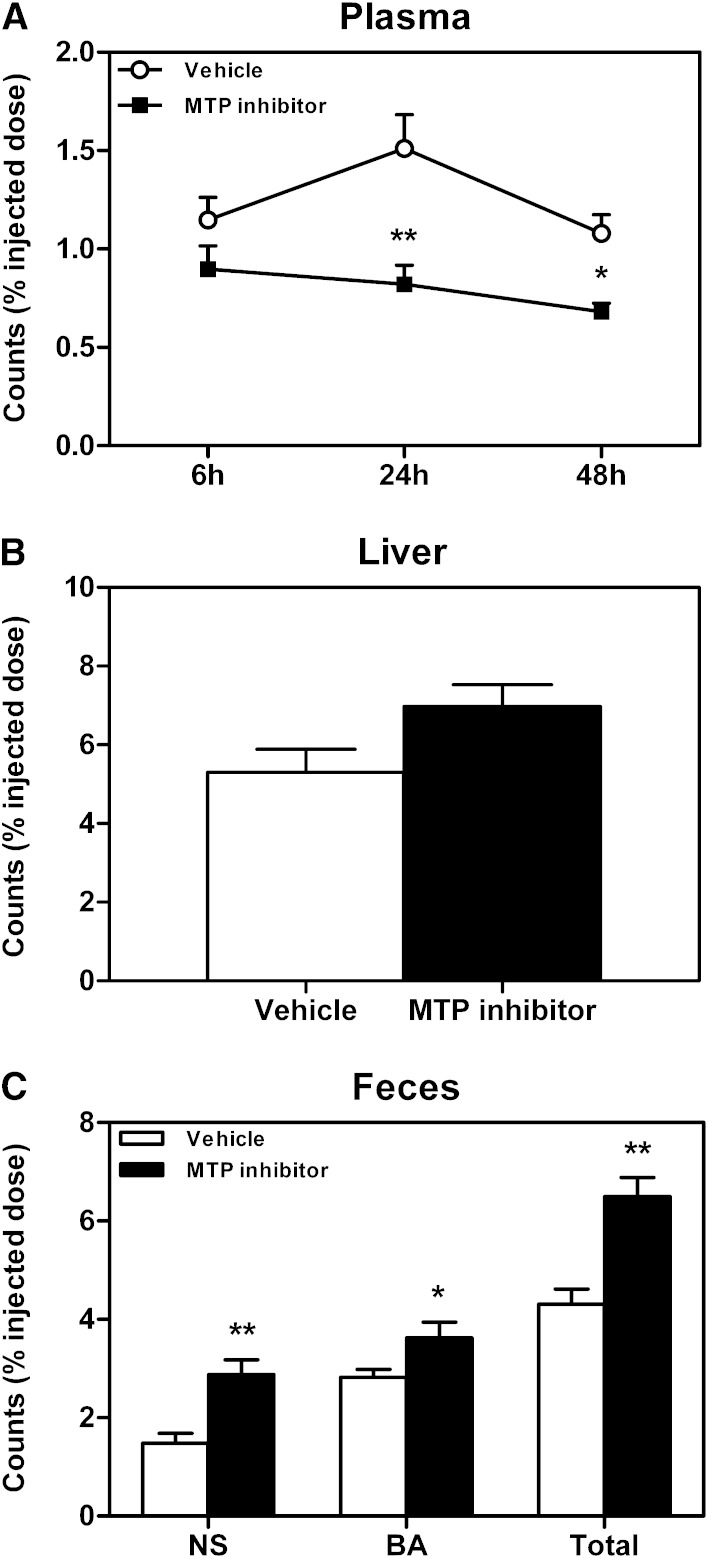
Next, a macrophage-to-feces RCT experiment was performed to determine whether the increase in mass fecal neutral sterol excretion upon systemic MTP inhibition would impact RCT. Plasma 3H-cholesterol levels were lower in MTP inhibitor-treated mice at 24 (P < 0.01) and 48 h (P < 0.05) after macrophage injection (Fig. 3A), while tracer recovery within the liver was not affected (Fig. 3B). However, systemic MTP inhibition significantly enhanced overall RCT, reflected by a higher fecal excretion of the macrophage-derived 3H-cholesterol tracer (P < 0.01, Fig. 3C). This increase was mainly attributable to a 2-fold higher excretion of 3H-cholesterol within the fecal neutral sterol fraction (P < 0.01, Fig. 3C), although tracer recovery within the fecal bile acid fraction was also significantly higher (P < 0.05, Fig. 3C).
Fig. 3.
Impact of systemic MTP inhibition on macrophage-to-feces RCT. Following 2 weeks of MTP inhibitor treatment, an in vivo macrophage-to-feces RCT experiment was performed as detailed in Materials and Methods. A: Cholesterol tracer appearance in plasma 6, 24, and 48 h after macrophage administration. B: Cholesterol tracer recovery within liver 48 h after macrophage injection. C: Cholesterol tracer appearance in feces collected continuously from 0 to 48 h after macrophage injection. Data are presented as means ± SEM, at least n = 6 for each group. Statistically significant differences from the control group are indicated as *P < 0.05, **P < 0.01.
