Abstract
Regulation of maturation in meiotically competent mammalian oocytes is a complex process involving the carefully coordinated exchange of signals between the somatic and germ cell compartments of the ovarian follicle via paracrine and cell-cell coupling pathways. This review highlights recent advances in our understanding of how such signaling controls both meiotic arrest and gonadotropin-triggered meiotic resumption in competent oocytes and relates them to the historical context. Emphasis will be on rodent systems, where many of these new findings have taken place. A regulatory scheme is then proposed that integrates this information into an overall framework for meiotic regulation that demonstrates the complex interplay between different follicular compartments.
I. Overview of Oocyte Maturation
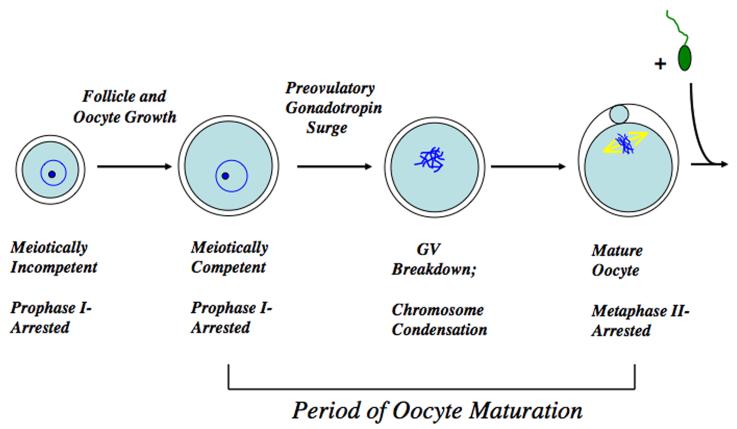
Meiosis is initiated in the mammalian oocyte during fetal development but at about the time of birth becomes arrested late in prophase I, at the late G2 phase of the cell cycle, and can persist in this state for many years. When a cohort of follicles is stimulated to initiate development, meiotic arrest is maintained throughout the period of oocyte and follicle growth, during which time the oocyte achieves competence to undergo meiosis (Fig. 1). Follicle and oocyte development is orchestrated by a bidirectional exchange of signals between the germ and somatic cell compartments; paracrine oocyte signals direct the differentiation of the mural and cumulus granulosa cells and their regulation of germ cell developmental competence (Gilchrist and Thompson, 2007; Su et al, 2009). Meiotically competent oocytes within Graafian follicles will then resume meiotic maturation in response to the preovulatory surge of gonadotropins, a response brought about, at least in part, by the generation of a transient spike in cAMP levels within the granulosa cells.
Figure 1.
Overview of oocyte maturation. The oocyte remains meiotically incompetent and in prophase I arrest during the period of follicular and oocyte growth. At the time of meiotic resumption the nucleus, or germinal vesicle, breaks down as the chromosomes condense and meiosis proceeds until the metaphase II stage that immediately follows extrusion of the first polar body. The MII arrest is maintained in healthy oocytes until successful fertilization is achieved, whereupon the oocyte completes maturation, maternal and paternal chromatin is combined, and embryonic development is initiated.
If oocytes are removed from non-stimulated Graafian follicles and placed in a suitable culture medium, they will undergo spontaneous, hormone-independent nuclear maturation, indicated by germinal vesicle breakdown (GVB), the first easily observed manifestation of maturation (Pincus and Enzmann, 1934; Edwards, 1965). The G2-to-M phase cell cycle progression, which occurs at the time of GVB in oocytes, depends on the activation of maturation promoting factor (MPF), a dimer comprised of CDK kinase and cyclin B subunits (Norbury and Nurse, 1992). MPF activation leads to a cascade of cellular phosphorylation through serine/threonine kinase activity (Murray and Kirschner, 1989), driving chromosome condensation and dissolution of the nuclear envelope. MPF activity is negatively controlled by cAMP through regulation of Wee1 kinases and cdc25 phosphatases (Han et al, 2005; Han and Conti, 2006; Solc et al, 2008; Zhang et al, 2008; Pirino et al, 2009).
Because of the phenomenon of spontaneous maturation, it is evident that the somatic compartment of the ovarian follicle imposes an inhibitory constraint upon the oocyte, maintaining it in meiotic arrest, and this meiotic arrest can be achieved in vitro in isolated oocytes by a number of inhibitory agents, including cAMP analogs, purines and phosphodiesterase inhibitors (Schultz, 1986), that block the activation of MPF (Choi et al, 1991). However, under these inhibitory conditions, addition of gonadotropin or other suitable ligand leads to the induction of GVB, but only in oocytes enclosed by granulosa cells (Downs et al, 1988). Thus, within the follicle, the somatic compartment controls both the maintenance of meiotic arrest and induction of meiotic resumption.
Once GVB has been triggered, the oocyte progresses meiotically through metaphase I (MI) and reaches metaphase II (MII), where it arrests for a second time. This second arrest point occurs at about the time of ovulation and is controlled by the oocyte itself, since by this time close association of the somatic and germ cell compartments has been eliminated through mucification and expansion of the cumulus granulosa cells. No further development is possible unless the oocyte is fertilized or activated parthenogenetically.
This review will focus on aspects of meiotic regulation surrounding the prophase I block, including maintenance of meiotic arrest and possible mechanisms of meiotic induction. Emphasis will be on studies using rodent oocytes, since many recent advances in our understanding of meiosis have occurred in these species. Other useful reviews on oocyte maturation include Zhang et al, 2009; Hsieh et al, 2009; Tsafriri and Motola, 2007; Lefevre et al, 2007; Kimura et al, 2007; Mehlmann, 2005; Tsafriri et al, 2005; Dekel, 2005).
II. In Vitro Models for Studying Mammalian Oocyte Maturation
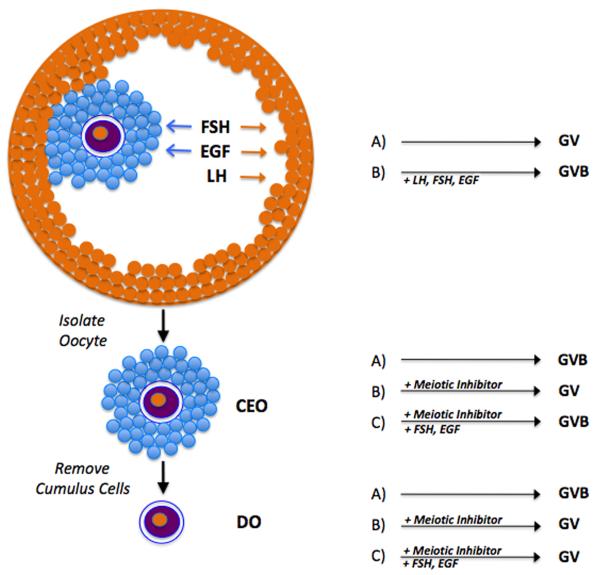
Illustrated in Fig. 2 are three different in vitro models commonly utilized to study oocyte maturation. There are pros and cons to each of these approaches, but information gleaned from all three has proven useful in delineating different aspects of meiotic regulation. The first is the most physiological and involves dissecting out intact preovulatory follicles. Because the follicle is intact, meiotic arrest is maintained naturally in culture without the need for supplementary inhibitors. Meiotic resumption can then be stimulated with gonadotropins or other agents such as EGF-like peptides. The second model system utilizes cumulus cell-enclosed oocytes (CEO) isolated from preovulatory follicles. Due to the reduced number of follicle cells, meiosis-inhibitory capability is lost, and these oocytes will undergo spontaneous maturation in vitro unless a meiotic inhibitor, such as a cAMP analog or phosphodiesterase inhibitor, is added to the medium. Under inhibitory conditions, meiotic resumption can be induced by gonadotropin or other stimulatory ligands. In the last system, the cumulus cells are removed to produce denuded oocytes (DO). DO will also undergo spontaneous maturation unless meiotic inhibitors are added, but, unlike CEO, will not respond to hormonal stimulation due to the absence of mediating follicle cells that possess the necessary receptors.
Figure 2.
In vitro model systems for studying oocyte maturation. The intact preovulatory follicle (top) maintains the oocyte in the germinal vesicle (GV) stage in the absence of hormonal stimulation. The addition of hormone (gonadotropins or EGF-like peptides) stimulates germinal vesicle breakdown (GVB) after binding to receptors on the follicle cells. Arrows show the cells responsive to each of the hormones. Removal of the cumulus-enclosed oocyte (CEO) results in spontaneous maturation in the absence of added inhibitor. If maintained in meiotic arrest with appropriate inhibitor, GVB can be induced by proper hormonal stimulation (FSH and EGF-like peptides, but not LH). Removal of cumulus cells produces a denuded oocyte (DO) that will also undergo spontaneous maturation, but is unresponsive to hormones if maintained in meiotic arrest.
It is important to understand the limitations of these different experimental approaches. While the study of isolated DO and CEO enables the examination of direct effects of certain agents on these target cells without the confounding influence of additional follicular tissue, the system is not completely physiological. On the other hand, it is sometimes difficult when using the more complex cultured follicle system or intact animal to identify the specific tissue(s) targeted by a particular treatment that mediate a meiotic response. Also, the literature is replete with studies demonstrating that the spontaneous meiotic maturation occurring when oocytes are removed from the follicle and cultured in permissive medium is not under the same regulation as oocytes induced to resume maturation by hormonal stimulation. Thus, one must be cautious when using the spontaneous maturation model, since some of the oocyte behaviors may not duplicate those elicited by meiosis-inducing stimuli in the intact follicle.
III. Maintenance of Meiotic Arrest
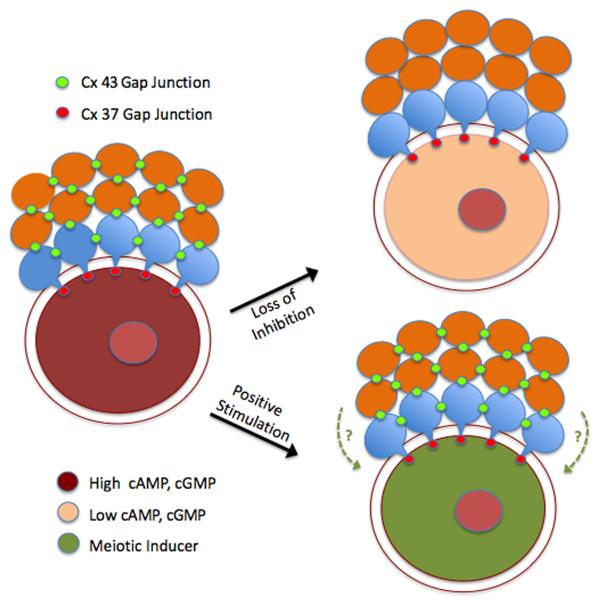
Folliculogenesis involves a close cooperative association between the developing germ and somatic cells. Bidirectional signaling between these two compartments ensures the proper differentiation of each cell type and successful development of the preovulatory follicle. This is exemplified by recent studies showing that, under the direction of oocyte-secreted factors, the surrounding cumulus granulosa cells carry out critical metabolic functions that the oocyte is unable to perform (Su et al, 2009). Granulosa cell products can then reach the oocyte by paracrine means or by direct transfer between the heterologous cells. During follicle growth, the oocyte is intimately coupled to the surrounding granulosa cells by gap junctional communication, enabling transmission of ions and small molecules through these intercellular membrane channels. Connexin (Cx) proteins are the fundamental units of gap junctions that provide for granulosa-granulosa coupling as well as granulosa-oocyte coupling. Cx43 is a major connexin present in gap junctions between somatic cells, while Cx37 junctions are restricted to the granulosa-oocyte interface (Kidder and Mhawi, 2002; Teilmann, 2005; see Fig. 2). While some Cx37 is expressed in granulosa cells away from the oocyte interface, homotypic Cx37 junctions exist primarily at this interface where they are controlled by the oocyte and mediate coupling between the two cell types (Veitch et al, 2004; see Fig. 3). The importance of these gap junctional proteins in folliculogenesis has been demonstrated by failure of normal development following knockout of either connexin in mice, with loss of Cx43 function severely restricting folliculogenesis (Juneja et al, 1999), while ablation of Cx37 function prevents both follicular development to the antral stage as well as normal oocyte growth and acquisition of meiotic competence (Simon et al, 1997; Carabatsos et al, 2000). Cx37 is probably responsible for mediating meiotic control in mice, since normal oogenesis occurred upon oocyte-targeted depletion of Cx43 (Gershon et al, 2008).
Figure 3.
Gap junction regulation of meiotic status. Homologous Cx 43 gap junctions couple mural granulosa cells (green) to one another and cumulus granulosa cells (blue) to one another, and heterologous gap junctions couple mural granulosa cells to cumulus granulosa cells. Heterologous Cx 37 gap junctions in the innermost layer of cumulus cells couple the somatic compartment of the follicle to the oocyte. Two different models exist for meiotic regulation. In the loss of inhibition model, loss of Cx 43 coupling terminates the flux of inhibitor (cAMP and cGMP) from mural to cumulus cells, thereby significantly reducing levels within the oocyte and leading to GVB. In the positive stimulation model, patent gap junctions mediate the flux of a positive stimulus into the oocyte from the surrounding follicle cells that can override elevated cyclic nucleotide levels, although contribution of a diffusible paracrine factor (dotted lines) originating from the granulosa cells cannot be discounted.
cAMP involvement
cAMP is an important physiological regulator of meiosis that maintains meiotic arrest by suppressing MPF activity through stimulation of cAMP-dependent protein kinase A (PKA). Many studies have confirmed that adenylate cyclase activators, cAMP analogs or phosphodiesterase (PDE) inhibitors reversibly suppress GVB and that inhibitors or antagonists of PKA can reverse meiotic arrest maintained by cAMP-elevating agents (Schultz, 1991). In addition, perturbation of PKA activity by knockdown of the type I regulatory subunit promoted oocyte maturation (Duncan et al, 2006). Moreover, a decrease in oocyte cAMP has been shown to precede GVB (Schultz et al, 1983, Vivarelli et al, 1983; Racowsky, 1984; Dekel et al, 1984; Sirard and First, 1988). Evidence has shown that stimulation of cAMP production in the granulosa cells can drive diffusion of the cyclic nucleotide into the oocyte via the gap junctional pathway (Bornslaeger and Schultz, 1985a; Webb et al, 2002). Consistent with this phenomenon is the proposal that granulosa cells maintain the oocyte in prophase I in vivo by supplying meiosis-arresting levels of cAMP through this coupling pathway (Gilula et al, 1978; Dekel et al, 1981).
Alternatively, substances may diffuse into the oocyte from the extracellular milieu that could impart an inhibitory influence to the oocyte, helping to maintain cAMP at meiosis-arresting levels. Once such compound is the purine base, hypoxanthine, a weak inhibitor of cAMP degradation (Downs et al, 1989) that was originally identified in porcine follicular fluid (Downs et al, 1985) and was subsequently measured in mouse follicular fluid at levels that maintain meiotic arrest (Eppig et al, 1985), though its efficacy is strain-dependent (Griffin et al, 2004). However, it is doubtful that hypoxanthine is the primary intrafollicular inhibitor responsible for maintaining meiotic arrest in situ due to its limited inhibitory potency; in fact, it may be released within the follicle as a consequence of follicular hypoxia. Nevertheless, its presence within the follicle implies potential participation in the overall negative regulation of meiosis, and, consequently, it has often been used in culture media to maintain meiotic arrest in vitro. Moreover, its inhibitory potential has been confirmed in numerous mammalian species (Downs, 1996).
