Abstract
Purpose.
To determine if nanoparticles delivering plasmids expressing Flt23k (an anti-VEGF intraceptor) can enhance murine cornea transplant survival and whether their effect is synergistic with steroid therapy.
Methods.
Biodegradable PLGA Flt23k loaded or blank nanoparticles were prepared using the emulsion solvent evaporation method. Graft survival, corneal neovascularization, and corneal lymphangiogenesis were compared among the Flt23k nanoparticles, blank nanoparticles, triamcinolone acetonide, and PBS groups following subconjunctival injection in mice that underwent penetrating keratoplasty. Graft survival, corneal neovascularization, and corneal lymphangiogenesis in a group treated with both nanoparticles and steroid therapy were also analyzed.
Results.
The Flt23k nanoparticle group showed less neovascularization, lymphangiogenesis, and graft failure compared with the PBS control group (P < 0.01). The 2-month graft survival rate was 20% in the Flt23k nanoparticle group with no grafts surviving in the PBS group. When the Flt23k nanoparticle was combined with steroid therapy, a significant increase in graft survival was seen compared with both steroid treatment alone (P < 0.05) and steroid combined with blank nanoparticle treatment (P < 0.05). The 2-month graft survival rate was 91.6% in the combination group compared with 47.6% in the triamcinolone-only group and 42.4% in the triamcinolone plus blank nanoparticle group.
Conclusions.
Anti-VEGF nanoparticles (Flt23k) have a significant effect on decreasing neovascularization and lymphangiogenesis, resulting in increased graft survival in penetrating keratoplasty. This beneficial effect is synergistically enhanced with steroid treatment.
Anti-VEGF nanoparticles (Flt23k) decreased neovascularization and lymphangiogenesis, resulting in increased graft survival in penetrating keratoplasty. This beneficial effect is synergistically enhanced with steroid treatment.
Introduction
Penetrating keratoplasty (PK) is one of the most common and successful organ transplant procedures worldwide, with survival rates as high as 95% in low-risk patient populations. Unfortunately, even with current immunosuppression techniques, rejection rates in high-risk patients can exceed 70%,1,2 with up to 28% of grafts requiring repeat transplantation.3 Graft rejection and repeat transplantation along with a growing demand has led to a global shortage of corneas available for transplant. For example, although there were approximately 2700 transplants in the United Kingdom (2008–2009), there is an estimated shortage of 500 corneas each year [www.uktransplant.org]. This shortage is even more pronounced in larger countries. NetraDaan.com, an organization focused on encouraging eye donations in India, estimated that although 35,000 corneas are collected each year, 150,000 would be required to meet the annual demand [www.Netradaan.com]. One potential way to alleviate this shortage is to improve the success rate of penetrating keratoplasty (PKP) through decreased transplant rejection.
Published risk factors for corneal graft rejection include corneal vascularization, regrafting, anterior synechiae, increased intraocular pressure, trauma, additional surgical procedures, herpetic infection, and active inflammation.3 Corneal vascularization is a recognized key risk factor for immunologic rejection4 and one of the most important causes of corneal graft failure.5 In addition, studies have shown that disrupting the lymph system may improve corneal graft survival by decreasing the immune response.6 Thus, inhibiting corneal angiogenesis and lymphangiogenesis has the potential to decrease graft failure rates.
Corneal angiogenesis and lymphangiogenesis are driven by vascular endothelial growth factor (VEGF), which binds and activates the tyrosine kinase receptors VEGFR1 (Flt-1) and VEGFR2 (KDR/Flk-1). Angiogenesis provides a potential route of entry for immune effector cells such as CD4+ alloreactive T lymphocytes and memory T lymphocytes to the corneal graft (efferent pathway).6 Conversely, lymphangiogenesis enables antigen presenting cells and antigenic materials to travel from the graft to the regional lymph nodes, thus inducing alloimmunization and subsequent graft rejection (afferent pathway).6 As a result, decreasing angiogenesis and lymphangiogenesis by inhibiting VEGF can potentially improve corneal graft survival through a diminished immune response.
Among the potential VEGF inhibitory strategies, anti-VEGF expressing nanoparticles are a promising approach capable of targeting VEGF intracellularly.2,7–9 The anti-VEGF intraceptor, Flt23k, is a recombinant construct of VEGF binding domains 2 and 3 of VEGFR-1 (vascular endothelial growth factor receptor-1, Flt-1) coupled with the endoplasmic reticulum (ER) retention signal sequence lysine-aspartic acid-glutamic acid-leucine (KDEL).8–10 KDEL is a peptide retention signal that binds ER retention receptors and prevents secretion of endogenous ER proteins coupled to KDEL. Flt23k intraceptors bind VEGF intracellularly with high affinity and sequester it within the ER, thereby inhibiting VEGF secretion and the VEGF autocrine loop.8–15
In a previous in vitro transfection study, the authors found that the Flt23k intraceptor could sequester VEGF and reduce neovascularization.2,7 In the current study, the authors utilized a murine penetrating keratoplasty model to investigate the effects of nanoparticles that delivered plasmids expressing the anti-VEGF intraceptor Flt23k on neovascularization and lymphangiogenesis in corneal grafts and the effect on overall corneal graft survival. The authors also investigated the synergistic effect of the Flt23k nanoparticle combined with triamcinolone acetonide, a long-acting glucocorticoid, which has been widely used to decrease graft rejection.
Materials and Methods
The experiments were performed in accordance with the regulations of ARVO and were approved by the Institutional Animal Care and Use Committee of the University of Utah.
Nanoparticles were conjugated to the RGD peptide (Gly-Arg-Gly-Asp-Ser-Pro-Lys) for surface functionalization and homing to αvβ3 integrin.8,9,16 Biodegradable PLGA (poly-L-lactide-co-glycolide) nanoparticles were used in this study as carriers of the Flt23k plasmid.8,10 RGD-conjugated Flt 23k plasmid loaded nanoparticles (Flt23k) and RGD-conjugated pCMV empty nanoparticles (blank nanoparticle) were used for the subconjunctival injections. The RGD peptide (Gly-Arg-Gly-Asp-Ser-Pro-Lys) binds integrin receptor αvβ3 with high affinity and allows neovascular targeting of Flt23k nanoparticles and blank nanoparticles.16 Particle size of the Flt nanoparticle and the blank nanoparticle was 270.2 nm and 221.4 nm, respectively.
