Abstract
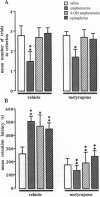
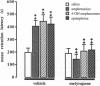
This study examined glucocorticoid-adrenergic interactions in modulating acquisition and memory storage for inhibitory avoidance training. Systemically (s.c.) administered amphetamine (1 mg/kg), but not epinephrine (0.1 mg/kg) or the peripherally acting amphetamine derivative 4-OH amphetamine (2 mg/kg), given to rats shortly before training facilitated acquisition performance in a continuous multiple-trial inhibitory avoidance (CMIA) task. Adrenocortical suppression with the 11beta-hydroxylase inhibitor metyrapone (50 mg/kg; s.c.), given to rats 90 min before training, did not block the effect of amphetamine and did not affect acquisition performance of otherwise untreated animals. Retention of CMIA and one-trial inhibitory avoidance was enhanced by either pre- or posttraining injections of amphetamine as well as 4-OH amphetamine and epinephrine. The finding that injections of amphetamine and epinephrine have comparable effects on memory is consistent with the view that amphetamine may modulate memory storage, at least in part, by inducing the release of epinephrine from the adrenal medulla. Metyrapone pretreatment blocked the memory-enhancing effects of amphetamine, 4-OH amphetamine, and epinephrine but did not affect retention performance of otherwise untreated animals. Posttraining injections of different doses of epinephrine (ranging from 0.0001 to 1.0 mg/kg) produced a dose-dependent memory enhancement for inhibitory avoidance training and metyrapone blocked the memory-enhancing effects of all these doses. These findings provide further evidence that the sympathoadrenal and adrenocortical systems are intimately coupled during processes of memory storage.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bohus B., de Kloet E. R. Adrenal steroids and extinction behavior: antagonism by progesterone, deoxycorticosterone and dexamethasone of a specific effect of corticosterone. Life Sci. 1981 Jan 26;28(4):433–440. doi: 10.1016/0024-3205(81)90090-4. [DOI] [PubMed] [Google Scholar]
- Borrell J., De Kloet E. R., Versteeg D. H., Bohus B. Inhibitory avoidance deficit following short-term adrenalectomy in the rat: the role of adrenal catecholamines. Behav Neural Biol. 1983 Nov;39(2):241–258. doi: 10.1016/s0163-1047(83)90910-x. [DOI] [PubMed] [Google Scholar]
- Borrell J., de Kloet E. R., Bohus B. Corticosterone decreases the efficacy of adrenaline to affect passive avoidance retention of adrenalectomized rats. Life Sci. 1984 Jan 2;34(1):99–104. doi: 10.1016/0024-3205(84)90336-9. [DOI] [PubMed] [Google Scholar]
- Cador M., Dulluc J., Mormède P. Modulation of the locomotor response to amphetamine by corticosterone. Neuroscience. 1993 Oct;56(4):981–988. doi: 10.1016/0306-4522(93)90144-5. [DOI] [PubMed] [Google Scholar]
- Chao H. M., Choo P. H., McEwen B. S. Glucocorticoid and mineralocorticoid receptor mRNA expression in rat brain. Neuroendocrinology. 1989 Oct;50(4):365–371. doi: 10.1159/000125250. [DOI] [PubMed] [Google Scholar]
- Cole B. J., Cador M., Stinus L., Rivier C., Rivier J., Vale W., Le Moal M., Koob G. F. Critical role of the hypothalamic pituitary adrenal axis in amphetamine-induced sensitization of behavior. Life Sci. 1990;47(19):1715–1720. doi: 10.1016/0024-3205(90)90344-q. [DOI] [PubMed] [Google Scholar]
- De Boer S. F., Koopmans S. J., Slangen J. L., Van der Gugten J. Plasma catecholamine, corticosterone and glucose responses to repeated stress in rats: effect of interstressor interval length. Physiol Behav. 1990 Jun;47(6):1117–1124. doi: 10.1016/0031-9384(90)90361-7. [DOI] [PubMed] [Google Scholar]
- Freo U., Holloway H. W., Kalogeras K., Rapoport S. I., Soncrant T. T. Adrenalectomy or metyrapone-pretreatment abolishes cerebral metabolic responses to the serotonin agonist 1-(2,5-dimethoxy-4-iodophenyl)-2-aminopropane (DOI) in the hippocampus. Brain Res. 1992 Jul 24;586(2):256–264. doi: 10.1016/0006-8993(92)91634-q. [DOI] [PubMed] [Google Scholar]
