Abstract
The effect of electron transport chain redox status on activity of the mitochondrial Ca2+-independent phospholipase A2 (iPLA2) has been examined. When oxidizing NAD-linked substrates, the enzyme is not active unless deenergization occurs. Uncoupler, rotenone, antimycin A, and cyanide are equally effective at upregulating the enzyme, while oligomycin is ineffective. Thenoyltrifluoroacetone causes deenergization and activates the enzyme, but only if succinate is the respiratory substrate. These findings show that the mitochondrial iPLA2 responds to the energetic state overall, rather than to the redox status of individual electron transport chain complexes. With NAD-linked substrates, and using rotenone to deenergize, iPLA2 activation can be reversed by adding succinate to reestablish a membrane potential. For this purpose, ascorbate plus N,N,N′N′-tetramethyl-phenylenediamine can be used instead of succinate and is equally effective. With succinate as substrate, the membrane potential can be reduced in a graded and stable fashion by adding increasing concentrations of malonate, which is a competitive inhibitor of succinate utilization. A partial and stable activation of the iPLA2 accompanies partial deenergization. These findings suggest that in addition to the several functions that have been proposed, the mitochondrial iPLA2 may help to coordinate local capillary blood flow with changing energy demands.
Keywords: membrane potential, redox status, electron transport chain inhibitors
Relationships between phospholipase activity in mitochondria and degradation of the structure and bioenergetic capacities of these organelles were already under investigation during the 1950s and 1960s [e.g., (1–3)]. Toward the end of that period Waite et al. (4) described an apparent relationship between mitochondrial swelling and the activity of an endogenous phospholipase A. Evidence for such a relationship grew stronger during the subsequent decade (5, 6), and during the same period the loss of coupled function which accompanied swelling became known as the mitochondrial permeability transition. There was a tendency to interpret results in that emerging area within the context of energy coupling mechanisms underlying the synthesis of ATP (7–9), as being related to the process of Ca2+ release from mitochondria (10, 11), or to the control of steroid hormone biogenesis in cell types having that capacity (12, 13).
Attempts to purify and characterize the phospholipase of interest led to the description of a low molecular weight phospholipase A2 that requires Ca2+ (14–16) and is subject to inhibition by a product, monolysocardiolipin (17). Its enzymatic properties, together with cloning and sequencing data, led to classification of the enzyme as a type IIA secretory phospholipase (16, 18). However, the occurrence of the enzyme in mitochondria from hepatocytes was later called into question, and it was thought ultimately that it is present primarily in the Kupfer cells of the liver (19, 20). This latter view raised again the question of the identity of the apparent phospholipase A2 activity in hepatocyte mitochondria and how it is related to the occurrence of the mitochondrial permeability transition and related phenomena.
The way in which phospholipase A2 activity might promote the permeability transition became more apparent as evidence accumulated showing that small amounts of FFAs promote opening of the permeability transition pore and related phenomena (21–25). The question of enzyme identity was illuminated, in part, when it was found that rat liver mitochondria contain a phospholipase A2 that does not require Ca2+, while appearing to be much larger than the type IIA secretory enzyme that was of interest before. Based on sensitivity of enzyme activity to bromoenol lactone (BEL), but not to other phospholipase inhibitors, the absence of a Ca2+ requirement and its apparent recognition by a commercial antibody raised against partial sequence from a phospholipase found in a macrophage-like cell line, we concluded that the predominant activity is a Ca2+-independent phospholipase A2 (iPLA2) (26, 27). Independent work has shown that an analogous enzyme is found in heart mitochondria (28), the mitochondria of kidney (29–31) and brain (32), and in those of lung and spleen (33).
The iPLA2 activity of rat liver mitochondria acting upon endogenous phospholipids is regulated in some manner by the energetic status of the organelle. That is to say, little or no activity is seen when mitochondria are intact and fully energized. The enzyme becomes active upon the addition of a protonophoric uncoupler, carbonyl cyanide m-chlorophenylhydrazone (CCCP), when the permeability transition pore is opened, or when the mitochondria are permeabilized by the pore-forming compound alamethicin (26, 27). Here we investigate the origin of this energy dependence in greater detail, including the relationship between activity and the redox status of individual respiratory chain complexes, the effect of partial deenergization on activity, and the reversibility of enzyme activation brought about by restoring the energetic state.
EXPERIMENTAL PROCEDURES
Preparation and incubation of mitochondria
Rats were maintained by University Laboratory Animal Resources, and all procedures were approved by the Institutional Animal Care and Use Committees of the Ohio State University and the Ohio Northern University. Briefly, the rats were euthanized by an overdose of Nembutal that was administered ip. When they were no longer responsive, the abdominal and chest cavities were opened with scissors; the liver was removed, and was then transferred to ice-cold isolation medium.
Rat liver mitochondria were prepared by a standard procedure in which BSA and EGTA were present in the homogenization medium but were absent from the medium used for washing (34). They were incubated at 25°C in medium containing 0.23 M mannitol, 0.07 M sucrose, and 3 mM HEPES (Na+) (pH 7.4), before the addition of respiratory substrates. Glutamate and malate (10 mM each), both Na+ salts, were normally used as the substrate, but in some cases respiration was supported by 10 mM of Na+ succinate, or by 6 mM ascorbic acid in the presence of 100 μM N,N,N′,N′-tetramethyl-phenylenediamine (TMPD). When succinate alone was the exogenous substrate, rotenone was present at 1 nmol/mg protein. For all substrates, as they were added to the media, the concentrations of mannitol and sucrose were reduced in proportion to maintain a total osmotic pressure of 300 mOsM.
Incubations were conducted in vessels that were open to the atmosphere, with stirring maintained to provide an availability of O2 throughout the time course (35). Because the experiments were conducted over relatively long time frames (up to 2 h), all incubations pertaining to a given experiment were conducted simultaneously to prevent variations arising from aging of the preparation. Cyclosporin A was always present to prevent occurrence of the permeability transition, and the effectiveness of this reagent was verified by monitoring swelling using a Brinkman probe colorimeter that was interfaced to a computer. Alternatively, swelling was sometimes monitored discontinuously using the same instrument, by moving the probe from vessel to vessel and manually recording the reading.
Changes in membrane potential were also of interest and were monitored in parallel with other parameters using a tetraphenylphosphonium cation (TPP+) electrode as previously described (35). For calculation of the potential in units of millivolts, the matrix volume was taken to be 1 μl/mg protein (36) and the fraction of TPP+ bound nonspecifically to mitochondrial components was accounted for (37). Expected redox states of complex 4 (cytochromes aa3) were verified by dual wavelength spectroscopy (38). Protein concentrations were determined by the Biuret reaction in the presence of 1% deoxycholate, while oxygen consumption was monitored with a Clark-type electrode.
Determination of iPLA2 activity
Recently distilled solvents were used throughout. Phospholipase activity was determined by monitoring the accumulation of FFAs derived from the endogenous phospholipids (34). To extract these products, a 3 ml aliquot of the incubation was added to 4 ml of cold methanol to which 5 μg of heptadecanoic acid (17:0) had previously been added as an internal standard. The aqueous methanol solution was mixed before addition of 8 ml CHCl3, and the resulting mixture was centrifuged to separate the organic and the aqueous phases. The aqueous phase (upper phase) was removed by aspiration, after which the lower organic phase was transferred to a 10 ml conical screw cap tube. This phase was brought to dryness under N2 and the FFAs were converted to fatty acid methyl esters (FAMEs) by reaction with diazomethane (39). During this procedure, the dried lipid phase was taken up in 1.1 ml of ether:methanol (10:1) to which 0.2 ml of the diazomethane solution was added. Reaction was allowed to continue for 15 min at room temperature. The samples were then brought to dryness and taken up in 1 ml hexane. Typically they were thereafter stored overnight at −80°C and under argon, prior to analysis by GLC.
To begin the analysis, the stored samples were dried and the lipids were taken up in 0.2 ml hexane. These solutions were applied to silica gel mini columns for the separation of FAMEs from other mitochondrial lipids (34). The columns were washed successively with 1.8 ml hexane, 1.8 ml CHCl3, and finally 2.0 ml hexane:ether (1:1). FAMEs were obtained in the hexane:ether wash. The hexane:ether solvent mixture was removed under N2 and the FAMEs were taken up in 12 μl of hexane. They were separated and quantitated using a GLC equipped with a capillary column and a computing integrator. Peak areas representing the original level of individual FFAs were converted to units of nanomoles per milligram of mitochondrial protein. To convert the areas to units of mass, they were compared with the area of the internal standard peak, which represented the 5 μg of heptadecanoic acid that was added to the original extract.