Development of Nanoparticles
Nanoparticles were prepared by the conventional water/oil/water double-emulsion solvent evaporation technique. Poly (L-lactide-co-glycolide) (PLGA) Resomer 503H (50:50; intravenous 0.32–0.44 dL/g; Boehringer Ingelheim Chemicals, Petersburg, VA), Nile red (Sigma-Aldrich, St. Louis, MO), and the Flt23k plasmid were used in the preparation of nanoparticles. PLGA polymer and Nile red were dissolved in dichloromethane and maintained in an ice bath. Aqueous plasmid solution was mixed with the polymer solution by sonication at 10 W for 1 minute to form a w/o emulsion. The primary w/o emulsion was transferred to a 2% aqueous poly vinyl alcohol solution maintained in an ice bath and sonicated for 3 minutes at a power input of 30 W to form w/o/w emulsion. The secondary emulsion was stirred at room temperature for 3 hours. The nanoparticle emulsion was further subjected to rotary evaporation at 40°C for 2 hours. The nanoparticles formed were centrifuged at 20,000g for 20 minutes. The supernatant was discarded and pellet of nanoparticles was redispersed in 25 mL of distilled water. The nanoparticles were again centrifuged for 20 minutes at 20,000g. Washing with distilled water was repeated once more. The final nanoparticle pellet attained was redispersed in 10 mL of distilled water, and the dispersion was frozen at −80°C. The frozen dispersion was subjected to lyophilization (Labconco lyophilizer; Labconco Corporation, Kansas City, MO). Blank nanoparticles were loaded with the plasmid devoid of the Flt23K gene using the procedures described above.
Nanoparticle drug content and particle size were characterized after lyophilization. Plasmid content of the nanoparticles was measured by dissolving 2 mg of nanoparticles in 1 mL dichloromethane. This dispersion was vortexed for 1 hour. After 1 hour, the dichloromethane was evaporated under nitrogen. One milliliter of water was added to the above film formed and vortexed for 2 hours. Plasmid content was measured by checking the absorbance at 260 nm. An absorbance of 1 at 260 nm is equivalent to 50 μg/mL of plasmid. This factor was used to calculate the concentration of plasmid in the nanoparticles. Particle size was measured using Malvern Nanosizer (Malvern Inc., Worcestershire, UK).
To prepare the RGD.Flt23k.NR nanoparticles, the nanoparticles were weighed and dispersed in 3-(N-Morpholino)-propane sulfonic acid buffer (MOPS buffer; Sigma-Aldrich) by vortexing for 15 minutes. Ethyl-3-(3-dimethylaminopropyl) carbodiimide HCl (EDAC) (Sigma-Aldrich) was added to the above dispersion and vortexed for 2 hours. The RGD peptide (Catalogue # G1269; Sigma-Aldrich) was dissolved in MOPS buffer and added dropwise to the above nanoparticle dispersion with aggravation. To allow adequate conjugation, the dispersion was vortexed for 12 hours. The dispersion was then centrifuged at 30,000g for 15 minutes at 4°C to generate a pellet of nanoparticles. The pellet was resuspended in water and lyophilized.
Assessment of Corneal Delivery of Nanoparticles
RGD-conjugated pCMV empty nanoparticles and Flt23k-loaded nanoparticles were injected in the subconjunctival space of 16 BALB/c mice eyes. For preparation of the flat mount, eyes were fixed in 4% paraformaldehyde for 1 hour immediately after enucleation. After washing, the cornea and conjunctiva were flat mounted using gel mount (Vectashield mounting medium with DAPI; Vector Laboratories Inc., Burlingame, CA) for Confocal Imaging (Olympus FluoView FV1000 confocal microscope; Olympus, Tokyo, Japan) (×100 and ×1000 objectives).
Corneal Transplantation
Penetrating corneal transplantation was done using male mice (8 to 12 weeks old) of the BALB/c strain as graft recipients and mice of the C57BL/6 strain as graft donors (The Jackson Laboratory, JAX Mice and Services, Bar Harbor, ME). Mice were anesthetized by intramuscular injection with ketamine (100 mg/kg) and xylazine (10 mg/kg). The donor cornea was marked with a 2 mm trephine, the anterior chamber was penetrated with a knife (ClearCut; Alcon, Inc., Fort Worth, TX) and the cornea was cut with microsurgical scissors (Vannas; Katena Products, Inc., Denville, NJ) and then placed in Balanced Salt Solution (BSS; Alcon Laboratories, Inc., Fort Worth, IN). The recipient mouse was anesthetized as described previously. One percent tropicamide ophthalmic solution was used to dilate the pupil, and 0.5% proparacaine ophthalmic solution was used to anesthetize the cornea. The recipient right cornea was marked with 1.5 mm trephine and removed by the same method as the donor cornea. Viscoelastic material (Healon; 1% sodium hyaluronate, Abbott Medical Optics, Inc., IL) was used during recipient cornea dissection. The donor graft was sutured into the recipient bed using interrupted sutures (11-0 nylon, CS160-6; Ethicon, Inc., Cornelia, GA). After the transplantation, the eye was covered with 0.5% erythromycin ophthalmic ointment and the lid was sutured with 8-0 coated vicryl (BV130-5; Ethicon, Inc.). Sutures remained in the recipient's eye for 1 week after transplantation.
Subconjunctival Injection of Anti-Angiogenic Agents
Nanoparticles were dispersed in MES buffer (10 μg/μL). Ten microliters of suspension of RGD-conjugated, Flt23k-loaded, nanopaticle (0.1 μg/μL of plasmid concentration), and RGD-conjugated, blank nanoparticle (0.1 μg/μL of plasmid concentration) of each group were injected in the subconjunctiva on the day of transplantation and at 4 weeks postoperatively. For the comparison groups, 10 μL of triamcinolone acetonide (Kenalog-40, 40 μg/μL; Bristol-Myers Squibb Company, New York, NY) and PBS (15 μL) were injected in the subconjunctival space on the day of transplantation and postoperatively at 1, 2, 3, and 4 weeks. To evaluate the possible synergistic effect of nanoparticles, the authors tested triamcinolone plus Flt23k nanoparticle group and triamcinolone plus blank nanoparticle group. In the triamcinolone plus Flt23k nanoparticle combination group, subconjunctival injection (10 μL) of triamcinolone was performed on the day of keratoplasty and postoperatively at 1, 2, 3, and 4 weeks, and Flt23k nanoparticles (10 μL) were added on the day of keratoplasty and postoperatively at 4 weeks using a gas-tight syringe (Hamilton Company, Reno, NV). In the combined triamcinolone plus blank nanoparticle group, subconjunctival injection of triamcinolone (10 μL) was performed on the day of keratoplasty and postoperatively at 1, 2, 3, and 4 weeks, and blank nanoparticles (10 μL) were added on the day of keratoplasty and postoperatively at 4 weeks.
