Abstract
A reliable, cryoprotective, xeno-free medium suitable for different cell types is highly desirable in regenerative medicine. There is danger of infection or allergic reaction with the use of fetal bovine serum (FBS), making it problematic for medical applications. The aim of the present study was to develop an FBS-free cryoprotective medium for human mesenchymal stromal cells (hMSCs; primary cells) and immortalized human osteoblasts (SAOS-2 cell line). Furthermore, we endeavored to eliminate or reduce the presence of dimethyl sulfoxide (DMSO) in the medium. Sericin, a sticky protein derived from the silkworm cocoon, was investigated as a substitute for FBS and DMSO in the freezing medium. Cell viability (24 hours after thawing, both hMSC and SAOS-2) and colony-forming ability (2 weeks after thawing, only for hMSCs) were both determined. The FBS-free medium with 1% sericin in 10% DMSO was found to be a suitable freezing medium for primary hMSCs, in contrast to immortalized human osteoblasts. Surprisingly, the storage of hMSCs in a cultivation medium with only 10% DMSO also provided satisfactory results. Any drop in DMSO concentration led to significantly worse survival of cells, with little improvement in hMSC survival in the presence of sericin. Thus, sericin may substitute for FBS in the freezing medium for primary hMSCs, but cannot substitute for DMSO.
Introduction
The use of cell cultures is indispensable in various research and medical applications, and several different types of stem cells are currently employed. Due to ethical concerns associated with the use of human embryonic stem cells, adult stem cells are commonly used. Among them, human mesenchymal stromal cells (hMSCs) have a long history of successful research and clinical applications. Human mesenchymal stromal cells are progenitor cells that can differentiate into different cell types such as osteoblasts, adipocytes, or chondrocytes.1,2 Historically, hMSCs were primarily isolated from bone marrow.3 Currently, other sources of hMSCs are also available, including adipose tissue,4 dental pulp,5 and fetal sources such as umbilical cord blood, placenta, and Wharton jelly.6–8
The many natural functions of hMSCs include the regulation of hematopoiesis, tissue self-renewal, differentiation, and immunomodulation.9 Therefore, hMSCs provide a great tool for cellular therapy and regenerative medicine. Approximately 300 clinical trials utilizing hMSCs have been completed or are in progress. These clinical trials are focused on the treatment of various clinical entities such as bone and connective tissue diseases and injuries,10 cardiovascular disorders,11 and autoimmune diseases,12 including graft versus host reactions13 (http://www.clinicaltrials.gov, 9.1.2014, keyword “mesenchymal stem cells”).
Orthopedics is one possible field of application where the osteogenic potential of hMSCs may be utilized. The use of primary cells for research has its disadvantages, namely, the great inter-individual variability among different batches. To improve the reproducibility of experiments, the use of established cell lines may be advantageous. SAOS-2 is a well-established osteoblastic cell line derived from primary osteosarcoma, and is widely used as an osteoblastic model.14
The storage of frozen cells is of critical importance in the standard cell culture laboratory. To minimize the lethal effect of freezing-thawing cycles, various cryoprotectants are added to the freezing medium. The protective mechanism of cryoprotective agents involves the reduced formation of ice crystals, which have the potential to mechanically destroy cells.15 Dimethyl sulfoxide (DMSO) is the most commonly used compound; however, high concentrations of DMSO are cytotoxic to eukaryotic cells.16,17 Therefore, other cryoprotectants are sometimes added to the freezing medium,18 and the optimal composition is still a matter of debate. Fetal bovine serum (FBS) is also often used as a cryoprotectant, although its protective mechanism remains unclear. If applied in the cultivation or cryopreservation of cells used for clinical purposes, anti-FBS antibodies of unknown clinical significance may be detected in the recipient.19 Additionally, the theoretical possibility of prion disease transmission remains, although it is possible to obtain FBS from countries where it is free of bovine spongiform encephalopathy. Above all, FBS is a poorly understood substance with inherent variation between sources and batches. Therefore, an alternative to FBS is desirable and is the subject of current research, for example, a study using only a culture medium with DMSO and dextran 40 for heart valve preservation has been published.20
Sericin has the potential to be used instead of FBS in the freezing media, as reported previously for different cell types such as human adipose tissue-derived stem cells,21 myeloma cell lines, ovarian cells, fibroblasts, keratinocytes and insect cell lines,18 rat insulinoma cell lines, and mouse hybridoma cell lines.22 To the best of our knowledge, the use of sericin in the cryopreservation of bone marrow-derived hMSCs has not been reported thus far. Sericin is a sticky protein derived from the silkworm (Bombyx mori) cocoon. It is the second major protein component (besides fibroin) of silk and is extracted from the cocoon by a degumming process. Sericin is rich in the amino acid serine and is water soluble.23 Sericin has a number of interesting properties that are the subject of contemporary research, such as the promotion of cell viability and collagen production at certain sericin concentrations,24 the acceleration of cell proliferation,25 the suppression of skin tumorigenesis,26 and colon carcinogenesis27 in murine models. A great advantage of sericin is that silk has been approved as a biomaterial by the US Food and Drug Administration (FDA).28
The aim of the present study was to develop an FBS-free and DMSO-free or DMSO-low-concentration medium for the cryopreservation of primary cells (hMSCs) and a cell line of human osteoblasts (SAOS-2). Various concentrations of DMSO (0%, 1%, 5%, 10%, and 100%) in the parallel presence or absence of sericin (1% or 5%) in the freezing medium were examined. These were tested against a standard freezing medium that contained 25% FBS and 10% DMSO.
