Abstract
Recently it has been shown that levels of circulating oxidized LDL immune complexes (ox-LDL-ICs) predict the development of diabetic retinopathy (DR). This study aimed to investigate whether ox-LDL-ICs are actually present in the diabetic retina, and to define their effects on human retinal pericytes versus ox-LDL. In retinal sections from people with type 2 diabetes, costaining for ox-LDL and IgG was present, proportionate to DR severity, and detectable even in the absence of clinical DR. In contrast, no such staining was observed in retinas from nondiabetic subjects. In vitro, human retinal pericytes were treated with native LDL, ox-LDL, and ox-LDL-IC (0–200 mg protein/l), and measures of viability, receptor expression, apoptosis, endoplasmic reticulum (ER) and oxidative stresses, and cytokine secretion were evaluated. Ox-LDL-IC exhibited greater cytotoxicity than ox-LDL toward retinal pericytes. Acting through the scavenger (CD36) and IgG (CD64) receptors, low concentrations of ox-LDL-IC triggered apoptosis mediated by oxidative and ER stresses, and enhanced inflammatory cytokine secretion. The data suggest that IC formation in the diabetic retina enhances the injurious effects of ox-LDL. These findings offer new insights into pathogenic mechanisms of DR, and may lead to new preventive measures and treatments.
Keywords: apoptosis, blood retinal barrier, diabetes, dyslipidemia, endoplasmic reticulum stress, immunohistochemistry, lipoprotein, oxidative stress, scavenger receptor
Diabetic retinopathy (DR) remains a leading cause of vision loss in working-age adults. Early disease is characterized by vascular abnormalities, including pericyte loss, basement membrane thickening, microaneurysm formation, and capillary leakage (1, 2). Pericytes are critical to vascular integrity (3), and their loss is considered an initiating event in DR.
Dyslipidemia has been implicated in DR, and associations between plasma lipoprotein profiles and disease severity have been observed in large cohort studies (4–6), but were not of sufficient strength to define risk for individuals. The term “dyslipidemia” may include qualitative as well as quantitative alterations in plasma lipoproteins. The qualitative abnormalities include modification by oxidation, but oxidized LDL (ox-LDL) constitutes only a small fraction of total plasma LDL, ranging from 0.001% in healthy people to 5% in the presence of cardiovascular disease (7). We therefore hypothesized that the dominant effects of lipoproteins in DR occur not in plasma, but rather after extravasation through leaking blood-retina barriers (BRBs) (8–15); the extent of this leakage may be more important than plasma concentrations of lipoproteins. We further hypothesized that extravasated intra-retinal lipoproteins undergo extensive modification (glycation, oxidation), rendering them cytotoxic (8). Thus in DR, as in atherosclerosis (16, 17), extravascular modified LDL could play an important role in disease progression; but in contrast to the artery, extravasation of LDL in the retina does not occur unless BRB leakage is present, and thus particularly affects people with diabetes.
Supporting these hypotheses, we demonstrated that apolipoprotein B and ox-LDL were present in diabetic human retinas, even before the development of clinical retinopathy, but were absent in nondiabetic retinas (8). Consistent with a general neurovascular retinal insult in DR (18), we also found evidence of a broad array of cytotoxic effects: “highly oxidized, glycated LDL” promoted oxidative stress, endoplasmic reticulum (ER) stress, inflammation and abnormal gene expression, and decreased viability, not only in retinal vascular cells (endothelial cells, pericytes), but also in Müller glia and retinal pigment epithelial cells (RPEs) (9–15).
It is now well-known that modification of LDL may render it immunogenic, leading to the formation of LDL immune complexes (LDL-ICs) (19). Recently, in the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) cohort, we found that increased levels of circulating ox-LDL-ICs predicted risk for severe nonproliferative DR (NPDR) and proliferative DR (PDR) in type 1 diabetes (20). Others have shown that plasma levels of antibodies to malondialdehyde-modified apolipoprotein B-100 are correlated with DR severity in type 2 diabetes (21). Further emphasizing the importance of ox-LDL-ICs, it is now established that in plasma, only a minor proportion of ox-LDL circulates free; 95% circulates in ICs (7, 19). For ox-LDL-ICs, as with ox-LDL, changes observed in plasma may provide only an indirect index of events in tissue. Thus in the retina, effects of ox-LDL-ICs could predominate over those of ox-LDL, and it is important to define whether IC formation alters the known toxicity of ox-LDL toward retinal cells. In the present study, we aimed to determine whether ox-LDL-ICs are indeed present in the diabetic retina, and to define their effects on human retinal pericytes versus ox-LDL.
MATERIALS AND METHODS
The study was approved by the Institutional Review Boards of the University of Oklahoma Health Sciences Center and the Medical University of South Carolina, and was conducted according to the principles of the Declaration of Helsinki. Written informed consent was obtained from each living participant.
Immunohistochemistry of human retinas
Retinas were obtained postmortem from 15 type 2 diabetic and 5 nondiabetic humans through the National Disease Research Interchange (NDRI) (Philadelphia, PA) as described previously (8). Subjects were grouped as follows: nondiabetic, diabetic without clinical DR, NPDR, and PDR (n = 5), based on the diagnosis provided by NDRI. Ages were similar across the groups (nondiabetic, 62.6 ± 14.9 years; diabetic without DR, 69.4 ± 9.9 years; NPDR, 70.6 ± 8.3 years; and PDR, 57 ± 6.5 years; mean ± SD, P > 0.05 by one-way ANOVA). The eyes were obtained and fixed in 10% neutral-buffered formalin within 12 h after death. For immunohistochemistry, retinal sections (5 μm) were incubated overnight with rabbit polyclonal anti-ox-LDL or goat polyclonal anti-human IgG antibody (Abcam, Cambridge, MA), followed by detection with fluorescence-conjugated anti-rabbit or -goat antibodies (Life Technologies, Carlsbad, CA) and confocal microscopy (Olympus, Japan) as described (8). Absence of nonspecific tissue binding by secondary antibodies was confirmed.
Human LDL preparation
Native LDL (N-LDL) was isolated by sequential ultracentrifugation (density 1.019–1.063) of fresh plasma pooled from 4 to 6 fasted healthy volunteers; ox-LDL was prepared as before (12). For preparation of insoluble ox-LDL-ICs, human ox-LDL antibodies were isolated using a two-step protocol involving affinity chromatography with immobilized protein G (Protein G-Sepharose 4 Fast Flow; Amersham-Pharmacia Biotech, Piscataway, NJ) and fractionation by affinity chromatography in Sepharose-linked ox-LDL (22). Isolated ox-LDL antibodies were centrifuged (90,000 g, 45 min) to eliminate aggregates, sterilized, and confirmed free of endotoxin using the Etoxate kit (detection limit 0.005–0.01 ng/ml; Sigma-Aldrich, St. Louis, MO). Subsequently, human ox-LDL-ICs were prepared (22), with the ratio of ox-LDL and IgG antibody approximately 1:3.3 (w/w).