Changes in FFA levels can usually be related to activity of a phospholipase A2 by considering the composition of products, because positional analysis data have shown which fatty acids are normally located at positions 1 and 2 within a glycerophospholipid molecule as defined by the stereospecific numbering system (sn-1 and sn-2 positions) of mitochondrial phospholipids (6, 26). For the present study, we used the sum of accumulating linoleic acid (18:2), arachidonic acid (20:4), and docosahexaenoic acid (22:6) as the indicator of phospholipase A2 activity. In sum, these fatty acids are present at a level of ∼170 nmol/mg protein and constitute 83% of the acyl groups esterified at the sn-2 position (6). The results of FFA analysis are reported as totals of the three specified above and are in units of nanomoles per milligram mitochondrial protein. Individual peaks were identified by their retention time, obtained by comparison to those of known components in a commercial standard mixture (Nu-Chek Prep, Elysian MN). Repetitive analysis of single samples has shown that the standard deviation for individual peak area values is approximately ±4% (34). During this study, we further determined that the standard deviation on maximal activity of the mitochondrial iPLA2, from rat to rat (animal variability), is ±16% .3
All experiments were repeated twice, and in most cases three times, with the data shown being representative.
RESULTS
Effects of electron transport inhibitors
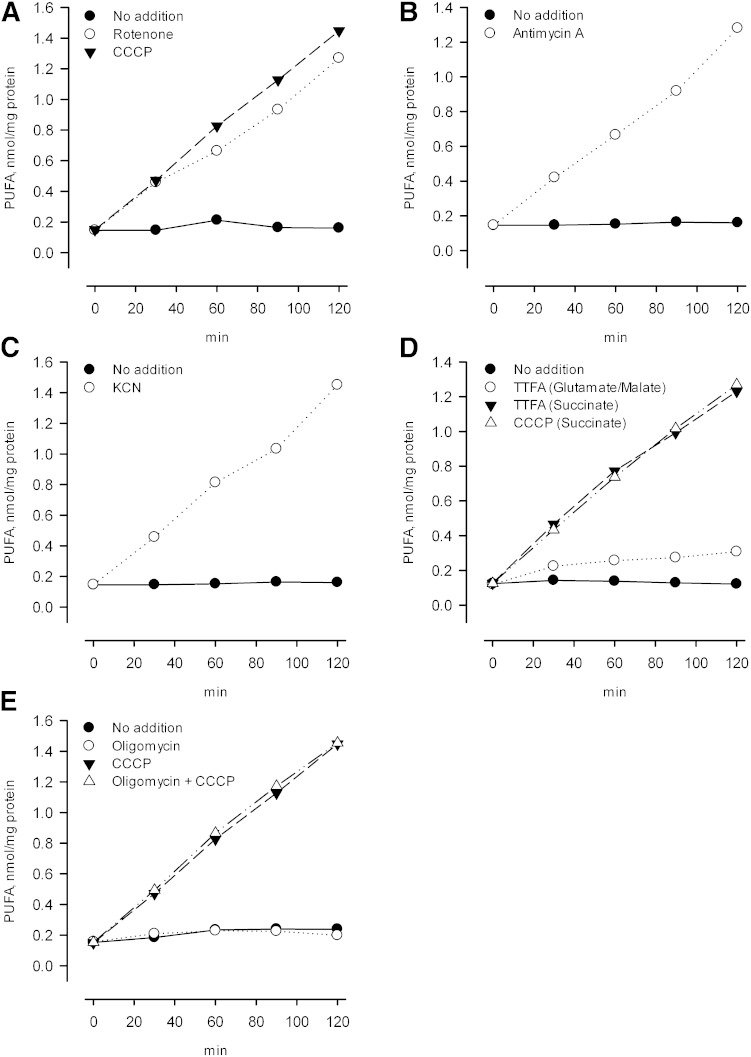
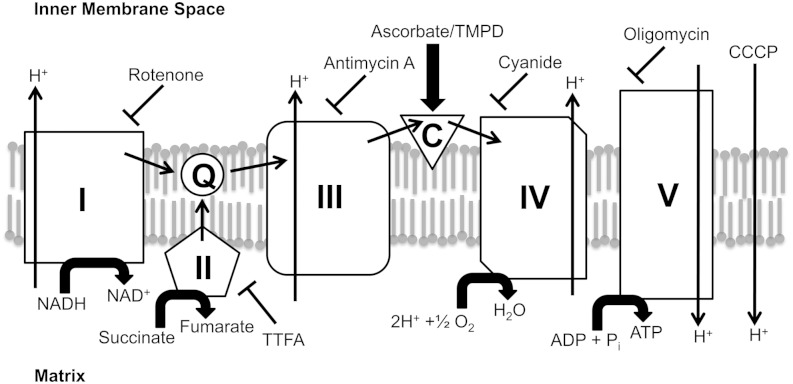
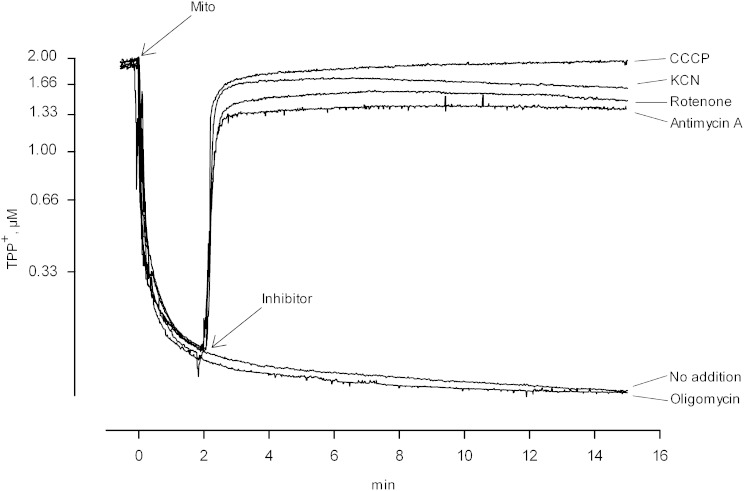
In principle, activation of the iPLA2 upon deenergizing mitochondria could reflect a direct effect of proton motive force on the conformational state of the enzyme, or an interaction of enzyme with some other mitochondrial component that is affected by the energetic state. Electron transport chain complexes are prominent possibilities of components that might interact with the iPLA2, with the interaction affecting activity, and with the nature of the interaction affected by a change in the redox status of the complex. To examine the effect of individual electron transport chain complexes on iPLA2 activity, we used well-established inhibitors that leave chain components in particular states of reduction, and compared these states to enzyme activity. Results are shown in Fig. 1. The inhibitor rotenone blocks electron transport through complex 1, leaving that complex reduced when NADH-linked substrates are present, while the other components become oxidized due to the presence of excess O2 (see Scheme 1and Table 1). In addition, rotenone leads to deenergization of mitochondria under these conditions, similar to the effect of the uncoupler CCCP (Fig. 2, rotenone and CCCP). As seen in Fig. 1A, in mitochondria oxidizing glutamate plus malate, the iPLA2 is active when rotenone is present, and the activity level is the same as when CCCP is present. These findings are consistent with the notion that the iPLA2 is activated by deenergization per se, but as already noted, mitochondrial components change their redox state upon deenergization, and it might be that it is the state of these that is actually affecting the enzyme. Among the various possibilities, Fig. 1A tends to rule out an important role for the redox state of complex 1 because it will be reduced when rotenone is present but (substantially) oxidized in the presence of CCCP.
Fig. 1.
Effect of electron transport inhibitors on mitochondrial iPLA2 activity. Rat liver mitochondria were incubated at 1.0 mg protein/ml in the mannitol/sucrose-based medium containing 10 mM each of glutamate and malate as respiratory substrates. The temperature was 25°C and cyclosporin A was added at the beginning of the incubation (0.5 nmol/mg protein). Other agents were added 2 min after mitochondria as follows. A: •, No addition; ○, rotenone (0.5 nmol/mg protein); ▾, the uncoupler CCCP (1 nmol/mg protein). B: •, No addition; ○, antimycin A (1 nmol/mg protein). C: •, No addition; ○, KCN (0.50 mM). D: •, Succinate (10 mM) was the respiratory substrate in absence of both glutamate and malate and rotenone was present from the beginning (0.5 nmol/mg protein). There was no further addition. ○, Glutamate and malate were the respiratory substrates and TTFA was added (50 μM). ▾, Same as • except that TTFA was added in addition to rotenone. △, Same as • except that the CCCP was added. E: Glutamate and malate were the respiratory substrates. •, No addition; ○, oligomycin was added (1 nmol/mg protein); ▾, the uncoupler CCCP was added; △, same as • except that oligomycin and CCCP were added.
