Abstract
Oxidative stress, hepatic steatosis, and mitochondrial dysfunction are key pathophysiological features of nonalcoholic fatty liver disease. A conjugated linoleic acid (CLA) mixture of cis9,trans11 (9,11-CLA) and trans10,cis12 (10,12-CLA) isomers enhanced the antioxidant/detoxifying mechanism via the activation of nuclear factor E2-related factor-2 (Nrf2) and improved mitochondrial function, but less is known about the actions of specific isomers. The differential ability of individual CLA isomers to modulate these pathways was explored in Wistar rats fed for 4 weeks with a lard-based high-fat diet (L) or with control diet (CD), and, within each dietary treatment, two subgroups were daily administered with 9,11-CLA or 10,12-CLA (30 mg/day). The 9,11-CLA, but not 10,12-CLA, supplementation to CD rats improves the GSH/GSSG ratio in the liver, mitochondrial functions, and Nrf2 activity. Histological examination reveals a reduction of steatosis in L-fed rats supplemented with both CLA isomers, but 9,11-CLA downregulated plasma concentrations of proinflammatory markers, mitochondrial dysfunction, and oxidative stress markers in liver more efficiently than in 10,12-CLA treatment. The present study demonstrates the higher protective effect of 9,11-CLA against diet-induced pro-oxidant and proinflammatory signs and suggests that these effects are determined, at least in part, by its ability to activate the Nrf2 pathway and to improve the mitochondrial functioning and biogenesis.
Keywords: nuclear factor E2-related factor-2, fatty acids, mitochondrial efficiency
Nonalcoholic fatty liver disease (NAFLD), which includes steatosis and its progression to nonalcoholic steatohepatitis (NASH), is a liver disorder of clinical significance and one of the most frequent hepatic lesions in Western countries. NAFLD has been described as the hepatic manifestation of metabolic syndrome (1), and although its pathogenesis is not fully understood, it is clinically characterized by ectopic lipid accumulation in the liver (steatosis), inflammation, oxidative stress (2), and mitochondrial dysfunction (3).
Mitochondrial function and nuclear factor E2-related factor-2 (Nrf2) have been recognized to play a key role in NAFLD pathogenesis (3, 4). In particular, defects in mitochondrial performance could contribute to the development of liver disease, and mitochondrial oxidative capacity has been considered a good predictor of liver disease (5). Mitochondria are fundamental organelles involved in providing energetic support through the chemiosmotic process of oxidative phosphorylation. In particular, they generate ATP by oxidizing nutrients (glucose, FAs, and some amino acids), and the energy generated by the electron transport is utilized to phosphorylate ADP to ATP. Electron transport and ATP synthesis are tightly coupled, but some of the energy generated by electron transport is uncoupled from ATP synthesis (6). Among the factors that affect the degree of mitochondrial coupling, the permeability of the mitochondrial inner membrane to hydrogen ions (leak) plays an important role. The mitochondrial inner membrane exhibits a basal proton leak pathway that has been estimated to contribute 20–25% to the basal metabolic rate in rats (7). In addition, FAs can act as natural uncouplers of oxidative phosphorylation by generating an FA-dependent proton leak pathway (8), which is a function of their unbound amount in the cell. Notably, an inducible proton leak has recently emerged as a major mechanism for the adjustment of the membrane potential to control mitochondrial reactive oxygen species (ROS) emission. By mildly uncoupling, the mitochondria can avoid the oversupply of electrons/reducing equivalents into the respiratory complexes and minimize the likelihood of electron interaction with oxygen (9). Oxidative stress results from the imbalance between ROS levels and the ability of antioxidant defenses to fully cope with ROS-mediated oxidative damage (10); therefore, either excessive ROS production or limited antioxidant defenses contribute to its occurrence in pathophysiological processes.
Cells have evolved several mechanisms to neutralize oxidative stress, and, among them, Nrf2 is considered the main mediator of cellular adaptation to redox stress (11). In normal conditions, the level of Nrf2 protein in the cell is maintained at very low levels by its inhibitor Keap1, which sequesters Nrf2 in the cytosol and facilitates its degradation via the proteasome. Under mild stress conditions, Nrf2 translocates to the nucleus, where it binds to antioxidant responsive elements and activates the transcription of a wide array of enzymes (phase 2 enzymes), the upregulation of which represents an adaptive response leading to improved antioxidant/detoxifying defenses [glutathione S-transferases (GSTs), NAD(P)H:quinone oxidoreductase (NQO1), and γ-glutamylcysteine ligase (GCL)] (12). Nrf2 also modulates genes involved in metabolic regulation, such as fibroblast growth factor 21 (FGF21), a liver-derived prolipolytic hormone, and PPARs, which play an important role in nutrient homeostasis (13). Notably, the link between metabolism, ROS homeostasis, and mitochondrial metabolism is further indicated by the role played by PPARγ coactivator-1 (PGC-1). PGC-1α plays an important role in mitochondrial biogenesis and in the regulation of genes responsible for ROS detoxification (14); whereas PGC-1β is involved in mitochondrial metabolism, and its activation exerted a protective effect from lipid overload (15).
Conjugated linoleic acid (CLA) is the collective name used to indicate a class of positional and geometric conjugated dienoic isomers of linoleic acid. Among the possible isomers, two [namely, cis9,trans11-octadecadienoic acid (9, 11) and trans10,cis12-octadecadienoic acid (10, 12), which represent ∼90% and 10% of the CLA found in ruminant meats and dairy products, respectively] exhibited characteristic biological activities (16). Consequently, although the isomeric CLA mixture (1:1) has been shown to trigger off the Nrf2 pathway (17, 18) and to modulate hepatic mitochondrial function (19), the efficacy of specific isomers on these mechanisms remains unclear.
The modulation of mitochondrial functions and the activation of the Nrf2 pathway have been suggested for the treatment of NAFLD (4, 20); therefore, drugs or natural molecules improving the mitochondrial function and Nrf2-activated defenses may prove useful in the treatment/prevention of NAFLD. We assumed that the CLA isomer showing an increased ability to improve mitochondrial function and the Nrf2 pathway would also ameliorate proinflammatory and pro-oxidant signs of nutritionally induced steatohepatitis. To test this hypothesis, Wistar rats were fed a normal diet [control diet (CD)] or a lard-based high-fat diet (L) for 4 weeks, and within each dietary treatment, two subgroups were administered cis9,trans11-CLA (9,11-CLA) or trans10,cis12-CLA (10,12-CLA) (30 mg/day). At the end of this period, proinflammatory [monocyte chemoattractant protein-1 (MCP-1), interleukin-1α (IL-1α), and TNF-α] and liver stress markers [alanine amino transferase (ALT) and γ-glutamyl transpeptidase (GGT)] were measured in the sera; mitochondrial functioning (protein mass, respiratory capacity, β-oxidation, proton leak, aconitase activity, and H2O2 yield), histological parameters, and oxidative stress markers [carbonylated proteins (PCs), and thiobarbituric acid reactive substances (TBARSs)] were analyzed; and the expression of cytoprotective genes (GCL, NQO1, and GST), metabolic genes (FGF21, PPARα, and PPARγ), and genes involved in sensing cellular stress, mitochondrial biogenesis, and metabolism (PGC-1α and PGC-1β) were evaluated via biochemical or RT-PCR analysis.
MATERIALS AND METHODS
Reagents
Chemicals were of reagent of higher grade from Sigma-Aldrich (St. Louis, MO), unless otherwise specified. Pure 9,11-CLA (#1245) and 10,12-CLA isomer (#1249) were purchased from Matreya LLC (Pleasant Gap, PA). A primary antibody list is shown in supplementary Table I. Peroxidase-conjugated secondary antibodies against rabbit or mouse IgGs were purchased from Dako or Abcam, respectively.
Animals and diets
Wistar rats (average weight 380 g) (Charles River, Calco, Como, Italy) were divided into two groups (n = 24 each) and individually caged in a temperature-controlled room (24°C), under a 12 h light/12 h dark cycle with free access to water. The first group was fed with a standard rodent diet (CD) (15.88 kJ gross energy/g: 60.4 carbohydrates, 29 protein, and 10.6% fat; Mucedola, Milan, Italy). The second one received L diet (20 kJ/g) in which 40% of metabolizable energy was obtained from lard; the remaining calories were starch (31%) and protein (29%). Each group was further divided in three subgroups (n = 8 each): the first received only CD or L diet, and the two groups were administered daily with 30 mg 9,11-CLA (C9, L9) or 10,12-CLA (C10, L10) isomer corresponding to ∼80 mg/kg body weight or to 0.78 g/day, when expressed in “human equivalent dose” (21). This dose was chosen because it is comparable with the reported daily intake of 9,11-CLA in humans (22) and comprises the contribution of trans9-C18:1, which is converted in the 9,11-isomer by liver desaturases (23).