Hepatocyte-specific MTP deficiency decreases plasma triglycerides
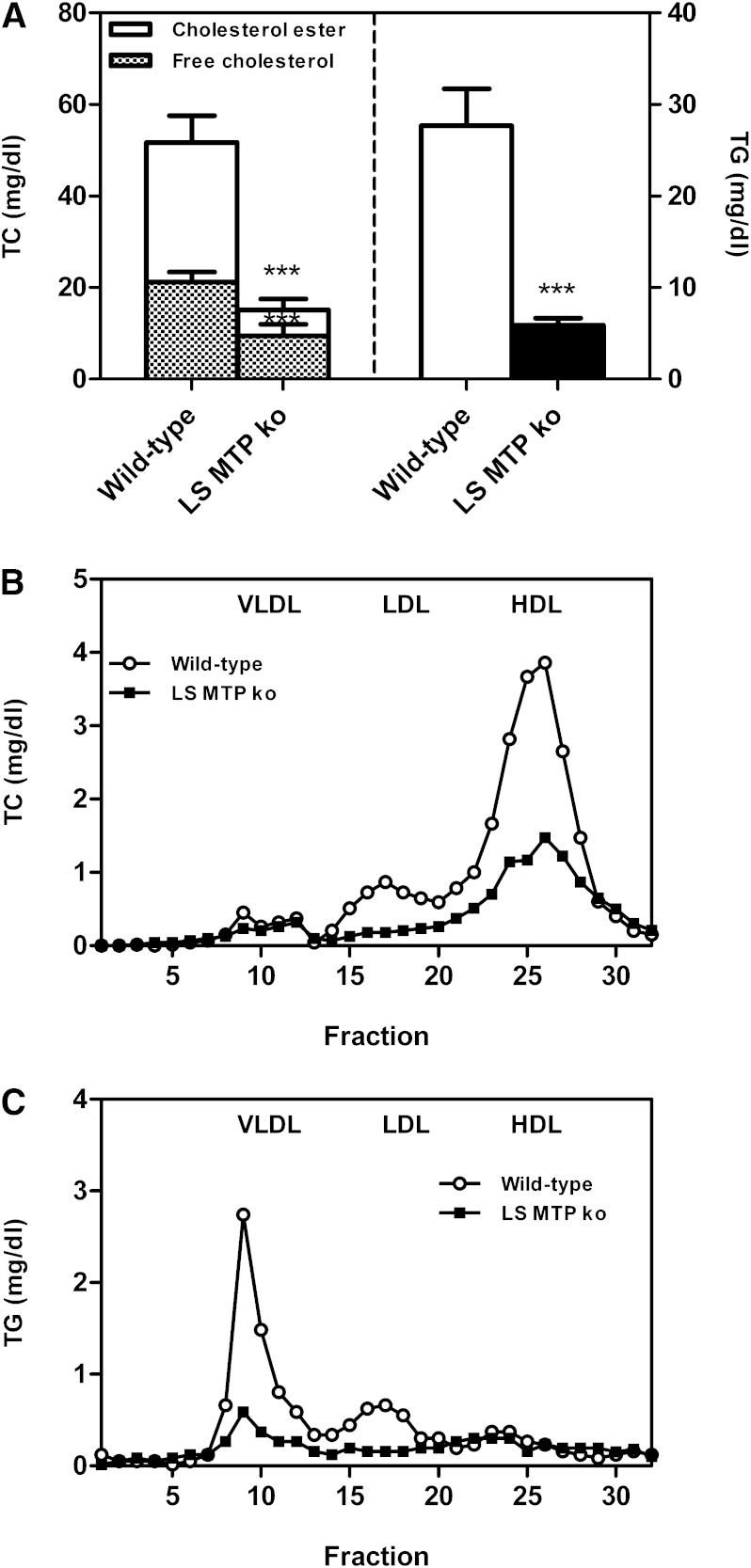
To determine whether the metabolic effects observed in mice treated with a systemic MTP inhibitor were attributable to inhibition of hepatic or intestinal MTP, a similar set of experiments was performed in L-Mttp−/− mice compared with control littermates. Plasma total cholesterol and triglyceride levels were significantly reduced in L-Mttp−/− mice (each P < 0.001, Fig. 4A). The decrease in total cholesterol was attributable to a decrease of both free cholesterol and cholesterol esters (each P < 0.001, Fig. 4A). FPLC analysis revealed lower VLDL, LDL, and HDL cholesterol levels in L-Mttp−/− mice (Fig. 4B), while triglycerides within the apoB-containing lipoproteins, VLDLs and LDLs, were substantially reduced (Fig. 4C).
Fig. 4.
Impact of hepatocyte-specific MTP deficiency (L-Mttp−/−) on plasma lipid levels and distribution. L-Mttp−/− mice were fasted for 4 h and a blood sample was taken. A: Plasma total cholesterol and triglyceride levels. Data are presented as means ± SEM. Statistically significant differences from control mice are indicated as ***P < 0.001. FPLC profiles of pooled plasma samples for the distribution of total cholesterol (B) and triglycerides (C) over the different lipoprotein subclasses as indicated. At least n = 6 for each group.