Clinical Evaluation of Rejection
The mice were examined and photographed weekly through postoperative week 8 under general anesthesia with intramuscular tribromoethanol (Avertin; made in our laboratory using 2,2,2-tribromoethanol and 2-methyl-2-butanol purchased from Sigma-Aldrich) using an operating microscope (SZX7; Olympus, Tokyo, Japan) with an attached camera (U-TVO.5XC-3, Japan). We evaluated clinical graft rejection according to grading system for orthotopic corneal graft opacity and neovascularization described by Sonoda and Streilein.17 The opacity grade (0–5) was used as follows17,18: 0-clear; 1-minimal, superficial (nonstromal) opacity, pupil margin and iris vessel readily visible through the cornea; 2-minimal, deep (stromal) opacity, pupil margin and iris vessel visible; 3-moderate stromal opacity, only pupil margin visible; 4-intense stromal opacity, only a portion of pupil margin visible; 5-maximum stromal opacity, anterior chamber not visible. Opacity grades 3 and above were considered a graft rejection. Mice with complications such as severe inflammation and hemorrhage were excluded from the study.
Analysis of Angiogenesis and Lymphangiogenesis
After planned injections and observation periods, mice eyes were harvested and the corneas were trimmed of any remaining limbus and iris. Immunohistochemical staining for vascular and lymphatic endothelial cells were performed on corneal flat mounts. Fresh corneas were dissected, rinsed in PBS for 30 minutes and fixed in 100% acetone (Sigma-Aldrich) for 20 minutes. After washing in PBST (0.1% Tween 20/PBS), nonspecific binding was blocked with 3% BSA/PBS for 3 nights at 4°C. Incubation with FITC-conjugated monoclonal antimouse CD31 antibody (558,738; BD Pharmingen, San Jose, CA) at a concentration of 1:500 and rabbit anti-LYVE-1(ab 14,917; Abcam Inc., Cambridge, MA) at a concentration 1:200 in 3% BSA/PBS at 4°C overnight was followed by 1:1000 goat antirabbit antibody-Alexa Fluor546 (A11071; Invitrogen Corporation, Carlsbad, CA) for 1 hour with subsequent washes in PBST at room temperature. Corneas were mounted with the antifading aqueous mounting medium (Gelmount; Biomeda, San Francisco, CA). After immunochemical staining for vascular endothelial cells and flat mounting of cornea, images of the corneal vasculature was captured using a camera attached to a fluorescent microscope (Carl Zeiss MicroImaging, Inc., Thornwood, NY). Neovascularization and lymphangiogenesis were quantified by setting a threshold level of fluorescence above which only vessels were captured and processed using Image J (National Institutes of Health, Bethesda, MD). The total corneal area was outlined using the innermost vessel of the limbal (rim of the cornea) arcade as the border and the grafted cornea area was outlined using the scar line between recipient and donor graft. A threshold level was set to divide the corneal image into neovascularized and non-neovascularized areas. The authors set and recorded the threshold of each image to minimize the margin of error. The threshold level to calculate neovascularization (total and graft) and lymphangiogenesis (total and graft) did not show a significant difference between the groups (P > 0.05). The total area of neovascularization and lymphangiogenesis was normalized to the total corneal area using pixel area. The graft neovascularization was normalized to the graft area.
Comparison of Each Group and Combination Group
The four groups analyzed included the Flt23k nanoparticle group, blank nanoparticle group, steroid group (triamcinolone acetonide), and PBS group. Graft survival, weekly graft opacification, neovascularization (NV) (total corneal NV = total NV area/total corneal area), graft NV (graft NV area/graft area), lymphangiogenesis (LY) (total corneal LY = total LY area/total corneal area) and only graft LY (graft LY area/graft area) were compared. In addition, the previously mentioned outcomes for the steroid-only group (triamcinolone injection) and combination groups (Flt23k nanoparticle plus triamcinolone group and blank nanoparticle plus triamcinolone group) were compared.
Statistical Analysis
Statistical analysis was performed using SPSS 11.5 (SPSS Inc., Chicago, IL). Postoperative survival of the allografts was analyzed using Kaplan-Meier survival curves and log rank test. Neovascularization and lymphangiogenesis of each group were compared with the corresponding control group using an unpaired two-tailed t-test and the Mann-Whitney U test.