Throughout the experimental period, body weights and food intakes were monitored daily to allow calculation of body-weight gain and gross energy intake. Spilled food was collected and used for food intake calculation. Gross energy density for rat chow or high-fat diet (HFD; 15.8 or 20.0 kJ/g, respectively) was determined by a bomb calorimeter (Parr adiabatic calorimeter; Parr Instruments Co., Moline, IL). At the end of the experimental period, the rats were anesthetized by an intraperitoneal injection of chloral hydrate (40 mg/100 g body weight) and then euthanized by decapitation. Blood was taken via inferior cava vein. Immediately after blood collection, liver was excised and either immediately processed for the isolation of mitochondria or cut in aliquots and frozen in liquid nitrogen and stored at −80°C for further processing or embedded in OCT compound for histological analysis. Visceral fat pad mass was removed and weighed.
All experiments were conducted in compliance with national guidelines for the care and use of research animals (D.L. 116/92, implementation of EEC directive 609/86). In particular, treatment, housing, and euthanizing of animals met the guidelines set by the Italian Health Ministry (Permission n. 176/2005A), and all procedures were approved by the Federico II University Ethical Committee for Animal Research.
Serum analysis
Total cholesterol and triglyceride concentration and ALT and GGT activities in serum were measured by using standard procedures. IL-1α, interleukin-10 (IL-10), TNF-α, and MCP-1 content in sera was measured by using commercially available ELISA kits (Thermo Scientific, Rockford, IL; RBMS627R and RBMS629R, Biovendor R and D, Brno, Czech Republic).
Histology and immunohistochemistry
After the animal was euthanized, small pieces of liver tissue were quickly embedded in OCT compound for cryosectioning with a cryostat. The sections were fixed in acetone and then stained with Sudan Black (24) or hematoxylin-eosin (HE). Immunohistochemical staining to detect macrophages on rat liver cryosections was performed utilizing mouse anti Rat CD68 IgGs and peroxidase-antiperoxidase (Dako) staining. The density of cells expressing CD68 was quantified by counting the number of positive cells from five randomly selected fields with a microscope with a calibrated ocular. Results were expressed as the number of CD68 positive cells per square millimeter.
Liver lipid content, redox status, and Nrf2-activated enzyme activities
Liver aliquots from the differently treated rats were also used to prepare cytosolic and nuclear extracts (17) to be used for Western blotting assays (supplementary Material). Total lipid content, redox status markers, and the activity of GST and NQO1 were measured as described in the supplementary Material.
Mitochondrial function
Mitochondria isolation and oxygen consumption (polarographically measured using a Clark-type electrode) were carried out as previously reported (25). Oxygen consumption was measured in the presence of substrates and ADP (state 3) and in the presence of substrates alone (state 4), and their ratio [respiratory control ratio (RCR)] was calculated. The rate of mitochondrial FA oxidation was assessed in the presence of palmitoyl-l-carnitine (40 μM). Carnitine-palmitoyl-transferase (CPT) system (CPT1 plus CPT2) and aconitase activity were measured spectrophotometrically (at 412 nm) (26, 27). The rate of mitochondrial H2O2 release was assayed by following the linear increase in fluorescence (ex 312 nm and em 420 nm) due to the oxidation of homovanillic acid in the presence of horseradish peroxidase (28).
Mitochondrial protein mass
Mitochondrial protein mass was assessed by evaluating the expression of cytochrome C in isolated mitochondria by Western blotting and by measuring the activity of a mitochondrial marker enzyme, citrate synthase (CS), in liver homogenate and isolated mitochondria (29). CS activity, measured in the homogenate and expressed per gram wet liver, reflects the product of mitochondrial protein mass and specific activity of the CS enzyme. To determine CS-specific activity, measurements were made in isolated mitochondria, and the results were expressed per milligram of mitochondrial proteins. Finally, mitochondrial protein mass, expressed as milligram per gram of wet liver, was calculated as the ratio between CS activity in the homogenate and isolated mitochondria.
Quantitative RT-PCR analysis
Total RNA was isolated from liver of rats fed with the different diets with or without CLA supplement by using the NucleoSpin RNA II kit (Macherey-Nagel), with an on-column DNase I step. RNA concentrations were given using a Qubit Fluorometer (Invitrogen). RNAs were then reverse transcribed using the SuperScript VILO MasterMix (Invitrogen). A total of 100 ng of cDNA and its dilution series to calculate the efficacy of primers were amplified by quantitative RT-PCR on an iCycler iQTM (Bio-Rad) using 300 nM gene-specific primers, Maxima SYBR Green/Fluorescein qPCR Master Mix (26) (Thermo Scientific), and the following PCR conditions: 1 cycle at 95°C for 10 min, and 40 cycles of 95°C for 15 s, 60°C for 30 s, and 72°C for 30 s. The expression level of β-actin gene was used as an internal control for normalization. Raw cycle threshold values (Ct values) obtained for target genes were subtracted from the Ct value obtained for the reference gene. The final graphical data were derived from the R = (Etarget)ΔCt_target (control - sample)/(Eref)ΔCt_ref (control - sample) formula (30). Universal Probe Library Assay Design Center (https://www.roche-applied-science.com/sis/rtpcr/upl/index.jsp?id=UP030000) was used for designing primers (supplementary Table II).
Statistical analysis
All data, obtained from triplicate analyses, were presented as mean ± standard error. Differences among groups were compared by ANOVA followed by the Newman-Keuls test to correct for multiple comparisons. Differences were considered statistically significant at P < 0.05. All analyses were performed using GraphPad Prism (GraphPad Software, San Diego, CA).
RESULTS
Diet supplementation with 9,11-CLA decreases proinflammatory cytokine release in CD-fed rats
The effects produced by the supplementation of individual CLA isomers on lipid metabolism (total cholesterol and triglycerides), liver damage marker enzymes (ALT and GGT), proinflammatory cytokines (IL-1α and TNF-α), chemokine (MCP-1), and IL-10 were measured in the serum of rats maintained on CD. No differences in total cholesterol, triglycerides, IL-10, MCP-1 levels, or GGT activity were found in the C9 and C10 groups; however, in the C9 animals, a significant decrease of IL-1α and TNF-α levels (27% and 35%, respectively) was found compared with the C10 and the CD rats. In contrast, the ALT level in the C10 rats was significantly higher (47%, P < 0.01) compared with the CD animals (Table 1). These results demonstrate that, due to the low doses used, the administration of individual isomers to CD-fed rats produces differential effects on blood serum parameters and that 9,11-CLA intake is accompanied by a mild but significant decrease in proinflammatory cytokine levels.
TABLE 1.
CLA isomer supplementations have different impacts on serum biochemical markers of CD-fed rats
| Serum Parameters | CD | C9 | C10 |
| Triglycerides (mg/dl) | 100 ± 4 | 106 ± 5 | 110 ± 6 |
| Total cholesterol (mg/dl) | 66 ± 3 | 62 ± 2 | 65 ± 2 |
| TNF-α (pg/ml) | 110 ± 10a | 71 ± 5b | 109 ± 12a |
| MCP-1 (ng/ml) | 3.3 ± 0.2 | 3.7 ± 0.2 | 3.8 ± 0.3 |
| IL-1α (pg/ml) | 56 ± 3a | 41 ± 1b | 51 ± 3a |
| IL-10 (pg/ml) | 56 ± 1 | 60 ± 4 | 57 ± 3 |
| ALT (U/l) | 42 ± 2a | 47 ± 2a | 62 ± 2b |
| GGT (U/l) | 0.54 ± 0.07 | 0.48 ± 0.04 | 0.56 ± 0.08 |
The results are expressed as the means ± SD from triplicate analyses from n = 7 animals/group. Differing superscript letters indicate statistically significant differences (P < 0.05).
Mitochondrial coupling efficiency is downregulated by 9,11-CLA
The increased mitochondrial state 3 and state 4 respiration rates measured in C10 rats using succinate or palmitoyl-carnitine (FA oxidation) as substrates (Fig. 1A, B) were further increased in C9 animals compared with controls. The CPT activity measured in the C9 and C10 rats (6.4 ± 0.5 or 6.2 ± 0.3 nmol/min/mg) was not different from that measured in the CD rats (6.0 ± 0.3 nmol/min/mg). All mitochondrial preparations, regardless of rat treatment, were able to produce RCRs of ≥6, indicating that the mitochondria were not damaged during the isolation procedure.