Hepatocyte-specific MTP deficiency does not alter intestinal cholesterol absorption and does not impact biliary or fecal sterol excretion
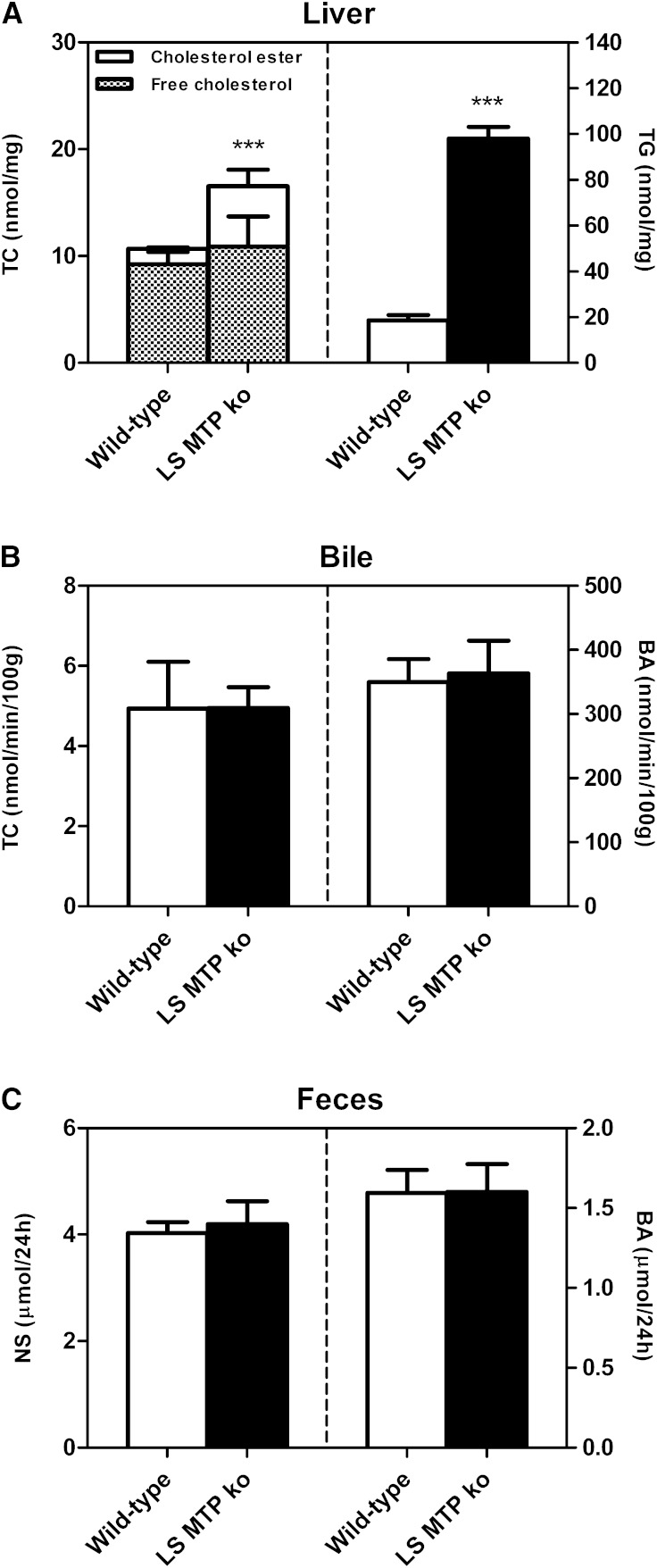
Body weight as well as liver weight of L-Mttp−/− mice was unchanged compared with wild-type controls (data not shown). Consistent with the results obtained in mice treated with the systemic MTP inhibitor, liver total cholesterol and cholesterol ester content was increased in L-Mttp−/− mice (P < 0.001, Fig. 5A). Also in the L-Mttp−/− mice, hepatic mRNA expression of the SREBP2 target genes, HMG-CoAR (P < 0.001, Table 1) and LDLR (P < 0.01, Table 1), was significantly lower, likely as a consequence of hepatic cholesterol accumulation. Hepatic triglyceride content was increased 5-fold (P < 0.001, Fig. 5A). Next, we performed a 30 min continuous bile cannulation experiment to determine the impact of hepatocyte-specific MTP deficiency on biliary secretion rates. Bile flow was unchanged compared with wild-type controls (data not shown), as were the biliary secretion rates of cholesterol (Fig. 5B), bile acids (Fig. 5B), and phospholipids (data not shown). In contrast to systemic MTP inhibition, eliminating MTP in the liver alone did not affect the fecal excretion of either neutral sterols or bile acids (Fig. 5C, Table 3). Consistent with these findings, in the L-Mttp−/− model, no changes in the intestinal expression of NPC1L1 were observed (Table 1) and also experimentally measured intestinal cholesterol absorption rates were identical in wild-type and L-Mttp−/− mice (52.2 ± 5.3% vs. 52.1 ± 5.5%, NS).
Fig. 5.
Impact of hepatocyte-specific MTP deficiency (L-Mttp−/−) on liver lipids and biliary as well as fecal sterol excretion. A: Hepatic contents of total cholesterol, free cholesterol, cholesterol esters, and triglycerides. L-Mttp−/− mice were fasted for 4 h, the liver was excised, and cholesterol as well as triglyceride contents were determined as detailed in Materials and Methods. B: Biliary secretion rates of total cholesterol and bile acids. Continuous bile cannulation experiments were performed for 30 min in L-Mttp−/− mice as described in Materials and Methods. C: Mass fecal neutral sterol and bile acid excretion levels. Feces from L-Mttp−/− mice were collected for a 24 h period, processed, and analyzed as detailed in Materials and Methods. Data are presented as means ± SEM, at least n = 6 for each group. Statistically significant differences from control mice are indicated as ***P < 0.001.
TABLE 3.