Results
Corneal Delivery of Nanoparticles
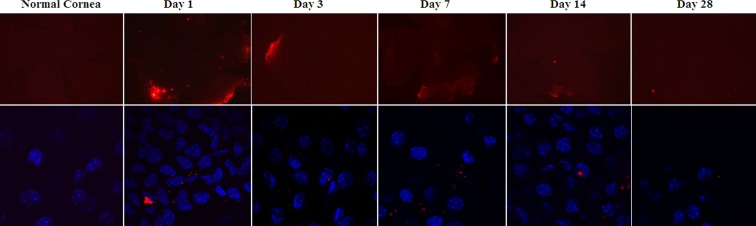
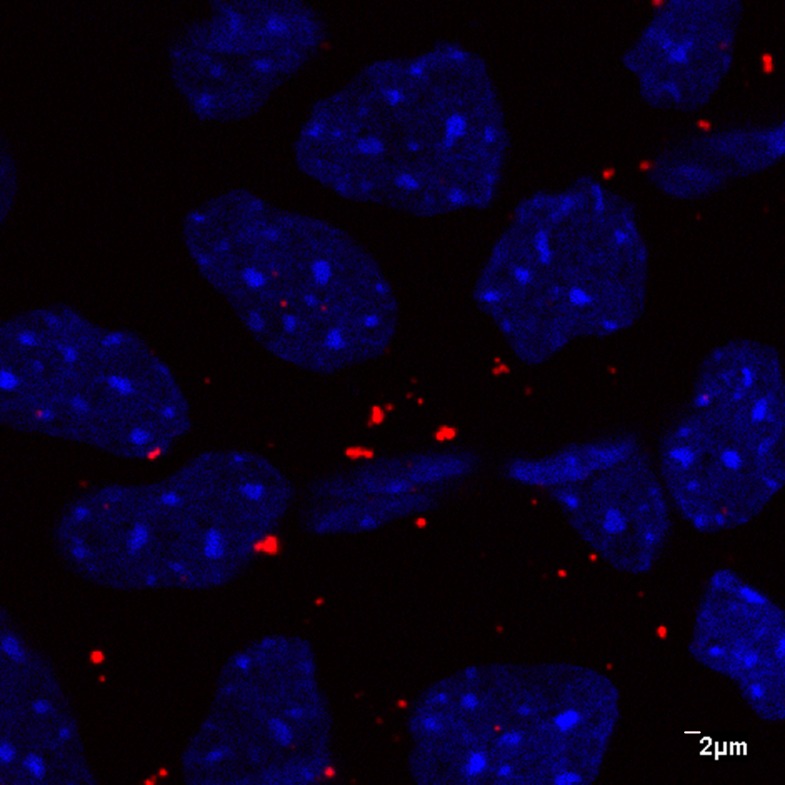
The size of the nanoparticles used in this study was 270.2 nm for the Flt nanoparticle and 221.4 nm for the blank nanoparticle. Figure 1 shows the presence of nanoparticles in the cornea as observed by fluorescence microscopy and confocal microscopy. The nanoparticles were present in the cornea and conjunctiva through week 4 after injection. It appears that there are some aggregated forms of the nanoparticles as well on the tissues. Figure 2 shows the expression of nanoparticles in the cornea as observed with a high magnification (×1000) lens and confocal microscopy. It appears that nanoparticles were present in the extracellular space as well as the intranuclear space.
Figure 1.
The expression of nanoparticles in the cornea as observed by fluorescence microscopy (upper row—objective lens ×2.5) and confocal microscope (lower row—objective lens ×100).
Figure 2.
The presence of nanoparticles in corneal tissue as observed by confocal microscope with high magnification (objective lens ×1000).
Single Treatment Comparisons
Graft Survival
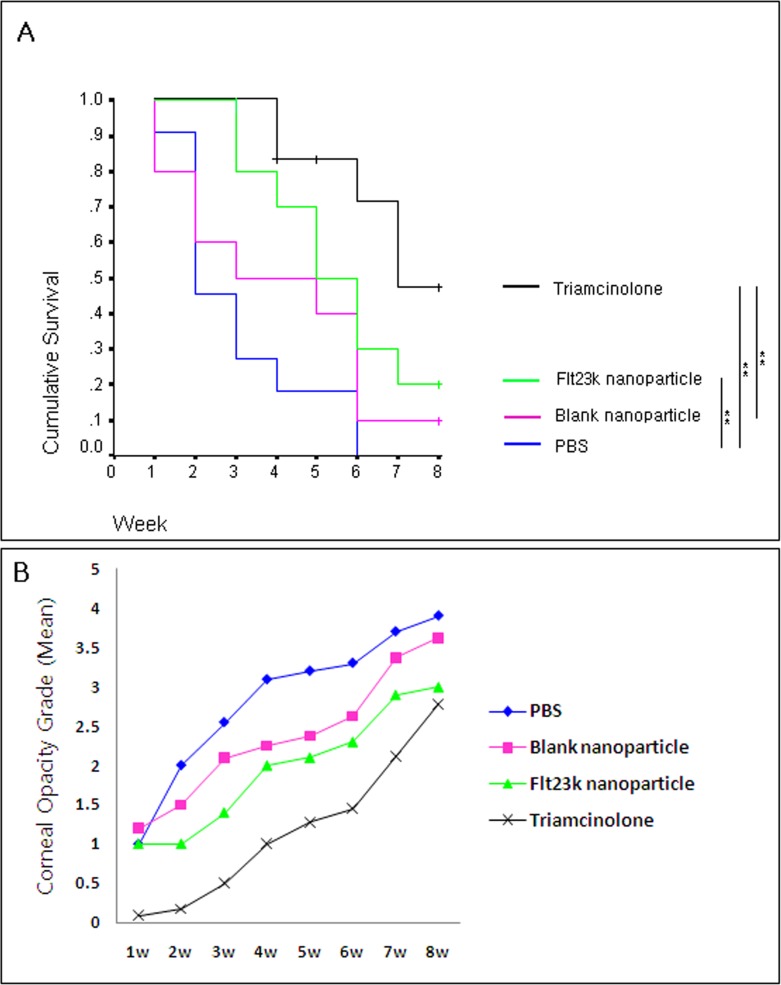
Both the Flt23k nanoparticle and triamcinolone groups showed improved graft survival compared with the PBS group (P = 0.009 and P = 0.000, respectively) (Fig. 3). Through the initial 8 weeks in the survival analysis, the Flt23k nanoparticle group did not show statistically significant difference in the survival curve when compared with the blank nanoparticle group. The Flt23k nanoparticle group showed better survival than blank nanoparticle group through the third week (P = 0.029). The survival of triamcinolone group was not statistically better than Flt23k nanoparticle group (P = 0.09). The 8-week graft survival rate was 20.0%, 47.6%, 10.0%, and 0% in Flt23k nanoparticle, triamcinolone, blank nanoparticle, and PBS groups, respectively.
Figure 3.
(A) Comparison of graft survival through 8 weeks postoperatively. Flt23k nanoparticle group showed better survival than the PBS group (P = 0.009) until 8 weeks postoperatively. Flt23k nanoparticle group showed better survival when compared with blank nanoparticle group until 3 weeks postoperatively (P = 0.029). (+Sensored data, *P < 0.05, **P < 0.01). (B) Comparison of graft opacity grade for each week. Through 2 to 5 weeks postoperatively, the Flt23k nanoparticle group showed decreased opacification compared with the PBS group (P < 0.05).
Graft Opacity
Corneal graft opacity results by week are presented in Figure 3. There was no significant difference in opacity between the triamcinolone and Flt23k nanoparticle groups from weeks 3 to 8. Through 2 to 5 weeks postoperatively, the Flt23k nanoparticle group showed decreased opacification compared with the PBS group (P < 0.05).
