Abstract
Purpose.
Optical coherence tomography (OCT) shows retinal nerve fiber layer (RNFL) thickening in optic nerve head (ONH) swelling, but does not provide information on acute axonal disruption. It was hypothesized that scanning laser polarimetry (SLP) compared with OCT might reveal the status of axon integrity and visual prognosis in acute RNFL swelling.
Methods.
Threshold perimetry, OCT, and SLP were used to prospectively study eyes with papilledema (24), optic neuritis (14), nonarteritic anterior ischemic optic neuropathy (NAION) (21), and ONH swelling (average RNFL value by OCT was above the 95th percentile of controls at presentation). Regional RNFL was judged reduced if the quadrant measurement was below the fifth percentile of controls.
Results.
At presentation, average RNFL by OCT was similar for eyes with papilledema and NAION (P = 0.97), and reduced for optic neuritis. Average RNFL by SLP was slightly increased for papilledema and optic neuritis, and reduced for NAION (P = 0.02) eyes. The RNFL by SLP was reduced in at least one quadrant in 1 eye with papilledema, 1 eye with optic neuritis, and in 13 eyes with NAION. In NAION eyes, quadrants with reduced SLP had corresponding visual field loss that did not recover. By one month, eyes with NAION showed RNFL thinning by OCT (7/17 eyes) and by SLP (14/16 eyes) in contrast to optic neuritis (by OCT, 0/12, P = 0.006; and by SLP, 1/12, P = 0.0004).
Conclusions.
OCT and SLP revealed different aspects of RNFL changes associated with ONH swelling. OCT revealed thickening due to edema. SLP revealed a decrease in retardance in eyes with axonal injury associated with visual field loss, which is unlikely to recover.
SLP seems to be predictive of regions of permanent axon dysfunction and visual field loss in eyes with optic disc edema.
Introduction
Optical coherence tomography (OCT) and scanning laser polarimetry (SLP) show loss of peripapillary retinal nerve fiber layer (RNFL) in glaucoma and at later time points after optic neuritis, nonarteritic anterior ischemic optic neuropathy (NAION), or traumatic optic neuropathy.1–11 Prior investigations, which used older methodologies to compensate for the corneal birefringence, have found that RNFL derived from SLP is not changed in eyes with edematous optic discs due to entities such as NAION.6,12 However, the RNFL thickness derived from OCT was shown to be increased with swelling of the optic discs due to intracranial hypertension.13–15 A previous study of optic neuritis using both OCT and SLP has shown that mild RNFL edema is better demonstrated by OCT. In this optic neuritis study, acute reduction in birefringence is generally transient in acute optic neuritis, mostly reflecting temporarily increased RNFL water content (Kupersmith M, et al. IOVS 2009;50:ARVO E-Abstract 5664). Additionally, minimal RNFL thinning has been detected by both OCT and SLP one month after acute optic neuritis, even though OCT still shows some degree of RNFL swelling.16
For this study, consideration was given to whether using both OCT and SLP to image the peripapillary RNFL might provide complementary data and further an understanding of the pathophysiologic mechanisms occurring within the RNFL, associated with different etiologies of ONH swelling, and whether permanent damage could be predicted. Optic disc edema in papilledema is due to intracranial hypertension and is associated with transmission of elevated pressure through the subarachnoid space to the optic nerve head at the level of the lamina cribosa, causing stasis of axoplasmic flow.17–19 Early in the course, vision is typically normal or mildly abnormal in most cases. Optic nerve head edema in optic neuritis is due to acute inflammation of the retrobulbar optic nerve with axoplasmic flow stasis and blockade at the level of the demyelinating lesion.20,21 In most cases, vision recovery is significant22 and RNFL loss, although common, is typically minimal.4 In contrast, optic disc edema due to NAION is presumably due to ischemia in the lamina and retrolaminar optic nerve, and visual field loss is commonly irreversible and permanent, but the visual acuity can improve one or two lines in up to 40% of patients.23,24 For this study, it was hypothesized that the papilledema, NAION, and optic neuritis might show distinctive differentiating features of the RNFL, revealed by SLP in comparison with OCT in the same eye. It was hypothesized that SLP would be relatively unchanged by intra-axonal swelling and extracellular edema associated with papilledema and optic neuritis, but would show decreased retardance in regions of early axon injury associated with corresponding disorganization of axoplasmic microtubules and decrease in function, especially in NAION. Such axonal injury would result in reduction of the retardation or normal delay of polarized light passing through the RNFL since SLP-derived RNFL thickness is proportional to the phase shift of polarized light induced by intact axons.25,26 In contrast, OCT would be expected to show RNFL thickening associated with optic disc edema, which would mask early axon injury until resolution of the edema would reveal RNFL atrophy.
Methods
Subjects who had active papilledema due to intracranial hypertension or optic disc edema due to new onset (within 21 days of vision loss) optic neuritis or NAION were prospectively included in this study. At baseline presentation, all eyes enrolled with NAION and optic neuritis showed optic disc swelling by clinical ophthalmoscopy. Inclusion criteria required that the average RNFL thickness by OCT in the affected eye(s) be greater than the 95th percentile of controls. Patients enrolled with papilledema had symptoms of intracranial hypertension, bilateral papilledema, and no optic atrophy seen with ophthalmoscopy. Each patient with papilledema had also undergone a brain magnetic resonance imaging (MRI) study with gadolinium and had lumbar puncture confirmation of raised intracranial pressure. Each patient with optic neuritis had undergone MRI with gadolinium of the brain and orbits. This research was conducted with New York Eye and Ear Infirmary Institutional Review Board approval. All subjects were treated in accordance with the Declaration of Helsinki.