Fecal excretion of neutral sterol and bile acid species in wild-type and L-Mttp−/− mice
| Wild-type | L-Mttp−/− | |
| Fecal output (g/24 h) | 0.76 ± 0.07 | 0.74 ± 0.03 |
| Neutral sterol species in feces | ||
| Cholesterol (nmol/24 h) | 3,435.09 ± 167.02 | 3,739.75 ± 399.07 |
| Dihydrocholesterol (nmol/24 h) | 219.52 ± 15.57 | 241.32 ± 21.22 |
| Coprostanol (nmol/24 h) | 378.21 ± 145.86 | 213.99 ± 90.91 |
| Bile acid species in feces | ||
| Allocholic acid (nmol/24 h) | 23.41 ± 1.85 | 31.31 ± 5.64 |
| α-Muricholic acid (nmol/24 h) | n.d. | n.d. |
| β-Muricholic acid (nmol/24 h) | 218.83 ± 30.02 | 241.86 ± 53.44 |
| ω-Muricholic acid (nmol/24 h) | 552.03 ± 59.69 | 483.89 ± 69.23 |
| Cholic acid (nmol/24 h) | 138.43 ± 16.72 | 175.44 ± 34.75 |
| Deoxycholic acid (nmol/24 h) | 599.22 ± 46.52 | 624.25 ± 43.97 |
| Hyodeoxycholic acid (nmol/24 h) | 57.55 ± 5.17 | 41.07 ± 3.36a |
Feces were collected over a 24 h period. Data are presented as means ± SEM, at least n = 6 for each group. n.d., not detected.
P < 0.05 versus control mice.
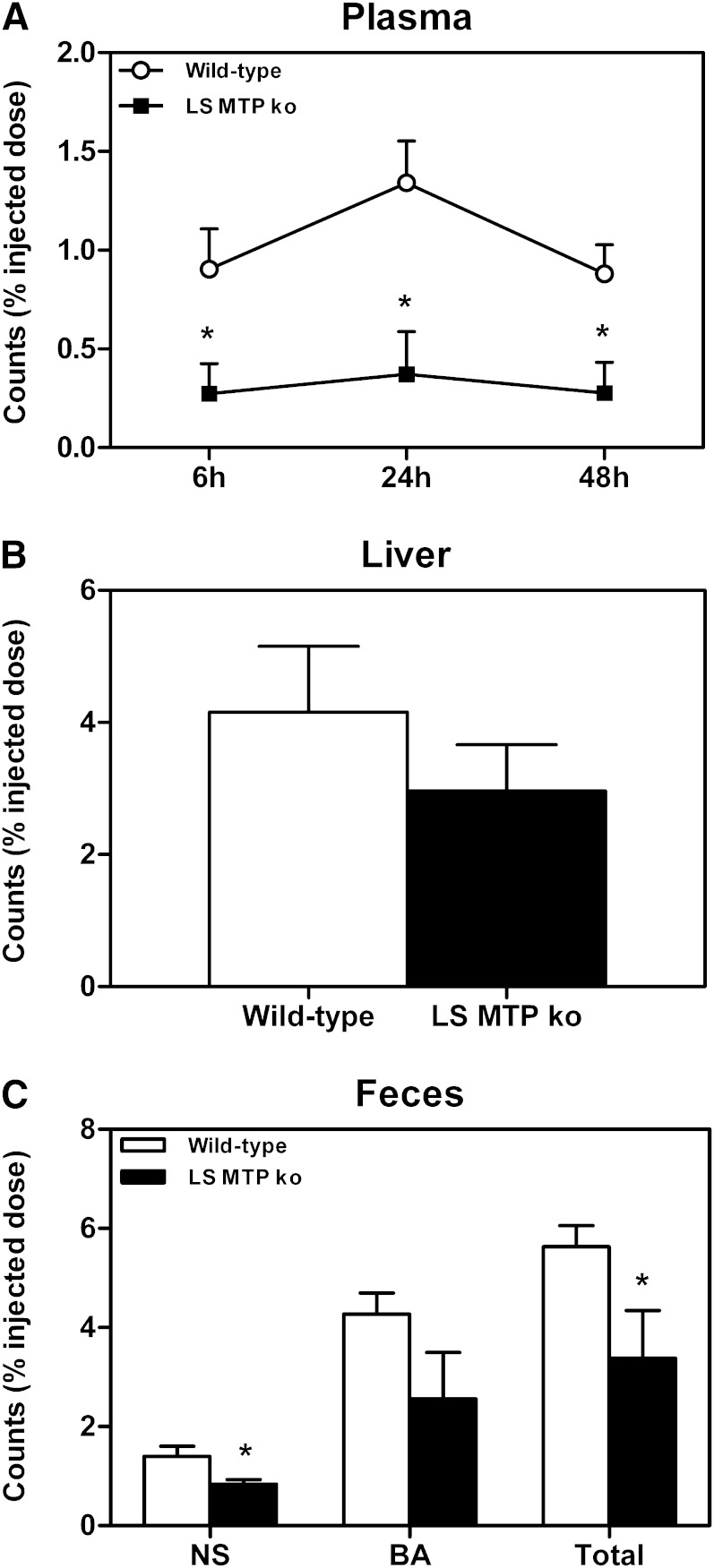
Hepatocyte-specific MTP deficiency decreases macrophage-to-feces RCT
Next, we investigated the contribution of hepatic MTP expression to the changes in RCT observed with the systemic MTP inhibitor and performed a macrophage-to-feces RCT experiment in the hepatocyte-specific MTP-deficient mice. Plasma 3H-cholesterol levels were lower in L-Mttp−/− mice at 6, 24, and 48 h after macrophage injection (each P < 0.05, Fig. 6A), while tracer recovery within the liver at 48 h after macrophage injection was unchanged (Fig. 6B). Most importantly, and in contrast to the results with systemic MTP inhibition, the total excretion of 3H-cholesterol originating from macrophages into the feces was decreased in L-Mttp−/− mice (P < 0.05, Fig. 6C). This was mainly attributable to a significantly lower recovery of the cholesterol tracer within the fecal neutral sterol fraction (P < 0.05, Fig. 6C), and there was a trend toward lower tracer recovery within the bile acid fraction as well (P = 0.12, Fig. 6C).