Materials and Methods
Cells and culture conditions
The human osteoblast-like cell line SAOS-2 (DSMZ, Braunschweig, Germany) was grown in McCoy's 5A medium without phenol red (PromoCell, Heidelberg, Germany) supplemented with 15% heat-inactivated FBS (PAA, Pasching, Austria), penicillin (20 U/mL; Sigma-Aldrich, St. Louis, MO, USA) and streptomycin (20 μg/mL; Sigma-Aldrich); standard culture medium for SAOS-2. The doubling time of SAOS-2 cells is 43 hours (see DSMZ catalogue). We did not study the population doubling time for our specific batches of hMSCs. However, as has been shown previously, the doubling time for cells cultured in the described conditions varies widely from 12–50 h for the first 43 doublings, and from 50–60 h after the 55th doubling.29 Human MSCs were obtained from patients undergoing diagnostic biopsies after providing informed, written consent. Bone marrow blood was aspirated from the posterior iliac crest and the mononuclear fraction was isolated by gradient centrifugation. The adherent cells were cultivated in α-MEM medium (Life Technologies, Carlsbad, CA, USA) with 10% heat inactivated FBS (PAA, Pasching, Austria), penicillin (20 U/mL; Sigma-Aldrich) and streptomycin (20 μg/mL; Sigma-Aldrich). The medium was changed twice weekly. Experiments were performed with hMSCs from five patients in passage numbers one to three. Both cell types were cultivated at 37°C and 5% CO2 atmosphere when not frozen.
Freezing media
The various types of freezing media consisted of a culture medium (CM) specific for the cells being tested (McCoy's 5A for SAOS-2 cells and α-MEM for hMSCs), 1%, 5%, 10%, or 100% DMSO (Sigma-Aldrich), 1% or 5% sericin (Wako, Osaka, Japan), and 25% FBS (PAA). The exact composition of the various freezing media and their abbreviations are shown in Table 1. As a standard freezing medium, CM with 10% DMSO and 25% FBS was used. Pure sericin was obtained from Wako (produced by Seiren Co., Tokyo, Japan). The sericin powder was diluted in phosphate-buffered saline at the stock concentration of 40%.
Table 1.
List of Tested Freezing Media for Both hMSCs and SAOS-2 Cells and Corresponding Abbreviations
| Full name of freezing medium | Abbreviation |
|---|---|
| culture medium+10% DMSO+25% FBS (standard) | CM+10D+25F |
| culture medium+10% DMSO | CM+10D |
| culture medium+10% DMSO+1% sericin | CM+10D+1S |
| culture medium+10% DMSO+5% sericin | CM+10D+5S |
| culture medium+5% DMSO | CM+5D |
| culture medium+5% DMSO+1% sericin | CM+5D+1S |
| culture medium+5% DMSO+5% sericin | CM+5D+5S |
| culture medium+1% DMSO | CM+1D |
| culture medium+1% DMSO+1% sericin | CM+1D+1S |
| culture medium+1% DMSO+5% sericin | CM+1D+5S |
| culture medium+1% sericin | CM+1S |
| culture medium+5% sericin | CM+5S |
| culture medium+25% FBS | CM+25F |
| culture medium only | CM only |
| DMSO only | DMSO only |
Cryopreservation of cells
Cells were collected in an exponential growth phase and counted using a Bürker chamber. SAOS-2 cells (1×105) or hMSCs (7×104) were centrifuged at 300 g for 7 min at room temperature (RT). The cells were washed in the appropriate culture medium without FBS and centrifuged once more. Pellets were resuspended in 0.5 mL of the corresponding freezing medium, and the cells were frozen in freezing tubes at −80°C in a CoolCell container (Biocision, Larkspur, CA, USA) at a cooling rate of 1°C/min. Frozen cells were transferred into liquid nitrogen (-196°C) and stored for 72 hours. The number of frozen cells (1×105 SAOS-2 cells and 7×104 hMSCs) was set at 100% for further calculations.