Each subject had complete clinical evaluation and threshold perimetry performed with the Humphrey Field Analyzer (Zeiss-Meditec, Inc., Dublin, CA) SITA 24-2 standard perimeter strategy using size III stimulus (expressed as mean deviation, in decibels). The peripapillary RNFL evaluation was performed with SLP in undilated eyes and with OCT, following pupil dilation. For SLP (GDx with enhanced corneal compensation research software, Zeiss-Meditec, Inc.), an elliptical annulus was adjusted to permit measurement of the same peripapillary RNFL region between examinations and eyes. The SLP retardation values were determined within the annulus, which was resampled into 64 circumferential sectors of derived RNFL thickness. The OCT (Stratus version 3; time domain OCT 4 Software, fast RNFL protocol, Zeiss-Meditec, Inc.), was used to obtain a set of three 3.4-mm diameter retinal scans, averaged together to provide the RNFL thickness at 256 points along the circumference of the circular peripapillary scan in each eye. For the HD-OCT (high definition, Cirrus spectral HD-OCT, Zeiss-Meditec, Inc.), a 6 × 6-mm optic disc volume scan was obtained, capturing a cube of data consisting of 200 A-scans from 200 linear B-scans (40,000 points). The time domain OCT was used early in the studies and spectral HD-OCT was used for later cases (a comparison of both methods typically showed that HD-OCT RNFL measures were reduced by 8–12 μm). Both OCT methods provided values for the average RNFL thickness and for the 12 clock-hour sectors around the peripapillary circumference. At least two scans were performed for each method on each eye, and only images centered on the optic disc with signal strength scores of six or greater were analyzed. The same OCT method was used for individuals who had images obtained at one month follow-up.
The RNFL thickness derived from SLP retardance and from OCT was analyzed and the average sampled for the entire peripapillary circumference for OCT and for SLP. Measurements were calculated for 4 quadrants (temporal, superior, nasal, and inferior) by averaging the values for equally distributed 12 clock-hour sectors for OCT (3 sectors per quadrant) and 64 circumferential measurements for SLP (16 measurements per quadrant). For SLP, the fifth and 95th percentiles of RNFL thickness (retardance) in normal controls were determined (provided by Zeiss-Meditec, Inc.; Table 1) for 32- and 65-year-olds (matched for age of papilledema/optic neuritis patients and NAION patients, respectively) to compare to our data. For OCT, the fifth and 95th percentiles of RNFL thickness in normal controls were determined (Table 1) (OCT 4 provided by University of Iowa, with mean age 53 years; and HD-OCT provided by Zeiss-Meditec, Inc., for 32- and 65-year-olds). For our study eyes, RNFL was judged abnormally thick by OCT for measurements greater than the 95th percentile of controls (applied separately to time domain OCT and HD-OCT data). Abnormal retardance by SLP or RNFL by OCT was determined for the entire circumference or for quadrant measurements if values were greater than the 95th percentile (swelling) or less than the fifth percentile (atrophy) of controls for normal 32-year-olds, for papilledema and optic neuritis, and for 65-year-olds for NAION eyes (Table 1). The values of SLP and OCT were compared across all affected eyes at presentation and, when applicable, at one month. A two-tailed Student's t-test and paired t-test were used and P values reported.
Table 1.
Lower and Upper Limits of Normal RNFL Thickness Measured in Micrometers
|
Age |
Average RNFL |
Temporal Quadrant |
Superior Quadrant |
Nasal Quadrant |
Inferior Quadrant |
|
| Scanning Laser Polarimetry (ECC) RNFL | ||||||
| 5th Percentile | 32 | 47 | 15 | 50 | 23 | 57 |
| 65 | 34 | 13 | 50 | 21 | 54 | |
| 95th Percentile | 32 | 73 | 41 | 94 | 58 | 98 |
| 65 | 71 | 39 | 91 | 56 | 94 | |
| Optical Coherence Tomography RNFL | ||||||
| Stratus OCT | ||||||
| 5th Percentile | 53 | 84 | 50 | 85 | 52 | 94 |
| 95th Percentile | 53 | 114 | 97 | 152 | 108 | 163 |
| Cirrus HD OCT | ||||||
| 5th Percentile | 32 | 82 | 52 | 86 | 52 | 84 |
| 65 | 76 | 52 | 87 | 52 | 84 | |
| 95th Percentile | 32 | 110 | 89 | 134 | 88 | 133 |
| 65 | 104 | 89 | 135 | 88 | 133 | |
ECC, enhanced corneal compensation.
Results
Patients with Papilledema from Idiopathic Intracranial Hypertension (IIH)
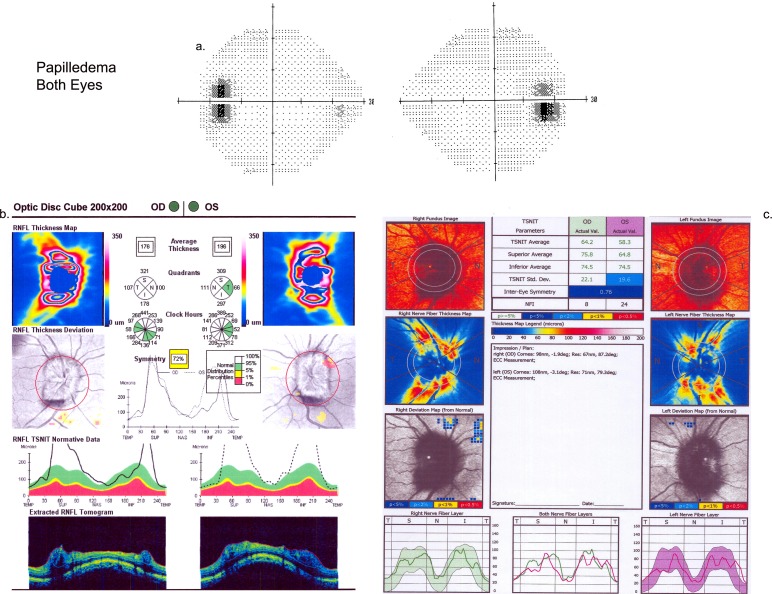
Twenty-four eyes (14 patients) with papilledema from IIH were evaluated (4 with HD-OCT, 10 with time domain OCT). Owing to our entry criteria, all eyes had average RNFL measurements by OCT greater than the 95th percentile of controls (Table 1; see Fig. 1b). None of the eyes had a definite abnormality in the visual field except for enlargement of the blind spot (Table 2; Fig. 1a). By OCT, every eye with papilledema had at least two quadrants thicker than the 95th percentile of controls (see distribution in Table 4a). By OCT, no eye had an RNFL quadrant that was less than the fifth percentile limit. The average RNFL thickness (Table 3) varied widely among eyes for both OCT (range, 118–523 μm) and SLP (range, 49–74 μm), owing to the wide range of papilledema grade.