Human retinal pericyte culture
Human retinal pericytes (Cambrex, Walkersville, MD) were cultured in EBM-2 medium (glucose 5.5 mM) supplemented with the EGM-2 SingleQuot kit (Lonza, Allendale, NJ). Cells of passages 3–9 (80–85% confluence) were pretreated with serum-free medium (SFM) for 18–24 h before being treated with lipoprotein preparations (50–200 mg protein/l). In some experiments, 50 mg/l monoclonal antibodies (Ancell Corp., Bayport, MN) were employed to block CD16, CD32, CD36, and CD64 receptors. Tunicamycin, 4-phenylbutyric acid (4-PBA), tauroursodeoxycholic acid (TUDCA), Tempol, and N-acetyl cysteine were obtained from Sigma-Aldrich.
Cell viability
Pericyte viability was determined in 96-well plates (1 × 104 cells/well) using the Cell Counting Kit-8 (CCK-8; Dojindo Molecular Technologies, Rockville, MD) (15).
Flow cytometry for cell surface receptors
Cultured pericytes were detached from the flask with trypsin, which was neutralized using a Lonza ReagentPack, per vendor's instruction (Lonza). Cells were then incubated with FITC-labeled anti-CD16, -CD32, -CD36, and -CD64 antibodies (R&D, Minneapolis, MN) for 25 min on wet ice and washed with PBS/2%BSA. Cells were resuspended in 300 μl fluorescent-activated cell sorting (FACS) solution, immediately followed by flow cytometry (FACS Calibur; BD Biosciences, San Jose, CA).
Phosphatidylserine externalization was measured using an annexin V-FITC Apoptosis Detection Kit II (BD Pharmingen, Franklin Lakes, NJ) according to the manufacturer's manual. In brief, pericytes were trypsinized, washed twice with cold PBS, and resuspended in a binding buffer [10 mM HEPES/NaOH, (pH 7.4), 140 mM NaCl, 2.5 mM CaCl2] at a density of 1 × 106 cells/ml. One hundred microliters of the cell suspension were transferred to a 5 ml tube to which 5 μl annexin V-FITC and 5 μl propidium iodide were added. After 20 min incubation at room temperature in the dark, an additional 400 μl of binding buffer was added, followed by immediate analysis by flow cytometry.
Caspase-3 activity was measured by a caspase-3 activity assay kit (Cell Signaling Technology, Danvers, MA) per manufacturer's instructions. Briefly, extracted pericyte lysates containing 10 μg protein were added to a black 96-well plate containing 200 μl fluorogenic substrate solution [25 mM HEPES (pH 7.5), 100 mM NaCl, 1 mM EDTA, 0.1% CHAPS, 10 mM DTT, 20 μM Ac-DEVD-AMC] and then incubated at 37°C for 2 h in the dark. Fluorescence was quantified by a VICTOR3 microplate reader (PerkinElmer, Waltham, MA), with excitation at 380 nm and emission at 460 nm.
Mitochondrial membrane potential
DiOC6(3) (Life Technologies) was used to evaluate the mitochondrial membrane potential (Δψm) changes (15). Pericytes were harvested and resuspended in 400 m l PBS containing 20 nM DiOC6(3) followed by 15 min incubation at 37°C in the dark and then analyzed by flow cytometry (FACS Calibur; BD Biosciences). The results are shown as the proportion of cells exhibiting low Δψm as indicated by reduced DiOC6(3) uptake.
Western blot
Cells were homogenized (Complete Lysis Buffer; Roche, Indianapolis, IN) and protein concentrations determined (BCA assay; Pierce, Rutherford, IL). Protein (30 μg) was resolved by SDS-PAGE and blotted with antibodies against 3-nitrotyrosine, glutathione peroxidase 1, 78 kDa glucose-regulated protein (GRP78), phospho-inositol requiring enzyme 1α (p-IRE1α) (all from Abcam), phospho-protein kinase-like ER kinase (p-PERK), C/EBP-homologous protein (CHOP), activated caspase-3, and cleaved poly ADP ribose polymerase (PARP) (all from Cell Signaling Technology). Blots were stripped and reblotted with antibody against β-actin for standardization.
Immunofluorescence for activating transcription factor 6 (ATF6) and terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) assay was performed as previously (15). In brief, pericytes were fixed (4% paraformaldehyde, 20 min), permeabilized, and incubated overnight at 4°C with primary antibody against ATF6 (Abcam), followed by the secondary antibody for 1 h. Apoptosis was assessed by the TUNEL assay (In-Situ-Cell-Death-Detection kit; Roche).
Measurement of intracellular reactive oxygen species
Intracellular reactive oxygen species (ROS) were measured by the dichlorodihydrofluorescein method using CM-H2DCFDA (Invitrogen, Carlsbad, CA) as per manufacturer's instructions. Pericytes were washed, incubated with 2 μM CM-H2DCFDA in phenol red-free medium (37°C, 20 min), and fluorescence was measured (VICTOR3; PerkinElmer).
Measurement of cytokines
Interleukin-6 (IL-6), soluble intercellular adhesion molecule-1 (sICAM-1), soluble vascular cell adhesion molecule-1 (sVCAM-1), pigment epithelium-derived factor (PEDF), and vascular endothelial growth factor (VEGF) were assayed in cell supernatants (DuoSet ELISA; R&D), per manufacturer's instructions.
Data analysis
Data are expressed as means ± SD. Statistical significance was determined by one-way ANOVA followed by Dunnett's post hoc test (Prism 5; GraphPad, La Jolla, CA). P ≤ 0.05 was considered significant.
RESULTS
Ox-LDL and IgG were present and colocalized in diabetic retina
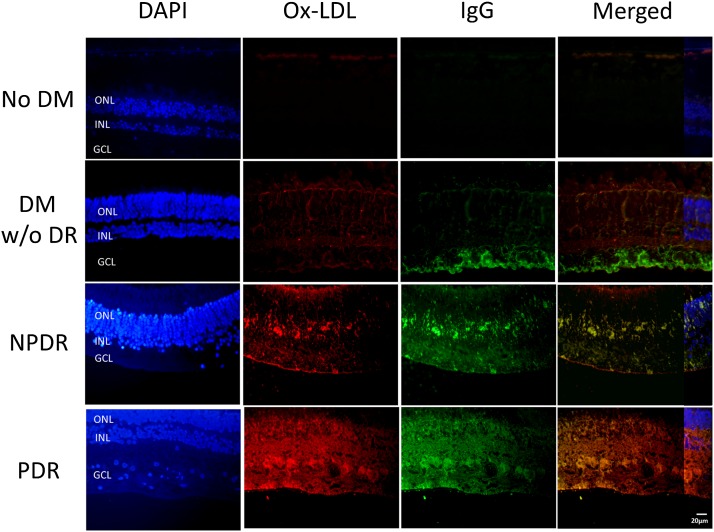
Figure 1 shows the representative ox-LDL and IgG immunostaining in retinal sections from nondiabetic and three categories of type 2 diabetic subjects (no clinical DR, NPDR, PDR). No signal was detectable in nondiabetic retinas. Staining for ox-LDL (red) and IgG (green) was observed in all three diabetic groups, increasing with DR severity. Colocalization of ox-LDL and IgG was clearly seen in the photos merging the two stains, consistent with the presence of ox-LDL-ICs in diabetic retina.