A portion of the cells prepared for freezing was plated without freezing onto 6-well tissue culture polystyrene (PS) plates (TPP, Trasadingen, Switzerland), and their viability was evaluated 24 h after plating in the same manner as frozen cells (see below). The cells processed without freezing were used as a positive control.
Thawing of cells
The thawing of cells was performed as quickly as possible (maximum of 3 min) in a 37°C water bath. After complete thawing, 1 mL of the appropriate culture medium (see above) heated to 37°C was immediately added to the cells, and these were centrifuged to remove the DMSO at 300 g for 7 min at RT. The resultant pellet was resuspended in the appropriate standard culture medium and cells were plated in 1 mL of CM onto 6-well PS plates. Additional culture medium was added to cells to obtain a total volume of 3 mL.
Assessment of cell viability
Considering evidence that cell death associated with cryopreservation occurs 6 h or more after thawing,30 we examined cell viability 24 h after thawing. Cell morphology was determined by light microscope with phase contrast. The number of living adherent cells was counted as the number of DAPI-stained (Sigma-Aldrich, 1:1000) cell nuclei. Living cells could easily be distinguished from dead cells, as live cells attached and spread on the plastic surface of the culture well, while dead cells did not attach and remained rounded. Ten immunofluorescence pictures from one well (resp. two wells of one freezing medium) for each experiment were recorded by microscope Eclipse Ti-S and DS-Qi1Mc Digital Cameras (Nikon, Tokyo, Japan) and analyzed by Cell Profiler (Broad Institute, http://www.cellprofiler.org) software. The number of living, adherent cells 24 h after plating was expressed as a percentage of frozen cells.
In order to examine the ability of hMSCs to form colonies, a colony-forming unit-fibroblast (CFU-F) assay was performed, as described previously.31 Thawed hMSCs (1.5 cells/cm2, i.e., 90 cells total) were plated onto 60 cm2 PS Petri dishes (TPP, Trasadingen, Switzerland) and cultivated for 2 weeks. The colonies formed were stained by Crystal violet (Sigma-Aldrich, 0.5% solution in methanol) and counted by naked eye. Cell clusters ≥2 mm in diameter were considered as CFU-Fs.
Statistical analyses
All data were obtained from at least three independent experiments. Each experiment was done in two biological parallels. The Wilcoxon signed-rank test was used to compare results from experimental media with results from the standard medium. Values of p<0.05 were considered statistically significant. Data are presented in box-plot graphs.