Figure 1.
(a) Threshold visual field for a patient with papilledema at presentation. (b) Cirrus output for spectral OCT (top to bottom: RNFL thickness map, RNFL thickness deviation from control database, RNFL thickness distribution compared to normal distributions, extracted RNFL tomogram) for the same patient with papilledema at presentation. (c) GDx output for SLP (top to bottom: fundus photo, RNFL thickness map, map of deviation RNFL from control database, RNFL thickness distribution) for the same patient with papilledema at presentation.
Table 2.
Patient Demographics, Visual Acuity, and Visual Field Data
|
Diagnosis |
Time Point of Data Collection |
Age, y, Mean ± SD (Range) |
Sex (F/M) |
LogMAR Visual Acuity, Mean ± SD (Range) |
Mean Deviation, dB, Mean ± SD (Range) |
| Papilledema | At presentation |
38.3 ± 12.3 (18–64) | 14/0 | 0.02 ± 0.05 (0.2 to 0) | −1.70 ± 1.90 (−5.99 to 0.13) |
| NAION | At presentation | 65. ± 13.0 (38–85) | 9/12 | 0.47 ± 0.65 (hand motion to 0) | −12.16 ± 7.59 (−32.00 to −2.00) |
| 1 mo | 0.44 ± 0.65 (hand motion to 0) | −14.65 ± 9.02 (−32.00 to −5.89) | |||
| 6 mo | 0.63 + 0.73 (hand motion to 0) | −12.36 + 14.00 (−32 to −1.93) | |||
| Compare 1 and 6 mo | P = 0.2 | P = 0.4 | |||
| Optic neuritis | At presentation | 29.4 ± 7.5 (16–43) | 12/2 | 0.56 ± 0.46 (1.5 to −0.1) | −15.10 ± 8.82 (−32.22 to −1.89) |
| 1 mo | 0.12 ± 0.43 (1.5 to −0.1) | −5.20 ± 10.22 (−34.28 to 2.09) | |||
| 6 mo | 0.04 + 0.34 (1 to −0.1) | −3.83 + 9.95 (−32 to 0.04) | |||
| Compare at presentation and 6 mo | P = 0.008 | P = 0.006 | |||
| Compare 1 and 6 mo | P = 0.08 | P = 0.09 |
Table 4a.
Number of Eyes (Out of 24 With Papilledema, 14 With Optic Neuritis, and 21 With NAION) With a. Quadrant RNFL Thickening at Presentation (>95th Percentile of Normal Eyes) and b. Quadrant RNFL Thinning at Presentation (<5th Percentile of Normal Eyes)
| a. | ||||||
|
Quadrant |
Papilledema OCT |
Papilledema SLP |
Optic Neuritis OCT |
Optic Neuritis SLP |
NAION OCT |
NAION SLP |
| Superior | 24 | 0 | 12 | 1 | 20 | 0 |
| Temporal | 16 | 11 | 3 | 2 | 16 | 4 |
| Nasal | 18 | 3 | 8 | 0 | 18 | 2 |
| Inferior | 24 | 0 | 9 | 0 | 17 | 1 |
Table 3.
Affected Eye RNFL Thickness in Micrometers at Presentation for OCT and SLP*
|
Diagnosis |
Imaging Test, No. of Eyes |
Average RFNL (Mean ± SD) |
Temporal Quadrant (Mean ± SD) |
Superior Quadrant (Mean ± SD) |
Nasal Quadrant (Mean ± SD) |
Inferior Quadrant (Mean ± SD) |
| Papilledema (24 eyes) | OCT: Stratus, 16; HD, 8 | 213 ± 100 | 152 ± 192 | 271 ± 145 | 187 ± 125 | 241 ± 92 |
| SLP | 59 ± 7 | 38 ± 10 | 73 ± 14 | 49 ± 12 | 75 ± 13 | |
| NAION (21 eyes) | OCT: HD, 21 | 214 ± 76 | 144 ± 61 | 298 ± 135 | 174 ± 85 | 244 ± 118 |
| SLP | 48 ± 9 | 30 ± 9 | 52 ± 15 | 41 ± 11 | 66 ± 17 | |
| Optic neuritis (14 eyes) | OCT: Stratus, 12; HD, 2 | 141 ± 21 | 83 ± 18 | 184 ± 34 | 121 ± 29 | 177 ± 33 |
| SLP | 55 ± 5 | 28 ± 9 | 74 ± 12 | 45 ± 7 | 71 ± 12 |
See “Methods” explaining use of HD and time domain OCT. For papilledema: 8 eyes, HD; and 16 eyes, time domain OCT. For NAION: eyes HD and time domain OCT.