Fig. 1.
Immunostaining for ox-LDL and IgG in human retinas. Immunohistochemistry for ox-LDL (red) and IgG (green) in retinal sections from four groups: nondiabetic (no DM), diabetic without clinical retinopathy (DM w/o DR), NPDR, and PDR. Merged images reveal colocalization of ox-LDL and IgG (yellow), suggesting formation of ox-LDL-ICs. ONL, outer nuclear layer; INL, inner nuclear layer; GCL, ganglion cell layer. DAPI staining was partially superimposed on the “merged” images to show the cell layers.
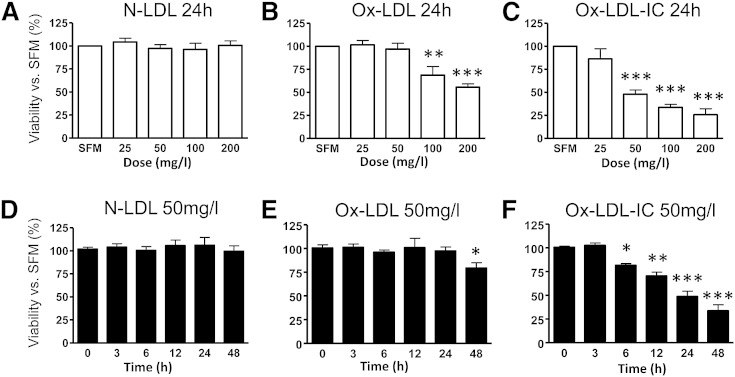
Ox-LDL-ICs caused greater reductions in pericyte viability than ox-LDL
As shown in Fig. 2, both ox-LDL-ICs and ox-LDL decreased pericyte viability in a dose-dependent (0–200 mg/l) and time-dependent (0–48 h) manner. There was a significant leftward shift of the dose-response relationship for ox-LDL-ICs versus ox-LDL, indicating much higher potency of ox-LDL-ICs in triggering cell death. At 50 mg/l, ox-LDL-ICs elicited cell death much earlier that ox-LDL (6 vs. 48 h).
Fig. 2.
Ox-LDL-ICs reduced pericyte viability (CCK-8) more than ox-LDL. A–C: Dose responses to N-LDL, ox-LDL, or ox-LDL-ICs (0–200 mg/l for 24 h). D–F: Time-course responses to 50 mg/l N-LDL, ox-LDL, or ox-LDL-ICs for 0–48 h. Data show percentage versus SFM or predose baseline (mean ± SD, n = 3). *P < 0.05, **P < 0.01, ***P < 0.001 versus SFM or predose.
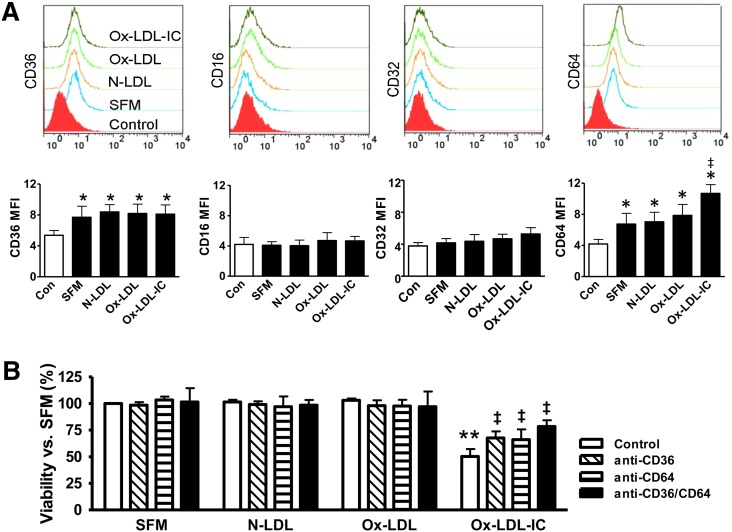
CD36 and CD64 receptors mediated effects of ox-LDL-ICs
To identify the receptors mediating ox-LDL-IC-induced toxicity, we examined the expression of CD36 (receptor for ox-LDL as well as other molecules and multi-molecular complexes), CD16 and CD32 (low-affinity IgG receptors, FcγRIII and FcγRII, respectively), and CD64 (high-affinity FcγRI). CD36 and CD64 were expressed on the pericyte surface, but neither CD16 nor CD32 was detectable (Fig. 3A). Ox-LDL-ICs, but not ox-LDL, induced CD64 upregulation versus SFM and N-LDL. To determine the role of CD36 and CD64, cells were pretreated with relevant blocking antibodies, and viability was determined. Both anti-CD36 and anti-CD64 attenuated ox-LDL-IC-induced cell death, and when combined, evidence suggesting additional protection was observed (P < 0.1 vs. anti-CD36 alone; Fig. 3B).
Fig. 3.
Pericyte surface receptors and effects of ox-LDL-ICs. A: Receptor expression in pericytes. Cells were treated for 6 h with 50 mg/l N-LDL, ox-LDL, ox-LDL-ICs, or SFM, and labeled with FITC-tagged antibodies against CD16, CD32, CD36, or CD64 for flow cytometry. IgG or IgM were used, as appropriate, as nonbinding baseline controls. Mean fluorescence intensities (MFIs) in response to N-LDL, ox-LDL, or ox-LDL-ICs were compared with nonbinding baseline control and SFM (mean ± SD, n = 3; *P < 0.05 vs. nonbinding baseline, indicative of receptor expression; ‡P < 0.05 vs. SFM). CD36 and CD64 were expressed, but not CD16 or CD32. Ox-LDL-ICs increased CD64 (but not CD36) expression versus SFM. Neither ox-LDL nor N-LDL altered CD64 or CD36 expression. B: Receptor-mediated cytotoxicity. Cell viability was measured by the CCK-8 assay after 24 h treatment with 50 mg/l N-LDL, ox-LDL, or ox-LDL-ICs versus SFM. Ox-LDL-ICs significantly reduced pericyte viability; the effects were attenuated by the blocking antibodies against CD36 and CD64. Blockade of both receptors together offered additional protective effects. Data are expressed as percentage of SFM control (mean ± SD, n = 3). **P < 0.01 versus SFM; ‡P < 0.05 versus ox-LDL-IC treatment alone.