Fig. 6.
Impact of hepatocyte-specific MTP deficiency (L-Mttp−/−) on macrophage-to-feces RCT. An in vivo macrophage-to-feces RCT experiment was performed in L-Mttp−/− mice as detailed in Materials and Methods. A: Cholesterol tracer appearance in plasma 6, 24, and 48 h after macrophage administration. B: Cholesterol tracer recovery within liver 48 h after macrophage injection. C: Cholesterol tracer appearance in feces collected continuously from 0 to 48 h after macrophage injection. Data are presented as means ± SEM, at least n = 6 for each group. Statistically significant differences from the control group are indicated as *P < 0.05.
DISCUSSION
This study investigated the consequences of differentially lowering MTP activity systemically with a pharmacological inhibitor or genetically in the liver only on cholesterol metabolism, with a specific focus on RCT. Our data demonstrate that decreasing intestinal and hepatic secretion of apoB-containing lipoproteins via inhibition of MTP increases RCT, while the absence of hepatocyte MTP expression has the opposite effect. These results indicate, on the one hand, that hepatic secretion of RCT-relevant cholesterol within apoB-containing lipoproteins contributes to RCT and, on the other hand, that upon pharmacological MTP inhibition, the decrease in intestinal cholesterol absorption has a relatively larger effect on RCT than the decrease in VLDL production.
MTP expression in the intestine is essential for chylomicron formation (4, 5, 10). Genetic absence of intestinal MTP consequently results in malabsorption of lipids with all its adverse effects, such as deficiency in fat-soluble vitamins (4, 10). In the present study, a systemic but not a hepatocyte-specific deficiency of MTP activity resulted in decreased intestinal cholesterol absorption and increased fecal sterol excretion, suggesting that the effect of chemical MTP inhibition on RCT is mainly mediated by the intestine by decreasing cholesterol absorption. Similar effects on RCT have been observed previously with the use of the NPC1L1 inhibitor, ezetimibe (25–27). These previous studies consistently demonstrated that targeting intestinal sterol absorption, and thereby the last step of RCT, is an effective means of increasing the fecal excretion of macrophage-derived cholesterol (25–27).
The major part of plasma cholesterol within apoB-containing lipoproteins originates from hepatic secretion of VLDLs (34). Thereby, hepatocyte MTP is considered to have a prime relevance for atherosclerotic CVD. Consequently, a number of therapeutic strategies were developed aiming to substantially decrease hepatic VLDL secretion, among these, antisense approaches targeting MTP as well as apoB (17, 35). However, only antisense therapeutics against apoB have been evaluated in human clinical trials (35). These have proven to possess a high efficacy to decrease circulating levels of pro-atherogenic lipoproteins that would be expected to translate into a substantial reduction in cardiovascular risk on the basis of epidemiological data (35). On the other hand, effects on HDL, a protective lipoprotein subclass for CVD development, have not been addressed. Specifically, the functionality of HDL particles represents an emerging concept in cardiovascular research (36, 37). A prime established atheroprotective function of HDLs is to mediate RCT (20, 38). In the present study, we demonstrate that a hepatocyte-specific lack in MTP decreases RCT, which would be expected to counteract some of the beneficial effects of decreasing hepatic VLDL production.
How a decrease in hepatic VLDL production, specifically, might cause a decrease in RCT is not immediately apparent. There are several possibilities for the rerouting of hepatic cholesterol when MTP activity is lacking. One would be a change in bile acid synthesis, which can be excluded on the basis of unchanged fecal bile acid excretion in the L-Mttp−/− mice. Another possibility would be a change in biliary cholesterol secretion, a pathway of high relevance for macrophage-to-feces RCT (39). However, the biliary secretion of cholesterol remained unaltered in all the models with decreased MTP activity investigated in our study, which also makes changes in this route unlikely. A third possibility, however, likely inversely linked to the biliary secretion pathway (22), could be an increased resecretion via ABCA1. This pathway also does not appear causative to explain our results, because, in addition to unchanged biliary sterol secretion, a decrease in HDL cholesterol and not an increase was noted in response to absent hepatic MTP, and no apparent changes in ABCA1 expression were observed.
