Abstract
cAMP plays a critical role in the control of oocyte maturation, as a high level of cAMP maintains oocyte arrest at the first meiotic prophase. Yet this study shows that pulsing meiotically arrested denuded oocytes (DO) with cAMP induces oocyte maturation through the activation of AMP-activated protein kinase (PRKA). Short term (3 h) pulsing of meiotically arrested oocytes with forskolin, an adenyl cyclase (AC) activator, increased oocyte cAMP, led to elevated AMP and induced oocyte meiotic resumption compared to oocytes continuously cultured in the control medium with or without forskolin. Western analysis showed that GV-stage oocytes after forskolin pulsing contained increased levels of phospho-acetyl CoA carboxylase (pACACA), a primary substrate of PRKA. Pulsing oocytes with the PDE-sensitive cAMP analog, 8-Bromo-cAMP (8-Br-cAMP), also increased pACACA and pPRKA levels in GV-stage oocytes and induced oocyte meiotic resumption. Moreover, the PRKA inhibitors, compound C and araA, prevented 8-Br-cAMP pulsing-induced maturation. The lack of effect on meiotic induction and PRKA activation when oocytes were pulsed with the PDE-resistant activators of cAMP-dependent protein kinase, Sp-cAMP-AM and Sp-5,6-DCI-cBIMPS, suggests that cAMP degradation is required for pulsing-induced maturation. Pulsing oocytes with the Epac (exchange protein directly activated by cAMP)-specific activator, 8-CPT-2′-O-Me-cAMP, had no stimulatory effect on oocyte maturation, suggesting Epac is not involved in the pulsing-induced maturation. Taken together, these data support the idea that a transient increase in oocyte cAMP can induce meiotic resumption via activation of PRKA.
INTRODUCTION
Fully-grown mammalian oocytes within healthy preovulatory follicles are arrested in the first meiotic prophase until the gonadotropin surge in vivo. However, the means by which meiotic resumption is regulated remains unclear. cAMP within the oocyte has been shown to play a critical role in maintaining oocyte meiotic arrest. High levels of cAMP prevent oocyte spontaneous maturation in vitro, while a decrease in oocyte cAMP is associated with the resumption of meiosis [Dekel et al, 1984; Schultz et al, 1983]. Currently, there are two models for how inhibitory levels of oocyte cAMP are maintained. The first is that cAMP diffuses from cumulus cells to the oocyte through the gap junctions that couple the two cell types [Dekel et al, 1981]. It follows that LH stimulation disrupts the interaction between oocyte and the follicle cells [Dekel et al, 1988], leading to a decrease in oocyte cAMP and subsequent re-entry into the cell cycle [Edry et al, 2006]. Alternatively, studies have shown that the oocyte can generate cAMP by the constitutive activity of the heterotrimeric G protein Gs [Mehlmann et al, 2002, Kalinowski et al, 2004; Freudzon et al, 2005] and, subsequently, type 3 adenyl cyclase (AC3) [Horner et al, 2003]. Inhibition of Gs by injection of follicle-enclosed oocytes with a Gs inhibitory antibody or a dominant-negative form causes meiotic resumption in the intact follicle [Mehlmann et al, 2002]. A later study showed that it is GPR3, an orphan member of the G protein-coupled receptor family that constitutively activates Gs protein and maintains oocyte cAMP levels [Mehlmann et al, 2004]. In rat oocytes, AC3 is the major cyclase isoform that produces cAMP, and mouse oocytes deficient in AC3 show defective meiotic arrest in vivo and accelerated spontaneous maturation in vitro [Horner et al, 2003], indicating the involvement of AC3 in the control of oocyte meiotic arrest. Therefore, based on these observations, to induce oocyte maturation, a positive signal should either reduce oocyte cAMP or overcome its inhibition.
Follicle-stimulating hormone (FSH) stimulates the maturation of cumulus cell-enclosed oocytes (CEO) in vitro [Downs et al, 1988]. FSH also produces a transient arrest of spontaneous oocyte maturation. Following FSH stimulation, the cAMP concentration transiently rises in oocyte-cumulus cell complexes [Schultz et al, 1983; Salustri et al, 1985] and a parallel increase is observed in oocytes [Salustri et al, 1985; Webb et al, 2002], suggesting that the increase in oocyte cAMP is responsible for the delay in meiotic resumption. It must be noted that FSH-treated CEO resume meiosis before oocyte cAMP decreases to basal levels [Salustri et al, 1985], suggesting the presence of an FSH-induced meiotic signal that overcomes the cAMP-regulated meiotic arrest.
Cyclic nucleotide phosphodiesterase (PDE) is the enzyme that degrades cAMP and is essential for driving oocyte meiotic resumption by lowering inhibitory cAMP levels. In rodents, oocyte cAMP hydrolysis is primarily carried out by PDE3A [Richard et al, 2001; Shitsukawa et al, 2001]. PDE3A activity increases in rodent oocytes before meiotic resumption in both spontaneous and gonadotropin-stimulated maturation [Richard et al, 2001], and inhibition of the oocyte PDE3 activity completely blocks oocyte maturation in vitro and in vivo [Wiersma et al, 1998; Conti et al, 2002]. PDE3A null oocytes lack cAMP-specific PDE activity, contain increased cAMP levels, and fail to undergo spontaneous maturation in vitro [Masciarelli et al 2004]. Recently, in mouse oocytes, protein kinase B (PKB/Akt) has been shown to regulate PDE3A activity by phosphorylation on the Ser290-292 sites, thus playing a role in regulation of oocyte meiotic induction [Han et al, 2006].
AMP, a product of ATP and cAMP degradation, is an important factor regulating AMP-activated protein kinase (PRKA) activity. PRKA is a cellular energy sensor composed of a catalytic α subunit, and regulatory β and γ subunits [Hardie and Hawley, 2001]. AMP allosterically binds to the β subunit and facilitates the activation of the enzyme [Davies et al, 1995; Hawley et al, 2002; Sanders et al, 2007]. It has been shown that PRKA activation in mouse oocytes provides a positive stimulus for meiotic resumption in vitro in response to a variety of nonphysiological and physiological signals [Chen et al, 2006; Chen and Downs, 2008]. Here, it is proposed that a transient increase of cAMP concentration in oocytes could provide a source of AMP, which activates PRKA, inducing oocytes to overcome meiotic inhibition and re-enter meiosis.
To test this hypothesis, denuded oocytes were pulsed with cAMP and then assessed for meiotic maturation and PRKA activation. Our results indicate that an increase in cAMP within the oocyte can induce meiotic resumption if the culture conditions allow subsequent cAMP degradation to AMP and activation of PRKA.
MATERIALS AND METHODS
Oocyte isolation and culture conditions
Animals were raised in the research colony of the principal investigator (SMD). All experiments were carried out with prior approval of the Marquette University Institutional Animal Care and Use Committee.
C57BL/6JxSJL/J Fl mice, 19-23 days old, were used for all experiments. Mice were primed with 5 IU equine choronic gonadotropin and killed 2 d later by cervical dislocation. Ovaries were removed and placed in the culture medium, and cumulus cell-enclosed oocytes (CEO) were obtained by puncturing large antral follicles with sterile needles. Denuded oocytes (DO) were prepared by repeated pipetting with a Pasteur pipette or by passage through mouth-operated small bore pipettes. Tubes were gassed with a humidified mixture of 5% CO2, 5% O2 and 90% N2 and placed in a water bath at 37°C for the duration of culture. For maturation kinetics experiments, oocytes were placed in 200-μl drops of medium under oil and cultured in a similarly gassed water-jacketed incubator.
The culture medium used was Eagle’s minimum essential medium with Earle’s salts (GIBCO), supplemented with 0.23 mM pyruvate, penicillin, streptomycin sulfate and 3 mg/ml crystallized lyophilized bovine serum albumin (ICN ImmunoBiologicals, Lisle, IL) and buffered with 26 mM bicarbonate.
Western Analysis
DO and oocyte-cumulus cell complexes were washed in phosphate-buffered saline (PBS, pH 7.4)/PVP (3 mg/ml) plus protease inhibitors (Protease Inhibitors Cocktail Tablets, 1 mM Na orthovanadate, 2 μg/ml pepstatin, 50 mM beta-Glycerophospate) and then added to an equal volume of 2x Laemmli’s buffer containing 20% beta-mercaptoethanol (BME). After heating at 95°C for 5 min, samples were stored frozen at −80°C until used for Western blotting. For Western analysis, proteins were electrophoresed on a 3-8% tris-acetate mini gel (Invitrogen) for 1 h at 150 V and then transferred to nitrocellulose at 100 V for 1 h. To obtain the sharpest bands for active PRKA (pPRKA) blots, samples were electrophoresed on a 4-12% bis-tris SDS mini gel (Invitrogen) for 50 min at 100 V and then in a semi-dry system transferred to nitrocellulose at 200 mA for 2.5 h at 4°C. Blots were blocked with 5% nonfat milk for 2 h at room temperature and then incubated with primary antibodies (anti-pPRKA or anti-pACACA, 1:250) overnight at 4°C, washed three times in Tris-buffered saline (TBS pH 7.4) and incubated with HRP-conjugated IgG (1:2000, in 5% nonfat milk) for 1 h at room temperature. After washing in TBS, detection was performed with Supersignal Western Dura Chemiluminescent Substrate (Pierce; Rockford, IL). Blots were stripped (7 μl/ml BME, 2% SDS, room temperature 30 min) and reprobed with ACACA antiserum (1:2000) as a loading control. Bands were quantified by UVP Biolmaging Systems (UVP, Inc.; Upland, CA).