Scheme 1.
TABLE 1.
Effects of electron transport chain inhibitors on mitochondrial parameters and iPLA2 activity
| Inhibitor | Reduction/Oxidation State | Membrane Potential | iPLA2 activity | ||||
| I | II | III | IV | Cyto a | |||
| Rotenone | Red. | Ox. | Ox. | Ox. | Ox. | <90 mV | Active |
| Rotenonea | Red. | Red. | Red. | Ox. | ND | High | Inactive |
| TTFA | Red. | Ox. | Red. | Ox. | Ox. | High | Inactive |
| TTFAa | Ox. | Red. | Ox. | Ox. | Ox. | <90 mV | Active |
| Antimycin A | Red. | Red. | Red. | Ox. | Ox. | <90 mV | Active |
| Antimycin Ab | Red. | Red. | Red. | Ox. | ND | High | Inactive |
| KCN | Red. | Red. | Red. | Red. | Red. | <90 mV | Active |
| CCCP | Ox. | Ox. | Ox. | Ox. | Ox. | <90 mV | Active |
| CCCPa | Ox. | Ox. | Ox. | Ox. | Ox. | <90 mV | Active |
| Oligomycin | Red. | Red. | Red. | Ox. | Ox. | High | Inactive |
The oxidation/reduction status of the major electron transport chain components in the presence of selected inhibitors, when glutamate/malate is the respiratory substrate. The status of cytochrome a was checked by dual wavelength spectroscopy (605–630 nm) to verify that the inhibitors were behaving as expected. Cyto a, cytochrome a; Red., reduction; Ox., oxidation; ND, not determined.
When succinate plus rotenone is the respiratory substrate.
When ascorbate plus TMPD is the respiratory substrate.
Fig. 2.
Effect of electron transport inhibitors on mitochondrial membrane potential. Incubation conditions were those described in the legend to Fig. 1 except that the media contained 2 μM of TPP+ to allow monitoring of mitochondrial membrane potential as described in Experimental Procedures. A downward deflection indicates a reduced concentration of TPP+ in the medium due to an increase in the fraction accumulated. When used, inhibitors were added at 2 min. Trace labeled No addition: glutamate and malate were the respiratory substrates and there was no further addition. Trace labeled Rotenone: same as trace labeled No addition, but rotenone was added at 0.5 nmol/mg protein. Trace labeled Antimycin A: same as trace labeled No addition except that antimycin A was added at 1 nmol/mg protein. Trace labeled KCN: same as trace labeled No addition except KCN was added at 0.5 mM. Trace labeled CCCP: same as trace labeled No addition except CCCP was added at 1 nmol/mg protein. Trace labeled Oligomycin: same as trace labeled No addition except oligomycin added at 1 nmol/mg protein. Mito, mitochondria.
To further probe for possible effects of redox state on iPLA2 activity, we examined the effects of antimycin A, which inhibits electron transport at complex 3 (Scheme 1). As seen in Fig. 1B, this agent also activates the enzyme. It is equally effective compared with rotenone in this regard, and in promoting loss of membrane potential (Fig. 2, antimycin A). Antimycin A, like rotenone, will cause a persistent reduction of complex 1 in the presence of an NAD+-linked substrate, and will also produce a persistent reduced state of complex 3. Thus it would seem that as with complex 1, there is also no special role for the redox state of complex 3 in regulating the iPLA2 because equal activity is seen when complex 3 is oxidized (rotenone present, antimycin absent) and when it is reduced (rotenone absent, antimycin A present).
Cyanide blocks electron transport at complex 4, resulting in reduction of the entire electron transport chain while also maintaining a state of deenergization (Fig. 2, KCN). Like the other inhibitors employed to this point, the iPLA2 is active when cyanide is present (Fig. 1C).
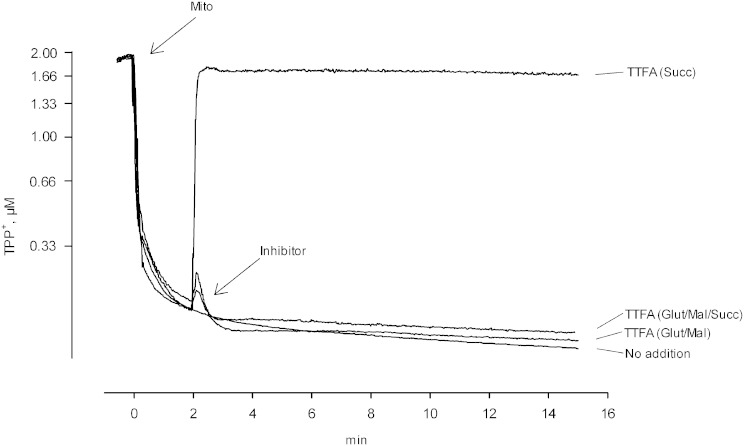
The enzyme is also active when succinate plus rotenone is used as substrate and thenoyltrifluoroacetone (TTFA) is present (Fig. 1D). The latter agent inhibits complex 2 (40, 41) and deenergizes mitochondria that are oxidizing succinate [Fig. 3, TTFA (succinate)]. It does not deenergize mitochondria when malate and glutamate are present together with succinate [Fig. 3, TTFA (glutamate/malate/succinate)] or when malate and glutamate are used alone [Fig. 3, TTFA (glutamate/malate)]. Likewise, TTFA does not activate the iPLA2 when NAD-linked substrates are present (Fig. 1D).
Fig. 3.
Effect of TTFA on mitochondrial membrane potential. Experiments are analogous to those shown in Fig. 2, but they examine the effects of TTFA as an inhibitor of complex 2. Trace labeled No addition: succinate was the respiratory substrate in the presence of rotenone (0.5 nmol/mg protein) and there was no further addition. Trace labeled TTFA (Glut/Mal): 10 mM glutamate and 10 mM malate were the respiratory substrates. TTFA (50 μM) was added where indicated. Trace labeled TTFA (Glut/Mal/Succ): glutamate, malate, and succinate were the substrates. TTFA (50 μM) was added. Trace labeled TTFA (Succ): same as the trace labeled No addition except that TTFA (50 μM) was added. Mito, mitochondria.
Thus, the iPLA2 in liver mitochondria becomes active upon deenergization, regardless of what redox states in the electron transport chain are maintained by the agents used to produce deenergization. Oligomycin, which inhibits complex 5 (the ATP synthase complex) and so leads to depletion of ATP in the matrix space, has no effect on iPLA2 activity (Fig. 1E) or on mitochondrial membrane potential under these conditions (Fig. 2, oligomycin).
