Abstract
Aging is a progressive process with degenerative changes of various tissues and organs. As a classic model organism in genetics and neurobiology, Caenorhabditis elegans is also a powerful system in aging and behavioral studies and can be used at both the molecular and organismal levels to evaluate potential therapeutics for age-related neurodegeneration, owing to its short life span, relative simplicity, and high degree of experimental tractability as well as significant conservation of disease genes and signaling pathways with humans. We attempt here to summarize the use of C. elegans models in exploring traditional Chinese medicine for potential remedies against aging and neurodegeneration.
Introduction
Aging and age-related diseases have become a worldwide concern, increasingly perplexing biomedical researchers. Aging is essentially a deteriorating process in various organs and tissues that is accompanied with increased oxidative damage, degenerative disorders, and behavioral deficits.1 As a major model organism in modern life sciences, Caenorhabditis elegans is powerful at both the molecular and organismal levels and has been used in a broad range of pioneering biomedical research. Although C. elegans is tiny in size, it contains about two-thirds of the potential counterparts of human disease genes; nearly one-third of its somatic cells are neurons.2 Together with its transparent body and short life span, this nematode model has provided a highly tractable system for studies of aging and neurobiology.
Traditional Chinese medicine has a long history of anti-aging practice, and hundreds of medicinal herbs have been used for hundreds of years to treat age-related diseases, including late-onset dementia. Although a growing number of investigations are devoted to Chinese medicine in aging-related conditions, studies using modern model organisms such as C. elegans and zebrafish are limited. Here we aim to present studies on traditional Chinese medicine focusing on aging and neurodegeneration using C. elegans models, including wild-type, transgenic, and drug-induced models, coupled with feasible experimental means.
Life Span Extension As an Unequivocal Anti-Aging Index
The decline of essential functions at both cellular and organismal levels is apparent during the aging process and ultimately causes the end of life. Therefore, life span as a quantitative indicator of aging is undoubtedly a gold standard in anti-aging studies. In this regard, the seemingly simple nematode C. elegans is a leading model due to its short and reproducible life span, ease of maintenance and genetic manipulation, and amenability to high-throughput screening.3 Life span determination of C. elegans can be achieved either on solid plates or in liquid culture by monitoring survival rates followed by statistical analysis. Life span experiments on solid agar plates with standard nematode growth medium (NGM) provide accurate counts of live/dead animals by repeated picking of the nematodes; however, the animals are also subjected to mechanical damage by the frequent transfer.4 In a typical solid life span assay, synchronized nematodes are grown on 35-mm plates seeded with Escherichia coli OP50 and treated from the L4 stage with herbal extracts. Live nematodes are transferred to fresh plates and counted every day until termination of egg laying and then every 2 days until all are dead.
The liquid life span assay of C. elegans is usually conducted in small volumes of liquid culture in a microplate format, which is relatively rapid, labor saving, and of low cost. Equipping the process with automated devices and image analysis software makes high-throughput analysis possible. Typically, batches of synchronized L1 nematodes are incubated until L4 in S medium containing E. coli OP50 as food and antibiotics to inhibit other bacteria. Before further incubation to young adulthood, 2′-deoxy-5-fluorouridine is added to prevent reproduction. Aliquots of the young adults are then dispensed to micro-plates, and herbal extracts are added to test life span–extending effects. The number of live nematodes is scored every 2 days until all are dead; before scoring, the plate is gently vibrated to stimulate movement of nematodes. The live/dead discrimination is based on mobility, shape, and pharyngeal pumping.