The idea that oocyte cAMP derives primarily from the granulosa cells was challenged by the finding that treatment of DO with the adenylate cyclase stimulator, forskolin, elicits suppression of spontaneous maturation in vitro in several rodent species in concert with an elevation in oocyte cAMP (Urner et al, 1983; Olsiewski and Beers, 1983; Ekholm et al, 1984; Racowsky, 1984, 1985). Though denudation of oocytes can leave remnants of the cumulus cells still attached to the oocyte (Eppig and Downs, 1984), Bornslaeger and Schultz (1985b) showed that enzymatic removal of the zona pellucida and most of the somatic cell processes produced an oocyte still capable of cAMP generation in response to forskolin, thus confirming an oocyte source of cAMP. Further evidence supporting oocyte-derived cAMP came from studies showing a transient delay in spontaneous maturation upon treatment of oocytes with nonhydrolyzable GTP analogs (Downs et al, 1992) or the Gs activator, cholera toxin (Dekel and Beers, 1978; Schultz et al, 1983b; Vivarelli et al, 1983; Downs et al, 1992), which suggested a G-protein-mediated mechanism, and in immunocytochemical localization of adenylate cyclase at the oolemma (Kuyt et al, 1988). Moreover, in the intact oocyte-cumulus cell complex, elevation of cAMP within the cumulus cells does not always lead to equilibration of cyclic nucleotide with the oocyte despite a patent coupling pathway (Schultz et al, 1983; Bilodeau et al, 1993).
Definitive evidence for an oocyte source of cAMP came from a study showing that both mouse and rat oocytes express the functional AC3 isoform of adenylate cyclase; in addition, mice lacking this isoform exhibited compromised meiotic arrest in vivo and accelerated spontaneous maturation in vitro (Horner et al, 2003). Using an intact mouse follicle system whereby injections could be directed into the oocyte, Mehlmann, Jaffe and colleagues showed that meiotic arrest could be interrupted by microinjection of antibodies to, or a dominant negative form of, Gs, demonstrating the presence of a G-protein-linked receptor at the oolemma (Mehlmann et al, 2002; Kalinowski et al, 2004). Subsequent studies determined that oocyte expression of the G-protein coupled receptor, PGR3, is essential for maintaining cAMP levels and meiotic arrest (Mehlmann et al, 2004; Hinckley et al, 2005; Ledent et al, 2005). GPR3 exhibits constitutive activity that is independent of granulosa cell influence (Freudzon et al, 2005). An additional functional receptor, GPR12, was also identified in both rat and mouse oocytes (Hinckley et al, 2005), but seems to be important only in the rat, since the knockout mouse exhibited no phenotype.
Maintenance of cyclic nucleotide levels in cells is maintained by a balance between synthesis, degradation and release from the cell. Major players in determining cyclic nucleotide concentration are the cyclic nucleotide phosphodiesterases (PDEs), a diverse group of enzymes that hydrolyze the 3′ phosphate bond of cyclic nucleotides. For example, in the human genome, twenty-one PDE-encoding genes and their corresponding proteins have been identified and characterized, with some specific for cAMP, some specific for cGMP, and others that recognize both cyclic nucleotides (Conti and Beavo, 2007). PDEs specific for cAMP show an interesting compartmentalization within the mammalian follicle. Milrinone and cilostamide, inhibitors of PDE3-specific isoforms, suppress spontaneous oocyte maturation in follicle-enclosed rat oocytes, but the PDE4-specific inhibitor, rolipram, has little effect even at very high concentrations; on the other hand, rolipram alone, but not PDE3 inhibitors, stimulates GVB in cultured follicles (Tsafriri et al, 1996). These results suggested that PDE3 resides in the oocyte, while PDE4 is present in the somatic compartment, and this was confirmed by in situ hybridization. Similar results have been reported from numerous laboratories (eg, Shitsukawa et al, 2001; Jensen et al, 2002; Mayes and Sirard, 2002; Nogueira et al, 2003; Thomas et al, 2004; Laforest et al, 2005). In 1998, Wiersma et al reported the exciting finding that injection of PDE3 inhibitors into superovulated rats and mice prevented meiotic resumption without affecting ovulation rate, strongly suggesting an essential need for oocyte PDE3 in mediating meiotic resumption via cAMP degradation. Indeed, PDE3A activity was shown to increase after gonadotropin stimulation prior to GVB (Richard et al, 2001). An absolute requirement for this PDE isoform was later proven when the same lab showed PDE3A-deficient mice were infertile due to an inability of the oocytes to resume maturation (Masciarelli et al, 2004).
Similar to the results with PDE isoforms, we reported differential regulation of PKA isozymes within the germ and somatic cell compartments of oocyte-cumulus cell complexes from mice (Downs and Hunzicker-Dunn, 1995). Only the type I regulatory PKA subunit was detected in oocytes by photoaffinity labeling of PKA regulatory subunits with 8-N3-[32P]cAMP, while both type I and II isoforms were identified in cumulus cells. In addition, by the use of site-selective analogs of cAMP, that in various combinations can selectively activate type I or II PKA, meiotic arrest was shown to be more pronounced in type I PKA-stimulated oocytes, while selective activation of type II PKA in oocyte-cumulus cell complexes more effectively promoted oocyte maturation and cumulus expansion. It was concluded that type I PKA in the oocyte mediates meiotic arrest, while type II PKA in the granulosa cells mediates maturation of the oocyte-cumulus cell complex. Similar conclusions were drawn from studies by Rodriguez et al (2002). However, more recent reports have demonstrated the presence of both type I and II regulatory PKA isoforms in mouse oocytes (Brown et al, 2002; Newhall et al, 2006), and in one of these studies (Newhall et al, 2006) a combination of site-selective cAMP analogs that preferentially stimulate type II PKA isoforms were presented as more potent in maintaining meiotic arrest when compared with a combination that selectively activated type I PKA, with the conclusion that type II PKA is the more relevant isoform performing this function. Nevertheless, this conclusion seems tenuous, since only one site-selective pairing was compared, and the dose response curves were nearly superimposable. It therefore seems safer to propose that activation of either isoform in the oocyte can suppress GVB.
Subcellular compartmentalization of PKA isozymes within the oocyte is facilitated by A-kinase anchoring proteins (AKAPs). AKAPs serve as scaffolding proteins that bind to PKAs and regulate their cellular localization, forming microdomains of phosphorylation signaling (Wong and Scott, 2004; Smith et al, 2006). AKAPs have been identified in rodent oocytes, and their localization is developmentally regulated (Brown et al, 2002; Kovo et al, 2002; Newhall et al, 2006; Webb et al, 2008). More significantly, disruption of AKAP-mediated anchoring of PKA by treatment with peptide inhibitors reverses meiotic arrest maintained by PDE inhibitor (Dekel, 2005; Newhall et al, 2006), demonstrating a vital role for AKAPs in meiotic arrest. However, AKAP1-null oocytes are more sensitive to the meiosis-arresting action of cAMP-elevating agents than wild type oocytes (Newhall et al, 2006), signifying the involvement of multiple AKAPs in meiotic regulation. Controlling PKA localization within the oocyte via AKAPs might help explain how meiotic resumption can be induced in the presence of elevated cAMP.
Control of MPF activity
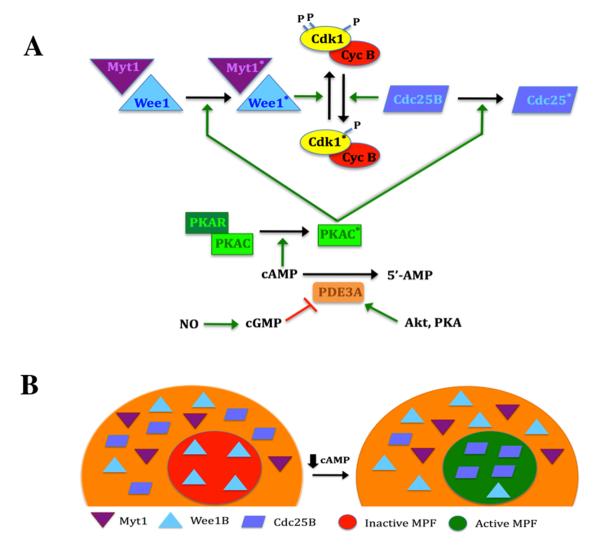
Ample pre-MPF (Cdk1 and cyclin B) exists in the meiotically competent mouse oocyte, and its activity is controlled by the phosphorylation state of Cdk1. Sustained levels of cAMP in the mouse oocyte persistently activate PKA, promoting phosphorylations that positively regulate Wee1 kinase (Han et al, 2005), and negatively regulate Cdc25B phosphatase (Lincoln et al, 2005; Pirino et al, 2009). These PKA substrates control MPF activity at two highly conserved phosphorylation sites (Thr14 and Tyr15) on the Cdk1 kinase of MPF; phosphorylation by the Wee1 family of kinases (Wee 1B and Myt1) inactivates the kinase, while dephosphorylation by the Cdc25 family, most notably Cdc25B, promotes activation (Fig. 4A). Thus, the control of MPF by PKA is indirect, brought about by phosphorylation of mediating proteins.
Figure 4.
Regulation of MPF activity in the mouse oocyte. A, Control of MPF by PKA. MPF activity is regulated by the phosphorylation state of the Cdk1 subunit. PKA activity is dependent on cAMP levels. This cyclic nucleotide binds to the regulatory subunit (PKAR), driving dissociation from, and activation of, the catalytic subunit (PKAC; asterisk denotes activated state). When sufficient cyclin B is present, MPF is maintained in the inactive state by the action of Wee1 and Myt1 kinases that are positively regulated by PKA. Dephosphorylation of Cdk1 triggers activation of MPF and is principally driven by Cdc25B that is negatively regulated by PKA. This response occurs when PKA activity is lowered by a decrease in cAMP, brought about by the action of PDE3. Increased PDE3 activity results from a drop in cGMP levels or active stimulation by Akt or perhaps PKA. B, Effect of Myt1/Wee1/Cdc25B localization on MPF activity. In the competent oocyte, pre-MPF accumulates in the nucleus. Under meiosis-arresting conditions, Wee1B is present in the germinal vesicle, but Myt1 and Cdc25B are restricted to the cytoplasm, and this is under PKA control. When oocyte cAMP levels are reduced, Wee1B exits the nucleus and Cdc25B translocates into the nucleus. The loss of inhibitory Wee1B and the entry of stimulatory Cdc25B promotes dephosphorylation of pre-MPF in the germinal vesicle and its subsequent activation that will drive GVB.
Localization of these regulatory proteins within the mouse oocyte plays a vital role in controlling MPF activity. During the development of meiotic competence in mouse oocytes, the components of MPF, Cdk1 and cyclin B, accumulate in the nucleus, where they will direct meiotic resumption (Mitra and Schultz, 1996). Indeed, meiotic regulation is significantly affected by the nuclear abundance of cyclin B (Marangos and Carroll, 2004). Zhang et al (2008) showed that phosphorylation of Cdc25B by PKA sequesters it in the cytoplasm, while a decrease in oocyte cAMP that drives inactivation of PKA leads to translocation of Cdc25B from the cytoplasm into the nucleus, activation of MPF and meiotic resumption (Fig. 4B). A recent study by Oh et al, (2010) confirms this mechanism and further clarifies contributions from Wee1B and Myt1 kinases. In the prophase I-arrested mouse oocyte, Wee1B is confined to the nucleus, while Myt1 and Cdc25B are present only in the cytoplasm. Under conditions that permit meiotic resumption, prior to germinal vesicle breakdown Cdc25B initially translocates to the nucleus, subsequently followed by the exodus of a significant fraction of Wee1B from the nucleus into the cytoplasm. The localization of Myt1 is not affected before meiotic resumption (Fig. 4B). All three components are required for optimal regulation of MPF activity, and it appears that removal from the nucleus of the inhibitory kinase combined with entry of the stimulatory phosphatase converts pre-MPF to active meiotic inducer (Oh et al, 2010; Fig. 3B). A similar role for cdc25C has been reported in human oocytes (Cunat et al, 2008).
Additional MPF regulation exists at the level of the anaphase-promoting complex (APC). Destruction of cyclin B inactivates MPF and requires polyubiquitination by the APC that targets it for proteosomal degradation (Murray, 2004). Reis et al (2006) demonstrated that cyclin B levels in mouse oocytes are restricted by the activity of APC that is maintained by the activator, cdh1. Germinal vesicle-stage oocytes contain active APC that is down-regulated by cdh1-targeted morpholinos, leading to reduced cyclin B degradation and promotion of meiotic resumption in milrinone-arrested oocytes. Further evidence for APC mediation of meiotic arrest was provided by Marangos et al (2007), who showed that depletion of the APC inhibitor, Emi1, delays entry of mouse oocytes into meiosis I and that microinjection of Emi1 accelerated meiotic resumption. In addition, inhibition of proteosome activity induces GVB in hypoxanthine-arrested mouse oocytes in vitro (Huo et al, 2004). Evidence also indicates that APC regulates reinitiation of meiosis in pig oocytes (Yamamuro et al, 2008).
cGMP involvement
Because the predominant PDE in oocytes controlling cAMP levels is cGMP-regulated PDE3A (Shitsukawa et al, 2001), a primary candidate for the physiological modulator of PDE3A activity is cGMP. This cyclic nucleotide has a myriad of functions in the mammalian ovary (reviewed by LaPolt et al, 2003). About three decades ago it was shown that ovarian cGMP levels decline significantly in rat (Ratner, 1976; Ratner and Sanborn, 1980) and hamster (Makris and Ryan, 1978; Hubbard, 1980) ovaries following LH stimulation. In addition, an inverse relationship exists between cAMP and cGMP levels (Hubbard, 1980). Exposure of hamster oocytes to the cGMP analog, 8-Br-cGMP, proved inhibitory to spontaneous maturation in cumulus cell-enclosed oocytes (CEO) but not denuded oocytes (DO), and this effect was reversed by LH treatment (Hubbard and Terranova, 1980). The authors raised several important points regarding a potential role for cGMP in meiotic regulation: (1) general PDE inhibitors such as IBMX may suppress maturation by their action on cGMP as well as cAMP; (2) cGMP action depends on the cumulus cells and may involve alterations in cell-cell coupling; and (3) LH may trigger meiosis reinitiation by lowering somatic cell cGMP levels.
A role for cGMP was further supported by work from other laboratories that demonstrated an inhibitory effect of cGMP analogs and cGMP PDE-specific inhibitors on oocyte maturation in several mammalian species (Downs et al, 1988; Tornell et al, 1991; Zhang et al, 2005; Wang et al, 2008) and a drop in oocyte cGMP levels prior to GVB (Tornell et al, 1990). In addition, blocking the flow of purine metabolism to guanyl compounds at the inosine monophosphate branchpoint with IMP dehydrogenase inhibitors, which would prevent the formation of GTP and, presumably, cGMP, induces meiotic resumption in mouse oocytes both in vivo (Downs and Eppig, 1997) and in vitro (Downs et al, 1986; Downs, 1990). The fact that these inhibitors trigger GVB in CEO but not DO is consistent with the idea that guanyl compounds such as cGMP are produced in the somatic compartment and then diffuse to the oocyte to contribute to meiotic arrest. Indeed, treatment of rat follicles with an inhibitor of soluble guanylate cyclase triggers GVB (Sela-Abramovich et al, 2008). Two important modifiers of guanylate cyclase are nitric oxide (soluble form; Denninger et al, 1999) and atrial natriuretic peptide (membrane-bound form; Waldman and Murad, 1989), and a vast literature indicates that each may play an important role in modulating oocyte cGMP levels and meiotic arrest (reviewed by Zhang et al, 2007).