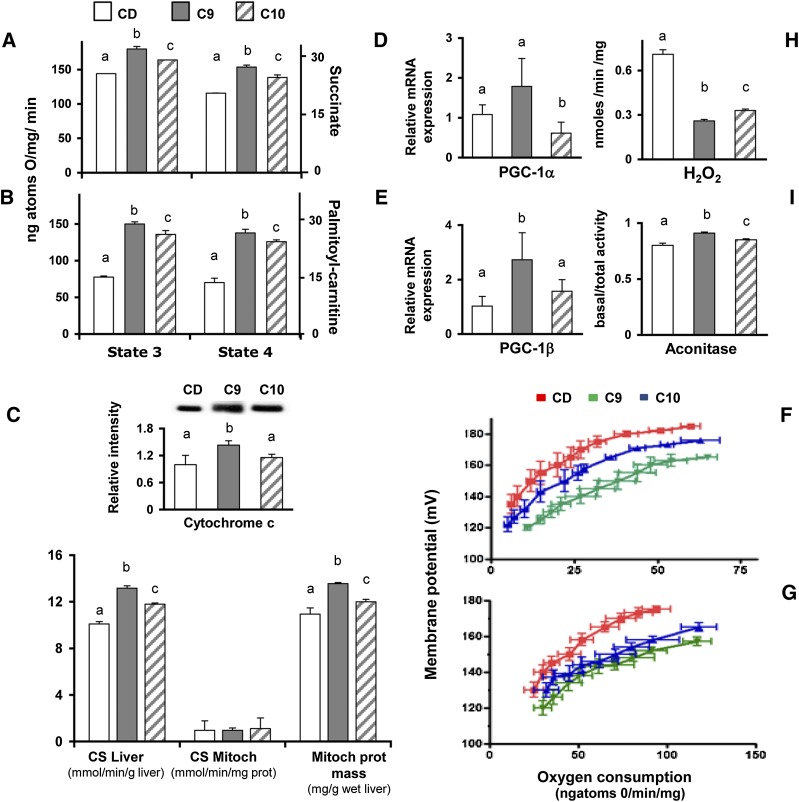
Fig. 1.
CLA isomer differently affects coupling efficiency in the liver mitochondria of chow-fed rats. The liver mitochondrial respiration rates were evaluated in the presence of succinate (A) or palmitoyl-carnitine (B) as substrates. CS activity was measured in liver homogenate and mitochondrial fractions of differently treated rats; the mitochondrial protein mass was then calculated as the ratio between CS activity in the homogenate and the isolated mitochondria (C) and representative immunoblot of cytochrome C levels in liver mitochondria from differently treated rats (C, upper insert). The bands were quantified using densitometric analysis from triplicate experiments, and the values were expressed as average fold increase (± SD) as compared with control (CD). The effect of individual CLA isomers on mitochondria biogenesis or metabolism was evaluated by measuring the mRNA expression PGC-1α (D) or PGC-1β (E), respectively. Basal and palmitate-induced proton leaks were measured in the isolated hepatic mitochondria from C9 (green), C10 (blue), or CD animals (red) (F, G). The effects of CLA supplementation on the intracellular H2O2 yield (H) and basal aconitase/total aconitase ratio were reported (I). The results are expressed as the means ± SD from triplicate analyses from n = 7 animals/group. Differing superscript letters indicate statistically significant differences (P < 0.05).
After 4 weeks of treatment, the CS-specific activity (per milligram protein in the isolated mitochondria) was invariable, whereas significantly higher CS activity (per gram of tissue homogenate) was found. Therefore, the significantly higher mitochondrial protein (calculated as CS activity/CS-specific activity) (Fig. 1C) and cytochrome C found in CLA-treated rats (C9 > C10) (Fig. 1C, insert) indicate that the improved oxidative capacity appears to be supported (at least in part) by an increased mitochondrial mass. In addition, the significant increase of PGC-1α and PGC-1β in C9 rats, as compared with CD or C10 animals (Fig. 1D, E), clearly indicates the 9,11-CLA ability to upregulate mitochondrial biogenesis and metabolism.
Mitochondrial proton leak was assessed via the titration of the steady respiratory rate (state) as a function of mitochondrial membrane potential in liver mitochondria. This titration curve is an indirect measurement of proton leak because a steady oxygen consumption rate (i.e., proton efflux rate) in nonphosphorylating mitochondria is equal to the proton influx rate due to proton leak.
To determine the individual effect of CLA isomers on mitochondrial leakage, both the basal and FFA-induced proton leakage were measured in differently treated animals. When the basal proton leakage was evaluated in the CD rat groups, the mitochondria from the CLA-treated animals exhibited increased proton leakage (C9 > C10); the mitochondria from the C9 rats exhibited the highest oxygen consumption to maintain the same membrane potential compared with the controls and C10 animals (Fig. 1F). In FFA-induced conditions, the mitochondria from the CLA-supplemented animals exhibited comparable kinetic curves (Fig. 1G). No changes were observed in the expression of uncoupling protein 2 (UCP2) (data not shown).
Next, the H2O2 yield in the mitochondria was measured. Aconitase is very sensitive to superoxide exposure; thus, its activity represents a valuable index of ROS-dependent injury. Interestingly, the H2O2 yield was significantly reduced in the mitochondria from the C9 and C10 groups (C9 > C10; P < 0.05) (Fig. 1H), and, similarly, a higher aconitase activity was found in the C9 and C10 rats compared with CD (C9 > C10) (Fig. 1I), thus supporting the link between mitochondrial leakage and redox status (8).
9,11-CLA, but not 10,12-CLA, administration activates the Nrf2 pathway
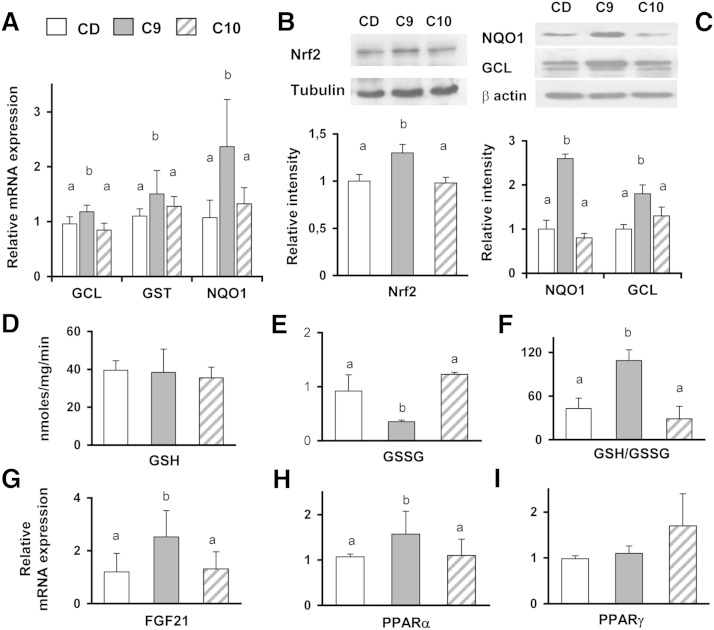
Significantly lower liver lipid contents were measured in the C9 and C10 rats maintained on CD (3.3 ± 0.1% and 3.1 ± 0.2%, respectively) compared with controls (4.0 ± 0.1%). The oxidative stress marker (PC and TBARS) levels measured in C9 and C10 animals were similar to the controls (data not shown). To investigate the ability of individual isomers to activate the Nrf2 pathway, the mRNA expression of antioxidant/detoxifying enzymes (GCL, GST, and NQO1) in the liver was compared between the groups maintained on CD. Interestingly, their levels were significantly elevated in C9 rats, but no difference in mRNA expression was found in the C10 animals compared with CD (Fig. 2A). No change in the GST or NQO1 enzyme activity was found in differently treated rats (data not shown); however, the activation of Nrf2 pathway in C9 rats was evidenced by enhanced translocation of Nrf2 into the nucleus (Fig. 2B) and the higher cytoplasmic levels of GCL (full-length form, 70 kDa) and NQO1, compared with the C10 or CD animals (Fig. 2C).
Fig. 2.
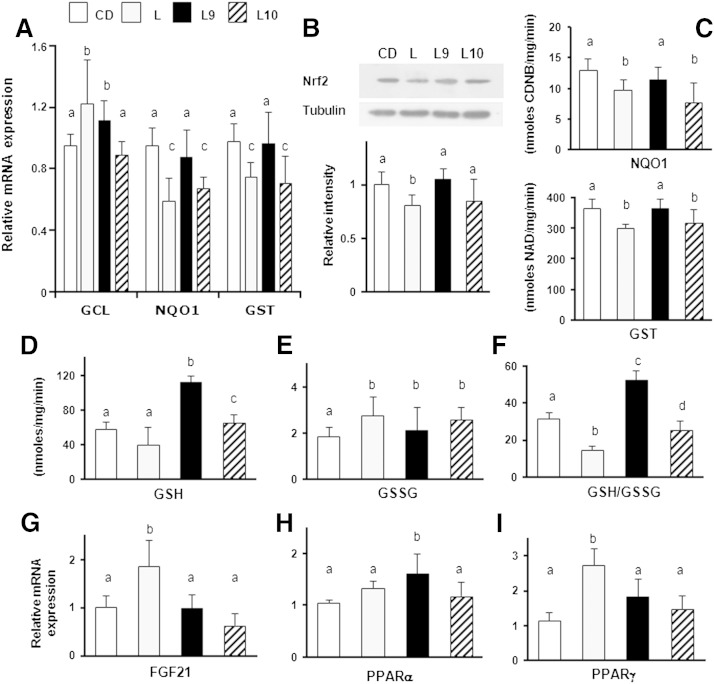
9,11-CLA improves redox status and the Nrf2-mediated antioxidant and metabolic detoxifying defenses in the livers of CD-fed rats. Nrf2 activation in the livers of CD-fed rats with or without the CLA supplement was examined via measuring the mRNA expression of the phase 2 enzymes (GCL, GST, and NQO1) (A) and of genes involved in lipid metabolism (FGF21, PPARα, and PPARγ) (G–I). Representative immunoblot showing Nrf2 translocation in nuclear extracts (B) and GCL and NQO1 protein expression cytoplasmic extracts of the livers from differently treated rats (C) are shown. The bands were quantified by densitometric analysis and normalized against tubulin or β-actin. The values were expressed as average fold increase as compared with controls (CD). Liver GSH (D), GSSG content (E), and GSH/GSSG ratio (F) measured in differently treated rats. The results are expressed as the means ± SD from triplicate analyses from n = 7 animals/group. Differing superscript letters indicate statistically significant differences (P < 0.05).