Table 4b.
| b. | ||||||
|
Quadrant |
Papilledema OCT |
Papilledema SLP |
Optic Neuritis OCT |
Optic Neuritis SLP |
NAION OCT |
NAION SLP |
| Superior | 0 | 0 | 0 | 0 | 0 | 11 |
| Temporal | 0 | 0 | 0 | 0 | 0 | 2 |
| Nasal | 0 | 0 | 0 | 0 | 0 | 1 |
| Inferior | 0 | 1 | 0 | 1 | 0 | 5 |
In contrast to OCT, the average SLP-derived RNFL measurement was greater than the 95th percentile of controls in only 1 of 24 eyes (Fig. 1c). By quadrant analysis (Table 4a), the SLP-derived RNFL did show abnormal thickening in some eyes, but in fewer quadrants than the corresponding OCT, and the thickening was most frequent in the temporal quadrant (Table 4a). By SLP (Table 4b), only one eye with papilledema had a quadrant measurement less than the fifth percentile of the age-matched control group, and this was an inferior quadrant (SLP-derived RNFL = 54 μm; lower fifth percentile for younger controls = 57 μm).
Patients with NAION
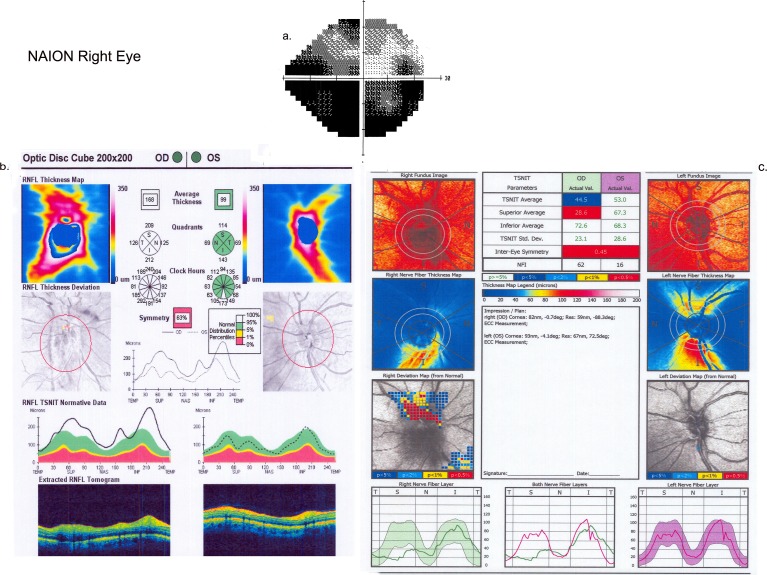
Twenty-one eyes with acute NAION associated with active disc swelling were evaluated (20 with HD-OCT, 1 with time domain OCT). The visual field and visual acuity deficits varied among patients, but were typical of NAION (Table 2; Fig. 2a). The average RNFL measurement by OCT also varied among eyes with acute NAION (range, 111–408 μm; Table 3) and for SLP (range, 22–64 μm), consistent with the variation in the severity of disc edema among NAION eyes studied. Owing to the entry criteria, all eyes measured by OCT had average RNFL measurements greater than the 95th percentile of age-matched controls (Fig. 2b). For OCT, regional thickening of the quadrant RNFL was observed at acute presentation (Table 4a). At presentation, no eyes tested by OCT had an average RNFL or quadrant RNFL measurement (that showed thinning) less than the fifth percentile of controls.
Figure 2.
(a) Visual field for the affected eye (OD) in a patient with NAION at presentation. (b) Cirrus output for spectral OCT for the same patient with NAION at presentation. (c) GDx output for SLP for the same patient with NAION at presentation.
In contrast to OCT, no eye with acute NAION had an average RNFL measurement derived from SLP retardance that was greater than the 95th percentile of controls at presentation. However, a small number of eyes showed regional thickening of RNFL by SLP (Table 4a). Note that no eyes tested by SLP had superior quadrant thickening of the RNFL, as was commonly observed with OCT. Of significance, the average SLP-derived RNFL value was below the fifth percentile of age-matched controls in two NAION eyes at presentation. Of even more significance was the fact that at the acute stage of NAION, SLP-derived RNFL measurements were below the fifth percentile of controls in at least one quadrant in 13 NAION eyes, or 62% (Fig. 2c). The abnormally low SLP-derived RNFL values predominantly affected the superior and inferior quadrants (Table 4b).
At presentation, the visual field defect predominantly affected the superior field in 5 eyes, the inferior field in 13 eyes, and the entire visual field in 1 eye, and equal superior and inferior partial defects in 2 eyes. In the 15 eyes with some degree of inferior field defect, 11 had SLP-derived RNFL values less than the fifth percentile of controls in the corresponding superior quadrant. Three of seven eyes with a superior visual field defect had SLP-derived RNFL values less than the fifth percentile of controls in the corresponding inferior quadrant. However, the eye with the entire visual field affected had no quadrants with retardance less than the fifth percentile of controls.
At one month after presentation, eyes with NAION continued to demonstrate impairment of visual acuity and visual field (Table 2). Of NAION eyes with adequate-quality OCT and SLP imaging, the average OCT RNFL measurements (Table 5) were smaller than at presentation but remained above the 95th percentile of controls in 5 of 17 eyes measured by OCT but in none of 16 eyes measured by SLP. By quadrants, the OCT RNFL value of one or more quadrants was greater than the 95th percentile of controls in nine eyes and in none for SLP-derived RNFL values. One or more RNFL quadrant measurements were less than the control fifth percentile for OCT in 7 of 17 eyes (41%) and in 14 of 16 eyes (88%) for SLP at one-month follow-up.
Table 5.
