Abstract
Purpose.
To examine the effect of obesity on the incidence of age-related eye disease.
Methods.
Participants of the Beaver Dam Eye Study were examined every 5 years over a 20-year period (1988–1990 through 2008–2010). Lens and fundus photographs were used to evaluate presence and severity of cataract and macular degeneration. Height and weight were measured at all examinations. Waist and hip circumference were measured at all examinations beginning at the first follow-up (1993–1995). Models of ocular outcomes over 15 years were stratified by sex and smoking status.
Results.
Overall, 2641 participants contributed 5567 person-visits to 15-year incidence analysis. Female nonsmokers had increased risk of late AMD associated with higher body mass index (BMI; hazard ratio [HR] per 2.5 kg/m2 1.31, 95% confidence interval [CI] 1.15–1.50, P < 0.001), waist to hip ratio (HR per 0.1 cm/cm 1.95, 95% CI 1.33–2.86, P < 0.001), waist circumference (HR per 5 cm 1.21, 95% CI 1.10–1.34, P < 0.001), and waist to height ratio (HR per 0.1 cm/cm 1.74, 95% CI 1.31–2.31, P < 0.001). Increased BMI was also associated with early AMD in female nonsmokers (HR 1.10, 95% CI 1.02–1.19, P = 0.02).
Conclusions.
Female nonsmokers had risk of late AMD associated with increasing measures of greater obesity and increased risk of early AMD associated with greater BMI.
Keywords: age-related macular degeneration, smoking, body mass index
Over 15 years of follow-up in the Beaver Dam Eye Study, female nonsmokers had increased risk of incident late AMD associated with increased body mass index, waist circumference, waist to hip ratio, and waist to height ratio.
Introduction
Measures of obesity, such as body mass index (BMI), waist to hip ratio (WHR), waist to height ratio (WHtR), and waist circumference (WC) have shown to be related to illness and mortality in many populations.1–4 It is advantageous to examine the effect of each of these measures as it has been shown that WHR, WHtR, or WC may be better predictors for illness or mortality than BMI, which has been commonly used as a predictor of risk.5–8 Some studies suggest that WC is a better indicator of abdominal visceral fat than BMI.9 Waist circumference may be useful in identifying health risks even in healthy weight elderly persons.10 It has also been reported that WHR was a better measure of adiposity-related diabetes risk than BMI in a study of 29,000 Chinese adults.11 As a practical matter, WHR, WHtR, and WC are more easily obtained as they do not require a scale to measure body weight.
In the Beaver Dam Eye Study (BDES), we have previously reported cross-sectional associations of obesity as it relates to prevalent cataract and AMD12 and 5-year incidence of cataract13 as well as the 5-year incidence of macular degeneration in a collaborative study.14 Of note, at the first BDES examination phase at which WHR was measured, it was more strongly associated with early AMD in women than was BMI.12
While the link between excess body fat and incidence of outcomes such as cardiovascular disease,15 type II diabetes,16 and hypertension are clear,17 reports of risk of age-related eye disease as related to obesity have shown to be inconsistent in strength as well as directionality of effect.12,14,18–23 Inconsistencies in study findings may be a result of differing subgroups used for analysis. Distribution of body fat and risk of disease associated with increased body mass, WC, WHR, or WhtR have been shown to differ by sex.24,25 Also, adiposity itself and its effect as a risk factor may be influenced by smoking status,26 an exposure that is linked to many age-related eye diseases.27–29
In the current report, we describe the associations in the BDES of several measures of adiposity with incident AMD, cataract, cataract extraction by sex, and smoking status1,18,26 taking advantage of the long-term follow-up of this population-based cohort.
Methods
A private census of Beaver Dam, Wisconsin, was performed from 1987 to 1988 to identify all eligible residents 43 years of age and older for study participation. At the baseline examination (1988–1990), 4926 participants were seen with 3722, 2962, 2375, and 1913 seen at the 5- (1993–1995), 10- (1998–2000), 15- (2003–2005), and 20-year (2008–2010) follow-up examinations, respectively. Average time between visits was 5.06 years with greater than 80% participation among survivors.30–32 Differences in baseline characteristics between survivors and nonsurvivors have been presented elsewhere.30–32 Participants were generally younger, had lower blood pressure, and had fewer comorbidities than nonparticipants. All data were collected with approval of the institutional review board from the University of Wisconsin-Madison. The work complied with the Health Insurance Portability and Accountability Act in conformity with all federal and state laws, and the study adhered to the tenets of the Declaration of Helsinki. Informed consent was obtained from each participant prior to examination.