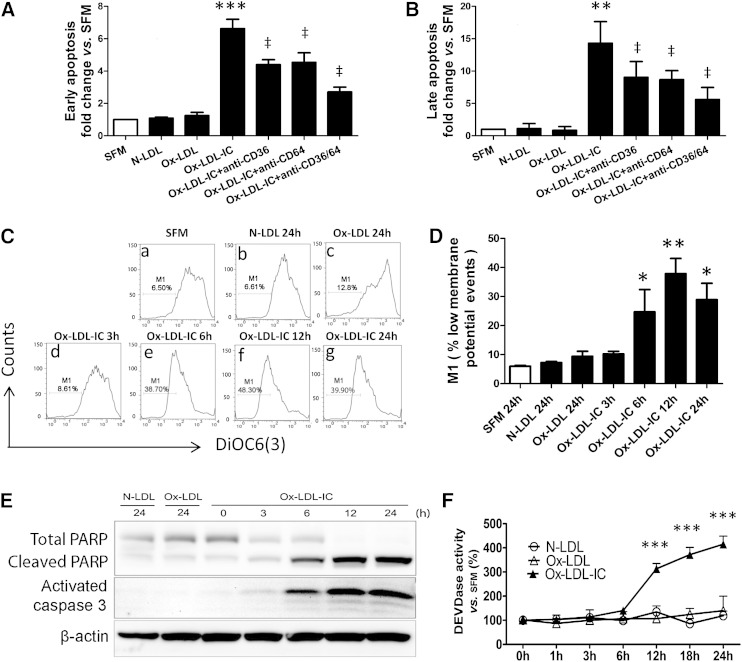
Ox-LDL-ICs caused pericyte death by apoptosis
Because 50 mg/l of ox-LDL was not lethal to pericytes over 24 h, this concentration was selected for additional studies. Flow cytometry showed that ox-LDL-IC dramatically increased apoptosis (annexin V-positive and propidium iodide-negative cells) versus SFM, N-LDL, or ox-LDL (Fig. 4A); apoptosis was attenuated by antagonism of CD36, CD64, or both combined (all P ≤ 0.05). There was a trend toward a greater effect by combined antagonism than by either alone (P = 0.06). TUNEL assay yielded similar results (Fig. 4B). Ox-LDL-ICs decreased Δψm (Fig. 4C, D). Ox-LDL-ICs also increased protein levels of both the cleaved PARP and activated caspase-3 (Fig. 4E), as well as caspase-3 activity (Fig. 4F). In contrast, for all endpoints, 50 mg/l ox-LDL produced minimal effects versus N-LDL. The data support apoptosis mediated by CD36 and CD64 receptors as the mechanism of pericyte death induced by ox-LDL-ICs, and demonstrate that ox-LDL-ICs have greater cytotoxicity than ox-LDL.
Fig. 4.
Ox-LDL-ICs were more potent in inducing pericyte apoptosis than ox-LDL. A, B: Early- and late-stage apoptosis. Effects of 50 mg/l N-LDL, ox-LDL, or ox-LDL-IC ± 1 h pretreatment with 50 mg/l blocking antibodies against CD36 and CD64, individually or combined. A: Early apoptosis: annexin V-positive/propidium iodide-negative cell numbers relative to SFM quantified by flow cytometry after 12 h treatment (mean ± SD, n = 3; ***P < 0.001 vs. SFM; ‡P < 0.05 vs. ox-LDL-IC treatment in absence of blocking antibodies). Treatment with combined antibodies produced greater attenuation of apoptosis than either individually. B: Late apoptosis: quantitative analysis of the TUNEL staining after 24 h treatment, data expressed relative to SFM (mean ± SD, n = 3). **P < 0.01 versus SFM; ‡P < 0.05 versus ox-LDL-IC treatment without blocking antibodies. C: The Δψm: pericytes were treated with SFM (a), 50 mg/l N-LDL (b), ox-LDL (each for 24 h) (c), or 50 mg/l ox-LDL-ICs (d–g) (3–24 h), and then analyzed by the DiOC6(3) method with flow cytometry. The percent total events (i.e., M1) denote low membrane potential corresponding to low fluorescence intensity, indicating mitochondrial dysfunction. D: Quantitative analysis of Δψm assay (mean ± SD, n = 3; *P < 0.05, **P < 0.01 vs. SFM). E: Cleaved PARP and activated caspase-3 by Western blot. Data are representative of three independent experiments. F: Caspase-3 activity using Ac-DEVD-AMC as a substrate (means± SD, n = 3; ***P < 0.001 vs. predose).
ER stress was implicated in ox-LDL-IC-induced apoptosis
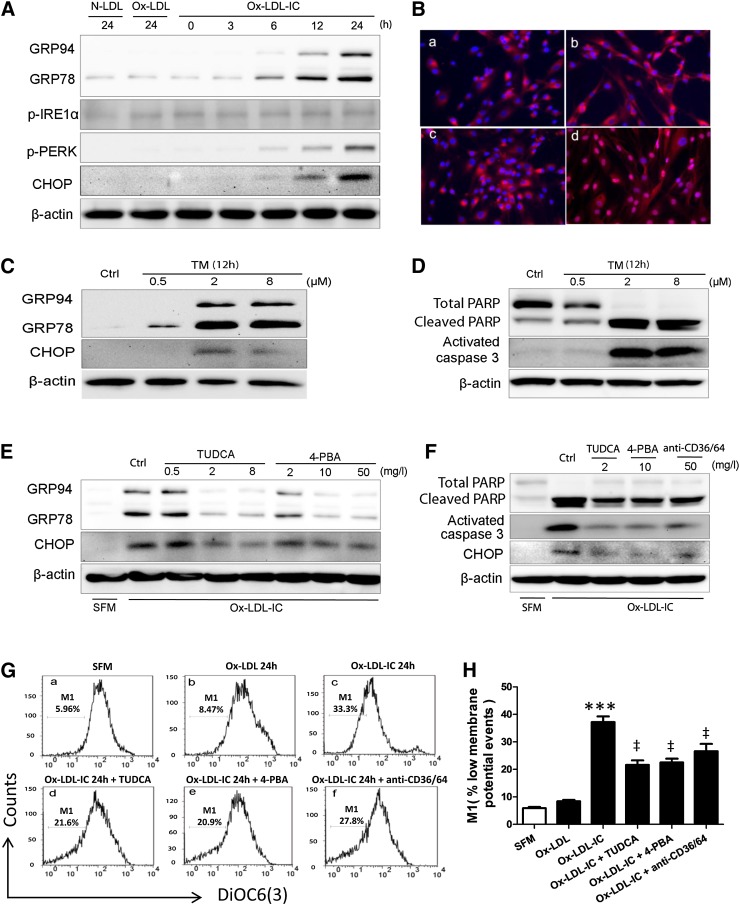
Upon treatment with ox-LDL-ICs, pericytes expressed higher levels of GRP78, an ER stress chaperone, PERK phosphorylation, and CHOP, a pro-apoptotic factor activated in ER stress (Fig. 5A). ATF6 nuclear translocation was increased (Fig. 5B). These findings indicate increased ER stress; however, phosphorylation of IRE1α was unchanged.
Fig. 5.