Reversibility and partial activation
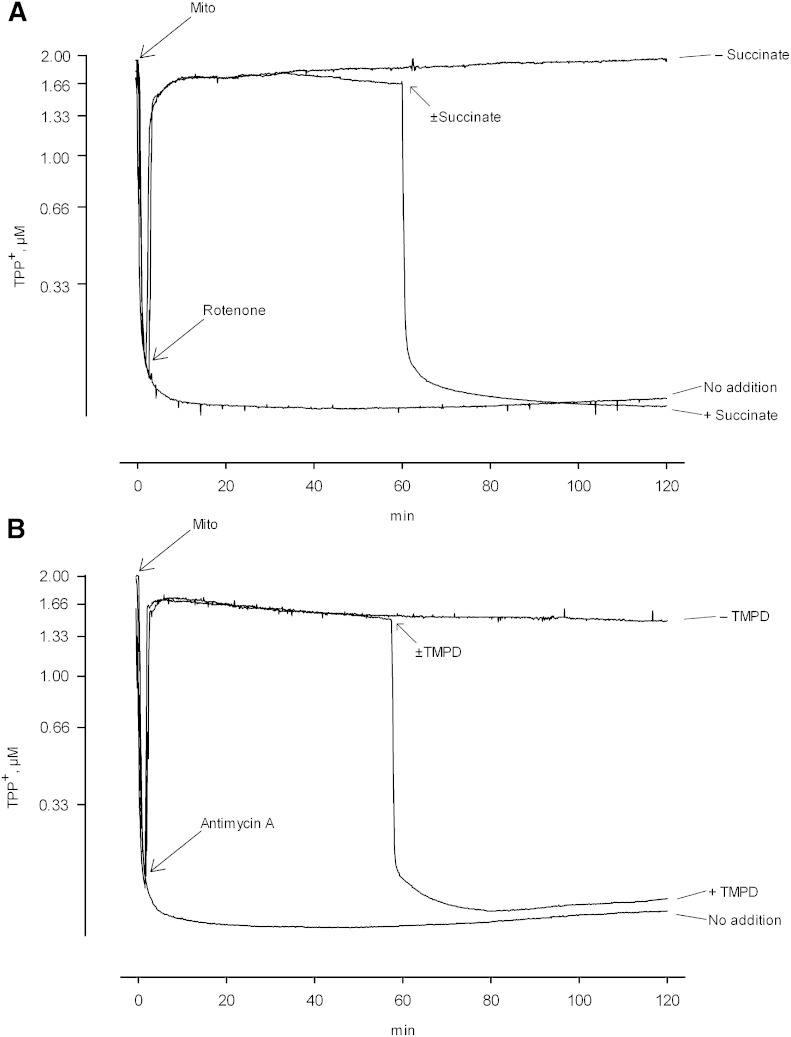
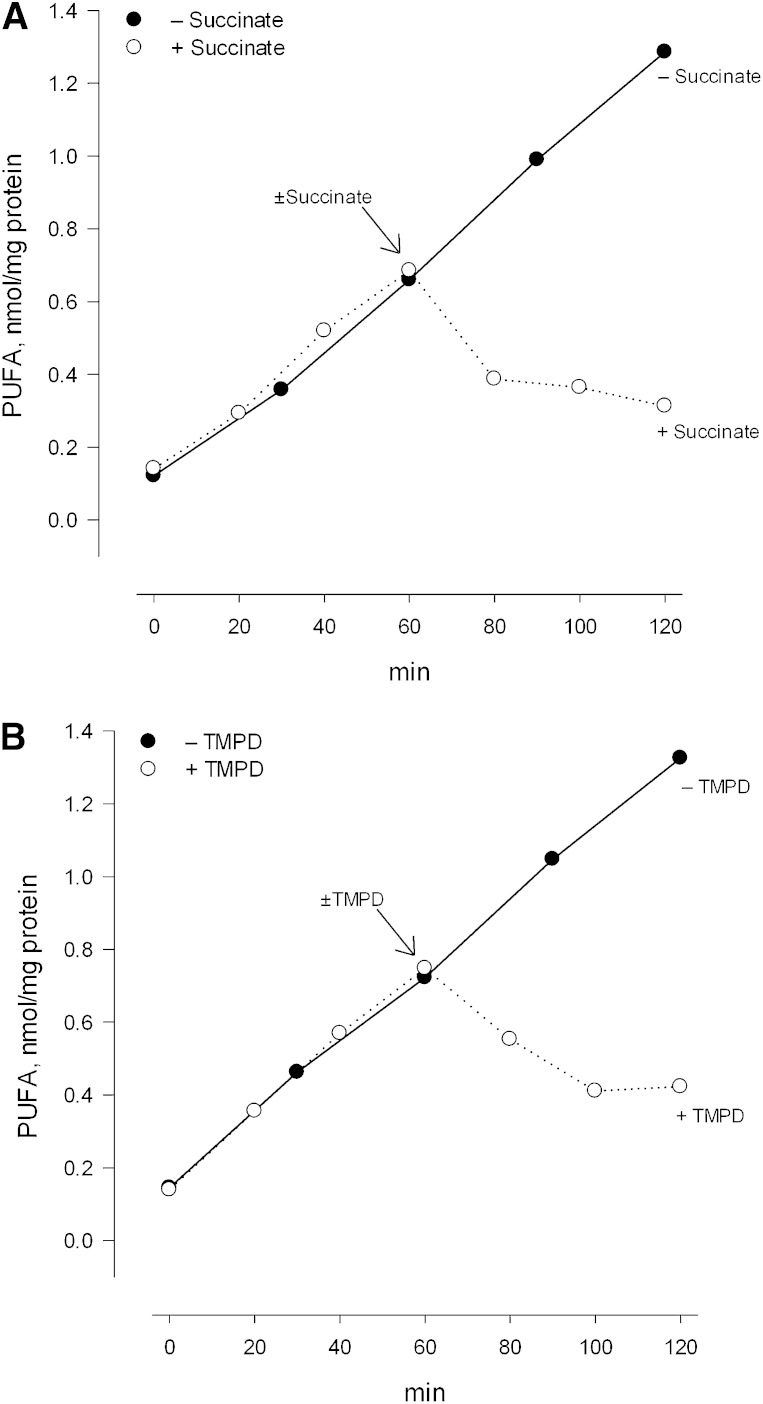
As further discussed below, one can imagine several physiological or pathophysiological processes wherein the energy dependence of mitochondrial iPLA2 activity might be of significance. These possibilities are perhaps best considered within the context of whether or not activation of the enzyme is reversible, and in light of a more detailed understanding of the relationship between the extent of deenergization and the magnitude of enzyme activity. To examine the potential for reversibility, mitochondria oxidizing glutamate plus malate were deenergized with rotenone and maintained in that state for a period of 60 min. Succinate was then added to reestablish electron flow below complex 1 and the incubation was continued. As seen in Fig. 4A, succinate addition reestablished a large membrane potential and prevented further accumulation of FFAs during a subsequent period (Fig. 5A). There was furthermore a reduction in the preexisting level of FFAs which occurred during further incubation. These findings were confirmed by using antimycin A to produce deenergization and subsequently adding the artificial electron carrier TMPD in the presence of ascorbic acid. These conditions reestablish electron flow to complex 4 and reestablished a significant membrane potential as expected (Fig. 4B). This membrane potential was also sufficient to thereafter limit activity of the iPLA2, and a reduction in the preexisting level of FFAs was again observed (Fig. 5B).4
Fig. 4.
Restoration of membrane potential in previously deenergized mitochondria. A: Mitochondria oxidizing glutamate and malate were deenergized with rotenone as shown, and were maintained in that condition for 1 h. At that point, 10 mM succinate was added, or not added, as indicated in the figure and the incubations were continued for an additional hour. B: Mitochondria oxidizing glutamate and malate were deenergized with antimycin A as shown, with 6 mM ascorbic acid having been present from the beginning. Deenergization was maintained for 1 h. At that point, 100 μM TMPD was added, or not added, as indicated in the figure and the incubations were continued for an additional 1 h. In both panels the media contained 2 μM TPP+ in order to monitor changes in membrane potential. Mito, mitochondria.
Fig. 5.
Effect of reenergization on iPLA2 activity. A: Conditions were the same as those described for Fig. 4A except that TPP+ was not present. ○, Succinate (10 mM) was added after 1 h of deenergization. •, Succinate was not added at 1 h. In both cases the incubations were continued for an additional hour. B: Conditions were the same as described for Fig. 4B except for the exclusion of TPP+. ○, TMPD (100 μM) was added after 1 h of deenergization. •, TMPD was not added. In both cases the incubations were continued for an additional 1 h.