Affected Eye RNFL Thickness at One and Six Months
|
Diagnosis |
Imaging Test |
Average RFNL |
Temporal Quadrant |
Superior Quadrant |
Nasal Quadrant |
Inferior Quadrant |
| NAION: No. of eyes RNFL thinned, 1 mo | OCT | 6/17 | 2/17 | 5/17 | 2/17 | 5/17 |
| SLP | 4/16 | 1/16 | 14/16 | 1/16 | 7/16 | |
| NAION | OCT | 111 ± 49 | 78 ± 50 | 123 ± 57 | 95 ± 36 | 149 ± 85 |
| Thickness, μm, mean ± SD (1 mo) | SLP | 43 ± 9 | 27 ± 9 | 44 ± 13 | 36 ± 12 | 61 ± 17 |
| NAION: No. of eyes RNFL thinned, 6 mo | OCT | 15/17 | 9/17 | 15/17 | 4/17 | 15/17 |
| NAION | ||||||
| Thickness, μm, mean ± SD (6 mo) P for 1 and 6 mo | OCT | 71 ± 9, P = 0.003 | 56 ± 10, P = 0.14 | 73 ± 19, P = 0.005 | 62 ± 11, P = 0.04 | 99 ± 34, P = 0.003 |
| Optic neuritis: No. of eyes RNFL thinned, 1 mo | OCT | 0/11 | 0/11 | 0/11 | 0/11 | 0/11 |
| SLP | 1/11 | 0/11 | 0/11 | 0/11 | 2/11 | |
| Optic neuritis | OCT | 127 ± 16 | 73 ± 13 | 164 ± 22 | 110 ± 19 | 160 ± 29 |
| Thickness, μm, mean ± SD (1 mo) | SLP | 52 ± 7 | 28 ± 8 | 70 ± 9 | 46 ± 11 | 70 ± 13 |
| Optic neuritis: No. of eyes* RNFL thinned, 6 mo | OCT | 1/12 | /12 | 1/12 | 1/12 | 1/12 |
| Optic neuritis | ||||||
| Thickness, μm, mean ± SD (6 mo) P for 1 and 6 mo | OCT | 96 ± 16, P = 0.0007 | 62 ± 15, P = 0.08 | 128 ± 21, P = 0.002 | 76 ± 17, P = 0.0007 | 120 ± 23, P = 0.004 |
One eye had all 4 quadrants thinned.
Patients with Acute Optic Neuritis
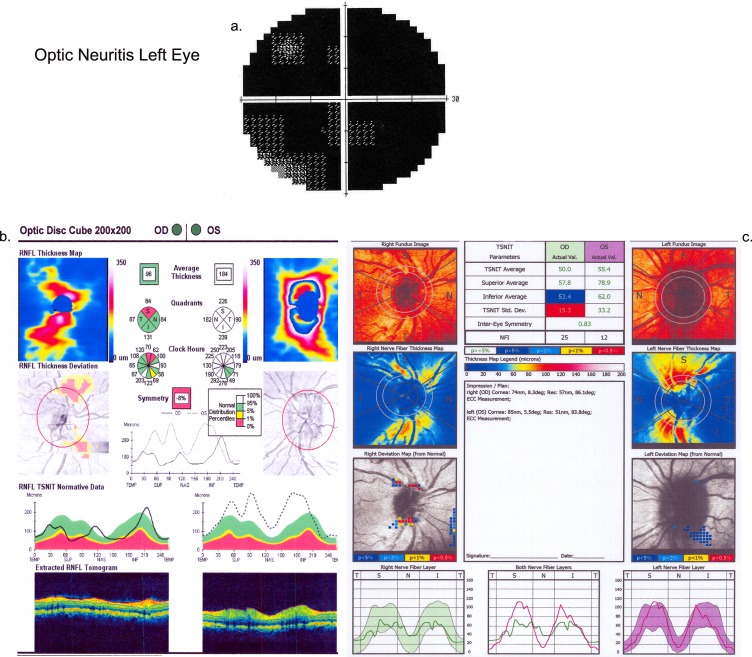
Fourteen eyes with optic neuritis in 14 patients were evaluated (1 HD-OCT, 12 time domain OCT). The degree of vision loss varied between patients and showed a pattern typical of optic neuritis (Table 2; example shown in Fig. 3a). The average OCT-derived RNFL measurement (Table 3) in optic neuritis showed less interindividual differences than for papilledema or NAION eyes (range, 121–180 μm) and for SLP-derived RNFL values (range 49–68 μm), owing to the lesser degree of optic disc edema in acute optic neuritis than in papilledema or acute NAION. Owing to the entry criteria, all eyes had OCT-derived average RNFL thickness measurement greater than the control 95th percentile (Fig. 3b). Regional OCT RNFL measurements showed abnormal thickness in four quadrants of three eyes, three quadrants of two eyes, two quadrants of six eyes, and one quadrant of two eyes (Table 4a). No affected eye had an OCT quadrant RNFL measurement less than the fifth percentile of controls, owing to the lack of atrophy at the acute presentation of vision loss.
Figure 3.
(a) Visual field of the affected eye of a patient with optic neuritis (OS) at presentation. (b) Cirrus output for spectral OCT for the same patient with optic neuritis (OS) at presentation. (c) GDx output for SLP for the same patient with optic neuritis (OS) at presentation.
In contrast to OCT, no eye had SLP-derived average RNFL value greater than the 95th percentile of controls at acute presentation (Fig. 3c). However, three eyes showed regional RNFL thickening by SLP in one quadrant (two temporal and one superior) greater than the control 95th percentile (Table 4a). At acute presentation, one eye had quadrant thinning (inferior quadrant = 50 μm), which was less than the fifth percentile of age-matched control eyes and had a corresponding superior visual field defect. Other than this one example, RNFL by SLP in acute optic neuritis did not show abnormal thinning in the corresponding area of visual field defect (Table 4b).