Participants were examined at the study site, in a nursing home, or in their homes. Protocols for measurements relevant to this analysis were the same across all visits.33,34 All examination components were performed by trained examiners. Self-reported smoking, drinking, medication, health, and lifestyle histories were obtained using a standard questionnaire. Ever-smoking was defined as having smoked at least 100 cigarettes in a lifetime; for purposes of this paper, persons were dichotomized as ever-smokers (smokers) or never smokers (nonsmokers). In smokers, the total pack-years smoked was defined as the number of cigarettes smoked per day divided by 20 and multiplied by the number of years smoked. Heavy drinking was defined as ever having consumed four or more servings of alcoholic beverages on a daily basis. A sedentary lifestyle was defined as working up a sweat less than three times a week. Participants were asked to bring all medications, which were being taken at the time of the examination, to the examination site to be recorded. Blood pressures were measured according to a modification of the Hypertension Detection and Follow-up Program protocol.35 Hypertension was defined as systolic blood pressure greater than 140 mm Hg, diastolic blood pressure greater than 90 mm Hg, or taking a blood pressure medication at the time of examination. Glycosylated hemoglobin levels were measured at each examination. Diabetes was defined as self-report confirmed by use of insulin or diet to control diabetes, self-report with glycosylated hemoglobin A1c level above 6.5%, or no self-report with glycosylated hemoglobin A1c above 7%.36
Height and weight were measured at all examinations on a standard scale while participants were not wearing shoes. Waist and hip circumference were measured at each examination beginning at the second examination (BDES2, 1993–1995). Circumference measurements were taken twice and the average of the two measurements was used for analysis. Body mass index was defined as weight in kilograms divided by the square of height in meters. Waist-to-hip ratio was calculated as the WC in centimeters divided by the hip circumference in centimeters. Waist-to-height ratio was calculated as the WC in centimeters divided by the height in centimeters.
Following pupil dilation, photographs of the lens were taken in accordance with study protocol and graded in masked fashion by experienced graders.33,34 Slit-lamp photographs were taken to determine the degree of nuclear sclerosis and retroillumination photographs were taken to grade presence and severity of cortical cataract and posterior subscapular cataract (PSC). Scores for nuclear sclerosis were based on a 5-step severity score determined by comparisons with standard photographs. Nuclear cataract was considered present with lens opacity greater than standard 3. Scores for cortical cataract and PSC were based on the amount of lens involved. Cortical cataract was considered present if greater than or equal to 5% of the lens was involved. Posterior subscapular cataract was considered present if greater than or equal to 5% of a given lens segment was involved.33 Presence of cataract surgery was determined by self-reported history and was corroborated by red reflex photographs.
Photographs of the retina were taken to determine presence and severity of lesions associated with AMD and the Wisconsin Age-related Maculopathy Grading System was used to assess the fundus photographs.34 Grading procedures, lesion descriptions, and detailed definitions for presence and severity have appeared elsewhere.37 Early AMD was defined by the presence of soft indistinct drusen or any type of drusen associated with pigmentary abnormality (i.e., retinal pigment epithelium depigmentation or increased retinal pigment). Late AMD was defined by the presence of neovascular macular degeneration or pure geographic atrophy (GA).
Statistical Analysis
For this analysis, the outcomes of interest were incidence of cataract, cataract surgery, and early or late AMD. The main risk factors were measures of body adiposity including BMI, WC, WHR, and WHtR that were measured beginning at the second examination (BDES2, 1993–1995). Participants were seen every 5 years from baseline, and data from each 5-year interval was combined to determine risk of the given outcome over a long-term 15-year follow-up. Incidence of the outcome was determined at the person level, combining data from both eyes. At the start of the interval, a person was considered at risk for the outcome if they were free of disease in both eyes. Incidence at the following examination (end of the 5-year interval) was determined if the person had disease in either eye. Persons were eligible to contribute to analysis until incidence of the outcome of interest or censoring occurred. To contribute to analysis in a given 5-year interval, a person must have had complete data on the risk factors of interest (BMI, WHR, WC, or WHtR) and the outcome (incident nuclear, cataract, cortical cataract, or PSC, cataract surgery, or early or late AMD) and all covariates included in the maximally adjusted model (age, sedentary lifestyle, diabetes, hypertension). All analyses were performed with stratification by sex and smoking status.
All models presented were fit using the discrete-time hazard model with complementary log-log link function and time varying predictors.38 Risk variables were updated and censoring was accounted for appropriately. In this way, change in the risk profile, for example being a nonsmoker and becoming a smoker, were updated at the beginning of each 5-year interval. P values represent a two-tailed test of significance with α equaling 0.05. Effects of body measures on risk of ocular outcomes were examined in an age adjusted model and a maximally adjusted model which included age, sedentary lifestyle, and presence of hypertension and diabetes. SAS version 9.3 (SAS Institute, Inc., Cary, NC, USA) was used for all analyses.
Results
In our preliminary analysis we examined the effects of BMI, WHR, WHtR, and WC on incidence of cataract, cataract surgery, and early and late AMD by sex and smoking status. Waist and hip circumference were measured beginning at the second examination (BDES2, 1993–1995); therefore, these models examine the risk of each outcome by the given risk factors over 15 years of follow-up (BDES2 to BDES5 2008–2010). Overall, there were 2641 participants (870 female nonsmokers, 640 female smokers, 368 male nonsmokers, and 763 male smokers contributing 1824, 1334, 803, and 1606 person-visits, respectively) contributing to this analysis for at least one outcome. Participant characteristics over all person-visits for those included and excluded from analysis are presented (Supplementary Table S1). Generally, persons who were excluded from analysis were older and had more comorbid conditions compared with those included.
For those included, female smokers tended to be younger than nonsmokers. There were no significant differences between female nonsmokers and smokers with respect to systolic or diastolic blood pressure, education level, BMI, WC, WHR, WHtR, heavy drinking, cardiovascular disease, hypertension, diabetes, having a sedentary lifestyle, or using vitamins. In males, nonsmokers tended to be older and have more years of education and smaller WC as compared with male smokers. Male smokers were more likely to have ever been a heavy drinker, have cardiovascular disease, or diabetes and were less likely to have a sedentary lifestyle (Table 1).