Next, GSH and GSSG concentrations were measured to evaluate the effects of CLA administration on liver redox status. The GSH content was not significantly different between the considered groups (Fig. 2D), but in C9, the GSSG concentration was ∼3-fold lower compared with the C10 and CD animals (P < 0.02) (Fig. 2E). Consequently, 9,11-CLA administration was associated with a significant increase of the GSH/GSSG ratio (P < 0.001; Fig. 2F) compared with other treatments.
In addition, because Nrf2 activation was demonstrated to induce the expression of metabolic genes (PPARs and FGF21) (13), we investigated whether the enhanced mRNA levels of the phase 2 enzyme genes were accompanied with increased expression of the PPARα, PPARγ, and FGF21 genes. The FGF21 and PPARα mRNA levels in the C9 rats were increased by ∼50% and 30% (P = 0.015 and 0.035, respectively) compared with the untreated group (Fig. 2G, H). The 10,12-CLA supplement tended to increase the PPARγ mRNA levels in the liver, compared with the CD or C9 treatment. However, this difference was not statistically significant (Fig. 2I).
9,11-CLA administration to L-fed rats ameliorates diet-induced alteration of blood parameters
As expected, despite the equal food intake, weight gain and visceral fat pad accumulation in L-fed rats was ∼2-fold higher than those of controls, and a significant lessening (∼50%) resulted from diet supplementation with both CLA isomers (supplementary Fig. I). Similarly, the blood hyperlipidemia and proinflammatory marker levels (TNF-α, IL-1α, and MCP-1), as well as hepatic enzyme activities, were significantly increased by the L diet. The data in Table 2 clearly indicate that 9,11-CLA is more efficacious to hamper the diet-induced alteration of biochemical and immunological parameters. In particular, the triglycerides and total cholesterol were greatly reduced in the L9 rats (26% and 21%, respectively) compared with the L10 rats (11 and 8%, respectively), and a more remarkable reduction of TNF-α and MCP-1 levels was found in the L9 animals (40%) than in the L10 animals (25%), compared with the L-fed rats. Notably, a significant reduction of ALT and GGT activities was measured in the L9 animals (P < 0.05), whereas no measurable effects were observed in the L10 rats. In contrast, despite the negligible effects on IL-10 levels produced by the intake of the L-diet alone, IL-10 was upregulated more by 10,12-CLA than by 9,11-CLA supplementation, compared with the control animals.
TABLE 2.
Differential efficacy of individual CLA isomers on HFD-induced biochemical serum marker changes
| Serum Parameters | L | L9 | L10 |
| Triglycerides (mg/dl) | 168 ± 9a | 125 ± 3b | 150 ± 3c |
| Total cholesterol (mg/dl) | 100 ± 4a | 79 ± 2b | 92 ± 4a |
| TNF-α (pg/ml) | 200 ± 7a | 112 ± 6b | 150 ± 10c |
| MCP-1 (ng/ml) | 6.3 ± 0.5a | 3.8 ± 0.2b | 4.8 ± 0.3c |
| IL-1α (pg/ml) | 121 ± 3a | 80 ± 1b | 76 ± 9b |
| IL-10 (pg/ml) | 56 ± 2a | 68 ± 3b | 86 ± 1c |
| ALT (U/l) | 71 ± 5a | 58 ± 2b | 71 ± 4a |
| GGT (U/l) | 0.71 ± 0.06a | 0.65 ± 0.06b | 0.76 ± 0.08a |
The results are expressed as the means ± SD from triplicate analyses from n = 7 animals/group. Differing superscript letters indicate statistically significant differences (P < 0.05).
Nutritional-induced liver steatosis and oxidative stress are more efficiently ameliorated via 9,11-CLA treatment
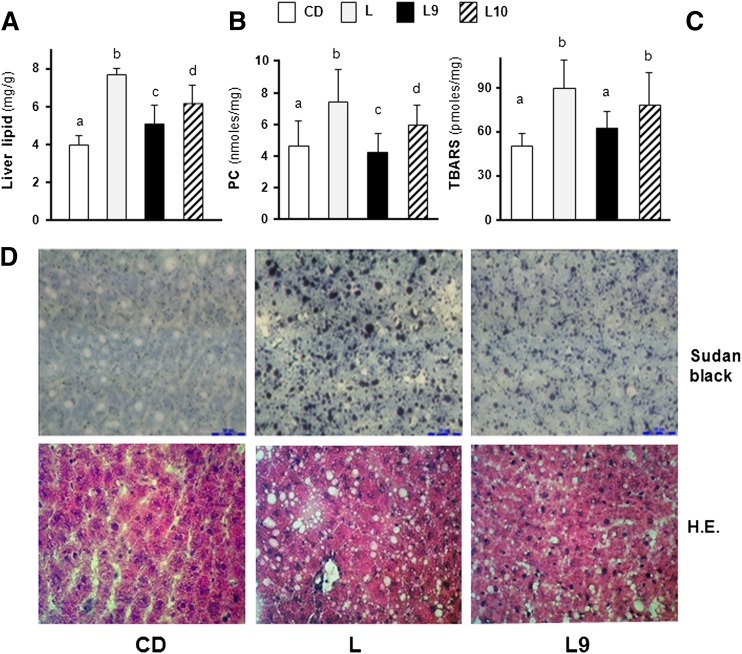
The liver lipid content and oxidative stress markers (PC and TBARS) were determined in differently treated rats. The lipid amount in the livers of the L-fed rats was significantly elevated compared with that in the CD-fed rats, and its amount resulted in ∼8% of the weight of the liver (Fig. 3A), resembling the level typically found in a steatosis (2). A significantly lower fat content was found in the livers of the L9 and L10 animals compared with the L-fed rats (33% or 20%, respectively) (Fig. 3A). Similarly, the diet-induced enhancement of oxidative stress markers (PC and TBARS) was more efficiently decreased by 9,11-CLA than by 10,12-CLA supplementation, and the values measured in the L9 rats were comparable with those found in the CD animals (Fig. 3B, C).
Fig. 3.
CLA supplement improves liver accumulation and histopathological signs in HFD-fed rats. The total lipid (A), PC (B), and TBARS (C) contents in the livers of differently treated rats are shown. The results are expressed as the means ± SD from triplicate analyses from n = 7 animals/group. Differing superscript letters indicate statistically significant differences (P < 0.05). Representative photomicrographs of liver sections from CD, L, and L9 rats (D) stained with Sudan Black (upper panels) or HE (×400) (lower panels).
To assess whether fatty infiltration occurred in the livers of differently treated rats, lipid-specific Sudan Black staining was performed. Our data revealed that lipid accumulation was not present in the hepatocytes of CD rats, as indicated by a weak reactivity to Sudan Black staining (Fig. 3D, upper panel). In the liver section of the L group, an intense pathological injury was observed with the presence of macrovesicular droplets. In the L9 and L10 groups, the pattern of lipid deposition showed a mixed reaction with micro- and macrosteatosis. This difference in the fatty degeneration of the hepatocytes between the CD, L, and L9 rats was also visible after HE staining (white vacuoles; Fig. 3D, lower panel). We were unable to determine the quantitative differences between the CLA-supplemented samples; thus, representative microphotographs of the liver sections from the L9-treated rats are shown (Fig. 3D). The results indicate that CLA treatment ameliorates diet-induced liver steatosis and that the associated oxidative stress signs are more efficiently dampened by 9,11-isomer rather than by 10,12-isomer supplementation. In addition, when histological evaluation of macrophage infiltration was carried out on liver sections, only minor differences in the number of CD68 positive cells were evidenced (supplementary Fig. II).