Neovascularization
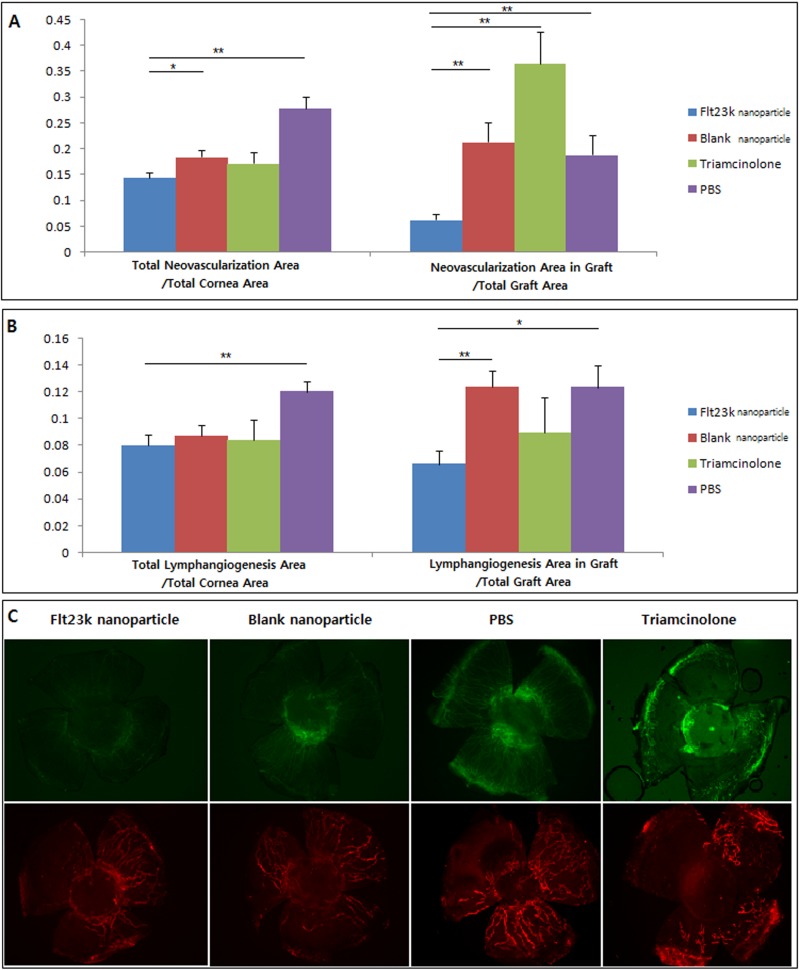
As shown in Figure 4, the Flt23k nanoparticle group (0.144 ± 0.009) demonstrated less total corneal neovascularization compared with the PBS group (0.277 ± 0.022) (P = 0.000) and the blank nanoparticle group (0.185 ± 0.013) (P = 0.019).The Flt23k nanoparticle group (0.062 ± 0.011) also showed less graft neovascularization than the PBS group (0.188 ± 0.038) (P = 0.009), the blank nanoparticle group (0.213 ± 0.038, P = 0.001), and the triamcinolone group (0.364 ± 0.061, P = 0.000).
Figure 4.
(A) The Flt23k nanoparticle group showed less total neovascularization compared with the PBS group (P = 0.000) and the blank nanoparticle group (P = 0.019). The Flt23k nanoparticle group showed less graft neovascularization than PBS group (P = 0.009), blank nanoparticle group (P = 0.001), and triamcinolone group (P = 0.000). Error bar is SEM. *P < 0.05, **P < 0.01. (B) The Flt23k nanoparticle group showed less total lymphangiogenesis compared with the PBS group (P = 0.002). The Flt23k nanoparticle group also showed less graft lymphangiogenesis compared with PBS group (P = 0.011) and blank nanoparticle group (P = 0.002). (C) Representative pictures of corneal neovascularization (upper row) and lymphangiogenesis (lower row) in each group.
Lymphangiogenesis
The Flt23k nanoparticle group (0.079 ± 0.008) developed less total lymphangiogenesis compared with the PBS group (0.119 ± 0.007, P = 0.002) (Fig. 3). With respect to graft lymphangiogenesis, the Flt23k nanoparticle group (0.065 ± 0.010) showed less graft lymphangiogenesis compared with the PBS group (0.123 ± 0.016, P = 0.011) and the blank nanoparticle group (0.123 ± 0.012, P = 0.002). There was no difference between the grafts treated with triamcinolone and the Flt23k nanoparticle.
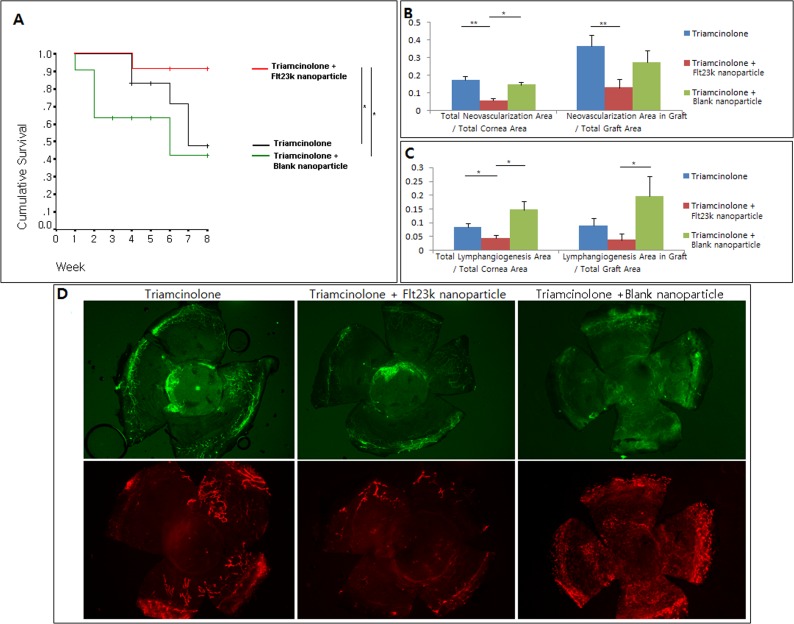
Combination Treatment Comparisons
Graft Survival
Figure 5 presents the results of the comparison between the combination groups (triamcinolone plus Flt23k nanoparticle, triamcinolone plus blank nanoparticle) and triamcinolone only group. The graft survival rate was 47.6% in the triamcinolone-only group, 42.4% in the triamcinolone plus blank nanoparticle group, and 91.6% in the triamcinolone plus Flt23k nanoparticle group. Graft survival of triamcinolone plus the Flt23k nanoparticle was higher than the triamcinolone-only group and triamcinolone plus blank nanoparticle group (P = 0.048, P = 0.020, respectively). There was no difference in graft survival between the triamcinolone-only group and triamcinolone plus blank nanoparticle group (P > 0.05).