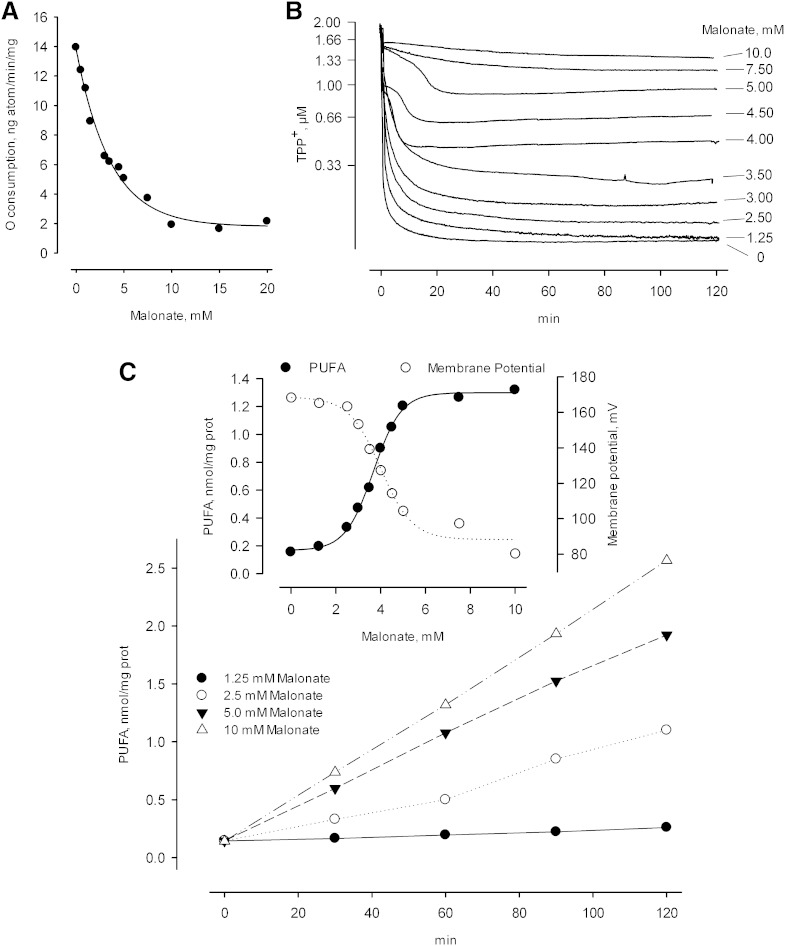
To investigate how a partial loss of membrane potential affects iPLA2 activity, methods were needed to generate various degrees of deenergization and to sustain these over an extended period. The activity of malonate as an inhibitor of succinate oxidation can be used in both regards, as illustrated in Fig. 6A, B. Increasing the concentration of malonate progressively decreases the succinate oxidase activity of respiring mitochondria with a half maximal effect seen at about 3 mM, and a maximal effect at about 15 mM, when the succinate concentration is 10 mM (Fig. 6A). As the succinate oxidase activity falls, the membrane potential also falls following a lag that is apparent at the lowest malonate concentrations employed. This lag in the concentration dependence can be attributed to the need for less than the available succinate oxidase activity to maintain a full potential when mitochondria are not under a significant load; but more importantly, it is seen that the partially reduced membrane potentials produced at intermediate levels of malonate are stable for periods of 2 h or longer when an open incubation vessel is employed (Fig. 6B). This time frame allowed us to compare activity to membrane potential when the latter parameter was suppressed to different degrees. As seen in Fig. 6C, there is a clear relationship between these parameters wherein a partially reduced membrane potential produces a partial activation of the iPLA2 that is maintained over time. In more detail, decreasing the membrane potential from about 170 mV to about 130 mV was sufficient to activate the iPLA2 to 1/2 of the maximal activity. Presuming that the same situations pertain in vivo, one can imagine that relatively modest energy loads would bring forth some activity of the iPLA2, which would return to the resting (no activity) level when the energy load was dissipated.
Fig. 6.
Effect of partial deenergization on iPLA2 activity. The medium contained 10 mM succinate plus rotenone (0.5 nmol/mg protein) as the respiratory substrate, with increasing concentrations of malonate present as indicated. Malonate was present from the beginning of incubations. A: Inhibition of basal succinate oxidation produced by increasing malonate. B: Decreasing membrane potential (decreasing TPP+ accumulation) produced by increasing malonate. TPP+ (2 μM) was present in these incubations. C: Partial and stable activation of iPLA2 produced by selected concentrations of malonate. •, 1.25 mM malonate; ○, 2.5 mM malonate; ▾, 5.0 mM malonate; △, 10 mM malonate. C (inset): •, iPLA2 activity as a function of malonate concentration and determined as the total PUFA levels found after 60 min of incubation. ○, Membrane potential determined at 60 min, as calculated from the data shown in (B).
Inhibition of induced activities by BEL
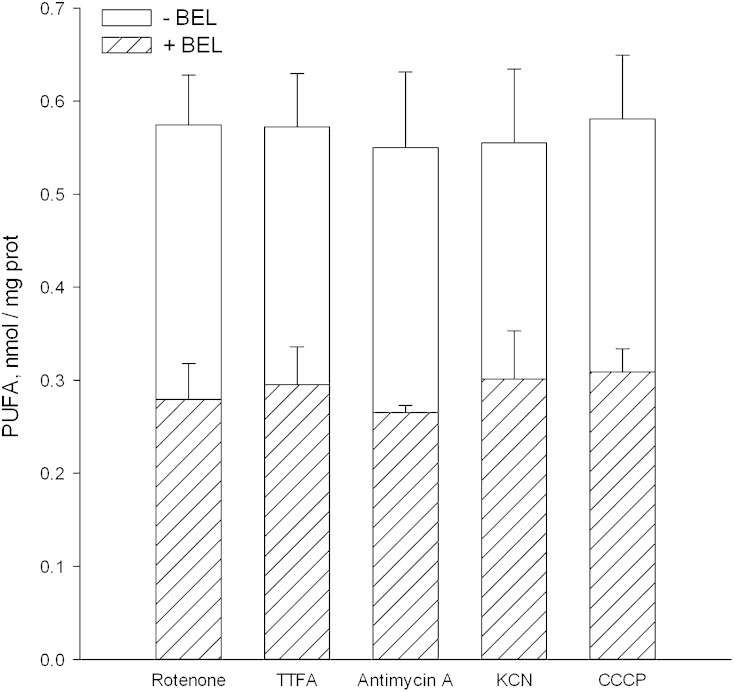
The effectiveness of BEL as an inhibitor of activity induced by the various inhibitors used here was also examined. As seen in Fig. 7, BEL was equally effective with all of them, while the fraction of activity inhibited was limited to ∼50%. The inhibited fraction was not increased by using higher levels of BEL, by making a second addition at 1 hr after the first, by using the (R) and (S) BEL enantiomers individually, or by including an excess of EGTA in the medium (data not shown). The failure of EGTA to alter the fraction of BEL-insensitive activity shows that the insensitive activity is not Ca2+ dependent.5
Fig. 7.
Effect of BEL on the iPLA2 activity of deenergized mitochondria. Conditions were as described in the legend to Fig. 1. Glutamate plus malate were the respiratory substrates when deenergization was produced by CCCP, rotenone, antimycin A, or KCN. When TTFA was employed, glutamate and malate were replaced by succinate plus rotenone as also described in the legend to Fig. 1. The racemic mixture of BEL was used at 7 μM, and when present was added 45 s after the mitochondria. Samples were taken at 1 h and each bar shown represents the activity seen in response to the indicated inhibitor of respiration. The total height of the bar (open plus hatched portions) represents the activity seen when BEL was absent. The hatched area shows the activity that remained when BEL was present. For each inhibitor examined, data were obtained from four separate preparations of mitochondria. The error bars shown are standard deviations of the mean values. When comparing activities seen in the absence and presence of BEL by the paired Student's t-test, BEL present values were lower than BEL absent values at a confidence value of 0.005. When one-way ANOVA was used to compare activities seen when the different deenergizers were employed (BEL absent or present), there were no significant differences at a level of 0.05.
DISCUSSION
As documented in the Introduction, there has been considerable effort directed at determining which proteins are responsible for releasing fatty acids from mitochondrial phospholipids. It is also important to understand how the process is regulated biochemically, as investigated here. In our preceding papers describing iPLA2 activity in liver mitochondria, we emphasized that deenergization is required to activate the enzyme (26, 27). Chemical uncoupling, pore formation, and ion transport loads were emphasized as the approaches to deenergization. We can now add to this group a number of electron transport inhibitors, all of which are equally effective, and as effective as the approaches used earlier. This is significant because each of the agents used produce well-understood states of the electron transport chain in which the individual complexes are highly reduced or highly oxidized during continued incubation. Thus, in light of this data, it seems unlikely that enzyme activity is controlled by association with electron transport chain components or with the interaction changing as the redox state of the interactive partner changes, as once seemed probable.
The question now becomes, what other consequences of deenergization might lead to activation of the iPLA2, and do so in a graded and reversible fashion? The possibilities include a direct sensing of membrane potential by the enzyme whereby changes in potential alter conformation with attendant changes in activity, changes in the state of covalent modification (phosphorylation for example) affecting activity, changes in the level of small molecule effectors which are normally bound by the enzyme controlling its activity, or possibly an altered physical state of phospholipids which renders the enzyme more or less able to associate and act upon individual molecules. No firm choice between these possibilities can be made at present, although some further points can be added.