Anti-pACACA antiserum was purchased from Upstate Biotechnology Inc. (Lake Placid, NY); anti-ACACA antibodies were generated in Dr. Hardie’s laboratory; anti- pPRKA antiserum was obtained from Cell Signaling Technology (Beverly, MA).
AMP/ATP Level Measurement
Denuded oocytes were cultured for varying periods in organ culture dishes containing 1 ml culture medium. After the designated culture times, individual oocytes were assayed for AMP as previously described [Chang et al, 2004].
cAMP Assay
The level of cAMP in denuded oocytes was determined using a Direct Cyclic AMP Enzyme Immunoassay Kit (Assay Designs, Ann Arbor, MI). Samples were washed in 3 mg/ml MEM/BSA containing 0.2 mM IBMX immediately after culture. Denuded oocytes (about 150 per group) in a volume of 10 μl were transferred to 90 μl of 0.1 N HCI, and stored in −80°C until assay. During the assay, all samples were acetylated according to the procedure of the kit. Plates were read by EL800 Universal Microplate Reader (BIO-TEK, VT), and data was reported by the software KCjunior (BJO-TEK, VT). The cAMP concentrations were determined by AssayZap V3 (Biosoft, Cambridge, UK).
Immunofluorescence
Oocytes were fixed with 4% formaldehyde for 1 h at 4°C and then permeabilized with 0.1% triton in blocking buffer (0.5% saponin in PBS, pH 7.4, plus 10% sheep serum) for 30 min. Oocytes were then washed free of triton and continuously blocked for another 90 min at room temperature. Oocytes were incubated overnight at 40C with primary antibody (rabbit anti-phospho-AMPKalpha-PT172, 1:100; Cell Signaling, Beverly, MA) and then washed in blocking buffer and incubated with FITC-conjugated sheep anti-rabbit antibody (1:1000; Cell Signaling) at room temperature for 1 h. Oocytes were then washed again and mounted on slides with Vectashield containing DAPI to stain chromatin. Images were viewed on a laser scanning confocal microscope (Carl Zeiss Co.) with a 40x objective. Digitally recorded images were exported by LSM Examiner (Carl Zeiss Co,.).
Statistical Analysis
Oocyte maturation experiments were repeated at least 3 times with at least 25 oocytes per group per experiment. Data are reported as mean percentage GVB ± SEM. Maturation frequencies were analyzed statistically by ANOVA followed by Duncan’s multiple range test, or paired comparisons were analyzed by Student’s t-test. For all statistical analyses, a P value less than 0.05 was considered significant.
RESULTS
Pulsing oocytes with endogenous cAMP induces oocyte maturation and AMPK activation
Apart from one experiment where pulsing time was varied, the experimental paradigm for this study was to pretreat, or pulse, DO for 3 h with either cAMP analogs or cAMP-elevating agents, wash out the pulsing agent, and then continue culture in meiotic-arresting medium before assessing the parameter in question. Initial experiments utilized the cAMP-elevating agent, forskolin, to test the effects of endogenous cAMP. Forskolin activates adenylate cyclase by directly interacting with the catalytic subunit of the enzyme, and has been shown to raise cAMP levels in mouse oocytes [Schultz et al, 1983; Urner et al, 1983]. Denuded oocytes were maintained in meiotic arrest with 1mM N6-monobutyryladenosine cAMP (mbcAMP), and exposed to increasing concentrations of forskolin. After washing out the forskolin, culture in mbcAMP was continued for 17-18 h before maturation assessment. Oocytes continuously cultured in mbcAMP-containing medium without forskolin for 20-21 h served as controls. Oocyte maturation was dose-dependently induced by forskolin pulsing, compared to oocytes cultured in mbcAMP alone (an increase from 17% to 34% GVB, Figure 1A), while continuous exposure to forskolin had no stimulatory effect.
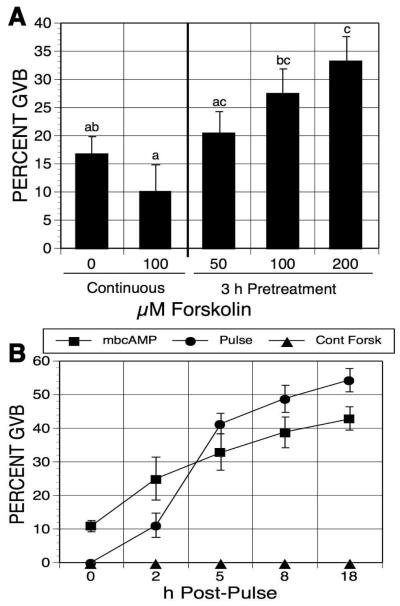
Figure 1.
Effects of forskolin-pulsing on oocyte meiotic resumption. (A) Forskolin dose response. Denuded oocytes (DO), maintained in meiotic arrest with 1mM mbcAMP, were pulsed with increasing concentrations of forskolin for 3 h, washed free of forskolin, and cultures were continued in mbcAMP alone for 17-18 h before assessment of GVB. Other oocytes were continuously cultured with or without 100 μM forskolin in the meiotic-arresting medium for 20-21 h. Groups with no common letter are significantly different. (B) Maturation kinetics. Oocytes were treated as in (A) for 3 h; then, post-pulse cultures were carried out in medium drops under oil, with meiotic maturation assessed after the designated time periods.
We next tested the time course of meiotic maturation after forskolin pulsing under these conditions. DO were cultured 3 h in 1 ml medium in culture tubes containing mbcAMP alone or mbcAMP plus 100 μM forskolin. The mbcAMP-treated oocytes were then transferred to the same medium, while oocytes pulsed in mbcAMP plus forskolin were split into two groups: one was transferred to the same medium, while the other was washed free of forskolin and transferred to mbcAMP-containing medium. All three secondary cultures were carried out in 200 μl medium under oil so periodic maturation assessment could be made after 2, 5, 8 and 18 h of culture. When cultured in mbAMP alone, maturation gradually increased from 11% GVB to 43% GVB (Fig. 1B). Continuous exposure to forskolin completely suppressed GVB. Oocytes pulsed with forskolin had a small increase (10%) in maturation between 0 and 2 h, but a 30% increase was manifest between 2 and 5 h, with more modest increases resuming thereafter. The frequency of maturation in pulsed oocytes surpassed that of the mbcAMP controls during the 2-5 h post-pulse period, but the difference was never greater than 12%.
3-isobutyl-1-methylxanthine (IBMX), a general PDE inhibitor, has been used to promote cAMP accumulation by preventing its degradation. Oocytes treated with both forskolin and IBMX would be expected to contain higher levels of cAMP than forskolin alone; consequently, pulsing oocytes with the two compounds together might be more effective in meiotic induction than pulsing with either agent alone. Thus, mbcAMP-arrested DO were pulsed with 100 μM forskolin plus 100 μM IBMX, washed, and returned to medium containing only mbcAMP for 17-18 h. Under these conditions, maturation was again stimulated, but to no greater extent than pulsing with forskolin alone (an increase of 17% from 37% to 54% GVB; Fig. 2A). Continuous exposure to forskolin plus IBMX reduced the maturation percentage by 29%.
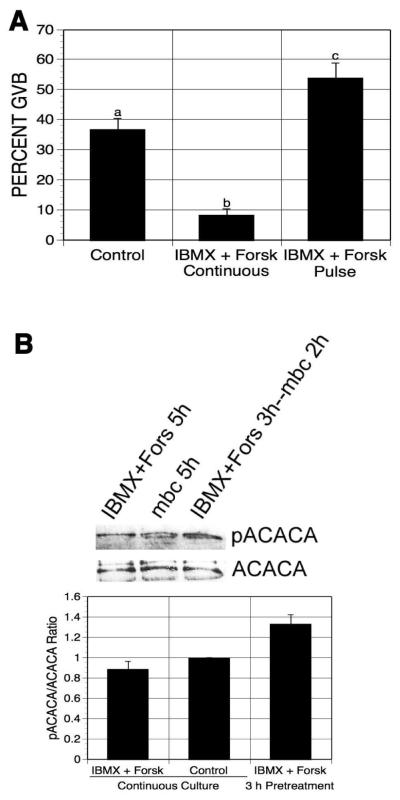
Figure 2.