Using the liquid life span assay, we have tested both aqueous and ethanolic extracts of Chinese herbs. For example, astragalan, a hot water–soluble polysaccharide from Astragalus membranaceus roots, is found to extend the adult life span of both wild-type and polyglutamine (polyQ) nematodes.5 Aqueous as well as organic fractions of the extracts from Damnacanthus officinarum roots and leaves are also shown to extend the life span of an HA759 strain nematode, a polyQ150-expressing model.6
Oxidative Stress Resistance As a Prognostic Indicator of Anti-Aging Capability
Enhanced resistance to oxidative stress has been shown to extend the C. elegans life span in several studies and thus can be used to assess the anti-aging potential of herbal extracts. Recently, we have used C. elegans to evaluate polysaccharides against paraquat- and hydrogen peroxide (H2O2)-induced oxidative stress. An example is the polysaccharide from Nostoc commune, which has been traditionally used in China as food and medicine. In vivo treatment with the Nostoc polysaccharide not only increases the activities of anti-oxidant enzymes (superoxide dismutase, catalase, and glutathione peroxidase) and decreases the content of lipid peroxidation product malondialdehyde, but also promotes the survival of a nematode population under paraquat-induced oxidative stress.7
Suppression of Neuronal Proteotoxicity As an Indication of Anti-Neurodegenerative Efficacy
In addition to aging studies, C. elegans is also a powerful yet humble model that offers a convenient in vivo system for investigation of neurological disorders given the molecular conservation in neuronal signaling pathways across species. Due to its short life span, C. elegans is particularly valuable for lifetime follow-up examinations of late-onset disorders, including several neuron-selective proteotoxic diseases. In fact, C. elegans has been engineered to express a number of neurodegeneration-associated human proteins such as amyloid-β peptide (Aβ) and polyglutamine (polyQ), whose aggregation in human brain is the definitive indicators of Alzheimer disease (AD) and Huntington disease (HD), respectively.
These transgenic nematode models of human neurodegenerative diseases can be readily exploited for studies of traditional medicine. For example, C. elegans strain HA759 expresses Htn-Q150 (a polyQ tract derived from human huntingtin) together with green fluorescent protein (GFP) strongly in ASH neurons, leading to progressive neurodegeneration and ASH neuronal death, and thus can be employed as a valid HD model.8
We have recently used this model in neuronal survival assay of herbal extracts and complex prescriptions. Synchronized L1 HA759 nematodes in liquid culture are treated with herbal extracts in 96-well plates at 15°C for 3 days. The nematodes are then collected and immobilized with sodium azide on an agarose pad. GFP-positive ASH neurons were scored with a fluorescence microscope, and the fraction of live ASH neurons was calculated.5 Using this assay, we found that astragalan and some fractions of D. officinarum extracts are effective in protection of the nematodes from polyQ-mediated ASH neurodegeneration.5,6
Rescue of Behavioral Deficits As a Necessity to Achieve Relief from Neurodegenerative Diseases