It is first of all clear that small molecule effectors involved in activation would have to be inhibitors rather than activators. This is because activation by opening the permeability transition pore, or by adding alamethicin, will be accompanied by the release of all matrix space components up to a molecular weight of approximately 1,500 (42). Thus potential activators will be diluted by about 103 when activation results from pore formation, rather than rising in concentration as one would expect if an activator was controlling. Likewise, control by phosphorylation/dephosphorylation would have to be such that the phosphorylated form was poorly active while activation occurred by dephosphorylation. This is because ATP (and other nucleotides) is released when the enzyme is activated by pore formation and would not be available to support a phosphorylated state. Also, arguing against a poorly activated phosphorylated form, which becomes active upon dephosphorylation, is the failure of oligomycin to produce activation. This is because this agent, by inhibiting ATP synthesis, should antagonize the phosphorylation. If dephosphorylation was proposed to activate the enzyme following loss of membrane potential, because ATP synthesis is interrupted, then one is left to wonder why oligomycin alone would not have the same effect. Thus, while several potential activating mechanisms can be ruled out, several others remain. At present we favor a direct effect of membrane potential on the enzyme because it is straightforward and supported by the failure of oligomycin to produce an activation, and because it does not conflict with any other available data on regulation of activity.
While deenergization is clearly required to observe activity in rat liver mitochondria, this may not be the case in mitochondria from all sources. Schnellmann and coworkers, examining kidney mitochondria from the rabbit, found that uncoupling was not required (31). Brustovetsky et al. (32) showed that similar to kidney mitochondria, there is a background level of iPLA2 activity observed in brain mitochondria without uncoupling. This level was increased by the presence of tBid plus full-length Bax, possibly relating to ROS formation, and without requiring the permeability transition (32). Williams and Gottlieb (28), who identified an iPLA2 in rabbit heart mitochondria, used an activity assay based upon an artificial and exogenous substrate, which itself may affect bioenergetic status. They also obtained their mitochondria from perfused hearts, which were subject to ischemia/reperfusion and/or ischemic preconditioning, which does indeed affect their biochemical properties [e.g., (43)]. Thus, it is not clear from that study if the heart mitochondrial enzyme is regulated similarly to the enzyme in liver mitochondria.
More recently, Gross and coworkers have described how multiple products can arise from the gene encoding iPLA2γ (44, 45). These studies stop short of revealing how activity is controlled at the protein level, but have identified new possibilities for investigation. Included are the possible significance of a nucleotide binding site located near to the active site (46), the activating effects of divalent cations, and the inhibitory effects of long chain acyl-CoA. The latter two of these possibilities were seen when the enzyme was overexpressed (47). Within the context of activation by divalent cations (Ca2+ and Mg2+), it can be recalled that maintaining liver mitochondria in a high ionic strength medium (KCl) also yields a relatively high activity of the iPLA2 (26). Thus, activating effects of electrolytes added to the medium should be evaluated within the context of how they affect ionic strength and the mitochondrial content of K+.
While it is clear that regulation of the mitochondrial iPLA2 is not completely understood at the protein level, it can also be said that the same is true regarding the physiological and pathophysiological functions of the enzymes. We originally demonstrated that activity of the mitochondrial iPLA2 promotes the permeability transition (26, 27), and this has been verified and extended by other reports (31, 48). That consequence of activity was expected, given the well-established effects of FFAs on the phenomenon (see Introduction). Thus to some degree, the question of mitochondrial iPLA2 functions reduces to functions of the permeability transition. Apoptotic and necrotic cell death are prominent among the known functions of the transition, and so the iPLA2 is likely involved in those processes as well. In addition, it has long been maintained that the permeability transition can be seen, in its most basic function, as a way to identify poorly functioning mitochondria within an intracellular population, and thereby to initiate their removal/replacement (35, 49, 50). The present findings remain consistent with that role because even a modest depolarization produces an acceleration of iPLA2 activity, which would raise the probability that the transition occurs in the affected mitochondrion (Fig. 6).
Numerous other functions of iPLA2 have been suggested, as summarized by others (33, 47, 51). Some of these reflect the generation of free arachidonic acid from phospholipids and its expected conversion to lipid signaling molecules, including prostaglandins, HETES, thromboxanes, and leukotrienes. Local circulation through capillaries is influenced by some of these, and in particular it is increased by prostaglandins of the E series [e.g., (52, 53)]. Given the graded response of iPLA2 activity to decreasing membrane potential (Fig. 6C), and the reversibility of activation once a full potential is restored (Fig. 5), one can imagine that this enzyme provides for a signaling mechanism between mitochondria and the local circulation as regards the adequacy of blood flow. If flow were not adequate to meet the needs for O2 and substrates, the membrane potential would decrease proportionately, iPLA2 activity would rise increasing the availability of arachidonate, and prostaglandins produced from some of this would cause dilation of nearby capillaries. Once the blood supply became sufficient, the membrane potential would rise and enzyme activity would return to a near zero value. With the availability of iPLA2 knockout mice (54–56), this possibility, and others, can be further investigated.
A final point to consider here is the partial inhibition of apparent iPLA2 activity produced by the mechanism-based inhibitor BEL. This was seen when BEL was used as a racemic mixture, and regardless of which electron transport inhibitor was used to bring about deenergization. More specifically, we found that BEL reduced activity by about 50% when it was assessed at 1 h after deenergization and the BEL had been added at the beginning of the incubation (Fig. 7). This partial sensitivity to BEL, together with the absence of an external Ca2+ requirement, provides evidence that an iPLA2 participates in the accumulation of FFAs that we observed. However, it also suggests that multiple enzymes are participating, or that the duration of BEL effects on mitochondrial iPLA2 is limited. It is now thought that iPLA2 activities are derived as splice variants from either the PNPLA8 gene (iPLA2γ) or from the PLA2G6 gene (iPLA2β) (57). Gross and coworkers have knocked out the gene producing iPLA2γ and have shown that this reduces activity against an exogenous substrate, but does not eliminate it in liver mitochondria (45, 48). These findings also allow for the participation of multiple enzymes in hydrolyzing mitochondrial phospholipids at the sn-2 position. In our first paper describing the presence of an iPLA2 in liver mitochondria, we found that BEL eliminated about 85% of the activity (26). To explain that in light of the present finding, we note that in the earlier paper the effectiveness of BEL was evaluated in media having a high ionic strength (KCl-based media). Total iPLA2 activity is higher in those media than in the mannitol-sucrose media used here, but the BEL-insensitive activity is about the same. Hence, a larger fraction of total activity is inhibited by BEL at high ionic strength. We did not use high ionic strength in this study because mitochondria swell and lose cytochrome c during extended exposure to KCl-based media (27, 58). Those changes are incompatible with goals which require that constant membrane potential be maintained on a time scale of hours. Further work will be required to establish the protein identity of the BEL-insensitive activity.
In conclusion, while regulation of the mitochondrial iPLA2 is not yet completely understood, data presented in this article contribute to our understanding of how mitochondria likely respond to and modulate iPLA2 activity under physiological and pathophysiological conditions that produce changes in energization. The reversibility of enzyme activity upon reenergization, as well as the graded nature of the activation with partial deenergization, fit well with models of signaling and regulatory mechanisms.
Footnotes
Abbreviations:
- BEL
- bromoenol lactone
- CCCP
- carbonyl cyanide m-chlorophenylhydrazone
- FAME
- fatty acid methyl ester
- iPLA2
- Ca2+-independent phospholipase A2
- ROS
- reactive oxygen species
- sn-1/2
- position 1 or 2 within a glycerophospholipid molecule as defined by the stereospecific numbering system
- TMPD
- N,N,N′N′-tetramethyl-phenylenediamine
- TPP+
- tetraphenylphosphonium cation
- TTFA
- thenoyltrifluoroacetone
Animal to animal variation was determined from six repeat experiments using different mitochondrial preparations, which examined iPLA2 after uncoupling with CCCP. The variation is similar when other approaches to deenergization are employed, as can be seen in Fig. 7.
We did not determine the destination of the FFAs that were lost upon reestablishing a membrane potential, which amounted to about 0.3 nmol/mg protein. We did notice, however, that the use of Percoll gradient purified mitochondria or the presence of BEL did not alter that behavior.