Table 1.
Participant Characteristics for Persons Included in Analysis by Sex and Smoking Status Over All Person-Visits
|
Females |
Males |
|||||
|
Mean ± SD orN(%) |
PValue* |
Mean ± SD orN(%) |
PValue* |
|||
|
Nonsmokers |
Smokers |
Nonsmokers |
Smokers |
|||
| Age, y | 67.1 ± 9.5 | 65.1 ± 8.5 | 0.001 | 65.0 ± 8.9 | 65.0 ± 8.5 | 0.03 |
| Systolic BP, mm Hg | 130.1 ± 19.3 | 128.4 ± 18.7 | 0.35 | 127.8 ± 17.2 | 130.2 ± 17.0 | 0.80 |
| Diastolic BP, mm Hg | 74.9 ± 10.3 | 74.1 ± 10.5 | 0.68 | 77.0 ± 10.6 | 76.2 ± 10.2 | 0.18 |
| Education, y | 12.6 ± 2.4 | 12.6 ± 2.1 | 0.07 | 13.6 ± 3.6 | 12.9 ± 2.9 | 0.001 |
| BMI, kg/m2 | 29.7 ± 5.8 | 29.5 ± 6.2 | 0.61 | 30.2 ± 4.7 | 30.6 ± 4.7 | 0.41 |
| WC, cm | 91.0 ± 15.9 | 91.8 ± 16.7 | 0.17 | 102.5 ± 12.1 | 105.0 ± 12.0 | 0.05 |
| WHR, cm/cm | 0.8 ± 0.1 | 0.8 ± 0.1 | 0.31 | 1.0 ± 0.1 | 1.0 ± 0.1 | 0.09 |
| WHtR, cm/cm | 0.6 ± 0.1 | 0.6 ± 0.1 | 0.42 | 0.6 ± 0.1 | 0.6 ± 0.1 | 0.08 |
| Pack-years† | 0 | 23.3 ± 21.9 | 0 | 30.7 ± 27.3 | ||
| Heavy drinker | ||||||
| No | 1778 (97.5) | 1185 (88.8) | 0.54 | 696 (86.7) | 397 (62.2) | 0.001 |
| Yes | 46 (2.5) | 149 (11.2) | 107 (13.3) | 241 (37.8) | ||
| Sedentary lifestyle | ||||||
| No | 545 (29.9) | 414 (31.0) | 0.15 | 265 (33.0) | 201 (31.5) | 0.04 |
| Yes | 1279 (70.1) | 920 (69.0) | 538 (67.0) | 437 (68.5) | ||
| Cardiovascular disease present | ||||||
| No | 1675 (92.0) | 1220 (91.7) | 0.97 | 707 (88.0) | 514 (81.0) | 0.04 |
| Yes | 146 (8.0) | 111 (8.3) | 96.0 (12.0) | 330 (20.6) | ||
| Hypertension present | ||||||
| No | 813 (44.6) | 663 (49.7) | 0.81 | 390 (48.6) | 711 (44.3) | 0.80 |
| Yes | 1011 (55.4) | 671 (50.3) | 413 (51.4) | 895 (55.7) | ||
| Diabetes present | ||||||
| No | 1666 (91.3) | 1202 (90.1) | 0.66 | 724 (90.2) | 1388 (86.4) | 0.05 |
| Yes | 158 (8.7) | 132 (9.9) | 79 (9.8) | 218 (13.6) | ||
| Vitamin use | ||||||
| No | 567 (31.1) | 436 (32.7) | 0.17 | 364 (45.3) | 746 (46.5) | 0.22 |
| Yes | 1257 (68.9) | 898 (67.3) | 439 (54.7) | 860 (53.6) | ||
BP, blood pressure.
Age adjusted.
Number of packs of cigarettes smoked per day divided by years smoked.
In female nonsmokers, there were no significant associations between any measure of adiposity and any cataract endpoint. However, increased BMI was associated with increased risk of early AMD in age adjusted and maximally adjusted models (for the maximally adjusted models HR per 2.5 kg/m2 1.10, 95% CI 1.02–1.19, P = 0.02). In female nonsmokers, each body measure was associated with increased risk of late AMD in the age adjusted and maximally adjusted models (BMI: HR per 2.5 kg/m2 1.31, 95% CI 1.15–1.50, P < 0.001; WC: HR per 5 cm 1.21, 95% CI 1.10–1.34, P < 0.001; WHR: HR per 0.1 cm/cm 1.95, 95% CI 1.33–2.86, P < 0.001; and WHtR: HR per 0.1 cm/cm 1.74, 95% CI 1.31–2.31, P < 0.001; Table 2). In male nonsmokers, increased BMI, WHR, and WHtR were associated with greater risk of cortical cataract and PSC in the age adjusted model, but these effects did not remain significant when adjusting for confounders in the maximally adjusted model (Table 2).
Table 2.