At one month after acute optic neuritis, all quadrants had OCT-derived RNFL values thinner than at presentation (Table 5), but still thicker than normal. Ten of 12 eyes with adequate quality imaging still showed RNFL thickening by OCT, with average RNFL measurements greater than the 95th percentile of controls. In contrast, none of the 12 eyes had SLP-derived average RNFL value greater than the control 95th percentile. RNFL thinning by SLP (below fifth percentile of controls) was found in only one quadrant in two eyes but in no eyes studied by OCT. At one month, only two eyes with optic neuritis had persistent visual field defects (Table 2). The eye showing a superior visual field defect on acute presentation continued to show this defect with a corresponding inferior quadrant SLP retardance at one month, and the RNFL was further reduced by an additional 5 μm from the 50 μm at presentation (the visual defect and thinning of the RNFL by both OCT and SLP were also observed at 6 months). The second optic neuritis eye that had a persistent inferior-quadrant defect at one month had no significant RNFL reduction by SLP. However, although in this eye the six-month visual field and OCT findings were normal, RNFL by SLP showed persistent thinning.
Comparison of Eyes with NAION, Papilledema, and Optic Neuritis
At presentation, eyes with NAION had greater RNFL thickening via OCT than eyes with optic neuritis (P = 0.008) but not more than eyes with papilledema (P = 0.97), a result consistent with the degree of optic disc edema in the three diagnostic groups at the time of presentation. In contrast to OCT, eyes with acute NAION measured with SLP had significantly lower average derived RNFL values than eyes with optic neuritis (P = 0.008) and papilledema (P = 0.003). Consistent with the predilection for ischemia of the superior ONH with NAION, quadrant analysis of RNFL by SLP revealed that the superior quadrant was significantly thinner in eyes with NAION compared to eyes with optic neuritis (P = 0.001) and papilledema (0.0001).
At one month, RNFL thickness measured by OCT (Table 5) was still not significantly different between NAION and optic neuritis eyes, presumably owing to incomplete resolution of optic disc edema and the lack of axon atrophy at this time point. However, for SLP at one month, eyes with NAION had persistent reduction in RNFL as compared to eyes with optic neuritis (P = 0.002), and this was mostly due to regional thinning in the superior quadrant (P = 0.0001), although there was a trend towards significant thinning in the inferior quadrant (P = 0.08). The same 11 NAION eyes having superior quadrant SLP-derived RNFL values below the fifth percentile of controls at acute presentation continued to have abnormal thinning at one month.
Discussion
The results of this study highlighted the differences in information revealed by OCT and SLP in the setting of three different causes of acute ONH swelling: NAION, optic neuritis, and papilledema. These differences may reflect different pathologic processes, associated with the propensity for recovery (e.g., papilledema and optic neuritis) or for permanent damage (e.g., NAION), and offer the potential for monitoring and directing treatment. One important finding of this study was that OCT reveals anatomic thickening of the peripapillary RNFL no matter what the etiology of the ONH swelling, which is useful for quantifying the degree of edema at presentation and over time. As algorithms for defining the borders of the RNFL in the setting of edema are improved upon, OCT should have a greater use in more severe cases of optic disc edema and elevation, for which present algorithms can fail. The disadvantage of OCT is that it is relatively insensitive for revealing acute axon damage at the time of acute presentation and even one month later. This failure of OCT to demonstrate axonal loss early or at presentation is likely due to two mechanisms: (1) permanent axonal loss or thinning, if occurring, may only start to reduce the thickness of the RNFL at this time point, particularly with a retrobulbar lesion; and (2) persistent edema, which thickens the RNFL at one month, masks a reduction in RNFL thickness.
The results for SLP were quite different than for OCT at acute presentation and at one month. Unlike OCT, SLP did not usually show a significant increase in RNFL thickness, derived from retardance measurements, for all three causes of ONH swelling.
RNFL swelling in the setting of optic disc edema, particularly for papilledema, is principally due to increased water content, which is at first due to intra-axonal edema from axoplasmic flow stasis and which then becomes extracellular as the process progresses. OCT-derived RNFL thickness measurement would be expected to reflect both intracellular and extracellular components, so it was expected to reveal a thickened RNFL regardless of mechanism or cause. In contrast, SLP does not directly measure the actual dimension of each retinal layer. The SLP retardance is primarily dependent upon the integrity of the parallel structural organization of axon plasma membranes, intracellular microtubules, and neurofilaments, which produce birefringence.25,26 The RNFL birefringence induces delay in one of two orthogonal beams of polarized light passing through the bundles of nerve fibers and this slowing, measured in the reflected light, is termed retardance. The retardance is used to calculate the RNFL thickness, which is proportional to the retardance. Therefore, SLP would not be expected to reveal a significant increase in RNFL thickness to the same extent as OCT in conditions of optic disc edema having intact axons with retained intra-axonal cellular organization.12,16 This suggests that the slight SLP-derived RNFL thickening between the affected and unaffected eyes transiently observed in acute optic neuritis (Kupersmith M, et al. IOVS 2009;50:ARVO E-Abstract 5664) is more likely due to an increase in intra-axonal water content, which dilates axons. This is similar to what occurs with papilledema.17–19 Increases in retardance could also be due in part to an increased density of organelles in response to the retrobulbar nonpermanent optic nerve injury. In contrast to the current study, a prior report, using a less sensitive fixed corneal compensation SLP method and no interocular comparison, has failed to demonstrate significant RNFL thickening in NAION.12
The most significant finding of this study was that the SLP showed a decrease in retardance of birefringence (resulting in a decrease in the derived RNFL thickness) in a large number of the NAION eyes at both the acute presentation and at one month, while optic disc edema was still present. The importance of this finding was that OCT did not show RNFL thinning or loss in any of the affected quadrants for any of the three disorders at presentation. The greatest disparity between OCT and SLP values for RNFL at presentation was seen in eyes with NAION, suggesting that in the setting of ischemia, optic disc edema, and visual field loss, SLP may be unique in revealing early disruption of intra-axonal microtubular and neurofilament structure, which may be an early sign of potentially permanent axon loss. Conversely, regional areas that are normal by SLP in acute NAION may not have yet developed the axonal changes associated with inevitable injury and might indicate a potential for recovery from ischemia in those locations. This delay in evolving to a more degenerated state might be an explanation for why more eyes with NAION did not show reduced SLP-derived RNFL reduction. Since significant permanent vision and axon loss is not as common in papilledema, due to intracranial hypertension or in acute optic neuritis, this may also help to explain why SLP-derived RNFL measurements were not found to show thinning on presentation as frequently as for eyes with NAION.