- Fuxe K., Cintra A., Härfstrand A., Agnati L. F., Kalia M., Zoli M., Wikström A. C., Okret S., Aronsson M., Gustafsson J. A. Central glucocorticoid receptor immunoreactive neurons: new insights into the endocrine regulation of the brain. Ann N Y Acad Sci. 1987;512:362–393. doi: 10.1111/j.1749-6632.1987.tb24974.x. [DOI] [PubMed] [Google Scholar]
- Gelowitz D. L., Kokkinidis L. The effects of amygdaloid stimulation on amphetamine-elicited locomotor sensitization. Brain Res Bull. 1993;32(6):561–565. doi: 10.1016/0361-9230(93)90155-5. [DOI] [PubMed] [Google Scholar]
- Gold P. E., Rose R. P., Spanis C. W., Hankins L. L. Retention deficit for avoidance training in hypophysectomized rats: time-dependent enhancement of retention performance with post-training ACTH injections. Horm Behav. 1977 Jun;8(3):363–371. doi: 10.1016/0018-506x(77)90010-1. [DOI] [PubMed] [Google Scholar]
- Gold P. E., Van Buskirk R. B. Facilitation of time-dependent memory processes with posttrial epinephrine injections. Behav Biol. 1975 Feb;13(2):145–153. doi: 10.1016/s0091-6773(75)91784-8. [DOI] [PubMed] [Google Scholar]
- Harvey S., Phillips J. G., Rees A., Hall T. R. Stress and adrenal function. J Exp Zool. 1984 Dec;232(3):633–645. doi: 10.1002/jez.1402320332. [DOI] [PubMed] [Google Scholar]
- Honkaniemi J., Pelto-Huikko M., Rechardt L., Isola J., Lammi A., Fuxe K., Gustafsson J. A., Wikström A. C., Hökfelt T. Colocalization of peptide and glucocorticoid receptor immunoreactivities in rat central amygdaloid nucleus. Neuroendocrinology. 1992 Apr;55(4):451–459. doi: 10.1159/000126156. [DOI] [PubMed] [Google Scholar]
- Ivens I. A., Janak P. H., Martinez J. L., Jr Microdialysis measurement of striatal dopamine levels in freely moving rats after cocaine or amphetamine treatment. Proc West Pharmacol Soc. 1992;35:165–169. [PubMed] [Google Scholar]
- Jones G. H., Mittleman G., Robbins T. W. Attenuation of amphetamine-stereotypy by mesostriatal dopamine depletion enhances plasma corticosterone: implications for stereotypy as a coping response. Behav Neural Biol. 1989 Jan;51(1):80–91. doi: 10.1016/s0163-1047(89)90686-9. [DOI] [PubMed] [Google Scholar]
- Krivanek J. A., McGaugh J. L. Facilitating effects of pre- and posttrial amphetamine administration on discrimination learning in mice. Agents Actions. 1969 Nov;1(2):36–42. doi: 10.1007/BF01977664. [DOI] [PubMed] [Google Scholar]
- Kuczenski R., Segal D. S. Regional norepinephrine response to amphetamine using dialysis: comparison with caudate dopamine. Synapse. 1992 Jun;11(2):164–169. doi: 10.1002/syn.890110210. [DOI] [PubMed] [Google Scholar]
- Kvetnanský R., Fukuhara K., Pacák K., Cizza G., Goldstein D. S., Kopin I. J. Endogenous glucocorticoids restrain catecholamine synthesis and release at rest and during immobilization stress in rats. Endocrinology. 1993 Sep;133(3):1411–1419. doi: 10.1210/endo.133.3.8396019. [DOI] [PubMed] [Google Scholar]
- Liang K. C., Juler R. G., McGaugh J. L. Modulating effects of posttraining epinephrine on memory: involvement of the amygdala noradrenergic system. Brain Res. 1986 Mar 12;368(1):125–133. doi: 10.1016/0006-8993(86)91049-8. [DOI] [PubMed] [Google Scholar]
- Martinez J. L., Jr, Ishikawa K., Liang K. C., Jensen R. A., Bennett C., Sternberg D. B., McGaugh J. L. 4-OH amphetamine enhances retention of an active avoidance response in rats and decreases regional brain concentrations of norepinephrine and dopamine. Behav Neurosci. 1983 Dec;97(6):962–969. doi: 10.1037//0735-7044.97.6.962. [DOI] [PubMed] [Google Scholar]
- Martinez J. L., Jr, Jensen R. A., Messing R. B., Vasquez B. J., Soumireu-Mourat B., Geddes D., Liang K. C., McGaugh J. L. Central and peripheral actions of amphetamine on memory storage. Brain Res. 1980 Jan 20;182(1):157–166. doi: 10.1016/0006-8993(80)90838-0. [DOI] [PubMed] [Google Scholar]
- Martinez J. L., Jr, Vasquez B. J., Rigter H., Messing R. B., Jensen R. A., Liang K. C., McGaugh J. L. Attenuation of amphetamine-induced enhancement of learning by adrenal demedullation. Brain Res. 1980 Aug 18;195(2):433–443. doi: 10.1016/0006-8993(80)90077-3. [DOI] [PubMed] [Google Scholar]