These considerations leave the most prominent phenotype, the decrease in hepatic VLDL production itself, as a likely explanation. In previous work, we demonstrated that HDL-derived cholesterol can be resecreted within VLDLs following hepatic uptake (24). In another study, where we tested the relevance of the biliary pathway for RCT, we found that, although biliary secretion plays the predominant role for RCT, around 25% of macrophage-derived counts in the fecal neutral sterol fraction were still excreted in Abcb4 knockout mice that had absent biliary cholesterol secretion (39). How can these data be integrated into a model? Recently, the concept of transintestinal cholesterol excretion (TICE), an intestinal pathway of cholesterol excretion from the body, is increasingly gaining interest (31, 40, 41). For TICE, a number of key points have not been addressed yet, including the lipoprotein substrates donating cholesterol for this pathway, their cellular uptake, the trafficking routes within the enterocyte, and the apical transporters mediating final excretion into the intestinal lumen (42). Regarding the apical transporters, a significant impact was shown only for ABCG5/G8, but models with absent TICE are lacking, which complicates studying the relevance of this pathway (31, 42). Although HDLs have not been formally excluded as a substrate for TICE, present data strongly suggest that apoB-containing lipoproteins serve as the main cholesterol donor particles for the pathway (43, 44). Conceivably, the decrease in RCT in the L-Mttp−/− mice could therefore be explained by a fraction of RCT-relevant cholesterol entering the liver on HDLs, then being resecreted within VLDLs and subsequently taken up by the intestine for excretion from the body via TICE. Such a concept would actually be consistent with results that suggested that the intestine contributes to RCT based on data obtained in short-term RCT experiments employing a bile-diverted mouse model (45), while yet another study supported the view that the major pathway for RCT occurs via biliary secretion (46). However, we would also like to point out that all these data, including the present study, were generated in mice, a predominant HDL species. The conclusions might therefore not be directly translatable into the human system.
In summary, systemic pharmacological inhibition of MTP activity and liver-specific deficiency in MTP activity have differential effects on cholesterol metabolism and RCT, with pharmacological inhibition of MTP activity increasing RCT and liver-specific absence of MTP activity decreasing RCT. These results indicate that the intestine contributes to RCT in two different ways, by absorbing more or less cholesterol secreted via the bile and by excreting RCT-relevant cholesterol that is secreted by hepatocytes within apoB-containing lipoproteins. In a therapeutic perspective, our data suggest that systemic or intestinal MTP inhibition might be preferable to not only lower plasma levels of pro-atherogenic apoB-containing lipoproteins but to also beneficially impact RCT, a major function of the anti-atherogenic HDL lipoprotein subclass.
Supplementary Material
Acknowledgments
The authors gratefully acknowledge Dr. David Gordon from Bristol-Myers Squibb (Pennington, NJ) for kindly providing the MTP inhibitor BMS-212122.
Footnotes
Abbreviations:
- FPLC
- fast protein liquid chromatography
- L-Mttp−/−
- liver-specific microsomal triglyceride transfer protein knockout
- MTP
- microsomal triglyceride transfer protein
- RCT
- reverse cholesterol transport
- TICE
- transintestinal cholesterol excretion
This work was supported by grants from the Netherlands Organization for Scientific Research (VIDI Grant 917-56-358 to U.J.F.T.), the Top Institute (TI) Food and Nutrition (to U.J.F.T.), and National Institutes of Health Grants DK046900 and HL095924 (to M.M.H.). The authors have no conflicts of interest to disclose.
The online version of this article (available at http://www.jlr.org) contains supplementary data in the form of two figures and text.
REFERENCES
- 1.Di Angelantonio E., Gao P., Pennells L., Kaptoge S., Caslake M., Thompson A., Butterworth A. S., Sarwar N., Wormser D., Saleheen D., et al. 2012. Lipid-related markers and cardiovascular disease prediction. JAMA. 307: 2499–2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Robinson J. G., Wang S., Jacobson T. A. 2012. Meta-analysis of comparison of effectiveness of lowering apolipoprotein B versus low-density lipoprotein cholesterol and nonhigh-density lipoprotein cholesterol for cardiovascular risk reduction in randomized trials. Am. J. Cardiol. 110: 1468–1476. [DOI] [PubMed] [Google Scholar]
- 3.Wetterau J. R., Lin M. C., Jamil H. 1997. Microsomal triglyceride transfer protein. Biochim. Biophys. Acta. 1345: 136–150. [DOI] [PubMed] [Google Scholar]
- 4.Berriot-Varoqueaux N., Aggerbeck L. P., Samson-Bouma M., Wetterau J. R. 2000. The role of the microsomal triglyceride transfer protein in abetalipoproteinemia. Annu. Rev. Nutr. 20: 663–697. [DOI] [PubMed] [Google Scholar]
- 5.Hussain M. M., Rava P., Walsh M., Rana M., Iqbal J. 2012. Multiple functions of microsomal triglyceride transfer protein. Nutr. Metab. (Lond). 9: 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chang B. H., Liao W., Li L., Nakamuta M., Mack D., Chan L. 1999. Liver-specific inactivation of the abetalipoproteinemia gene completely abrogates very low density lipoprotein/low density lipoprotein production in a viable conditional knockout mouse. J. Biol. Chem. 274: 6051–6055. [DOI] [PubMed] [Google Scholar]