9,11-CLA supplement highly reduces the efficiency of mitochondrial coupling in L-fed rats
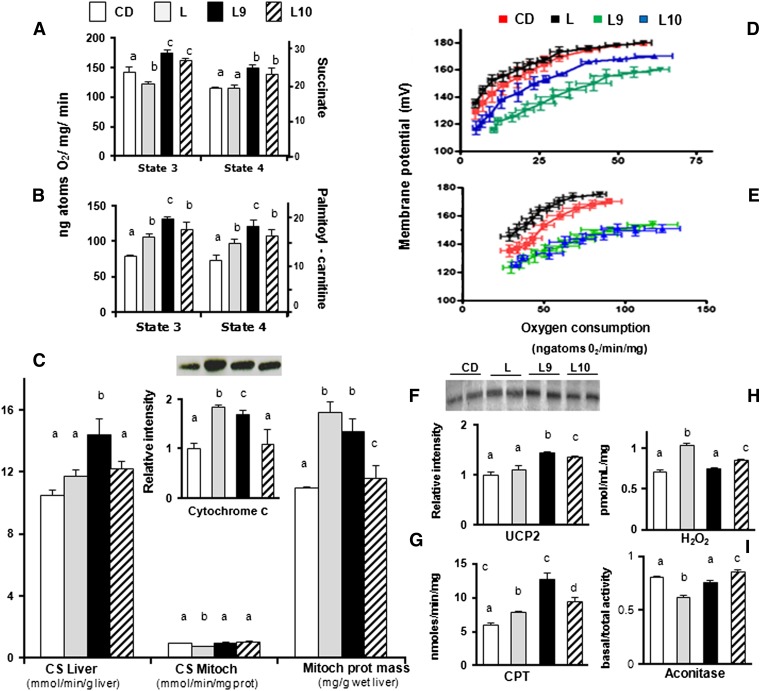
The state 4 mitochondrial oxygen consumption in the L-fed animals in the presence of succinate was similar to that of CD group but increased in the presence of palmitoyl-carnitine. In addition, the oxygen consumption at state 3 was significantly decreased in the presence of succinate and significantly increased in the presence of palmitoyl-carnitine (Fig. 4A, B). Notably, compared with the CD-fed animals, a significant increase in oxygen consumption (at both states 3 and 4) was measured independently from the substrate in the L-fed animals supplemented with individual CLA isomers (L9 > L10) (Fig. 4B).
Fig. 4.
CLA isomers differently affect coupling efficiency in the liver mitochondria of L-fed rats. The liver mitochondrial respiration rates were evaluated in the presence of succinate (A) or palmitoyl-carnitine (B) as substrates. CS activity was measured in the liver homogenate and mitochondrial fractions of differently treated rats; the mitochondrial protein mass was then calculated as the ratio between CS activity in the homogenate and isolated mitochondria (C). The basal and palmitate-induced proton leak from L (black), L9 (green), L10 (blue), or CD animals (red) (D, E) were measured in isolated hepatic mitochondria. Representative immunoblot of cytochrome C and UCP2 levels in liver mitochondria (C and F, insert, respectively) from differently treated rats; the bands were quantified using densitometric analysis from triplicate experiments, and the values were expressed as average fold increase (± SD) as compared with CD (F). The CPT activity (G), intracellular H2O2 yield (H), and basal aconitase/total aconitase ratios were reported (I). The results are expressed as the means ± SD from triplicate analyses from n = 7 animals/group. Differing superscript letters indicate statistically significant differences (P < 0.05).
The mitochondrial protein mass in the L and L9 rats, calculated by CS activity/CS-specific activity or cytochrome C levels (Fig. 4C or insert, respectively), was significantly higher compared with that in CD or L10. In the L rats, the marked increase of mitochondrial protein mass was accompanied by a decreased state 3 respiration rate in the presence of succinate; this suggests that the increased mitochondrial protein mass in these animals was a compensatory mechanism in response to the diet-mediated injury. Figure 4D shows that the basal proton leak in the CLA rats increased (L9 > L10) compared with the CD and L rats. Regarding the FFA-induced proton leak among the four analyzed groups, the L rats had the lowest proton leak, and L9 and L10 had the highest proton leak (Fig. 4E). The UCP2 protein content was increased in L10 and further increased in L9 compared with the L and CD rats (Fig. 4F). The CPT activity in the L9 and L10 rats (12.7 ± 0.9 and 9.5 ± 0.5 nmol/mg/min, respectively) was significantly higher than that in the L (7.8 ± 0.2 nmol/mg/min) or CD (6.0 ± 0.3 nmol/mg/min) rats. The mitochondrial CPT activity was significantly higher in the L9 and L10 rats (32% and 43%) compared with the L group, and it was also higher (23% and 5%, respectively) as compared with the L animals (Fig. 4G). The H2O2 yield was increased in the L rats compared with the control animals and was significantly decreased in the L9 and L10 animals (L9 > L10) compared with the L group (Fig. 4H). Similarly, a lower aconitase activity was found in the L rats compared with the CD group but was significantly higher in the L9 and L10 animals than in the L rats (Fig. 4I). By contrast, the higher PGC-1α transcription levels produced by HFD intake were influenced by CLA intake in a nonsignificant way, while minor alterations of PGC-1β mRNA were observed in the differently treated rats (supplementary Fig. III).
9,11-CLA preserves Nrf2-mediated cytoprotection and ameliorates liver redox status in L-fed animals
PPARγ and FGF21 upregulation of mRNA levels has been previously associated with hepatic steatosis (31, 32); however, owing to the complex interaction between FGF21, PPARα, and PPARγ pathways, their transcriptional levels were used as extra indicators of NAFLD progression or to substantiate the reported links with Nrf2 activation rather than to provide a comprehensive picture of the CLA impact on their expression. To confirm the ability of the 9,11-isomer to enhance the expression of phase 2 enzymes (GCL, GST, and NQO1) and of nonantioxidant genes (FGF21, PPARα, and PPARγ) downstream of the Nrf2 activation, their mRNA levels were measured in the liver of rats maintained on the L diet.
We found that in the L10 animals, the GST and NQO1 levels (similar to the L-fed group) were significantly reduced compared with CD-fed animals, whereas the GCL levels were not markedly influenced by dietary treatment. In contrast, 9,11-CLA administration hindered the diet-induced decline of NQO1 and GST expression and increased GCL expression at levels comparable with those of the CD group (Fig. 5A). The cytoprotective effect of 9,11-CLA was further indicated by the increased Nrf2 translocation into the nucleus (Fig. 5B) and by the NQO1 and GST activities. In fact, these enzymatic activities in the L9 rats were similar to those measured in the CD fed rats, whereas they were significantly reduced in the L and L10 groups (Fig. 5C).
Fig. 5.
9,11-CLA improves the redox status and Nrf2-mediated antioxidant detoxifying defenses in the livers of L-fed rats. Nrf2 activation in the livers of CD- or L-fed rats supplemented or not with CLA supplement was examined by measuring the mRNA expression of downstream genes (GCL, GST, and NQO1) (A) and of genes involved in lipid metabolism (FGF21, PPARα, and PPARγ) (G–I). Representative immunoblot showing Nrf2 levels in nucleus (B) and the effects of the different treatments on enzyme GST and NQO1 activity are shown (C). GSH (D), GSSG content (E), and GSH/GSSG ratio (F) were measured in the livers of differently treated rats. The results are expressed as the means ± SD from triplicate analyses from n = 7 animals/group. Differing superscript letters indicate statistically significant differences (P < 0).
The GSH concentration was markedly increased (∼2-fold) in the liver of the L9 rats, but no significant difference between the L, C10, and CD animals was found (Fig. 5D). The GSSG concentrations in the L, L9, and L10 rat liver were significantly higher than in the CD rats (Fig. 5E), and when the GSH/GSSG ratio was calculated, its levels in the L9 rats were markedly higher (40%) than that found in CD-fed rats; the GSH/GSSG values in L and L10 animals were significantly lower (54% and 20%, respectively) than those found in the CD rats (Fig. 5F). Higher FGF21 and PPARγ mRNA levels (∼2-fold) were found in the liver of the L-fed rats compared with the liver of the CD-fed animals; however, these levels were significantly downregulated in the CLA-treated rats (Fig. 5G, I). Notably, the PPARα mRNA amount was significantly higher in the L9 animals, whereas its transcriptional levels in the L or L10 animals were comparable with those found in the CD group (Fig. 5H). Therefore, it could be hypothesized that enhanced GSH content and preserved detoxifying defenses are responsible for improved redox-buffering capacity in the livers of the 9,11-treated rats.
DISCUSSION
Most of the animal studies aimed at investigating the CLA effects on liver metabolism have used mixtures (1:1) of 9,11- and 10,12-isomers, and, despite the detrimental effect associated to 10,12-CLA intake, only minor effects were evidenced in animals fed with a 9,11-CLA-enriched diet (33). The presented results are the first to indicate that the dietary supplement of 9,11-CLA to young rats activates Nrf2-mediated cytoprotective defenses and improves mitochondrial function more efficiently than 10,12-CLA. Notably, such biological activities also persist in an animal model of nutritionally induced steatosis, leading to the improvement of typical pathological signs such as mitochondrial dysfunction and the enhancement of proinflammatory and pro-oxidant markers. Our observations indicate that the beneficial effects elicited by 9,11-CLA are, at least in part, mediated by its ability to modulate mitochondria ROS emission and Nrf2 activation.