The observed swelling of the ONH and the OCT findings demonstrated that RNFL thickening in papilledema18,19 and optic neuritis16 is predominantly due to axonal blockade in the optic nerve in the retrobulbar space or at the level of the lamina. In NAION, ischemia of the optic nerve in the laminar and retrolaminar regions27 appears to cause permanent injury to axons, which would be associated with loss of mitochondria and microtubules, defects in the axonal membranes, as well as axonal blockade. During the acute and subacute stage of NAION, the axons may succumb to the ischemia and die or they may survive. At this time it is not clear whether any aspect of reduction in SLP retardance is reversible or if the significant decrease is always associated with eventual atrophy of the affected RFNL. This is the subject of an ongoing study to better define how well SLP provides prognostic information in NAION. In acute inner retinal ischemia due to central retinal artery occlusion (a more profound cause of ischemia to the inner retina), all eyes have marked diffuse reduction in retardance at presentation and have sustained permanent injury.28 Normally, the retardance is proportional to the RNFL thickness and decreases with RNFL or axonal thinning29 or microtubule loss.30 Loss or disruption of the structures that are birefringent accounts for the reduction in retardation and the calculated RNFL thinning. Thus, SLP retardance reduction suggests an acute disruption in the intra-axonal structures, such as microtubules or axonal membrane, rather than an absolute loss or thinning of RNFL at presentation in NAION eyes. The retardance reduction in NAION was most often regional, and most often affecting the quadrant corresponding to the visual field defect. Factors that determine the frequency by which SLP affects retardance in acute NAION include the extent of ischemia and intracellular disorganization of microtubules within a given RNFL bundle at the time of imaging. Axonal loss and thinning was readily apparent with OCT (41% of eyes) at one month in NAION eyes, while at that later time point SLP demonstrated an even greater decrease in retardance in 88% of affected eyes. The same quadrants with reduced retardance at baseline had persistent reduction at one month, supporting the lack of reversibility of acute axonal injury within the SLP-derived RNFL in NAION. In contrast, at one month, only 18% (two) of eyes with optic neuritis showed reduced retardation while none, using our criteria for OCT, had RNFL thinning. Our conclusion that SLP does not frequently show retardance reduction in eyes with optic neuritis at presentation differs from a report on nine eyes with optic neuritis.31 However, of the four eyes reported that had a decrease in retardance and four that had an increase, all were within our established limits of retest variability and standard deviation.16 In our optic neuritis eyes, SLP-measured RNFL thinning was not observed at presentation even when interocular comparison was performed (data not shown).
Papilledema due to raised intracranial pressure, optic neuritis, and NAION causes swelling or thickening of the peripapillary RNFL, principally due to increased water content within axons and in the interstitial tissue, which is better demonstrated by OCT than by SLP. However, in the setting of swelling of the optic disc and RNFL, SLP appears to better reflect injury within the axons of the RNFL, particularly due to ischemia, that leads to permanent injury and persistent vision loss. Our findings also suggest that SLP could be used to measure early axonal injury, which may help determine which patients may be more likely to benefit from treatments that preserve axons.
Disclosure: M.J. Kupersmith, None; R. Kardon, None; M. Durbin, Zeiss Meditec Inc. (F, E); M. Horne, Zeiss Meditec Inc. (F, E); J. Shulman, None
Supported by National Eye Institute Grant 3U10EY017281-01A1S1 and grants from Veterans Health Administration (Merit Grant; Rehabilitation Division) and Research to Prevent Blindness, New York, New York.
References
- 1. Medeiros F, Zangwill L, Bowd C, Weinreb R. Comparison of the GDx VCC scanning laser polarimeter, HRT II confocal scanning laser ophthalmoscope, and Stratus OCT optic coherence tomography for the detection of glaucoma. Arch Ophthalmol. 2004;122:827–837. [DOI] [PubMed] [Google Scholar]
- 2. Pro M, Pons M, Liebmann J, et al. Imaging of the optic disc and retinal nerve fiber layer in acute optic neuritis. J Neurol Sci. 2006;250:114–119. [DOI] [PubMed] [Google Scholar]
- 3. Trip A, Schlottmann P, Jones S, et al. Retinal nerve fiber layer axonal loss and visual dysfunction in optic neuritis. Ann Neurol. 2005;58:383–391. [DOI] [PubMed] [Google Scholar]
- 4. Costello F, Coupland S, Hodge W, et al. Quantifying axonal loss after optic neuritis with ocular coherence tomography. Ann Neurol. 2006;59:963–969. [DOI] [PubMed] [Google Scholar]
- 5. Steel DH, Waldock A. Measurement of the retinal nerve fiber layer with scanning laser polarimetry in patients with previous demyelinating optic neuritis. J Neurol Neurosurg Psychiatry. 1988;64:505–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Colen T, van Everdinagen J, Lemij H. Axonal loss in a patient with anterior ischemic optic neuropathy as measured with scanning laser polarimetry. Am J Ophthalmol. 2000;130:847–850. [DOI] [PubMed] [Google Scholar]