Effects of pulsing oocytes with forskolin and IBMX on oocyte maturation and PRKA activation. (A) DO were cultured for 21-22 h in 1 mM mbcAMP or mbcAMP plus forskolin (100 μM) and IBMX (100 μM); a third group was pulsed 3 h with forskolin plus IBMX, washed free of these agents and then cultured a further 17-18 h in mbcAMP alone before assessing meiotic status. Groups with no common letter are significantly different. (B) DO were exposed to the same treatments as in (A) except that GV-stage oocytes were collected after 5 h and extracts were processed for western analysis (300 oocytes per lane), using antibody to phospho-ACACA. The mean pACACA/ACACA ratio of four blots is shown, normalized to the mbcAMP alone group.
Active AMPK (PRKA) specifically phosphorylates Ser79 of acetyl-CoA carboxylase (ACACA); thus, western analysis using anti-phospho-ACACA antibody was performed to indirectly detect PRKA activity in the pulsed oocytes.. Cellular extracts were obtained from GV-stage oocytes after pulsing with IBMX plus forskolin and 2 h additional culture in mbcAMP-containing medium, or after 5 h continuous treatment in mbcAMP alone (control) or mbcAMP plus IBMX and forskolin, and were then processed for western analysis. The intensity of pACACA bands was quantified and normalized to ACACA levels. pACACA band intensity was increased by 33% in oocytes after pulsing with IBMX plus forskolin compared to oocytes continuously cultured in mbcAMP-containing medium (Fig. 2B), indicating that PRKA was activated in the pulsed oocytes before meiotic resumption. Continuous exposure to forskolin plus IBMX had no effect on band intensity.
To determine if the meiosis-inducing action of forskolin would occur under different inhibitory conditions, the dose response experiment was repeated in 4mM hypoxanthine (HX)-supplemented medium. Although meiotic arrest was maintained to a lesser extent (58% GVB), maturation was again stimulated by forskolin pulsing, resulting in a 26% increase to 84% GVB; Fig. 2A). On the other hand, continuous exposure to HX plus forskolin resulted in a significant reduction in maturation (14% GVB).
The time course of meiotic induction by forskolin pulsing in HX-arrested oocytes was next tested by means identical to those used earlier with mbcAMP-arrested oocytes. When cultured in HX alone, a low level of maturation was maintained throughout the secondary culture period (an increase from 9% at 0 h to 30% GVB after 18 h). Inhibition in oocytes exposed continuously to HX plus forskolin was more pronounced, with a small increase only after 8 h (from 0% to 13% GVB; Fig. 3B). Oocytes pulsed with HX plus forskolin and washed free of the forskolin exhibited an increase in maturation compared to oocytes cultured only in HX, and this was first manifested between 2 and 5 h of culture post-pulse with a 26% increase by 18 h (Fig. 3B).
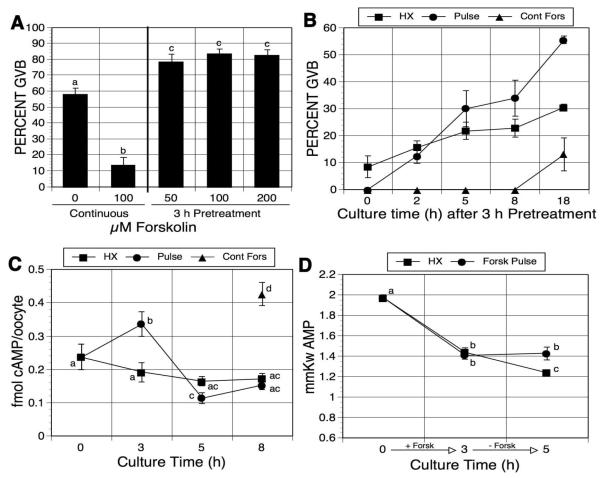
Figure 3.
Effects of forskolin pulsing on oocyte maturation and cAMP and AMP concentrations in hypoxanthine (HX)-arrested oocytes. (A) Forskolin dose response. Hypoxanthine-arrested DO were pulsed 3 h with increasing concentrations of forskolin, washed free of forskolin and returned to HX-containing medium; alternatively, they were cultured continuously in HX alone or HX plus 100 μM forskolin. GVB was assessed after a total of 21-22 h. (B) Maturation kinetics. After 3 h pretreatment with HX plus 100 μM forskolin, DO were washed and cultured in HX alone. Meiotic resumption was determined at the indicated culture times after pulsing. Maturation kinetics were also determined for oocytes continuously cultured in HX alone or HX plus 100 μM forskolin (Fors). Time 0 h represents 3 h of culture. (C) cAMP assay. Fresh DO were collected (time 0) or DO were cultured in 4 mM HX-containing medium in the presence (Fors pulse) or absence (HX) of 100 μM forskolin. Oocytes in the HX group were collected after 3, 5 and 8 h of culture. Oocytes in the Fors pulse group were washed free of forskolin after 3 h and returned to HX-containing medium. Oocytes from this group were collected immediately after the forskolin pulse or 2 or 5 h after washing out the forskolin and subsequent culture in HX (3, 5 and 8 total h of culture, respectively). An additional group of oocytes was continuously cultured in HX plus forskolin for the entire 8-h period (Cont Fors). Only GV-stage oocytes were assayed for cAMP. Results shown here represent the mean ± SEM of 7 experiments. Groups with no common letter are significantly different. (D) AMP assay. Fresh oocytes were collected or DO were cultured in medium containing HX in the presence or absence of forskolin. Oocytes treated with HX alone were collected after 3 and 5 h of culture. Oocytes treated with HX plus forskolin were collected 3 h after forskolin pulsing and 2 h after pulsed oocytes were washed free of forskolin and returned to HX medium (5 h total culture time). Groups with no common letter are significantly different.
To confirm that forskolin-pulsed oocytes produced a transient increase in cAMP, cyclic nucleotide concentrations were measured by direct cAMP enzyme immunoassay. After pulsing with 100 μM forskolin in 4 mM HX, oocytes were collected or washed free of forskolin and cultured in HX-containing medium for an additional 2 or 5 h before assay. A parallel group of oocytes was continuously cultured in the HX-containing medium for the same treatment time, and an additional group was maintained in the presence of HX plus forskolin for 8 h. HX, a weak PDE inhibitor [Downs et al, 1989], maintained intra-oocyte cAMP concentration at similar levels (0.17-0.19 fmol/oocyte) during the culture (Fig. 3C). After forskolin treatment for 3 h, oocyte cAMP concentration was elevated from 0.24 fmol/oocyte to 0.33 fmol/oocyte and the concentration was even higher after 8 h of forskolin treatment (0.43 fmol/oocyte). When oocytes were cultured 2 h in the control medium after forskolin pulsing, the cAMP concentration was dramatically decreased to 0.11 fmol/oocyte, with no further decrease after 3 additional hours of culture in HX-containing medium.
To examine the effects of forskolin pulsing on the generation of AMP, individual oocytes were collected and assayed for AMP. Freshly isolated oocytes contained 1.70 mmol AMP per kg wet wt of oocyte (Fig. 3D). After 3 h culture in medium containing HX or HX plus forskolin, oocyte AMP concentration was decreased to 1.44 or 1.41 mmol per kg, respectively. Oocytes cultured in HX for 5 h exhibited a further decline in AMP concentration to 1.24 mmole per kg; however, the 3-h pulse with forskolin prevented this further decline with AMP maintained at 1.43 mmole per kg. The results are consistent with production of AMP as cAMP levels decline following forskolin pulsing.
Pulsing oocytes with 8-Br-cAMP induces oocyte maturation and AMPK activation
8-Br-cAMP, a PDE sensitive cAMP analog, can be metabolized to 8-Br-AMP. Oocytes were pulsed with increasing concentrations of 8-Br-cAMP in mbcAMP-containing medium, washed, and cultured in mbcAMP alone for an additional 17-18 h before assessing GVB. Oocytes continuously cultured in the meiosis-arresting medium without 8-Br-cAMP for 20-21 h served as controls. As shown in Fig. 4A, the maturation percentage was induced in 6 mM 8-Br-cAMP-pulsed oocytes (a 22% increase), compared to the control groups. Continuous culture in 8-Br-cAMP had no effect on the maturation percentage.