Results
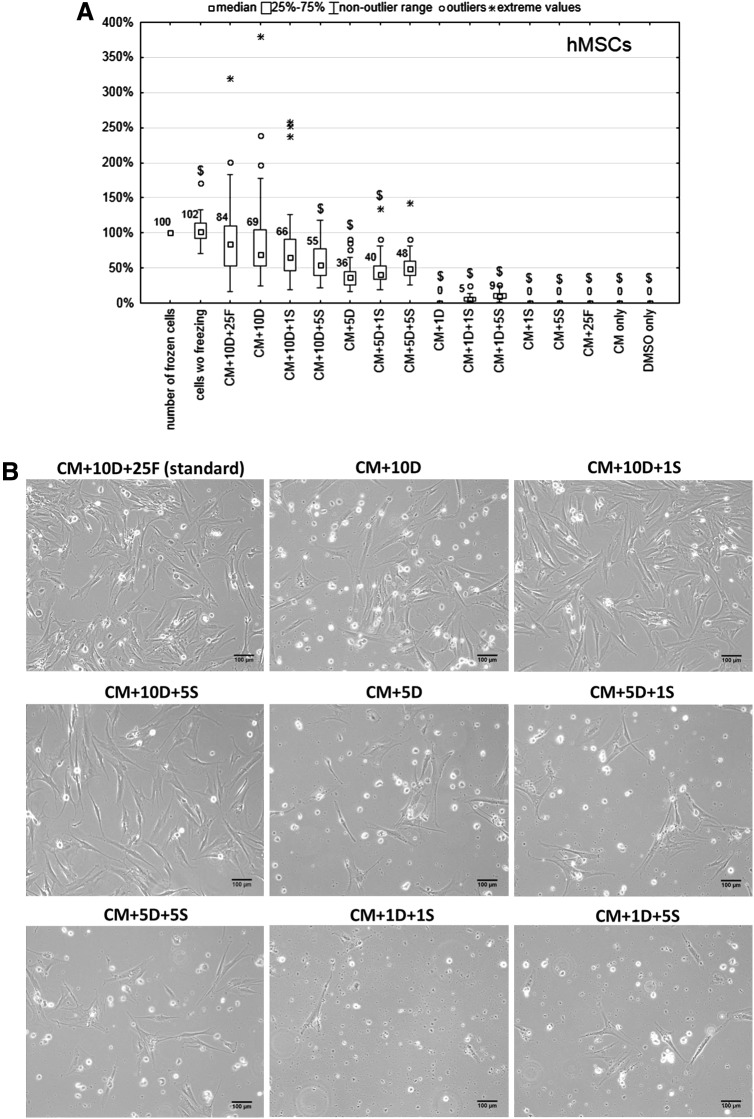
Survival of hMSCs after thawing
The influence of different freezing media on the viability and morphology of hMSCs is shown in Figure 1. The highest number of cells survived after thawing from the standard freezing medium (CM+10D+25F, 84%; Fig. 1A). Similar numbers of cells survived when frozen with 1% sericin as a substitute for FBS (CM+10D+1S, 66%; p=0.238), and when both FBS and sericin were omitted (CM+10D, 69%%; p=0.248). Significantly fewer cells survived when 5% sericin was used (55% surviving cells; p=0.012) instead of 1% sericin (CM+10D+5S).
FIG. 1.
(A) Percentage of surviving hMSCs 24 h after thawing from different freezing media. Number of frozen cells was set as 100%. CM, α-MEM; DMSO, dimethyl sulfoxide; FBS, fetal bovine serum. $p<0.05 compared to the standard freezing medium (CM+10% DMSO+25% FBS). (B) Phase contrast images of hMSCs cultivated 24 h after thawing from different freezing media.
When the concentration of DMSO in the freezing medium was decreased to 5%, no significant difference was observed between standard freezing medium and medium containing 5% DMSO and 5% sericin (84% surviving cells from CM+10D+25F vs. 48% from CM+5D+5S, with no significant difference, p=0.504). However, this statistical result could be caused by the low number of data points, so less importance is attributed to this result.
In the freezing medium containing only 1% DMSO, the presence of 1% and 5% sericin appeared to help some hMSCs survive (5% in CM+1D+1S and 9% in CM+1D+5S), in contrast to no surviving cells detected in CM+1D. When DMSO was not a component of the freezing medium (CM+1S, CM+5S, CM+25F, CM only), or when it was the only component (100% DMSO), all hMSCs were dead 24 hours after thawing.
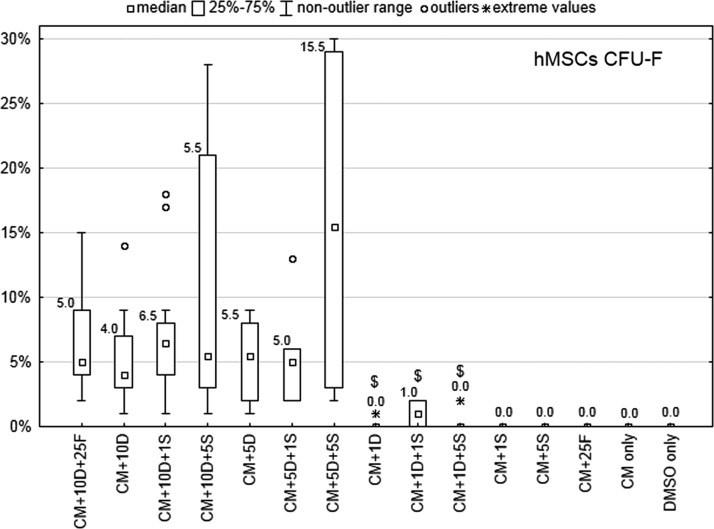
Ability of hMSCs to form colonies
In the CFU-F assay, formed colonies were counted after 2 weeks of hMSC cultivation (Fig. 2). The colony-forming ability of hMSCs seemed to be best preserved with CM+5D+5S (15.5 colonies per 90 plated cells), however this was not significantly different from cells frozen in the standard medium (5.0 colonies per 90 cells, p=0.116). Also, no significantly different results (compared to standard medium CM+10D+25F) were detected for hMSCs frozen in other media except for media with 1% DMSO, where a maximum of 1% cells survived. No colonies were observed when freezing media without DMSO or only 100% DMSO were used.