Two recent papers have solidified the importance of cGMP in meiotic regulation in mouse oocytes. In the first, Norris et al (2009), using FRET-based cyclic nucleotide sensors, provided convincing evidence that oocyte cAMP levels are maintained by granulosa cell cGMP that is transmitted through gap junctions to suppress oocyte PDE3A activity; further, follicular LH stimulation suppresses cGMP production by the granulosa cells, leading to a decrease in oocyte cGMP and meiotic resumption. In the second study, Vaccari et al (2009) report a number of important findings: (1) the cGMP-specific PDE, PDE5, is preferentially expressed in the granulosa compartment; (2) cGMP suppresses cAMP hydrolysis in oocyte extracts; (3) LH stimulation of cultured follicles leads to reduced oocyte cGMP before GVB that requires mediation by EGF-like peptides (see below) and is prevented by PDE5 inhibitors; and (4) microinjection of cGMP-specific PDE into oocytes overcomes meiotic arrest in PDE3A-dependent fashion. These data therefore provide convincing evidence that meiotic arrest depends, at least in part, on cGMP supplied by the granulosa compartment that reaches the oocyte through gap junctions to restrict PDE3A activity and maintain elevated oocyte cAMP.
IV. Mechanisms of Meiotic Induction
cAMP plays an important role in maintaining the prophase I arrest in oocytes, but this cyclic nucleotide has a dual role in meiotic regulation, since it also mediates the meiosis-inducing action of gonadotropin in granulosa cells. Dekel (1988) proposed an explanation for such a paradox in that maturation is blocked when oocyte cAMP is maintained above an inhibitory threshold but that meiotic resumption results from a transient pulse of elevated cAMP within the granulosa cells. Indeed, transient pulsing of follicles or CEOs with cAMP analogs or cAMP-elevating agents triggers meiotic resumption (Tsafriri et al, 1972; Hillensjo et al, 1978; Dekel et al, 1981; Downs et al, 1988). Although it has been known for many years that the preovulatory surge of gonadotropins is the initial stimulus for meiotic resumption in vivo (Lindner et al, 1974; Tsafriri et al, 1982), only recently have some of the downstream transduction pathways mediating gonadotropin action been uncovered.
Mitogen-Activated Protein Kinase
Mitogen-activated protein kinase (MAPK), known also as extracellular-regulated kinase (ERK), comprises a family of Ser/Thr kinases that are optimally activated upon phosphorylation on threonine and tyrosine residues (Seger and Krebs, 1995; Chen et al, 2001). They are turned on by phosphorylation signal cascades initiated by a panoply of external signals and transduction pathways. Two isoforms are expressed in mammalian oocytes, ERK1 and ERK2, that are actively engaged in meiotic regulation. Activity is controlled by the upstream kinase, MEK, which, in turn, is regulated further upstream in vertebrate oocytes by the MEK kinase, MOS, a product of the cmos protooncogene (Sagata et al, 1988). On the other hand, somatic cell MEK is regulated by Raf isoforms (Chen et al, 2001).
Perturbation of these two upstream regulatory sites has profound effects on meiotic progression that implicate ERK1/2 in the mechanisms regulating oocyte maturation. Because upstream regulation of ERKs by MOS is unique to the oocyte, eliminating MOS by knockout or other means prevents activation of ERK specifically in the germ cell, thereby allowing analysis of germ cell-specific functions. In oocytes from Mos knockout mice, meiotic maturation progresses normally but oocytes fail to block in MII, demonstrating an essential role for ERKs in mediating this second check point (Colledge et al, 1994; Hashimoto et al, 1994). ERK activity is unaltered and MPF fluctuates normally during maturation up until MII, when MPF activity drops prematurely (Araki et al, 1996; Choi et al, 1996; Verlhac et al, 1996).
Important tools that have emerged for elucidating ERK participation in oocyte maturation are the MEK inhibitors, PD98059 and U0126 (Alessi et al, 1995; Dudley et al, 1995; Favata et al, 1998; Davies et al, 2000). These agents have been shown to prevent ERK stimulation and meiotic induction in isolated mouse cumulus cell-enclosed oocytes (Leonardsen et al, 2000; Su et al, 2001, 2002) or follicles (Su et al, 2003). Similar results were obtained in the rat (Sela-Abramovich et al, 2005; Motola et al, 2008) and numerous non-rodent mammalian species (reviewed by Fan and Sun, 2004); yet these inhibitors have little effect on spontaneous maturation (Fan et al, 2003; Tong et al, 2003).
Oocyte ERK1/2 activity is not required for GVB in rodent oocytes, since activity increases only after GVB, whether maturation occurs spontaneously (Verlhac et al, 1993; Lu et al, 2001; Tan et al, 2001) or is hormonally-induced (Su et al, 2002; Sela-Abramovich et al, 2005). This relationship is not as clear in domestic species, since ERK1/2 is activated prior to GVB in spontaneously maturing equine, porcine and bovine oocytes (Fissore et al, 1996; Goudet et al, 1998; Inoue et al, 1998). That ERK activity within the rodent oocyte is not necessary for meiotic resumption is supported by the finding that in mouse oocytes lacking MOS, FSH-induced maturation in hypoxanthine-arrested cumulus cell-enclosed oocytes was not compromised; nevertheless, U0126 suppressed meiotic induction in granulosa cell-coupled oocytes (Su et al, 2002). These results suggest that cumulus/mural granulosa cell ERK is necessary for the in vitro induction of maturation by agents acting through the somatic compartment. Consistent with this is the finding that cumulus cell ERK1/2 activation, as assessed by western analysis of its phosphorylation status, precedes GVB in meiotically induced oocytes (Su et al, 2002). However, in the mouse, oocyte paracrine signaling is absolutely required for the cumulus cell ERK1/2 response and meiotic induction, demonstrating that the oocyte plays a crucial role in regulating its own meiotic maturation (Su et al, 2003).
Until recently, the conclusion that granulosa cell ERK stimulation mediated hormone-induced oocyte maturation was based primarily on two important criteria: (1) granulosa cell ERK activity increased prior to GVB and (2) MEK inhibitors suppressed both this activity and meiotic resumption. Although PD98059 and U0126 are relatively specific kinase inhibitors (Davies et al, 2000; Bain et al, 2007), a question of specificity in mouse oocytes was recently raised. Both MEK inhibitors prevented meiotic induction in cumulus-free mouse oocytes induced to resume maturation even though such induction was not mediated by an increase in ERK1/2 activity; the inhibitors were equally effective in similarly treated Mos−/− oocytes (LaRosa and Downs, 2005). This study, therefore, demonstrated a direct inhibitory effect of MEK inhibitors on the oocyte by means unrelated to ERK1/2 activation and rendered somewhat tenuous earlier conclusions drawn concerning ERK1/2 involvement in meiotic induction.
However, a new study by JoAnne Richards’ lab (Fan et al, 2009) has put to rest any doubt regarding the importance of ERK1/2 in meiotic induction. Since two isoforms of ERK exist in the oocyte, knocking out both was essential to determine unequivocally whether the kinase is required for meiotic induction. And since Erk2-null mice are embryonic lethal (Aouadi et al, 2006), ERK2 function could not be eliminated at the organism level. The authors accomplished the double knockout through conditional ablation of ERK2 function in granulosa cells by crossing Erk2fl/fl mice with Cyp19-Cre transgenic mice, which knocks out ERK2 activity in granulosa cells, and then crossing these F1 mice into the Erk1−/− background. Activation of ERK1/2 in the granulosa cells that normally occurs following LH stimulation was thereby completely eliminated, and, as a consequence, a myriad of post-LH ovulatory follicular responses was prevented, including meiotic resumption, cumulus expansion, ovulation and luteinization, while FSH-dependent effects on follicle growth were extended (Fan et al, 2009). These results show the critical role Erk1/2 plays in the preovulatory response to LH, but, most relevant to this review, demonstrate the absolute requirement for Erk1/2 in meiotic induction, at least in the mouse.
EGF-like peptides
In 1985, Dekel and Sherizly demonstrated stimulation of GVB in cultured rat preovulatory follicles by epidermal growth factor (EGF), and a similar action in cultured mouse CEOs was confirmed three years later (Downs et al, 1988). Moreover, EGF proved to be the most potent of ten growth factors tested for stimulation of meiotic induction and cumulus expansion (Downs, 1989). In the next several years, studies by Roy and Greenwald (1990; 1991a,b) suggested a link between gonadotropin stimulation of granulosa cells and EGF-mediated events: (1) FSH-stimulated DNA synthesis was mimicked by cAMP and EGF; (2) FSH triggered EGF synthesis; and (3) FSH-stimulated DNA synthesis was blocked by EGF antiserum. Numerous labs subsequently demonstrated a meiosis-inducing action by members of the EGF growth factor family (eg, Brucker et al, 1991; Das et al, 1992; Goud et al, 1998;Tsafriri et al, 1989; Prochazka et al, 2000), but more than a decade passed before expression of EGF-like peptides by mural and cumulus granulosa cells in response to gonadotropin stimulation was reported (Espey and Richards, 2002; Sekiguchi et al, 2004; Park et al, 2004; Ashkenazi et al, 2005; Ben-Ami et al, 2006).
A seminal study by Conti and colleagues (Park et al, 2004) determined that the preovulatory actions of LH are, indeed, mediated by members of the EGF growth factor family--not by EGF, but by amphiregulin and epiregulin, and, to a lesser extent beta-cellulin. This study also showed that these peptides act through the granulosa cells, where they bind to the EGF receptor (Shimada et al, 2006). Subsequent studies confirmed the potent meiosis-inducing action of these EGF-like peptides on the somatic compartment to stimulate GVB (reviewed by Hsieh et al, 2009) and that knockout of EGF-like peptides in mice compromised meiotic resumption (Hsieh et al, 2007). In addition, it appears that protein kinase C (PKC) mediates, at least in part, the increase in EGF-like peptides in cumulus granulosa cells in response to gonadotropin stimulation (Downs and Chen, 2008; Chen et al, 2008), consistent with a stimulatory action of PKC activators on meiotic resumption (reviewed by Downs et al, 2001). Romero and Smitz (2009) recently reported that optimal induction of meiotic resumption in cultured mouse follicles by epiregulin required hCG, but these experiments were conducted in follicles grown in vitro that were not exposed to hormonal priming in vivo, which may affect their responsiveness (cf, Fujinaga et al, 1994).
Shimada et al (2006) carried out an extensive analysis of the dynamics of EGF-like peptides during the preovulatory period and proposed an elaborate signaling cascade in which EGF-like peptides and prostaglandin E2 provide paracrine communication between the mural and cumulus granulosa cells as well as autocrine stimulation among each individual granulosa cell sub-type. Gonadotropin or mural granulosa-derived PGE2 elicits expression of EGF-like peptides in the cumulus mass by a p38MAPK-dependent process that act in autocrine fashion to trigger ERK1/2-dependent gene transcription. These gene products then mediate the actions of gonadotropins on oocyte maturation, cumulus expansion and ovulation (Shimada et al, 2006; Conti et al, 2006).
Gap junction regulation
The gap junctional coupling pathway is an important conduit through which regulatory molecules are exchanged between the follicular somatic and germ cell compartments, and this interface between cumulus cells and the oocyte is intimately linked to meiotic regulation (Carabatsos et al, 2000). While it is generally agreed that this coupling pathway plays a critical role in maintaining meiotic arrest, there is less of a consensus on how gap junction dynamics relate to meiotic resumption. More than thirty years ago, the attractive hypothesis was proffered that gonadotropin stimulation terminated coupling between the oocyte and granulosa cell compartments, thereby preventing the transfer of inhibitory molecules (eg, cAMP) to the oocyte and triggering GVB (Gilula et al,1978; Dekel and Beers, 1978). Since that time, considerable evidence has been generated to support this idea. Ultrastructural studies revealed that significant alteration of gap junctions occurred at the time of GVB (Larsen et al, 1986, 1987) or commitment to undergo GVB (Racowsky et al, 1989; Wert and Larsen, 1990). Racowsky and Baldwin (1989) showed that when the cumulus cell-enclosed oocyte was physically separated from the mural granulosa cell wall in the intact hamster preovulatory follicle, meiotic arrest was compromised. GVB can also be initiated by chemical interruption of the coupling pathway in rat follicles (Piontkewitz and Dekel, 1993; Sela-Abramovich et al, 2006) or arrested mouse CEOs (Downs, 2001). Furthermore, gonadotropin stimulation causes a rapid phosphorylation of Cx43 (Lau et al, 1992; Granot and Dekel, 1994; Shimada et al, 2001; Kalma et al, 2004; Sela-Abramovich et al, 2005, 2006) that is associated with gap junction closure and appears to be dependent on EGF-like peptides (Lau et al, 1992; Leithe and Rivedal, 2004).
A critical issue regarding gap junction involvement in meiotic regulation, in addition to when, is where the uncoupling occurs. There are two likely sites where uncoupling could elicit a meiotic response: the mural/cumulus granulosa interface and the cumulus/oocyte interface (See Fig. 4). Although several studies have shown a decrease in metabolic or dye coupling between the oocyte and cumulus cells at the time of GVB, others showed that the coupling does not significantly decline prior to GVB (reviewed by Downs, 1995). Furthermore, coupling inhibitors can block the meiosis-promoting action of gonadotropin (Fagbohun and Downs, 1991; Coskun and Lin, 1994; Downs, 2001) or cumulus cells Vozzi et al, 2001) in isolated CEOs, indicating a need for patent gap junctions in meiotic induction. However, since in the latter model system the oocyte is not coupled to the mural granulosa component of the follicle and requires inhibitory agents to maintain meiotic arrest, it may not accurately reflect conditions in situ. Furthermore, uncoupling the cumulus mass from the mural granulosa could bring about GVB by terminating the flow of inhibitory molecules to the oocyte despite extant communication with the cumulus cells (Larsen et al, 1987).
With this question in mind, Norris et al, (2008) examined gap junction closure in cultured mouse follicles, using two-photon microscopy to monitor diffusion of a microinjected gap junction permeant tracer. They reported that, following LH exposure, gap junction permeability within the mural granulosa decreased prior to GVB in association with ERK1/2-dependent phosphorylation of Cx43, but the oocyte was not uncoupled from the cumulus mass before meiotic resumption. Antibody to Cx43 and gap junction inhibitor both blocked coupling and stimulated GVB. Thus, the data support the conclusion that LH triggers GVB in the follicle by sequestering the oocyte-cumulus cell mass from the rest of the follicle, thereby preventing gap junction-mediated inhibitory input from the mural granulosa cells such as cGMP (Norris et al, 2008, 2009). Nevertheless, although the data convincingly support such a mechanism, they do not prove it is the primary means by which LH works in this capacity in vivo. Indeed, treatment of LH-stimulated follicles with a low dose of the ERK1/2 inhibitor, U0126, prevented gap junction closure but had no effect on meiotic induction (Norris et al, 2008). This supports the alternative possibility that a positive stimulus mediates hormone-induced maturation as opposed to simply isolating the oocyte from the somatic compartment (cf, Downs et al, 1988). Recent evidence from the porcine system also suggests that meiotic resumption does not result simply from loss of gap junctional communication (Sasseville et al, 2009).
The mural/cumulus isolation mechanism also fails to take into account the role of EGF-like peptides in meiotic resumption. The rapid expression of these peptides following gonadotropin stimulation and their potent meiosis-inducing action on the cumulus-enclosed oocyte are commensurate with a paracrine action of mural granulosa-derived peptides that diffuse to the CEO and stimulate GVB and cumulus expansion (Shimada et al, 2006; Panigone et al, 2008). ERK1/2 appears to mediate actions of the EGF-like peptides but to also have functions upstream of EGFR activation. Preventing activation of EGFR blocks--but only partially--ERK1/2 activation, yet meiotic induction in mouse follicles is prevented (Panigone et al, 2008). Thus, under these conditions, coupling between the cumulus and mural granulosa compartments may be abolished (cf, Norris et al, 2008), but meiotic induction cannot occur in the absence of EGFR activation. Hence, although the oocyte may be isolated from a major source of inhibitory input (eg, cGMP), this may not be the sole mechanism regulating meiotic resumption; rather, a positive stimulus for maturation triggered by EGF-like peptides in the CEO is implicated (Fig. 4).