Liver disease is closely associated with hepatic inflammation and with increased serum levels of TNF-α (34) or MCP-1 (35); therefore, our data showing lower serum TNF-α and IL-1α levels in C9 rats, along with increased ALT levels in C10 animals, are indicative of the higher protective effect elicited by 9,11-CLA. In order to compare the preventive effect of the individual CLA isomer on the typical signs of nutritionally induced steatosis, 9,11-CLA or 10-12-CLA was orally administered to rats maintained on an L diet for 4 weeks. A short-treatment L diet has been previously demonstrated to greatly affect liver homeostasis (36); however, the marked increase of ALT levels found in the serum of the L-fed rats was comparable with that expected in the steatosis rather than the steatohepatitis (37). In this experimental condition, the pathological signs were improved by both individual CLA isomer supplements; however, 9,11-CLA administration more effectively downregulates pathological changes in the liver (macro- and microvesicular hepatic steatosis and oxidative stress) and in the serum (ALT, GGT, proinflammatory cytokines, and chemokines) compared with 10,12-CLA treatment. These results are consistent with the reported different health benefits elicited by individual CLA isomers (14), but the favorable effects exhibited by 10,12-CLA are not in complete agreement with a recent review showing its steatogenic activity (33, 38). The explanation for this discrepancy can be attributed to dosage as well as the different treatments or animal models used. Indeed, when low 10,12-CLA doses were administered (similar with those used in the present study), no harmful consequences were observed in mice livers (39). The presented data showing the higher efficacy of 9,11-CLA are not unexpected; although few data show its anti-inflammatory activity in animal models of steatosis, 9,11-CLA was shown to suppress the release of proinflammatory cytokines (primarily TNF-α) in several in vitro and in vivo experimental models (40). It is well established that under the influence of inflammatory signals macrophages accumulate, although to a different extent, in both adipose and liver. Notably, the lack of macrophage accumulation in liver is consistent with data reporting that adipose tissue represents the preferential site of macrophage infiltration during the first 6 weeks of dietary intake of HFD (41). Consequently, it can be concluded that in our experimental conditions the liver does not actively contribute to the proinflammatory environment produced by L treatment.
Oxidative stress, in addition to its causal role in the upregulation of proinflammatory cytokine and hepatocellular injury markers (higher ALT and GGT), plays a central role in the transition from steatosis to NASH and represents a characteristic sign of animal (42) and human NAFLD (43). Data showing the protective effects displayed by CLA administration to L-fed rats agree with the recently reported ability of CLA to improve liver status activity (17), while the more marked antioxidant effect of 9,11-isomer is consistent with data showing the ability of a CLA mixture enriched in 9,11-isomer to reduce the ROS yield/improve redox status (44).
Mitochondria are the major source of ROS and also constitute a major target of cumulative oxidative stress. It is thus conceivable that the generation of ROS damages the area surrounding their site of production. Because mitochondrial dysfunction is central to NASH physiopathology (45), protein mass, oxidative capacity, and energy efficiency were evaluated in hepatic mitochondria from differently treated rats.
To the best of our knowledge, this is the first study aimed at investigating the differential in vivo impact of purified CLA isomers on mitochondrial efficiency, and presented data show that CLA supplementation improves mitochondrial function in the liver in both nonstressing (CD diet) and stressing conditions (L diet). In particular, we demonstrated that 9,11-CLA improves FA oxidation rate and reduces ROS yield (by inducing proton leak, which plays a major role in the control of mitochondrial ROS emission), as indicated by the decline in H2O2 release and increase of basal aconitase/total aconitase ratio (8).
The results confirm the association between HFD-induced ectopic fat storage in the liver and alterations in the mitochondrial compartment (3). The L rats exhibited reduced respiratory capacity and increased oxidative stress in their liver mitochondria even if the ability to utilize fat as a metabolic fuel was elevated. The marked increase of mitochondrial protein mass accompanied by a reduced respiratory capacity suggests that increased mitochondrial protein mass is a compensatory mechanism in response to the diet-mediated injury in L-fed animals. The increased mitochondrial FA oxidation rate observed in the fatty liver can be due to an increased hepatic uptake and synthesis of FFA caused by an HFD and/or enhanced CPT1 activity (46), which would further increase the entry of long-chain FFA into the mitochondria. However, this increase in lipid oxidation is apparently not sufficient to handle the increased load of hepatic FFAs, the result being that the remaining FFAs are converted into triglycerides that are partly stored in the cytoplasm, causing steatosis. An increase in mitochondrial energy efficiency, as shown by the decrease in the induced proton leak in L rats, also contributes to the fat accumulation observed in L rats. Moreover, in L-fed rats, increased mitochondrial oxidative stress parameters (H2O2 production and aconitase inhibition) can be due to the concomitant increase in FA oxidation rate (which enhances the generation of NAD and Flavin Adenine Dinucleotide, Reduced forms and thus electron delivery to the respiratory chain) and respiratory chain impairment (as indicated by the decrease in succinate state 3 oxygen consumption, which would partially block electron flow within the respiratory chain). Furthermore, a decreased proton leak can contribute to excessive ROS formation (8) in these animals. Indeed, the highest increase in respiratory capacity, mitochondrial protein mass, oxidation rate, and CPT system activity exhibited by the rats supplemented with 9,11-CLA could play a role in protecting against diet-induced steatosis. These effects, along with the more marked decline in the efficient utilization of substrates through a stimulation of both the basal and FA-induced proton leak (9,11 > 10,12) would lead to increased fat burn. The significant increase of CS activity, cytochrome C expression, PGC-1α and β, and mRNA levels in the C9 group, when compared with C10 or CD rats, altogether indicate for the first time the capabilities of 9,11-CLA to improve mitochondrial biogenesis.
The Nrf2-mediated adaptive response triggered by mild oxidative stress has been reported as a key protective mechanism (12). We provide evidence that the 9,11-isomer (at a low dose of 80 mg/kg body weight), but not 10,12-CLA, improves liver redox status (higher GSH/GSSG ratio and reduced PC and TBARS) cytoprotective defenses through a typical adaptive response. The upregulation of mRNA/protein expression of well-characterized Nrf2 target genes (GCL, NQO1, and GST) produced by 9,11-CLA administration in nonstressing conditions (CD diet) explains, at least in part, the protective activity exhibited by this isomer against the pro-oxidant effects accompanying stressing conditions (L diet). As is consistent with a previous study (36), short-time administration of HFD reduces the expression of Nrf2 target genes (NQO1 and GST) and their enzyme activity. By contrast, the upregulation of the level of GCL, which is a rate-limiting enzyme in GSH synthesis, likely represents a compensatory mechanism in response to diet-induced oxidative stress (lower GSH/GSSG ratio and enhanced oxidative stress markers). In particular, enhanced GSH and GSH/GSSG found in L9 rats (in the presence of GCL mRNA levels comparable with that of L animals) may partly depend on the improved NADH supply resulting from the increased FA oxidation in mitochondria and by the enhanced conversion of GSSG to GSH (by GSH reductase) downstream the Nrf2 activation (47). Notably, the presented data showing the positive association of Nrf2 activation with PGC-1α levels are consistent with literature data reporting the link between these two important mechanisms (48) and suggest that 9,11-CLA improves ROS detoxification capacities via different mechanisms.
Our results showing the ability of 9,11-CLA to increase PPARα mRNA levels in CD-fed rats are consistent with those in the literature (49). Moreover, the concomitant upregulation of Nrf2 activation and PPARα mRNA levels in C9 rats likely involves signaling from both PPARα and Nrf2; however, the cross talk between these pathways warrants further elucidation. Nevertheless, based on the positive association between PPARα expression and improved redox status in steatotic livers (50), the involvement of PPARα in the beneficial effects produced by 9,11-CLA supplement to HFD-fed rats may be hypothesized. In addition, according to the reported role of ROS in the transcriptional induction of PPARα and PGC-1α (44), it can be hypothesized that their concurrent activation in the liver of C9 rats may be consequential to the generation of mild oxidative stress.
As enhanced mitochondrial uncoupling (essentially increased UCP2 expression) was associated with PPARα induction (51, 52), its involvement in the different efficacy of CLA isomers, as well as in modulating mitochondrial function or UCP2 protein expression in L-fed rats (L9 > L10), may be hypothesized. As is consistent with the reported modulatory role of Nrf2 in metabolic pathways (13), the increase of PPARα and FGF21 mRNA expression in C9 may be a consequence of the Nrf2-mediated upregulation of phase 2 enzymes; in contrast, the marked decline of FGF21 and PPARγ mRNA levels in the L9 and L10 groups is indicative of their protective effects (19, 20). The inhibitory activity displayed by 9,11-CLA administration on FGF21 overexpression induced by the steatogenic diet may be explained on the basis of the reported role of the Nrf2-mediated hepatic antioxidant and detoxification upon NAFLD progression (4), whereas the mechanisms underlying the beneficial effects of 10,12-CLA (reduced liver steatosis in L-fed rats) remain to be clarified. PPARγ is highly expressed in adipose tissue, where activation plays a major role in promoting FA uptake into adipocytes, thereby reducing FA delivery to the liver (51). The presented results showing a lack of influence by 9,11-CLA on PPARγ expression in CD-fed rats are dissimilar to those reporting the positive correlation between Nrf2 and PPARγ in adipose tissue (2, 19) and to data showing the FGF21 upregulation by PPARγ agonists in adipocytes (52, 53). These discrepancies may be explained by the effect elicited by CLA on liver PPARγ. This effect may be secondary to those induced in adipose tissue; therefore, the negligible difference observed caused by any alteration may be too subtle for detection.