- 7.Raabe M., Veniant M. M., Sullivan M. A., Zlot C. H., Bjorkegren J., Nielsen L. B., Wong J. S., Hamilton R. L., Young S. G. 1999. Analysis of the role of microsomal triglyceride transfer protein in the liver of tissue-specific knockout mice. J. Clin. Invest. 103: 1287–1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Raabe M., Flynn L. M., Zlot C. H., Wong J. S., Veniant M. M., Hamilton R. L., Young S. G. 1998. Knockout of the abetalipoproteinemia gene in mice: reduced lipoprotein secretion in heterozygotes and embryonic lethality in homozygotes. Proc. Natl. Acad. Sci. USA. 95: 8686–8691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Josekutty J., Iqbal J., Iwawaki T., Kohno K., Hussain M. M. 2013. Microsomal triglyceride transfer protein inhibition induces endoplasmic reticulum stress and increases gene transcription via Ire1α/cJun to enhance plasma ALT/AST. J. Biol. Chem. 288: 14372–14383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gregg R. E., Wetterau J. R. 1994. The molecular basis of abetalipoproteinemia. Curr. Opin. Lipidol. 5: 81–86. [DOI] [PubMed] [Google Scholar]
- 11.Tietge U. J., Bakillah A., Maugeais C., Tsukamoto K., Hussain M., Rader D. J. 1999. Hepatic overexpression of microsomal triglyceride transfer protein (MTP) results in increased in vivo secretion of VLDL triglycerides and apolipoprotein B. J. Lipid Res. 40: 2134–2139. [PubMed] [Google Scholar]
- 12.Liao W., Kobayashi K., Chan L. 1999. Adenovirus-mediated overexpression of microsomal triglyceride transfer protein (MTP): mechanistic studies on the role of MTP in apolipoprotein B-100 biogenesis. Biochemistry. 38: 10215. [DOI] [PubMed] [Google Scholar]
- 13.Iqbal J., Parks J. S., Hussain M. M. 2013. Lipid absorption defects in intestine-specific microsomal triglyceride transfer protein and ATP-binding cassette transporter A1-deficient mice. J. Biol. Chem. 288: 30432–30444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xie Y., Newberry E. P., Young S. G., Robine S., Hamilton R. L., Wong J. S., Luo J., Kennedy S., Davidson N. O. 2006. Compensatory increase in hepatic lipogenesis in mice with conditional intestine-specific Mttp deficiency. J. Biol. Chem. 281: 4075–4086. [DOI] [PubMed] [Google Scholar]
- 15.Cuchel M., Meagher E. A., du Toit Theron H., Blom D. J., Marais A. D., Hegele R. A., Averna M. R., Sirtori C. R., Shah P. K., Gaudet D., et al. 2013. Efficacy and safety of a microsomal triglyceride transfer protein inhibitor in patients with homozygous familial hypercholesterolaemia: a single-arm, open-label, phase 3 study. Lancet. 381: 40–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cuchel M., Bloedon L. T., Szapary P. O., Kolansky D. M., Wolfe M. L., Sarkis A., Millar J. S., Ikewaki K., Siegelman E. S., Gregg R. E., et al. 2007. Inhibition of microsomal triglyceride transfer protein in familial hypercholesterolemia. N. Engl. J. Med. 356: 148–156. [DOI] [PubMed] [Google Scholar]
- 17.Lee R. G., Fu W., Graham M. J., Mullick A. E., Sipe D., Gattis D., Bell T. A., Booten S., Crooke R. M. 2013. Comparison of the pharmacological profiles of murine antisense oligonucleotides targeting apolipoprotein B and microsomal triglyceride transfer protein. J. Lipid Res. 54: 602–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Linsel-Nitschke P., Tall A. R. 2005. HDL as a target in the treatment of atherosclerotic cardiovascular disease. Nat. Rev. Drug Discov. 4: 193–205. [DOI] [PubMed] [Google Scholar]
- 19.Wang X., Rader D. J. 2007. Molecular regulation of macrophage reverse cholesterol transport. Curr. Opin. Cardiol. 22: 368–372. [DOI] [PubMed] [Google Scholar]
- 20.Annema W., Tietge U. J. 2012. Regulation of reverse cholesterol transport - a comprehensive appraisal of available animal studies. Nutr. Metab. (Lond). 9: 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dikkers A., Tietge U. J. 2010. Biliary cholesterol secretion: more than a simple ABC. World J. Gastroenterol. 16: 5936–5945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Annema W., Dikkers A., de Boer J. Freark, Gautier T., Rensen P. C., Rader D. J., Tietge U. J. 2012. ApoE promotes hepatic selective uptake but not RCT due to increased ABCA1-mediated cholesterol efflux to plasma. J. Lipid Res. 53: 929–940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yamamoto S., Tanigawa H., Li X., Komaru Y., Billheimer J. T., Rader D. J. 2011. Pharmacologic suppression of hepatic ATP-binding cassette transporter 1 activity in mice reduces high-density lipoprotein cholesterol levels but promotes reverse cholesterol transport. Circulation. 124: 1382–1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wiersma H., Nijstad N., Gautier T., Iqbal J., Kuipers F., Hussain M. M., Tietge U. J. 2010. Scavenger receptor BI (SR-BI) facilitates hepatic very low density lipoprotein (VLDL) production in mice. J. Lipid Res. 51: 544–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Maugeais C., Annema W., Blum D., Mary J-L., Tietge U. J. 2013. rHDL administration increases reverse cholesterol transport in mice, but is not additive on top of ezetimibe or cholestyramine treatment. Atherosclerosis. 229: 94–101. [DOI] [PubMed] [Google Scholar]