The capacity to control its own behavior is an essential feature of an animal, but there is a gradual decrease in behavioral coordination as aging progresses or as age-related degeneration develops. Therefore, rescue of behavioral phenotypes is an important step in alleviating late-onset neurodegenerative diseases. C. elegans has been engineered to manifest aging-related behavioral deficits, including locomotory and sensory impairments.9 For example, the transgenic C. elegans strain CL2355 with pan-neuronal Aβ expression presents a phenotype of olfactory defect and thus can be used in chemotaxis assay to test its response to odorants, providing indirect evidence of Aβ neurotoxicity.10 The strain CL4176 with inducible muscle-specific Aβ expression displays a rapid paralysis phenotype and thus can be used to monitor Aβ-mediated toxicity and behavioral impairments.11 Using these models, Ginkgo biloba leaf extract EGb 761 is found to rescue chemotactic defects and paralysis phenotypes.12
PolyQ-mediated neurotoxicity in ASH neurons also makes the aforementioned HA759 nematodes defective in osmotic avoidance behavior.13,14 Therefore, this model can be used in an osmotic avoidance assay to test neuroprotective functions of herbal extracts. Typically age-synchronized L1 nematodes are incubated with an extract in S medium for 3 days at 15°C and then transferred to an assay plate. The nematodes and an attractant (usually diluted butanedione) are spotted on opposite sides of the plate, which is divided into two areas by glycerol. The nematodes are scored separately 90 min later in the two areas, and the osmotic avoidance behavior is quantified by an aversion index, which results from the number of nematodes with dead ASH neurons passing through the glycerin barrier. Using this assay we have assessed the neuroprotective effects of water-soluble polysaccharides and other extracts from a variety of traditional herbs. For example, the n-butanol fraction of D. officinarum extracts is found to increase the avoidance capacity of HA759 nematodes to high osmolarity.6
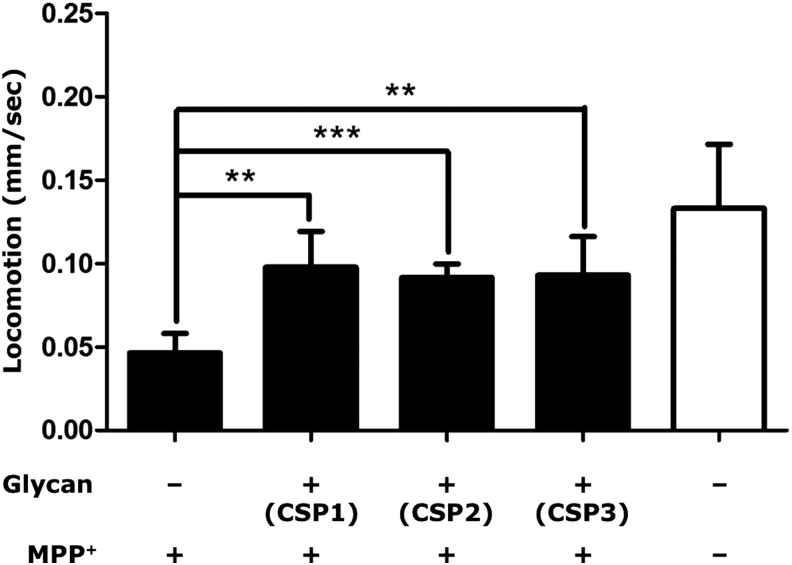
In addition to transgenic models, chemically induced C. elegans models are also readily available. For example, the neurotoxic compound 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) and its active metabolite 1-methyl-4-phenylpyridine (MPP+) are known to induce PD symptoms.15 Therefore, we have used MPP+-induced nematode models to test herbal extracts for potential anti-PD activities. Synchronized L1 wild-type nematodes were treated with a mixture of MPP+ and herbal extracts in 96-well plates for 48 hr at 20°C and then transferred to fresh 35-mm plates. The locomotory patterns of nematodes were analyzed from recorded movies with Worm Tracker & Track Analyzer software for speed of locomotion and by visual counting for frequencies of reversals and body bends.16,17 Using this assay, we have found that the polysaccharides from Chaenomeles speciosa are able to improve MPP+-induced mobility defects in C. elegans (Fig. 1).
FIG. 1.
Pharmacologic suppression of 1-methyl-4-phenylpyridine (MPP+)-induced locomotion defect in Caenorhabditis elegans by polysaccharides from Chaenomeles speciosa. Synchronized L1 wild-type nematodes were treated with MPP+ (200 μg/mL) and polysaccharides (150 μg/mL) for 48 hr at 20°C, and locomotion of the nematodes was analyzed from recorded movies on fresh plates. CPS1–3, fractions of C. speciosa polysaccharides from anion-exchange chromatography eluted with H2O, 1 M NaCl and 2 M NaCl, respectively. (**) p<0.01; (***) p<0.001.