Body Measures and Incidence of Any Cataract, Cataract Surgery, and Early or Late AMD Over 15 Years (Beaver Dam Eye Study [BDES] 2 to BDES5) by Sex and Smoking Status
|
Incidence |
Sex |
Risk Factor |
Nonsmokers |
Smokers |
||||||||||
|
Age Adjusted |
Maximally Adjusted |
Age Adjusted |
Maximally Adjusted |
|||||||||||
|
HR |
95% CI |
PValue |
HR |
95% CI |
P value |
HR |
95% CI |
PValue |
HR |
95% CI |
PValue |
|||
| Cataract surgery | Female | BMI* | 1.02 | (0.96, 1.09) | 0.44 | 1.02 | (0.95, 1.09) | 0.59 | 1.06 | (1.00, 1.13) | 0.05 | 1.03 | (0.97, 1.09) | 0.38 |
| WC† | 1.01 | (0.97, 1.06) | 0.59 | 1.00 | (0.96, 1.05) | 0.91 | 1.06 | (1.01, 1.10) | 0.01 | 1.03 | (0.98, 1.08) | 0.20 | ||
| WHR‡ | 1.06 | (0.90, 1.26) | 0.49 | 1.03 | (0.86, 1.22) | 0.76 | 1.21 | (1.01, 1.45) | 0.04 | 1.08 | (0.89, 1.32) | 0.42 | ||
| WHtR‡ | 1.05 | (0.92, 1.20) | 0.50 | 1.02 | (0.89, 1.17) | 0.78 | 1.19 | (1.04, 1.36) | 0.01 | 1.10 | (0.95, 1.27) | 0.21 | ||
| Male | BMI* | 1.09 | (0.96, 1.24) | 0.17 | 1.09 | (0.97, 1.24) | 0.16 | 0.97 | (0.89, 1.06) | 0.55 | 0.97 | (0.89, 1.07) | 0.57 | |
| WC† | 1.07 | (0.97, 1.18) | 0.17 | 1.08 | (0.98, 1.18) | 0.14 | 1.00 | (0.93, 1.07) | 0.97 | 1.00 | (0.93, 1.07) | 0.99 | ||
| WHR‡ | 1.24 | (0.87, 1.78) | 0.23 | 1.28 | (0.89, 1.84) | 0.18 | 1.07 | (0.80, 1.42) | 0.65 | 1.06 | (0.80, 1.42) | 0.67 | ||
| WHtR‡ | 1.26 | (0.92, 1.74) | 0.15 | 1.28 | (0.93, 1.74) | 0.12 | 1.01 | (0.79, 1.27) | 0.97 | 1.00 | (0.79, 1.27) | 0.99 | ||
| Nuclear cataract | Female | BMI* | 0.96 | (0.89, 1.03) | 0.29 | 0.96 | (0.89, 1.04) | 0.32 | 0.95 | (0.88, 1.03) | 0.20 | 0.95 | (0.87, 1.03) | 0.20 |
| WC† | 1.97 | (0.93, 1.02) | 0.30 | 0.97 | (0.92, 1.03) | 0.29 | 0.98 | (0.92, 1.03) | 0.39 | 0.98 | (0.92, 1.03) | 0.40 | ||
| WHR‡ | 1.97 | (0.80, 1.17) | 0.73 | 0.96 | (0.78, 1.18) | 0.68 | 1.13 | (0.90, 1.41) | 0.31 | 1.15 | (0.91, 1.46) | 0.25 | ||
| WHtR‡ | 0.92 | (0.79, 1.07) | 0.27 | 0.91 | (0.76, 1.08) | 0.26 | 0.94 | (0.79, 1.12) | 0.52 | 0.94 | (0.79, 1.13) | 0.54 | ||
| Male | BMI* | 0.98 | (0.86, 1.13) | 0.82 | 0.97 | (0.84, 1.12) | 0.69 | 0.93 | (0.84, 1.02) | 0.13 | 0.86 | (0.70, 1.05) | 0.14 | |
| WC† | 1.00 | (0.91, 1.10) | 0.97 | 0.99 | (0.89, 1.09) | 0.78 | 0.98 | (0.92, 1.05) | 0.61 | 0.99 | (0.92, 1.06) | 0.71 | ||
| WHR‡ | 0.92 | (0.64, 1.33) | 0.67 | 0.88 | (0.60, 1.29) | 0.50 | 1.11 | (0.83, 1.47) | 0.48 | 1.13 | (0.84, 1.52) | 0.41 | ||
| WHtR‡ | 0.90 | (0.64, 1.28) | 0.57 | 0.87 | (0.61, 1.24) | 0.43 | 0.95 | (0.74, 1.21) | 0.67 | 0.96 | (0.74, 1.24) | 0.74 | ||
| Cortical cataract or PSC | Female | BMI* | 1.04 | (0.98, 1.11) | 0.21 | 1.03 | (0.96, 1.11) | 0.37 | 0.95 | (0.88, 1.02) | 0.14 | 0.93 | (0.86, 1.01) | 0.08 |
| WC† | 1.02 | (0.97, 1.06) | 0.42 | 1.01 | (0.96, 1.06) | 0.80 | 0.99 | (0.94, 1.04) | 0.73 | 0.98 | (0.93, 1.04) | 0.56 | ||
| WHR‡ | 1.13 | (0.96, 1.33) | 0.16 | 1.08 | (0.90, 1.29) | 0.40 | 1.20 | (0.97, 1.48) | 0.09 | 1.20 | (0.96, 1.50) | 0.12 | ||