- McCarty R., Gold P. E. Plasma catecholamines: effects of footshock level and hormonal modulators of memory storage. Horm Behav. 1981 Jun;15(2):168–182. doi: 10.1016/0018-506x(81)90026-x. [DOI] [PubMed] [Google Scholar]
- McEwen B. S. Glucocorticoid-biogenic amine interactions in relation to mood and behavior. Biochem Pharmacol. 1987 Jun 1;36(11):1755–1763. doi: 10.1016/0006-2952(87)90234-6. [DOI] [PubMed] [Google Scholar]
- McGaugh J. L., Introini-Collison I. B., Nagahara A. H. Memory-enhancing effects of posttraining naloxone: involvement of beta-noradrenergic influences in the amygdaloid complex. Brain Res. 1988 Apr 12;446(1):37–49. doi: 10.1016/0006-8993(88)91294-2. [DOI] [PubMed] [Google Scholar]
- Nicoll R. A. The coupling of neurotransmitter receptors to ion channels in the brain. Science. 1988 Jul 29;241(4865):545–551. doi: 10.1126/science.2456612. [DOI] [PubMed] [Google Scholar]
- Oitzl M. S., de Kloet E. R. Selective corticosteroid antagonists modulate specific aspects of spatial orientation learning. Behav Neurosci. 1992 Feb;106(1):62–71. doi: 10.1037//0735-7044.106.1.62. [DOI] [PubMed] [Google Scholar]
- Pauly J. R., Robinson S. F., Collins A. C. Chronic corticosterone administration enhances behavioral sensitization to amphetamine in mice. Brain Res. 1993 Aug 27;620(2):195–202. doi: 10.1016/0006-8993(93)90156-h. [DOI] [PubMed] [Google Scholar]
- Reul J. M., de Kloet E. R. Two receptor systems for corticosterone in rat brain: microdistribution and differential occupation. Endocrinology. 1985 Dec;117(6):2505–2511. doi: 10.1210/endo-117-6-2505. [DOI] [PubMed] [Google Scholar]
- Roozendaal B., Koolhaas J. M., Bohus B. Attenuated cardiovascular, neuroendocrine, and behavioral responses after a single footshock in central amygdaloid lesioned male rats. Physiol Behav. 1991 Oct;50(4):771–775. doi: 10.1016/0031-9384(91)90016-h. [DOI] [PubMed] [Google Scholar]
- Sandi C., Rose S. P. Corticosterone enhances long-term retention in one-day-old chicks trained in a weak passive avoidance learning paradigm. Brain Res. 1994 May 30;647(1):106–112. doi: 10.1016/0006-8993(94)91404-4. [DOI] [PubMed] [Google Scholar]
- Silva M. T. Extinction of a passive avoidance response in adrenalectomized and demedullated rats. Behav Biol. 1973 Nov;9(5):553–562. doi: 10.1016/s0091-6773(73)80050-1. [DOI] [PubMed] [Google Scholar]
- Sternberg D. B., Isaacs K. R., Gold P. E., McGaugh J. L. Epinephrine facilitation of appetitive learning: attenuation with adrenergic receptor antagonists. Behav Neural Biol. 1985 Nov;44(3):447–453. doi: 10.1016/s0163-1047(85)90856-8. [DOI] [PubMed] [Google Scholar]
- Strashimirov D., Bohus B. Effect of 2-methyl-1,2-bis-3-pyridl-1-propanone (SU-4885) on adrenocortical secretion in normal and hypophysectomized rats. Steroids. 1966 Feb;7(2):171–180. doi: 10.1016/0039-128x(66)90024-9. [DOI] [PubMed] [Google Scholar]
- Strupp B. J., Bunsey M., Levitsky D., Kesler M. Time-dependent effects of post-trial amphetamine treatment in rats: evidence for enhanced storage of representational memory. Behav Neural Biol. 1991 Jul;56(1):62–76. doi: 10.1016/0163-1047(91)90291-w. [DOI] [PubMed] [Google Scholar]
- Van Eekelen J. A., Jiang W., De Kloet E. R., Bohn M. C. Distribution of the mineralocorticoid and the glucocorticoid receptor mRNAs in the rat hippocampus. J Neurosci Res. 1988 Sep;21(1):88–94. doi: 10.1002/jnr.490210113. [DOI] [PubMed] [Google Scholar]
- WEIL-MALHERBE H., AXELROD J., TOMCHICK R. Blood-brain barrier for adrenaline. Science. 1959 May 1;129(3357):1226–1227. doi: 10.1126/science.129.3357.1226. [DOI] [PubMed] [Google Scholar]
- Wyndham C. H., Williams C. G., Morrison J. F., Watson M. The maximum oxygen intakes of men working on industrial tasks. Int Z Angew Physiol. 1966 Apr 28;22(2):141–148. doi: 10.1007/BF00698149. [DOI] [PubMed] [Google Scholar]
- de Nicola A. F., Dahl V. Acute effects of SU-4885 and its reduced derivative (SU-5236) on the adrenocortical secretion of the rat. Endocrinology. 1971 Nov;89(5):1236–1241. doi: 10.1210/endo-89-5-1236. [DOI] [PubMed] [Google Scholar]