In conclusion, our findings indicate that the amelioration of NAFLD signs in response to 9,11-CLA intake is clearly multifactorial and involves plasma-membrane/cytoplasmic (Nrf2-Keap1), nuclear (PPARα), and mitochondrial biogenesis (PGC1-α), as well as proton leak pathways. Our study indicates the ability of dietary 9,11-CLA doses to improve Nrf2- and mitochondrial-mediated cytoprotections and highlights the efficacy of CLA to reduce oxidative stress conditions induced by an HFD. In addition, although the more marked protection exhibited by 9,11-CLA cannot be attributed to a single regulatory mechanism, its beneficial effects are nevertheless likely associated with the improvement of mitochondrial functioning and the adaptive response activated downstream of the Nrf2 pathway. Further experiments with different times and/or dosages are necessary to confirm the therapeutic role of 9,11-CLA for the treatment/prevention of liver disease.
Supplementary Material
Acknowledgments
The authors thank Dr. Emilia De Santis for her skillful management of the animal house.
Footnotes
Abbreviations:
- ALT
- alanine aminotransferase
- C9
- control diet + 9,11-CLA
- C10
- control diet + 10,12-CLA
- CD
- control diet
- CLA
- conjugated linoleic acid
- 9
- 11-CLA, cis9,trans11-CLA
- 10
- 12-CLA, trans10,cis12-CLA
- CPT
- carnitine-palmitoyl-transferase
- CS
- citrate synthase
- FGF21
- fibroblast growth factor 21
- GCL
- γ-glutamylcysteine ligase
- GGT
- γ-glutamyl transpeptidase
- GST
- glutathione S-transferase
- HE
- hematoxylin-eosin
- HFD
- high-fat diet
- IL-1α
- interleukin-1α IL-10, interleukin-10
- L
- lard-based high-fat diet
- L9
- lard-based high-fat diet + 9,11-CLA
- L10
- lard-based high-fat diet + 10,12-CLA
- MCP-1
- monocyte chemoattractant protein-1
- NAFLD
- nonalcoholic fatty liver disease
- NASH
- nonalcoholic steatohepatitis
- NQO1
- NAD(P)H:quinone oxidoreductase 1
- Nrf2
- nuclear factor E2-related factor-2
- PC
- carbonylated protein
- PGC-1
- PPARγ coactivator-1
- ROS
- reactive oxygen species
- TBARS
- thiobarbituric acid reactive substance
- UCP2
- uncoupling protein 2
The online version of this article (available at http://www.jlr.org) contains supplementary data in the form of two tables, three figures, and Materials.
REFERENCES
- 1.Paredes A. H., Torres D. M., Harrison S. A. 2012. Nonalcoholic fatty liver disease. Clin. Liver Dis. 16: 397–419. [DOI] [PubMed] [Google Scholar]
- 2.Reddy J. K., Rao M. S. 2006. Lipid metabolism and liver inflammation. II. Fatty liver disease and fatty acid oxidation. Am. J. Physiol. Gastrointest. Liver Physiol. 290: G852–G858. [DOI] [PubMed] [Google Scholar]
- 3.Mantena S. K., King A. L., Andringa K. K., Eccleston H. B., Bailey S. M. 2008. Mitochondrial dysfunction and oxidative stress in the pathogenesis of alcohol- and obesity-induced fatty liver diseases. Free Radic. Biol. Med. 44: 1259–1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bataille A. M., Manautou J. E. 2012. Nrf2: a potential target for new therapeutics in liver disease. Clin. Pharmacol. Ther. 92: 340–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Szendroedi J., Roden M. 2009. Ectopic lipids and organ function. Curr. Opin. Lipidol. 20: 50–56. [DOI] [PubMed] [Google Scholar]
- 6.Stucki J. W. 1980. The optimal efficiency and the economic degrees of coupling of oxidative phosphorylation. Eur. J. Biochem. 109: 269–283. [DOI] [PubMed] [Google Scholar]
- 7.Rolfe D. F., Brown G. C. 1997. Cellular energy utilization and molecular origin of standard metabolic rate in mammals. Physiol. Rev. 77: 731–758. [DOI] [PubMed] [Google Scholar]
- 8.Skulachev V. P. 1991. Fatty acid circuit as a physiological mechanism of uncoupling of oxidative phosphorylation. FEBS Lett. 294: 158–162. [DOI] [PubMed] [Google Scholar]
- 9.Mailloux R. J., Harper M. E. 2011. Uncoupling proteins and the control of mitochondrial reactive oxygen species production. Free Radic. Biol. Med. 51: 1106–1115. [DOI] [PubMed] [Google Scholar]
- 10.Finkel T., Holbrook N. J. 2000. Oxidants, oxidative stress and the biology of ageing. Nature. 408: 239–247. [DOI] [PubMed] [Google Scholar]
- 11.Niture S. K. R. K., Jaiswal A. K. 2014. Regulation of Nrf2-an update. Regulation of Nrf2—an update. Free Radic. Biol. Med. 66: 36–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Osburn W. O., Kensler T. W. 2008. Nrf2 signaling: an adaptive response pathway for protection against environmental toxic insults. Mutat. Res. 659: 31–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chartoumpekis D. V., Kensler T. W. 2013. New player on an old field; the keap1/Nrf2 pathway as a target for treatment of type 2 diabetes and metabolic syndrome. Curr. Diabetes Rev. 9: 137–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.St-Pierre J., Drori S., Uldry M., Silvaggi J. M., Rhee J., Jäger S., Handschin C., Zheng K., Lin J., Yang W., et al. 2006. Suppression of reactive oxygen species and neurodegeneration by the PGC-1 transcriptional coactivators. Cell. 127: 397–408. [DOI] [PubMed] [Google Scholar]
- 15.Bellafante E., Murzilli S., Salvatore L., Latorre D., Villani G., Moschetta A. 2013. Hepatic-specific activation of peroxisome proliferator-activated receptor γ coactivator-1β protects against steatohepatitis. Hepatology. 57: 1343–1356. [DOI] [PubMed] [Google Scholar]
- 16.Churruca I., Fernández-Quintela A, Portillo M. P. 2009. Conjugated linoleic acid isomers: differences in metabolism and biological effects. Biofactors. 35: 105–111. [DOI] [PubMed] [Google Scholar]
- 17.Bergamo P., Maurano F., Rossi M. 2007. Phase 2 enzyme induction by conjugated linoleic acid improves lupus-associated oxidative stress. Free Radic. Biol. Med. 43: 71–79. [DOI] [PubMed] [Google Scholar]
- 18.Bergamo P., Gogliettino M., Palmieri G., Cocca E., Maurano F., Stefanile R., Balestrieri M., Mazzarella G., David C., Rossi M. 2011. Conjugated linoleic acid protects against gliadin-induced depletion of intestinal defenses. Mol. Nutr. Food Res. 55: S248–S256. [DOI] [PubMed] [Google Scholar]
- 19.Pereira A. F., Sá L. L., Reis F. H., Cardoso F. C., Alberici R. M., Prado I. M., Eberlin M. N., Uyemura S. A., Curti C., Alberici L. C. 2012. Administration of a murine diet supplemented with conjugated linoleic acid increases the expression and activity of hepatic uncoupling proteins. J. Bioenerg. Biomembr. 44: 587–596. [DOI] [PubMed] [Google Scholar]
- 20.Rolo A. P., Teodoro J. S., Palmeira C. M. 2012. Role of oxidative stress in the pathogenesis of nonalcoholic steatohepatitis. Free Radic. Biol. Med. 52: 59–69. [DOI] [PubMed] [Google Scholar]
- 21.Reagan-Shaw S., Nihal M., Ahmad N. 2008. Dose translation from animal to human studies revisited. FASEB J. 22: 659–661. [DOI] [PubMed] [Google Scholar]
- 22.Gebauer S. K., Chardigny J. M., Jakobsen M. U., Lamarche B., Lock A. L., Proctor S. D., Baer D. J. 2011. Effects of ruminant trans fatty acids on cardiovascular disease and cancer: a comprehensive review of epidemiological, clinical, and mechanistic studies. Adv. Nutr. 2: 332–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Turpeinen A. M., Mutanen M., Aro A., Salminen I., Basu S., Palmquist D. L., Griinari J. M. 2002. Bioconversion of vaccenic acid to conjugated linoleic acid in humans. Am. J. Clin. Nutr. 76: 504–510. [DOI] [PubMed] [Google Scholar]
- 24.Humasson G. H. 1972. Staining lipids and carbohydrates. In Animal Tissue Techniques. D. Kennedy and R. B. Park, editors. W. H. Freeman, San Francisco, CA. 45–47. [Google Scholar]