Conclusions
Anti-aging is in some sense an abstract concept, and therefore to determine anti-aging capacities we need to quantify phenotypic changes recognized as aging. In this respect, C. elegans is a useful model not only in anti-aging itself but also in aging-related diseases and has been successfully used for in vivo investigation of traditional Chinese medicine as summarized here. Furthermore, C. elegans is also a powerful system for studying mechanisms of action at both molecular and organismal levels.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (grant no. 81274048), Guangdong Provincial Department of Science and Technology (grant no. 2012B090600025), the Fundamental Research Funds for the Central Universities (grants nos. 20103060101000167 and 2012306020205), and Guangdong Pharmaceutical University.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Blagosklonny MV. Prospective treatment of age-related diseases by slowing down aging. Am J Pathol 2012;181:1142–1146 [DOI] [PubMed] [Google Scholar]
- 2.Sluder AE, Baumeister R. From genes to drugs: Target validation in Caenorhabditis elegans. Drug Discov Today Technol 2004;1:171–177 [DOI] [PubMed] [Google Scholar]
- 3.Swierczek NA, Giles AC, Rankin CH, Kerr RA. High-throughput behavioral analysis in C. elegans. Nat Methods 2011;8:592–598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shoyama T, Shimizu Y, Suda H. Decline in oxygen consumption correlates with lifespan in long-lived and short-lived mutants of Caenorhabditis elegans. Exp Gerontol 2009;44:784–791 [DOI] [PubMed] [Google Scholar]
- 5.Zhang H, Pan N, Xiong S, Zou S, Li H, Xiao L, Cao Z, Tunnacliffe A, Huang Z. Inhibition of polyglutamine-mediated proteotoxicity by Astragalus membranaceus polysaccharide through the DAF-16/FOXO transcription factor in Caenorhabditis elegans. Biochem J 2012;441:417–424 [DOI] [PubMed] [Google Scholar]
- 6.Yang X, Zhang P, Wu J, Xiong S, Jin N, Huang Z. The neuroprotective and lifespan-extension activities of Damnacanthus officinarum extracts in Caenorhabditis elegans. J Ethnopharmacol 2012;141:41–47 [DOI] [PubMed] [Google Scholar]
- 7.Li H, Xu J, Liu Y, Ai S, Qin F, Li Z, Zhang H, Huang Z. Antioxidant and moisture-retention activities of the polysaccharide from Nostoc commune. Carbohydr Polym 2011;83:1821–1827 [Google Scholar]
- 8.Gidalevitz T, Ben-Zvi A, Ho KH, Brignull HR, Morimoto RI. Progressive disruption of cellular protein folding in models of polyglutamine diseases. Science 2006;311:1471–1474 [DOI] [PubMed] [Google Scholar]
- 9.Liu J, Zhang B, Lei H, Feng Z, Liu J, Hsu AL, Xu XZ. Functional aging in the nervous system contributes to age-dependent motor activity decline in C. elegans. Cell Metab 2013;18:392–402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dosanjh LE, Brown MK, Rao G, Link CD, Luo Y. Behavioral phenotyping of a transgenic Caenorhabditis elegans expressing neuronal amyloid-beta. J Alzheimers Dis 2010;19:681–690 [DOI] [PubMed] [Google Scholar]
- 11.Xin L, Yamujala R, Wang Y, Wang H, Wu WH, Lawton MA, Long C, Di R. Acetylcholineestarase-inhibiting alkaloids from Lycoris radiata delay paralysis of amyloid beta-expressing transgenic C. elegans CL4176. PLoS One 2013;8:e63874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wu Y, Wu Z, Butko P, Christen Y, Lambert MP, Klein WL, Link CD, Luo Y. Amyloid-beta-induced pathological behaviors are suppressed by Ginkgo biloba extract EGb 761 and ginkgolides in transgenic Caenorhabditis elegans. J Neurosci 2006;26:13102–13113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hilliard MA, Apicella AJ, Kerr R, Suzuki H, Bazzicalupo P, Schafer WR. In vivo imaging of C. elegans ASH neurons: Cellular response and adaptation to chemical repellents. EMBO J 2005;24:63–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kunitomo H, Sato H, Iwata R, Satoh Y, Ohno H, Yamada K, Iino Y. Concentration memory-dependent synaptic plasticity of a taste circuit regulates salt concentration chemotaxis in Caenorhabditis elegans. Nat Commun 2013;4:2210. [DOI] [PubMed] [Google Scholar]
- 15.Braungart E, Gerlach M, Riederer P, Baumeister R, Hoener MC. Caenorhabditis elegans MPP+ model of Parkinson's disease for high-throughput drug screenings. Neurodegener Dis 2004;1:175–183 [DOI] [PubMed] [Google Scholar]
- 16.Ramot D, Johnson BE, Berry TL, Jr, Carnell L, Goodman MB. The Parallel Worm Tracker: A platform for measuring average speed and drug-induced paralysis in nematodes. PLoS One 2008;3:e2208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Qiao L, Luo S, Liu Y, Li X, Wang G, Huang Z. Reproductive and locomotory capacities of Caenorhabditis elegans were not affected by simulated variable gravities and spaceflight during the Shenzhou-8 mission. Astrobiology 2013;13:617–625 [DOI] [PMC free article] [PubMed] [Google Scholar]