Propagating the signal from cumulus to oocyte
At this time, the nature of the putative positive signal is very much a matter of conjecture. Numerous laboratories have investigated the possibility of a diffusible meiotic inducer of granulosa origin by coculturing meiotically arrested oocytes with granulosa cells under a variety of conditions. Studies have shown that cocultured cells can alter the medium in such a way that DO are stimulated to resume maturation; this has been interpreted as due to active secretion of a diffusible factor that can act on the oocyte to trigger a meiotic response (reviewed by Downs et al, 2006). But we have been unable to consistently demonstrate this effect to a degree that would support such a mechanism in vivo. Confounding interpretation are several caveats inherent in these types of experiments that include the use of small medium volumes, oil overlays, and oocytes denuded of their granulosa cell investment. In some instances, the positive coculture effect observed may be explained by somatic cell-mediated alterations in the levels of culture medium components such as energy substrates and meiotic inhibitors like hypoxanthine (Downs et al, 2006). It should be noted, however, that the transzonal cumulus cell processes that traverse the zona pellucida to make contact with the oolemma are ideally suited for depositing secreted products in close proximity to the oocyte, which would make possible oocyte exposure to high concentrations of a paracrine factor at these focal points (Albertini et al, 2001). Such a mechanism is difficult to test experimentally.
One type of paracrine factor that has garnered considerable scrutiny in the last decade are two closely related sterols, termed meiosis-activating sterol (MAS), derived from follicular fluid and bull testicular tissue, that were shown to stimulate meiotic resumption in hypoxanthine-arrested mouse oocytes (Byskov et al, 1995). Since then, many studies have confirmed a meiosis-inducing action in a variety of species and have reported other beneficial effects on development; nevertheless, the accumulated data have failed to identify MAS as an obligatory mediator of meiotic resumption, and its role in this capacity has been seriously challenged (Tsafriri et al, 2005). Steroids are a second type of paracrine factor suggested as a meiosis-inducing agent within the follicle. Despite earlier reports to the contrary, a recent reexamination of this question has generated data supporting a meiosis-inducing action of androgens on arrested mouse oocytes in vitro (reviewed by Deng et al, 2009). However we have been unable to duplicate these results in the mouse (Downs, unpublished), and Tsafriri and colleagues have been unsuccessful in rats, leading to the conclusion that these compounds are not obligatory mediators of meiotic induction in vivo (Tsafriri and Motola, 2007). Although androgens can induce meiosis in fish and frogs and certain mammalian species, particularly the pig, Li et al (2009) speculate that throughout evolution androgens have become increasingly less important for this function and have been replaced by gonadotropins.
As mentioned earlier, numerous studies using chemical gap junctional blockers have implicated this coupling pathway in the hormone induction within CEO in vitro. Nevertheless, the chemical nature of the putative positive agent that can traverse the cumulus-oocyte gap junctions remains enigmatic. One appealing candidate, calcium, can induce meiotic resumption (Homa et al, 1993), and calcium or a calcium-releasing agent has been shown to cross these gap junctions (Mattioli et al, 1998; Webb et al, 2002). Evidence is strongest in the spontaneously maturing mouse oocyte model, where the involvement of PKC and the PI3 kinase signaling pathway have been implicated (Lefevre et al, 2007). However, calcium does not appear to be the agent responsible for transducing the maturation signal generated by gonadotropin stimulation (Webb et al, 2002b; Mehlmann et al, 2006). Clearly, if gap junction-mediated transfer of a positive stimulus is important for meiotic induction, the agent in question needs to be identified.
Whatever the positive signal received by the mouse oocyte, cGMP-sensitive PDE3A likely mediates its action. The drop in oocyte cGMP that occurs prior to GVB would release an inhibition on the enzyme, increasing its activity. But it has also been shown that protein kinase B/Akt stimulates meiotic resumption in mouse oocytes in a PDE3A-dependent manner (Han et al, 2006). Thus, the putative positive signal may act by turning on the PI3 transduction pathway, activating Akt and PDE3A, and thereby driving GVB. It is possible that a fall in cGMP acts cooperatively with Akt activation to rapidly trigger cAMP degradation.
It is also possible that a transient rise in cAMP within the oocyte could stimulate increased PDE3A activity. We recently found that cAMP levels in mouse oocytes from primed mice injected with an ovulatory dose of hCG were significantly elevated 1 h post-hCG (Chen and Downs, unpublished), well before meiosis was reinitiated. This observation is consistent with our study in which pulsing immature denuded mouse oocytes with high concentrations of cAMP produced a meiosis-inducing stimulus in inhibitor-containing medium (Chen et al, 2009). Although PDE activity was not measured, the results support the possibility that the enzyme can be activated by high oocyte cAMP levels, since (1) cAMP falls rapidly thereafter in vivo prior to GVB and (2) AMP-activated protein kinase is activated in oocytes pulsed with cAMP in vitro (see section below).
IV. AMP-Activated Protein Kinase and Meiotic Resumption
It is generally accepted that negative regulation of meiotic maturation by cAMP occurs via stimulation of PKA; thus, it follows that increased PDE3A activity leads to a drop in oocyte cAMP levels and subsequent GVB principally through inactivation of PKA. However, PDE cleaves cAMP into 5′-AMP, a compound that has been largely ignored in oocyte physiology, presumed to be an inactive byproduct of the enzyme in oocytes. Yet 5′-AMP is an important regulator of a serine/threonine kinase termed AMP-activated protein kinase, or AMPK, that has been implicated in the regulation of meiotic induction in mouse oocytes.
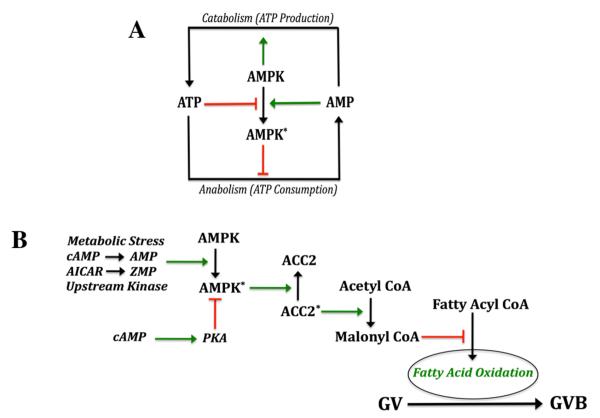
AMPK is a member of the AMPK/SNF1 protein kinase family whose members can be found in a diverse array of organisms (eg, mammals, plants and yeast) and are typically activated in response to nutritional or environmental stress. AMPK controls the activity of important rate-limiting enzymes of carbohydrate and fat metabolism, acting as a type of “fuel gauge” in response to changes in cellular energy charge (Hardie et al, 2006). Thus, as a result of stresses that compromise cellular energy levels, AMPK becomes activated, and a principal function is to conserve energy by shutting down energy-depleting pathways and activating energy-generating pathways (Fig. 5A). These activities are controlled at the level of post-translational modification and also gene transcription (Hardie, 2003).
Figure 5.
AMPK in metabolism and oocyte maturation. A, general metabolic control. High levels of ATP suppress AMPK activity while elevated AMP has the opposite effect. Activation of AMPK (asterisk) suppresses anabolic pathways that consume ATP and stimulates catabolic pathways that generate ATP. B, proposed contribution of AMPK to meiotic resumption in the mouse oocyte. PKA can phosphorylate AMPK and maintain it in the inactive state. A number of different stimuli can activate AMPK, including stress, AMP produced by cAMP degradation, conversion of AICAR to the AMP analog, ZMP, and upstream kinases such as LKB1 and CaMKKβ. Active AMPK phosphorylates, and inactivates, mitochondria-associated acetyl CoA carboxylase II. This enzyme catalyzes the conversion of acetyl CoA to malonyl CoA, an important negative regulator of fatty acid transport into the mitochondrion, and, thus, fatty acid oxidation. Since fatty acid oxidation appears to be a vital component of meiotic induction in mouse oocytes, the positive action of AMPK on oocyte maturation is likely due, at least in part, to activation of fatty acid oxidation (see Downs et al, 2009),
AMPK is a heterotrimeric protein with a catalytic α subunit and β and γ regulatory subunits, and its activity is sensitively controlled within cells by the AMP/ATP ratio (reviewed by Sanz, 2009). Since the level of ATP in cells is in the millimolar range while that of AMP is much lower, small increases in AMP or larger decreases in ATP significantly increase the AMP/ATP ratio and bring about activation of the enzyme (Hardie et al, 1998). AMP activates AMPK allosterically but also by promoting phosphorylation of its catalytic α subunit at T172 by an upstream kinase and suppressing its inactivation by dephosphorylation (Hardie and Carling, 1997; Sanders et al, 2007). AMPKα can also be negatively regulated at S485/491 by elevated cAMP, presumably via PKA (Hurley et al, 2006), and suppressing AMPK activity could help mediate meiotic arrest brought about by high levels of cAMP in the oocyte. AMP does not accumulate appreciably in cells due to the activity of adenylate kinase, but it is possible that, after the gonadotropin surge, PDE activity within oocytes generates enough AMP from cAMP degradation to activate AMPK that, in turn, can provoke a positive stimulus for oocyte maturation.
We have shown that both α1 and α2 isoforms of the catalytic subunit of AMPK are present in extracts from oocytes and oocyte-cumulus cell complexes (Downs et al, 2002). Also, the AMPK activator, AICAR, is a potent stimulator of GVB, and this meiotic induction is associated with increased AMPK activity. AICAR is unable to reverse the inhibitory action of MPF inhibitors, indicating a site of action upstream of MPF. Subsequent studies have established that an increase in AMPK precedes GVB and is required for meiotic induction by AICAR (Chen et al, 2006), different stresses (LaRosa and Downs, 2006, 2007) and the more physiological stimulators, FSH and amphiregulin (Chen et al, 2008); furthermore, specific AMPK inhibitors can prevent such meiotic induction. Interestingly, recent work from our lab shows that cAMP pulsing of the oocyte itself can also induce maturation, in concert with activation of AMPK (Chen et al, 2009), supporting the idea that PDE-generated AMP can activate AMPK. AMPK may act by turning on fatty acid oxidation, which appears to be important for meiotic resumption in the mouse (Downs et al, 2009). Although the accumulated data support a role for AMPK in meiotic induction in the mouse, it remains to be determined if its participation is an absolute requirement. A potential scheme is presented in Fig. 5B that shows how AMPK might participate in meiotic resumption in mouse oocytes.
Work from other labs suggests that the meiosis-inducing action of AMPK may be unique to the mouse. When cow and pig oocytes were treated with AMPK activators, meiotic maturation was suppressed instead of stimulated (Bilodeau-Geoseels et al, 2007; Mayes et al, 2007; Tosca et al, 2007). In addition, work in progress in our lab comparing mouse and rat oocytes indicates profound differences in a number of meiotic behaviors, most notably a failure of rat oocyte AMPK to stimulate meiotic resumption and differences in localization of active AMPK throughout the maturation period from GVB to MII.
The participation of AMPK in reproduction is an important consideration, especially considering its potential relationship with certain reproductive complications. A common treatment for both polycystic ovary syndrome and type II diabetes is metformin, a drug that has recently been shown to act through activation of AMPK (Zhou et al, 2001). Thus, it is possible that alterations in AMPK function can lead to reproductive anomalies and this kinase holds promise as a potential therapeutic target (eg, Ruderman and Prentki, 2004; Kahn et al, 2005; Viollet et al, 2009; Zhang et al, 2009). Indeed, recent use of a diabetic mouse model has shown that AMPK can ameliorate some of the symptoms of diabetes, including lesions in normal meiotic maturation (Ratchford et al, 2007).
An additional regulatory role for AMPK that has recently been uncovered is that of mitotic cell polarity and division (Williams and Brenman, 2008) that has important implications for the malignant phenotype (Kuhajda, 2008; Jansen et al, 2009). Current work in progress in our lab shows that active AMPK localizes with condensed chromatin and the meiotic spindle throughout maturation; thus, it is likely that this kinase plays an important role throughout the entire period of oocyte maturation, from meiotic resumption to MII.
V. Concluding remarks
The recent work on EGF-like peptides and cGMP serve to emphasize the complex and highly integrated nature of meiotic regulation. Carefully timed paracrine and gap junctional signaling between the mural and cumulus granulosa cells as well as between the somatic and germ cell compartments is essential for the successful control of oocyte development from early growth through terminal maturation prior to fertilization. This complexity is inherent in the challenge to those studying the process.
Figure 1 incorporates results from recent studies into an integrated scheme for meiotic regulation in the mouse follicle. It is not meant to be all-inclusive, and emphasis is on those aspects for which a reasonable body of evidence exists in the litereature. Since not all potential chemical and pathway participants could be discussed in this review due to space constraints, this diagram should be viewed as a simplified version of reality. Certain aspects are speculative and remain to be proven, but with the rapid advances in molecular and technical tools in recent years, the rate of important new findings can only accelerate. Some important unanswered questions remain, including: (1) How are signal transduction pathways integrated between the somatic and germ cell compartments to communicate the meiosis-inducing signal? (2) To what extent are gap junction uncoupling and positive stimulation utilized to achieve meiotic resumption? (3) What is the chemical nature of the putative positive stimulus? and (4) What differences exist between species in meiotic regulation?
It is becoming increasingly clear that what occurs mechanistically in one species is not necessarily duplicated in another. It is already known that significant differences exist between species in the sensitivity of oocytes to meiotic inhibitors, the timing of meiotic maturation, and even maturation status at the time of ovulation. While we know that LH is the initial upstream positive regulator of GVB, and MPF is the downstream meiotic trigger, the intermediate hormonal/metabolic/biochemical activity manifested in granulosa cells and oocytes to reach that physiological endpoint may exhibit considerable variation between species. It therefore remains incumbent upon us to resist sweeping generalizations based on data from a limited number of species. Because of this, it is possible, if not likely, that no oocyte from a given species mimics completely the transduction systems implemented by another species.
Figure 6.