Ox-LDL-ICs induced greater ER stress than ox-LDL in pericytes. A: GRP94, GRP78, p-IRE1α, p-PERK, and CHOP expression by Western blot after treatment with 50 mg/l N-LDL, ox-LDL, or ox-LDL-ICs. B: ATF6 nuclear translocation at 12 h [SFM (a), N-LDL (b), ox-LDL (c), and ox-LDL-ICs (d) all at 50 mg/l). C, D: GRP94, GRP78, CHOP, cleaved PARP, and activated caspase-3 expression after treatment with tunicamycin (TM, an ER stress inducer) for 12 h. E: Pretreatment with ER stress inhibitors TUDCA and 4-PBA for 1 h reduced the expression of GRP94, GRP78, and CHOP as induced by ox-LDL-ICs (50 mg/ml, 12 h). F–H: Cells were pretreated with TUDCA (2 mg/l), 4-PBA (10 mg/l), or anti-CD36 + CD64 (50 mg/l each) for 1 h, followed by exposure to 50 mg/ml ox-LDL-ICs for 24 h. F: Expression of cleaved PARP, activated caspase-3, and CHOP by Western blot. G: The Δψm assay: pericytes were treated as indicated, followed by percent M1 determination as above. H: Quantitative analysis of the Δψm data (mean ± SD; ***P < 0.001 vs. SFM, ‡P < 0.05 vs. ox-LDL-IC). All experiments were confirmed on three separate occasions. The data implicate ER stress in ox-LDL-IC-induced apoptosis in pericytes: effects of ox-LDL-ICs differ significantly from ox-LDL at this concentration.
Tunicamycin (an ER stress inducer) was used to determine whether ER stress was sufficient to induce apoptosis. After 12 h exposure, increased GRP78 and CHOP confirmed ER stress (Fig. 5C), while caspase-3 activation and PARP cleavage confirmed apoptosis (Fig. 5D). The ER stress inhibitors, 4-PBA and TUDCA, were also employed prior to ox-LDL-IC challenge (Fig. 5E–H). They attenuated ER stress (Fig. 5E), reduced apoptosis (Fig. 5F), and mitigated mitochondrial dysfunction (Fig. 5G, H). Again, CD36 and CD64 blockade inhibited apoptosis and improved mitochondrial function (Fig. 5F–H). The data support a role for ER stress in ox-LDL-IC-induced apoptosis, implicating CHOP.
Increased intracellular ROS by ox-LDL-ICs preceded ER stress
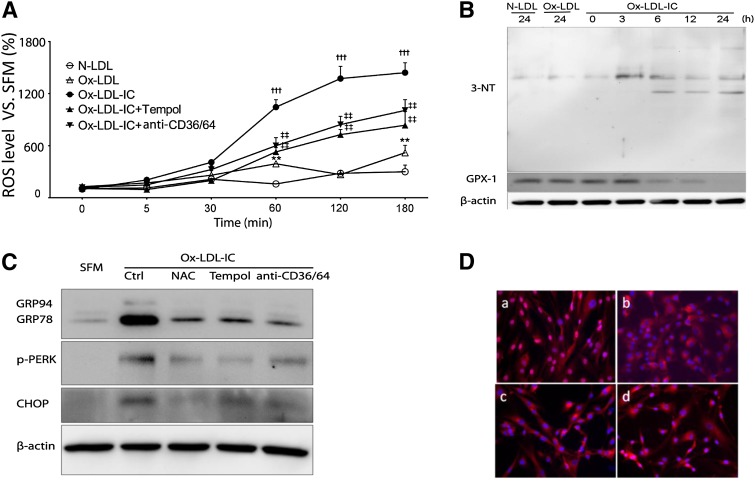
Ox-LDL-ICs significantly increased intracellular ROS versus N- or ox-LDL in a time-dependent fashion (Fig. 6A). The increase was attenuated by pretreatment with the antioxidant Tempol, and by blockade of CD36 and CD64. Ox-LDL-ICs also increased levels of 3-nitrotyrosine-positive proteins, and decreased glutathione peroxidase 1, whereas N- and ox-LDL did not (Fig. 6B). Antioxidants Tempol and N-acetyl cysteine decreased ER stress markers (Fig. 6C, D), suggesting that oxidative stress is upstream of ER stress. Again, blockade of CD36/CD64 was protective.
Fig. 6.
Oxidative stress was proximal to ox-LDL-IC-induced ER stress in pericytes LDL. Cells were exposed to 50 mg/l N-LDL, ox-LDL, or ox-LDL-ICs for various durations, with or without 1 h pretreatment with the antioxidants Tempol (10 mM) or N-acetyl cysteine (NAC; 100 μM), or combined anti-CD36 + CD64 blocking antibodies (50 mg/l) (n = 3 for all experiments). A: Intracellular ROS levels measured by the dichloro-dihydrofluorescein method. Data relative to SFM baseline (mean ± SD; **P < 0.01 vs. N-LDL, †††P < 0.001 vs. N-LDL and ox-LDL, ‡‡P < 0.01 vs. ox-LDL-ICs). B: Increased expression of 3-nitrotyrosine (3-NT) and decreased expression of glutathione peroxidase 1 (GPX-1) following exposure to ox-LDL-ICs. In both experiments (A) and (B), the effects of ox-LDL-ICs differ significantly from ox-LDL; the latter produced minimal changes. C: Effects of antioxidants and receptor antagonism on ox-LDL-IC-induced ER stress. Cells were pretreated with N-acetyl cysteine, Tempol, or combined anti-CD36 + CD64 for 1 h, then exposed to ox-LDL-ICs for 12 h. ER stress markers GRP94, GRP78, p-PERK, and CHOP were determined by Western blot. D: ATF6 nuclear translocation induced by ox-LDL-ICs (12 h) (a) was inhibited by pretreatment with NAC (b), Tempol (c), or anti-CD36 + CD64 combined (d). Overall, data suggest that inhibition of oxidative stress decreases both ER stress and apoptosis.
Ox-LDL-ICs had greater effects on pericyte cytokine secretion than ox-LDL
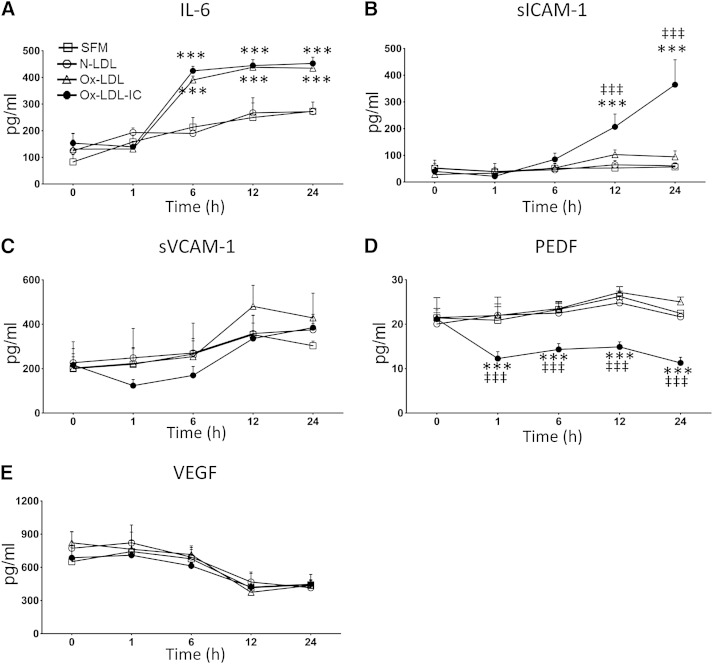
Secretion of cytokines related to inflammation, oxidative stress, and angiogenesis was determined (Fig. 7). Compared with SFM, ox-LDL-ICs significantly increased cellular secretion of IL-6 and sICAM-1, decreased PEDF, and had no effect on sVCAM-1 or VEGF. Ox-LDL affected only IL-6, while N-LDL was without effect.