- 25.Lionetti L., Crescenzo R., Mollica M. P., Tasso R., Barletta A., Liverini G., Iossa S. 2004. Modulation of hepatic mitochondrial energy efficiency with age. Cell. Mol. Life Sci. 61: 1366–1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Alexson S. E. H., Nedergaard J. 1988. A novel type of short- and medium chain acyl-CoA hydrolases in brown adipose tissue mitochondria. J . Biol. Chem. 263: 13564–13571. [PubMed] [Google Scholar]
- 27.Hausladen A., Fridovich I. 1996. Measuring nitric oxide and superoxide: rate constants for aconitase reactivity. Methods Enzymol. 269: 37–41. [DOI] [PubMed] [Google Scholar]
- 28.Barja G. 1998. Mitochondrial free radical production and aging in mammals and birds. Ann. N. Y. Acad. Sci. 854: 224–238. [DOI] [PubMed] [Google Scholar]
- 29.Srere P. A. 1969. Citrate synthase. Methods Enzymol. 13: 3–5. [Google Scholar]
- 30.Pfaffl M. W. 2001. A new mathematical model for relative quantification in realtime RT-PCR. Nucleic Acids Res. 29: e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.La Cour Poulsen L., Siersbæk M., Mandrup S. 2012. PPARs: fatty acid sensors controlling metabolism. Semin. Cell Dev. Biol. 23: 631–639. [DOI] [PubMed] [Google Scholar]
- 32.Zhang Y. K., Yeager R. L., Tanaka Y., Klaassen C. D. 2010. Enhanced expression of Nrf2 in mice attenuates the fatty liver produced by a methionine- and choline-deficient diet. Toxicol. Appl. Pharmacol. 245: 326–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vyas D., Kadegowda A. K., Erdman R. A. 2012. Dietary conjugated linoleic acid and hepatic steatosis: species-specific effects on liver and adipose lipid metabolism and gene expression. J. Nutr. Metab. 2012: 932928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Abiru S., Migita K., Maeda Y., Daikoku M., Ito M., Ohata K., Nagaoka S., Matsumoto T., Takii Y., Kusumoto K., et al. 2006. Serum cytokine and soluble cytokine receptor levels in patients with non-alcoholic steatohepatitis. Liver Int. 26: 39–45. [DOI] [PubMed] [Google Scholar]
- 35.Baeck C., Wehr A., Karlmark K. R., Heymann F., Vucur M., Gassler N., Huss S., Klussmann S., Eulberg D., Luedde T., et al. 2012. Pharmacological inhibition of the chemokine CCL2 (MCP-1) diminishes liver macrophage infiltration and steatohepatitis in chronic hepatic injury. Gut. 61: 416–426. [DOI] [PubMed] [Google Scholar]
- 36.Tanaka Y., Aleksunes L. M., Yeager R. L., Gyamfi M. A., Esterly N., Guo G. L., Klaassen C. D. 2008. NF-E2-related factor 2 inhibits lipid accumulation and oxidative stress in mice fed a high-fat diet. J. Pharmacol. Exp. Ther. 325: 655–664. [DOI] [PubMed] [Google Scholar]
- 37.Engl J., Sturm W., Sandhofer A., Kaser S., Tschoner A., Tatarczyk T., Weiss H., Tilg H., Patsch J. R., Ebenbichler C. F. 2008. Effect of pronounced weight loss on visceral fat, liver steatosis and adiponectin isoforms. Eur. J. Clin. Invest. 38: 238–244. [DOI] [PubMed] [Google Scholar]
- 38.Shen W., Chuang C-C., Martinez C., Reid T., Brown J. M., Xi L., Hixson L., Hopkins R., Starnes J., McIntosh M. 2013. Conjugated linoleic acid reduces adiposity and increases markers of browning and inflammation in white adipose tissue of mice. J. Lipid Res. 54: 909–922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Reynolds C. M., Roche H. M. 2010. Conjugated linoleic acid and inflammatory cell signalling. Prostaglandins Leukot. Essent. Fatty Acids. 82: 199–204. [DOI] [PubMed] [Google Scholar]
- 40.Stanton M. C., Chen S. C., Jackson J. V., Rojas-Triana A., Kinsley D., Cui L., Fine J. S., Greenfeder S., Bober L. A., Jenh C. H. 2011. Inflammatory signals shift from adipose to liver during high fat feeding and influence the development of steatohepatitis in mice. J. Inflamm. (Lond.). 8: 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Oliveira C. P., Costa Gayotto L. C., Tatai C., Della Bina B. I., Janiszewski M., Lima E. S., Abdalla D. S., Lopasso F. P., Laurindo F. R., Laudanna A. A. 2002. Oxidative stress in the pathogenesis of nonalcoholic fatty liver disease, in rats fed with a choline-deficient diet. J. Cell. Mol. Med. 6: 399–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Seki S., Kitada T., Yamada T., Sakaguchi H., Nakatani K., Wakasa K. 2002. In situ detection of lipid peroxidation and oxidative DNA damage in non-alcoholic fatty liver diseases. J. Hepatol. 37: 56–62. [DOI] [PubMed] [Google Scholar]
- 43.Choi J. S., Koh I. U., Jung M. H., Song J. 2007. Effects of three different conjugated linoleic acid preparations on insulin signalling, fat oxidation and mitochondrial function in rats fed a high-fat diet. Br. J. Nutr. 98: 264–275. [DOI] [PubMed] [Google Scholar]
- 44.Serviddio G., Sastre J., Bellanti F., Viña J., Vendemiale G., Altomare E. 2008. Mitochondrial involvement in non-alcoholic steatohepatitis. Mol. Aspects Med. 29: 22–35. [DOI] [PubMed] [Google Scholar]
- 45.Lee Y., Wang M. Y., Kakuma T., Wang Z. H., Babcock E., McCorkle K., Higa M., Zhou Y. T., Unger R. H. 2001. Liporegulation in diet-induced obesity. The antisteatotic role of hyperleptinemia. J. Biol. Chem. 276: 5629–5635. [DOI] [PubMed] [Google Scholar]
- 46.Harvey C. J., Thimmulappa R. K., Singh A., Blake D. J., Ling G., Wakabayashi N., Fujii J., Myers A., Biswal S. 2009. Nrf2-regulated glutathione recycling independent of biosynthesis is critical for cell survival during oxidative stress. Free Radic. Biol. Med. 46: 443–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Baldelli S., Aquilano K., Ciriolo M. R. 2013. Punctum on two different transcription factors regulated by PGC-1α: nuclear factor erythroid-derived 2-like 2 and nuclear respiratory factor 2. Biochim. Biophys. Acta. 1830: 4137–4146. [DOI] [PubMed] [Google Scholar]
- 48.Moya-Camarena S. Y., Vanden Heuvel J. P., Belury M. A. 1999. Conjugated linoleic acid activates peroxisome proliferator-activated receptor α and β subtypes but does not induce hepatic peroxisome proliferation in Sprague-Dawley rats. Biochim. Biophys. Acta. 1436: 331–342. [DOI] [PubMed] [Google Scholar]
- 49.Tailleux A., Wouters K., Staels B. 2012. Roles of PPARs in NAFLD: potential therapeutic targets. Biochim. Biophys. Acta. 1821: 809–818. [DOI] [PubMed] [Google Scholar]
- 50.Patterson A. D., Shah Y. M., Matsubara T., Krausz K. W., Gonzalez F. J. 2012. Peroxisome proliferator-activated receptor alpha induction of uncoupling protein 2 protects against acetaminophen-induced liver toxicity. Hepatology. 56: 281–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nakatani T., Tsuboyama-Kasaoka N., Takahashi M., Miura S., Ezaki O. 2002. Mechanism for peroxisome proliferator-activated receptor-alpha activator-induced up-regulation of UCP2 mRNA in rodent hepatocytes. J. Biol. Chem. 277: 9562–9569. [DOI] [PubMed] [Google Scholar]
- 52.Muise E. S., Azzolina B., Kuo D. W., El-Sherbeini M., Tan Y., Yuan X., Mu J., Thompson J. R., Berger J. P., Wong K. K. 2008. Adipose fibroblast growth factor 21 is up-regulated by peroxisome proliferator-activated receptor gamma and altered metabolic states. Mol. Pharmacol. 74: 403–412. [DOI] [PubMed] [Google Scholar]
- 53.Zhang X., Yeung D. C., Karpisek M., Stejskal D., Zhou Z. G., Liu F., Wong R. L., Chow W. S., Tso A. W., Lam K. S., et al. 2008. Serum FGF21 levels are increased in obesity and are independently associated with the metabolic syndrome in humans. Diabetes. 57: 1246–1253. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.