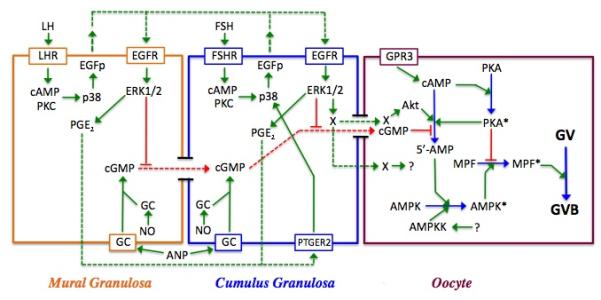
Model for meiotic regulation in the mouse. Shown are the three cell types within the follicle--mural and cumulus granulosa cells and the oocyte—and the interactions between them. Red lines depict inhibitory processes; green lines are stimulatory; and dotted lines represent agents that diffuse between cells. Active enzymes within the oocyte are denoted by an asterisk. In the oocyte, PGR3-dependent cAMP production activates PKA, which negatively regulates MPF. cGMP produced within the granulosa cells by soluble or membrane bound guanylate cyclase (GC) is transmitted through gap junctions to the oocyte where it suppresses PDE3 activity and helps to maintain meiotic arrest by sustaining high cAMP levels and PKA activity. Soluble GC can be stimulated by nitric oxide (NO), while membrane-bound GC can be stimulated by atrial natriuretic peptide (ANP). LH stimulation of the mural granulosa cells initiates a signal transduction cascade that results in p38MAPK-dependent production of EGF-like peptides (EGFp) that act in autocrine fashion to stimulate the phosphorylation and activation of ERK1/2. ERK1/2 then drives the synthesis of prostaglandin E2 that diffuses to cumulus granulosa cells, binds to the prostaglandin receptor PTGER2 and triggers the synthesis of EGFp (see Shimada et al, 2006). FSH can mimic this action in cumulus cells. ERK1/2 terminates gap junction coupling between the two granulosa compartments and blocks cGMP transfer to the oocyte; it also generates a positive signal (X) for maturation that can be transmitted through gap junctions or possibly diffuses to the oocyte. PDE3 activity in the oocyte is increased via protein kinase B (Akt), the removal of cGMP inhibition, and perhaps also by a transient increase in oocyte cAMP and PKA activity that occurs following gonadotropin stimulation of the granulosa cells. The resulting decrease in cAMP levels leads to inactivation of PKA and elimination of its block to MPF activation. AMPK is activated in immature mouse oocytes in response to gonadotropin stimulation and helps drive MPF activation and GVB, most likely through indirect means. Its activation may result from AMP generated by PDE3 and/or perhaps by an upstream kinase (AMPKK), whose identity has not yet been identified.
REFERENCES
- Albertini DF, Combelles CMH, Benecci E, Carabatsos MJ. Cellular basis for paracrine regulation of ovarian follicle development. Reproduction. 2001;121:647–653. doi: 10.1530/rep.0.1210647. [DOI] [PubMed] [Google Scholar]
- Alessi DR, Cuenda A, Cohen P, Dudley DT, Saltiel AR. PD98059 is a specific inhibitor of activation of mitogen-activated protein kinase kinase in vitro and in vivo. J Biol Chem. 1995;270:27489–27494. doi: 10.1074/jbc.270.46.27489. [DOI] [PubMed] [Google Scholar]
- Araki K, Naito K, Haraguchi S, Suzuki R, Yokoyama M, Inoue M, Aizawa S, Toyoda Y, Sato E. Meiotic abnormalities of c-MOS knockout mouse oocytes: Activation after first meiosis or entrance into third meiotic metaphase. Biol Reprod. 1996;55:1315–1324. doi: 10.1095/biolreprod55.6.1315. [DOI] [PubMed] [Google Scholar]
- Ashkenazi H, Cao X, Motola S, Popliker M, Conti M, Tsafriri A. Epidermal growth factor family members: mediators of the ovulatory response. Endocrinology. 2005;146:77–84. doi: 10.1210/en.2004-0588. [DOI] [PubMed] [Google Scholar]
- Ben-Ami I, Freimann S, Armon L, Dantes A, Ron-El R, Amsterdam A. Novel function of ovarian growth factors: combined studies by DNA microarray, biochemical and physiological approaches. Mol Hum Reprod. 2006;12:413–419. doi: 10.1093/molehr/gal045. [DOI] [PubMed] [Google Scholar]
- Bilodeau S, Fortier MA, Sirard MA. Effect of adenylate cyclase stimulation on meiotic resumption and cyclic AMP content of zona-free and cumulus-enclosed bovine oocytes in vitro. J Reprod Fertil. 1993;97:5–11. doi: 10.1530/jrf.0.0970005. [DOI] [PubMed] [Google Scholar]
- Bilodeau-Goeseels S, Sasseville M, Guillemette C, Richard FJ. Effects of adenosine monophosphate-activated kinase activators on bovine oocyte nuclear maturation in vitro. Mol Reprod Dev. 2007;74:1021–1034. doi: 10.1002/mrd.20574. [DOI] [PubMed] [Google Scholar]
- Bornslaeger EA, Schultz RM. Regulation of mouse oocyte maturation: effect of elevating cumulus cell cAMP on oocyte cAMP levels. Biol Reprod. 1985a;33:698–704. doi: 10.1095/biolreprod33.3.698. [DOI] [PubMed] [Google Scholar]
- Bornslaeger EA, Schultz RM. Adenylate cyclase activity in zona-free mouse oocytes. Exp Cell Res. 1985b;156:277–281. doi: 10.1016/0014-4827(85)90282-4. [DOI] [PubMed] [Google Scholar]
- Brown RL, Ord T, Moss SB, Williams CJ. A-kinase anchor proteins as potential regulators of protein kinase A function in oocytes. Biol Reprod. 2002;67:981–987. doi: 10.1095/biolreprod.101.003046. [DOI] [PubMed] [Google Scholar]
- Brucker C, Alexander NJ, Hodgen GD, Sandow BA. Transforming growth factor-alpha augments meiotic maturation of cumulus cell-enclosed mouse oocytes. Mol Reprod Dev. 1991;28:94–98. doi: 10.1002/mrd.1080280115. [DOI] [PubMed] [Google Scholar]
- Byskov AG, Andersen CY, Nordholm L, Thogersen H, Xia G, Wassmann O, Andersen JV, Guddal E, Roed T. Chemical structure of sterols that activate oocyte meiosis. Nature. 1995;374:559–562. doi: 10.1038/374559a0. [DOI] [PubMed] [Google Scholar]
- Carabatsos MJ, Sellitto C, Goodenough DA, Albertini DF. Oocyte-granuolosa cell heterologous gap junctions are required for the coordination of nuclear and cytoplasmic meiotic competence. Dev Biol. 2000;226:167–179. doi: 10.1006/dbio.2000.9863. [DOI] [PubMed] [Google Scholar]
- Chen J, Hudson E, Chi MM, Chang AS, Moley KH, Hardie DG, Downs SM. AMPK regulation of mouse oocyte meiotic resumption in vivo. Dev Biol. 2006;291:227–238. doi: 10.1016/j.ydbio.2005.11.039. [DOI] [PubMed] [Google Scholar]
- Chen J, Chi M, Moley K, Downs SM. cAMP pulsing of denuded mouse oocytes increases meiotic resumption via activation of AMP-activated protein kinase. Reproduction. 2009 doi: 10.1530/REP-08-0535. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Downs SM. AMP-activated protein kinase is involved in hormone-induced moue oocyte meiotic maturation. Dev Biol. 2008;313:47–57. doi: 10.1016/j.ydbio.2007.09.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z, Gibson TB, Robinson F, Silvestro L, Pearson G, Xu B, Wright A, Vanderbilt C, Cobb MH. MAP Kinases. Chem Rev. 2001;101:2449–2476. doi: 10.1021/cr000241p. [DOI] [PubMed] [Google Scholar]
- Choi T, Aioki F, Mori M, Yamashita M, Naganama Y, Kohmoto K. Activation of p34cdc2 protein kinase activity in meiotic and mitotic cell cycles in mouse oocytes and embryos. Development. 1991;113:789–795. doi: 10.1242/dev.113.3.789. [DOI] [PubMed] [Google Scholar]
- Choi T, Fukasawa K, Zhou R, Tessarollo L, Borror K, Resau J, Vande Woude GF. The MOS/mitogen-activated protein kinase (MAPK) pathway regulates the size and degradation of the first polar body in maturing mouse oocytes. Proc Natl Acad Sci USA. 1996;93:7032–7035. doi: 10.1073/pnas.93.14.7032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colledge WH, Carlton MB, Udy GB, Evans JM. Disruption of c-mos causes parthenogenetic development of unfertilized mouse eggs. Nature. 1994;370:65–68. doi: 10.1038/370065a0. [DOI] [PubMed] [Google Scholar]
- Conti M, Beavo J. Biochemistry and physiology of cyclic nucleotide phosphodiesterases: essential components in cyclic nucleotide signaling. Ann Rev Biochem. 2007;76:481–511. doi: 10.1146/annurev.biochem.76.060305.150444. [DOI] [PubMed] [Google Scholar]
- Conti M, Hsieh M, Park JY, Su YQ. Role of the epidermal growth factor network in ovarian follicles. Mol Endocrinol. 2009;20:715–723. doi: 10.1210/me.2005-0185. [DOI] [PubMed] [Google Scholar]
- Cunat S, ANahory T, Berthenet C, Hedon B, Franckhauser C, Fernandez A, Hammah S, Lamb NJC. The cell cycle control protein cdc25C is present, and phosphorylated on serine 214 in the transition from germinal vesicle to metaphase II in human oocyte meiosis. Mol Reprod Dev. 2008;75:1176–1184. doi: 10.1002/mrd.20853. [DOI] [PubMed] [Google Scholar]
- Das K, Phipps W, Hensleig H, Tagatz G. Epidermal growth factor in human follicular fluid stimulates mouse oocyte maturation in vitro. Fertil Steril. 1992;57:895–901. doi: 10.1016/s0015-0282(16)54977-2. [DOI] [PubMed] [Google Scholar]
- Davies RP, Reddy H, Caivano M, Cohen P. Specificity and mechanism of action of some commonly used protein kinase inhibitors. Biochem J. 2000;351:95–105. doi: 10.1042/0264-6021:3510095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dekel N. Regulation of oocyte maturation. The role of cAMP. Ann NY Acad Sci. 1988;541:211–216. doi: 10.1111/j.1749-6632.1988.tb22258.x. [DOI] [PubMed] [Google Scholar]
- Dekel N. Cellular, biochemical and molecular mechanisms regulating oocyte maturation. Mol Cell Endocrinol. 2005;234:19–25. doi: 10.1016/j.mce.2004.09.010. [DOI] [PubMed] [Google Scholar]
- Dekel N, Beers WH. Rat oocyte maturation in vitro: relief of cyclic AMP inhibition by gonadotropins. Proc Natl Acad Sci USA. 1978;75:4369–4373. doi: 10.1073/pnas.75.9.4369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dekel N, Lawrence TS, Gilula NB, Beers WH. Modulation of cell-to-cell communication in the cumulus-oocyte complex and the regulation of oocyte maturation by LH. Dev Biol. 1981;86:356–362. doi: 10.1016/0012-1606(81)90193-7. [DOI] [PubMed] [Google Scholar]
- Dekel N, Aberdam E, Sherizly I. Spontaneous maturation in vitro of cumulus-enclosed rat oocytes is inhibited by forskolin. Biol Reprod. 1984;30:537–543. doi: 10.1095/biolreprod31.2.244. [DOI] [PubMed] [Google Scholar]
- Dekel N, Sherizly I. Epidermal growth factor induces maturation of rat follicle-enclosed oocytes. Endocrinology. 1985;116:406–409. doi: 10.1210/endo-116-1-406. [DOI] [PubMed] [Google Scholar]
- Deng J, Carbajal L, Evaul K, Rasar M, Jamnongjit M, Hammes SR. Nongenomic steroid-triggered oocyte maturation: of mice and frogs. Steroids. 2009;74:595–601. doi: 10.1016/j.steroids.2008.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denninger JW, Marletta MA. Guanylate cyclase and the NO/cGMP signaling pathway. Biochim Biophys Acta. 1999;1411:334–350. doi: 10.1016/s0005-2728(99)00024-9. [DOI] [PubMed] [Google Scholar]
- Downs SM. Specificity of epidermal growth factor action on maturation of the murine oocyte and cumulus oophorus in vitro. Biol Reprod. 1989;41:371–379. doi: 10.1095/biolreprod41.2.371. [DOI] [PubMed] [Google Scholar]
- Downs SM. Stimulation of parthenogenesis in mouse ovarian follicles by inhibitors of inosine monophosphate dehydrogenase. Biol Reprod. 1990;43:427–436. doi: 10.1095/biolreprod43.3.427. [DOI] [PubMed] [Google Scholar]
- Downs SM. Ovulation 2: control of the resumption of meiotic maturation in mammalian oocytes. In: Grudzinskas JG, Yovich JL, editors. Gametes—The Oocyte. Cambridge University Press; 1995. pp. 150–192. [Google Scholar]
- Downs SM. Regulation of meiotic arrest and resumption in mammalian oocytes. In: Filicori M, Flamigni C, editors. The Ovary: Regulation, Dysfunction and Treatment. Elsevier Science; 1996. [Google Scholar]
- Downs SM, Coleman DL, Eppig JJ. Hypoxanthine is the principal inhibitor of murine oocyte maturation in a low molecular weight fraction of porcine follicular fluid. Proc Natl Acad Sci USA. 1985;82:454–458. doi: 10.1073/pnas.82.2.454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downs SM, Hunzicker-Dunn M. Differential regulation of oocyte maturation and cumulus expansion in the mouse oocyte-cumulus cell complex by site-selective analogs of cyclic adenosine monophosphate. Dev Biol. 1995;172:72–85. doi: 10.1006/dbio.1995.0006. [DOI] [PubMed] [Google Scholar]
- Downs SM, Coleman DL, Eppig JJ. Maintenance of murine oocyte meiotic arrest: uptake and metabolism of hpoxanthine and adenosine by cumulus cell-enclosed and denuded oocytes. Dev Biol. 1986;117:174–183. doi: 10.1016/0012-1606(86)90359-3. [DOI] [PubMed] [Google Scholar]
- Downs SM, Eppig JJ. Induction of mouse oocyte maturation in vivo by perturbants of purine metabolism. Boil Reprod. 1987;36:431–437. doi: 10.1095/biolreprod36.2.431. [DOI] [PubMed] [Google Scholar]
- Downs SM, Daniel SAJ, Eppig JJ. Induction of maturation in cumulus cell-enclosed mouse oocytes by follicle-stimulating hormone and epidermal growth factor: evidence for a positive stimulus of somatic cell origin. J Exp Zool. 1988;245:86–96. doi: 10.1002/jez.1402450113. [DOI] [PubMed] [Google Scholar]
- Downs SM, Daniel SAJ, Bornslaeger EA, Hoppe PC, Eppig JJ. Maintanance of meiotic arrest in mouse oocytes by purines: modulation of cAMP levels and cAMP phosphodiesterase activity. Gamete Res. 1989;23:323–334. doi: 10.1002/mrd.1120230309. [DOI] [PubMed] [Google Scholar]
- Downs SM, Buccione R, Eppig JJ. Modulation of meiotic arrest in mouse oocytes by guanyl nucleotides and modifiers of G-proteins. J Exp Zool. 1992;262:391–404. doi: 10.1002/jez.1402620405. [DOI] [PubMed] [Google Scholar]
- Downs SM, Cottom J, Hunzicker-Dunn M. Protein kinase C and meiotic regulation in isolated mouse oocytes. Mol Reprod Dev. 2001;58:101–115. doi: 10.1002/1098-2795(200101)58:1<101::AID-MRD13>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- Downs SM, Gilles R, Vanderhoef C, Humpherson PG, Leese HJ. Differential response of cumulus cells-enclosed and denuded mouse oocytes in a meiotic induction model system. Mol Reprod Dev. 2006;73:379–389. doi: 10.1002/mrd.20416. [DOI] [PubMed] [Google Scholar]