Figure 5.
(A) The Flt23k nanoparticle plus triamcinolone group increased graft survival compared with the triamcinolone group and triamcinolone plus blank nanoparticle group (P = 0.048, P = 0.020, respectively). +Sensored data, *P < 0.05, **P < 0.01. (B) The Flt23k nanoparticle plus triamcinolone group showed less total neovascularization compared with triamcinolone group (P = 0.000) and triamcinolone plus blank nanoparticle group (P = 0.028). The Flt23k nanoparticle plus triamcinolone group showed less graft neovascularization compared with triamcinolone group (P = 0.008). (C) The Flt23k nanoparticle plus triamcinolone group showed significantly less total lymphangiogenesis compared with triamcinolone group (P = 0.043) and triamcinolone plus blank nanoparticle group (P = 0.014). The Flt23k nanoparticle plus triamcinolone group showed less graft lymphangiogenesis compared with the triamcinolone plus blank nanoparticle group (P = 0.028). (D) Representative pictures of neovascularization (upper row) and lymphangiogenesis (lower row) in each group.
Neovascularization
As shown in Figures 5B and 5D, the triamcinolone plus Flt23k nanoparticle combination group demonstrated significantly less total neovascularization compared with both the triamcinolone-only group (5.5% vs. 17.2% of total corneal area, P = 0.000) and the triamcinolone plus blank nanoparticle group (5.5% vs. 14.5% of total corneal area, P = 0.028). The triamcinolone plus Flt23k nanoparticle combination group also showed less graft neovascularization compared with triamcinolone-only group (12.8% vs. 36.4%, P = 0.008) and tendency of less graft neovascularization compared with triamcinolone plus blank nanoparticle group (12.8% vs. 27.2%, P = 0.077).
Lymphangiogenesis
The triamcinolone plus the Flt23k nanoparticle combination group demonstrated significantly less total lymphangiogenesis compared with both triamcinolone-only group (4.3% vs. 8.3% of total corneal area, P = 0.043) and triamcinolone plus blank nanoparticle group (4.3% vs. 14.7% of total cornea area, P = 0.014) (Fig. 5). Although the triamcinolone plus Flt23k nanoparticle combination group trended toward decreased graft lymphangiogenesis when compared with triamcinolone group (3.7% vs. 8.9% of graft area), this did not reach statistical significance (P = 0.160). The triamcinolone plus Flt23k nanoparticle group showed significantly less lymphangiogenesis compared with triamcinolone plus blank nanoparticle group (3.7% vs. 19.6% of graft area, P = 0.028).
Discussion
In corneal transplantation, corneal angiogenesis and lymphangiogenesis driven by VEGF is a key pathogenic process and determinant of corneal graft rejection.19 Although the relative importance of angiogenesis and lymphangiogenesis in graft rejection is currently unclear, inhibiting these processes through the VEGF pathway may represent a potential treatment option to protect corneal grafts and improve corneal transplant success rates.19–23
Nanoparticles are defined as particles with a diameter of less than 1 μm, consisting of various biological materials, such as natural or synthetic polymers.24 Potential advantages of nanoparticle therapeutics include enhanced or targeted cell and tissue uptake, reduced clearance, and sustained release.24–27 Through targeted delivery of nanoparticles, such as subconjunctival injection, potential systemic effects of the drug can be reduced while optimizing the desired pharmacological effects in target tissues.24–26
In this study, the authors found that Flt23k nanoparticles increased graft survival more than PBS through postoperative week 8 and increased graft survival compared with a blank nanoparticle through postoperative week 3. We found that the Flt23k nanoparticle decreased neovascularization and lymphangiogenesis compared with both blank nanoparticle and PBS. As corneal vascularization is a risk factor for transplant rejection, improvements to block angiogenesis have the potential to enhance outcomes in corneal transplantation.
Although the Flt23k nanoparticle group showed better 8-week survival compared with the PBS group and better 3-week survival compared with the blank nanoparticle group, when compared with triamcinolone, the Flt23k nanoparticle group did not improve graft survival. When neovascularization and lymphangiogenesis of the Flt23k nanoparticle group was compared with triamcinolone, the Flt23k nanoparticle group did not show a significant difference in neovascularization and lymphangiogenesis. Flt23k nanoparticle treatment alone was not significantly better than triamcinolone treatment, which is currently widely used after PK.
However, when the authors tested the combination of Flt23k nanoparticles with steroid treatment (triamcinolone), they found a synergistic effect of the combined treatments. The authors found a substantially increased graft survival with combined treatment of triamcinolone plus Flt23k nanoparticle compared with treatment with only triamcinolone or triamcinolone plus blank nanoparticle. This finding suggests that the treatment combination of the Flt23k nanoparticle and steroids can lead to significantly better graft survival than steroid therapy alone. This is a promising result as a potential treatment to decrease graft rejection as steroid treatment is still the mainstay of treatment.
Previous studies28,29 have not blocked VEGF-A intracellularly, which may explain the lack of synergistic effects in increasing graft survival for treatment combination. The authors' findings suggest the potential importance of autocrine signaling in corneal angiogenesis and eventual graft survival since we blocked VEGF-A intracellularly. Furthermore, as steroid treatment affects multiple immunologic and inflammatory pathways, the increased graft survival seen with steroid and Flt23k nanoparticle combination treatment provides additional support for the idea that while angiogenesis and lymphangiogenesis play a key role in corneal graft survival and outcomes, they are not the only determining factors.