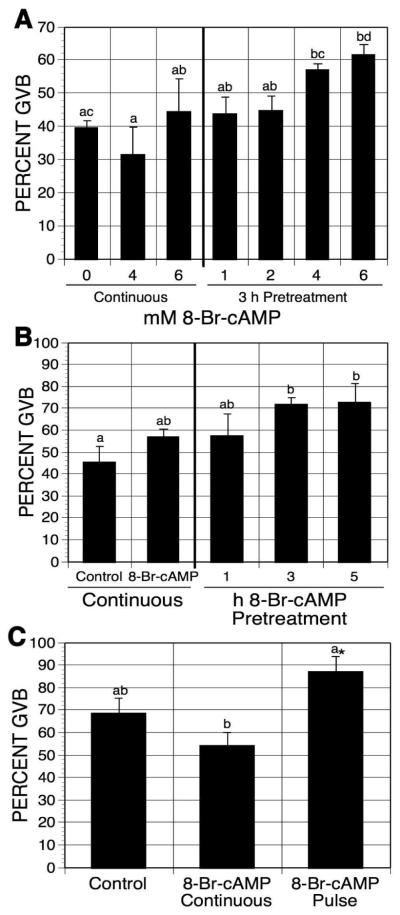
Figure 4.
Effects of 8-Br-cAMP pulsing on oocyte maturation. (A) Dose response effect of 8-Br-cAMP treatment. DO were pretreated for 3 h in 1 mM mbcAMP with increasing concentrations of 8-Br-cAMP before they were washed free of 8-Br-cAMP and cultured 17-18 h in mbcAMP alone. Separate sets of oocytes were cultured for 21-22 h in either mbcAMP alone or mbcAMP plus 4 or 6 mM 8-Br-cAMP. Groups with no common letter are significantly different. (B) Time-dependent effect of 8-Br-cAMP pretreatment. DO were pulsed with 6 mM 8-Br-cAMP for the indicated times before culture in 1 mM mbcAMP. The total culture time (including the pulsing time) was 20-21 h. Oocytes continuously cultured in mbcAMP or mbcAMP plus 6 mM 8-Br-cAMP for 20-21 h served as controls. Groups with no common letter are significantly different. (C) Effect of 8-Br-cAMP pulsing on HX-arrested oocytes. DO cultured in 4 mM HX were pulsed 3 h with 6 mM 8-Br-cAMP, washed free of the cyclic nucleotide and cultured 17-18 additional h in HX alone. Other oocytes were continuously cultured 21-22 h in HX alone or HX plus 8-Br-cAMP. Note that although the pulse group was not significantly different from the HX continuous group by ANOVA, it was by Student’s t-test, as indicated by the asterisk.
To establish how pulsing time affects meiotic induction, mbcAMP-arrested oocytes were pretreated with 6mM 8-Br-cAMP for 1, 3, or 5 h before subsequent culture in mbcAMP-arresting medium. The total culture time was 20-21 h. Figure 4B shows that a pulse time of 3 or 5 h, but not 1 h, significantly induced meiotic maturation compared to oocytes not exposed to 8-Br-cAMP (an increase of 26%).
When hypoxanthine-arrested oocytes were similarly pulsed with 6 mM 8-Br-cAMP, maturation was stimulated by 18.5% (Fig. 4B). Continuous exposure to 8-Br-cAMP was again without effect (Fig. 4C).
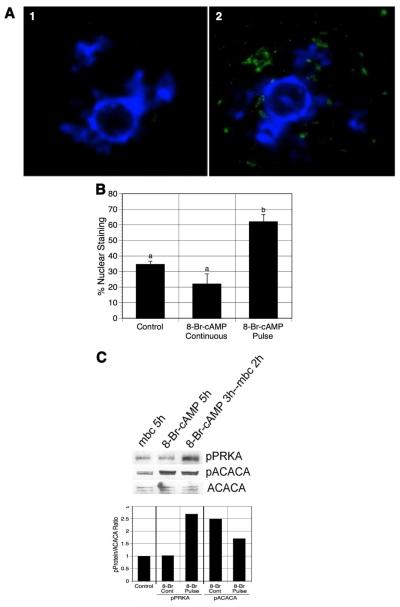
To examine PRKA activation in pulsed oocytes, mbcAMP-arrested DO were exposed to 8-Br-cAMP, washed, and then returned to mbcAMP-containing medium for 2 h. These oocytes were fixed and stained with antibody to phospho-PRKA for detection by immunofluorescence. Oocytes cultured 5 h in mbcAMP alone or mbcAMP plus 8-Br-cAMP were also processed in this manner. Fig. 5A shows the germinal vesicle area from two oocytes from the pulsed group, each stained with DAPI (blue) for DNA and with FITC (green) for active PRKA. In the oocyte to the left, PRKA has not been stimulated; however, punctate staining for PRKA is apparent in the oocyte to the right, indicating active kinase.
Figure 5.
Effect of 8-Br-cAMP pretreatment on PRKA activation. (A) Fluorescent nuclear staining for phospho-PRKA. mbcAMP-arrested DO were pretreated with 6 mM 8-Br-cAMP, washed free of 8-Br-cAMP, and then cultured 2 h in mbcAMP alone. GV-stage oocytes were processed for immunofluorescent staining using anti-phospho-PRKA antibody and FITC-labeled secondary antibody (green). DNA was stained with DAPI (blue). Panel 1 shows an oocyte with unstimulated PRKA, while the oocyte in panel 2 has active PRKA, indicated by punctate staining within the GV. Oocytes not exposed to primary antibody had only a faint diffuse staining throughout the oocyte and never displayed nuclear staining (not shown). (B) Quantification of germinal vesicle staining for phospho-PRKA. The frequency of nuclear (germinal vesicle) staining was compared in oocytes continuously cultured in mbcAMP or mbcAMP plus 8-br-cAMP with oocytes pulsed 3 h in mbcAMP plus 8-Br-cAMP followed by 2 h in mbcAMP alone. Groups with no common letter are significantly different. (C) Western analysis of 8-Br-cAMP-pulsed oocytes. DO treated identically to those in (B) above were processed for western analysis for pPRKA and pACACA (500 oocytes per lane). The mean pPRKA/ACACA and pACACA/ACACA ratios from 2 blots are shown, normalized to the mbcAMP alone group. Note that pPRKA increases only after an 8-Br-cAMP pulse, while pACACA levels increase after either a pulse or continuous exposure to 8-Br-cAMP. shown are the mean values for two blots.
The frequency of nuclear staining for the three groups was next quantified, with these data presented in Fig. 5B. A mean of 34% of oocytes cultured 5h in mbcAMP contained nuclei that stained positively for active PRKA, while the addition of 8-Br-cAMP had no significant effect (22%). Pulsing with 8-Br-cAMP, however, produced a significant increase in oocytes with positive nuclear staining (62%).
Western analysis was performed to detect pPRKA or pACACA in 8-Br-cAMP-pulsed GV-stage oocytes. GV-stage oocytes were pulsed with mbcAMP plus 6 mM 8-Br-cAMP, washed, and cultured in mbcAMP alone for 2 h before they were collected for analysis. GV-stage oocytes were also collected after 5 h culture in mbcAMP-containing medium with or without 8-Br-cAMP. The blots were probed with either anti-pPRKA or anti-pACACA antibody. The intensity of the pPRKA band in 8-Br-cAMP pulsed oocytes was dramatically enhanced compared to the other two groups (Fig. 5C), indicating PRKA activation in the pulsed oocytes before meiotic maturation, and this is further indicated by quantitation of band intensity. Accordingly, the pACACA levels in the pulsed oocytes were also increased compared to the oocytes cultured in mbcAMP-containing medium. However, surprisingly, oocytes continuously cultured with 8-Br-cAMP contained high levels of pACACA but relatively low levels of pPRKA. The inconsistency of the two phosphorylated protein levels suggests that other mechanisms, besides active PRKA, may act to regulate ACACA phosphorylation on the Ser79 site.
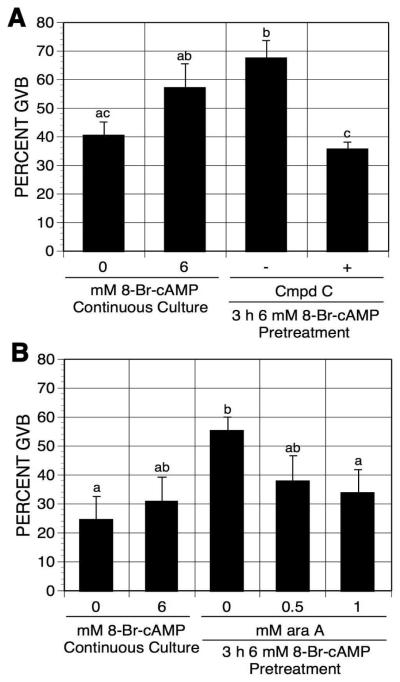
To test whether PRKA activity is required for pulsing-induced maturation, pulsed oocytes were exposed to PRKA inhibitors. Compound C, a small molecular weight PRKA inhibitor [Zhou et al, 2001], and araA, a precursor of araATP (a competitive inhibitor of PRKA, [Henin et al, 1996]), have been shown to block meiotic maturation via inhibition of PRKA activation [Chen et al, 2006]. After pretreatment with 6 mM 8-Br-cAMP, oocytes were washed and continuously cultured in the presence or absence of compound C or araA in mbcAMP-arresting medium for an additional 17-18 h. The two PRKA inhibitors, compound C and araA, both eliminated 8-Br-cAMP pulsing-induced maturation (Fig. 6A,B), thereby implicating PRKA activation in the cAMP-pulsing-induced meiotic resumption.