Fig. 7.
Ox-LDL-ICs exert greater effects on pericyte cytokine secretion than ox-LDL. Pericytes were treated with 50 mg/l N-LDL, ox-LDL, or ox-LDL-ICs versus SFM for up to 24 h. Supernatants were serially harvested to measure the levels of IL-6, sICAM-1, sVCAM-1, PEDF, and VEGF by ELISA. Data are expressed as mean ± SD, n = 3. ***P < 0.001 versus SFM, ‡‡‡P < 0.001 versus ox-LDL. Ox-LDL-ICs significantly increased pericyte secretion of IL-6 and sICAM-1, and decreased secretion of PEDF. No changes were observed for sVCAM-1 or VEGF. In contrast, while ox-LDL increased cellular secretion of IL-6, it had no effect on other cytokines.
DISCUSSION
We present the first evidence for the presence/formation of ox-LDL-ICs in human diabetic retinas. Immunostaining for ox-LDL, detectable prior to the onset of clinical DR, confirmed earlier observations (8), while costaining for IgG supported the concept of ox-LDL-IC formation in the retina. We therefore compared effects of ox-LDL-ICs versus ox-LDL (and N-LDL) on human retinal capillary pericytes in vitro.
We found that ox-LDL-ICs exerted greater toxicity toward retinal capillary pericytes than ox-LDL; low concentrations, at which ox-LDL itself was almost without effect, induced oxidative and ER stresses, apoptosis, increased secretion of inflammatory cytokines, and reduced secretion of a key anti-angiogenic factor, PEDF. The scavenger receptor CD36 and Fcγ receptor I (CD64) were implicated.
Previously, we demonstrated the presence of intra-retinal apolipoprotein B and ox-LDL, attributing it to vascular leakage (no LDL was detectable in the absence of diabetes) (8). Such leakage is likely: in diabetes the BRB becomes permeable to particles that are much larger than LDL (23). Intra-retinal ox-LDL was associated with apoptosis, and in proliferative DR, with macrophage infiltration (8). Consistent with a generalized retinal injury (18), in vitro-modified LDL was toxic toward Müller cells and RPEs as well as toward vascular cells (9–15). In the present work, we have demonstrated that formation of ox-LDL-ICs might further enhance the toxic effects of ox-LDL, and explored some of the mechanisms involved.
Our current findings complement our recent clinical observations in the type 1 diabetes DCCT/EDIC cohort that increased plasma ox-LDL-ICs predict DR progression years in advance (20). In plasma, ox-LDL represents only a small fraction of total LDL (7), and also, most ox-LDL is incorporated in ICs (19). In atheromatous plaque, ox-LDL concentration may be as much as 70-fold higher than in plasma (16). Human plasma has considerable antioxidant capacity, and therefore, LDL oxidation is most likely to happen in the extravascular compartment, and circulating ox-LDL may be derived largely through “reflux” from the sub-endothelial space back into plasma (24). It should also be noted that estimates of intra-plaque ox-LDL concentrations represent averages of a nonuniform distribution; local concentrations could be much higher. This consideration also applies to the retina, given the patchy distribution of apolipoprotein B and ox-LDL seen in our earlier study (8). Overall, we consider the concentrations of ox-LDL and ox-LDL-ICs employed in our present work (10–30% of total plasma LDL) to be conservative estimates of local intra-retinal concentrations in diabetes.
We now demonstrate colocalization of immunoglobulins and ox-LDL, providing strong circumstantial evidence for the presence of intra-retinal ox-LDL-ICs. We observed this colocalization in all three diabetic patient groups, including the “no clinical DR” group, consistent with a role for ox-LDL-ICs in initiating retinal injury. While ox-LDL was found throughout the retina in diabetes, IgG appeared initially in the inner ganglion cell layer (i.e., in subjects with no clinical DR), but permeated all layers in those with overt DR. This is consistent with intra-retinal ox-LDL-IC formation, and suggests that ox-LDL-ICs may contribute strongly to the propagation of DR, including eventually to macrophage activation in PDR.
We recognize that intra-retinal immunoglobulins may bind to antigens other than ox-LDL. Antibodies against vascular cells (25), collagens (26), and phospholipids (27) have been reported in patients with DR. Furthermore, because the retina is lipid-rich, oxidation of other retinal lipids or lipid-protein structures could yield epitopes similar to modified LDL. Finally, BRB leakage in DR implies a general leakage of plasma immunoglobulins into the retina.
Ox-LDL-ICs were more potent than ox-LDL in reducing human retinal pericyte viability via caspase-dependent apoptosis, as measured by phosphatidylserine exposure, TUNEL staining, Δψm, activated caspase-3, and PARP. Apoptosis was mitigated by cyclosporine A, an inhibitor of mitochondrial permeability transition pore opening, and Z-VAD-FMK, a pan-caspase inhibitor (data not shown). While these effects are similar to those of (non-immune-complexed) modified LDL (8, 15), ox-LDL-ICs exhibited toxic effects at much lower concentrations.
To define receptor mechanisms, we hypothesized that putative receptor(s) could be related to ox-LDL and/or IgG. We assessed cell surface expression of the scavenger receptor CD36 (28) and three IgG Fc receptors: high-affinity CD64 (FcγRI), low-affinity CD32 (FcγR II), and CD16 (FcγR III) (29). Only CD36 and CD64 were detectable in pericytes. Ox-LDL-ICs induced CD64 membrane expression, suggesting that IC-pericyte interaction may mediate toxicity. Toxicity was attenuated by blockade of CD36 or CD64, and more effectively when both were blocked simultaneously. Cellular ROS levels, ER stress, and apoptosis were likewise attenuated by CD36/CD64 blockade. Compared with mesangial cells (30), two differences were noted: first, CD36 mediated the interaction with ox-LDL-ICs in pericytes, but not in mesangial cells; second, blockade of CD16 inhibited ox-LDL-IC effects in mesangial cells, but expression of this receptor was undetectable in pericytes. CD64 played a role in both cells.
ER stress has been implicated in DR, and can be elicited by ox-LDL (12, 15, 31, 32). We found ox-LDL-ICs to be more potent inducers of ER stress than ox-LDL, increasing expression of GRP78, activating ER stress sensors PERK and ATF6, and increasing CHOP, a pro-apoptotic protein triggered by ER stress. We did not observe alterations of p-IRE1α, suggesting that it may not be involved; however expression of this protein was low and so this tentative conclusion merits further investigation. ER stress was manifest 3–6 h after treatment, peaking at 12 h; whereas mitochondrial dysfunction and apoptosis were manifest after 6 h, with the peak at 24 h. The data suggest that ER stress precedes apoptosis, with CHOP serving as the link between the two (33). Induction of ER stress by tunicamycin was sufficient to cause apoptosis, whereas ER stress inhibitors, TUDCA and 4-PBA, attenuated mitochondrial dysfunction and apoptosis elicited by ox-LDL-ICs. The data suggest that ER stress mediated apoptosis induced by ox-LDL-ICs in pericytes. While (non-immune-complexed) ox-LDL exhibited similar effects to those of ox-LDL-ICs in pericytes [and in other human retinal cell types including RPEs (14) and Müller cells (12), as well as in mouse retina after intravitreal administration of ox-LDL (34)], it did so only at much higher concentrations.