| WHtR‡ | 1.07 | (0.94, 1.23) | 0.29 | 1.04 | (0.89, 1.21) | 0.62 | 0.97 | (0.83, 1.14) | 0.72 | 0.95 | (0.80, 1.13) | 0.56 | ||
| Male | BMI* | 1.13 | (1.00, 1.26) | 0.04 | 1.08 | (0.96, 1.22) | 0.18 | 1.06 | (0.98, 1.15) | 0.15 | 1.05 | (0.97, 1.14) | 0.22 | |
| WC† | 1.11 | (1.01, 1.21) | 0.03 | 1.07 | (0.98, 1.18) | 0.14 | 1.04 | (0.98, 1.11) | 0.21 | 1.04 | (0.97, 1.11) | 0.28 | ||
| WHR‡ | 1.54 | (1.07, 2.23) | 0.02 | 1.43 | (0.97, 2.12) | 0.07 | 1.18 | (0.90, 1.53) | 0.23 | 1.15 | (0.88, 1.50) | 0.30 | ||
| WHtR‡ | 1.52 | (1.12, 2.06) | 0.01 | 1.37 | (1.00, 1.88) | 0.05 | 1.26 | (1.00, 1.58) | 0.05 | 1.24 | (0.98, 1.57) | 0.08 | ||
| Early AMD | Female | BMI* | 1.09 | (1.01, 1.17) | 0.03 | 1.10 | (1.02, 1.19) | 0.02 | 1.06 | (0.98, 1.15) | 0.18 | 1.07 | (0.98, 1.17) | 0.14 |
| WC† | 1.02 | (0.97, 1.08) | 0.44 | 1.03 | (0.97, 1.09) | 0.33 | 1.03 | (0.97, 1.10) | 0.31 | 1.04 | (0.97, 1.11) | 0.29 | ||
| WHR‡ | 0.95 | (0.78, 1.16) | 0.61 | 0.96 | (0.78, 1.19) | 0.71 | 0.96 | (0.75, 1.21) | 0.72 | 0.93 | (0.72, 1.20) | 0.59 | ||
| WHtR‡ | 1.05 | (0.89, 1.23) | 0.57 | 1.07 | (0.90, 1.27) | 0.45 | 1.12 | (0.92, 1.36) | 0.25 | 1.14 | (0.93, 1.41) | 0.21 | ||
| Male | BMI* | 0.89 | (0.75, 1.06) | 0.18 | 0.90 | (0.75, 1.07) | 0.23 | 0.98 | (0.89, 1.07) | 0.61 | 1.00 | (0.90, 1.10) | 0.92 | |
| WC† | 0.93 | (0.82, 1.05) | 0.22 | 0.93 | (0.82, 1.06) | 0.29 | 1.02 | (0.94, 1.10) | 0.67 | 1.04 | (0.96, 1.13) | 0.38 | ||
| WHR‡ | 0.91 | (0.57, 1.44) | 0.68 | 0.93 | (0.58, 1.48) | 0.76 | 1.19 | (0.85, 1.66) | 0.31 | 1.29 | (0.92, 1.81) | 0.15 | ||
| WHtR‡ | 0.74 | (0.48, 1.14) | 0.17 | 0.75 | (0.47, 1.19) | 0.22 | 1.06 | (0.81, 1.40) | 0.65 | 1.14 | (0.86, 1.52) | 0.35 | ||
| Late AMD | Female | BMI* | 1.28 | (1.13, 1.45) | 0.001 | 1.31 | (1.15, 1.50) | 0.001 | 0.99 | (0.85, 1.16) | 0.91 | 0.99 | (0.81, 1.21) | 0.93 |
| WC† | 1.19 | (1.09, 1.29) | 0.001 | 1.21 | (1.10, 1.34) | 0.001 | 0.95 | (0.85, 1.05) | 0.32 | 0.94 | (0.81, 1.10) | 0.45 | ||
| WHR‡ | 1.82 | (1.28, 2.59) | 0.001 | 1.95 | (1.33, 2.86) | 0.001 | 0.68 | (0.45, 1.01) | 0.06 | 0.65 | (0.38, 1.12) | 0.12 | ||
| WHtR‡ | 1.63 | (1.27, 2.10) | 0.001 | 1.74 | (1.31, 2.31) | 0.001 | 0.79 | (0.57, 1.12) | 0.19 | 0.78 | (0.49, 1.25) | 0.30 | ||
| Male | BMI* | 0.90 | (0.66, 1.23) | 0.50 | 0.86 | (0.61, 1.20) | 0.36 | 1.19 | (0.94, 1.51) | 0.15 | Cannot estimate | |||
| WC† | 0.95 | (0.74, 1.22) | 0.69 | 0.92 | (0.69, 1.21) | 0.53 | 1.18 | (1.00, 1.40) | 0.05 | Cannot estimate | ||||
| WHR‡ | 1.42 | (0.47, 4.30) | 0.54 | 1.38 | (0.43, 4.45) | 0.59 | 1.57 | (0.85, 2.90) | 0.15 | Cannot estimate | ||||
| WHtR‡ | 0.94 | (0.40, 2.25) | 0.90 | 0.84 | (0.33, 2.18) | 0.73 | 1.90 | (0.99, 3.66) | 0.05 | Cannot estimate | ||||
Maximally adjusted model includes age (age and age squared for cataract outcomes), sedentary lifestyle, hypertension, and diabetes. PSC, posterior subscapular cataract.
BMI per 2.5 kg/m2.
Waist circumference per 5 cm.
WHR and WHtR per 0.1 cm/cm.