FIG. 2.
Number of hMSCs colonies formed after 2 weeks cultivation of cells thawed from different freezing media (CFU-F assay). CM, α-MEM; DMSO, dimethyl sulfoxide; FBS, fetal bovine serum. $p<0.05 compared to the standard freezing medium (CM+10% DMSO+25% FBS).
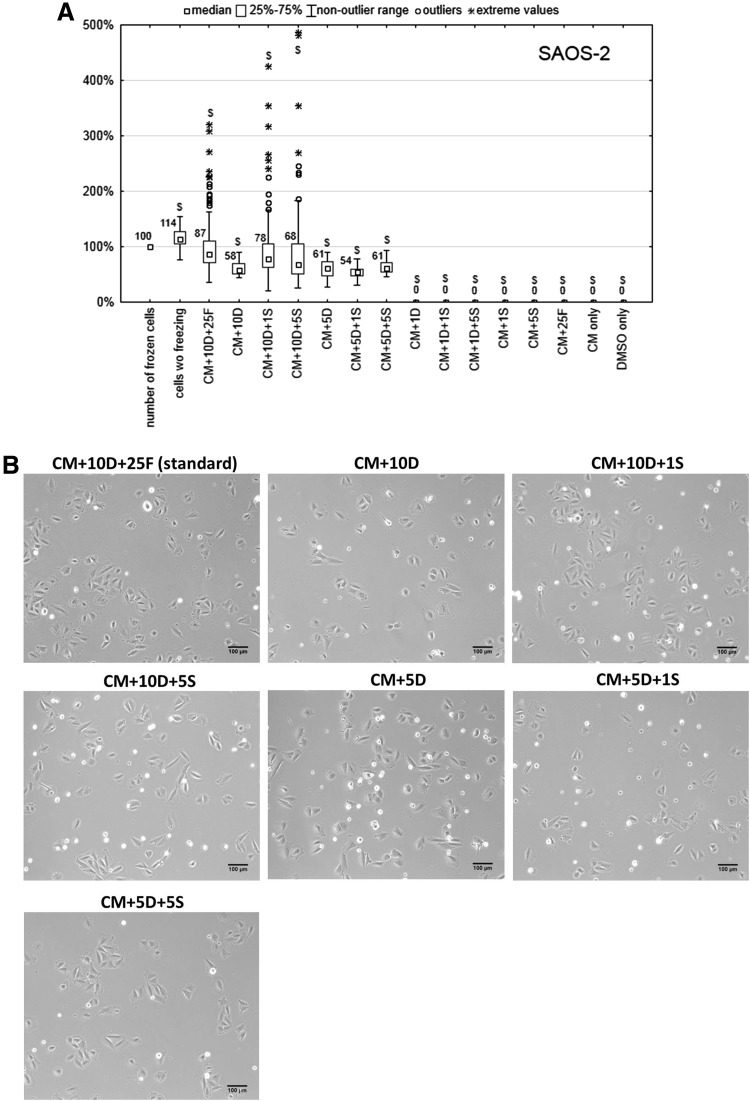
Survival of osteoblasts after thawing
The viability of osteoblasts thawed from different freezing media varied as well. Their morphology and number after 24 h of cultivation are presented in Figure 3. Eighty-seven percent of frozen osteoblasts survived 24 h after thawing from the standard freezing medium (CM+10D+25F). The next best-performing medium was CM+10D+1S (78% surviving cells), however the difference between this medium and the standard medium (CM+10D+25F) was statistically significant, p=0.009. Also other tested media using 10% DMSO without FBS (CM+10D, 58% surviving cells; CM+10D+5S, 68% surviving cells) were significantly worse (p=0.001 for both media) than the standard freezing medium (CM+10D+25F).
FIG. 3.
(A) Percentage of surviving SAOS-2 cells 24 h after thawing from different freezing media. Number of frozen cells was set as 100%. CM, McCoy's 5A medium; DMSO, dimethyl sulfoxide; FBS, fetal bovine serum; $p<0.05 compared to the standard freezing medium (CM+10% DMSO+25% FBS). (B) Phase contrast images of SAOS-2 cells cultivated 24 h after thawing from different freezing media.