Figure 6.
Effects of compound C (A) and araA (B) on 8-Br-cAMP pulsing-induced maturation. After 3 h pulsing with mbcAMP plus 6 mM 8-Br-cAMP, DO were washed free of 8-Br-cAMP and cultured in mbcAMP alone or mbcAMP plus either compound C (A) or araA (B) for 17-18 h. Oocytes continuously cultured in mbcAMP or mbcAMP plus 6 mM 8-Br-cAMP served as controls. Groups with no common letter are significantly different.
Pulsing with PDE-resistant cAMP analogs does not induce meiotic resumption
To rule out the possibility that transiently increasing cAMP activates cAMP-dependent protein kinase (PRKACA) that triggers meiotic resumption by means unrelated to PRKA activation, oocytes were pulsed with PDE-resistant cAMP analogs in 1 mM mbcAMP-containing medium. The cAMP analogs, cAMP acetoxymethyl ester, Sp-isomer (Sp-cAMP-AM) and 5,6-Dichloro-1-beta-D-ribofuranosylbenzimidazole-cAMP, Sp-isomer (Sp-5,6-DCI-cBIMPS), are activators of PRKACA, and both are resistant to PDE degradation [Dostman et al, 1990; Sandberg et al, 1991]. DO were pretreated with increasing concentrations of Sp-cAMP-AM or Sp-5,6-DCI-cBIMPS in mbcAMP-arresting medium, washed free of the compound and continuously cultured in the control medium for 17-18 h before determining maturation percentage. Neither Sp-cAMP-AM nor Sp-5,6-DCI-cBIMPS pretreatment induced oocyte maturation, compared to the continuously cultured groups (Fig. 7A, B). Both of these agents were used at effective doses, since continuous exposure to either augmented the mbcAMP-induced meiotic arrest by >15%.
Figure 7.
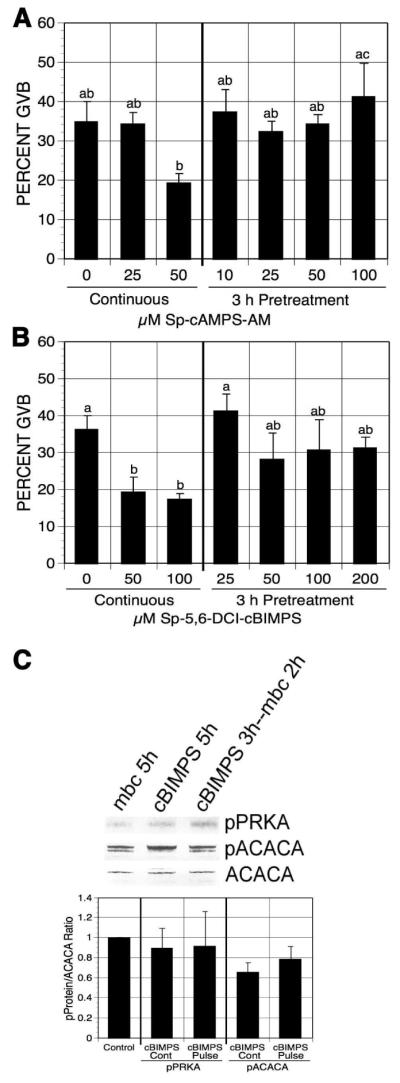
Effects of PDE-resistant cAMP analog pulsing on oocyte maturation. mbcAMP-arrested DO were pretreated 3 h with increasing concentrations of Sp-cAMP-AM (A) or SP-5,6-DCI-cBIMPS (B) and then washed and cultured in mbcAMP alone for 17-18 h before assessment of GVB. Oocytes continuously cultured in mbcAMP or mbcAMP plus Sp-cAMP-AM (A) or SP-5,6-DCI-cBIMPS (B) for 20-21 h served as controls. Groups with no common letter are significantly different. (C) Western analysis of pPRKA and pACACA in SP-5,6-DCI-cBIMPS-pulsed GV-stage oocytes. After 3 h pretreatment in 1 mM mbcAMP plus 6 mM SP-5,6-DCI-cBIMPS, DO were washed and cultured 2 h in mbcAMP. DO cultured in mbcAMP or mbcAMP plus 200 μM SP-5,6-DCI-cBIMPS for 5 h served as controls. The mean pPRKA/ACACA and pACACA/ACACA ratios from 4 blots are shown, normalized to the mbcAMP alone group.
Western analysis was carried out to detect pPRKA and pACACA in Sp-5,6-DCI-cBIMPS pulsed GV-stage oocytes. Little phosphorylation on the Thr172 site of PRKA or the Ser79 site of ACACA was observed after 3 h of Sp-5,6-DCI-cBIMPS pulsing plus 2 h culture in the control medium (Fig. 7C), indicating negligible activation of PRKA in oocytes after PDE-resistant cAMP analog pulsing.
Pulsing oocytes with an Epac-specific activator does not induce meiotic resumption
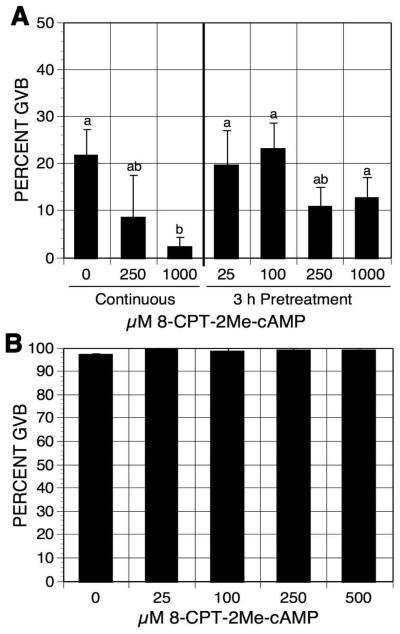
cAMP signaling can be PRKACA-dependent or PRKACA-independent. Epac (exchange protein directly activated by cAMP), another target of cAMP [de Rooij et al, 1998; Enserink et al, 2002], is a guanine nucleotide-exchange factor for the Ras-like small GTPase Rapl and Rap2. To test whether transiently increasing cAMP levels within oocytes induces meiotic resumption through the activation of Epac, oocytes were pulsed with the Epac-specific activator 8-(4-Chlorophenylthio)-2-O-methyladenosine 3′,5′-cyclic monophosphate (8-CPT-2′-O-Me-cAMP) [Enserink et al, 2002]. Pulsing oocytes with increasing concentrations of 8-CPT-2′-O-Me-cAMP had no effect on oocyte meiotic maturation status compared to the continuously cultured controls (Fig. 8A). Yet 8-CPT-2′-O-Me-cAMP potentiated the meiosis-arresting effect of mbcAMP in the continuous cultures, indicating that oocytes are permeable to the compound. The results also suggest that activation of Epac may contribute to oocyte meiotic arrest. To further examine this possibility, DO were cultured for 4 h in inhibitor-free medium with increasing concentrations of 8-CPT-2′-O-Me-cAMP. 8-CPT-2′-O-Me-cAMP at the concentration of 500 μM did not show any inhibitory effect on oocyte spontaneous maturation (Fig. 8B), suggesting that Epac activation, though augmenting the effect of PRKACA, does not play an important role in cAMP-regulated oocyte meiotic arrest.
Figure 8.