Oxidative stress, an established risk factor for DR (35), may operate partly through induction of ER stress. In the current study, ox-LDL-ICs induced oxidative and nitrosative stresses and decreased the expression of glutathione peroxidase at much lower concentrations than previously observed with ox-LDL (12, 14, 15). It also reduced PEDF levels, perhaps further compromising antioxidant capacity (13). Attenuation of oxidative stress mitigated ox-LDL-IC-induced ER stress, suggesting that oxidative stress was, at least partly, responsible for the ER stress.
Pericytes play an important role in maintaining vascular integrity (3), and alterations in their secretory behavior may contribute to DR. We previously showed that ox-LDL modulates pericyte cytokine secretion, enhancing inflammation and oxidative stress (13). We now demonstrate that ox-LDL-ICs produced even more robust pro-inflammatory and pro-angiogenic effects, increasing IL-6 and sICAM-1 and decreasing PEDF at concentrations where ox-LDL had little effect.
In summary, our findings support the presence (and probable formation) of ox-LDL-ICs in human diabetic retina, beginning before the clinical onset of DR, and accumulating to a degree proportionate to DR severity. In vitro, ox-LDL-ICs induced more pronounced toxic effects to pericytes than ox-LDL, mediated via CD36 and CD64 receptors. Oxidative stress and subsequent ER stress mediated apoptosis. Ox-LDL-ICs altered the secretory pattern of pericytes, potentially contributing to inflammation and angiogenesis in diabetic retina. Conceivably, therapeutic strategies to reduce IC-mediated retinal injury could include plasma lipid lowering, reduction of oxidative stress and ox-LDL production (e.g., antioxidants and glycemic control), antagonism of CD36 and CD64 receptors, inhibition of IgG synthesis (e.g., rituximab), and anti-inflammatory treatments (e.g., corticosteroids). In practice, the benefits of reducing IC-mediated complications need clearly to outweigh the risks inherent in immuno-suppression, which has the potential for severe side effects. The development of new therapeutic agents to prevent BRB leakage could be the most effective means to reduce LDL extravasation and IC formation in the retina.
Acknowledgments
The NDRI provided valued assistance in obtaining human retinal specimens.
Footnotes
Abbreviations:
- ATF6
- activating transcription factor 6
- BRB
- blood-retina barrier
- CCK-8
- Cell Counting Kit-8
- CHOP
- C/EBP-homologous protein
- DCCT/EDIC
- Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications
- DR
- diabetic retinopathy
- ER
- endoplasmic reticulum
- FACS
- fluorescent-activated cell sorting
- GRP78
- 78 kDa glucose-regulated protein
- IC
- immune complex
- IL-6
- interleukin-6
- NDRI
- National Disease Research Interchange
- N-LDL
- native LDL
- NPDR
- nonproliferative diabetic retinopathy
- ox-LDL
- oxidized LDL
- ox-LDL-IC
- oxidized LDL immune complex
- PARP
- poly ADP ribose polymerase
- 4-PBA
- 4-phenylbutyric acid
- PDR
- proliferative diabetic retinopathy
- PEDF
- pigment epithelium-derived factor
- p-IRE1α
- phospho-inositol requiring enzyme 1α p-PERK, phospho-protein kinase-like endoplasmic reticulum kinase
- ROS
- reactive oxygen species
- RPE
- retinal pigment epithelial cell
- SFM
- serum-free medium
- sICAM-1
- soluble intercellular adhesion molecule-1
- sVCAM-1
- soluble vascular cell adhesion molecule-1
- TUDCA
- tauroursodeoxycholic acid
- TUNEL
- terminal deoxynucleotidyl transferase dUTP nick end labeling
- VEGF
- vascular endothelial growth factor
- Δψm
- mitochondrial membrane potential
This work was supported by the Oklahoma Center for the Advancement of Science and Technology (HR08-067), by the Centers of Biomedical Research Excellence Program of the National Center for Research Resources (P20 RR 024215), by the American Diabetes Association Clinical Translational Research Grant 7-12-CT46, and by the LINJO fund. The authors declare no duality of interest associated with this manuscript.
REFERENCES
- 1.Cogan D. G., Toussaint D., Kuwabara T. 1961. Retinal vascular patterns. IV. Diabetic retinopathy. Arch. Ophthalmol. 66: 366–378. [DOI] [PubMed] [Google Scholar]
- 2.Kern T. S., Engerman R. L. 1995. Vascular lesions in diabetes are distributed non-uniformly within the retina. Exp. Eye Res. 60: 545–549. [DOI] [PubMed] [Google Scholar]
- 3.Hammes H. P. 2005. Pericytes and the pathogenesis of diabetic retinopathy. Horm. Metab. Res. 37(Suppl 1): 39–43. [DOI] [PubMed] [Google Scholar]
- 4.Lloyd C. E., Klein R., Maser R. E., Kuller L. H., Becker D. J., Orchard T. J. 1995. The progression of retinopathy over 2 years: the Pittsburgh Epidemiology of Diabetes Complications (EDC) Study. J. Diabetes Complications. 9: 140–148. [DOI] [PubMed] [Google Scholar]
- 5.Lyons T. J., Jenkins A. J., Zheng D., Lackland D. T., McGee D., Garvey W. T., Klein R. L. 2004. Diabetic retinopathy and serum lipoprotein subclasses in the DCCT/EDIC cohort. Invest. Ophthalmol. Vis. Sci. 45: 910–918. [DOI] [PubMed] [Google Scholar]
- 6.van Leiden H. A., Dekker J. M., Moll A. C., Nijpels G., Heine R. J., Bouter L. M., Stehouwer C. D., Polak B. C. 2002. Blood pressure, lipids, and obesity are associated with retinopathy: the Hoorn study. Diabetes Care. 25: 1320–1325. [DOI] [PubMed] [Google Scholar]
- 7.Holvoet P., De Keyzer D., Jacobs D. R., Jr 2008. Oxidized LDL and the metabolic syndrome. Future Lipidol. 3: 637–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wu M., Chen Y., Wilson K., Chirindel A., Ihnat M. A., Yu Y., Boulton M. E., Szweda L. I., Ma J-X., Lyons T. J. 2008. Intraretinal leakage and oxidation of LDL in diabetic retinopathy. Invest. Ophthalmol. Vis. Sci. 49: 2679–2685. [DOI] [PubMed] [Google Scholar]
- 9.Song W., Barth J. L., Lu K., Yu Y., Huang Y., Gittinger C. K., Argraves W. S., Lyons T. J. 2005. Effects of modified low-density lipoproteins on human retinal pericyte survival. Ann. N. Y. Acad. Sci. 1043: 390–395. [DOI] [PubMed] [Google Scholar]
- 10.Song W., Barth J. L., Yu Y., Lu K., Dashti A., Huang Y., Gittinger C. K., Argraves W. S., Lyons T. J. 2005. Effects of oxidized and glycated LDL on gene expression in human retinal capillary pericytes. Invest. Ophthalmol. Vis. Sci. 46: 2974–2982. [DOI] [PubMed] [Google Scholar]