The influence of various DMSO concentrations (0%, 1%, 5%, 10%, and 100%) on osteoblast freezing was investigated as well. SAOS-2 thawed from all tested media lacking DMSO (CM+1S, CM+5S, CM+25F, only CM) were all dead after 24 h of cultivation. When SAOS-2 cells were cryopreserved in media with 1% DMSO (CM+1D, CM+1D+1S, CM+1D+5S), no cells survived after thawing regardless of sericin concentration. Freezing cells in 100% DMSO was also lethal. In freezing media containing 5% DMSO (CM+5D, CM+5D+1S, CM+5D+5S), the number of surviving cells was approximately 60%, which was lower (p=0.001 for all media) than survival after thawing from the standard medium.
Discussion
In the present study, we performed a series of experiments to determine whether sericin can serve as a substitute for FBS in freezing media for primary hMSCs and the immortalized osteoblastic cell line SAOS-2. We also investigated the effects of various DMSO concentrations on these cells and explored whether sericin can substitute for DMSO. In examining the role of sericin substituted for DMSO, our findings with MSCs are in agreement with Baust et al.,32 who determined that the best DMSO concentration for the cryopreservation of other human cells—fibroblasts, keratinocytes, hepatic and renal cells, is 10%. We confirm that a 10% DMSO concentration is optimal both for the freezing of primary hMSCs and SAOS-2 cell line, and that sericin cannot compensate for a 5% decrease of DMSO. Some encouraging results were observed with CM+5D+5S regarding both hMSCs viability and CFU-F assay. However, due to the small number of experiments and great variability between acquired data (different patients), lower importance should be attributed to this result. Nevertheless, it cannot be excluded that a decrease in DMSO preferentially kills more differentiated cells with less self-renewing ability (SAOS-2), while the less mature, proliferating cells may be salvaged by sericin (hMSCs).
When media with no DMSO were used (CM, CM+1S, CM+5S), all hMSC and SAOS-2 cells were dead 24 h after thawing. When the DMSO concentration was 1%, sericin (1% and 5%) potentiated the survival of primary hMSCs; however, 5% and 9% surviving cells cannot be considered a satisfactory result. Additionally, no SAOS-2 cells survived when the DMSO concentration was decreased to 1%. As the aim of this part of the study was to investigate sericin as a substitute for DMSO, we did not explore DMSO concentrations higher than 10%.
While sericin did not prove a suitable substitute for DMSO in freezing media, it could substitute for FBS during the freezing of hMSCs, in contrast to SAOS-2 cells. No significant difference in hMSC viability was observed between the standard freezing medium (CM+10D+25F, 84% surviving cells) and CM+10D+1S (66% surviving cells). Similar results were obtained using 10% DMSO only (CM+10D, 69% surviving cells). Most studies to date have not found sericin superior to FBS in cryopreservative experiments.21,22,33 One exception where a freezing medium containing sericin performed better than a medium with FBS was a study using Chinese-hamster ovary cells frozen in PBS, supplemented with 1% sericin, 0.5% maltose, 0.3% proline, 0.3% glutamine, and 10% DMSO.18 Thus, our results are in agreement with the majority of data published thus far. Conversely, we observed that freezing hMSCs in 10% DMSO only is not significantly worse than a standard freezing medium containing FBS. This finding has not yet been published for these cells, but may be of interest for future research and clinical applications. Surprisingly, we found that sericin is not a suitable substitute for FBS in freezing osteoblastic cell lines.
There are some limitations to the present study. The number of experiments was limited, which may explain why some results did not reach statistical significance. This is best illustrated by results related to the colony-forming capacity of hMSCs (CFU-F), which essentially paralleled the results of cell viability after 24 hours (Figs. 1A and 2). The number of experiments was limited primarily by the requirement of sufficiently large batches of hMSCs necessary to run all the investigations in parallel, which was not always achieved after 1–2 passages (relatively young cells). A further limitation is that relatively low concentrations of frozen cells were used (1×105 for SAOS-2 cells and 7×104 for hMSCs). This was also due to batch sizes of hMSCs and the necessity to run all experiments in parallel. However, even considering these limitations, we can conclude that 1% sericin could substitute for 25% FBS in the freezing solution for primary hMSCs in contrast to SAOS-2 osteoblastic cells. Moreover, hMSCs could be cryopreserved in a growth medium containing only 10% DMSO with satisfactory results.