In addition to chelating any external Ca2+, in media containing external Na+, EGTA depletes mitochondria of endogenous Ca2+ through action of the Na+/Ca2+ antiporter [see (10, 11) for review].
This research was supported by the Ellie Kovalcik Research Foundation, the Institute for Mitochondrial Biology (Ohio State University), and the Department of Chemistry and Biochemistry (Ohio Northern University).
REFERENCES
- 1.Hunter F. E., Jr, Ford L. 1955. Inactivation of oxidative and phosphorylative systems in mitochondria by preincubation with phosphate and other ions. J. Biol. Chem. 216: 357–369. [PubMed] [Google Scholar]
- 2.Wojtczak L., Lehninger A. L. 1961. Formation and disappearance of an endogenous uncoupling factor during swelling and contraction of mitochondria. Biochim. Biophys. Acta. 51: 442–456. [DOI] [PubMed] [Google Scholar]
- 3.Chappell J. B., Crofts A. R. 1965. Calcium ion accumulation and volume changes of isolated liver mitochondria. Calcium ion-induced swelling. Biochem. J. 95: 378–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Waite M., Van Deenen L. L. M., Ruigrok T. J. C., Elbers P. F. 1969. Relation of mitochondrial phospholipase A activity to mitochondrial swelling. J. Lipid Res. 10: 599–608. [PubMed] [Google Scholar]
- 5.Pfeiffer D. R., Kauffman R. F., Lardy H. A. 1978. Effects of N-ethylmaleimide on the limited uptake of Ca2+, Mn2+, and Sr2+ by rat liver mitochondria. J. Biol. Chem. 253: 4165–4171. [PubMed] [Google Scholar]
- 6.Pfeiffer D. R., Schmid P. C., Beatrice M. C., Schmid H. H. O. 1979. Intramitochondrial phospholipase activity and the effects of Ca2+ plus N-ethylmaleimide on mitochondrial function. J. Biol. Chem. 254: 11485–11494. [PubMed] [Google Scholar]
- 7.Haworth R. A., Hunter D. R. 1979. The Ca2+-induced membrane transition in mitochondria. II. Nature of the Ca2+ trigger site. Arch. Biochem. Biophys. 195: 460–467. [DOI] [PubMed] [Google Scholar]
- 8.Hunter D. R., Haworth R. A. 1979. The Ca2+-induced membrane transition in mitochondria. I. The protective mechanisms. Arch. Biochem. Biophys. 195: 453–459. [DOI] [PubMed] [Google Scholar]
- 9.Hunter D. R., Haworth R. A. 1979. The Ca2+-induced membrane transition in mitochondria. III. Transitional Ca2+ release. Arch. Biochem. Biophys. 195: 468–477. [DOI] [PubMed] [Google Scholar]
- 10.Gunter T. E., Pfeiffer D. R. 1990. The mechanisms by which mitochondria transport calcium. Am. J. Physiol. 258: C755–C786. [DOI] [PubMed] [Google Scholar]
- 11.Pfeiffer D. R., Gunter T. E., Eliseev R., Broekemeier K. M., Gunter K. K. 2001. Release of Ca2+ from mitochondria via the saturable mechanisms and the permeability transition. IUBMB Life. 52: 205–212. [DOI] [PubMed] [Google Scholar]
- 12.Pfeiffer D. R., Kuo T. H., Tchen T. T. 1976. Some effects of Ca2+, Mg2+, and Mn2+ on the ultrastructural, light-scattering properties, and malic enzyme activity of adrenal cortex mitochondria. Arch. Biochem. Biophys. 176: 556–563. [DOI] [PubMed] [Google Scholar]
- 13.Pfeiffer D. R., Tchen T. T. 1975. The activation of adrenal cortex mitochondrial malic enzyme by Ca2+ and Mg2+. Biochemistry. 14: 89–96. [DOI] [PubMed] [Google Scholar]
- 14.Waite M., Sisson P. 1971. Partial purification and characterization of the phospholipase A2 from rat liver mitochondria. Biochemistry. 10: 2377–2383. [DOI] [PubMed] [Google Scholar]
- 15.De Winter J. M., Vianen G. M., Van den Bosch H. 1982. Purification of rat liver mitochondrial phospholipase A2. Biochim. Biophys. Acta. 712: 332–341. [DOI] [PubMed] [Google Scholar]
- 16.Aarsman A. J., de Jong J. G. N., Arnoldussen E., Neys F. W., van Wassenaar P. D., Van den Bosch H. 1989. Immunoaffinity purification, partial sequence, and subcellular localization of rat liver phospholipase A2. J. Biol. Chem. 264: 10008–10014. [PubMed] [Google Scholar]
- 17.Reers M., Pfeiffer D. R. 1987. Inhibition of mitochondrial phospholipase A2 by mono- and dilysocardiolipin. Biochemistry. 26: 8038–8041. [DOI] [PubMed] [Google Scholar]
- 18.Van Schaik R. H. N., Verhoeven N. M., Neijs F. W., Aarsman A. J., Van den Bosch H. 1993. Cloning of the cDNA for 14 kDa group II phospholipase A2 from rat liver. Biochim. Biophys. Acta. 1169: 1–11. [DOI] [PubMed] [Google Scholar]
- 19.Inada M., Tojo H., Kawata S., Tarui S., Okamoto M. 1991. Preferential distribution of group-II-like phospholipase A2 in mononuclear phagocytic cells in rat spleen and liver. Eur. J. Biochem. 197: 323–329. [DOI] [PubMed] [Google Scholar]
- 20.Hatch G. M., Vance D. E., Wilton D. C. 1993. Rat liver mitochondrial phospholipase A2 is an endotoxin-stimulated membrane-associated enzyme of Kupffer cells which is released during liver perfusion. Biochem. J. 293: 143–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Medvedev B. I., Severina E. P., Gogvadze V. G., Chukhlova E. A., Evtodienko Y. V. 1985. Participation of endogenous fatty acids in Ca2+ release activation from mitochondria. Gen. Physiol. Biophys. 4: 549–556. [PubMed] [Google Scholar]
- 22.Hunter D. R., Haworth R. A., Southard J. H. 1976. Relationship between configuration, function, and permeability in calcium-treated mitochondria. J. Biol. Chem. 251: 5069–5077. [PubMed] [Google Scholar]
- 23.Roman I., Gmaj P., Nowicka C., Angielski S. 1979. Regulation of Ca2+ efflux from kidney and liver mitochondria by unsaturated fatty acids and Na+ ions. Eur. J. Biochem. 102: 615–623. [DOI] [PubMed] [Google Scholar]
- 24.Bernardi P., Broekemeier K. M., Pfeiffer D. R. 1994. Recent progress on regulation of the mitochondrial permeability transition: a cyclosporin sensitive pore in the inner mitochondrial membrane. J. Bioenerg. Biomembr. 26: 509–517. [DOI] [PubMed] [Google Scholar]
- 25.Broekemeier K. M., Pfeiffer D. R. 1995. Inhibition of the mitochondrial permeability transition by cyclosporin A during long time frame experiments: relationship between pore opening and the activity of mitochondrial phospholipases. Biochemistry. 34: 16440–16449. [DOI] [PubMed] [Google Scholar]
- 26.Broekemeier K. M., Iben J. R., LeVan E. G., Crouser E. D., Pfeiffer D. R. 2002. Pore formation and uncoupling initiate a Ca2+-independent degradation of mitochondrial phospholipids. Biochemistry. 41: 7771–7780. [DOI] [PubMed] [Google Scholar]
- 27.Gadd M. E., Broekemeier K. M., Crouser E. D., Kumar J., Graff G., Pfeiffer D. R. 2006. Mitochondrial iPLA2 activity modulates the release of cytochrome c from mitochondria and influences the permeability transition. J. Biol. Chem. 281: 6931–6939. [DOI] [PubMed] [Google Scholar]
- 28.Williams S. D., Gottlieb R. A. 2002. Inhibition of mitochondrial calcium-independent phospholipase A2 (iPLA2) attenuates mitochondrial phospholipid loss and is cardioprotective. Biochem. J. 362: 23–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Blum J. L., Kinsey G. R., Monian P., Sun B., Cummings B. S., McHowat J., Schnellmann R. G. 2011. Profiling of fatty acids released during calcium-induced mitochondrial permeability transition in isolated rabbit kidney mitochondria. Toxicol. In Vitro. 25: 1001–1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kinsey G. R., McHowat J., Beckett S., Schnellmann R. G. 2007. Identification of calcium-independant phospholipase A2gamma in mitochondria and its role in mitochondrial oxidative stress. Am. J. Physiol. Renal Physiol. 292: F853–F860. [DOI] [PubMed] [Google Scholar]