- 11.Diffley J. M., Wu M., Sohn M., Song W., Hammad S. M., Lyons T. J. 2009. Apoptosis induction by oxidized glycated LDL in human retinal capillary pericytes is independent of activation of MAPK signaling pathways. Mol. Vis. 15: 135–145. [PMC free article] [PubMed] [Google Scholar]
- 12.Wu M., Yang S., Elliott M. H., Fu D., Wilson K., Zhang J., Du M., Chen J., Lyons T. 2012. Oxidative and endoplasmic reticulum stresses mediate apoptosis induced by modified LDL in human retinal Müller cells. Invest. Ophthalmol. Vis. Sci. 53: 4595–4604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang S. X., Wang J. J., Dashti A., Wilson K., Zou M. H., Szweda L., Ma J. X., Lyons T. J. 2008. Pigment epithelium-derived factor mitigates inflammation and oxidative stress in retinal pericytes exposed to oxidized low-density lipoprotein. J. Mol. Endocrinol. 41: 135–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Du M., Wu M., Fu D., Yang S., Chen J., Wilson K., Lyons T. J. 2013. Effects of modified LDL and HDL on retinal pigment epithelial cells: a role in diabetic retinopathy? Diabetologia 56: 2318–2328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fu D., Wu M., Zhang J., Du M., Yang S., Hammad S. M., Wilson K., Chen J., Lyons T. J. 2012. Mechanisms of modified LDL-induced pericyte loss and retinal injury in diabetic retinopathy. Diabetologia. 55: 3128–3140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nishi K., Itabe H., Uno M., Kitazato K. T., Horiguchi H., Shinno K., Nagahiro S. 2002. Oxidized LDL in carotid plaques and plasma associates with plaque instability. Arterioscler. Thromb. Vasc. Biol. 22: 1649–1654. [DOI] [PubMed] [Google Scholar]
- 17.Ylä-Herttuala S., Palinski W., Rosenfeld M. E., Parthasarathy S., Carew T. E., Butler S., Witztum J. L., Steinberg D. 1989. Evidence for the presence of oxidatively modified low density lipoprotein in atherosclerotic lesions of rabbit and man. J. Clin. Invest. 84: 1086–1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Antonetti D. A., Klein R., Gardner T. W. 2012. Diabetic retinopathy. N. Engl. J. Med. 366: 1227–1239. [DOI] [PubMed] [Google Scholar]
- 19.Truman J. P., Al Gadban M. M., Smith K. J., Jenkins R. W., Mayroo N., Virella G., Lopes-Virella M. F., Bielawska A., Hannun Y. A., Hammad S. M. 2012. Differential regulation of acid sphingomyelinase in macrophages stimulated with oxidized LDL and oxidized LDL immune complexes: role in phagocytosis and cytokine release. Immunology. 136: 30–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lopes-Virella M. F., Baker N. L., Hunt K. J., Lyons T. J., Jenkins A. J., Virella G. 2012. High concentrations of AGE-LDL and oxidized LDL in circulating immune complexes are associated with progression of retinopathy in type 1 diabetes. Diabetes Care. 35: 1333–1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fredrikson G. N., Anand D. V., Hopkins D., Corder R., Alm R., Bengtsson E., Shah P. K., Lahiri A., Nilsson J. 2009. Associations between autoantibodies against apolipoprotein B-100 peptides and vascular complications in patients with type 2 diabetes. Diabetologia. 52: 1426–1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Virella G., Atchley D., Koskinen S., Zheng D., Lopes-Virella M. F., Group D. E. R. 2002. Proatherogenic and proinflammatory properties of immune complexes prepared with purified human oxLDL antibodies and human oxLDL. Clin. Immunol. 105: 81–92. [DOI] [PubMed] [Google Scholar]
- 23.Qaum T., Xu Q., Joussen A. M., Clemens M. W., Qin W., Miyamoto K., Hassessian H., Wiegand S. J., Rudge J., Yancopoulos G. D., et al. 2001. VEGF-initiated blood-retinal barrier breakdown in early diabetes. Invest. Ophthalmol. Vis. Sci. 42: 2408–2413. [PubMed] [Google Scholar]
- 24.Itabe H., Obama T., Kato R. 2011. The dynamics of oxidized LDL during atherogenesis. J. Lipids. 2011: 418313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jones D. B., Wallace R., Frier B. M. 1992. Vascular endothelial cell antibodies in diabetic patients. Association with diabetic retinopathy. Diabetes Care. 15: 552–555. [DOI] [PubMed] [Google Scholar]
- 26.Balashova L. M., Zaitseva N. S., Teplinskaia L. E., Koval'chuk L. V., Gankovskaia L. V., Obraztsova E. N., Aronskind M. S. 2000. Antibodies to types II and IV collagens, tumor necrosis factor-alpha and circulating immune complexes in lacrimal fluid and serum of patients with diabetic retinopathy and different stages. Vestn. Oftalmol. 116: 31–34. [PubMed] [Google Scholar]
- 27.Gargiulo P., Goldberg J., Romani B., Schiaffini R., Ciampalini P., Faulk W. P., McIntyre J. A. 1999. Qualitative and quantitative studies of autoantibodies to phospholipids in diabetes mellitus. Clin. Exp. Immunol. 118: 30–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li W., Febbraio M., Reddy S. P., Yu D. Y., Yamamoto M., Silverstein R. L. 2010. CD36 participates in a signaling pathway that regulates ROS formation in murine VSMCs. J. Clin. Invest. 120: 3996–4006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ravetch J. V., Kinet J. P. 1991. Fc receptors. Annu. Rev. Immunol. 9: 457–492. [DOI] [PubMed] [Google Scholar]
- 30.Abdelsamie S. A., Li Y., Huang Y., Lee M-H., Klein R. L., Virella G., Lopes-Virella M. F. 2011. Oxidized LDL immune complexes stimulate collagen IV production in mesangial cells via Fc gamma receptors I and III. Clin. Immunol. 139: 258–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li J., Wang J. J., Yu Q., Wang M., Zhang S. X. 2009. Endoplasmic reticulum stress is implicated in retinal inflammation and diabetic retinopathy. FEBS Lett. 583: 1521–1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jing G., Wang J. J., Zhang S. X. 2012. ER stress and apoptosis: a new mechanism for retinal cell death. Exp. Diabetes Res. 2012: 589589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lin J. H., Walter P., Yen T. S. 2008. Endoplasmic reticulum stress in disease pathogenesis. Annu. Rev. Pathol. 3: 399–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang J, Wu M., Fu D., Yang S., Elliott M. H., Du M., Wilson K. W., Lyons T. J. 2011. Acceleration of retinopathy by intravitreal injection of modified low density lipoproteins in-STZ diabetic mice (Abstract A123 in ARVO 2011 Annual Meeting. Fort Lauderdale, FL, May 1–5, 2011). [Google Scholar]
- 35.Brownlee M. 2001. Biochemistry and molecular cell biology of diabetic complications. Nature. 414: 813–820. [DOI] [PubMed] [Google Scholar]