Conclusion
In the present study, we performed a systematic evaluation of sericin as a replacement for FBS or DMSO in the freezing of hMSCs and SAOS-2. It can be concluded that 1% sericin may substitute for FBS in the freezing medium of hMSCs when 10% DMSO is preserved. Moreover, these stem cells could be frozen in 10% DMSO as the sole component of a normal culture medium. Conversely, sericin cannot serve as a substitute for FBS in the freezing medium of the osteoblastic cell line SAOS-2, and furthermore the medium alone with 10% DMSO is not sufficient for this cell line. Thus, our results also show that different freezing formulas should be evaluated for different cell types to find the most satisfactory results.
Acknowledgments
Special thanks to Blanka Bilkova for technical assistance.
Author Disclosure Statement
The authors have no competing financial interests to report.
This work was supported by: Charles University in Prague, First Faculty of Medicine—projects PRVOUK-P24/LF1/3 and PRVOUK-P27/LF1/1, Faculty of Medicine in Pilsen—PRVOUK-P36/LFP and Faculty of Science—project GAUK no. 501212 and grant SVV-2013-267205; by the grant NT 13531 from Ministry of Public Health, Czech Republic and by the project ED2.1.00/03.0076 from European Regional Development Fund.
References
- 1.Pittenger MF, Mackay AM, Beck SC, et al. . Multilineage potential of adult human mesenchymal stem cells. Science 1999;284:143–147 [DOI] [PubMed] [Google Scholar]
- 2.Pytlik R, Stehlik D, Soukup T, et al. . The cultivation of human multipotent mesenchymal stromal cells in clinical grade medium for bone tissue engineering. Biomaterials 2009;30:3415–3427 [DOI] [PubMed] [Google Scholar]
- 3.Jiang YH, Jahagirdar BN, Reinhardt RL, et al. . Pluripotency of mesenchymal stem cells derived from adult marrow. Nature 2002;418:41–49 [DOI] [PubMed] [Google Scholar]
- 4.Zuk PA, Zhu M, Ashjian P, et al. . Human adipose tissue is a source of multipotent stem cells. Mol Biol Cell 2002;13:4279–4295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gronthos S, Brahim J, Li W, et al. . Stem cell properties of human dental pulp stem cells. J Dental Res 2002;81:531–535 [DOI] [PubMed] [Google Scholar]
- 6.Lee OK, Kuo TK, Chen WM, et al. . Isolation of multipotent mesenchymal stem cells from umbilical cord blood. Blood 2004;103:1669–1675 [DOI] [PubMed] [Google Scholar]
- 7.Abumaree MH, Al Jumah MA, Kalionis B, et al. . Phenotypic and functional characterization of mesenchymal stem cells from chorionic villi of human term placenta. Stem Cell Rev Rep 2013;9:16–31 [DOI] [PubMed] [Google Scholar]
- 8.Montanucci P, Basta G, Pescara T, et al. . New simple and rapid method for purification of mesenchymal stem cells from the human umbilical cord Wharton jelly. Tissue Eng Pt A 2011;17:2651–2661 [DOI] [PubMed] [Google Scholar]
- 9.Si YL, Zhao YL, Hao HJ, et al. . MSCs: Biological characteristics, clinical applications and their outstanding concerns. Age Res Rev 2011;10:93–103 [DOI] [PubMed] [Google Scholar]
- 10.Kon E, Filardo G, Roffi A, et al. . Bone regeneration with mesenchymal stem cells. Clin Cases Mineral Bone Metab 2012;9:24–27 [PMC free article] [PubMed] [Google Scholar]
- 11.Elnakish MT, Hassan F, Dakhlallah D, et al. . Mesenchymal stem cells for cardiac regeneration: Translation to bedside reality. Stem Cells Intl 2012;2012:646038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Figueroa FE, Carrion F, Villanueva S, et al. . Mesenchymal stem cell treatment for autoimmune diseases: A critical review. Biol Res 2012;45:269–277 [DOI] [PubMed] [Google Scholar]
- 13.Ball LM, Bernardo ME, Roelofs H, et al. . Multiple infusions of mesenchymal stromal cells induce sustained remission in children with steroid-refractory, grade III-IV acute graft-versus-host disease. Br J Haematol 2013;163:501–509 [DOI] [PubMed] [Google Scholar]
- 14.Rodan SB, Imai Y, Thiede MA, et al. . Characterization of a human osteosarcoma cell-line (Saos-2) with osteoblastic properties. Cancer Res 1987;47:4961–4966 [PubMed] [Google Scholar]