Effects of 8-CPT-2Me-cAMP on oocyte maturation. (A) DO were pulsed with increasing concentrations of 8-CPT-2Me-cAMP in 1 mM mbcAMP for 3 h. Oocytes were then washed and cultured in mbcAMP for 17-18 h before assessment of GVB. DO continuously cultured in mbcAMP or mbcAMP plus 8-CPT-2Me-cAMP served as controls. Groups with no identical letter are significantly different. (B) DO were cultured in inhibitor-free medium plus increasing concentrations of 8-CPT-2Me-cAMP. After 3 h of culture, the frequency of oocytes resuming maturation was determined
DISCUSSION
Sustaining elevated levels of oocyte cAMP is important in maintaining meiotic arrest. Yet in this study we have shown that a transient increase of cAMP levels in denuded oocytes can stimulate meiotic resumption through the activation of PRKA. Short-term elevation of intra-oocyte cAMP concentrations by forskolin induced oocyte maturation, and this was associated with active PRKA as determined by western analysis. Meiotic induction and AMPK activation were also stimulated by pulsing oocytes with 8-Br-cAMP, a PDE-sensitive cAMP analog, and the meiosis-inducing effect was blocked by PRKA inhibitors. On the other hand, pulsing oocytes with the PDE-resistant cAMP analogs, Sp-cAMP-AM or Sp-5,6-DCI-cBIMPS, had no effect on oocyte maturation and PRKA activation. Nor did pulsing with the Epac specific activator, 8-CPT-2′-O-Me-cAMP, positively influence maturation, suggesting that the meiosis-inducing effect of cAMP pulsing does not involve Epac
Intra-oocyte cAMP levels are regulated by the activity of adenyl cyclase and phosphodiesterase. Forskolin, an adenyl cyclase activator, has been shown to increase cAMP levels in mouse oocytes [Schultz et al, 1983; Urner et al, 1983]. Consistent with this, after 3 h of forskolin treatment, oocyte cAMP levels were increased from 0.24 fmol/oocyte to 0.34 fmol/oocyte. HX is a meiotic inhibitor present in the follicular fluid [Downs et al, 1985; Eppig et al, 1985] that maintains meiotic arrest by preventing cAMP degradation [Downs et al, 1989]. Oocyte cAMP decreased by 20% after 3 h in HX, and stabilized 2 h later after a further 10% decline. This inability to completely prevent cAMP degradation renders HX a relatively weak meiotic inhibitor. When pulsed oocytes were washed free of forskolin and cultured in HX-containing medium for 2 h, a dramatic drop in cAMP (from 0.34 fmol/oocyte to 0.11 fmol/oocyte) was observed (Fig. 3A). Moreover, these oocytes contained a significantly higher level of AMP (1.43 mmole per kg) when compared to oocytes continuously cultured in HX alone (1.24 mmole per kg, Fig. 3B); thus, the decrease in cAMP can be accounted for, at least in part, by an increase in AMP. Moreover, these changes were occurring at the same time the maturation percentage in pulsed oocytes was beginning to rise above that in control oocytes. This is consistent with the activation of PRKA, as determined by western analysis, and accompanying stimulatory action on meiotic resumption. These data therefore demonstrate that cAMP pulsing can generate a stimulus to induce oocyte maturation and that PRKA is activated before GVB. It must be noted, however, that oocyte cAMP levels within DO decreased below the initial concentration 2 h after forskolin pulsing, which may result in some inactivation of PRKACA and contribute to the meiotic resumption by a means unrelated to PRKA activation..
AMP activates PRKA by allosterically binding to the enzyme and preventing phosphatase-mediated dephosphorylation [Davies et al, 1995; Sanders et al, 2007]. 8-Br-cAMP, a PDE-sensitive cAMP analog, can be degraded to 8-Br-AMP, which is a partial agonist at the allosteric binding site of AMP [Davies et al, 1989] and can activate PRKA at low concentrations. Pulsing oocytes with 6mM 8-Br-cAMP activated PRKA in GV-stage oocytes and induced meiotic maturation in mbcAMP-arrested, as well as hypoxanthine-arrested, oocytes. Two PRKA inhibitors, compound C and araA, that prevent PRKA-induced meiotic maturation in vitro in mouse oocytes [Chen et al, 2006], blocked 8-Br-cAMP pulsing-induced maturation, further implicating PRKA activity in the meiotic response.
Acetyl CoA carboxylase (ACACA), as an important substrate of PRKA, becomes phosphorylated on Ser79 and thereby is inactivated [Ha et al, 1994]. Studies have shown that ACACA phosphorylation on Ser79 corresponds to PRKA activation in rat muscle, liver, adipose tissue [Ruderman et al, 2003] and mouse oocytes [Chen et al, 2006]. Therefore, ACACA phosphorylation has been widely used as an indicator of PRKA activity. However, in this study, oocytes continuously cultured with 8-Br-cAMP contained a high level of pACACA but relatively low levels of pPRKA, suggesting ACACA can be phosphorylated by means other than active PRKA. One possible candidate is active PRKACA. Haystead et al. [1990] reported that activation of PRKACA by forskolin, IBMX or cAMP analogs (N6-butyryl cAMP plus 8-thiomethyl cAMP), leads to ACACA Ser79 phosphorylation and inactivation in isolated rat adipocytes. However, the mechanism of the observation is still not known, as PRKACA does not directly phosphorylate ACACA at the Ser 79 site [Ha et al, 1994]. It is important to mention that the other cAMP analogs used in this study failed to increase pACACA levels in the pulsed oocytes, making it unlikely that this response is simply due to an increase in pPRKACA activity. In our experimental system, apart from this exception, pACACA levels were correlated with pPRKA in oocytes and directly related to the extent of meiotic induction. How persistent 8-Br-cAMP treatment results in ACACA phosphorylation remains unclear in this system. Nevertheless, it should be noted there was a trend towards increased meiotic resumption when oocytes were continuously cultured in medium containing high levels of 8-Br-cAMP, raising the possibility that the phosphorylation state of ACACA influences meiotic regulation. Indeed, results of preliminary experiments suggest that suppression of ACACA activity, which would occur under PRKA activation, can lead to meiotic resumption in mouse oocytes.
Apart from PRKA activation, it was possible that cAMP pulsing-induced maturation is due to a transient hyperactivation of PRKACA that stimulates additional pathways capable of inducing meiotic resumption. To exclude this possibility, oocytes were pulsed with the PDE-resistant PRKACA activators, Sp-cAMP-AM and Sp-5,6-DCI-cBIMPS. Pulsing with these cAMP analogs had no effect on oocyte maturation or, in the case of Sp-5,6-DCL-cBIMPS, PRKA activation, suggesting that degradation of cAMP to AMP is required for pulsing-induced maturation. That these analogs were able to activate PRKACA is suggested by their inhibitory effect on maturation upon continuous exposure. Hence, PDE degradation of cAMP appears to be essential for pulsing-induced maturation.
Casting further doubt on a PRKACA-mediated mechanism in response to cAMP pulsing are the results of a recent study showing inhibition of PRKA by forskolin and IBMX (Hurley et al, 2006). In mouse oocytes, persistent activation of PRKACA by forskolin or cAMP analogs negatively regulates both PRKA and meiotic maturation. Hence, PRKA may be one of the substrates for PRKACA in mouse oocytes, and modulation of its phosphorylation state is a potential mechanism whereby PRKACA negatively regulates GVB.
It is well accepted that cAMP exerts its effect mainly through the activation of PRKACA. However, Rooij et al. [1998] report that cAMP also directly regulates Epac. Epac proteins are guanine nucleotide-exchange factors (GEFs) for the small GTPases, Rapl and Rap2, that have been implicated in various cellular processes such as integrin-mediated cell adhesion and cell-cell junction formation, the control of insulin secretion and neurotransmitter release [Bos, 2006]. cAMP has been shown to regulate oocyte maturation through the activation of PRKACA, but the possible contribution of Epac activation to such regulation is still unknown. Pulsing oocytes with an Epac specific activator, 8-CPT-2′-O-Me-cAMP, had no effect on meiotic maturation. Moreover, 8-CPT-2′-O-Me-cAMP at 500 μM failed to arrest oocyte spontaneous maturation after 4 h of culture, although the cAMP analog was taken up by the oocyte, as shown by its augmentation of mbcAMP-maintained arrest. These data indicate that Epac does not play a major role in either cAMP-mediated meiotic arrest or cAMP pulsing-induced meiotic resumption.
Pulsing oocytes with forskolin or cAMP analogs exposes them to brief periods of elevated cAMP, and the results of previous studies suggest that this may be a common component of meiotic resumption in mammals. In mice, treatment of CEO with FSH or exposure of follicles to forskolin caused a transient rise in oocyte cAMP, with meiotic resumption occurring before cyclic nucleotides fell below the pretreatment levels [Salustri et al, 1985; Hashimoto et al, 1985]. Similar results were seen in follicle-enclosed rabbit and hamster oocytes exposed to hCG or forskolin [Hubbard, 1985, 1986; Yoshimura et al, 1992a,b]. In ovine and porcine oocytes, cAMP was transiently elevated by the gonadotropin surge, and did not decrease below the fresh, non-stimulated levels in the early stages of maturation [Moor and Heslop, 1981]. Whether these brief fluxes in oocyte cAMP were causally related to meiotic resumption was not examined in these reports.
If the oocyte cAMP concentration in freshly isolated oocytes is sufficient to maintain meiotic arrest, our data support the idea that meiosis can resume without cAMP falling significantly below this level. Nevertheless, a decrease in cAMP is necessary for meiotic resumption to occur, since mouse oocytes lacking PDE fail to undergo GVB in vivo or in vitro [Masciarelli et al, 2004]. In addition, PDE activity in mouse oocytes was recently observed to increase before meiotic maturation in vivo [Han et al, 2006], and it is possible that increased oocyte cAMP could provide a signal for such increased PDE activity. In the present study, the dramatic drop in oocyte cAMP following forskolin pulsing in vitro is certainly consistent with this possibility. Therefore, increased AMP may be generated in gonadotropin-stimulated GV-stage oocytes that could activate PRKA and contribute to meiotic induction. PRKA becomes activated in mouse oocytes in vivo prior to GVB [Chen and Downs, 2008], but the mechanism is not yet known.