In females smokers, BMI, WC, WHR, and WHtR were associated with increased risk of cataract surgery in the age adjusted models; however, these results were attenuated and not significant in the maximally-adjusted model. In male smokers, increased WHtR was associated with increased risk of cortical cataract or PSC in the age adjusted model, although this did not remain significant when adjusting for other risk factors. Greater WC and WHtR were associated with increased risk of late AMD in male smokers when adjusting for age. There was not significant power in this subgroup to calculate the effect of any body measure on incidence of late AMD in the maximally adjusted model (Table 2).
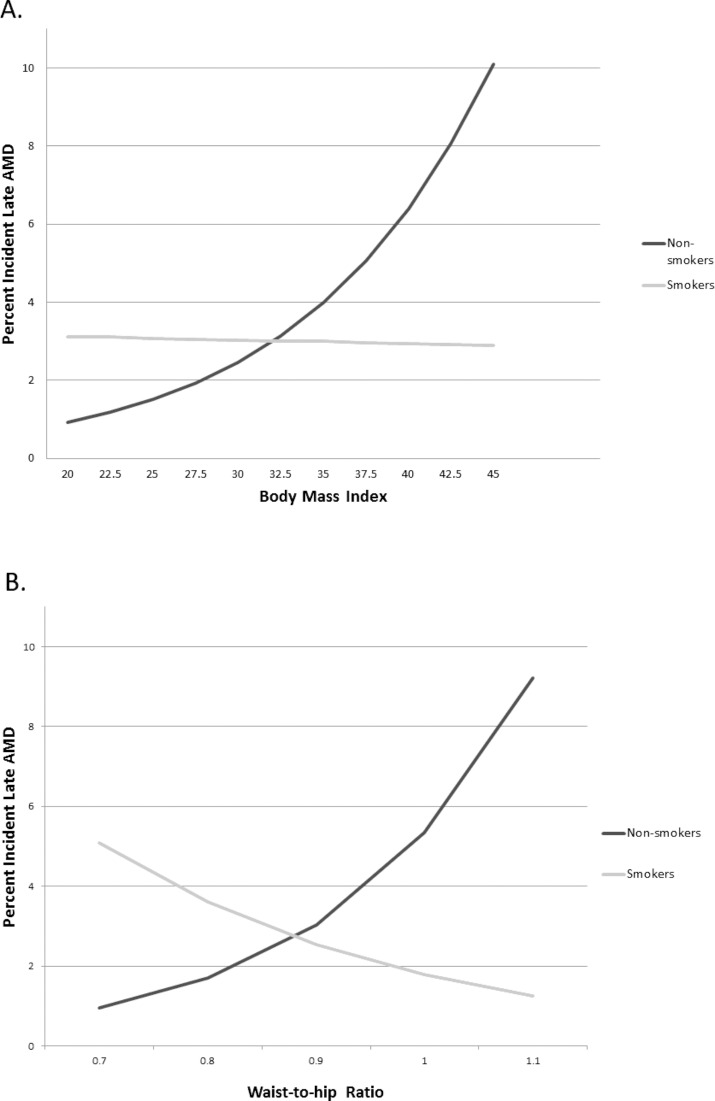
To illustrate the effect of BMI and WHR on risk of late AMD in females, results of the age-adjusted model are presented in the Figure. Incidence of late AMD in female smokers remained constant as BMI increased, while in nonsmokers the incidence of late AMD increased with increase in BMI. For nonsmokers, the risk of late AMD increased significantly with greater WHR (P < 0.001), while incidence of late AMD in smokers decreased with increasing WHR, although the effect was not significant (P = 0.12).
Figure.
(A) Age-adjusted incidence of late AMD in women by BMI and smoking status. (B) Age-adjusted incidence of late AMD in women by waist to hip ratio and smoking status.
Discussion
We examined the effect of measures of adiposity on risk of eye disease, including cataract, cataract surgery, and AMD and found different measures of obesity to be associated with increased risk of late AMD in females who were nonsmokers. This is important because understanding the impact of obesity on health outcomes is critical at a time when obesity rates continue to increase across the globe. Inconsistencies in reporting of the link between obesity and eye disease may be a result of differences in population structure; therefore, research is needed to identify how obesity measures impact risk in various subgroups.
It has been shown that the risk of adverse health outcomes due to increased BMI may be modified by the effect of current and former smoking. Adams et al.1 showed that the effect of BMI on mortality was more pronounced in those without a history of smoking, while this effect was masked when grouping smokers and nonsmokers together in analysis. In another study it was found that both sex and smoking status modified the relationship between measures of obesity and risk of early and late AMD, although they found the effect in men only, which highlights the importance of subgroup analysis in order to gain insight into the complex relationships between these risk factors.18 Chiolero et al.26 points out that smoking may be linked to other risk health behaviors such as being sedentary, having increased alcohol intake, or having an unhealthy diet; therefore a higher BMI would not pose an increased risk beyond these factors, which are captured by smoking status.
Our findings are consistent with previous reports by Klein et al.12,20 as well as those by Chakravarthy and Seddon.19,23 While Tomany14 did not find a relationship between BMI and late AMD in a collaborative, multicohort study, that report did not stratify by sex or smoking status and may have attenuated any possible associations.