- 15.Baust JG, Gao DY, Baust JM. Cryopreservation: An emerging paradigm change. Organogenesis. 2009;5:90–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Qi WD, Ding DL, Salvi RJ. Cytotoxic effects of dimethyl sulphoxide (DMSO) on cochlear organotypic cultures. Hearing Res 2008;236:52–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang X, Hua TC, Sun DW, et al. . Cryopreservation of tissue-engineered dermal replacement in Me2SO: Toxicity study and effects of concentration and cooling rates on cell viability. Cryobiology 2007;55:60–65 [DOI] [PubMed] [Google Scholar]
- 18.Sasaki M, Kato Y, Yamada H, et al. . Development of a novel serum-free freezing medium for mammalian cells using the silk protein sericin. Biotechnol Appl Bioc 2005;42:183–188 [DOI] [PubMed] [Google Scholar]
- 19.Sundin M, Ringden O, Sundberg B, et al. . No alloantibodies against mesenchymal stromal cells, but presence of anti-fetal calf serum antibodies, after transplantation in allogeneic hematopoietic stem cell recipients. Haematologica 2007;92:1208–1215 [DOI] [PubMed] [Google Scholar]
- 20.Brockbank KG, Heacox AE, Schenke-Layland K. Guidance for removal of fetal bovine serum from cryopreserved heart valve processing. Cells Tissues Organs 2011;193:264–273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Miyamoto Y, Oishi K, Yukawa H, et al. . Cryopreservation of human adipose tissue-derived stem/progenitor cells using the silk protein sericin. Cell Transplant 2012;21:617–622 [DOI] [PubMed] [Google Scholar]
- 22.Toyosawa T, Oumi Y, Ogawa A, et al. . Novel serum-free cryopreservation of mammalian cells using sericin. Anima Cell Tech 2009;15:41–45 [Google Scholar]
- 23.Aramwit P, Siritientong T, Srichana T. Potential applications of silk sericin, a natural protein from textile industry by-products. Waste Manage Res 2012;30:217–224 [DOI] [PubMed] [Google Scholar]
- 24.Aramwit P, Kanokpanont S, Nakpheng T, et al. . The effect of sericin from various extraction methods on cell viability and collagen production. Int J Mol Sci 2010;11:2200–2211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Terada S, Takada N, Itoh K, et al. . Silk protein sericin improves mammalian cell culture. Cell Technol Cell Products 2007:397–401 [Google Scholar]
- 26.Zhaorigetu S, Yanaka N, Sasaki M, et al. . Silk protein, sericin, suppresses DMBA-TPA-induced mouse skin tumorigenesis by reducing oxidative stress, inflammatory responses and endogenous tumor promoter TNF-alpha. Oncol Rep 2003;10:537–543 [PubMed] [Google Scholar]
- 27.Sasaki M, Kato N, Watanabe H, et al. . Silk protein, sericin, suppresses colon carcinogenesis induced by 1,2-dimethylhydrazine in mice. Oncol Rep 2000;7:1049–1052 [DOI] [PubMed] [Google Scholar]
- 28.Cao Y, Wang B. Biodegradation of silk biomaterials. Int J Mol Sci 2009;10:1514–1524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Soukup T, Mokry J, Karbanova J, et al. . Mesenchymal stem cells isolated from the human bone marrow: Cultivation, phenotypic analysis and changes in proliferation kinetics. Acta Med 2006;49:27–33 [PubMed] [Google Scholar]
- 30.Baust JM. Molecular mechanisms of cellular demise associated with cryopreservation failure. Cell Preserv Technol 2002;1:17–31 [Google Scholar]
- 31.Colter DC, Sekiya I, Prockop DJ. Identification of a subpopulation of rapidly self-renewing and multipotential adult stem cells in colonies of human marrow stromal cells. Proc Natl Acad Sci USA 2001;98:7841–7845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Baust JM, Van Buskirk R, Baust JG. Modulation of the cryopreservation cap: Elevated survival with reduced dimethyl sulfoxide concentration. Cryobiology 2002;45:97–108 [DOI] [PubMed] [Google Scholar]
- 33.Ohnishi K, Murakami M, Morikawa M, et al. . Effect of the silk protein sericin on cryopreserved rat islets. J Hepato-Bil-Pan Sci 2012;19:354–360 [DOI] [PubMed] [Google Scholar]