- 31.Kinsey G. R., McHowat J., Beckett S., Schnellmann R. G. 2007. Role of Ca2+-independent phospholipase A2gamma in Ca2+-induced mitochondrial permeability transition. J. Pharmacol. Exp. Ther. 321: 707–715. [DOI] [PubMed] [Google Scholar]
- 32.Brustovetsky T., Antonsson B., Jemmerson R., Dubinsky J. M., Brustovetsky N. N. 2005. Activation of calcium-independant phospholipase A (iPLA) in brain mitochondria and release of apoptogenic factors by BAX and truncated BID. J. Neurochem. 94: 980–994. [DOI] [PubMed] [Google Scholar]
- 33.Jaburek M., Jezek J., Zelenka J., Jezek P. 2013. Antioxidant activity by a synergy of redox-sensitive mitochondrial phospholipase A2 and uncoupling protein-2 in lung and spleen. Int. J. Biochem. Cell Biol. 45: 816–825. [DOI] [PubMed] [Google Scholar]
- 34.Broekemeier K. M., Schmid P. C., Schmid H. H. O., Pfeiffer D. R. 1985. Effects of phospholipase A2 inhibitors on ruthenium red-induced Ca2+ release from mitochondria. J. Biol. Chem. 260: 105–113. [PubMed] [Google Scholar]
- 35.Broekemeier K. M., Klocek C. K., Pfeiffer D. R. 1998. Proton selective substate of the mitochondrial permeability transition pore: regulation by the redox state of the electron transport chain. Biochemistry. 37: 13059–13065. [DOI] [PubMed] [Google Scholar]
- 36.Igbavboa U., Pfeiffer D. R. 1991. Transient induction of the mitochondrial permeability transition by uncoupler plus a Ca(2+)-specific chelator. Biochim. Biophys. Acta. 1059: 339–347. [DOI] [PubMed] [Google Scholar]
- 37.Marcinkeviciute A., Mildaziene V., Crumm S., Demin O., Hoek J. B., Kholodenko B. N. 2000. Kinetics and control of oxidative phosphorylation in rat liver mitochondria after chronic ethanol feeding. Biochem. J. 349: 519–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chance B., Williams G. R. 1955. Respiratory enzymes in oxidative phosphorylation. II. Difference spectra. J. Biol. Chem. 217: 395–407. [PubMed] [Google Scholar]
- 39.Schlenk H., Gellerman J. L. 1960. Esterification of fatty acids with diazomethane on a small scale. Anal. Chem. 32: 1412–1414. [Google Scholar]
- 40.Ingledew W. J., Ohnishi T. 1977. The probable site of action of thenoyltrifluoroacetone on the respiratory chain. Biochem. J. 164: 617–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nelson B. D., Norling B., Persson B., Ernster L. 1971. Effect of thenoyltrifluoroacetone on the interaction of succinate dehydrogenase and cytochrome b in ubiquinone-depleted submitochondrial particles. Biochem. Biophys. Res. Commun. 44: 1312–1320. [DOI] [PubMed] [Google Scholar]
- 42.Pfeiffer D. R., Gudz T. I., Novgorodov S. A., Erdahl W. L. 1995. The peptide mastoparan is a potent facilitator of the mitochondrial permeability transition. J. Biol. Chem. 270: 4923–4932. [DOI] [PubMed] [Google Scholar]
- 43.Giedt R. J., Pfeiffer D. R., Matzavinos A., Kao C. Y., Alevriadou B. R. 2012. Mitochondrial dynamics and motility inside living vascular endothelial cells: role of bioenergetics. Ann. Biomed. Eng. 40: 1903–1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mancuso D. J., Jenkins C. M., Sims H. F., Cohen J. M., Yang J., Gross R. W. 2004. Complex transcriptional and translational regulation of iPLAgamma resulting in multiple gene products containing dual competing sites for mitochondrial or peroxisomal localization. Eur. J. Biochem. 271: 4709–4724. [DOI] [PubMed] [Google Scholar]
- 45.Mancuso D. J., Jenkins C. M., Gross R. W. 2000. The genomic organization, complete mRNA sequence, cloning, and expression of a novel human intracellular membrane-associated calcium-independent phospholipase A(2). J. Biol. Chem. 275: 9937–9945. [DOI] [PubMed] [Google Scholar]
- 46.Wolf M. J., Gross R. W. 1996. Expression, purification, and kinetic characterization of a recombinant 80-kDa intracellular calcium-independent phospholipase A2. J. Biol. Chem. 271: 30879–30885. [DOI] [PubMed] [Google Scholar]
- 47.Moon S. H., Jenkins C. M., Liu X., Guan S., Mancuso D. J., Gross A. 2012. Activation of mitochondrial calcium-independent phospholipase A2γ (iPLA2γ) by divalent cations mediating arachidonate release and production of downstream eicosanoids. J. Biol. Chem. 287: 14880–14895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Moon S. H., Jenkins C. M., Kiebish M. A., Sims H. F., Mancuso D. J., Gross R. W. 2012. Genetic ablation of calcium-independent phospholipase A(2)γ (iPLA(2)γ) attenuates calcium-induced opening of the mitochondrial permeability transition pore and resultant cytochrome c release. J. Biol. Chem. 287: 29837–29850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lemasters J. J., Nieminen A-L., Qian T., Trost L. C., Elmore S. P., Nishimura Y., Crowl R. M., Cascio W. E., Bradham C. A., Brenner D. A., et al. 1998. The mitochondrial permeability transition in cell death: a common mechanism in necrosis, apoptosis and autophagy. Biochim. Biophys. Acta. 1366: 177–196. [DOI] [PubMed] [Google Scholar]
- 50.Skulachev V. P. 1998. Uncoupling: new approaches to an old problem of bioenergetics. Biochim. Biophys. Acta. 1363: 100–124. [DOI] [PubMed] [Google Scholar]
- 51.Song H., Ramanadham S., Bao S., Hsu F. F., Turk J. 2006. A bromoenol lactone suicide substrate inactivates group VIA phospholipase A2 by generating a diffusible bromomethyl keto acid that alkylates cysteine thiols. Biochemistry. 45: 1061–1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hao C. M., Breyer M. D. 2008. Physiological regulation of prostaglandins in the kidney. Annu. Rev. Physiol. 70: 357–377. [DOI] [PubMed] [Google Scholar]
- 53.Venuto R. C., O'Dorisio T., Stein J. H., Ferris T. F. 1975. Uterine prostaglandin E secretion and uterine blood flow in the pregnant rabbit. J. Clin. Invest. 55: 193–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mancuso D. J., Sims H. F., Han X., Jenkins C. M., Gaun S. P., Yang K., Moon S. H., Pietka T., Abumrad N. A., Schlesinger P. H., et al. 2007. Genetic ablation of calcium-independent phospholipase A2gamma leads to alterations of mitochondrial lipid metabolism and function resulting in a deficient mitochondrial phenotype. J. Biol. Chem. 282: 34611–34622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bao S., Song H., Wohltmann M., Ramanadham S., Jin W., Bohrer A., Turk J. 2006. Insulin secretory response and phospholipid composition of pancreatic islets from mice that do not express group VIA phospholipase A2 and effects of metabolic stress on glucose homeostasis. J. Biol. Chem. 281: 20958–20973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bao S., Miller D. J., Ma Z., Wohltmann M., Eng G., Ramandham S., Moley K., Turk J. 2004. Male mice that do not express group VIA phospholipase A2 produce spermatozoa with impaired motility and have greatly reduced fertility. J. Biol. Chem. 279: 38194–38200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dennis E. A., Cao J., Hsu Y-H., Margroti V., Kokotos G. 2011. Phospholipase A2 enzymes: physical structure, biological function, disease implication, chemical inhibition, and therapeutic intervention. Chem. Rev. 111: 6130–6185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Crouser E. D., Gadd M. E., Julian M. W., Huff J. E., Broekemeier K. M., Robbins K. A., Pfeiffer D. R. 2003. Quantitation of cytochrome c release from rat liver mitochondria. Anal. Biochem. 317: 67–75. [DOI] [PubMed] [Google Scholar]