- 26.Briand F., Naik S. U., Fuki I., Millar J. S., Macphee C., Walker M., Billheimer J., Rothblat G., Rader D. J. 2009. Both the peroxisome proliferator-activated receptor delta agonist, GW0742, and ezetimibe promote reverse cholesterol transport in mice by reducing intestinal reabsorption of HDL-derived cholesterol. Clin. Transl. Sci. 2: 127–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sehayek E., Hazen S. L. 2008. Cholesterol absorption from the intestine is a major determinant of reverse cholesterol transport from peripheral tissue macrophages. Arterioscler. Thromb. Vasc. Biol. 28: 1296–1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Khatun I., Zeissig S., Iqbal J., Wang M., Curiel D., Shelness G. S., Blumberg R. S., Hussain M. M. 2012. Phospholipid transfer activity of microsomal triglyceride transfer protein produces apolipoprotein B and reduces hepatosteatosis while maintaining low plasma lipids in mice. Hepatology. 55: 1356–1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dikkers A., de Boer J. F., Annema W., Groen A. K., Tietge U. J. 2013. Scavenger receptor BI and ABCG5/G8 differentially impact biliary sterol secretion and reverse cholesterol transport in mice. Hepatology. 58: 293–303. [DOI] [PubMed] [Google Scholar]
- 30.Wiersma H., Gatti A., Nijstad N., Oude Elferink R. P., Kuipers F., Tietge U. J. 2009. Scavenger receptor class B type I mediates biliary cholesterol secretion independent of ATP-binding cassette transporter g5/g8 in mice. Hepatology. 50: 1263–1272. [DOI] [PubMed] [Google Scholar]
- 31.van der Veen J. N., van Dijk T. H., Vrins C. L., van Meer H., Havinga R., Bijsterveld K., Tietge U. J., Groen A. K., Kuipers F. 2009. Activation of the liver X receptor stimulates trans-intestinal excretion of plasma cholesterol. J. Biol. Chem. 284: 19211–19219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.de Boer J. F., Annema W., Schreurs M., van der Veen J. N., van der Giet M., Nijstad N., Kuipers F., Tietge U. J. 2012. Type I diabetes mellitus decreases in vivo macrophage-to-feces reverse cholesterol transport despite increased biliary sterol secretion in mice. J. Lipid Res. 53: 348–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Iqbal J., Li X., Chang B. H., Chan L., Schwartz G. J., Chua S. C., Jr, Hussain M. M. 2010. An intrinsic gut leptin-melanocortin pathway modulates intestinal microsomal triglyceride transfer protein and lipid absorption. J. Lipid Res. 51: 1929–1942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chan D. C., Barrett P. H., Watts G. F. 2006. Recent studies of lipoprotein kinetics in the metabolic syndrome and related disorders. Curr. Opin. Lipidol. 17: 28–36. [DOI] [PubMed] [Google Scholar]
- 35.Visser M. E., Witztum J. L., Stroes E. S., Kastelein J. J. 2012. Antisense oligonucleotides for the treatment of dyslipidaemia. Eur. Heart J. 33: 1451–1458. [DOI] [PubMed] [Google Scholar]
- 36.Duffy D., Rader D. J. 2009. Update on strategies to increase HDL quantity and function. Nat. Rev. Cardiol. 6: 455–463. [DOI] [PubMed] [Google Scholar]
- 37.Fisher E. A., Feig J. E., Hewing B., Hazen S. L., Smith J. D. 2012. High-density lipoprotein function, dysfunction, and reverse cholesterol transport. Arterioscler. Thromb. Vasc. Biol. 32: 2813–2820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rosenson R. S., Brewer H. B., Jr, Davidson W. S., Fayad Z. A., Fuster V., Goldstein J., Hellerstein M., Jiang X. C., Phillips M. C., Rader D. J., et al. 2012. Cholesterol efflux and atheroprotection: advancing the concept of reverse cholesterol transport. Circulation. 125: 1905–1919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nijstad N., Gautier T., Briand F., Rader D. J., Tietge U. J. 2011. Biliary sterol secretion is required for functional in vivo reverse cholesterol transport in mice. Gastroenterology. 140: 1043–1051. [DOI] [PubMed] [Google Scholar]
- 40.Temel R. E., Brown J. M. 2012. Biliary and nonbiliary contributions to reverse cholesterol transport. Curr. Opin. Lipidol. 23: 85–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.van der Velde A. E., Vrins C. L., van den Oever K., Kunne C., Oude Elferink R. P., Kuipers F., Groen A. K. 2007. Direct intestinal cholesterol secretion contributes significantly to total fecal neutral sterol excretion in mice. Gastroenterology. 133: 967–975. [DOI] [PubMed] [Google Scholar]
- 42.Tietge U. J., Groen A. K. 2013. Role the TICE?: advancing the concept of transintestinal cholesterol excretion. Arterioscler. Thromb. Vasc. Biol. 33: 1452–1453. [DOI] [PubMed] [Google Scholar]
- 43.Vrins C. L., Ottenhoff R., van den Oever K., de Waart D. R., Kruyt J. K., Zhao Y., van Berkel T. J., Havekes L. M., Aerts J. M., van Eck M., et al. 2012. Trans-intestinal cholesterol efflux is not mediated through high density lipoprotein. J. Lipid Res. 53: 2017–2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Le May C., Berger J. M., Lespine A., Pillot B., Prieur X., Letessier E., Hussain M. M., Collet X., Cariou B., Costet P. 2013. Transintestinal cholesterol excretion is an active metabolic process modulated by PCSK9 and statin involving ABCB1. Arterioscler. Thromb. Vasc. Biol. 33: 1484–1493. [DOI] [PubMed] [Google Scholar]
- 45.Temel R. E., Sawyer J. K., Yu L., Lord C., Degirolamo C., McDaniel A., Marshall S., Wang N., Shah R., Rudel L. L., et al. 2010. Biliary sterol secretion is not required for macrophage reverse cholesterol transport. Cell Metab. 12: 96–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Xie P., Jia L., Ma Y., Ou J., Miao H., Wang N., Guo F., Yazdanyar A., Jiang X. C., Yu L. 2013. Ezetimibe inhibits hepatic Niemann-Pick C1-like 1 to facilitate macrophage reverse cholesterol transport in mice. Arterioscler. Thromb. Vasc. Biol. 33: 920–925. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.