The higher incidence of AMD associated with more adipose tissue in older nonsmoking females may reveal a true increase in susceptibility, which is masked by smoking behavior in other analyses. Further study of this observation is warranted because if confirmed, intervention on this characteristic is possible. To what extent measures of body shape and body size not affecting risk of AMD in males could be attributable to a different smoking intensity by sex (male smokers tended to have more pack-years than female smokers in this cohort), or a changing effect of adiposity measures as related to age is yet unknown and is beyond the scope of this manuscript. The relationship between age, sex, body shape, body size, and high-risk habits such as smoking is quite complex and warrants further research.
While our study has many strengths, including 15 years of long-term follow-up using standardized protocols and fundus and lens photographs to assess AMD and cataract status in a well-established population with a low rate of attrition, it also has shortcomings. First, waist and hip measurements were not taken at the baseline examination; therefore, analyses involving these measures are limited to 15 years, which somewhat limits the statistical power to find an effect. Second, the population is limited to persons of white European descent, and these findings may not be generalizable to persons of other races/ethnicites. There are constitutional factors related to obesity that we have not considered (e.g., genes associated with adiposity, ethnicity, adipokines). Studying their effect may further the understanding of the role of systemic factors and age-related eye disease.
In summary, we find little evidence to support the notion of adverse effects of adiposity on the lens. However, we found that risk of late AMD in female nonsmokers increased significantly with increased measures of body adiposity, which highlights the importance of further investigation and corroboration of this finding.
Acknowledgments
Supported by National Institutes of Health, Bethesda, Maryland, United States, Grant EY06594 (BEKK, RK). The National Eye Institute, Bethesda, Maryland, United States, provided funding for entire study, including collection and analyses of data. Additional support was provided by an unrestricted grant from Research to Prevent Blindness, New York, New York, United States.
Disclosure: K.P. Howard, None; B.E.K. Klein, None; K.E. Lee, None; R. Klein, None
References
- 1. Adams KF, Schatzkin A, Harris TB, et al. Overweight, obesity, and mortality in a large prospective cohort of persons 50 to 71 years old. N Engl J Med. 2006; 355: 763–778 [DOI] [PubMed] [Google Scholar]
- 2. Britton KA, Massaro JM, Murabito JM, Kreger BE, Hoffmann U, Fox CS. Body fat distribution, incident cardiovascular disease, cancer, and all-cause mortality. J Am Coll Cardiol. 2013; 62: 921–925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Chen Z, Yang G, Offer A, et al. Body mass index and mortality in China: a 15-year prospective study of 220,000 men. Int J Epidemiol. 2012; 41: 472–481 [DOI] [PubMed] [Google Scholar]
- 4. Cuevas A, Alvarez V, Olivos C. The emerging obesity problem in Latin America. Expert Rev Cardiovasc Ther. 2009; 7: 281–288 [DOI] [PubMed] [Google Scholar]
- 5. Bener A, Yousafzai MT, Darwish S, Al-Hamaq AO, Nasralla EA, Abdul-Ghani M. Obesity index that better predict metabolic syndrome: body mass index, waist circumference, waist hip ratio, or waist height ratio. J Obes. 2013; 2013: 269038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cristo Rodriguez Perez MD, Cabrera De LA, Aguirre-Jaime A, et al. The waist to height ratio as an index of cardiovascular risk and diabetes [in Spanish]. Med Clin (Barc). 2010; 134: 386–391 [DOI] [PubMed] [Google Scholar]
- 7. Lee CM, Huxley RR, Wildman RP, Woodward M. Indices of abdominal obesity are better discriminators of cardiovascular risk factors than BMI: a meta-analysis. J Clin Epidemiol. 2008; 61: 646–653 [DOI] [PubMed] [Google Scholar]
- 8. Noble RE. Waist-to-hip ratio versus BMI as predictors of cardiac risk in obese adult women. West J Med. 2001; 174: 240–241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Rankinen T, Kim SY, Perusse L, Despres JP, Bouchard C. The prediction of abdominal visceral fat level from body composition and anthropometry: ROC analysis. Int J Obes Relat Metab Disord. 1999; 23: 801–809 [DOI] [PubMed] [Google Scholar]
- 10. Wannamethee SG, Shaper AG, Morris RW, Whincup PH. Measures of adiposity in the identification of metabolic abnormalities in elderly men. Am J Clin Nutr. 2005; 81: 1313–1321 [DOI] [PubMed] [Google Scholar]
- 11. Qin L, Corpeleijn E, Jiang C, et al. Physical activity, adiposity, and diabetes risk in middle-aged and older Chinese population: the Guangzhou Biobank Cohort Study. Diabetes Care. 2010; 33: 2342–2348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Klein BE, Klein R, Lee KE, Jensen SC. Measures of obesity and age-related eye diseases. Ophthalmic Epidemiol. 2001; 8: 251–262 [DOI] [PubMed] [Google Scholar]