We have presented the unique finding that direct pulsing of arrested DO with forskolin or PDE-sensitive cAMP analogs can stimulate meiotic resumption in a PRKA-dependent manner. Nevertheless, while we have recently shown that PRKA plays an important role in hormone-induced maturation of mouse oocytes [Chen and Downs, 2008], several experimental results in the present study suggest that cAMP pulsing alone within oocytes may not be the principal means by which PRKA activation and meiotic resumption are controlled in mice: (1) high levels of cAMP were required to stimulate maturation; (2) a cAMP pulse time greater than 1 h was required for meiotic induction; (3) the kinetics of maturation following cAMP pulsing were slower than that observed in vivo; and (4) the extent of meiotic induction following a cAMP pulse was limited, never exceeding 32%. These findings are reminiscent of the response of mouse oocytes to different stresses, in which a lower level of PRKA activity resulted in a more subdued meiotic response when compared to the PRKA activator, AICAR [LaRosa and Downs, 2006]. It therefore appears that the extent of PRKA activation made possible by cAMP pulsing alone is limited and likely cannot account completely for the meiotic induction resulting from more physiological stimuli. It should also be emphasized that our experiments were carried out with DO, an artifactual situation far removed physiologically from conditions in vivo. Nevertheless, these results show that the meiosis-inducing action of cAMP pulsing is not restricted to the somatic compartment and demonstrate a potential signaling function for cAMP within the oocyte beyond simply stimulation of PRKACA and maintenance of meiotic arrest. Though perhaps not absolutely required for meiotic resumption, cAMP pulsing may increase the efficiency by which meiotic resumption is achieved. Yet the extent to which such a signaling function is manifested in situ remains to be determined.
Acknowledgments
This work was supported by a grant from the NIH (R01 40392) to SMD.
Footnotes
There is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
REFERENCES
- Bos JL. Epac proteins: multi-purpose cAMP targets. Trends Biochem Sci. 2006;31:680–686. doi: 10.1016/j.tibs.2006.10.002. [DOI] [PubMed] [Google Scholar]
- Chang AS, Chi MM, Moley KH. Maternal hyperglycemia adversely affects preovulatory oocyte metabolism. Fertil Steril. 2004;82:S269–S269. [Google Scholar]
- Chen J, Hudson E, Chi MM, Chang AS, Moley KH, Hardie DG, Downs SM. AMPK regulation of mouse oocyte meiotic resumption in vitro. Dev Biol. 2006;29:227–238. doi: 10.1016/j.ydbio.2005.11.039. [DOI] [PubMed] [Google Scholar]
- Chen J, Downs SM. AMP-activated protein kinase is involved in hormone-induced mouse oocyte meiotic maturation in vitro. Dev Biol. 2008;313:47–57. doi: 10.1016/j.ydbio.2007.09.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conti M, Andersen CB, Richard F, Mehats C, Chun S-Y, Horner K, Jin C, Tsafriri A. Role of cyclic nucleotide signaling in oocyte maturation. Mol Cell Endocr. 2002;187:153–159. doi: 10.1016/s0303-7207(01)00686-4. [DOI] [PubMed] [Google Scholar]
- Davies SP, Carling D, Hardie DG. Tissue distribution of the AMP-activated protein kinase, and lack of activation by cyclic-AMP-dependent protein kinase, studied using a specific and sensitive peptide assay. Eur J Biochem. 1989;186:123–128. doi: 10.1111/j.1432-1033.1989.tb15185.x. [DOI] [PubMed] [Google Scholar]
- Davies SP, Helps NR, Cohen PTW, Hardie DG. 5′-AMP inhibits dephosphorylation, as well as promoting phosphorylation, of the AMP-activated protein kinase. Studies using bacterially expressed human protein phosphatase-2C[alpha] and native bovine protein phosphatase-2Ac. FEBS Lett. 1995;377:421–425. doi: 10.1016/0014-5793(95)01368-7. [DOI] [PubMed] [Google Scholar]
- Dekel N, Aberdam E, Sherizly I. Spontaneous maturation in vitro of cumulus-enclosed rat oocytes is inhibited by forskolin. Biol Reprod. 1984;31:244–250. doi: 10.1095/biolreprod31.2.244. [DOI] [PubMed] [Google Scholar]
- Dekel N, Lawrence TS, Gilula NB, Beers WH. Modulation of cell-to-cell communication in the cumulus-oocyte complex and the regulation of oocyte maturation by LH. Dev Biol. 1981;86:356–362. doi: 10.1016/0012-1606(81)90193-7. [DOI] [PubMed] [Google Scholar]
- Dekel N. Regulation of Oocyte Maturation. The Role of cAMP. Ann NY Acad Sci. 1988;541:211–216. doi: 10.1111/j.1749-6632.1988.tb22258.x. [DOI] [PubMed] [Google Scholar]
- de Rooij J, Zwartkruis FJT, Verheijen MHG, Cool RH, Nijman SMB, Wittinghofer A, Bos JL. Epac is a Rap1 guanine-nucleotide-exchange factor directly activated by cyclic AMP. Nature. 1998;396:474–477. doi: 10.1038/24884. [DOI] [PubMed] [Google Scholar]
- Dostmann WR, Taylor SS, Genieser HG, Jastorff B, Doskeland SO, Ogreid D. Probing the cyclic nucleotide binding sites of cAMP-dependent protein kinases I and II with analogs of adenosine 3′,5′-cyclic phosphorothioates. J Biol Chem. 1990;265:10484–10491. [PubMed] [Google Scholar]
- Downs SM, Coleman DL, Ward-Bailey PF, Eppig JJ. Hypoxanthine is the Principal Inhibitor of Murine Oocyte Maturation in a Low Molecular Weight Fraction of Porcine Follicular Fluid. Proc Natl Acad Sci. 1985;82:454–458. doi: 10.1073/pnas.82.2.454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downs SM, Daniel SAJ, Bornslaeger EA, Hoppe PC, Eppig JJ. Maintenance of meiotic arrest in mouse oocytes by purines: Modulation of cAMP levels and cAMP phosphodiesterase activity. Gamete Res. 1989;23:323–334. doi: 10.1002/mrd.1120230309. [DOI] [PubMed] [Google Scholar]
- Downs SM, Daniel SA, Eppig JJ. Induction of maturation in cumulus cell-enclosed mouse oocytes by follicle-stimulating hormone and epidermal growth factor: Evidence for a positive stimulus of somatic cell origin. J Exp Zool. 1988;245:86–96. doi: 10.1002/jez.1402450113. [DOI] [PubMed] [Google Scholar]
- Edry I, Sela-Abramovich S, Dekel N. Meiotic arrest of oocytes depends on cell-to-cell communication in the ovarian follicle. Mol Cell Endocr. 2006;252:102–106. doi: 10.1016/j.mce.2006.03.009. [DOI] [PubMed] [Google Scholar]
- Enserink JM, Christensen AE, de Rooij J, van Triest M, Schwede F, Genieser HG, Doskeland SO, Blank JL, Bos JL. A novel Epac-specific cAMP analogue demonstrates independent regulation of Rap1 and ERK. Nat Cell Biol. 2002;4:901–906. doi: 10.1038/ncb874. [DOI] [PubMed] [Google Scholar]
- Eppig JJ, Ward-Bailey PF, Coleman DL. Hypoxanthine and adenosine in murine ovarian follicular fluid: concentrations and activity in maintaining oocyte meiotic arrest. Biol Reprod. 1985;33:1041–1049. doi: 10.1095/biolreprod33.5.1041. [DOI] [PubMed] [Google Scholar]
- Freudzon L, Norris RP, Hand AR, Tanaka S, Saeki Y, Jones TLZ, Rasenick MM, Berlot CH, Mehlmann LM, Jaffe LA. Regulation of meiotic prophase arrest in mouse oocytes by GPR3, a constitutive activator of the Gs G protein. J. Cell Biol. 2005;171:255–265. doi: 10.1083/jcb.200506194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ha J, Daniel S, Broyles SS, Kim KH. Critical phosphorylation sites for acetyl-CoA carboxylase activity. J Biol Chem. 1994;269:22162–22168. [PubMed] [Google Scholar]