- Downs SM, Chen J. EGF-like peptides mediate FSH-induced maturation of cumulus cell-enclosed mouse oocytes. Mol Reprod Dev. 2008;75:105–114. doi: 10.1002/mrd.20781. [DOI] [PubMed] [Google Scholar]
- Downs SM, Mosey JL, Klinger J. Fatty acid oxidation and meiotic resumption in mouse oocytes. Mol Reprod Dev. 2009;76:844–853. doi: 10.1002/mrd.21047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudley DT, Pang L, Decker SJ, Bridges AJ, Saltiel AR. A synthetic inhibitor of the mitogen-activated protein kinase cascade. Proc Natl Acad Sci USA. 1995;92:7686–7689. doi: 10.1073/pnas.92.17.7686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan FE, Moss SB, Williams CJ. Knockdown of the cAMP-dependent protein kinase (PKA) type Iα regulatory subunit in mouse oocytes disrupts meiotic arrest and results in meiotic spindle defects. Dev Dyn. 2006;235:2961–2968. doi: 10.1002/dvdy.20930. [DOI] [PubMed] [Google Scholar]
- Edwards RG. Maturation in vitro of mouse, sheep, cow, pig, rhesus monkey and human ovarian oocytes. Nature. 1965;208:349–351. doi: 10.1038/208349a0. [DOI] [PubMed] [Google Scholar]
- Ekholm C, Hillensjo T, Magnusson C, Rosberg S. Stimulation and inhibition of rat oocyte meiosis by forskolin. Biol Reprod. 1984;30:537–543. doi: 10.1095/biolreprod30.3.537. [DOI] [PubMed] [Google Scholar]
- Eppig JJ, Downs SM. Chemical signals that regulate mammalian oocyte maturation. Biol Reprod. 1984;30:1–11. doi: 10.1095/biolreprod30.1.1. [DOI] [PubMed] [Google Scholar]
- Eppig JJ, Ward-bailey PF, Coleman DL. Hypoxanthine and adenosine in murine ovarian follicular fluid: concentrations and activity in maintaining oocyte meiotic arrest. Biol Reprod. 1985;33:1041–1049. doi: 10.1095/biolreprod33.5.1041. [DOI] [PubMed] [Google Scholar]
- Espey LL, Richards JS. Temporal and spatial patterns of ovarian gene transcription following an ovulatory dose of gonadotropin in the rat. Biol Reprod. 2002;67:1662–1670. doi: 10.1095/biolreprod.102.005173. [DOI] [PubMed] [Google Scholar]
- Faerge I, Terry B, Kalous J, Wahl P, Lessl M, Ottesen JL, Hyttel P, Grondahl C. Resumption of meiosis-activating sterol has a different signal transduction pathway than spontaneous resumption of meiosis in denuded mouse oocytes cultured in vitro. Biol Reprod. 2001;65:1751–1758. doi: 10.1095/biolreprod65.6.1751. [DOI] [PubMed] [Google Scholar]
- Fan HY, Tong C, Lian L, Li SW, Gao WX, Cheng Y, Chen DY, Schatten H, Sun QY. Characterizaton of ribosomal S6 protein kinase p90rsk during meiotic maturation and fertilization in pig oocytes: MAPK-associated activation and localization. Biol Reprod. 2003;68:968–977. doi: 10.1095/biolreprod.102.008839. [DOI] [PubMed] [Google Scholar]
- Fan HY, Sun QY. Involvement of mitogen-activated protein kinase cascade during oocyte maturation and fertilization in mammals. Biol Reprod. 2004;70:535–547. doi: 10.1095/biolreprod.103.022830. [DOI] [PubMed] [Google Scholar]
- Fan H-Y, Liu Z, Shimada M, Sterneck E, Johnson PF, Hedrick SM, Richards JS. MAPK3/1 (ERK1/2) in ovarian granulosa cells are essential for female fertility. Science. 2009;324:938–941. doi: 10.1126/science.1171396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Favata MF, Horiuchi KY, Manos EF, Daulerio AJ, Stradley DA, Feeser WS, Van Dyk DE, Pitts WJ, Earl RA, Hobbs F, Copeland RA, Magolda RL, Scherle RA, Trzaskos JM. Identification of a novel inhibitor of mitogen-activated protein kinase kinase. J Biol Chem. 1998;273:18623–18632. doi: 10.1074/jbc.273.29.18623. [DOI] [PubMed] [Google Scholar]
- Fissore RA, He CI, VandeWoude GF. Potential role of mitogen-activated protein kinase during meiosis resumption in bovine oocytes. Biol Reprod. 1996;55:1261–1270. doi: 10.1095/biolreprod55.6.1261. [DOI] [PubMed] [Google Scholar]
- Freudzon L, Norris RP, Hand AR, Tanaka S, Saeki Y, Jones TLZ, Rasenick MM, Berlot CH, Mehlmann LM, Jaffe LA. Regulation of meiotic prophase arrest in mouse oocytes by GPR3, a constitutive activator of the Gs G protein. J Cell Biol. 2005;171:255–265. doi: 10.1083/jcb.200506194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujinaga H, Yamoto M, Shikone T, Nakano R. FSH and LH up-regulate epidermal growth factor receptors in rat granulosa cells. J Endocrinol. 1994;140:171–177. doi: 10.1677/joe.0.1400171. [DOI] [PubMed] [Google Scholar]
- Gershon E, Plaks V, Aharon I, Galiani D, Reizel Y, Sela-Abramovich S, Granot I, Winterhager E, Dekel N. Oocyte-directed depletion of connexin43 using the Cre-LoxP system leads to subfertility in female mice. Dev Biol. 2008;313:1–12. doi: 10.1016/j.ydbio.2007.08.041. [DOI] [PubMed] [Google Scholar]
- Gilchrist RB, Thompson JG. Oocyte maturation: emerging concepts and technologies to improve developmental potential in vitro. Theriogenology. 2007;67:6–15. doi: 10.1016/j.theriogenology.2006.09.027. [DOI] [PubMed] [Google Scholar]
- Gilula NB, Epstein ML, Beers WH. Cell-to-cell communication and ovulation, a study of the cumulus-oocyte complex. J Cell Biol. 1978;78:58–75. doi: 10.1083/jcb.78.1.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goud PT, Goud AP, Qian C, Laverge H, Van der Elst J, De Sutter P, Dhont M. In-vitro maturation of human germinal vesicle stage oocytes: role of cumulus cells and epidermal growth factor in the culture medium. Hum Reprod. 1998;13:1638–1644. doi: 10.1093/humrep/13.6.1638. [DOI] [PubMed] [Google Scholar]
- Goudet G, Belin F, Bezard J, Gerard N. Maturation-promoting factor (MPF) and mitogen activated protein kinase (MAPK) expression in relation to oocyte competence for in-vitro maturation in the mare. Mol Hum Reprod. 1998;4:563–570. doi: 10.1093/molehr/4.6.563. [DOI] [PubMed] [Google Scholar]
- Granot I, Dekel Phosphorylaton and expression of connexin-43 ovarian gap junction protein are regulated by luteinizing hormone. J Biol Chem. 1994;269:30502–30509. [PubMed] [Google Scholar]
- Griffin AM, Grondahl C, Fleming SD. Action of hypoxanthine and meiosis-activating sterol on oocyte maturation in the mouse is strain specific. Reprod BioMed Online. 2004;8:673–681. doi: 10.1016/s1472-6483(10)61648-3. [DOI] [PubMed] [Google Scholar]
- Han SJ, Chen R, Paronetto MP, Conti M. Wee1B is an oocyte-specific kinase nvolved in the control of meiotic arrest in the mouse. Curr Biol. 2005;15:1670–1676. doi: 10.1016/j.cub.2005.07.056. [DOI] [PubMed] [Google Scholar]
- Han SJ, Conti M. New pathways from PKA to the Cdc2/cyclin B complex in oocytes: Wee1B as a potential PKA substrate. Cell Cycle. 2006;5:227–231. doi: 10.4161/cc.5.3.2395. [DOI] [PubMed] [Google Scholar]
- Han SJ, Vaccari S, Nedachi T, Andersen CB, Kovacina KS, Roth RA, Conti M. Protein kinase B/Akt phosphorylation of PDE3A and its role in mammalian oocyte maturation. EMBO J. 2006;25:5716–5725. doi: 10.1038/sj.emboj.7601431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardie DG. Minireview: The AMP-activated protein kinase cascade: the key sensor of cellular energy status. Endocrinology. 2003;144:5179–5183. doi: 10.1210/en.2003-0982. [DOI] [PubMed] [Google Scholar]
- Hardie DG, Carling D, Carlson M. The AMP-activated SNF1 protein kinase subfamily: metabolic sensors of the eukaryotic cell? Ann Rev Biochem. 1998;67:821–855. doi: 10.1146/annurev.biochem.67.1.821. [DOI] [PubMed] [Google Scholar]
- Hardie DG, Hawley SH, Scott JW. AMP-activated protein kinase—development of the energy sensor concept. J Physiol. 2006;574:7–15. doi: 10.1113/jphysiol.2006.108944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto N, Watanabe N, furuta Y, Tamemoto H, Sagata N, Yokoyama M, Okazaki K, Nagayoshi M, Takeda N, Ikawa Y, Aizawai S. Parthenogenetic activation of oocytes in c-mos-deficient mice. Nature. 1994;379:68–71. doi: 10.1038/370068a0. [DOI] [PubMed] [Google Scholar]
- Hinckley M, Vaccari S, Horner K, Chen R, Conti M. The G-protein-coupled receptors GPR3 and GPR12 are involved in cAMP signaling and maintenance of meiotic arrest in rodent oocytes. Dev Biol. 2005;287:249–261. doi: 10.1016/j.ydbio.2005.08.019. [DOI] [PubMed] [Google Scholar]
- Horner K, Livera G, Hinckley M, Trinh K, Storm D, Conti M. Rodent oocytes express an active adenylyl cyclase required for meiotic arrest. Dev Biol. 2003;258:385–396. doi: 10.1016/s0012-1606(03)00134-9. [DOI] [PubMed] [Google Scholar]
- Hsieh M, Lee D, Panigone S, Horner K, Chen R, Theologis A, Lee DC, Threadgill DW, Conti M. Luteinizing hormone-dependent activation of the epidermal growth factor network is essential for ovulation. Mol Cell Biol. 2007;27:1914–1924. doi: 10.1128/MCB.01919-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh H, Zamah AM, Conti M. Epidermal growth factor-like growth factors in the follicular fluid: role in oocyte development and maturation. Sem Reprod Med. 2009;27:52–61. doi: 10.1055/s-0028-1108010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbard CJ. Ovarian cAMP and cGMP fluctuations in the hamster during the oestrous cycle. J Reprod Fertil. 1980;59:351–355. doi: 10.1530/jrf.0.0590351. [DOI] [PubMed] [Google Scholar]
- Hubbard CJ. The effect in vivo of alterations in gonadotropins and cyclic nucleotides on oocyte maturation in the hamster. Life Sci. 1983;33:1695–1702. doi: 10.1016/0024-3205(83)90726-9. [DOI] [PubMed] [Google Scholar]
- Hubbard CJ, Terranova PF. Inhibitory action of cyclic guanosine 5′-phosphoric acid (GMP) on oocyte maturation: dependence on an intact cumulus. Biol Reprod. 1980;26:628–632. doi: 10.1095/biolreprod26.4.628. [DOI] [PubMed] [Google Scholar]
- Huo L-J, Fan H-Y, Zhong Z-S, Chen D-Y, Schatten H, Sun Q-Y. Ubiquitin-proteasome pathway modulates mouse oocyte meiotic maturation and fertilizaton via regulation of MAPK cascade and cyclin B1 degradation. Mech Dev. 2004;121:1275–1287. doi: 10.1016/j.mod.2004.05.007. [DOI] [PubMed] [Google Scholar]
- Inoue M, Naito K, Nakayama T, Sato E. Mitogen-activated protein kinase translocates into the germinal vesicle and induces germinal vesicle breakdown in porcine oocytes. Biol Reprod. 1998;58:130–136. doi: 10.1095/biolreprod58.1.130. [DOI] [PubMed] [Google Scholar]
- Jansen M, Ten JKlooster JP, Offerhaus HJ, Clevers H. LKB1 and AMPK family signaling: the intimate link between cell polarity and energy metabolism. Physiol Rev. 2009;89:777–798. doi: 10.1152/physrev.00026.2008. [DOI] [PubMed] [Google Scholar]
- Jensen JT, Schwinof KM, Zelinski-Wooten MB, Conti M, DePaolo LV, Stouffer RL. Phosphodiesterase 3 inhibitors selectively block the spontaneous resumption of meiosis by macaque oocytes in vitro. Hum Reprod. 2002;17:2079–2084. doi: 10.1093/humrep/17.8.2079. [DOI] [PubMed] [Google Scholar]
- Juneja SC, Barr KJ, Enders GC, Kidder GM. Defects in the germ line and gonads of mice lacking connexin43. Biol Reprod. 1999;60:1263–1270. doi: 10.1095/biolreprod60.5.1263. [DOI] [PubMed] [Google Scholar]
- Kahn BB, Alquier T, Carling D, Hardie DG. AMP-activated protein kinase: ancient energy gauge provides clues to modern understanding of metabolism. Cell Metab. 2005;1:15–25. doi: 10.1016/j.cmet.2004.12.003. [DOI] [PubMed] [Google Scholar]
- Kalinowski RR, Berlot CH, Jones TL, Ross LF, Jaffe LA, Mehlmann LM. Maintenance of meiotic prophase arrest in vertebrate oocytes by a Gs protein-mediated pathway. Dev Biol. 2004;267:1–13. doi: 10.1016/j.ydbio.2003.11.011. [DOI] [PubMed] [Google Scholar]
- Kalma Y, Ganot I, Galiani D, Barach A, Dekel N. Luteinizing hormone-induced connexin 43 down-regulation: inhibition of translation. Endocrinology. 2004;145:1617–1624. doi: 10.1210/en.2003-1051. [DOI] [PubMed] [Google Scholar]
- Kidder GM, Mhawi AA. Gap junctions and ovarian folliculogenesis. Reproduction. 2002;123:613–620. doi: 10.1530/rep.0.1230613. [DOI] [PubMed] [Google Scholar]
- Kimura N, Hoshino Y, Totsukawa K, Sato E. Cellular and molecular events during oocyte maturation in mammals: molecules of cumulus-oocyte complex matrix and signaling pathways regulating meiotic progression. Soc Reprod Fertil Suppl. 2007;63:327–342. [PubMed] [Google Scholar]
- Kovo M, Schillace RV, Galiani D, Josefsberg LB, Carr DW, Dekel N. Developmental regulation of cAMP-dependent protein kinas A (PKA) and A-kinase anchoring proteins (AKAPs) during meiosis in rat oocytes. Mol Cell Endocrinol. 2002;192:105–113. doi: 10.1016/s0303-7207(02)00084-9. [DOI] [PubMed] [Google Scholar]
- Kuhajda FP. AMP-activated protein kinase and human cancer: cancer metabolism revisited. Int J Obesity. 2008;32(Suppl 4):S36–41. doi: 10.1038/ijo.2008.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuyt JR, Kruip TA, de Jong-Brink M. Cytochemical localization of adenylate cyclase in bovine cumulus-oocyte complexes. Exp Cell Res. 1988;174:139–145. doi: 10.1016/0014-4827(88)90149-8. [DOI] [PubMed] [Google Scholar]
- Lau A, Kanemitsu M, Kurata W, Danesh S, Boynton A. Epidermal growth factor disrupts gapjunctional communication and induces phosphorylation of connexin 43 on serine. Mol Biol Cell. 1992;3:865–874. doi: 10.1091/mbc.3.8.865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laforest MF, Pouliot E, Gueguen L, Richard FJ. Fundamental significance of specific phosphodiesterases in the control of spontaneous meiotic resumption in porcine oocytes. Mol Reprod Dev. 2005;70:361–372. doi: 10.1002/mrd.20203. [DOI] [PubMed] [Google Scholar]