The authors also found that Flt23k nanoparticles inhibited lymphangiogenesis. Although previous studies suggest that lymphangiogenesis is mediated primarily by VEGF-C and VEGF-D,6,30 others, including the present study, suggest a potential role of VEGF-A in inflammatory lymphangiogenesis.5,6,31,32 VEGF-A may stimulate VEGFR2 on lymphatic endothelial cells and induce lymphangiogenesis directly. There may also be a relation between VEGF-A stimulated lymphangiogenesis and macrophage recruitment in inflammatory lymphangiogensis. Based on our results, the Flt23k nanoparticle may block the lymphangiogenesis directly by blocking VEGF-A or indirectly by decreasing leukocyte infiltration from blood vessels, macrophage migration, and activation caused by decreased VEGF-VEGFR1 signaling, thereby decreasing the VEGF-C and VEGF-D secreted by macrophages.5,6,31–33
By using nanoparticles to deliver an anti-VEGF plasmid to the subconjunctival space, the authors found sustained expression in conjunctiva and cornea for 4 weeks after injection. Larger (>200 nm) nonbiodegradable nanoparticles can be largely retained at the site of administration for at least 2 months.26 In the case of biodegradable nanoparticles, they will likely be retained in the periocular spaces until they reach a much smaller dimension that is suitable for clearance by systemic or lymphatic circulation.26,34 These factors and characteristics are significant for treatments that have the potential to improve corneal transplant outcomes through decreased treatment frequency, reduced side effects, and improved delivery to target tissues.
Owing to their prolonged existence in the conjunctiva and precorneal areas, although we only injected at 4 week intervals, our results showed less neovascularization and less lymphangiogenesis in the corneas of the Flt23k nanoparticle treatment group compared with both blank nanoparticle group and PBS group. The Flt23k nanoparticle treatment group showed significantly better graft survival than PBS group. However, in the comparison of graft survival between the Flt23k nanoparticle group and the blank nanoparticle group, the Flt23k nanoparticle group showed significantly better graft survival than blank nanoparticle group through 3 weeks postoperatively (P = 0.029). But after 4 weeks postoperatively, the Flt23k nanoparticle did not show improved graft survival compared with the blank nanoparticle group (P = 0.08 at 4 weeks and P = 0.29 at 8 weeks) despite injection of the Flt23k nanoparticles at 4 weeks postoperatively. The authors believe the reason why Flt23k nanoparticle did not show better graft survival compared with the blank nanoparticle after 4 weeks postoperatively was because of the decreasing concentration over 4 weeks. As a result, the authors recommend that the injection interval should be determined by the clinical basis to maintain the graft clarity, not by the nanoparticle's histologic existence in the cornea. Although their existence is evident from the authors' confocal microscopic result through 4 weeks after injection, once the graft becomes opaque because of a low concentration of nanoparticles, reinjection cannot recover corneal clarity. This 4-week interval was effective in decreasing neovascularization and lymphangiogenesis, and this interval may ultimately need to be lengthened to make this a clinically feasible treatment option. However, further research should be done to increase the retention time of the Flt23k nanoparticle in the periocular space to prevent graft failure.
In graft neovascularization of the single treatment comparisons (Fig. 4A), an unexpected increase in neovascularization in the triamcinolone group was observed. In our study, neovascularization and lymphangiogenesis in the grafts were positively correlated with neovascularization and lymphangiogenesis in total cornea (P = 0.003 in neovascularization, P = 0.000 in lymphangiogensis, respectively). Even though graft neovascularization was increased in the triamcinolone group, total neovascularization was not increased compared with the two control groups (blank nanoparticle and PBS). As a result, the authors theorize that this is caused by the distribution of neovascularization and does not represent an increase of total blood vessel area.
In conclusion, this study demonstrates the decreased neovascularization and lymphangiogenesis effects of anti-VEGF nanoparticles (Flt23k intraceptor) and their potential role in improving graft outcomes, both as a single treatment and especially when combined with steroid therapy. This study also provides support for using medium-term anti-VEGF therapy with steroids to improve corneal transplant outcomes.
Acknowledgments
The authors thank Jackie Simonis and Thomas Olsen for their assistance with this study.
Footnotes
Supported by National Institutes of Health Grant EY017182 (BKA), US DOD PRMRP Grant W81XWH-07-1-0439 (BKA), and NEI Grant 5R01EY017182 (BKA).
Disclosure: Y.K. Cho, None; H. Uehara, None; J.R. Young, None; P. Tyagi, None; U.B. Kompella, None; X. Zhang, None; L. Luo, None; N. Singh, None; B. Archer, None; B.K. Ambati, None
References
- 1. Santos LN, de Moura LR, Fernandes BF, Cheema DP, Burnier MN., Jr. Histopathological study of delayed regraft after corneal graft failure. Cornea. 2011;30:167–170. [DOI] [PubMed] [Google Scholar]
- 2. Jani PD, Singh N, Jenkins C, et al. Nanoparticles sustain expression of Flt intraceptors in the cornea and inhibit injury-induced corneal angiogenesis. Invest Ophthalmol Vis Sci. 2007;48:2030–2036. [DOI] [PubMed] [Google Scholar]
- 3. Weisbrod DJ, Sit M, Naor J, Slomovic AR. Outcomes of repeat penetrating keratoplasty and risk factors for graft failure. Cornea. 2003;22:429–434. [DOI] [PubMed] [Google Scholar]
- 4. Rocher N, Behar-Cohen F, Pournaras JA, et al. Effects of rat anti-VEGF antibody in a rat model of corneal graft rejection by topical and subconjunctival routes. Mol Vis. 2011;17:104–112. [PMC free article] [PubMed] [Google Scholar]
- 5. Cursiefen C, Chen L, Borges LP, et al. VEGF-A stimulates lymphangiogenesis and hemangiogenesis in inflammatory neovascularization via macrophage recruitment. J Clin Invest. 2004;113:1040–1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cursiefen C, Chen L, Dana MR, Streilein JW. Corneal lymphangiogenesis: evidence, mechanisms, and implications for corneal transplant immunology. Cornea. 2003;22:273–281. [DOI] [PubMed] [Google Scholar]