- Han SJ, Vaccari S, Nedachi T, Andersen CB, Kovacina KS, Roth RA, Conti M. Protein kinase B/Akt phosphorylation of PDE3A and its role in mammalian oocyte maturation. EMBO J. 2006;25:5716–5725. doi: 10.1038/sj.emboj.7601431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardie DG, Hawley SA. AMP-activated protein kinase: the energy charge hypothesis revisited. BioEssays. 2001;23:1112–1119. doi: 10.1002/bies.10009. [DOI] [PubMed] [Google Scholar]
- Hashimoto N, Kishimoto T, Nagahama Y. Induction and Inhibition of Meiotic Maturation in Follicle-enclosed Mouse Oocytes by Forskolin. Development, Growth & Differentiation. 1985;27:709–716. doi: 10.1111/j.1440-169X.1985.00709.x. [DOI] [PubMed] [Google Scholar]
- Hawley SA, Gadalla AE, Olsen GS, Hardie DG. The Antidiabetic Drug Metformin Activates the AMP-Activated Protein Kinase Cascade via an Adenine Nucleotide-Independent Mechanism. Diabetes. 2002;51:2420–2425. doi: 10.2337/diabetes.51.8.2420. [DOI] [PubMed] [Google Scholar]
- Haystead TAJ, Moore F, Cohen P, Hardie DG. Roles of the AMP-activated and cyclic-AMP-dependent protein kinases in the adrenaline-induced inactivation of acetyl-CoA carboxylase in rat adipocytes. Eur J Biochem. 1990;187:199–205. doi: 10.1111/j.1432-1033.1990.tb15295.x. [DOI] [PubMed] [Google Scholar]
- Henin N, Vincent MF, Van den Berghe G. Stimulation of rat liver AMP-activated protein kinase by AMP analogues. Biochim et Biophys Acta (General Subjects) 1996;1290:197–203. doi: 10.1016/0304-4165(96)00021-9. [DOI] [PubMed] [Google Scholar]
- Horner K, Livera G, Hinckley M, Trinh K, Storm D, Conti M. Rodent oocytes express an active adenylyl cyclase required for meiotic arrest. Dev Biol. 2003;258:385–396. doi: 10.1016/s0012-1606(03)00134-9. [DOI] [PubMed] [Google Scholar]
- Hubbard CJ. The effects of forskolin and LH on cAMP changes and maturation in the follicle-enclosed oocytes of hamsters. Acta Endocrinol. 1985;110:413–420. doi: 10.1530/acta.0.1100413. [DOI] [PubMed] [Google Scholar]
- Hubbard CJ. Cyclic AMP changes in the component cells of Graafian follicles: Possible influences on maturation in the follicle-enclosed oocytes of hamsters. Dev Biol. 1986;118:343–351. doi: 10.1016/0012-1606(86)90003-5. [DOI] [PubMed] [Google Scholar]
- Kalinowski RR, Berlot CH, Jones TLZ, Ross LF, Jaffe LA, Mehlmann LM. Maintenance of meiotic prophase arrest in vertebrate oocytes by a Gs protein-mediated pathway. Dev Biol. 2004;267:1–13. doi: 10.1016/j.ydbio.2003.11.011. [DOI] [PubMed] [Google Scholar]
- LaRosa C, Downs SM. Stress stimulates AMP-activated protein kinase and meiotic resumption in mouse oocytes. Biol Reprod. 2006;74:585–592. doi: 10.1095/biolreprod.105.046524. [DOI] [PubMed] [Google Scholar]
- Masciarelli S, Horner K, Liu C, Park SH, Hinckley M, Hockman S, Nedachi T, Jin C, Conti M, Manganiello V. Cyclic nucleotide phosphodiesterase 3A-deficient mice as a model of female infertility. J Clin Invest. 2004;114:196–205. doi: 10.1172/JCI21804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehlmann LM, Saeki Y, Tanaka S, Brennan TJ, Evsikov AV, Pendola FL, Knowles BB, Eppig JJ, Jaffe LA. The Gs-Linked Receptor GPR3 Maintains Meiotic Arrest in Mammalian Oocytes. Science. 2004;306:1947–1950. doi: 10.1126/science.1103974. [DOI] [PubMed] [Google Scholar]
- Mehlmann LMJ, Teresa LZ, Jaffe LA. Meiotic Arrest in the Mouse Follicle Maintained by a Gs Protein in the Oocyte. Science. 2002;297:1343–1345. doi: 10.1126/science.1073978. [DOI] [PubMed] [Google Scholar]
- Moor RM, Heslop JP. Cyclic AMP in mammalian follicle cells and oocytes during maturation. J Exp Zool. 1981;216:205–209. doi: 10.1002/jez.1402160125. [DOI] [PubMed] [Google Scholar]
- Richard FJ, Tsafriri A, Conti M. Role of Phosphodiesterase Type 3A in Rat Oocyte Maturation. Biol Reprod. 2001;65:1444–1451. doi: 10.1095/biolreprod65.5.1444. [DOI] [PubMed] [Google Scholar]
- Ruderman NB, park H, Kaushik VK, Dean D, Constant S, Saha AK. AMPK as a metabolic switch in rat muscle, liver and adipose tissue after exercise. Acta Physiol Scand. 2003;178:435–442. doi: 10.1046/j.1365-201X.2003.01164.x. [DOI] [PubMed] [Google Scholar]
- Salustri A, Petrungaro S, De Felici M, Conti M, Siracusa G. Effect of follicle-stimulating hormone on cyclic adenosine monophosphate level and on meiotic maturation in mouse cumulus cell- enclosed oocytes cultured in vitro. Biol Reprod. 1985;33:797–802. doi: 10.1095/biolreprod33.4.797. [DOI] [PubMed] [Google Scholar]
- Sandberg M, Butt E, Nolte C, Fischer L, Halbrugge M, Beltman J, Jahnsen T, Genieser HG, Jastorff B, Walter U. Characterization of Sp-5,6-dichloro-1-beta-D-ribofuranosylbenzimidazole- 3′,5′-monophosphorothioate (Sp-5,6-DCl-cBiMPS) as a potent and specific activator of cyclic-AMP-dependent protein kinase in cell extracts and intact cells. Biochem. J. 1991;279:521–527. doi: 10.1042/bj2790521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders MJ, Grondin PO, Hegarty BD, Snowden MA, Carling D. Investigating the mechanism for AMP activation of the AMP-activated protein kinase cascade. Biochem J. 2007;403:139–148. doi: 10.1042/BJ20061520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz RM, Montgomery RR, Belanoff JR. Regulation of mouse oocyte meiotic maturation: Implication of a decrease in oocyte cAMP and protein dephosphorylation in commitment to resume meiosis. Dev Biol. 1983;97:264–273. doi: 10.1016/0012-1606(83)90085-4. [DOI] [PubMed] [Google Scholar]
- Shitsukawa K, Andersen CB, Richard FJ, Horner AK, Wiersma A, van Duin M, Conti M. Cloning and Characterization of the Cyclic Guanosine Monophosphate-Inhibited Phosphodiesterase PDE3A Expressed in Mouse Oocyte. Biol Reprod. 2001;65:188–196. doi: 10.1095/biolreprod65.1.188. [DOI] [PubMed] [Google Scholar]
- Urner F, Herrmann WL, Baulieu EE, Schorderet-Slatkine S. Inhibition of denuded mouse oocyte meiotic maturation by forskolin, an activator of adenylate cyclase. Endocrinology. 1983;113:1170–1172. doi: 10.1210/endo-113-3-1170. [DOI] [PubMed] [Google Scholar]
- Webb RJ, Marshall F, Swann K, Carroll J. Follicle-Stimulating Hormone Induces a Gap Junction-Dependent Dynamic Change in [cAMP] and Protein Kinase A in Mammalian Oocytes. Dev Biol. 2002;246:441–454. doi: 10.1006/dbio.2002.0630. [DOI] [PubMed] [Google Scholar]
- Wiersma A, Hirsch B, Tsafriri A, Hanssen RGJM, Van de Kant M, Kloosterboer HJ, Conti M, Hsueh AJW. Phosphodiesterase 3 Inhibitors Suppress Oocyte Maturation and Consequent Pregnancy without Affecting Ovulation and Cyclicity in Rodents. J Clin.Invest. 1998;102:532–537. doi: 10.1172/JCI2566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimura Y, Nakamura Y, Ando M, Jinno M, Oda T, Karube M, Koyama N, Nanno T. Stimulatory role of cyclic adenosine monophosphate as a mediator of meiotic resumption in rabbit oocytes. Endocrinology. 1992a;131:351–356. doi: 10.1210/endo.131.1.1319321. [DOI] [PubMed] [Google Scholar]
- Yoshimura Y, Nakamura Y, Oda T, Ando M, Ubukata Y, Karube M, Koyama N, Yamada H. Induction of meiotic maturation of follicle-enclosed oocytes of rabbits by a transient increase followed by an abrupt decrease in cyclic AMP concentration. J Reprod Fertil. 1992b;95:803–812. doi: 10.1530/jrf.0.0950803. [DOI] [PubMed] [Google Scholar]
- Zhou G, Myers R, Li Y, Chen Y, Shen X, Fenyk-Melody J, Wu M, Ventre J, Doebber T, Fujii N, Musi N, Hirshman MF, Goodyear LJ, Moller DE. Role of AMP-activated protein kinase in mechanism of metformin action. J Clin Invest. 2001;108:1167–1174. doi: 10.1172/JCI13505. [DOI] [PMC free article] [PubMed] [Google Scholar]