- 13. Klein BE, Klein R, Lee KE. Diabetes, cardiovascular disease, selected cardiovascular disease risk factors, and the 5-year incidence of age-related cataract and progression of lens opacities: the Beaver Dam Eye Study. Am J Ophthalmol. 1998; 126: 782–790 [DOI] [PubMed] [Google Scholar]
- 14. Tomany SC, Wang JJ, Van LR, et al. Risk factors for incident age-related macular degeneration: pooled findings from 3 continents. Ophthalmology. 2004; 111: 1280–1287 [DOI] [PubMed] [Google Scholar]
- 15. National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation. 2002; 106: 3143–3421 [PubMed] [Google Scholar]
- 16. Kahn SE, Hull RL, Utzschneider KM. Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature. 2006; 444: 840–846 [DOI] [PubMed] [Google Scholar]
- 17. Wilson PW, D'Agostino RB, Sullivan L, Parise H, Kannel WB. Overweight and obesity as determinants of cardiovascular risk: the Framingham experience. Arch Intern Med. 2002; 162: 1867–1872 [DOI] [PubMed] [Google Scholar]
- 18. Adams MK, Simpson JA, Aung KZ, et al. Abdominal obesity and age-related macular degeneration. Am J Epidemiol. 2011; 173: 1246–1255 [DOI] [PubMed] [Google Scholar]
- 19. Chakravarthy U, Wong TY, Fletcher A, et al. Clinical risk factors for age-related macular degeneration: a systematic review and meta-analysis. BMC Ophthalmol. 2010; 10: 31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Klein R, Deng Y, Klein BE, et al. Cardiovascular disease, its risk factors and treatment, and age-related macular degeneration: Women's Health Initiative Sight Exam ancillary study. Am J Ophthalmol. 2007; 143: 473–483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Park S, Kim T, Cho SI, Lee EH. Association between cataract and the degree of obesity. Optom Vis Sci. 2013; 90: 1019–1027 [DOI] [PubMed] [Google Scholar]
- 22. Sabanayagam C, Wang JJ, Mitchell P, et al. Metabolic syndrome components and age-related cataract: the Singapore Malay eye study. Invest Ophthalmol Vis Sci. 2011; 52: 2397–2404 [DOI] [PubMed] [Google Scholar]
- 23. Seddon JM, Cote J, Davis N, Rosner B. Progression of age-related macular degeneration: association with body mass index, waist circumference, and waist-hip ratio. Arch Ophthalmol. 2003; 121: 785–792 [DOI] [PubMed] [Google Scholar]
- 24. Perissinotto E, Pisent C, Sergi G, Grigoletto F. Anthropometric measurements in the elderly: age and gender differences. Br J Nutr. 2002; 87: 177–186 [DOI] [PubMed] [Google Scholar]
- 25. Regitz-Zagrosek V, Lehmkuhl E, Weickert MO. Gender differences in the metabolic syndrome and their role for cardiovascular disease. Clin Res Cardiol. 2006; 95: 136–147 [DOI] [PubMed] [Google Scholar]
- 26. Chiolero A, Faeh D, Paccaud F, Cornuz J. Consequences of smoking for body weight, body fat distribution, and insulin resistance. Am J Clin Nutr. 2008; 87: 801–809 [DOI] [PubMed] [Google Scholar]
- 27. Galor A, Lee DJ. Effects of smoking on ocular health. Curr Opin Ophthalmol. 2011; 22: 477–482 [DOI] [PubMed] [Google Scholar]
- 28. Klein R, Cruickshanks KJ, Nash SD, et al. The prevalence of age-related macular degeneration and associated risk factors. Arch Ophthalmol. 2010; 128: 750–758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ye J, He J, Wang C, et al. Smoking and risk of age-related cataract: a meta-analysis. Invest Ophthalmol Vis Sci. 2012; 53: 3885–3895 [DOI] [PubMed] [Google Scholar]
- 30. Klein R, Klein BE, Lee KE. Changes in visual acuity in a population. The Beaver Dam Eye Study. Ophthalmology. 1996; 103: 1169–1178 [DOI] [PubMed] [Google Scholar]
- 31. Klein R, Klein BE, Lee KE, Cruickshanks KJ, Chappell RJ. Changes in visual acuity in a population over a 10-year period: the Beaver Dam Eye Study. Ophthalmology. 2001; 108: 1757–1766 [DOI] [PubMed] [Google Scholar]
- 32. Klein R, Klein BE, Lee KE, Cruickshanks KJ, Gangnon RE. Changes in visual acuity in a population over a 15-year period: the Beaver Dam Eye Study. Am J Ophthalmol. 2006; 142: 539–549 [DOI] [PubMed] [Google Scholar]
- 33. Klein BE, Klein R, Linton KL, Magli YL, Neider MW. Assessment of cataracts from photographs in the Beaver Dam Eye Study. Ophthalmology. 1990; 97: 1428–1433 [DOI] [PubMed] [Google Scholar]
- 34. Klein R, Davis MD, Magli YL, Segal P, Klein BE, Hubbard L. The Wisconsin age-related maculopathy grading system. Ophthalmology. 1991; 98: 1128–1134 [DOI] [PubMed] [Google Scholar]
- 35. Klein R, Klein BE. The Beaver Dam Eye Study. Manual of Operations, Revised. Springfield: National Technical Information Service; 1991. [Google Scholar]
- 36. Sahakyan K, Lee KE, Shankar A, Klein R. Serum cystatin C and the incidence of type 2 diabetes mellitus. Diabetologia. 2011; 54: 1335–1340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Klein R, Klein BE, Jensen SC, Meuer SM. The five-year incidence and progression of age-related maculopathy: the Beaver Dam Eye Study. Ophthalmology. 1997; 104: 7–21 [DOI] [PubMed] [Google Scholar]
- 38. Singer JD, Willett JB. Applied Longitudinal Data Analysis: Modeling Change and Event Occurrence. New York: Oxford University Press; 2003. [Google Scholar]