- 7. Singh N, Jani PD, Suthar T, Amin S, Ambati BK. Flt-1 intraceptor induces the unfolded protein response, apoptotic factors, and regression of murine injury-induced corneal neovascularization. Invest Ophthalmol Vis Sci. 2006;47:4787–4793. [DOI] [PubMed] [Google Scholar]
- 8. Singh SR, Grossniklaus HE, Kang SJ, Edelhauser HF, Ambati BK, Kompella UB. Intravenous transferrin, RGD peptide and dual-targeted nanoparticles enhance anti-VEGF intraceptor gene delivery to laser-induced CNV. Gene Ther. 2009;16:645–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sundaram S, Trivedi R, Durairaj C, Ramesh R, Ambati BK, Kompella UB. Targeted drug and gene delivery systems for lung cancer therapy. Clin Cancer Res. 2009;15:7299–7308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sundaram S, Roy SK, Ambati BK, Kompella UB. Surface-functionalized nanoparticles for targeted gene delivery across nasal respiratory epithelium. FASEB J. 2009;23:3752–3765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gerber HP, Malik AK, Solar GP, et al. VEGF regulates haematopoietic stem cell survival by an internal autocrine loop mechanism. Nature. 2002;417:954–958. [DOI] [PubMed] [Google Scholar]
- 12. Broggini M, Marchini SV, Galliera E, et al. Aplidine, a new anticancer agent of marine origin, inhibits vascular endothelial growth factor (VEGF) secretion and blocks VEGF-VEGFR-1 (flt-1) autocrine loop in human leukemia cells MOLT-4. Leukemia. 2003;17:52–59. [DOI] [PubMed] [Google Scholar]
- 13. Lee S, Chen TT, Barber CL, et al. Autocrine VEGF signaling is required for vascular homeostasis. Cell. 2007;130:691–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lee TH, Seng S, Sekine M, et al. Vascular endothelial growth factor mediates intracrine survival in human breast carcinoma cells through internally expressed VEGFR1/FLT1. PLoS Med. 2007;4:e186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wagner EM, Sanchez J, McClintock JY, Jenkins J, Moldobaeva A. Inflammation and ischemia-induced lung angiogenesis. Am J Physiol Lung Cell Mol Physiol. 2008;294:L351–357. [DOI] [PubMed] [Google Scholar]
- 16. Eliceiri BP, Cheresh DA. The role of alphav integrins during angiogenesis: insights into potential mechanisms of action and clinical development. J Clin Invest. 1999;103:1227–1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sonoda Y, Streilein JW. Orthotopic corneal transplantation in mice–evidence that the immunogenetic rules of rejection do not apply. Transplantation. 1992;54:694–704. [DOI] [PubMed] [Google Scholar]
- 18. Plskova J, Kuffova L, Holan V, Filipec M, Forrester JV. Evaluation of corneal graft rejection in a mouse model. Br J Ophthalmol. 2002;86:108–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hos D, Bock F, Dietrich T, et al. Inflammatory corneal (lymph)angiogenesis is blocked by VEGFR-tyrosine kinase inhibitor ZK 261991, resulting in improved graft survival after corneal transplantation. Invest Ophthalmol Vis Sci. 2008;49:1836–1842. [DOI] [PubMed] [Google Scholar]
- 20. Dastjerdi MH, Saban DR, Okanobo A, et al. Effects of topical and subconjunctival bevacizumab in high-risk corneal transplant survival. Invest Ophthalmol Vis Sci. 2010;51:2411–2417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Albuquerque RJ, Hayashi T, Cho WG, et al. Alternatively spliced vascular endothelial growth factor receptor-2 is an essential endogenous inhibitor of lymphatic vessel growth. Nat Med. 2009;15:1023–1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Dietrich T, Bock F, Yuen D, et al. Cutting edge: lymphatic vessels, not blood vessels, primarily mediate immune rejections after transplantation. J Immunol. 2010;184:535–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Yamagami S, Dana MR. The critical role of lymph nodes in corneal alloimmunization and graft rejection. Invest Ophthalmol Vis Sci. 2001;42:1293–1298. [PubMed] [Google Scholar]
- 24. Sahoo SK, Dilnawaz F, Krishnakumar S. Nanotechnology in ocular drug delivery. Drug Discov Today. 2008;13:144–151. [DOI] [PubMed] [Google Scholar]
- 25. Kompella UB, Bandi N, Ayalasomayajula SP. Subconjunctival nano- and microparticles sustain retinal delivery of budesonide, a corticosteroid capable of inhibiting VEGF expression. Invest Ophthalmol Vis Sci. 2003;44:1192–1201. [DOI] [PubMed] [Google Scholar]
- 26. Amrite AC, Kompella UB. Size-dependent disposition of nanoparticles and microparticles following subconjunctival administration. J Pharm Pharmacol. 2005;57:1555–1563. [DOI] [PubMed] [Google Scholar]
- 27. Qaddoumi MG, Ueda H, Yang J, Davda J, Labhasetwar V, Lee VH. The characteristics and mechanisms of uptake of PLGA nanoparticles in rabbit conjunctival epithelial cell layers. Pharm Res. 2004;21:641–648. [DOI] [PubMed] [Google Scholar]
- 28. Unal M, Yucel I. Evaluation of topical ciclosporin 0.05% for prevention of rejection in high-risk corneal grafts. Br J Ophthalmol. 2008;92:1411–1414. [DOI] [PubMed] [Google Scholar]
- 29. Dana R. Comparison of topical interleukin-1 vs tumor necrosis factor-alpha blockade with corticosteroid therapy on murine corneal inflammation, neovascularization, and transplant survival (an American Ophthalmological Society thesis). Trans Am Ophthalmol Soc. 2007;105:330–343. [PMC free article] [PubMed] [Google Scholar]
- 30. Alitalo K, Tammela T, Petrova TV. Lymphangiogenesis in development and human disease. Nature. 2005;438:946–953. [DOI] [PubMed] [Google Scholar]
- 31. Nagy JA, Vasile E, Feng D, et al. Vascular permeability factor/vascular endothelial growth factor induces lymphangiogenesis as well as angiogenesis. J Exp Med. 2002;196:1497–1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Sato Y. VEGFR1 for lymphangiogenesis: an alternative signaling pathway? Arterioscler Thromb Vasc Biol. 2008;28:604–605. [DOI] [PubMed] [Google Scholar]
- 33. Ferrara N. Vascular endothelial growth factor: basic science and clinical progress. Endocr Rev. 2004;25:581–611. [DOI] [PubMed] [Google Scholar]
- 34. Amrite ACEH, Singh SR, Kompella UB. Effect of circulation on the disposition and ocular tissue distribution of 20 nm nanoparticles after periocular administration. Molecular Vision. 2008;14:150–159. [PMC free article] [PubMed] [Google Scholar]