- LaPolt PS, Leung K, ishimaru R, Tafoya MA, Chen JY. Roles of cyclic GMP in modulating ovarian functions. Reprod BioMed Online. 2003;6:15–23. doi: 10.1016/s1472-6483(10)62051-2. [DOI] [PubMed] [Google Scholar]
- LaRosa C, Downs SM. MEK inhibitors block AICAR-induced maturation in mouse oocytes by a MAPK-independent mechanism. Mol Reprod Dev. 2005;70:235–245. doi: 10.1002/mrd.20200. [DOI] [PubMed] [Google Scholar]
- LaRosa C, Downs SM. Stress stimulates AMP-activated protein kinase and meiotic resumption in mouse oocytes. Biol Reprod. 2006;74:585–592. doi: 10.1095/biolreprod.105.046524. [DOI] [PubMed] [Google Scholar]
- LaRosa C, Downs SM. Meiotic induction by heat stress in mouse oocytes: involvement of AMP-activated protein kinase and MAPK family members. Biol Reprod. 2007;76:476–486. doi: 10.1095/biolreprod.106.057422. [DOI] [PubMed] [Google Scholar]
- Larsen WJ, Wert S, Brunner GD. A dramatic loss of cumulus cell gap junctions is correlated with germinal vesicle breakdown in rat oocytes. Dev Biol. 1986;113:517–521. doi: 10.1016/0012-1606(86)90187-9. [DOI] [PubMed] [Google Scholar]
- Larsen WJ, Wert S, Brunner GD. Differential modulation of rat follicle cell gap junction populations at ovulation. Dev Biol. 1987;122:61–71. doi: 10.1016/0012-1606(87)90332-0. [DOI] [PubMed] [Google Scholar]
- Ledent C, Demeestere I, Blum D, Petermans J, Hamalainen T, Smits G, Vassart G. Premature ovarian aging in mice deficient for Gpr3. Proc Natl Acad Sci USA. 2005;102:8922–8926. doi: 10.1073/pnas.0503840102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefevre B, Pesty A, Courtot AM, Martins CV, Broca O, Denys A, Arnault E, Poirot C, Avazeri N. The phosphoinositide-phospholipase C (PI-PLC) pathway in the mouse oocyte. Crit Rev Euk Gene Exp. 2007;17:259–269. doi: 10.1615/critreveukargeneexpr.v17.i4.10. [DOI] [PubMed] [Google Scholar]
- Leithe E, Rivedal E. Epidermal growth factor regulates ubiqitination, internalization and proteosome-dependent degradation of conexin 43. J Cell Sci. 2004;117:1211–1220. doi: 10.1242/jcs.00951. [DOI] [PubMed] [Google Scholar]
- Leonardsen L, Wiersma A, Baltsen M, Byskov AG, Andersen CY. Regulation of spontaneous and induced resumption of meiosis in mouse oocytes by different intracellular pathways. J Reprod Fertil. 2000;120:377–383. doi: 10.1530/jrf.0.1200377. [DOI] [PubMed] [Google Scholar]
- Li M, Schatten H, Sun QY. Androgen receptor’s destiny in mammalian oocytes: a new hypothesis. Mol Hum Reprod. 2009;15:149–154. doi: 10.1093/molehr/gap006. [DOI] [PubMed] [Google Scholar]
- Lincoln AJ, Wickramasinghe D, Stein P, Schultz RM, Palko ME, De Miguel MP, Tessarollo L, Donovan PJ. Cdc25b phosphatase isr equired for resumption of meiosis during oocyte maturation. Nat Genet. 2002;30:446–449. doi: 10.1038/ng856. [DOI] [PubMed] [Google Scholar]
- Lu Q, Smith GD, Chen DY, Yang Z, Han ZM, Schatten H, Sun QU. Phosphorylation of mitogen-activated protein kinase is regulated by protein kinase C, cyclic 3′,5′-adenosine monophosphate, and protein phosphatase modulators during meiosis resumption in rat oocytes. Biol Reprod. 2001;64:1444–1450. doi: 10.1095/biolreprod64.5.1444. [DOI] [PubMed] [Google Scholar]
- Marangos P, Carroll J. The dynamics of cyclin B1 distribution during meiosis I in mouse oocytes. Reproduction. 2004;128:153–162. doi: 10.1530/rep.1.00192. [DOI] [PubMed] [Google Scholar]
- Marangos P, Verschuren EW, Chen R, Jackson PK, Carroll J. Prophase I arrest and progression to metaphase I in mouse oocytes are controlled by Emi1-dependent regulation of APCCdh1. J Cell Biol. 2007;176:65–75. doi: 10.1083/jcb.200607070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masciarelli S, Horner K, Liu C, Park SH, Hinckley M, Hockman S, Nedachi T, Jin C, Conti M, Manganiello V. Cyclic nucleotide phosphodiesterase 3A-deficient mice as a model of female infertility. J Clin Invest. 2004;114:196–205. doi: 10.1172/JCI21804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattioli M, Gioia L, Barboni B. Calcium elevation in sheep cumulus-oocyte complexes after luteinizing hormone stimulation. Mol Reprod Dev. 1998;50:361–369. doi: 10.1002/(SICI)1098-2795(199807)50:3<361::AID-MRD13>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Mayes MA, Sirard MA. Effect of type 3 and type 4 phosphodiesterase inhibitors on the maintenance of bovine oocytes in meiotic arrest. Biol Reprod. 2002;66:180–184. doi: 10.1095/biolreprod66.1.180. [DOI] [PubMed] [Google Scholar]
- Mayes MA, Laforest MF, Guillemette C, Gilchrist RB, Richard FJ. Adenosine 5′-monophosphate kinase-activated protein kinase (PRKA) activators delay meiotic resumption in porcine oocytes. Biol Reprod. 2007;76:589–597. doi: 10.1095/biolreprod.106.057828. [DOI] [PubMed] [Google Scholar]
- Mehlmann LM. Stops and starts in mammalian oocytes: recent advances in understanding the regulation of meiotic arrest and oocyte maturation. Reproduction. 2005;130:791–799. doi: 10.1530/rep.1.00793. [DOI] [PubMed] [Google Scholar]
- Mehlmann LM, Jones TL, Jaffe LA. Meiotic arrest in the mouse follicle maintained by a Gs protein in the oocyte. Science. 2002;297:1343–1345. doi: 10.1126/science.1073978. [DOI] [PubMed] [Google Scholar]
- Mehlmann LM, Kalinowski RR, Ross LF, Parlow AF, Hewlett EL, Jaffe LA. Meiotic resumption in response to luteinizing hormone is independent of a Gi family G protein or calcium in the mouse oocyte. Dev Biol. 2006;299:345–355. doi: 10.1016/j.ydbio.2006.07.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehlmann LM, Sacki Y, Tanaka S, Brennan TJ, Evsikov AV, Pendola FL, Knowles BB, Eppig JJ, Jaffe LA. The Gs-linked receptor PGR3 maintains meiotic arrest in mammalian oocytes. Science. 2004;306:1947–1950. doi: 10.1126/science.1103974. [DOI] [PubMed] [Google Scholar]
- Mitra J, Schultz RM. Regulation of the acquisition of meiotic competence in the mouse: changes in the subcellular localization of cdc2, cyclin B1, cdc25C and wee1, and in the concentration of these proteins and their transcripts. J Cell Sci. 1996;109:2407–2415. doi: 10.1242/jcs.109.9.2407. [DOI] [PubMed] [Google Scholar]
- Motola S, Cao X, Popliker M, Tsafriri A. Involvement of mitogen-activated protein kinase (MAPK) pathway in LH- and meiosis-activating sterol (MAS)-induced maturation in rat and mouse oocytes. Mol Reprod Dev. 2008;75:1533–1541. doi: 10.1002/mrd.20899. [DOI] [PubMed] [Google Scholar]
- Murray AW, Kirschner MW. Dominoes and clocks: the union of two views of the cell cycle. Science. 1989;246:614–621. doi: 10.1126/science.2683077. [DOI] [PubMed] [Google Scholar]
- Newhall KJ, Criniti AR, Cheah CS, Smith KC, Kafer KE, Burkart AD, McKnight GS. Dynamic anchoring of PKA is essential during oocyte maturation. Curr Biol. 2006;16:321–327. doi: 10.1016/j.cub.2005.12.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nogueira D, Albano C, Adriaenssens T, Cortvrindt R, Bourgain C, Devroey P, Smitz J. Human oocytes reversibly arrested in prophase I by phosphodiesterase type 3 inhibitor in vitro. Biol Reprod. 2003;69:1042–1052. doi: 10.1095/biolreprod.103.015982. [DOI] [PubMed] [Google Scholar]
- Norbury C, Nurse P. Animal cell cycles and their control. Ann Rev Biochem. 1992;61:441–470. doi: 10.1146/annurev.bi.61.070192.002301. [DOI] [PubMed] [Google Scholar]
- Norris RP, Freudzon M, Mehlmann LM, Cowan AE, Simon AM, Paul DL, Lampe PD, Jaffe LA. Luteinizing hormone causes MAP kinase-dependent phosphorylation and closure of connexin 43 gap junctions in mouse ovarian follicles: one of two paths to meiotic resumption. Development. 2008;135:3229–3238. doi: 10.1242/dev.025494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norris RP, Ratzan WJ, Freudzon M, Mehlmann LM, Krall J, Movsesian MA, Wang H, Hengming K, Nikolaev O, Jaffe LA. Cyclic GMP from the surrounding somatic cells regulates cyclic AMP and meiosis in the mouse oocyte. Development. 2009;136:1869–1878. doi: 10.1242/dev.035238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh JS, Han SJ, Conti M. Wee1B, Myt1, and Cdc25 function in distinct compartments of the mouse oocyte to control meiotic resumption. J Cell Biol. 2010 doi: 10.1083/jcb.200907161. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsiewski PJ, Beers WH. cAMP synthesis in the rat oocyte. Dev Biol. 1983;100:287–293. doi: 10.1016/0012-1606(83)90223-3. [DOI] [PubMed] [Google Scholar]
- Panigone S, Hsieh M, Fu M, Persani L, Conti M. Luteinizing hormone signaling in preovulatory follicles involves early activation of the epidermal growth factor receptor pathway. Mol Endocrinol. 2008;22:924–936. doi: 10.1210/me.2007-0246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J-Y, Su Y-Q, Ariga M, Law E, Jin S-EC, Conti M. EGF-like growth factors as mediators of LH action in the ovulatory follicle. Science. 2004;303:682–684. doi: 10.1126/science.1092463. [DOI] [PubMed] [Google Scholar]
- Pincus G, Enzmann EV. Can mammalian eggs undergo normal development in vitro? Proc Natl Acad Sci USA. 1934;20:121–122. doi: 10.1073/pnas.20.2.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piontkewitz Y, Dekel N. Heptanol, an alkanol that blocks gap junctions, induces oocyte maturation. Endocrine J. 1993;1:365–372. [Google Scholar]
- Pirino G, Wescott MP, Donovan PJ. Protein kinase A regulates resumption of meiosis by phosphorylaton of Cdc25B in mammalian oocytes. Cell cycle. 2009;8:665–670. doi: 10.4161/cc.8.4.7846. [DOI] [PubMed] [Google Scholar]
- Prochazka R, Srsen V, Nagyova E, Miyano T, Flechon JE. Developmental regulation of effect of epidermal growth factor on porcine oocyte-cumulus cell complexes: nuclear maturation, expansion, and F-actin remodeling. Mol Reprod Dev. 2000;56:63–73. doi: 10.1002/(SICI)1098-2795(200005)56:1<63::AID-MRD8>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- Racowsky C. Effect of forskolin in the spontaneous maturation and cyclic AMP content of rat oocyte-cumulus cell complexes. J Reprod Fert. 1984;72:107–116. doi: 10.1530/jrf.0.0720107. [DOI] [PubMed] [Google Scholar]
- Racowsky C. Effect of forskolin on the spontaneous maturation and cyclic AMP content of hamster oocyte-cumulus complexes. J Exp Zool. 1985;234:87–96. doi: 10.1002/jez.1402340111. [DOI] [PubMed] [Google Scholar]
- Ratchford AM, Chang AS, Chi M, Sheridan R, Moley KH. Maternal diabetes adversely affects AMP-activated protein kinase activity and cellular metabolism in murine oocytes. Am J Physiol Endocrinol Metab. 2007;293:E1198–1206. doi: 10.1152/ajpendo.00097.2007. [DOI] [PubMed] [Google Scholar]
- Ratner A. The effects of follicle stimulating hormone and luteinizing hormone upon cyclic AMP and cyclic GMP levels in rat ovaries in vitro. Endocrinology. 1976;99:1496–1500. doi: 10.1210/endo-99-6-1496. [DOI] [PubMed] [Google Scholar]
- Ratner A, Sanborn CR. Effect of endogenous LH secretion on ovarian cyclic AMP and cyclic GMP levels in the rat. Life Sci. 1980;26:439–495. doi: 10.1016/0024-3205(80)90163-0. [DOI] [PubMed] [Google Scholar]
- Reis A, Chang H-Y, Levasseur M, Jones KT. APCcdh1 activity in mouse oocytes prevents entry into the first meiotic division. Nature Cell Biol. 2006;8:539–540. doi: 10.1038/ncb1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richard FJ, Tsafriri A, Conti M. Role of phosphodiesterase type 3A in rat oocyte maturation. Biol Reprod. 2001;65:1444–1451. doi: 10.1095/biolreprod65.5.1444. [DOI] [PubMed] [Google Scholar]
- Rodriguez KF, Petters RM, Crosier AE, Farin CE. Roles of gene transcription and PKA subtype activation in maturation in murine oocytes. Reproduction. 2002;123:799–806. doi: 10.1530/rep.0.1230799. [DOI] [PubMed] [Google Scholar]
- Romero S, Smitz J. Epiregulin can effectively mature isolated cumulus-oocyte complexes, but fails as a substitute for the hCG/epidermal growth factor stimulus on cultured follicles. Reproduction. 2009;137:997–1005. doi: 10.1530/REP-08-0523. [DOI] [PubMed] [Google Scholar]
- Ruderman N, Prentki M. AMP kinase and malonyl-CoA: targets for therapy of the metabolic syndrome. Nature Rev. 2004;3:340–351. doi: 10.1038/nrd1344. [DOI] [PubMed] [Google Scholar]
- Sanders MJ, Grondin PO, Hegarty BD, Snowden MA, Carling D. Investigating the mechanism for AMP activation of the AMP-activated protein kinase cascade. Biochem J. 2007;403:139–148. doi: 10.1042/BJ20061520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanz P. AMP-activated protein kinase: structure and regulation. Curr Prot Pep Sci. 9:478–492. doi: 10.2174/138920308785915254. [DOI] [PubMed] [Google Scholar]
- Sagata N, Oskarsson M, Copeland T, Brumbaugh J, Vande Woude GF. Function of c-mos proto-oncogene product in meiotic maturation in Xenopus oocytes. Nature. 1988;335:519–525. doi: 10.1038/335519a0. [DOI] [PubMed] [Google Scholar]
- Sasseville M, Gagnon MC, Guillemette C, Sullivan R, Gilchrist RB, Richard RJ. Regulation of gap junctions in porcine cumulus-oocyte complexes: contributions of granulosa cell contact, gonadotropins, and lipid rafts. Mol Endocrinol. 2009;23:700–710. doi: 10.1210/me.2008-0320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz RM. Molecular aspects of mammalian oocyte growth and maturation. In: Rossant J, Pedersen RA, editors. Experimental Approaches to Mammalian Embryonic Development. Cambridge University Press; Cambridge: 1986. pp. 195–237. [Google Scholar]