- 7. DeLeon-Ortega J, Carroll K, Arthur S, Girkin C. Correlations between retinal nerve fiber layer and visual fields in eyes with non-arteritic anterior ischemic optic neuropathy. Am J Ophthalmol. 2007;143:288–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Danesh-Meyer H, Carroll S, Ku J, et al. Correlation of retinal nerve fiber layer measured by scanning laser polarimeter to visual field in ischemic optic neuropathy. Arch Ophthalmol. 2006;124:1720–1726. [DOI] [PubMed] [Google Scholar]
- 9. Contreras I, Rebolleda G, Noval S, Munoz-Negrete F. Optic disc evaluation by optical coherence tomography in nonarteritic anterior ischemic optic neuropathy. Invest Ophthalmol Vis Sci. 2007;48:4087–4092. [DOI] [PubMed] [Google Scholar]
- 10. Bellusci C, Savini G, Carbonelli M, Carelli V, Sadun A, Barboni P. Retinal nerve fiber layer thickness in nonarteritic anterior ischemic optic neuropathy. Graefes Arch Clin Exp Ophthalmol. 2008;246:641–648. [DOI] [PubMed] [Google Scholar]
- 11. Meier F, Bernasconi P, Stürmer J, Caubergh M, Landau K. Axonal loss from acute optic neuropathy documented by scanning laser polarimetry. Br J Ophthalmol. 2002;86:285–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Banks M, Robe-Collignon N, Rizzo J, Pasquale L. Scanning laser polarimetry of edematous and atrophic optic nerve heads. Arch Ophthalmol. 2003;121:484–490. [DOI] [PubMed] [Google Scholar]
- 13. Karam E, Hedges T. Optical coherence tomography of the retinal nerve fibre layer in mild papilloedema and pseudopapilloedema. Br J Ophthalmol. 2005;89:294–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Rebolleda G, Munoz-Negrete F. Follow-up of mild papilledema in idiopathic intracranial hypertension with optical coherence tomography. Invest Ophthalmol Vis Sci. 2009;50:5197–5200. [DOI] [PubMed] [Google Scholar]
- 15. Scott C, Kardon R, Lee A, Frisen L, Wall M. Diagnosis and grading of papilledema in patients with raised intracranial pressure using optical coherence tomography vs clinical expert assessment using a clinical staging scale. Arch Ophthalmol. 2010;128:705–711. [DOI] [PubMed] [Google Scholar]
- 16. Kupersmith M, Mandel G, Anderson S, Meltzer D, Kardon R. Baseline and one month changes in the peripapillary retinal nerve fiber layer in acute optic neuritis: relation to baseline vision and MRI. J Neurol Sci. 2011;308:117–123. [DOI] [PubMed] [Google Scholar]
- 17. Tso M, Fine B. Electron microscopic study of human papilledema. Am J Ophthalmol. 1976;82:424–434. [DOI] [PubMed] [Google Scholar]
- 18. Hayreh S. Pathogenesis of optic disc oedema in raised intracranial pressure. Trans Ophthalmol Soc UK. 1976;96:404–407. [PubMed] [Google Scholar]
- 19. Hayreh S. Evolution and early diagnosis of optic disc oedema in raised intracranial pressure. Trans Ophthalmol Soc UK. 1976;96:408–411. [PubMed] [Google Scholar]
- 20. Prineas J. The neuropathology of multiple sclerosis. : Vinken P, Bruyn G, Klawans G, et al. , eds. Handbook of Clinical Neurology. Vol 47. Amsterdam: Elsevier; 1985: 212–257.
- 21. Henderson A, Barnett M, Parratt J, Prineas J. Multiple sclerosis; distribution of inflammatory cells in newly forming lesions. Ann Neurol. 2009;66:739–753. [DOI] [PubMed] [Google Scholar]
- 22. Beck R, Cleary P, Anderson M, Kupersmith M, et al. A randomized, controlled trial of corticosteroids in the treatment of acute optic neuritis. N Engl J Med. 1992;326:581–588. [DOI] [PubMed] [Google Scholar]
- 23. The Ischemic Optic Neuropathy Decompression Trial Research Group. Optic nerve decompression surgery for nonarteritic anterior ischemic optic neuropathy (NAION) is not effective and may be harmful. JAMA. 1995;273:625–632. [PubMed] [Google Scholar]
- 24. Hayreh S, Zimmerman B. Nonarteritic anterior ischemic optic neuropathy: natural history of visual outcome. Ophthalmology. 2008;115:298–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Zhou Q, Knighton R. Light scattering and form of parallel cylindrical arrays that represent cellular organelles of the retinal nerve fiber layer. Appl Opt. 1997;36:2273–2285. [DOI] [PubMed] [Google Scholar]
- 26. Huang X-R, Bagga H, Greenfield D, Knighton R. Variation of peripapillary retinal nerve fiber layer birefringence in normal human subjects. Invest Ophthalmol Vis Sci. 2004;45:3073–3080. [DOI] [PubMed] [Google Scholar]
- 27. Arnold A. Pathogenesis of nonarteritic anterior ischemic optic neuropathy. J Neuroophthalmol. 2003;23:157–163. [DOI] [PubMed] [Google Scholar]
- 28. Foroozian R, Buono L, Savino P, Sergott R. Scanning laser polarimetry of the retinal nerve fiber layer in central retinal artery occlusion. Ophthalmology. 2003;110:715–718. [DOI] [PubMed] [Google Scholar]
- 29. Weinreb R, Dreher A, Coleman A, Quigley H, Shaw B, Reiter K. Histopathologic validation of Fourier-ellipsometry measurements of retinal nerve fiber layer thickness. Arch Ophthalmol. 1990;108:557–560. [DOI] [PubMed] [Google Scholar]
- 30. Huang X-R, Knighton R. Microtubules contribute to the birefringence of the retinal nerve fiber layer. Invest Ophthalmol Vis Sci. 2005;46:4588–4593. [DOI] [PubMed] [Google Scholar]
- 31. Garas A, Simó M, Holló G. Nerve fiber layer and macular thinning measured with different imaging methods during the course of acute optic neuritis. Eur J Ophthalmol. 2011;21:473–483. [DOI] [PubMed] [Google Scholar]