- Schultz RM. Meiotic maturation of mammalian oocytes. In: Wassarman PM, editor. Elements of Mammalian fertilizaton. CRC Press; 1991. pp. 77–104. [Google Scholar]
- Schultz RM, Montgomery RR, Belanoff JR. Regulation of mouse oocyte maturation: implication of a decrease in oocyte cAMP and protein dephosphorylation in commitment to resume meiosis. Dev Biol. 1983a;97:264–273. doi: 10.1016/0012-1606(83)90085-4. [DOI] [PubMed] [Google Scholar]
- Schultz RM, Montgomery RR, Ward-Bailey PF, Eppig JJ. Regulation of oocyte maturation in the mouse: possible roles of intercellular communication, cAMP, and testosterone. Dev Biol. 1983b;95:294–304. doi: 10.1016/0012-1606(83)90030-1. [DOI] [PubMed] [Google Scholar]
- Seger R, Krebs EG. The MAPK signaling cascade. FASEB J. 1995;9:726–735. [PubMed] [Google Scholar]
- Sela-Abramovich S, Chorev E, Galiani D, Dekel N. Mitogen-activated protein kinase mediates luteinizing hormone-induced breakdown of communication and oocyte maturation in rat ovarian follicles. Endocrinology. 2005;146:1236–1244. doi: 10.1210/en.2004-1006. [DOI] [PubMed] [Google Scholar]
- Sela-Abramovich S, Edry I, Galiani D, Nevo N, Dekel N. Disruption of gap junctional communication within the ovarian follicle induces oocyte maturation. Endocrinology. 2006;147:2280–2286. doi: 10.1210/en.2005-1011. [DOI] [PubMed] [Google Scholar]
- Sela-Abramovich S, Galiani D, Nevo N, Dekel N. Inhibition of rat oocyte maturation and ovulation by nitric oxide: mechanism of action. Biol Reprod. 2008;78:1111–1118. doi: 10.1095/biolreprod.107.065490. [DOI] [PubMed] [Google Scholar]
- Shimada M, Maeda T, Terada T. Dynamic changes of connexin-43, gap junctional protein, in outer layers of cumulus cells are regulated by PKC and PI 3-kinase during meiotic resumption in porcine oocytes. Biol Reprod. 2001;64:1255–1263. doi: 10.1095/biolreprod64.4.1255. [DOI] [PubMed] [Google Scholar]
- Shimada M, Hernandez-Gonzalez I, Gonzalez-Robayna I, Richards JS. Paracrine and autocrine regulation of epidermal growth factor-like factors in cumulus oocyte complexes and granulosa cells: key roles for prostaglandin synthase 2 and progesterone receptor. Mol Endocrinol. 2006;20:1352–1365. doi: 10.1210/me.2005-0504. [DOI] [PubMed] [Google Scholar]
- Shitsukawa k, Andersen CB, Richard FJ, Horner AK, Wiersma A, van Duin M, Conti M. Cloning and characterization of the cyclic guanosine monophosphate-inhibited phosphodiesterase PDE3A expressed in mouse oocyte. Biol Reprod. 2001;65:188–196. doi: 10.1095/biolreprod65.1.188. [DOI] [PubMed] [Google Scholar]
- Simon AM, Goodenough DA, Li E, Paul DL. Female infertility in mice lacking connexin37. Nature. 1997;385:525–529. doi: 10.1038/385525a0. [DOI] [PubMed] [Google Scholar]
- Sirard MA, First NL. In vitro inhibition of oocyte nuclear maturation in the bovine. Biol Reprod. 1988;39:229–234. doi: 10.1095/biolreprod39.2.229. [DOI] [PubMed] [Google Scholar]
- Smith FD, Langeberg LK, Scott JD. The where’s and when’s of kinase anchoring. Trends Biochem Sci. 2006;31:316–323. doi: 10.1016/j.tibs.2006.04.009. [DOI] [PubMed] [Google Scholar]
- Solc P, Saskova A, Baran V, Kubelka M, Schultz RM, Motlik J. CDC25A phosphatase controls meiosis I progression in mouse oocytes. Dev Biol. 2008;317:260–269. doi: 10.1016/j.ydbio.2008.02.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su YQ, Rubinstein S, Luria A, Lax Y, Breitbart H. Involvement of MEK-mitogen-activated protein kinase pathway in follicle-stimulating hormone-induced but not spontaneous meiotic resumption of mouse oocytes. Biol Reprod. 2001;65:358–365. doi: 10.1095/biolreprod65.2.358. [DOI] [PubMed] [Google Scholar]
- Su YQ, Wigglesworth K, Pendola FL, O’Brien MJ, Eppig JJ. Mitogen-activated protein kinase (MAPK) activity in cumulus cells is essential for gonadotropin-induced oocyte meiotic resumption and cumulus expansion in the mouse. Endocrinology. 2002;143:2221–2232. doi: 10.1210/endo.143.6.8845. [DOI] [PubMed] [Google Scholar]
- Su YQ, Denegre JM, Wigglesworth K, Pendola FL, O’Brien MJ, Eppig JJ. Oocyte-dependent activation of mitogen-activated protein kinase (ERK1/2) in cumulus cells is required for the maturation of the mouse oocyte-cumulus cell complex. Dev Biol. 2003;263:126–138. doi: 10.1016/s0012-1606(03)00437-8. [DOI] [PubMed] [Google Scholar]
- Su YQ, Sugiura K, Eppig JJ. Mouse oocyte control of granulosa cell development and function: paracrine regulation of cumulus cell metabolism. Semin Reprod Med. 2009;27:32–42. doi: 10.1055/s-0028-1108008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan X, Chen DY, Yang Z, Han ZM, Schatten H, Sun QY. Phosphorylation of p90rsk during meiotic maturation and parthenogenetic activation of rat oocytes: correlation with MAP kinases. Zygote. 2001;9:269–276. doi: 10.1017/s0967199401001290. [DOI] [PubMed] [Google Scholar]
- Teilmann SC. Differential expression and localization of connexin-37 and connexin-43 in follicles of different stages in the 4-week-old mouse ovary. Mol Cell Endocrinol. 2005;234:27–35. doi: 10.1016/j.mce.2004.10.014. [DOI] [PubMed] [Google Scholar]
- Thomas RE, Thompson JG, Armstrong DT, Gilchrist RB. Effect of specific phosphodiesterase isoenzyme inhibitors during in vitro maturation of bovine oocytes on meiotic and developmental capacity. Biol Reprod. 2004;71:1142–1149. doi: 10.1095/biolreprod.103.024828. [DOI] [PubMed] [Google Scholar]
- Tong C, Fan HY, Chen da Y, Song XF, Schatten H, Sun QY. Effects of MEK inhibitor U0126 on meiotic progression in mouse oocytes: microtubule organization, asymmetric division and metaphase II arrest. Cell Res. 2003;13:375–383. doi: 10.1038/sj.cr.7290183. [DOI] [PubMed] [Google Scholar]
- Tornell J, Billig H, Hillensjo T. Resumption of rat oocyte meiosis is paralleled by a decrease in guanosine 3′,5′-cyclic monophosphate (cGMP) and is inhibited by microinjection of cGMP. Acta Physiol Scand. 1990;139:511–517. doi: 10.1111/j.1748-1716.1990.tb08953.x. [DOI] [PubMed] [Google Scholar]
- Tornell J, Billig H, HIllensjo T. Regulation of oocyte maturation by changes in ovarian levels of cyclic nucleotides. Human Reprod. 1991;6:411–422. doi: 10.1093/oxfordjournals.humrep.a137351. [DOI] [PubMed] [Google Scholar]
- Tosca L, Uzbekova S, Chabrolle C, Dupont J. Possible role of 5’AMP-activated protein kinase in the metformin-mediated arrest of bovine oocytes at the germinal vesicle stage during in vitro maturation. Biol Reprod. 2007;77:452–465. doi: 10.1095/biolreprod.107.060848. [DOI] [PubMed] [Google Scholar]
- Tsafriri A, Vale W, Hsueh AJ. Effects of transforming growth factors and inhibin-related proteins on rat preovulatory graafian follicles in vitro. Endocrinology. 1989;125:1857–1862. doi: 10.1210/endo-125-4-1857. [DOI] [PubMed] [Google Scholar]
- Tsafriri A, Chun SY, Zhang R, Hsueh AJW, Conti M. Oocyte maturation involves compartmentalization and opposing changes of cAMP levels in follicular somatic and germ cells: studies using selective phosphodiesterase inhibitors. Dev Biol. 1995;178:393–402. doi: 10.1006/dbio.1996.0226. [DOI] [PubMed] [Google Scholar]
- Trafriri A, Cao X, Ashkenazi H, Motola S, Popliker M, Pomerantz SH. Resumption of oocyte meiosis in mammals: on models, meiosis activating sterols, steroids and EGF-like factors. Mol Cell Endocrinol. 2005;234:37–45. doi: 10.1016/j.mce.2004.09.009. [DOI] [PubMed] [Google Scholar]
- Tsafriri A, Motola S. Are steroids dispensable for meiotic resumption in mammals? Trends Endocrinol Metab. 2007;18:321–327. doi: 10.1016/j.tem.2007.08.005. [DOI] [PubMed] [Google Scholar]
- Urner F, Herrmann WL, Baulieu EE, Schorderet-Slatkine S. Inhibition of denuded mouse oocyte meiotic maturation by forskolin, an activator of adenylate cyclase. Endocrinology. 1983;113:1170–1172. doi: 10.1210/endo-113-3-1170. [DOI] [PubMed] [Google Scholar]
- Vaccari S, Weeks JL, II, Hsieh M, Menniti FS, Conti M. Cyclic GMP signaling is involved in the luteinizing hormone-dependent meiotic maturation of mouse oocytes. Biol Reprod. 2009 doi: 10.1095/biolreprod.109.077768. DOI: 10.1095/biolreprod.109.077768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veitch GI, Gittens JEI, Shao Q, Laird DW, Kidder GM. Selective assembly of connexin37 into heterocellular gap junctions at the oocyte/granulosa cell interface. J Cell Sci. 2004;117:2699–2707. doi: 10.1242/jcs.01124. [DOI] [PubMed] [Google Scholar]
- Verlhac MH, Kubiak JZ, Weber M, Geraud G, Colledge WH, Evans MJ, Maro B. Mos is required for MAP kinase activation and is involved in microtubule organization during meiotic maturation in the mouse. Development. 1996;122:815–822. doi: 10.1242/dev.122.3.815. [DOI] [PubMed] [Google Scholar]
- Verlhac MH, Pennart HD, Maro B, Cobb MH, Clarke HJ. MAP kinase becomes stably activated at metaphase and is associated with microtubule-organizing centers during meiotic maturation of mouse oocytes. Dev Biol. 1993;158:330–340. doi: 10.1006/dbio.1993.1192. [DOI] [PubMed] [Google Scholar]
- Viollet B, Lantier L, Devin-Leclerc J, Hebrard S, Amouyal C, Mounier R, foretz M, Andreelli F. Targeting the AMPK pathway for the treatment of type 2 diabetes. Front Biosci. 2009;14:3380–3400. doi: 10.2741/3460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vivarelli E, Conti M, De Felici M, Siracusa G. Meiotic resumption and intracellular cAMP levels in mouse oocytes treated with compounds which act on cAMP metabolism. Cell Differ. 1983;12:271–276. doi: 10.1016/0045-6039(83)90023-4. [DOI] [PubMed] [Google Scholar]
- Vozzi C, Formenton A, Chanson A, Senn A, Sahil R, Shaw P, Nicod P, Germond M, Haefliger JA. Involvement of connexin 43 in meiotic maturation of bovine oocytes. Reproduction. 2001;122:619–628. [PubMed] [Google Scholar]
- Waldman SA, Murad F. Atrial natriuretic peptides: receptors and second messengers. Bioessays. 1989;10:16–19. doi: 10.1002/bies.950100105. [DOI] [PubMed] [Google Scholar]
- Wang S, Ning G, Chen X, Yang J, Ouyand H, Zhang H, Tai P, Muy X, Zhou B, Zhang M, Xia G. PDE5 modulates oocyte spontaneous maturaton via cGMP-cAMP but not cGMP-PKG signaling. Front Biosci. 2008;13:7087–7095. doi: 10.2741/3212. [DOI] [PubMed] [Google Scholar]
- Webb RJ, Marshall F, Swann K, Carroll J. Follicle-stimulating hormone induces a gap junction-dependent dynamic change in [cAMP] and protein kinase A in mammalian oocytes. Dev Biol. 2002a;246:441–454. doi: 10.1006/dbio.2002.0630. [DOI] [PubMed] [Google Scholar]
- Webb RJ, Bains H, Cruttwell C, Carroll J. Gap-junctional communication in mouse cumulusoocyte complexes: implications for the mechanism of meiotic maturation. Reproduction. 2002b;123:41–52. doi: 10.1530/rep.0.1230041. [DOI] [PubMed] [Google Scholar]
- Webb RJ, Tinworth L, Thomas GMH, Zaccolo M, Carroll J. Developmentally acquired PKA localization in mouse oocytes and embryos. Dev Biol. 2008;317:36–45. doi: 10.1016/j.ydbio.2008.01.045. [DOI] [PubMed] [Google Scholar]
- Wert S, Larsen WJ. Preendocytotic alterations in cumulus cell gap junctions precede meiotic resumption in the rat cumulus-oocyte complex. Tissue Cell. 1990;22:827–851. doi: 10.1016/0040-8166(90)90047-d. [DOI] [PubMed] [Google Scholar]
- Williams T, Brenman JE. LKB1 and AMPK in cell polarity and division. Trends Cell Biol. 2008;18:193–198. doi: 10.1016/j.tcb.2008.01.008. [DOI] [PubMed] [Google Scholar]
- Wong W, Scott JD. AKAP signaling complexes: focal points in space and time. Nat Rev Mol Cell Biol. 2004;5:959–970. doi: 10.1038/nrm1527. [DOI] [PubMed] [Google Scholar]
- Yamamuro T, Kano K, Naito K. Functions of FZR1 and CDC20, activators of the anaphase-promoting complex, during meiotic maturation of swine oocytes. Biol Reprod. 79:1202–1209. doi: 10.1095/biolreprod.108.070326. [DOI] [PubMed] [Google Scholar]
- Zhang M, Tao Y, Xia G, Xie H, Hong H, Wang F, Lei L. Atrial natriuretic peptide negatively regulates follicle-stimulating hormone-induced porcine oocyte maturation and cumulus expansion via cGMP-dependent protein kinase pathway. Theriogenology. 2005;64:902–916. doi: 10.1016/j.theriogenology.2004.12.012. [DOI] [PubMed] [Google Scholar]
- Zhang M, Xia G, Zhou B, Wang C. Gonadotropin-controlled mammal oocyte meiotic resumption. Front Biosci. 2007;12:282–296. doi: 10.2741/2064. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Zhang Z, Xu X-Y, Li X-S, Yu M, Yu A-M, Zong Z-H, Yu B-Z. Protein kinase A modulates Cdc25B activity during meiotic resumption of mouse oocytes. Dev Dyn. 2008;237:3777–3786. doi: 10.1002/dvdy.21799. [DOI] [PubMed] [Google Scholar]
- Zhang M, Ouyang H, Xia G. The signal pathway of gonadotrphins-induced mammalian oocyte meiotic resumption. Mol Hum Reprod. 2009a;15:399–409. doi: 10.1093/molehr/gap031. [DOI] [PubMed] [Google Scholar]
- Zhang BB, Zhou G, Li C. AMPK: an emerging drug target for diabetes and the metabolic syndrome. Cell Metab. 2009b;9:407–416. doi: 10.1016/j.cmet.2009.03.012. [DOI] [PubMed] [Google Scholar]
- Zhou G, Myers R, Li Y, Chen Y, Shen X, Fenyk-Melody J, Wu M, Ventre J, Doebbeer T, Fujii N, Musi N, Hirshman MF, Goodyear LJ, Moller DE. Role of AMP-activated protein kinase in mechanism of metformin action. J Clin Invest. 2001;108:1167–1174. doi: 10.1172/JCI13505. [DOI] [PMC free article] [PubMed] [Google Scholar]